Abstract
Older adults recall less episodically rich autobiographical memories (AM), however, the neural basis of this effect is not clear. Using functional MRI, we examined the effects of age during search and elaboration phases of AM retrieval. Our results suggest that the age-related attenuation in the episodic richness of AMs is associated with difficulty in the strategic retrieval processes underlying recovery of information during elaboration. First, age effects on AM activity were more pronounced during elaboration than search, with older adults showing less sustained recruitment of the hippocampus and ventrolateral prefrontal cortex (VLPFC) for less episodically rich AMs. Second, there was an age-related reduction in the modulation of top-down coupling of the VLPFC on the hippocampus for episodically rich AMs. In sum, the present study shows that changes in the sustained response and coupling of the hippocampus and PFC underlie age-related reductions in episodic richness of the personal past.
Keywords: Aging, fMRI, Autobiographical memory, Hippocampus, Prefrontal Cortex, Episodic Memory Retrieval, Effective Connectivity
The main effect of healthy aging on autobiographical memory (AM) retrieval is attenuation in episodic richness, which refers to a decrease in the ratio of specific episodic details compared to broad semantic information. Although this behavioral effect has been observed in several studies (Levine, Svoboda, Hay, Winocur, & Moscovitch, 2002;P. Piolino, Desgranges, Benali, & Eustache, 2002; St. Jacques & Levine, 2007) its neural mechanisms are largely unknown. In particular, it is unknown when the age effect occurs during retrieval. A memory cue (Where did I see these data before?) triggers effortful search process guided by semantic knowledge of one’s own life (…the Cognitive Neuroscience Society meeting? …Society for Neuroscience?), which eventually lead to successful recovery of a target memory (in an SfN poster…). Memory for the target might be elaborated by recovering additional episodic details (…it was early in the morning…). In the case of AM, search and elaboration processes can take as long as 15–30 seconds, which allows the use of functional MRI (fMRI) to disentangle the activations associated with these two phases (e.g., Addis, Wong, & Schacter, 2007; Daselaar, et al., 2008). The present fMRI study investigated age-effects on search and elaboration processes during AM retrieval.
The age-related reduction in episodic richness could occur early during retrieval, while one is searching for the target memory, or late during retrieval, while one elaborates upon recovered information. Elaboration processes might be more sensitive to aging because they depend on an interaction between the recovery of specific details mediated by the hippocampus and strategic control processes mediated by the prefrontal cortex (PFC), and both processes, and their associated brain regions, are known to decline with aging (for a review see Dennis & Cabeza, 2008). For example, fMRI studies have shown that hippocampal activity related to recollection is attenuated by aging, such that older adults rely more on familiarity processes associated with other medial temporal lobe (MTL) regions (e.g., Cabeza, et al., 2004; Daselaar, Fleck, Dobbins, Madden, & Cabeza, 2006). Aging also involves changes in frontal activation, with under-recruitment observed during memory conditions that lack environmental support (e.g., Logan, Sanders, Snyder, Morris, & Buckner, 2002; Paxton, Barch, Racine, & Braver, 2008), as well as compensatory over-recruitment (Cabeza, 2002). Furthermore, episodic richness is mediated by activations that occur late during AM retrieval (Cabeza & St. Jacques, 2007; Svoboda, McKinnon, & Levine, 2006), and relies on the recruitment of additional self-initiated retrieval processes (Daselaar, et al., 2008). In contrast, an age-related reduction in early search processes might be less likely because AM search tends to be guided by semantic memory (Conway & Pleydell-Pearce, 2000), which is a memory function relatively well preserved in older adults (Craik & Jennings, 1992). In sum, aging may have less impact on the search strategy and cue specification processes in AM that are guided by semantic information, but have greater impact on the elaboration of AM which involves online working memory processes that interact with the recovery of episodic information.
To investigate the neural basis of age-related differences in episodic richness during search and elaboration phases of AM retrieval we used a self-paced design in which young and older adults searched for an AM elicited by a generic cue word, pressed a key when the memory was found, and then elaborated on the memory until the end of the trial. After scanning, participants verbally described the memories they recalled in the scanner and objective analysis of these descriptions were used to determine episodic richness. We investigated the hypothesis that age-related declines in episodic richness are associated with reduced recovery of specific details mediated by the hippocampus and strategic control processes mediated by the PFC during elaboration.
1. Methods
1.1. Participants
There were seventeen young (18 – 35 years of age) and sixteen older participants (60 – 75 years of age), who were healthy, right-handed and without history of neurological or psychiatric episodes. All participants reported that they were not taking medication known to affect cognitive function, and older adult participants were screened for uncontrolled hypertension. Participants gave written informed consent for a protocol approved by the Duke University Institutional Review Board. One young adult and one older adult were excluded due to symptoms of depression as indicated by scores > 13 on the Beck Depression Inventory (BDI; Beck & Steer, 1978). Furthermore, two young adults and one older adult were excluded from the analyses because of problems with completing the task as instructed. Thus, the reported results are based on data from fourteen young (7 females; mean Age = 24.43, SD = 3.73) and fourteen older (6 females; mean Age = 64.21, SD = 2.86) participants.
Demographic and psychometric data (see Table 1) were obtained in a separate session within one week of the scanning session. All participants had obtained at least a secondary school education (12 years). Participants scored a minimum of 28 on the Mini-Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975) and a maximum of 11 on the BDI. There were no age-related differences in the number of years of education, MMSE, BDI, the Weschler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999), verbal fluency (FAS), or categorical fluency (animal names and supermarket items).
Table 1.
Participant Variables by Age Group
| M |
SD |
t (26) |
|||
|---|---|---|---|---|---|
| Young | Older | Young | Older | Y vs. O | |
| Age (years) | 24.43 | 64.21 | 3.73 | 2.86 | 31.79** |
| Education (years) | 16.50 | 17.43 | 2.28 | 1.91 | 1.17 |
| MMSE | 28.71 | 28.50 | 0.61 | 0.76 | −0.82 |
| BDI | 3.93 | 5.07 | 3.29 | 3.47 | 0.89 |
| WASI-Full IQ | 122.36 | 126.00 | 7.66 | 10.07 | 1.08 |
| WASI-Verbal IQ | 120.93 | 123.79 | 7.87 | 7.37 | 0.99 |
| WASI-Performance IQ | 118.14 | 122.00 | 7.49 | 11.79 | 1.03 |
| Verbal Fluency | 47.43 | 42.79 | 10.87 | 16.12 | −0.89 |
| Categorical Fluency | 48.79 | 41.21 | 13.55 | 11.78 | −1.58 |
p < .05,
p < .0001
1.2. Materials
Memory cues consisted of 60 emotionally arousing words selected from the affective norms for English words (ANEW) database (Bradley & Lang, 1999), such that there were 30 positive (Valence mean = 7.93, SD = 0.45; Arousal Mean = 5.96; SD = 0.83) and 30 negative (Valence Mean = 2.17, SD = 0.52; Arousal Mean = 6.00; SD = 1.03) words that were equally arousing. In order to create auditory cues the words were recorded in a female voice and constrained to an equal duration of 1 s.
1.3. Procedure
The procedure was similar to Daselaar et al. (2008) (also see Greenberg, et al., 2005). During scanning participants were asked to search for AMs triggered by the auditory cue words. Participants were instructed to retrieve an AM that was specific to time and place. They indicated when a specific AM was found by making a response on the button-box and then continued to elaborate on the retrieved event in as much detail as possible for the rest of the trial. Thirty seconds following the onset of the auditory cue participants were given auditory instructions to rate the amount of emotion (−4 = negatively arousing to +4 = positively arousing) and reliving (1= low to 8=high) associated with the memory on an 8-point scale. Rating responses were self-paced (up to 6 s) and separated by at least 0.5 s. Participants underwent extensive training to familiarize them with the timing of the trials. There were 6 functional runs, with 10 memory cues in each run (5 positive words and 5 negative word), and an inter-trial interval at least 1.5 to 7.5 s. Participants were instructed to keep their eyes closed for the duration of each run.
Immediately following the scanning session participants were asked to provide a short title for the memory retrieved during scanning and to answer additional questions on a subset of the autobiographical memory questionnaire (e.g., Rubin, Schrauf, & Greenberg, 2003). Participants were asked to date when the event had occurred (e.g., within the last day to > 10 years ago), to indicate the amount of vividness or how clearly the event was remembered, the perspective or whether the memory was seen through their own eyes or through the eyes of an outside observer, the significance of the memory, and the physiological response during retrieval (e.g., heart pounding, etc.). Also, given that AM comprises many different types of events (Brewer, 1986; St. Jacques & Cabeza, in press) we asked participants to indicate whether the type of memory retrieved was a unique event (referring to a particular time and place), repeated event (memory for an event with multiple occurrences), extended event (occurring longer than one day), or semantic information (long-standing facts about one’s own life).
Within two days of the scanning session, participants returned for an additional session in which they were cued by the title they had provided following the scanning session and asked to verbally recall the memories retrieved during scanning. The relatively short amount of time between scanning and verbal recall along with the use of idiosyncratic event titles helped to minimize potential changes in the properties of the memories between the two sessions. Participants were instructed to freely recall for at least one minute and were not provided with retrieval support from the experimenter. Following transcription each memory was coded for the number of episodic details and semantic details (similar to Levine, et al., 2002). Raters were blind to the age-group of the participant. Episodic details reflected information that was specific to time and place, whereas semantic details reflected long-standing factual information about one’s self or the world. Details were tallied for each category and the proportion of episodic details to the total details (episodic + semantic) was calculated for each memory in each subject as an estimate of the amount of episodic re-experiencing unbiased with respect to differences in protocol length.
1.4. fMRI Methods
1.4.1. Image Acquisition
Scanning was conducted using a 4T GE magnet. Auditory stimuli were presented using headphones and behavioral responses were recorded using an eight-button fiber optic response box (Resonance Technology, Northridge, CA). Head motion was minimized using foam pads and a headband. Anatomical scanning started with a T1-weighted sagittal localizer series, and then 3D fast spoiled gradient echo recalled (SPGR) structural images were acquired in the coronal plane (2562 matrix, TR = 12.3 ms, TE = 5.4 ms, flip angle = 20°, FOV = 240, 68 slices, 1.9 mm slice thickness). Coplanar functional images were subsequently acquired using an inverse spiral sequence (642 image matrix, TR = 2000 ms, TE = 6 ms, FOV = 240, flip angle = 60°, 34 slices, 3.8 mm slice thickness, and 3.8 × 3.8 × 3.8 mm voxel size).
1.4.2. Image Processing
Image processing and analyses were performed using Statistical Parameter Mapping software in Matlab (SPM5; Wellcome Department of Imaging Neuroscience). Functional images were corrected for slice acquisition order, realigned to correct for motion artifacts, and then spatially normalized to a standard stereotactic space, using the template implemented in SPM5. No re-sampling was conducted during normalization. Subsequently, the functional images were spatially smoothed using an 8 mm isotropic Gaussian kernel. Coordinates are reported in Talairach space using a transformation from the Montreal Neurological Institute coordinates (Brett, Christoff, Cusack, & Lancaster, 2001).
1.4.3. fMRI Analyses
To account for the fact that we used a self-paced paradigm in which participants indicated when they recalled a specific event, we implemented a flexible fMRI design in the context of the general linear model (GLM). The design distinguished six components in each trial: four transient and two sustained regressors. Transient regressors included the memory cue (immediately at onset of the trial), response-related decision processes (750 msec before the response indicating a memory was recalled), and the two ratings (second and third response). Sustained regressors included the memory search period (from trial onset to response) and the elaboration period (from response to the first rating). The transient regressors were modeled by convolving a canonical hemodynamic response function with a vector representing period onsets, while the sustained regressors were modeled by convolving the hemodynamic response function with a vector of a length that varied with that of the search and elaboration phases on each trial. In order to account for differences in the timing of activations due to the self-paced design, the response indicating that a memory was accessed determined the duration of the memory access period as well as the onsets of the response and elaboration periods. The use of the self-paced design reduced potential issues of multicollinearity between the regressors, and as an additional check we directly evaluated this by calculating the variance inflation factor (VIF; J. Cohen, Cohen, West, & Aiken, 2003). For each regressor in each run and each participant, we extracted R2, the squared multiple correlation between a single regressor and the other regressors, using Design Magic (http://www.matthijs-vink.com/tools.html) and computed the VIF (1/(1−R2)). The standard cut-off value of 10 (J. Cohen, et al., 2003) was used to exclude potential runs where multicollinearity between the regressors was an issue (e.g., Scheibe, et al., 2006). On the basis of these results, only 4 runs were potentially problematic and these runs were excluded from the analysis. Data were high pass filtered using a cutoff of 128 Hz, and global effects were removed (non-proportional scaling). Head motion was assessed prior to pre-processing, and no individual moved more than 3 mm in any direction, in any run.
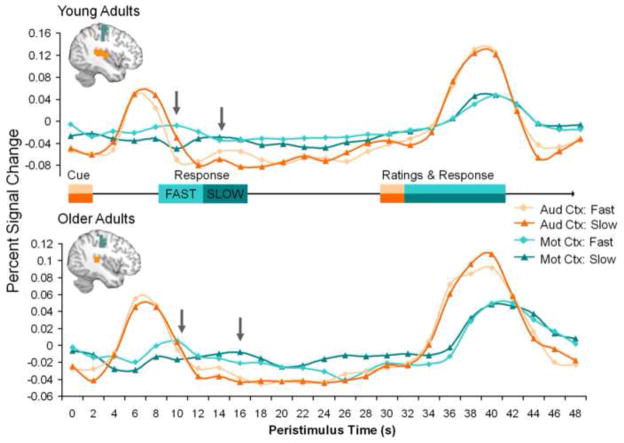
To assess the validity of our flexible fMRI design (i.e., accuracy of the regressors and potential age-related differences in the timing of the hemodynamic response function), we investigated the time courses for fast and slow responses with respect to the self-paced button press indicating that a memory was formed. First, fast and slow responses were determined with respect to the mean reaction time in each subject. Second, a GLM was created in which trial onsets time-locked to the cue were modeled with a Finite Impulse Response basis set of peristimulus time bins of 2 s duration (equal to the TR) within each participant. The resulting parameter estimates were subsequently averaged across subjects for each peristimulus time bin, yielding estimates of fMRI signal change across the whole 48 s trial period for both fast and slow bins. Finally, for the actual validation, we used a region of interest (ROI) approach, focusing on auditory and motor cortex defined using the Talairach Daemon Atlas (Lancaster, Summerin, Rainey, Freitas, & Fox, 1997; Lancaster, et al., 2000) implemented with PickAtlas software (Maldjian, Laurienti, Kraft, & Burdette, 2003). The clusters showing significant (p < 0.005, cluster size > 10) cue-related activity in the auditory cortex or response-related activity in the motor cortex based on the GLM analysis were defined as ROIs. These results show that, in both age groups, cue-related activity was not modulated by response time, whereas the peak of response-related activity shifted depending on whether it was a fast or a slow response (see Figure 1). Furthermore, the young and older adults showed similar peaks in auditory cortex, and a similar average peak response in the motor cortex. These findings are important because they potentially suggest that age-related differences in other brain regions, such as the hippocampus and the frontal lobes, may not be simply due to a global effect of aging on the hemodynamic response.
Figure 1.
Time Courses for Fast and Slow Responses. The time courses for fast and slow responses were nearly identical in the auditory cortex (aud ctx, orange/peach lines), and did not differ between age groups. In contrast, the time course for fast and slow responses was very different in the motor cortex (mot ctx, blue lines). The differences in peak latency in the motor cortex (gray arrows) for fast trials (light colors) and slow trials (dark colors) shows similar peaks in each age group, although there was a trend for slower responses in the older adults.
1.4.4. Age-Related Effects During AM Retrieval
In order to determine the impact of aging on search and elaboration phases of AM retrieval we examined both age-invariant effects and age-related differences in each phase. For assessing age-invariant effects associated with the search and elaboration phases, a conjunction map was created thresholding each age group’s random effects separately for each contrast (i.e., Elaboration or Search vs. implicit baseline) at p = .005 and 10 voxels and then exclusively masking with the age-related differences at p = .005. This procedure yielded an activation map containing only those voxels that showed age-invariant effects during the search and elaboration phases. We assessed brain areas showing age-related differences by using a two sample t-test to compare young and older adults separately during the search and elaboration phases (p = .005, with a cluster size > 10 voxels), and then inclusively masked this statistical image with the corresponding statistical map for the group of interest at p = .005. Thus, the resulting pattern of age-related differences in activity had to pass two hurdles: 1) the activation should be significant within one group, and 2) the activation should be significantly larger within one group compared to the other group. The threshold for whole-brain analysis was chosen to control for both Type I and Type II errors rates (Lieberman & Cunningham, 2009). Given the a priori role of the hippocampus in AM retrieval (Cabeza & St. Jacques, 2007; Maguire, 2001; McDermott, Szpunar, & Christ, 2009; Spreng, Mar, & Kim, 2009; Svoboda, et al., 2006) and our predictions regarding its involvement in aging, we conducted an additional ROI analysis (p = .01) to examine both age-invariant and age-related differences using the Talairach Daemon Atlas Labels (Lancaster, et al., 1997; Lancaster, et al., 2000) implemented with PickAtlas software (Maldjian, et al., 2003).
1.4.5. Hippocampal-PFC Coupling
In order to examine the effective connectivity between the hippocampus and PFC, we used dynamic causal modeling (DCM) implemented in SPM5 (Friston, et al., 2003). First, we created two volumes of interest (VOIs) in the hippocampus and VLPFC, based on the peaks from the age-related differences during elaboration and the sensitivity of these regions to episodic richness, using a sphere with an 8 mm radius, which were adjusted for the task design (i.e., other contrasts in the model) in order to isolate the specific contrast of interest. Second, for each individual subject and replicated over the six sessions, we created a model space that included iterations for the location of the driving input and modulatory inputs by episodic richness on possible intrinsic connections between each region. Episodic richness was determined as those trials with a greater proportion of episodic to semantic details (i.e., > 50%). Plausible models included combinations of the following manipulations: driving inputs (in the hippocampus, VLPFC or both regions), intrinsic connections (reciprocal, bottom-up only, top-down only) and modulatory inputs (reciprocal, bottom-up only, top-down only). The final model space included 27 models that were estimated for each participant and replicated over the six sessions. Third, we used a Bayesian Model Selection (BMS) implemented in SPM8 to select an optimal model separately within each age-group using random effects (Stephan, Penny, Daunizeau, Moran, & Friston, 2009). BMS involves calculating the probabilities of each of the models given the data and bases statistical inference on the value of the exceedance probability, which refers to the probability that a particular model is more likely than other models in the space. Finally, to assess group effects, the resulting individual participant coefficients were submitted to a two-sample t-test at p < .05.
2. Results
2.1. Behavioral Results
Participants were able to recall an event matching the cue on more than 95% of trials (see Table 2 for mean behavioral scores, standard deviations, t-scores, p-values, and effect sizes). There were no age-related differences in the online ratings of reliving or emotional arousal. However, older adults were slower to make emotional ratings and there was a trend for slower retrieval of AMs compared to young adults.
Table 2.
Mean behavioral responses by Age Group
| M |
SD |
t (26) |
Effect Size |
|||
|---|---|---|---|---|---|---|
| Young | Older | Young | Older | Y vs. O | Cohen’s D | |
| Scanning | ||||||
| % AMs | 0.99 | 0.97 | 0.02 | 0.03 | 1.45 | 0.57 |
| Reaction Time (s) | 6.55 | 8.68 | 2.11 | 3.62 | −1.90 | −0.72 |
| Reliving | ||||||
| Rating | 5.03 | 5.26 | 1.07 | 0.93 | −0.62 | −0.23 |
| Reaction Time (s) | 2.49 | 2.96 | 0.60 | 0.66 | −2.00 | −0.75 |
| Emotion | ||||||
| Rating | 2.53 | 2.28 | 0.33 | 0.37 | 1.74 | 0.71 |
| Reaction Time (s) | 2.14 | 2.67 | 0.67 | 0.63 | −2.09 * | −0.81 |
| Post-Scanning Ratings | ||||||
| Vividness | 4.69 | 4.70 | 0.78 | 1.01 | −0.42 | −0.01 |
| Significance | 3.00 | 3.23 | 0.79 | 0.95 | −0.68 | −0.26 |
| Physiological Response | 2.31 | 1.73 | 0.99 | 0.77 | 1.74 | 0.65 |
| Perspective | ||||||
| Own Eyes | 5.78 | 5.44 | 0.83 | 0.91 | −1.02 | 0.39 |
| Observer | 2.37 | 2.38 | 0.92 | 0.90 | 0.05 | −0.01 |
| Date of Memory (%) | ||||||
| day | 0.05 | 0.03 | 0.04 | 0.04 | 0.92 | 0.50 |
| week | 0.09 | 0.06 | 0.06 | 0.05 | 1.50 | 0.54 |
| month | 0.12 | 0.06 | 0.08 | 0.07 | 2.29 * | 0.80 |
| year | 0.26 | 0.15 | 0.09 | 0.08 | 3.20 *** | 1.29 |
| 5 years | 0.25 | 0.13 | 0.11 | 0.09 | 2.93 ** | 1.19 |
| 10 years | 0.14 | 0.15 | 0.08 | 0.11 | −0.16 | −0.10 |
| > 10 years | 0.09 | 0.41 | 0.06 | 0.19 | −6.07 **** | −2.27 |
| Memory Type (%) | ||||||
| Unique | 0.81 | 0.62 | 0.15 | 0.19 | 2.96 ** | 1.11 |
| Repeated | 0.14 | 0.24 | 0.09 | 0.14 | −2.22 * | −0.85 |
| Extended | 0.05 | 0.12 | 0.06 | 0.15 | −1.66 | −0.61 |
| Fact | 0.002 | 0.02 | 0.01 | 0.03 | −1.93 | −0.80 |
| Verbal Recall | ||||||
| Episodic Richness (%) | 0.72 | 0.51 | 0.09 | 0.17 | 4.05 **** | 1.54 |
| Total Number of Details | 17.10 | 19.15 | 3.22 | 7.02 | −0.99 | 0.38 |
p < .05,
p < .01,
p < .005,
p < .0005
As predicted the older adults showed a reduction in the episodic richness, the proportion of episodic details to the total number of episodic and semantic details, during the retrieval of AMs, t (26) = 4.05, p < .0005. There were no differences between the age-groups in the overall number of episodic and semantic details recalled, t (26) = 0.99, p = .33, suggesting that the reported differences cannot be explained by group differences in verbosity. The values of the episodic richness scores indicated that memories retrieved by the older adults (mean = 0.51 SD = 0.17) were 20% less episodically rich compared to young adults (mean =0.72, SD = 0.09), and the effect size, d = 1.54, is large according to Cohen’s standards (J. Cohen, 1988). The large effect size of the age-related differences in episodic richness compared to the small effect size of the reliving ratings (d = −.23) suggests that the latter were not sensitive enough to detect age effects (also see Rubin & Schulkind, 1997). Consistent with the finding of an age-related reduction in episodic richness, the older adults reported that they recalled a smaller proportion of unique AMs and a greater proportion of repeated AMs and semantic memories compared to young adults. There were no age-related differences in post-scan ratings of vividness, significance of the memory, physiological responses or memory perspective. Although there were no age-related differences in the proportion of memories retrieved from the most recent periods (i.e., day, week), older adults retrieved more remote memories (i.e., > 10 years), and young adults recalled more events from other periods (i.e., month, year, 5 years). The age-related difference in episodic richness could be related to the observed age-related differences in remoteness (Cabeza & St. Jacques, 2007; Moscovitch, et al., 2005). However, there was no intra-individual correlation between remoteness and episodic richness when collapsed across age, r = −.04, t (26) = −1.20, p = .24, or separately in young adults, r = −.05, t (13) = −.03, p = .54, or older adults, r = −.05, t (13) = −1.32, p = .21, which suggests that more remote memories were not necessarily less episodically rich. This result might seem unexpected, because remote memories are sometimes associated with less episodic richness (Alvarez & Squire, 1994; Moscovitch, et al., 2005). However, in the present study, remote memories were also self-selected, very accessible, and potentially more detailed than if retrieval was constrained to a specific lifetime and remote period (e.g., G. Cohen & Faulkner, 1988; Dijkstra & Kaup, 2005; Rubin & Schulkind, 1997); thus, it is not so surprising that these remote events would vary in episodic richness. In sum, the behavioral results show that the episodic richness of AMs was greatly reduced in older adults. Below, we turn to the fMRI results to elucidate the potential neural bases underlying this age-related reduction in episodic richness.
2.2. fMRI Results
2.2.1 Age-invariant effects during AM retrieval
During AM retrieval several age-invariant effects were observed during the search phase (see Table 3). During search, both young and older adults recruited a number of the regions frequently found in AM retrieval (e.g., Cabeza & St. Jacques, 2007; Svoboda, et al., 2006) including the left VLPFC, left hippocampus, bilateral temporopolar cortex, bilateral retrosplenial cortex, and left parahippocampal cortex. During elaboration, a subset of these age-invariant search regions continued to remain online in both groups including the left parahippocampal cortex and the left retrosplenial cortex, and there was additional age-invariant recruitment of the left ventral parietal cortex (VPC). However, the pattern of age-related differences during elaboration suggested that the overall amount of activity recruited by the older adults was reduced when compared to the young adults.
Table 3.
Age-Invariant Effects
| Region | BA | H | Young | Older | Voxels | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | T | x | y | z | T | ||||
| Search | |||||||||||
| Ventrolateral PFC | 45 | L | −45 | 19 | 17 | 8.22 | −41 | 23 | 13 | 5.55 | 276 |
| 47 | L | −33 | 22 | −4 | 7.14 | −48 | 29 | −11 | 5.34 | 276 | |
| Anterior Cingulate | 32 | L | −7 | 20 | 41 | 10.47 | −15 | 13 | 38 | 6.50 | 48 |
| Temporopolar Cortex | 38/21 | L | −52 | 3 | −16 | 6.06 | −52 | 3 | −19 | 4.61 | 73 |
| 38/21 | R | 52 | −1 | −16 | 6.71 | 56 | 3 | −19 | 5.06 | 75 | |
| * Hippocampus | -- | L | −26 | −22 | −9 | 3.70 | −19 | −26 | −8 | 4.35 | 21 |
| Parahippocampus | -- | L | −19 | −37 | −8 | 3.43 | −15 | −37 | −8 | 6.49 | 39 |
| Retrosplenial Cortex | 30 | L | −22 | −51 | 10 | 5.37 | −11 | −54 | 10 | 5.34 | 83 |
| 30 | R | 15 | −44 | 2 | 4.91 | 11 | −47 | 2 | 5.86 | 18 | |
| Cuneus | 17/18 | R | 4 | −94 | 5 | 5.81 | 11 | −94 | 5 | 5.34 | 20 |
| Midbrain | L | −4 | −33 | −14 | 4.83 | −7 | −30 | −14 | 5.00 | 39 | |
| Elaboration | |||||||||||
| Retrosplenial Cortex | 29/23 | L | −7 | −58 | 13 | 9.24 | −11 | −54 | 13 | 3.72 | 50 |
| * Hippocampus | -- | L | −26 | −15 | −19 | 4.71 | −26 | −19 | −15 | 4.12 | 6 |
| Parahippocampus | -- | L | −26 | −37 | −11 | 6.82 | −26 | −30 | −14 | 4.17 | 19 |
| Ventral Parietal Cortex | 39 | L | −48 | −71 | 32 | 5.25 | −48 | −68 | 24 | 3.88 | 20 |
Talaraich Coordinates Reported. BA, Brodmann’s Area; H, Hemisphere.
Region of Interest
2.2.2. Age-related differences during AM retrieval
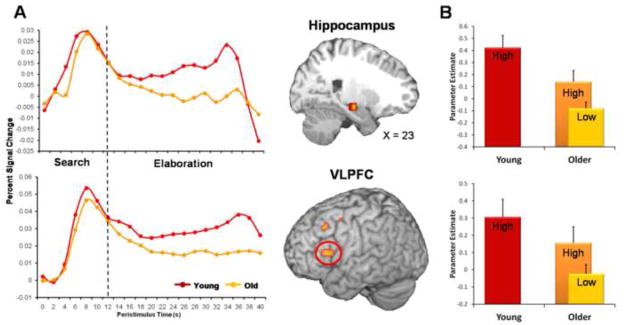
Age-related differences were primarily observed during elaboration (see Table 4). Compared to the age-invariant activity observed during the search phase, older adults showed less activity in the right hippocampus and left VLPFC (see Figure 2-A). The reduction in recruitment of the right hippocampus and VLPFC suggests that fewer details may have been generated during elaboration in the older adults. Consistent with this idea, there was an age-related reduction in the additional recruitment of the right VPC, suggesting less internal attention driven by the recovery of memory details (Cabeza, Ciaramelli, Olson, & Moscovitch, 2008). Furthermore, there were additional age-related reductions observed in the recruitment of the left posterior cingulate, a region associated episodic memory retrieval and self-referential processes (Buckner & Carroll, 2007; Wagner, Shannon, Kahn, & Buckner, 2005), and left DLPFC, associated with manipulation of the products of retrieval (Petrides, 2005). These results suggest an alteration in the recruitment of the AM retrieval network during elaboration of AMs in older adults, which might contribute to reductions in episodic richness. In sum, these findings are consistent with the idea that aging strongly impacts the rich elaboration of AMs.
Table 4.
Age-Related Differences
| Region | BA | H | x | y | z | T | Voxels |
|---|---|---|---|---|---|---|---|
| Search | |||||||
| Young > Older | |||||||
| Dorsomedial PFC | 8/32 | L | −7 | 20 | 44 | 4.39 | 47 |
| Older > Young | |||||||
| No Significant Voxels | |||||||
| Elaboration | |||||||
| Young > Older | |||||||
| Ventrolateral PFC | 45 | L | −56 | 19 | 10 | 3.34 | 11 |
| Dorsolateral PFC | 9 | L | −33 | 24 | 37 | 3.96 | 18 |
| Dorsomedial PFC | 8/6 | C | 0 | 21 | 55 | 3.54 | 11 |
| Premotor Cortex | 6 | L | −41 | −1 | 46 | 3.15 | 11 |
| Middle Temporal Cortex | 21 | R | 56 | −12 | −12 | 3.44 | 15 |
| Ventral Parietal Cortex | 40/22 | R | 52 | −43 | 16 | 4.42 | 22 |
| * Hippocampus | -- | R | 26 | −15 | −12 | 3.01 | 6 |
| Posterior Cingulate | 31 | L | −11 | −46 | 34 | 5.44 | 175 |
| Older > Young | |||||||
| No Significant Voxels | |||||||
Talaraich Coordinates Reported. BA, Brodmann’s Area; H, Hemisphere.
Region of Interest
Figure 2.
Age Effects During Autobiographical Memory (AM) Retrieval. (A) Age-invariant activity was observed in the right hippocampus and left ventrolateral prefrontal cortex (VLPFC) during search, but older adults showed reductions in this region during elaboration. Dotted line represents the peak of the average motor cortex response for the button press. Clusters of activation show areas where, compared to young adults, older adults had less recruitment during elaboration. (B) Age effects in the right hippocampus and left VLPFC were attenuated for highly episodic rich AMs. High and low refer to episodic richness.
We conducted two ancillary analyses in order to confirm that the age-related reduction during elaboration was linked to older adults’ overall reduction in episodic richness and difficulty in engaging strategic retrieval processes. First, we examined activity only on those trials associated with higher episodic richness, which included AMs containing more episodic details than semantic details (i.e., > 50% episodic richness). Thus, this additional analysis included the majority of AMs recalled in young adults (81%), but only a subset of the memories recalled by older adults (55%). The fMRI analysis revealed that when both groups recalled more episodically rich AMs there were fewer age-related differences during elaboration. Along with the regions previously showing age-invariant activity across all trials during elaboration (see Table 3), episodically rich AMs were also associated with age-invariant effects in the right hippocampus and left VLPFC (see Figure 2-B). These results suggest that the aforementioned age-related reductions in the right hippocampus and left VLPFC were related to older adults’ overall difficulty in elaboration of episodically rich AMs. A few regions continued to show age-related reductions during the elaboration of episodically rich AMs, including the left posterior cingulate, left DLPFC, and dorsomedial PFC (see Table 4). Additional age-related differences were also observed in the left anterior PFC (Brodmann’s Area 9; Talairach coordinates: X = −15, Y = 53, Z = 32; T = 3.31, 11 voxels). In sum, these findings show that older adults recruited the right hippocampus and left VLPFC to a greater extent, which was equivalent to activation levels in young adults, on those trials in which they recalled more episodically rich AMs.
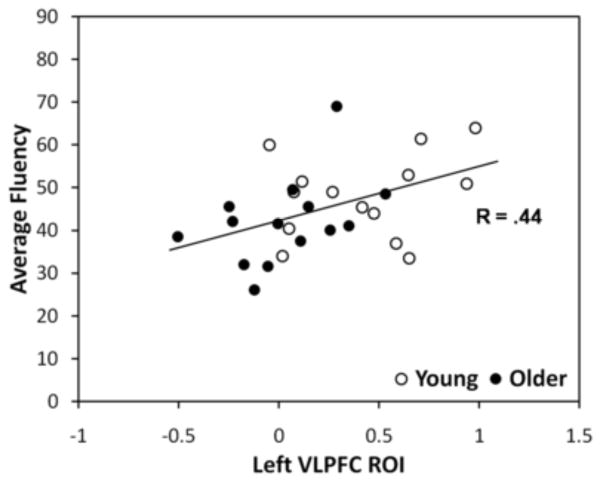
Second, we examined whether individual differences in strategic retrieval processes could account for group differences in the recruitment of the VLPFC during elaboration of AMs. Phonological and categorical fluency are associated with strategic retrieval processes involving the VLPFC (e.g., Gold & Buckner, 2002), and performance on these measures may account for age-related differences in the recall of AMs (Addis, Sacchetti, Ally, Budson, & Schacter, 2009). Further, in the current study we found a significant correlation between average fluency scores and the mean response in the VLPFC (see Figure 3), R = .44, p < .05. Based on these observations, we included phonological and categorical fluency scores as covariates at the second level using an ANCOVA model to determine whether individual differences on strategic retrieval processes could account for the age-related differences in the VLPFC during elaboration. The results of the covariate analysis revealed that including categorical and phonological fluency reduced the age-related differences in the VLPFC, such that it was no longer significant at the specified threshold. This result suggests that the recruitment of the VLPFC may reflect strategic retrieval or controlled processes that operate during the elaboration of AMs and which are reduced in older adults. In contrast, the fluency measures did not account for the age-related effects observed in the hippocampus or the posterior cingulate, although it did somewhat reduce the spatial extent of age-related differences in the DLPFC (from 18 to 8 voxels). In sum, these results support the interpretation that older adults rely less on strategic retrieval processes, as reflected by age-related reductions in the VLPFC during elaboration, which potentially contribute to the reduced episodic richness of AM.
Figure 3.
Relationship Between Fluency and Ventrolateral Prefrontal Cortex (VLPFC). There was a significant correlation between the average fluency score (phonological and categorical) and the mean response in the VLPFC during elaboration. Activity in the VLPFC is expressed as a Beta Estimate. ROI = Region of Interest.
2.2.3. Hippocampal-PFC Coupling
The analyses above indicate that the right hippocampus and left VLPFC were the only regions showing both significant age-related reductions during elaboration and sensitivity to episodic richness. Given that these two brain regions may interact during episodic retrieval, our next fMRI analyses investigated the effect of aging on the coupling between these regions. It is important to note that the level of activity is not directly dependent upon co-activation among brain regions (Nyberg, et al., 1996; Stephan, 2004). Although the right hippocampus and left VLPFC showed less activity during elaboration in the older adults, there was sufficient variance within each of these regions for effective connectivity analysis, as indicated by the observation of age-invariant effects here for highly episodically rich AMs.
We used DCM in order to distinguish potential age-related differences in the direction of the hippocampal-PFC coupling (i.e., bottom-up vs. top-down) and the influence of episodic richness. Bayesian Model Selection revealed the identical optimal model within each age group (for means and SD of parameters see Table 5). A DCM (see Figure 4) with a driving input on the VLPFC, reciprocal intrinsic connections between the hippocampus and the VLPFC, and modulation of episodic richness on the top-down influence of the VLPFC on the hippocampus, was the best model in both young adults (exceedance probability = 0.63) and older adults (exceedance probability = 0.15). These results show that in both age-groups it is the top-down rather than the bottom-up influence of the PFC on the hippocampus that modulates the episodic richness of AMs, which suggest that strategic retrieval processes importantly contribute to the recall of specific details during elaboration. Although the identical model was optimal in both age groups, there were age-related differences in the strength of the model parameters. There was a significant age-related reduction in the modulation of episodic richness by the top-down influence of the VLPFC on hippocampus, t (26) = 2.89, p < .01. Additionally, there were also age-related reductions in the driving input of the VLPFC, t (26) = 3.29, p < .01. These results suggest that the age-related reduction in episodic richness is associated with a decrease in the top-down modulation of the hippocampus by the VLPFC.
Table 5.
Parameters in the Dynamic Causal Model
| Driving Input (C ) | VLPFC to HIPP (A) | HIPP to VLPFC (A) | Episodic Richness (B) | ||
|---|---|---|---|---|---|
| Young | Mean | 0.016 | 0.089 | 0.047 | 0.101 |
| SD | 0.013 | 0.083 | 0.050 | 0.100 | |
| Older | Mean | 0.003 | 0.043 | 0.018 | 0.008 |
| SD | 0.007 | 0.050 | 0.040 | 0.067 |
Figure 4.
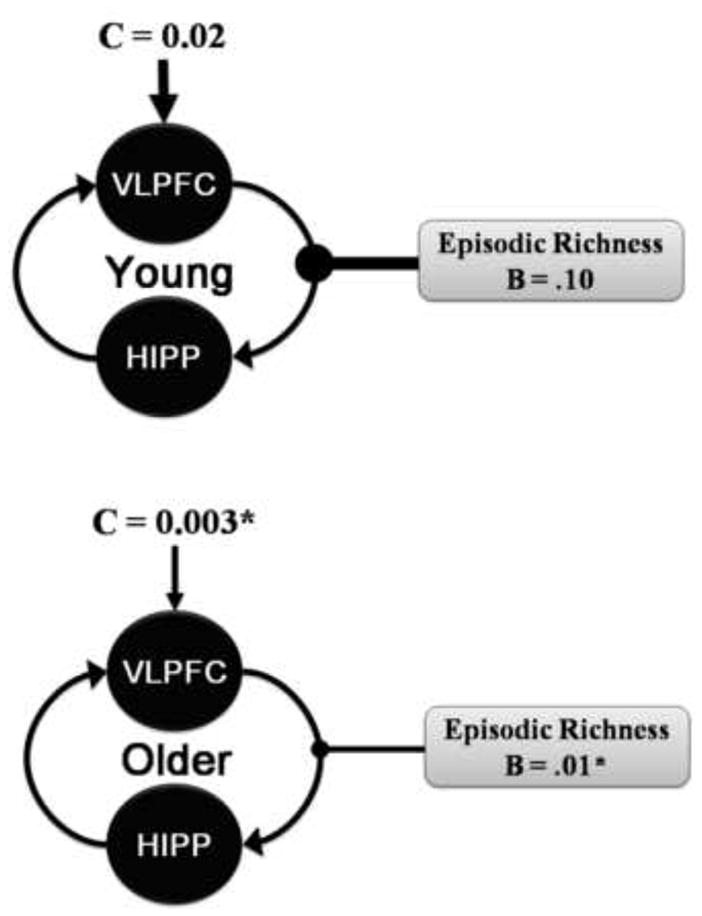
Age-Related Differences in the Dynamic Causal Model (DCM). A similar DCM was observed in both young and older adults. However, older adults showed a reduction in modulation of episodic richness by top-down influence of the VLPFC on hippocampus. There was also an age-related reduction in the driving input on the VLPFC. B = value of the modulatory input, C = value of the driving input.
3. Discussion
The results of the present study suggest that the age-related attenuation in the episodic richness of AMs is associated with difficulty in the strategic retrieval processes that guide the recovery of information during elaboration. There were two main findings supporting this idea. First, age-related differences in functional activations were greater during elaboration than during search in the hippocampus and VLPFC. The age-related differences were attenuated for highly episodically rich AMs and when individual differences in strategic retrieval processes were included as covariates in the analysis. Second, there was an age-related reduction in the amount of top-down modulation of the VLPFC on the hippocampus by episodic richness during elaboration. These findings are discussed in separate sections below.
3.1. Age-Related Effects During Autobiographical Memory Retrieval
To our knowledge this is the first fMRI study to examine age-invariant and age-related differences in search and elaboration processes underlying AM retrieval. We found that aging had less of an impact on functional activations during the initial search process, but a greater impact on later elaboration processes. The results of the present study are consistent with evidence from event-related potential studies showing that aging effects later retrieval processes in episodic memory (Trott, Friedman, Ritter, & Fabiani, 1997; Trott, Friedman, Ritter, Fabiani, & Snodgrass, 1999; Wegesin, Friedman, Varughese, & Stern, 2002) (although see Li, Morcom, & Rugg, 2004; Mark & Rugg, 1998), and to functional neuroimaging studies showing age-invariant effects in the retrieval network (Duverne, Habibi, & Rugg, 2008; Maguire & Frith, 2003; Morcom, Li, & Rugg, 2007), but they extend these findings by linking the age-related effects to both spatial and temporal components of retrieval.
The current results show preservation in older adults in the recruitment of search processes relying on semantic information (i.e., temporopolar cortices) and to the access of the memory trace (i.e., hippocampus and retrosplenial cortices; Botzung, Denkova, Ciuciu, Scheiber, & Manning, 2008; Conway, Pleydell-Pearce, & Whitecross, 2001; Daselaar, et al., 2008). A subset of the age-invariant regions observed during search continued to remain online during elaboration, in the left hippocampus, left parahippocampal cortex and left retrosplenial cortex. Conjointly, these regions have been linked to scene construction, the ability to generate a complex and coherent scene or event, which is commonly engaged across a number of tasks including AM (Hassabis, Kumaran, Vann, & Maguire, 2007; Hassabis & Maguire, 2007). Thus, both age groups are potentially able to envision the context of the AM.
Older adults, however, had deficits in the recruitment of additional regions that support the rich elaboration of the constructed scene. First, although age-invariant activity was observed in the left hippocampus, older adults showed a reduction in the additional recruitment of the right hippocampus. In contrast to the current findings, Maguire and Frith (2003) found that aging led to a more bilateral pattern of hippocampal activity on an AM recognition task. Several methodological differences in the current study could potentially account for the observed differences such as the trial design (segregation of search and elaboration processes vs. no segregation of retrieval processes), the methodology to elicit memory (generic cues vs. pre-scan interview), the greater number of memories recalled (60 vs. 24), and the nature of retrieval (recall vs. true/false decision). Moreover, several studies have found bilateral recruitment of the hippocampus during AM retrieval in young adults (e.g., Addis, Moscovitch, Crawley, & McAndrews, 2004; Piefke, Weiss, Zilles, Markowitsch, & Fink, 2003; P. Piolino, et al., 2004), and previous functional neuroimaging studies of AM have linked the recruitment of bilateral or right hippocampus to greater episodic richness (e.g., P. Piolino, et al., 2008; Viard, et al., 2007) (also see Graham, Lee, Brett, & Patterson, 2003; Svoboda, et al., 2006). Further, damage to the hippocampus can affect the detailed recall of AMs (e.g., St-Laurent, Moscovitch, Levine, & McAndrews, 2009; Steinvorth, Levine, & Corkin, 2005). Consistent with these ideas, the age-related reduction in the right hippocampus was also coupled with reductions in posterior regions that support the rich elaboration of AMs, such as the posterior cingulate (Addis, et al., 2004; Levine, et al., 2004) (also see Levine, Svoboda, Turner, Mandic, & Mackey, In Press) and the VPC (e.g., Levine, et al., 2004) (Berryhill, Phuong, Picasso, Cabeza, & Olson, 2007). Further, we found age-invariant activity in the right hippocampus when elaboration was restricted to highly episodically rich AMs, suggesting that this region is sensitive to variability in episodic richness.
Second, there was an age-related reduction in the sustained response in the left VLPFC, as well as in the additional recruitment of left DLPFC during elaboration. Lateral ventral and dorsal PFC regions work conjointly during AM retrieval (Cabeza & St. Jacques, 2007) through the controlled recovery and manipulation of information, respectively (Petrides, 2005) (also see Miller & Cohen, 2001). Supporting these roles, during elaboration the recovery of highly episodic rich AMs was associated with age-invariant VLPFC recruitment, whereas DLPFC continued to show an age-related reduction. Further, individual differences in strategic retrieval ability accounted for the age-related effects in the VLPFC, but had less effect on the DLPFC. Together, these results suggest that the VLPFC potentially contributes to age-related reductions in episodic richness via its role in strategic retrieval processing, whereas the DLFPC might contribute to additional age-related differences in the controlled manipulation of recovered information.
In sum, the results of the present study underscore the importance of decomposing the time course of retrieval processes when examining age-related effects by showing a differential pattern of functional activation across search versus elaboration processes in AM retrieval, particularly in the recruitment of the hippocampus and the PFC.
3.2. Age-Related Effects on Top-Down Modulation
The findings from the effective connectivity analysis suggest that, despite a similar pattern of coupling between the hippocampus and VLPFC during elaboration, there was an age-related reduction in top-down modulation associated with episodic richness. The PFC is associated with strategic, controlled processes involving the recovery of information (Petrides, 2005) (also see Miller & Cohen, 2001), which importantly contributes to the recall of episodically rich AMs (e.g., McKinnon, Black, Miller, Moscovitch, & Levine, 2006; Smith, Souchay, & Conway, 2010). Here we show that individual differences in strategic retrieval ability as measured by categorical and phonological fluency accounted for age-related effects in the VLPFC during elaboration. There are several lines of evidence suggesting that aging impacts the strategic, controlled processes mediated by the frontal lobes, possibly as the result of an age-related deficit in dopamine function (for a review see West, 1996). According to the resources deficit theory of cognitive aging (Craik & Byrd, 1982) older adults are impaired on tasks that require self-initiated behavior such that providing environmental support (i.e., retrieval cues) can attenuate age-deficits. Consistent with this latter idea, providing additional retrieval cues during AM recall increases episodic richness (e.g., Levine, et al., 2002). The present findings emphasize that age-related attenuations during elaboration are the result of the interaction between the PFC and hippocampus (cf. West, 1996). The hippocampus and PFC work together during memory retrieval (Moscovitch, 1992), and interactions between these regions are particularly important during more complex recall (for a review see Simons & Spiers, 2003) and contribute to the rich recollection of AMs across the lifespan (Viard, et al., 2010). Further, lesion evidence shows that disconnecting these regions results in AM retrieval deficits (Levine, et al., 1998).
The age-related reduction in the top-down modulation of the PFC on the hippocampus for episodically rich AMs is potentially one factor leading to behavioral differences during AM recall (Levine, et al., 2002; P. Piolino, et al., 2002; P. Piolino, et al., 2006; St. Jacques & Levine, 2007). Previous studies have associated the age-related reduction in the episodic richness of AMs with a more general aging phenomenon that tends to impair memory for specific details, but not memory for global information, and which has been associated with deficits in frontal function (for a review see Craik & Grady, 2002). However, to our knowledge, this is the first study to link the age-related reduction in episodic richness to a decline in the top-down influence of the PFC. Although older adults may recall less episodic information in their AMs, it is important to note that less reliance on specific details may infuse older adults’ AMs with a richer narrative that imparts more wisdom or serves a different purpose (Bluck, 2003; James, Burke, Austin, & Hulme, 1998; Labouvie-Vief, 1982; Mergler & Goldstein, 1983) when compared to the AMs of young adults. In sum, the findings demonstrate that elaboration involves the interaction between the recovery of details mediated by the hippocampus and controlled strategic retrieval processes directed by the PFC, and they suggest that older adults recall less episodically rich AM because of a reduction in top-down modulation.
4. Conclusions
In the present fMRI study, we examined age-related differences in episodic richness on the spatiotemporal dynamics of AM retrieval. Age-related reductions were primarily observed during the later elaboration processes of AM retrieval, with older adults showing a reduction in the recruitment of the hippocampus and PFC. Linking these results to the behavioral reductions in episodic richness in older adults, we found that functional activity in these regions was attenuated for AMs that were more episodically detailed. Further, we found an age-related reduction in the top-down modulation of the PFC on the hippocampus by episodic richness, possibly reflecting fewer controlled processes operating on the recovery of information in the hippocampus. In sum, the present findings suggest that the age-related deficit in the episodic richness of AMs is associated with an overall reduction in the rich elaboration of these memories, where older adults show less top-down modulation of the PFC on the hippocampus.
Acknowledgments
We thank Amanda Miles for help with scanning, Nicole Dautel for help with coding and Philip A. Kragel for help with the analysis. This work was supported by the National Institute of Aging [RO1 AG023123 to DCR and RC, and RO1 AG019731 and AG023770 to RC], and to the Philip Jackson Baugh, Myra and William Waldo Boone, and Leadership in an Aging Society Graduate Fellowships awarded to PLS.
Footnotes
Disclosure Statement
The authors certify that they have no actual or potential conflicts of interest regarding the research reported in this paper. The experimental protocol employed in the present study was approved for ethical treatment of human participants by the Institutional Review Board at Duke University Medical Center, and the experimental data were collected with the understanding and written consent of each participant.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14(6):752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Addis DR, Sacchetti DC, Ally BA, Budson AE, Schacter DL. Episodic simulation of future events is impaired in mild Alzheimer’s disease. Neuropsychologia. 2009;47:2660–2671. doi: 10.1016/j.neuropsychologia.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45(7):1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: a simple network model. Proceedings of the National Academy of Sciences USA. 1994;91(15):7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Steer R. Beck Depression Inventory. Philadelphia: Center for Cognitive Therapy; 1978. [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: bilateral damage causes impaired free recall of autobiographical memory. Journal of Neuroscience. 2007;27(52):14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluck S. Autobiographical memory: exploring its functions in everyday life. Memory. 2003;11(2):113–123. doi: 10.1080/741938206. [DOI] [PubMed] [Google Scholar]
- Botzung A, Denkova E, Ciuciu P, Scheiber C, Manning L. The neural bases of the constructive nature of autobiographical memories studied with a self-paced fMRI design. Memory. 2008;16(4):351–363. doi: 10.1080/09658210801931222. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words (ANEW) Gainesville, FL: The NIMH Center for the Study of Emotion and Attention, University of Florida; 1999. [Google Scholar]
- Brett M, Christoff K, Cusack R, Lancaster JL. Using the Talaraich atlas with the MNI template. Neuroimage. 2001;13(6):S85. [Google Scholar]
- Brewer WF. What is autobiographical memory? In: Rubin DC, editor. Autobiographical Memory. NY: Cambridge UP; 1986. pp. 25–49. [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Science. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology & Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nature Reviews Neuroscience. 2008;9(8):613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral Cortex. 2004;14(4):364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cabeza R, St Jacques PL. Functional neuroimaging of autobiographical memory. Trends in Cognitive Sciences. 2007;11(5):219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Cohen G, Faulkner D. Life span changes in autobiographical memory. In: Gruneberg MM, Morris PE, Sykes RN, editors. Practical aspects of memory: Current research and issues. Vol. 1. Chichester, UK: Wiley; 1988. pp. 277–282. [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychological Review. 2000;107(2):261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW, Whitecross SE. The neuroanatomy of autobiographical memory: A slow wave cortical potential study of autobiographical memory. Journal of Memory & Language. 2001;45:493–524. [Google Scholar]
- Craik FIM, Byrd M. Aging and cognitive deficits: The role of attentional resources. In: Craik FIM, Trehub SE, editors. Aging and Cognitive Processes. NY: Plenum; 1982. pp. 191–211. [Google Scholar]
- Craik FIM, Grady CL. Aging, memory, and frontal lobe functioning. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. London: Oxford University Press; 2002. pp. 528–540. [Google Scholar]
- Craik FIM, Jennings JM. Human memory. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. Hillsdale, NJ: Erlbaum; 1992. pp. 51–110. [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of Healthy Aging on Hippocampal and Rhinal Memory Functions: An Event-Related fMRI Study. Cerebral Cortex. 2006;16(12):1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC. The spatiotemporal dynamics of autobiographical memory: neural correlates of recall, emotional intensity, and reliving. Cerebral Cortex. 2008;18(1):217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 3. Mahwah, NJ: Lawrence Erlbaum Associates; 2008. pp. 1–54. [Google Scholar]
- Dijkstra K, Kaup B. Mechanisms of autobiographical memory retrieval in younger and older adults. Memory & Cognition. 2005;33(5):811–820. doi: 10.3758/bf03193076. [DOI] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg MD. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiology of Aging. 2008;29(12):1902–1916. doi: 10.1016/j.neurobiolaging.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35(4):803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Graham KS, Lee AC, Brett M, Patterson K. The neural basis of autobiographical and semantic memory: new evidence from three PET studies. Cognitive, Affective, & Behavioral Neuroscience. 2003;3(3):234–254. doi: 10.3758/cabn.3.3.234. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, LaBar KS. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43(5):659–674. doi: 10.1016/j.neuropsychologia.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proceedings of the National Academy of Sciences USA. 2007;104(5):1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends in Cognitive Science. 2007;11(7):299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- James LE, Burke DM, Austin A, Hulme E. Production and perception of “verbosity” in younger and older adults. Psychology & Aging. 1998;13(3):355–367. doi: 10.1037//0882-7974.13.3.355. [DOI] [PubMed] [Google Scholar]
- Labouvie-Vief G. Growth and aging in life span perspective. Human Development. 1982;25(1):65–79. [PubMed] [Google Scholar]
- Lancaster JL, Summerin JL, Rainey L, Freitas CS, Fox PT. The Talairach Daemon, a database server for Talairach Atlas Labels. Neuroimage. 1997;5:4–S633. [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talaraich atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Black SE, Cabeza R, Sinden M, McIntosh AR, Toth JP, Tulving E, Stuss DT. Episodic memory and the self in a case of isolated retrograde amnesia. Brain. 1998;121(Pt 10):1951–1973. doi: 10.1093/brain/121.10.1951. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychology & Aging. 2002;17(4):677–689. [PubMed] [Google Scholar]
- Levine B, Svoboda E, Turner GR, Mandic M, Mackey A. Behavioral and functional neuroanatomical correlates of anterograde autobiographical memory in isolated retrograde amnesic patient M.L. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2008.12.026. In Press. [DOI] [PubMed] [Google Scholar]
- Levine B, Turner GR, Tisserand D, Hevenor SJ, Graham SJ, McIntosh AR. The functional neuroanatomy of episodic and semantic autobiographical remembering: a prospective functional MRI study. Journal of Cognitive Neuroscience. 2004;16(9):1633–1646. doi: 10.1162/0898929042568587. [DOI] [PubMed] [Google Scholar]
- Li J, Morcom AM, Rugg MD. The effects of age on the neural correlates of successful episodic retrieval: an ERP study. Cognitive, Affecitive, & Behavioral Neuroscience. 2004;4(3):279–293. doi: 10.3758/cabn.4.3.279. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4(4):423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33(5):827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. Philosophical Transacations of the Royal Society in London B: Biolological Sciences. 2001;356(1413):1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126(Pt 7):1511–1523. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroantomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. (WFU Pickatlas, version 1232.1233) [DOI] [PubMed] [Google Scholar]
- Mark RE, Rugg MD. Age effects on brain activity associated with episodic memory retrieval. An electrophysiological study. Brain. 1998;121(Pt 5):861–873. doi: 10.1093/brain/121.5.861. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Szpunar KK, Christ SE. Laboratory-based and autobiographical retrieval tasks differ substantially in their neural substrates. Neuropsychologia. 2009;47(11):2290–2298. doi: 10.1016/j.neuropsychologia.2008.12.025. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Black SE, Miller B, Moscovitch M, Levine B. Autobiographical memory in semantic dementia: implication for theories of limbic-neocortical interaction in remote memory. Neuropsychologia. 2006;44(12):2421–2429. doi: 10.1016/j.neuropsychologia.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergler NL, Goldstein MD. Why are there old people. Senescence as biological and cultural preparedness for the transmission of information. Humal Development. 1983;26(2):72–90. doi: 10.1159/000272872. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: increased cortical recruitment with matched performance. Cerebral Cortex. 2007;17(11):2491–2506. doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and Working-with-Memory. Journal of Cognitive Neuroscience. 1992;4(3):257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, Nadel L. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. Journal of Anatomy. 2005;207(1):35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, McIntosh AR, Cabeza R, Nilsson LG, Houle S, Habib R, Tulving E. Network analysis of positron emission tomography regional cerebral blood flow data: ensemble inhibition during episodic memory retrieval. Journal of Neuroscience. 1996;16(11):3753–3759. doi: 10.1523/JNEUROSCI.16-11-03753.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cerebral Cortex. 2008;18(5):1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2005;360(1456):781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126(Pt 3):650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Benali K, Eustache F. Episodic and semantic remote autobiographical memory in ageing. Memory. 2002;10(4):239–257. doi: 10.1080/09658210143000353. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Clarys D, Guillery-Girard B, Taconnat L, Isingrini M, Eustache F. Autobiographical memory, autonoetic consciousness, and self-perspective in aging. Psychology & Aging. 2006;21(3):510–525. doi: 10.1037/0882-7974.21.3.510. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Hubert V, Bernard F, Matuszewski V, Chételat G, Baron JC, Eustache F. Reliving lifelong episodic autobiographical memories via the hippocampus: A correlative resting PET study in healthy middle-aged subjects. Hippocampus. 2008;18(5):445–459. doi: 10.1002/hipo.20406. [DOI] [PubMed] [Google Scholar]
- Piolino P, Giffard-Quillon G, Desgranges B, Chetelat G, Baron JC, Eustache F. Re-experiencing old memories via hippocampus: a PET study of autobiographical memory. Neuroimage. 2004;22(3):1371–1383. doi: 10.1016/j.neuroimage.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Schrauf RW, Greenberg DL. Belief and recollection of autobiographical memories. Memory & Cognition. 2003;31(6):887–901. doi: 10.3758/bf03196443. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Schulkind MD. Distribution of important and word-cued autobiographical memories in 20-, 35-, and 70-year-old adults. Psychology & Aging. 1997;12(3):524–535. doi: 10.1037//0882-7974.12.3.524. [DOI] [PubMed] [Google Scholar]
- Scheibe C, Wartenburger I, Wustenberg T, Kathmann N, Villringer A, Heekeren HR. Neural correlates of the interaction between transient and sustained processes: a mixed blocked/event-related fMRI study. Human Brain Mapping. 2006;27(7):545–551. doi: 10.1002/hbm.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews Neuroscience. 2003;4(8):637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Smith SJ, Souchay C, Conway MA. Overgeneral autobiographical memory in Parkinson’s disease. Cortex. 2010;46(6):787–793. doi: 10.1016/j.cortex.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- St-Laurent M, Moscovitch M, Levine B, McAndrews MP. Determinants of autobiographical memory in patients with unilateral temporal lobe epilepsy or excisions. Neuropsychologia. 2009;47(11):2211–2221. doi: 10.1016/j.neuropsychologia.2009.01.032. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Cabeza R. Neural correlates of autobiographical memory. In: Bauer P, Ghetti S, editors. Origins and Development of Recollection: Perspectives from Psychology and Neuroscience. in press. [Google Scholar]
- St Jacques PL, Levine B. Ageing and autobiographical memory for emotional and neutral events. Memory. 2007;15(2):129–144. doi: 10.1080/09658210601119762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinvorth S, Levine B, Corkin S. Medial temporal lobe structures are needed to re-experience remote autobiographical memories: evidence from H.M. and W.R. Neuropsychologia. 2005;43(4):479–496. doi: 10.1016/j.neuropsychologia.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Stephan KE. On the role of general system theory for functional neuroimaging. Journal of Anatomy. 2004;205:443–470. doi: 10.1111/j.0021-8782.2004.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Daunizeau J, Moran RJ, Friston KJ. Bayesian model selection for group studies. Neuroimage. 2009;46(4):1004–1017. doi: 10.1016/j.neuroimage.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44(12):2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott CT, Friedman D, Ritter W, Fabiani M. Item and source memory: differential age effects revealed by event-related potentials. Neuroreport. 1997;8(15):3373–3378. doi: 10.1097/00001756-199710200-00036. [DOI] [PubMed] [Google Scholar]
- Trott CT, Friedman D, Ritter W, Fabiani M, Snodgrass JG. Episodic priming and memory for temporal source: event-related potentials reveal age-related differences in prefrontal functioning. Psychology & Aging. 1999;14(3):390–413. doi: 10.1037//0882-7974.14.3.390. [DOI] [PubMed] [Google Scholar]
- Viard A, Lebreton K, Chetelat G, Desgranges B, Landeau B, Young A, De La Sayette V, Eustache F, Piolino P. Patterns of hippocampal-neocortical interactions in the retrieval of episodic autobiographical memories across the entire life-span of aged adults. Hippocampus. 2010;20(1):153–165. doi: 10.1002/hipo.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard A, Piolino P, Desgranges B, Chetelat G, Lebreton K, Landeau B, Young A, De La Sayette V, Eustache F. Hippocampal Activation for Autobiographical Memories over the Entire Lifetime in Healthy Aged Subjects: An fMRI Study. Cerebral Cortex. 2007;17(10):2453–2467. doi: 10.1093/cercor/bhl153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Science. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Wegesin DJ, Friedman D, Varughese N, Stern Y. Age-related changes in source memory retrieval: an ERP replication and extension. Cognitive Brain Research. 2002;13(3):323–338. doi: 10.1016/s0926-6410(01)00126-4. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120(2):272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]