Abstract
Opiate abuse increases the risk for human immunodeficiency virus (HIV) infection, while both opiates and HIV may impact the immune and nervous systems. To model potential interactions between opiate drugs and HIV on the brain, neurometabolite levels were evaluated in simian immunodeficiency virus (SIV)-infected macaques with or without chronic morphine administration. Over the course of the study, 58% of these SIV-infected animals progressed to acquired immune deficiency syndrome (AIDS). Brain extracts from four brain regions were evaluated with proton magnetic resonance spectroscopy. Animals with AIDS had lower N-acetyl-aspartate in all four brain regions (p≤0.05) as well as lower frontal gray matter total creatine (p=0.03), lower frontal white matter (p= 0.003) and caudate (p=0.002) glutamate, and higher frontal white matter myo-inositol (p=0.05) than the healthier non-AIDS macaques. Morphine-dependent animals had higher levels of myo-inositol in the putamen (p=0.003), especially those with AIDS. In the animals with AIDS, those with morphine dependence had higher total creatine in the frontal white matter (p=0.04) than those treated with saline, which in turn had lower creatine than saline-injected animals without AIDS (p=0.04), leading to an interaction between the effects of morphine and AIDS on total creatine in this brain region (ANOVA p=0.02). The majority of these brain metabolites correlated with viral counts indicating more severe metabolite abnormalities in animals with higher viral loads or set points. Collectively, these findings suggest that chronic morphine may protect against the neurotoxic effect of AIDS and reinforce the importance of maintaining a low viral load in AIDS.
Keywords: AIDS, Opiates, SIV, Neurometabolite, Viral load
Introduction
Opiate abuse is a major co-morbidity in human immuno-deficiency virus (HIV-1)-infected individuals and enhances the risk for HIV transmission (CDC 2002). However, little is known about possible interactions between HIV-1 and opiates in the brain. The simian (SIV) model is well suited for the study of HIV-1 and drug use interactions since primates can have drugs administered in similar routes as those in humans, and SIV is virologically closest to HIV-1 and hence produces similar neuropathology (McClure et al. 1990).
Opiate-dependent individuals may experience severe withdrawal if they abruptly reduce or stop using the drug. Therefore, relapse to opiate use or continued usage of opiates, including synthetic opiates such as methadone, are highly prevalent in those infected with HIV. The interactive effects between opiates and HIV on the brain are made more complex by the roles of opiate receptors in both the immune and nervous systems. While there is evidence that opiates can directly modulate HIV expression in the periphery (Peterson et al. 1990), epidemiological data and studies using animal models regarding various interactions between opiates and HIV infection on the course of various opportunistic infections and progression to AIDS are often contradictory (Donahoe and Vlahov 1998). Many of these discrepancies may be related to differences in health status of the infected individual (e.g., with or without AIDS), the stage of opiate dependence (actively using, withdrawing, or recovering), and the different degree of physiological stress associated with those states.
Proton magnetic resonance spectroscopy (1HMRS) can be used to monitor in vivo changes in brain metabolites in HIV patients; therefore, several groups that study animal models of HIV/AIDS have used in vivo or ex vivo 1HMRS to evaluate brain metabolite changes associated with SIV infection. These studies demonstrated various brain metabolite abnormalities that varied by brain region and with the duration of infection (Greco et al. 2004; Kim et al. 2005; Ratai et al. 2009). In general, brain metabolite abnormalities in SIV are similar to those found in HIV-infected individuals. For instance, SIV-infected animals had lower N-acetyl-aspartate (NAA) to total creatine (CR) ratios, suggesting damaged neuronal integrity, and NAA/CR tended to decrease with progressing disease severity (Lentz et al. 2008a, b). Markers of advanced disease such as higher plasma viral load (Fuller et al. 2004) were associated with lower NAA/CR and higher total choline to tCR ratios (CHO/CR), which indicated that more severe SIV infection was associated with more neuronal injury and greater cell membrane turnover (Fuller et al. 2004; Greco et al. 2004). Likewise, in patients infected with HIV, higher plasma viral loads were associated with higher concentrations of frontal white matter choline compounds and myo-inositol (MI, a glial marker), and those with more severe dementia also tended to show lower NAA (Barker et al. 1995; Chang et al. 1999; Meyerhoff et al. 1999; Chang et al. 2002). Altogether, these studies demonstrate a progression from glial activation early in SIV or HIV infection to neuronal injury in later stages of the brain infection (Chang et al. 2004). Fewer studies evaluated the effects of opiate on brain metabolite levels. One 1HMRS study of healthy heroin users (mostly poly-substance users) reported lower frontal gray matter NAA relative to controls (Haselhorst et al. 2002); other case reports of heroin users that developed leukoencephalopathy found apparent lower levels of brain NAA and choline with elevated lactate based on visual inspection (Bartlett and Mikulis 2005; Offiah and Hall 2008). The aim of the current study was to use ex vivo 1HMRS to evaluate potential interactions between chronic and daily opiate administration (i.e., opiate dependence) and SIV disease severity on brain metabolites in a group of SIV-infected animals.
Methods
Animals
Experiments were conducted according to standards in the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at Emory University. Animals included herein were part of a larger study of 3–5-year-old, male Indian-strain rhesus macaques (Macaca mulatta) from the colonies at the Yerkes National Primate Research Center (YNPRC), Atlanta, GA. They were housed individually at YNPRC as previously described (Donahoe et al. 2009).
After acclimation to the area and testing procedures, 5–7-year-old monkeys were randomly divided into two groups: one group (n=15) received morphine diluted in saline and the control group (n=14) received saline via injections into the outer flank of a hind limb. Increasing morphine dosages were given such that monkeys received 1 mg of morphine per kilogram of body weight every 6 h for 2 days, which was followed by 2 mg/kg for 2 days and then maintained at 3 mg/kg every 6 h for 4 1/2 months. The morphine dose was then lowered to 2 mg/kg to avoid anorexia in the animals. Control monkeys received injections of saline at the same volume-to-weight ratios. Two weeks after initiation of the morphine or saline injections, all monkeys were inoculated (i.v.) with 10,000 TCID50 of SIVsmm9 (Fultz et al. 1986). These animals were maintained on morphine for variable times, up to 4 years, until they were sacrificed.
The animals were monitored daily by the Clinical Veterinary Staff at YNPRC for changes in health and behavior. Animals received discretionary clinical care by veterinarians, including treatment with analgesics, antibiotics, intravenous fluids, etc. Blood was regularly collected by venipuncture of the femoral vein from ketamine-anesthetized animals to monitor their health. Branched chain DNA signal amplification was performed to quantify viral mRNA in plasma samples (log10 SIV mRNA copies per milliliter) for determination of viral load and set points (Donahoe et al. 2009). All animals were SIV infected; however, for analysis purposes, animals were classified as non-AIDS or AIDS (if their CD4+ T-cell count fell below 200/μl of blood or if they had an AIDS-defining opportunistic infection).
Tissue collection and assay
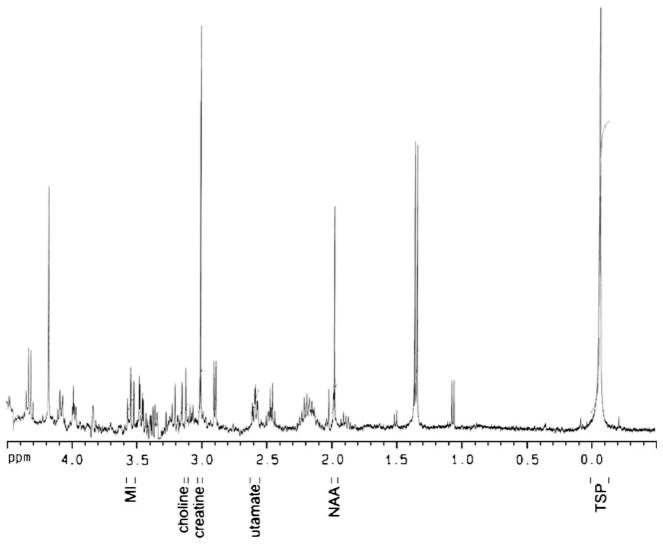
To avoid undue suffering from SIV-related complications, animals were euthanized by a barbiturate overdose if they had dramatic weight loss, documented opportunistic infection, lasting anorexia, intractable diarrhea, progressive neurologic signs, significant cardiac and/or pulmonary signs, pain or distress unresponsive to analgesics, or any other serious illness. Monkeys were exsanguinated, saline perfused, and their brains were quickly removed and dissected. Brain tissue from the frontal gray matter, frontal white matter, caudate, and putamen, were flash frozen and stored at −80°C. Sections of frozen brain tissue were homogenized in five volumes 0.04 M HClO4 (based on wet weight) and centrifuged twice. Supernatants were combined and 3-(trimethylsilyl)-propionic acid–D4, sodium salt (TSP) was added (final concentration 2.5 mM). A 0.425-ml sample was analyzed on a Bruker AVANCE 400 MHz spectrometer (9.4 T) with a 5-mm QNP probe at room temperature. The acquisition parameters were: 30° pulse (6-μs duration), 8,251 Hz spectral width, 0.251 Hz/point, repetition time 6 s, 128 averages, and total acquisition time 12.8 min per spectrum. Since published T1 values of NAA for similar extracts at high fields are 2–3 s (Sokol et al. 2004), the chosen repetition time and flip angle result in a “saturation” of longitudinal magnetization of 0.7–2% only, and therefore would minimize T1 effects as well as potential differential T1 effects (amongst groups). Signal areas were determined using integrals of the peak area referenced to TSP, following Fourier transformation, phasing, and baseline correction. Peaks were identified by comparison to an identically prepared brain extract that had been “spiked” with known quantities of the metabolites of interest and literature references. Peak areas representing NAA, glutamate, choline compounds (Cho), CR, and myo-inositol (MI) concentrations were measured (Fig. 1). Extracts from each brain region for each animal were measured in duplicates and their values averaged and reported as an estimated millimolar (mM) concentration in the tissue based on the TSP concentration in the extract and the dilution factor from the extraction process.
Fig. 1.
Typical 1HMRS spectrum from frontal gray matter with metabolites of interest labeled [MI myo-inositol, NAA N-acetyl-aspartate, TSP the internal standard: 3-(trimethylsilyl)-propionic acid]. The small vertical bars next to each metabolite indicate integration ranges for determination of peak areas
Statistical analyses were performed using StatView (SAS Institute Inc., Cary, NC, USA). All analyses of viral load measures were log transformed and Spearman correlations were used to account for skewed distributions of viral data. Clinical and brain metabolite concentrations for each region were analyzed for the four study groups (SIV+saline n=7, SIV+morphine n=10, AIDS+saline n=7, and AIDS+ morphine n=5) by two-way ANOVA, with morphine dependence and AIDS status as independent variables and using type III sums of squares ANOVA models due to the unbalanced sample sizes. To minimize multiple comparisons, post hoc t tests were conducted only for those variables with significant (p<0.05) ANOVA or interactive effects or trends (p<0.1) for the same.
Results
Clinical measures
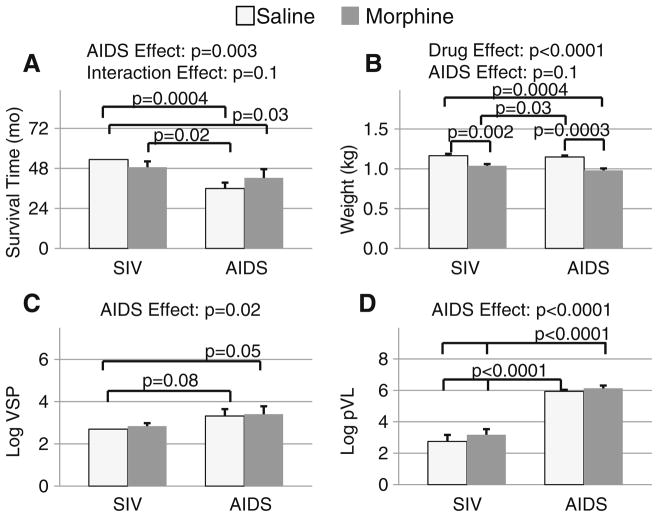
At the end of the study, seven of 14 (50%) saline-treated animals had progressed to AIDS, and only five of 15 (33%) morphine-dependent animals had progressed to AIDS (χ2= 0.8; p=0.4) (Fig. 2). Because animals that developed serious illnesses were euthanized to minimize suffering, only those who remained relatively healthy completed the study. Therefore, the animals with AIDS had significantly shorter survival times than those without AIDS (38.6±3.0 vs. 50.6± 2.1 months; p=0.003, Fig. 2a). However, there was a trend for an interaction (ANOVA p=0.1) between disease status and morphine administration on survival such that morphine-dependent animals with AIDS survived 6 months longer (42.3±5.3 months) than saline-treated animals with AIDS (35.9±3.6 months), while saline-treated animals without AIDS survived 5 months longer (53.3±0 months) than morphine-dependent animals without AIDS (48.7± 3.5 months). Post hoc tests indicated that saline-treated monkeys with AIDS had shorter survival times than both saline-treated non-AIDS (p=0.0004) and morphine-dependent (p=0.02) non-AIDS animals. However, amongst morphine-dependent animals, those with AIDS did not have significantly different survival times from the healthier non-AIDS animals. Due to the study design, the survival time of the animals was highly correlated with the animals’ age at time of death (r=0.91, p<0.0001); therefore, animals that developed AIDS were younger (10.0±0.3 years) at necropsy than those who had not developed AIDS (11.4±0.3 years) resulting in an effect of AIDS (p=0.0008) but not opiate treatment on the animals’ terminal age. Morphine-dependent monkeys weighed 12% less than those who received saline (p<0.0001), and there was a trend (p=0.1) for the AIDS animals to weigh less than the non-AIDS animals (Fig. 2b). As might be expected, the animals that developed AIDS had higher viral set points (p=0.02; Fig. 2c) and higher plasma viral loads (p<0.0001; Fig. 2d), but these viral measures were not altered by the chronic morphine dependence in the current study.
Fig. 2.
a Survival time, b body weight, c viral set point, and d plasma viral load (log10 SIV mRNA copies per milliliter), with significant findings by two-way ANOVA (AIDS status by drug treatment). Significant and trend level post hoc t test p values are also noted
AIDS effects on brain metabolites
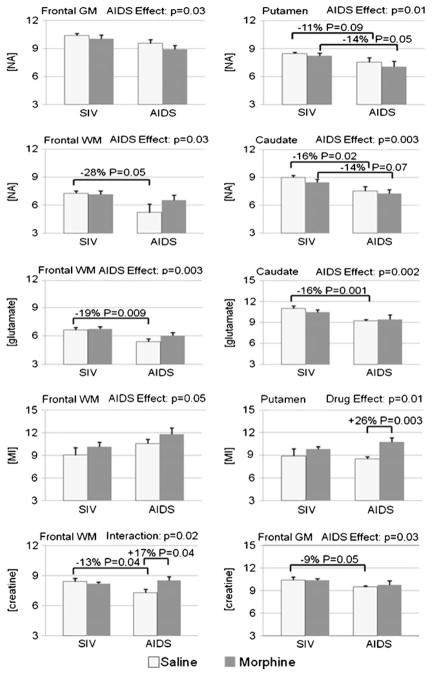
Consistent with prior studies, there were regional variations in the concentrations of the metabolites measured (Table 1 and Fig. 3). Therefore, further analyses were performed separately for each brain region. The animals with AIDS had lower NAA in all four brain regions relative to the healthier SIV-infected animals without AIDS regardless of their morphine treatment status (caudate −14%, p=0.003; frontal gray matter −9%, p=0.03; frontal white matter −20%, p= 0.03; putamen −12%, p=0.009). Furthermore, saline-treated AIDS animals had lower NAA concentration in the frontal white matter (−28%, p=0.05) and the caudate (−16%, p= 0.02) relative to saline-treated animals without AIDS, with a similar trend in the putamen (−11%, p=0.09). In the morphine-dependent animals, those with AIDS also had lower NAA than healthier non-AIDS animals in the putamen (−14%, p=0.05), with a similar trend in the caudate (−14%, p=0.07). Glutamate was also 16% lower (p=0.003) in the frontal white matter and 13% lower (p=0.002) in the caudate of all animals with AIDS compared to those without AIDS. The effect of AIDS on lower glutamate was particularly evident in the saline-treated AIDS versus non-AIDS animals (−19%, p=0.009 in white matter; −16%, p=0.001 in the caudate), whereas there was no difference in glutamate between the morphine-treated groups with and without AIDS. Frontal gray matter total creatine also was lower (−8%, p=0.03) in AIDS animals, with only the saline-treated animals showing significantly lower levels (−9%, p=0.05). In addition, compared to non-AIDS animals, those with AIDS showed higher MI in the frontal white matter (+14%, p=0.05) and a trend in the caudate, regardless of morphine status. Lastly, frontal gray matter glutamate tended to be lower (−7%, p=0.10) in the animals with AIDS than those without AIDS.
Table 1.
Brain metabolite concentrations (mM, mean±SEM and p values from two-way ANOVA between morphine exposure and AIDS status) and their correlations (p values) with plasma viral load (pVL) and viral set point (VSP)
| Brain region | Metabolite | Drug status | SIV without AIDS | SIV with AIDS | Drug effect | AIDS effect | Interaction effect | pVL | VSP |
|---|---|---|---|---|---|---|---|---|---|
| Frontal white matter | NA | Morphine | 7.16±0.38 | 6.53±0.55 | n.s. | 0.03 | n.s. | 0.01 | 0.05 |
| Saline | 7.26±0.27 | 5.22±0.92 | |||||||
| Glutamate | Morphine | 6.73±0.26 | 6.01±0.35 | n.s. | 0.003 | n.s. | 0.004 | 0.002 | |
| Saline | 6.63±0.26 | 5.38±0.31 | |||||||
| Creatine | Morphine | 8.20±0.17 | 8.54±0.35 | 0.08 | n.s. | 0.02 | 0.09. | n.s. | |
| Saline | 8.41±0.32 | 7.31±0.35 | |||||||
| Choline | Morphine | 1.04±0.06 | 1.06±0.03 | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Saline | 1.01±0.09 | 1.03±0.09 | |||||||
| myo-Inositol | Morphine | 10.17±0.63 | 11.81±0.84 | n.s. | 0.05 | n.s. | n.s. | n.s. | |
| Saline | 9.07±0.98 | 10.58±0.59 | |||||||
| Frontal gray matter | NA | Morphine | 10.04±0.43 | 8.92±0.42 | n.s. | 0.03 | n.s. | 0.01 | 0.01 |
| Saline | 10.37±0.25 | 9.56±0.41 | |||||||
| Glutamate | Morphine | 10.45±0.27 | 9.79±0.59 | n.s. | n.s. | n.s. | 0.02 | 0.03 | |
| Saline | 10.15±0.46 | 9.44±0.38 | |||||||
| Creatine | Morphine | 10.38±0.23 | 9.79±0.57 | n.s. | 0.03 | n.s. | 0.02 | 0.005 | |
| Saline | 10.43±0.38 | 9.50±0.17 | |||||||
| Choline | Morphine | 0.97±0.06 | 0.96±0.12 | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Saline | 0.91±0.10 | 1.11±0.09 | |||||||
| myo-Inositol | Morphine | 9.97±0.29 | 9.97±0.69 | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Saline | 8.96±0.73 | 10.62±0.43 | |||||||
| Putamen | NA | Morphine | 8.24±0.26 | 7.08±0.57 | n.s. | 0.009 | n.s. | 0.01 | 0.02 |
| Saline | 8.47±0.14 | 7.55±0.48 | |||||||
| Glutamate | Morphine | 9.74±0.42 | 9.39±0.57 | n.s. | n.s. | n.s. | 0.04 | 0.03 | |
| Saline | 9.56±0.36 | 9.08±0.18 | |||||||
| Creatine | Morphine | 12.70±0.33 | 12.91±0.68 | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Saline | 12.27±0.46 | 12.04±0.41 | |||||||
| Choline | Morphine | 1.00±0.09 | 0.93±0.06 | n.s. | n.s. | n.s. | n.s. | 0.01 | |
| Saline | 0.93±0.10 | 0.90±0.10 | |||||||
| myo-Inositol | Morphine | 9.84±0.31 | 10.79±0.52 | 0.01 | n.s. | n.s. | n.s. | n.s. | |
| Saline | 8.92±0.98 | 8.53±0.32 | |||||||
| Caudate | NA | Morphine | 8.46±0.35 | 7.25±0.42 | n.s. | 0.003 | n.s. | 0.001 | 0.01 |
| Saline | 9.00±0.23 | 7.54±0.46 | |||||||
| Glutamate | Morphine | 10.44±0.36 | 9.42±0.68 | n.s. | 0.002 | n.s. | 0.001 | 0.01 | |
| Saline | 10.99±0.35 | 9.19±0.23 | |||||||
| Creatine | Morphine | 12.12±0.29 | 12.08±0.48 | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Saline | 12.14±0.56 | 11.88±0.40 | |||||||
| Choline | Morphine | 1.04±0.08 | 1.25±0.14 | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Saline | 1.12±0.10 | 1.13±0.04 | |||||||
| myo-Inositol | Morphine | 9.23±0.52 | 10.33±1.08 | n.s. | 0.08 | n.s. | n.s. | 0.02 | |
| Saline | 7.90±0.84 | 9.91±0.98 |
Fig. 3.
Metabolite concentrations (mM, mean±SEM) for measures with significant findings by two-way ANOVA (AIDS status by drug treatment). Significant and trend level post hoc t test p values are also noted. The top figures illustrate predominantly neuronal indicators, while the lower figures illustrate predominantly glial or global indicators
Morphine effects and interactions with AIDS status on brain metabolites
The MI concentration in the putamen was higher (+16%, p= 0.01) in morphine-dependent animals, predominantly due to high MI in the animals with AIDS (+26%, p=0.003) (Table 1, pFig. 2). Frontal white matter showed a trend for higher total creatine (+6%, =0.08) in morphine-dependent animals, reaching significance in the morphine-treated animals with AIDS compared to saline-treated AIDS animals (+17%, p=0.04). Analysis of frontal white matter creatine also revealed an interaction (ANOVA p =0.02) between AIDS status and morphine dependence, such that saline-treated animals with AIDS had lower total creatine (−13%, p=0.04) than those without AIDS, while, as noted above, the AIDS animals treated with morphine had higher creatine than those with saline treatment (which is normalized relative to animals without AIDS).
Correlations between brain metabolites and virological measures
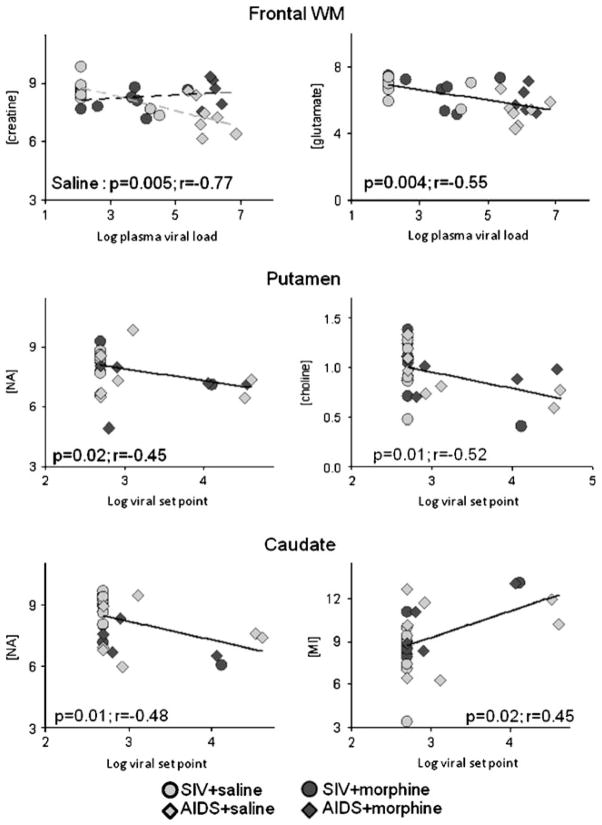
Most of the metabolite changes correlated with log plasma viral load (pVL) and log viral set point (VSP) (Table 1 and Fig. 4). Specifically, across all animals, VSP correlated negatively with NAA concentrations in the caudate (r=−0.48, p=0.01), frontal gray matter (r=−0.48, p=0.01), frontal white matter (r=−0.37, p=0.05), and putamen (r=−0.45, p=0.02). Higher VSP also was associated with lower glutamate in the caudate (r=−0.52, p=0.01), frontal gray matter (r=−0.40, p=0.03), frontal white matter (r=−0.57, p=0.002), and putamen (r=−0.41, p=0.03) as well as with lower frontal gray matter creatine (r=−0.53, p=0.005) and lower choline concentrations in the putamen (r=−0.53, p=0.01). Higher caudate MI was associated with higher VSP (r=0.44, p=0.02).
Fig. 4.
Representative correlations between brain metabolite concentrations (mM) and viremia (log10 SIV mRNA copies per milliliter) are illustrated
Likewise, across all animals, higher pVL was associated with lower NAA concentrations in the caudate (r=−0.62, p =0.001), frontal gray matter (r=−0.53, p=0.01), frontal white matter (r=−0.47, p=0.01), and putamen (r=−0.49, p =0.01) as well as lower glutamate in the caudate (r=−0.67, p=0.001), frontal gray matter (r=−0.45, p=0.02), frontal white matter (r=−0.55, p=0.004), and putamen (r=−0.39, p=0.04). PVL showed further negative correlations with frontal gray matter creatine (r=−0.46, p=0.02) with a similar trend in the frontal white matter (r=−0.32, p=0.09). However, choline and MI concentrations showed no associations with pVL. Since an interaction between morphine dependence and AIDS status was observed for frontal white matter creatine, further post hoc correlations with viral measures were performed. PVL strongly correlated with frontal white matter creatine in the saline-treated animals (r=−0.77, p=0.005), but not in the morphine-treated animals regardless of AIDS status.
Discussion
Consistent with prior studies, the brains of human or non-human primate subjects with AIDS showed more severe neurometabolite abnormalities than those that had not developed AIDS. These neurometabolite abnormalities reflect the severity of neuronal injury and glial activation, and were more severe in our animals with higher viral load or viral set point. The morphine-dependent animals, especially those with AIDS, had higher concentrations of the glial marker MI and the lowest neuronal marker NAA in the putamen, suggesting a greater glial response and more neuronal injury in this brain region. However, the interactive effect between morphine dependence and AIDS status on frontal white matter total creatine suggests a protective effect of morphine on cellular metabolism in this brain region of the animals with AIDS.
Clinical measures
As expected from the deleterious effects of SIV, animals that progressed to AIDS had significantly shorter survival times and higher plasma viral loads and higher viral set points. We did not observe a difference in survival times or viral measures between the morphine-dependent and saline-treated animals; however, there was a trend for an interaction effect between AIDS status and morphine treatment. In prior studies that included many of the animals herein, fewer morphine-dependent animals progressed to AIDS (Donahoe and Vlahov 1998; Donahoe et al. 2009); however, our sample size is probably too small to demonstrate a significant group difference. The higher viral set point leading to AIDS as well as the higher plasma viral loads in AIDS animals was also expected; however, findings from in vitro studies that showed dose-dependent amplification of HIV viral expression with acute morphine administration in chronically infected promonocytes co-cultured with human brain cells (Peterson et al. 1990) was not corroborated in our in vivo AIDS model of chronic morphine dependence. Since animals were inoculated at approximately the same age, the age at death and survival time were highly correlated and therefore difficult to disassociate in terms of their effect on the findings. Future studies that include a wider age range would be of interest since the human HIV population includes children as well as elderly subjects that use opiates medically or illicitly.
AIDS effects on brain metabolites
Similar to other ex vivo 1HMRS reports (González et al. 2000; Lentz et al. 2008a), more and greater metabolite abnormalities are observed with more severe SIV disease. We observed indications of neuronal damage (lower NAA and glutamate) in all brain regions in our AIDS animals, which is in agreement with prior ex vivo studies (Lentz et al. 2008a, b). Also consistent with prior in vivo findings (Greco et al. 2004; Ratai et al. 2009), our study suggests greater glial response (elevated MI) in the frontal white matter of animals with AIDS. In contrast to prior in vivo reports of elevated choline compounds in acute or sub-acute SIV infection models (Fuller et al. 2004; Greco et al. 2004), however, our animals had longer periods of infection and did not show changes in choline compounds. The similar levels of choline compounds across our four groups of animals, all with more than 4 years of SIV infection, suggest that elevated choline may be an early response to SIV infection, and that choline levels may stabilize after prolonged periods of infection.
Morphine effects and interaction between AIDS and morphine on brain metabolites
Elevated MI in the putamen of morphine-dependent animals is likely a reflection of the neuroglial response to morphine in SIV-infected animals. In the putamen, morphine-treated animals with AIDS not only had the highest MI but also the lowest neuronal marker NAA amongst the four subject groups. Therefore, some of the glial response may reflect ongoing repair to neuronal injury or may be contributing to neuronal injury. This is in agreement with a review of clinical and autopsy findings in heroin users with HIV, which suggested that heroin users are more vulnerable to HIV-related inflammation involving microglial up-regulation (Everall 2004).
In addition to the independent AIDS and morphine effects, we observed an interactive effect between AIDS status and morphine dependence on total creatine in the frontal white matter, showing morphine treatment might have normalized or prevented the lower creatine in saline-treated animals with AIDS. These findings suggest a protective effect of morphine maintenance on total creatine in the frontal white matter region. Creatine is a marker of cellular energy stores that is synthesized predominantly in oligodendrocytes, astrocytes, and neurons (Tachikawa et al. 2004) and is closely tied to mitochondrial high-energy phosphate shuttle functioning (Wallimann 2007). Therefore, lower creatine in saline-treated animals with AIDS suggests an oxidative stress-associated failure of the bioenergetic system similar to that observed in several neurodegenerative disorders including Parkinson’s, Huntington’s, and Alzheimer’s diseases (Beard and Braissant 2010) or reduced availability of arginine, glycine, or cystine for creatine synthesis due to their use for glutathione or nitric oxide production during oxidative stress. Therefore, the normalized creatine in morphine-dependent animals with AIDS may reflect a more effective and neuroprotective response to the bioenergetic demands of neuroinflammation. Future studies using a variety of MRS methods (i.e., 1H, 31P, and 13C) might elucidate if the observed total creatine changes are more dependent on creatine or phosphocreatine and if there are differences in the synthesis of creatine. While interactive effects between chronic HIV infection and marijuana use were observed in the same brain region, showing normalization of brain glutamate (Chang et al. 2006), studies of other drugs of abuse typically suggest exacerbated damage when HIV is a co-morbid condition with drugs of abuse. For instance, HIV and methamphetamine showed additive effects on lower neuronal (NA) and elevated glial (MI) metabolite levels (Chang et al. 2005). Similarly, HIV-positive alcoholics had greater neuronal injury (lower NAA and creatine) relative to non-alcoholic HIV-positive participants (Pfefferbaum et al. 2005). Our findings, however, may not be applicable to HIV-infected heroin-addicted individuals since our animals were carefully monitored and maintained on morphine to minimize physiological stress and other withdrawal effects, as well as other potential confounds such as poly-substance abuse, variable purity of the heroin, and commonly occurring co-infections like hepatitis C or B viruses. Future studies of methadone maintenance in HIV-infected individuals may lead to new insights regarding the possible interactive or protective effects of chronic opiate administration on HIV-associated brain injury.
Correlations between brain metabolites and viral measures
Similar to other studies, we also observed that animals with higher plasma viral load had lower neuronal markers (Fuller et al. 2004). HIV/SIV disease progression, as well as markers of disease state, such as CD4+ T cells and viral load, can be altered by opiate exposure (Donahoe and Vlahov 1998; Donahoe et al. 2009). Our finding of lower creatine in the frontal white matter in association with higher pVL only in saline-treated animals suggests that frontal white matter neuronal metabolism may be particularly vulnerable to the consequences of higher viral loads, but that morphine maintenance may be protective in this respect.
Several metabolite concentrations also correlated with VSP. In prior studies, AIDS progression rates were slower and survival times longer in morphine-dependent monkeys, while viral titers were unaffected by morphine dependence (Donahoe et al. 2009). Similarly, we report comparable pVL and VSP between morphine-dependent and saline-treated animals; in addition, animals with higher VSP had lower NAA and glutamate indicative of more neuronal injury or loss. Therefore, despite potentially slower disease progression and longer survival times in morphine-dependent monkeys with a high VSP (Donahoe et al. 2009), morphine-dependent animals were not protected from the neuronal damage associated with a high VSP. Lower frontal gray matter creatine also was associated with higher VSP, again suggesting a disruption of neuronal metabolism with high VSPs. The correlation between the glial marker MI in the caudate and VSP suggests greater glial response with higher viremia. In contrast, choline concentrations in the putamen were inversely related to VSP. Since choline concentrations increase with peak viremia and decline subsequently (Ratai et al. 2009), the choline levels in our animals might reflect levels after that decline, possibly reflecting cellular damage associated with the high VSP.
This study is the first to evaluate the combined and independent effects of AIDS and chronic morphine administration in the brain metabolites of SIV-infected macaques. The interactive effect between morphine treatment and AIDS on frontal white matter total creatine suggests that chronic but stable morphine administration might confer a protective response in the animals with AIDS. However, in animals without AIDS, chronic morphine did not appear to alter the brain metabolites significantly. These findings, therefore, suggest that chronic opiate administration such as those used for pain management may be relatively safe for HIV-infected individuals. Although morphine dependence led to only few and subtle effects on brain metabolites, SIV-infected animals with either early or ongoing high viral loads had lower neuronal markers (or greater neuronal injury); these findings suggest that effective viral suppression is essential to prevent brain injury in HIV patients.
While the sample size of the current study is relatively small and many results would not withstand more stringent statistical corrections for multiple comparisons, the present findings warrant further investigation with larger sample sizes in both animal models and human populations. In addition, future animal models should also evaluate different dosages or routes of administration of opiate-based treatments for their potential protective or neurotoxic effects. Other study designs might include the evaluation of opiate withdrawal, which might increase stress levels, or how opiate-associated reductions in pain may reduce stress and might indirectly affect neurometabolite concentrations in SIV-infected animals. Such studies would be useful models for HIV patients who use or abuse opiates.
In addition to these animal studies, a well-controlled study is needed in HIV-infected individuals who are maintained on chronic opiates, such as those on methadone maintenance or other chronic opiate medications, to further determine whether opiates might have interactive or additive effects on the brain (e.g., brain metabolite levels or cognitive function). Furthermore, changes in brain metabolites due to disease progression, treatments, aging, and associated viral or immunological measures would ideally be monitored in vivo on a high-field animal system; however, this possibility was not available during the course of the study. Finally, correlations with other clinical variables, such as viral loads, other immune markers, and neurocognitive assessments, would further elucidate the possible relationships between brain metabolites alterations and these variables in the setting of HIV and chronic opiate administration.
Acknowledgments
We thank the Clinical Vet Staff at Yerkes National Primate Research Center. This study was supported by the National Institute on Drug Abuse (1K01-DA021203, R01-DA010440, 2K24-DA16170; 1K02-DA16991) and the National Center for Research Resources to the Yerkes National Primate Research Center (RR000165) and the New England Primate Research Center (RR000168).
Footnotes
Portions of these data were presented at the International Society for Magnetic Resonance in Medicine 14th Scientific Meeting Seattle, Washington, USA May 2006.
Guarantors of the work: Christine C. Cloak, Linda Chang, and Robert M. Donahoe
Conflicts of interest The authors have no conflicts of interest to disclose.
Contributor Information
Christine C. Cloak, Email: cloak@hawaii.edu, Department of Medicine, Neuroscience and MRI Research, University of Hawaii, 1356 Lusitana St #713, Honolulu, HI 96813, USA
Linda Chang, Department of Medicine, Neuroscience and MRI Research, University of Hawaii, 1356 Lusitana St #713, Honolulu, HI 96813, USA.
Shawn P. O’Neil, Harvard Medical School, Southborough, MA, USA
Thomas M. Ernst, Department of Medicine, Neuroscience and MRI Research, University of Hawaii, 1356 Lusitana St #713, Honolulu, HI 96813, USA
Daniel C. Anderson, Yerkes National Primate Research Center, Emory University, Atlanta, GA, USA
Robert M. Donahoe, Department of Pathology, School of Medicine, University of Utah, Salt Lake City, UT, USA
References
- Barker PB, Lee RR, McArthur JC. AIDS dementia complex: evaluation with proton MR spectroscopic imaging. Radiology. 1995;195:58–64. doi: 10.1148/radiology.195.1.7892496. [DOI] [PubMed] [Google Scholar]
- Bartlett E, Mikulis DJ. Chasing “chasing the dragon” with MRI: leukoencephalopathy in drug abuse. Br J Radiol. 2005;78:997–1004. doi: 10.1259/bjr/61535842. [DOI] [PubMed] [Google Scholar]
- Beard E, Braissant O. Synthesis and transport of creatine in the CNS: importance for cerebral functions. J Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.06935.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- CDC CfDCaP. Centers, fDCaP. Rockville MD: CDC; 2002. Drug-associated HIV transmission continues in the United States. Fact Sheet. [Google Scholar]
- Chang L, Ernst T, Leonido-Yee M, Walot I, Singer E. Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology. 1999;52:100–108. doi: 10.1212/wnl.52.1.100. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Witt M, Ames N, Jocivich J, Speck O, Gaiefsky M, Walot I, Miller E. Relationships among cerebral metabolites, cognitive function and viral loads in antiretroviral-naïve HIV patients. Neuroimage. 2002;17:1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, Kolson D, Schifitto G, Jarvik JG, Miller EN, Lenkinski R, Gonzalez G, Navia BA. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004;23:1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry. 2005;162(2):361–9. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Cloak C, Yakupov R, Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J Neuroimmune Pharmacol. 2006;1(1):65–76. doi: 10.1007/s11481-005-9005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J Neuroimmunol. 1998;83:77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, O’Neil SP, Marsteller FA, Novembre FJ, Anderson DC, Lankford-Turner P, McClure HH. Probable deceleration of progression of Simian AIDS affected by opiate dependency: studies with a rhesus macaque/SIVsmm9 model. J Acquir Immune Defic Syndr. 2009;50:241–249. doi: 10.1097/QAI.0b013e3181967354. [DOI] [PubMed] [Google Scholar]
- Everall IP. Interaction between HIV and intravenous heroin abuse? J Neuroimmunol. 2004;147:13–15. doi: 10.1016/j.jneuroim.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Fuller RA, Westmoreland SV, Ratai E, Greco JB, Kim JP, Lentz MR, He J, Sehgal PK, Masliah E, Halpern E, Lackner AA, Gonzalez RG. A prospective longitudinal in vivo 1H MR spectroscopy study of the SIV/macaque model of neuroAIDS. BMC Neurosci. 2004;5:10. doi: 10.1186/1471-2202-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz PN, McClure HM, Anderson DC, Swenson RB, Anand R, Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys) Proc Natl Acad Sci. 1986;83:5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González RG, Cheng LL, Westmoreland SV, Sakaie KE, Becerra LR, Lee PL, Masliah E, Lackner AA. Early brain injury in the SIV-macaque model of AIDS. AIDS. 2000;14(18):2841–9. doi: 10.1097/00002030-200012220-00005. [DOI] [PubMed] [Google Scholar]
- Greco JB, Westmoreland SV, Ratai EM, Lentz MR, Sakaie K, He J, Sehgal PK, Masliah E, Lackner AA, Gonzalez RG. In vivo 1H MRS of brain injury and repair during acute SIV infection in the macaque model of neuroAIDS. Magn Reson Med. 2004;51:1108–1114. doi: 10.1002/mrm.20073. [DOI] [PubMed] [Google Scholar]
- Haselhorst R, Dursteler-MacFarland KM, Scheffler K, Ladewig D, Muller-Spahn F, Stohler R, Seelig J, Seifritz E. Frontocortical N-acetylaspartate reduction associated with long-term i.v. heroin use. Neurology. 2002;58:305–307. doi: 10.1212/wnl.58.2.305. [DOI] [PubMed] [Google Scholar]
- Kim JP, Lentz MR, Westmoreland SV, Greco JB, Ratai EM, Halpern E, Lackner AA, Masliah E, Gonzalez RG. Relationships between astrogliosis and 1H MR spectroscopic measures of brain choline/creatine and myo-inositol/creatine in a primate model. AJNR. 2005;26:752–759. [PMC free article] [PubMed] [Google Scholar]
- Lentz MR, Lee V, Westmoreland SV, Ratai EM, Halpern EF, Gonzalez RG. Factor analysis reveals differences in brain metabolism in macaques with SIV/AIDS and those with SIV-induced encephalitis. NMR Biomed. 2008a;21:878–887. doi: 10.1002/nbm.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz MR, Westmoreland SV, Lee V, Ratai EM, Halpern EF, Gonzalez RG. Metabolic markers of neuronal injury correlate with SIV CNS disease severity and inoculum in the macaque model of neuroAIDS. Magn Reson Med. 2008b;59:475–484. doi: 10.1002/mrm.21556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure HM, Anderson DC, Ansari AA, Fultz PN, Klumpp SA, Schinazi RF. Nonhuman primate models for evaluation of AIDS therapy. Ann NY Acad Sci. 1990;616:287–298. doi: 10.1111/j.1749-6632.1990.tb17849.x. [DOI] [PubMed] [Google Scholar]
- Meyerhoff D, Bloomer C, Cardenas V, Norman D, Weiner M, Fein G. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology. 1999;52:995–1003. doi: 10.1212/wnl.52.5.995. [DOI] [PubMed] [Google Scholar]
- Offiah C, Hall E. Heroin-induced leukoencephalopathy: characterization using MRI, diffusion-weighted imaging, and MR spectroscopy. Clin Radiol. 2008;63:146–152. doi: 10.1016/j.crad.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH., Jr Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990;4:869–873. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Cortical NAA deficits in HIV infection without dementia: influence of alcoholism comorbidity. Neuropsychopharmacology. 2005;30:1392–1399. doi: 10.1038/sj.npp.1300723. [DOI] [PubMed] [Google Scholar]
- Ratai EM, Pilkenton SJ, Greco JB, Lentz MR, Bombardier JP, Turk KW, He J, Joo CG, Lee V, Westmoreland S, Halpern E, Lackner AA, Gonzalez RG. In vivo proton magnetic resonance spectroscopy reveals region specific metabolic responses to SIV infection in the macaque brain. BMC Neurosci. 2009;10:63. doi: 10.1186/1471-2202-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol M, Przybyszewski WM, Matlas B. Investigation of metabolic changes in irradiated rat brain tissue by means of 1H NMR in vitro relaxation study. Solid State Nucl Magn Reson. 2004;25:53–60. doi: 10.1016/j.ssnmr.2003.03.013. [DOI] [PubMed] [Google Scholar]
- Tachikawa M, Fukaya M, Terasaki T, Ohtsuki S, Watanabe M. Distinct cellular expressions of creatine synthetic enzyme GAMT and creatine kinases uCK-Mi and CK-B suggest a novel neuron–glial relationship for brain energy homeostasis. Eur J Neurosci. 2004;20:144–160. doi: 10.1111/j.1460-9568.2004.03478.x. [DOI] [PubMed] [Google Scholar]
- Wallimann T. Introduction—creatine: cheap ergogenic supplement with great potential for health and disease. Subcell Biochem. 2007;46:1–16. doi: 10.1007/978-1-4020-6486-9_1. [DOI] [PubMed] [Google Scholar]