Abstract
One element of the reticular activating system (RAS) is the pedunculopontine nucleus (PPN), which projects to the thalamus to trigger thalamocortical rhythms and the brainstem to modulate muscle tone and locomotion. The PPN is a posterior midbrain site known to induce locomotion in decerebrate animals when activated at 40–60 Hz, and has become a target for DBS in disorders involving gait deficits. We developed a research program using brainstem slices containing the PPN to study the cellular and molecular organization of this region. We showed that PPN neurons preferentially fire at gamma band frequency (30–60 Hz) when maximally activated, accounting for the effects of electrical stimulation. In addition, we developed the P13 midlatency auditory evoked potential, which is generated by PPN outputs, in freely moving rats. This allows the study of PPN cellular and molecular mechanisms in the whole animal. We also study the P50 midlatency auditory evoked potential, which is the human equivalent of the rodent P13 potential, allowing us to study PPN-related processes detected in vitro, confirmed in the whole animal, and tested in humans. Previous findings on the P50 potential in PD suggest that PPN output in this disorder is overactive. This translational research program led to the discovery of a novel mechanism of sleep–wake control based on electrical coupling, pointing the way to a number of new clinical applications in the development of novel stimulants (e.g., modafinil) and anesthetics. In addition, it provides methods for monitoring therapeutic efficacy of DBS in humans and animal models.
Keywords: Arousal, Electrical coupling, Gamma band activity, Locomotion, Modafinil, Pedunculopontine nucleus
Introduction
There is little question that the use of deep brain stimulation (DBS) has brought dramatic and lasting benefits to patients with a number of neurological disorders. A number of reviews in this volume address the disorders targeted, the methods employed, and the successes and failures of DBS. A new target for therapy is the pedunculopontine nucleus (PPN), especially for addressing the gait and rigidity present in some Parkinson’s patients (Mazzone et al. 2008). While early reports reported significant effects on gait and freezing [Mazzone et al. (2005), n = 2, F = 10 Hz; Plaha and Gill (2005), n = 2, F = 20–25 Hz; Stefani et al. (2007), n = 6, F = 25 Hz], more recent reports have shown only modest effects [Ferraye et al. (2010), n = 6, F = 15–25 Hz; Moro et al. (2010), n = 6, F = 5,070 Hz]. While the variability in the results can be ascribed to patient variability, the use of differing parameters and stimulation locations make comparisons difficult. Much of the impetus for this new direction comes from studies performed as long ago as the 1960s, and it helps this new potential therapeutic avenue to review some of these results.
Stimulation of the PPN
In 1966, low amplitude (10–100 µA), long duration (0.5–1.0 s) pulses delivered at 40–60 Hz were reported to induce locomotion in the precollicular–postmammillary transected cat when applied to a region of the posterior midbrain (Shik et al. 1966). The region was located in what the old atlases called the lateral cuneiform nucleus and was named the “Mesencephalic Locomotor Region (MLR)”. Subsequent work revealed that other points within the posterior midbrain could also be stimulated to induce locomotion, including a point in the inferior colliculus, in the cuneiform, and in the PPN, among others (Beresovskii and Bayev 1988; Coles et al. 1989; Garcia-Rill et al. 1987; Sinnamon 1984). Our efforts were aimed at the PPN because we could identify the stimulation sites by processing the tissue from stimulated animals for choline acetyltransferase (ChAT) immunocytochemistry (Garcia-Rill et al. 1987), or NADPH diaphorase histochemistry (Garcia-Rill and Skinner 1988), which selectively label cholinergic PPN neurons. The lowest threshold sites were located dorsal to the brachium conjunctivum, in the lateral (but not medial) cuneiform nucleus, but which contained cholinergic neurons of the PPN. We were the first to use neuroactive agents injected into the PPN to induce locomotion (Garcia-Rill et al. 1985). We also found locomotion-related PPN neurons, such that some cells were active in relation to left–right alternation, while others were related to the duration of the stepping episode (Garcia-Rill and Skinner 1988). Later studies localized their stimulation sites to the cuneiform nucleus dorsal to the brachium conjunctivum, but they did not label PPN cells in the stimulated animals (Takakusaki et al. 2003). This led to the erroneous localization of the PPN as within, but not dorsal to the brachium conjunctivum. The fact is that cholinergic PPN neurons are present well dorsal to the brachium conjunctivum, in the region these authors reported positive effects on locomotion (Garcia-Rill et al. 1987; Garcia-Rill and Skinner 1988). These details aside, there is little doubt that there are multiple regions in the posterior midbrain that when stimulated can induce locomotion in the decerebrate animal. As far as the PPN is concerned, and because of the multiplicity of sites, we concluded that this was not a locomotion-specific region. We were concerned by the fact that locomotion was never instantaneous, but took several seconds to ensue, prompting us to propose that this region “recruited” locomotion, rather than induced it. We wondered why 40–60 Hz stimulation, but not lower or higher frequencies, was required to elicit stepping. In reports of DBS of the PPN, only one used stimulation frequencies in this range, and to good effect (Moro et al. 2010). We also wondered why a known sleep-related structure participated in driving locomotion.
Arousal and sleep
The PPN is most active during waking and paradoxical sleep (Steriade and McCarley 1990), is part of the reticular activating system (RAS) that modulates ascending projections through the thalamus and descending projections through the pons and medulla, and is composed of different populations of cholinergic, glutamatergic and GABAergic neurons (Wang and Morales 2009). Intracellular recordings of PPN neurons in vivo identified six categories of thalamic projecting PPN cells distinguished by their firing properties relative to ponto-geniculo-occipital (PGO) wave generation (Steriade et al. 1990b). Some of these neurons had low rates of spontaneous firing (<10 Hz), but most had high rates of tonic firing (20–80 Hz). PPN neurons increase firing during REM sleep (“REM-on”), or both waking and REM sleep (“Wake/REM-on”), but decrease during slow-wave sleep (SWS) (Sakai et al. 1990; Steriade et al. 1990a; Datta and Siwek 2002), suggestive of increased excitation during activated states. Stimulation of the PPN potentiates the appearance of fast (20–40 Hz) oscillations in the cortical EEG, outlasting stimulation by 10–20 s (Steriade et al. 1991). Injections of glutamate into the PPN increase waking and paradoxical sleep (Datta et al. 2001a), while injections of NMDA increases only waking (Datta et al. 2001b), and injections of a kainate receptor agonist increases only paradoxical sleep (Datta et al. 2002). In addition, the PPN has recently been found to exhibit electrical coupling (Garcia-Rill et al. 2007), along with groups of cells in one of its ascending targets, the parafascicular intralaminar nucleus, that indirectly participates in cortical activation, and in one of its descending targets, the subcoeruleus nucleus, that participates in paradoxical or REM sleep regulation (Heister et al. 2007; Garcia-Rill et al. 2008).
Cellular physiology
This aspect of PPN physiology has been reviewed previously (Reese et al. 1995; Scarnati and Florio 1997). Previous studies on the PPN reported the presence of three cell types based on electrophysiological criteria (Leonard and Llinas 1990; Kamondi et al. 1992; Takakusaki and Kitai 1997), including the presence of LTS currents in type I cells (non-cholinergic), of A currents in type II cells (2/3 cholinergic), and of both A + LTS currents in type III neurons (1/3 cholinergic). In addition, type II neurons exhibit a persistent sodium conductance (Leonard and Llinas 1990). We recently found that almost all PPN neurons exhibit gamma band activity when depolarized, as well as gamma band membrane oscillations, but not at higher frequencies (Simon et al. 2010). In addition, electrical and pharmacological activation of the PPN induced gamma band population responses. These results suggest that the PPN can impart gamma band activity on its targets when maximally activated. This represents a novel mechanism for the induction of activated states, waking and paradoxical sleep, by PPN efferents.
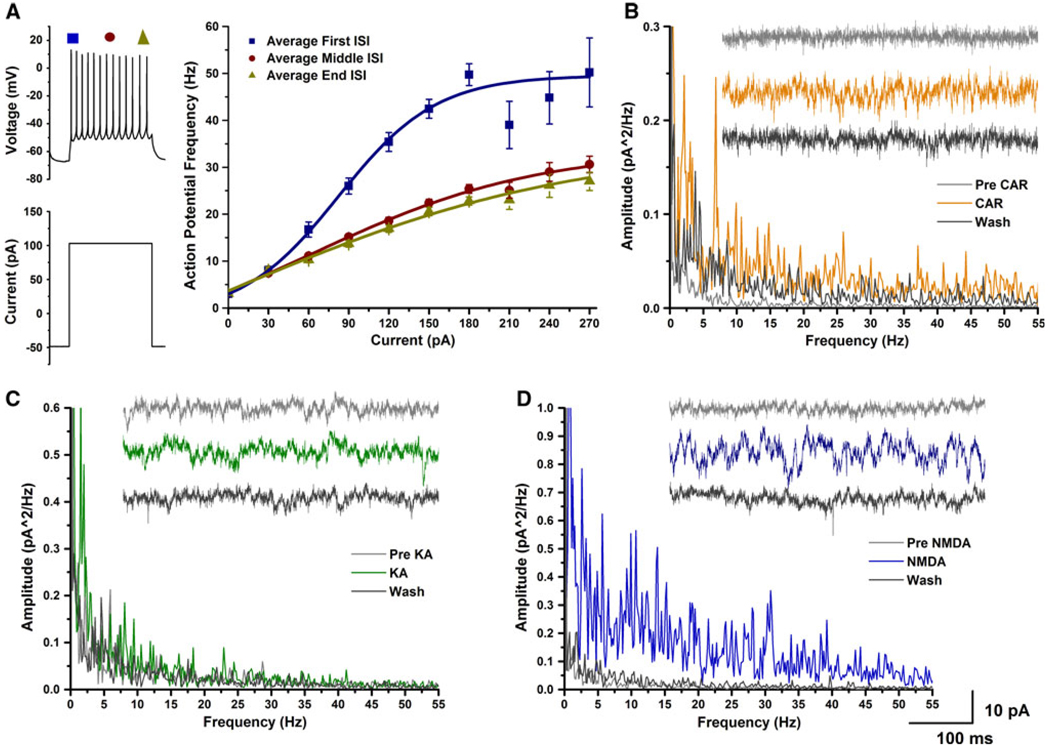
Figure 1 illustrates these findings. Whole-cell patch clamp recordings were performed in PPN neurons. Cells were recorded at −60 mV in current clamp and increasing depolarization current steps were used to induce gamma band frequency firing of action potentials (Fig. 1a). This protocol used nine current steps with an increase of 30 pA for each step. Each step was 500 ms in duration with 2.5-s interval between steps. The final current step was 280 pA greater than the current injection required for holding the cell at −60 mV. During the current steps, the cells were depolarized and fired action potentials when above threshold. Firing frequency was determined by measuring the interspike interval (ISI) between the first two, middle two, and final two action potentials in each current step, and converted to frequency in Hertz. Instantaneous ISI (converted to frequency in Hertz) measures revealed a smooth transition across the current step always reaching a plateau at gamma band frequency. Higher amplitude current injections increased the amplitude of the depolarization and the frequency of action potentials until the cells fired maximally at gamma frequency (Fig. 1a). Only three cells (6%) fired below gamma frequency during the beginning of the stimulus. The average maximal firing frequency occurred during beginning of the 180 pA current step, when the average firing frequency of the recorded neurons was 50 ± 2 Hz (mean ± SE). Following the initial peak in firing frequency, the firing rate of these neurons decreased during the middle and end of the stimulus, but the firing rate was still within the gamma range (peak middle ISI, 31 ± 2 Hz; peak end ISI, 27 ± 2 Hz). Briefly, these experiments demonstrate that almost all PPN neurons are able to fire action potentials maximally at gamma band frequency when electrically stimulated using current steps, and exhibited gamma band membrane oscillations. PPN cells thus appear to be limited to firing at this frequency band. We have preliminary data showing that this plateau in firing is due to the presence of P/Q calcium channels that open at depolarized membrane potentials to “fix” the firing rate at gamma band. These results may explain why it is necessary to stimulate the PPN at 40–60 Hz to induce locomotion in the decerebrate animal, basically, because that is the preferred firing frequency of the maximally activated PPN cell.
Fig. 1.
Gamma band activity in whole-cell recorded PPN cells. a Increasing steps of current (increase of 30 pA per step, each step was 500 ms in duration, 2.5 s latency between each step) caused cells to fire action potentials at higher frequencies. We measured the interspike interval at the beginning (blue symbols), middle (brown symbols), and the end (dark green symbols) of the current step. This cell fired maximally at 50 Hz, which is within the gamma frequency range, at the beginning of the step, then decreased to 25–30 Hz during the rest of the step. b Power spectrum and 1-s recordings of a PPN neuron prior to CAR (gray), during CAR peak effect (light brown), and following wash (black). Application of CAR increased the power of the oscillations at almost every frequency (light brown line in power spectrum), but there were also specific peaks in the theta and gamma range. c Power spectrum and 1-s recordings of a PPN cell prior to KA (light gray), during KA peak effect (green), and following wash (black). Application of KA induced oscillations in the theta range (green line in power spectrum). d Power spectrum and 1-s recordings of a whole-cell patched PPN neuron prior to NMDA (light gray), during the NMDA peak effect (blue), and following wash (black). Application of NMDA induced oscillations, however, the oscillations were not at a specific frequency and the power was increased at almost every frequency (including gamma)
Furthermore, in single cells, application of the cholinergic agonist carbachol and the glutamatergic agonist NMDA increased the frequency of membrane oscillations at both theta and gamma frequencies, while another glutamatergic agonist, kainic acid, induced membrane oscillations mainly in the theta range (Fig. 1b–d).
Population responses
We also developed a method for recording population responses in the PPN, given that the nucleus has multiple cell and transmitter types, and such a technique would allow recording the net output from the population as a whole. We used an interface chamber to record PPN population responses such as those that have recently been reported in cortex (Metherate and Cruikshank 1999; Sukov and Barth 2001; Cunningham et al. 2004), hippocampus (Fisahn et al. 1998), and cerebellum (Middleton et al. 2008). The electrode was inserted into the pars compacta in the posterior PPN, with the tip approximately in the middle of the thickness of the slice in order to record from as large a number of neurons as possible. Our population response studies showed that the PPN is also capable of producing gamma frequency oscillations in population responses when pharmacologically stimulated with the cholinergic agonist carbachol (CAR, which induced activation at specific peaks), the glutamatergic receptor agonist NMDA (which induced overall increases in activity), and the kainate receptor agonist kainic acid (KA, which induced overall activation at lower and somewhat at higher frequencies). This was the only difference between single cell and population response results, namely, i.e., the lack of activation of higher frequencies by KA on the single cells tested, with some activation by KA at higher frequencies in the population recordings. A larger sample of cells may reveal if this is indeed a major difference or merely a sampling issue. It may also be that subpopulations of cells may be activated to fire at different frequencies, or have different types of calcium channels that modulate their firing frequency, e.g., N type calcium channels that may drive theta/alpha firing versus P/Q type channels that drive gamma band firing. These possibilities are currently being tested.
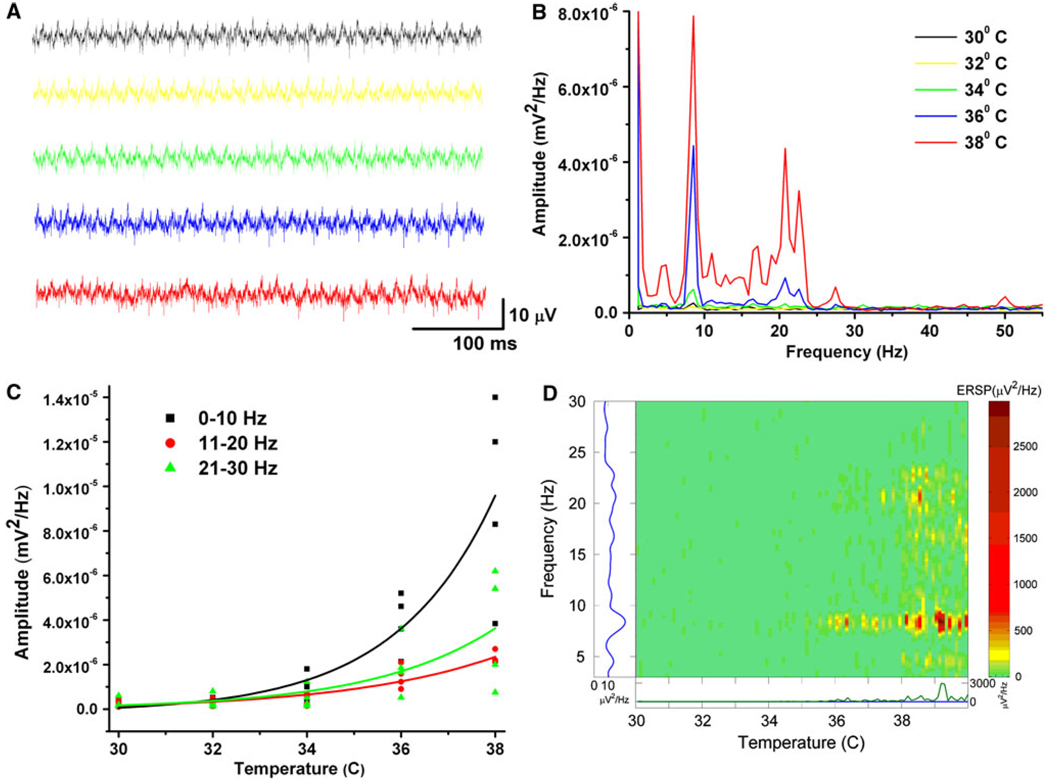
Previous studies on the mechanisms behind gamma band activity in the cortex used slice recordings at body temperature (37°C) instead of the usual 30°C. Figure 2 shows our results using population responses to assess the effects of neuroactive agents as the temperature of the slices was changed. Figure 2a shows sample activity during different temperatures, whereas Fig. 2b shows a power spectrum at each temperature. Figure 2c is a graph of the increase in activity across frequency bands (0–10, 11–20 and 21–30 Hz) with increasing temperature, showing that activity at all frequencies tends to increase with temperature. Figure 2d is an event-related spectral perturbation (ERSP), or running power spectrum, showing that spontaneous population activity increased with increasing temperature, and gamma band activity ensued especially at temperatures around normal body temperature (36–38°C). In fact, spontaneous gamma band oscillations occur consistently at 37°C, but have to be induced electrically (current steps, Fig. 1a) or chemically (pharmacological agents, Fig. 1b–d) at lower temperatures.
Fig. 2.
Effects of temperature on spontaneous PPN population responses. a Population recordings from PPN at various temperatures, at 30°C (black), 32°C (yellow), 34°C (green), 36°C (blue), and 38°C (red). All records are 500 ms in duration. b Power spectrum of population recordings at 30–38°C showing little, if any, spontaneous activity from 30–34°C. At 36°C, peaks of activity were evident in the theta and gamma ranges. At 38°C, the same peaks increased in amplitude, especially at higher frequencies. c Graph of the responses of four slices to changes in temperature from 30 to 38°C showing marked increases at most frequencies with increasing temperature. d Event-related spectral perturbation (ERSP) of spontaneous population activity across temperature. Spontaneous activity in the theta and gamma ranges increased significantly at 36°C and higher
As mentioned above, we discovered the presence of electrical coupling in the PPN (Garcia-Rill et al. 2007). We detected dye coupling between pairs of cells or injected both cells in order to visualize points of contact. The PPN cells were then determined to be electrically coupled by applying current steps to one cell that were evident in the other cell in the presence of TTX, an effect blocked by gap junction blockers. We also detected protein levels and mRNA of the neuronal gap junction protein connexin 36 (Cx 36) (not shown). We punched (1 mm diameter punches) the nucleus in question and analyzed the tissue for Cx 36 protein. Our study showed that Cx 36 was present at high levels early in development (10 days of age) and decreased dramatically during the developmental decrease in REM sleep (30 days). The decrease was evident in all of the RAS nuclei analyzed (PPN, subcoeruleus-SubC, and intralaminar thalamus/parafascicular nucleus-Pf) (Garcia-Rill et al. 2007).
Modafinil is approved for use in treating excessive sleepiness in narcolepsy, daytime sleepiness due to obstructive sleep apnea, and to shift work sleep disorder, and is also prescribed in a number of neuropsychiatric conditions. Many publications on this agent acknowledge that the mechanism of action of modafinil is unknown, although there is general agreement that it increases glutamatergic, adrenergic and histaminergic transmission and decreases GABAergic transmission (Ballon and Feifel 2006). Modafinil was recently found to increase electrical coupling between cortical interneurons, thalamic reticular neurons, and inferior olive neurons (Urbano et al. 2007). Following pharmacologic blockade of connexin permeability, modafinil restored electrotonic coupling. The effects of modafinil were counteracted by the gap junction blocker mefloquine. These authors proposed that modafinil may be acting in a wide variety of cerebral areas by increasing electrotonic coupling in such a way that the high input resistance typical of GABAergic neurons was reduced. Basically, electrical coupling may contribute to action potential synchronization and network oscillations, to coordination and reinforcement of IPSPs, and to coincidence detection in inhibitory networks (Landisman and Connors 2005). We confirmed the fact that modafinil appears to increase electrical coupling in the RAS by showing that, in the absence of action potentials or fast synaptic transmission, modafinil decreased the input resistance of electrically coupled RAS cells, an effect blocked by the gap junction antagonists carbenoxolone or mefloquine (Garcia-Rill et al. 2007). These effects were evident in the absence of changes in resting membrane potential or of changes in the amplitude of induced EPSCs. Modafinil and these blockers produced their effects without changing membrane potential or affecting other conductances (Heister et al. 2007; Garcia-Rill et al. 2007; Urbano et al. 2007), an important issue since mefloquine and carbenoxolone are thought to have a number of unspecific actions, although on differing mechanisms.
We hypothesized that increased electrical coupling of GABAergic RAS neurons by modafinil may decrease their input resistance and, consequently, GABA release, thus disinhibiting output cells, perhaps accounting for its stimulant properties. These findings in general suggest that increasing electrical coupling may promote states of synchronization of sleep–wake rhythms, thus controlling changes in state through increased coherence, especially at gamma band frequency. The presence of electrical coupling in the RAS, which may act with known transmitters to generate ensemble activity, provides new and exciting directions for the field of sleep–wake control. The idea is that the two mechanisms work together, such that gamma oscillations, arising from intrinsic membrane properties (probably, P/Q type calcium currents), are synchronized by electrical coupling so that the population as a whole can achieve coherence at specific frequencies, very much as occurs in the cortex (Pedroarena and Llinas 1997).
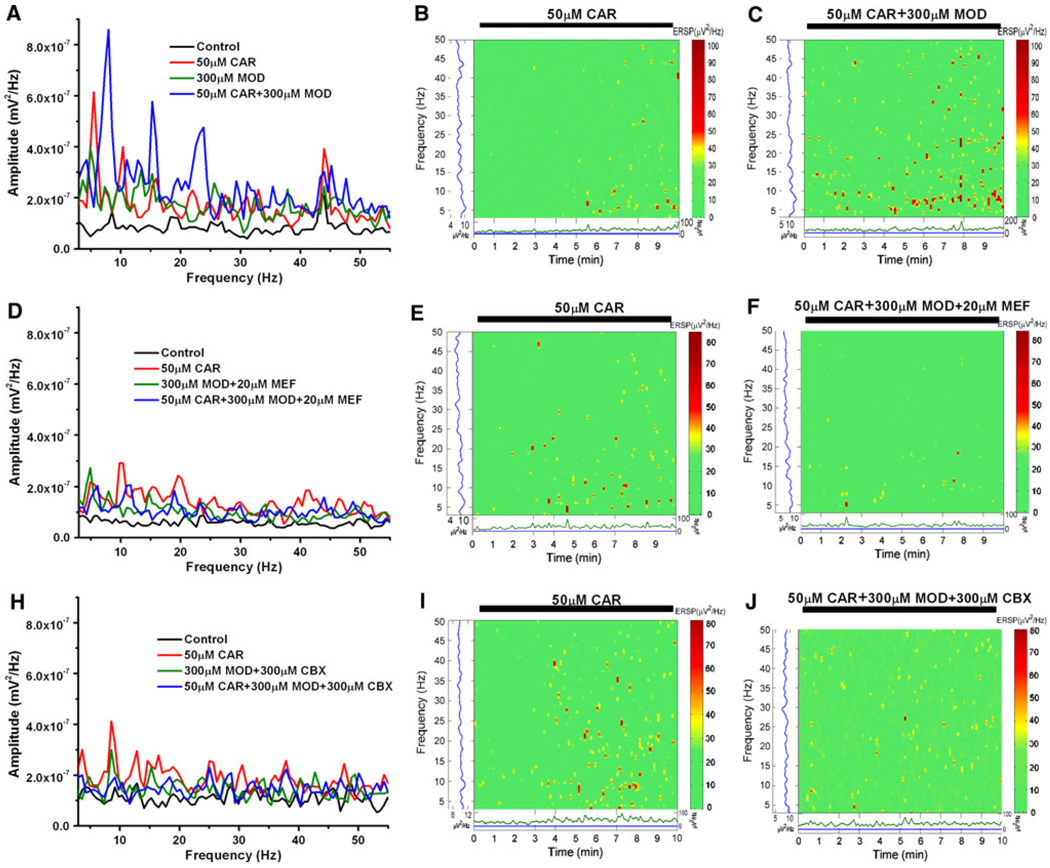
Figure 3 shows our results using population recordings to detect the response of the PPN population at large to the stimulant modafinil. Cholinergic input into the PPN was tested using different concentrations of cholinergic agonist CAR. We applied different concentrations of CAR to the PPN and discovered that rhythmic oscillations could be induced by CAR in a dose-dependent manner (not shown). CAR induced peaks of activity at theta and gamma band (Fig. 3a, b). Significantly, when we applied modafinil, the agent by itself did not produce a specific response, but when CAR was reapplied after 20 min of exposure to modafinil, the response to CAR was amplified (Fig. 3a, c). The increased coherence enabled by modafinil is evident in the event-related spectral perturbations (ERSPs) generated from the population responses (Fig. 3c). We also showed that the effect of modafinil was blocked by either of the gap junction blockers mefloquine (Fig. 3d–f) or carbenoxolone (Fig. 3h–j). In general, these results suggest that (a) gamma band activity appears to be part of the intrinsic membrane properties of PPN neurons, (b) the population as a whole generates gamma band activity spontaneously and under the influence of specific transmitters, and (c) modafinil appears to amplify the response to some transmitters.
Fig. 3.
Effects of modafinil and gap junction blockers on PPN population responses to CAR. a Power spectrum before (black line control), during minute 5 after CAR application (red line), after 20 min of MOD (green line), and 5 min after reapplication of CAR in the presence of MOD (blue line). Note amplification of the CAR response (red line) after application of MOD (blue line). b ERSP of 10 min exposure to CAR, note theta and gamma band activity induced. c ERSP of 10 min CAR exposure after 20 min of MOD, note increase in theta and gamma band activity. d Power spectrum before (black line control), during minute 5 after CAR application (red line), after 20 min of MOD + MEF (green line), and 5 min after reapplication of CAR in the presence of MOD + MEF (blue line). e ERSP of 10 min exposure to CAR, note theta and gamma band activity induced. f ERSP of 10 min CAR exposure after 20 min of MOD + MEF, note decreases in theta and gamma band activity. g Power spectrum before (black line control), during minute 5 after CAR application (red line), after 20 min of MOD + CBX (green line), and 5 min after reapplication of CAR in the presence of MOD + CBX (blue line). h ERSP of 10 min exposure to CAR, note theta and gamma band activity induced. i ERSP of 10 min CAR exposure after 20 min of MOD + CBX, note decreases in theta and gamma band activity
PPN output in freely moving animals
We wanted to develop a method for measuring the net effects of PPN activation, in addition to the single cell and population response levels, to the level of the whole animal. Sensory stimulation is known to activate PPN neurons, especially auditory input (Reese et al. 1995). In the rat, the waveforms following an auditory stimulus are the brainstem auditory evoked response (BAER), the P7 potential at a 6–8 ms latency, the P13 potential at 11–14 ms latency, and the P25 potential at 23–27 ms latency. The midlatency auditory evoked P13 potential appears to be the rodent equivalent of the human P50 potential and the feline “wave A” (Garcia-Rill and Skinner 2001). It is the only midlatency auditory evoked response that has the same three characteristics as the human P50 potential and feline “wave A” as described below (Miyazato et al. 1995). Since the P13 potential can be elicited after decerebration, or after ablation of the primary auditory cortex bilaterally, it appears to have a subcortical origin (Reese et al. 1995). Convincing evidence for the subcortical origin of the vertex-recorded P13 potential is that injections of various neuroactive agents known to inhibit PPN neurons, when injected into the posterior midbrain, will reduce or block the P13 potential, while not affecting the primary auditory P7 potential. Noradrenergic, gabaergic, serotonergic and cholinergic agents are all known to inhibit PPN neurons (Reese et al. 1995), and injection of each of these agents in the region of the PPN reduced or blocked the P13 potential in a dose-dependent manner (Miyazato et al. 1999a). Moreover, interventions which modulate arousal such as various anesthetics, head injury and ethanol, all selectively reduce or block the P13 potential in a dose-dependent manner (Miyazato et al. 1999b).
We determined if injections of modafinil into the PPN would affect the amplitude of the P13 potential in the awake, freely moving rat, and if any induced stimulant effect could be blocked by pretreatment, locally at the level of the PPN, with gap junction antagonists (Beck et al. 2008). We found that modafinil, when injected into the PPN, increased arousal levels in a dose-dependent manner as determined by the amplitude of the P13 potential in the rat, an effect blocked by the gap junction antagonist mefloquine, suggesting that one mechanism by which modafinil increased arousal may have been by increasing electrical coupling. Additional studies showed that carbenoxolone, another gap junction blocker, also negated the stimulant action of modafinil. This further confirmed that a novel mechanism of sleep–wake control includes gap junctions (Garcia-Rill et al. 2007). The fact that anesthetics like halothane and propofol block gap junctions and also induce anesthesia, make this finding relevant for the field of anesthesia. These results demonstrate the potential for measuring PPN output in the freely moving animal using the P13 potential.
PPN output in humans
The P50 potential is a midlatency auditory, click stimulus-evoked, response recorded from the vertex that occurs at a latency of 40–70 ms in the human. The P50 potential has three main characteristics: (a) it is present during waking and rapid eye movement (REM) sleep, but not during deep slow-wave sleep (Erwin and Buchwald 1986a) [i.e. it is sleep state-dependent, occurring during cortical electroencephalographic (EEG) synchronization of fast oscillations, mainly in the low gamma band range (20–40 Hz); but not during cortical synchronization of slow oscillations, <10 Hz]; (b) is blocked by the cholinergic antagonist scopolamine (i.e. may be mediated, at least in part, by cholinergic neurons) (Buchwald et al. 1991); and (c) undergoes rapid habituation at stimulation rates greater than 2 Hz [i.e. is not manifested by a primary afferent pathway, but perhaps by multi-synaptic, low security synaptic elements of the reticular activating system (RAS)] (Erwin and Buchwald 1986b). The P50 potential, but none of the earlier latency primary auditory evoked potentials, thus diminishes and disappears with progressively deep stages of sleep and then reappears during REM sleep (Kevanishvili and von Specht 1979). This suggests that at least one generator of the P50 potential is functionally related to states of arousal, prompting the idea that the potential is generated, at least in part, by cholinergic mesopontine cells, especially in the pedunculopontine nucleus (PPN), which are known to be preferentially active during waking and REM sleep, but inactive during slow-wave sleep (Steriade and McCarley 1990).
The P50 potential has been shown to exhibit characteristic abnormalities, especially in habituation, in various psychiatric and neurological disorders, all of which are marked, and even presaged, by sleep disorders. In general terms, the P50 potential is upregulated (increased amplitude and/or decreased sensory gating) in disorders that are marked by upregulation of RAS output, and downregulated in disorders marked by decreased RAS output. Using a paired stimulus paradigm, schizophrenic patients did not inhibit the response to the second click stimulus under conditions in which normal subjects do show such reduced responsiveness, i.e., there was a decrease in sensory gating (Adler et al. 1982). Schizophrenic patients show such sleep-related symptoms as reduced slow-wave sleep, reduced REM sleep latency, exaggerated startle response and hallucinations (reviewed in Reese et al. 1995). We reported the presence of decreased habituation of the P50 potential in another disorder marked by abnormalities of arousal and excitability, posttraumatic stress disorder (PTSD), in both male combat veterans and female rape victims (Skinner et al. 1999). PTSD is also marked by such sleep–wake state-related abnormalities as increased REM sleep drive, hyperarousal, hallucinations and exaggerated startle response. A similar decrease in sensory gating of the P50 potential was observed in patients with depression (Garcia-Rill et al. 2002). Studies on a neurodegenerative disorder suggest that sensory gating of this potential is also decreased in Huntington’s Disease (HD), which is marked by sleep dysregulation and exaggerated startle response (Uc et al. 2003). The P50 potential also appears to undergo changes in amplitude as a result of other pathological states, being reduced in amplitude in patients with Alzheimer’s disease (AD) (Buchwald et al. 1989), autism (Buchwald et al. 1992) and narcolepsy (Boop et al. 1994), which is characterized by daytime somnolence, cataplexy, sleep paralysis and hypnagogic hallucinations.
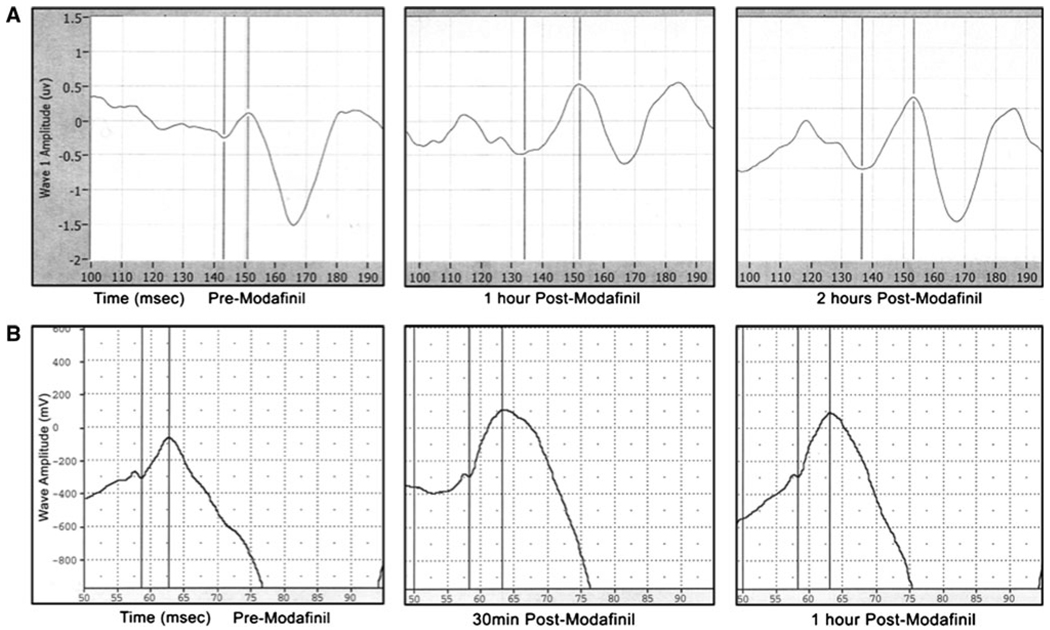
Figure 4a shows recordings of the P50 potential in a normal control before, and 1 and 2 h after ingestion of modafinil. There was an increase in the amplitude of the P50 potential after oral administration of modafinil. Figure 4b shows recordings of the P13 potential in a control rat before, and 30 min and 1 h after oral modafinil. A similar increase in P13 potential amplitude was observed after oral administration of modafinil. These results serve to emphasize the fact that P50 or P13 potential amplitude can be used as a measure of level of arousal, and is increased after administration of the stimulant modafinil.
Fig. 4.
Effects of modafinil on the P50 potential in humans and the P13 potential in rodents. a Panels showing P50 potential recordings in a control individual before (left panel), 1 h after (middle panel), and 2 h after (right panel) oral administration of 100 mg modafinil. The vertical lines are cursors used to calculate the amplitude of the P50 potential from the preceding Nb or baseline, to the peak of the potential ~50 ms after the stimulus (delivered at 100 ms). b Panels showing P13 potential recordings in a control rat before (left panel), 30 min after (middle panel), and 1 h after (right panel) oral administration of 4 mg/kg modafinil. The vertical lines are cursors used to calculate the amplitude of the P13 potential from the preceding Nb or baseline, to the peak of the potential ~13 ms after the stimulus (delivered at 50 ms)
We carried out a study of the P50 potential in PD patients (Teo et al. 1997). We showed, in 27 PD patients, that, as far as amplitude was concerned (amplitude of the first response of a pair of stimuli administered 250 ms apart), the PD group as a whole had 40% higher P50 potential amplitude, but it was not statistically significant because there was a stage-dependent increase, i.e., stage 3 patients had a 10% increase, stage 4 patients had a 42% increase, and stage 5 patients had a 55% increase. We concluded that this represented a trend towards increased P50 potential amplitude in late stage PD. More significantly, habituation (amplitude of the second response as percent of the first response of a pair) decreased significantly in the PD group as a whole (45 vs. 16% in controls, p < 0.001). In addition, there was a statistically significant decrease in habituation with severity, i.e., stage 3, 30 versus control 16%, not significant; stage 4, 50 versus control 16%, p < 0.05; stage 5, 70 versus control 16%, p < 0.01. This provided convincing evidence of a decrease in habituation (indicative of a decrease in sensory gating) and a trend towards increased amplitude (indicative of an increase in level of arousal) in a fairly large population of PD subjects. These results suggest that, if the PPN is overactive in PD, DBS may decrease or block PPN activity to mediate its salutary effects on gait. (Section “Introduction” addresses the use of short-duration, high-frequency stimulation in DBS that would tend to inactivate neurons in the region.) The P50 potential provides a non-invasive method for measuring PPN output in humans in disease states, and for monitoring the effects of treatment, including DBS, in these disorders. Interestingly, when we measured the P50 potential in PD patients who received bilateral pallidotomy that alleviated their motor symptoms, the amplitude and habituation of the P50 potential was within normal levels (Teo et al. 1998). Also, we should note that animal studies subsequently confirmed that the PPN is overactive in animal models of PD (Orieux et al. 2001; Breit et al. 2000).
Conclusions
The studies described represent a coordinated program of research spanning cellular electrophysiology, systems neurobiology, and human neurophysiology, all aimed at investigating PPN functions that have a number of clinical applications, including DBS of the PPN. A major target of this effort is the new stimulant modafinil, which is being used for the treatment of a number of disorders, despite the fact that its mechanism of action was unknown until recently. Our work confirmed previous studies in other brain regions that its mechanism of action is to increase electrical coupling in RAS centers. Our findings in slices showed that the effect of modafinil was blocked by two different gap junction blockers, each of which has side effects but only one effect in common. This allowed us to test the effects of modafinil in the whole animal, and specifically on a process subserved by the PPN, the sleep state-dependent P13 potential. Its effect was similarly blocked by the same two gap junction blockers, confirming that the arousing effects of this agent could be due to increased electrical coupling. Further studies on the sleep state-dependent P50 potential in humans showed that this agent induced similar increases in arousal. Our P50 potential results on patients with PD showed that the PPN is overactive when considering its amplitude and habituation in these patients, so that the beneficial effects of DBS may be to downregulate PPN output. That is, the use of such short-duration (60–100 µs) pulses in DBS rather than the long duration (500 µs) pulses used in animal locomotion studies may actually be inactivating the PPN. Moreover, the large size of the stimulating electrodes (1–2 mm) used in humans is of concern considering that only small diameter (100 µm) wires are used in animal preparations. It is not clear how much damage is produced by the large electrodes. Our previous results do show that the optimal frequency of stimulation for inducing locomotion in animals is 40–60 Hz, and the closer DBS frequencies come to that number, the more likely the predictable result.
Recent studies showed that single cell and population activity recordings in the PPN exhibit activation at gamma band range (responses plateau at this level and do not keep increasing with increasing depolarization or stimulation). Moreover, when these cells are depolarized, the responses persist at gamma band frequency, suggesting that the system is geared to fire, when activated by arousing stimuli, at gamma band frequencies. This may explain why stimulation of the PPN to induce locomotion in the decerebrate animal required 40–60 Hz pulse frequency. Our observation suggests that a similar mechanism to that in the cortex for achieving temporal coherence is present in the PPN, and perhaps its subcortical targets such as the intralaminar/parafascicular and subcoeruleus nuclei. We suggest that, rather than participating in the temporal binding of sensory events, gamma band activity generated in the PPN may help stabilize coherence related to arousal, providing a stable activation state during waking and paradoxical sleep. Much work is needed to support this speculation, but the intriguing findings described here certainly provide a starting point for such investigations. The translational research potential of this observation is considerable, allowing us to study basic arousal mechanisms and how they impact higher-level cognitive function related to sensory perception and binding.
Acknowledgments
This manuscript is dedicated to the special issue on the Rome meeting in May, 2010. The study is supported by USPHS awards from NIH R05 NS20246, P20 RR20146, and UL1 RR29884.
References
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- Ballon JS, Feifel D. A systematic review of Modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- Beck P, Odle A, Wallace-Huitt T, Skinner RD, Garcia-Rill E. Modafinil increases arousal determined by P13 potential amplitude: an effect blocked by gap junction antagonists. Sleep. 2008;31:1647–1654. doi: 10.1093/sleep/31.12.1647. (subject of an editorial commentary) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresovskii VK, Bayev KV. New locomotor regions of the brainstem revealed by means of electrical stimulation. Neuroscience. 1988;26:863–869. doi: 10.1016/0306-4522(88)90105-4. [DOI] [PubMed] [Google Scholar]
- Boop FA, Garcia-Rill E, Dykman R, Skinner RD. The P1: insights into attention and arousal. J Pediatr Neurosurg. 1994;20:57–62. doi: 10.1159/000120765. [DOI] [PubMed] [Google Scholar]
- Breit S, Bouali-Benazzouz R, Benabid A, Benazzouz A. Unilateral lesion of the nigrostriatal pathway induces an increase of neuronal activity of the pedunculopontine nucleus, which is reversed by the lesion of the subthalamic nucleus in the rat. Eur J Neurosci. 2000;14:1833–1842. doi: 10.1046/j.0953-816x.2001.01800.x. [DOI] [PubMed] [Google Scholar]
- Buchwald JS, Erwin R, Van Lancker D, Cummings JL. Midlatency auditory evoked responses: differential abnormality of P1 in Alzheimer’s disease. Electroencephalogr Clin Neurophysiol. 1989;74:378–384. doi: 10.1016/0168-5597(89)90005-1. [DOI] [PubMed] [Google Scholar]
- Buchwald JS, Rubinstein EH, Schwafel J, Strandburg RJ. Midlatency auditory evoked responses: differential effects of a cholinergic agonist and antagonist. Electroencephalogr Clin Neurophysiol. 1991;80:303–309. doi: 10.1016/0168-5597(91)90114-d. [DOI] [PubMed] [Google Scholar]
- Buchwald JS, Erwin R, Van Lancker D, Guthrie D, Schwafel J, Tanguay P. Midlatency auditory evoked responses: P1 abnormalities in adult autistic subjects. Electroencephalogr Clin Neurophysiol. 1992;84:164–171. doi: 10.1016/0168-5597(92)90021-3. [DOI] [PubMed] [Google Scholar]
- Coles SK, Iles JF, Nicolopoulos-Stournaras N. The mesencephalic center controlling locomotion in the rat. Neuroscience. 1989;28:149–157. doi: 10.1016/0306-4522(89)90239-x. [DOI] [PubMed] [Google Scholar]
- Cunningham MO, Whittington MA, Bibbig A, Roopun A, LeBeau FE, Vogt A, Monyer H, Buhl EH, Traub RD. A role for fast rhythmic bursting neurons in cortical gamma oscillations in vitro. Proc Nat Acad Sci. 2004;101:7152–7157. doi: 10.1073/pnas.0402060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep–wake cycle in the freely moving rats. J Neurosci Res. 2002;70:79–82. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- Datta S, Spoley EE, Patterson EH. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Am J Physiol Regul Integr Comp Physiol. 2001a;280:R752–R759. doi: 10.1152/ajpregu.2001.280.3.R752. [DOI] [PubMed] [Google Scholar]
- Datta S, Patterson EH, Spoley EE. Excitation of the pedunculopontine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J Neurosci Res. 2001b;66:109–116. doi: 10.1002/jnr.1202. [DOI] [PubMed] [Google Scholar]
- Datta S, Spoley EE, Mavanji VK, Patterson EH. A novel role of pedunculopontine tegmental kainate receptors: a mechanism of rapid eye movement sleep generation in the rat. Neuroscience. 2002;114:157–164. doi: 10.1016/s0306-4522(02)00250-6. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Buchwald JS. Midlatency auditory evoked responses: differential effects of sleep in the human. Electroencephalogr Clin Neurophysiol. 1986a;65:383–392. doi: 10.1016/0168-5597(86)90017-1. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Buchwald JS. Midlatency auditory evoked responses: differential recovery cycle characteristics. Electroencephalogr Clin Neurophysiol. 1986b;64:417–423. doi: 10.1016/0013-4694(86)90075-1. [DOI] [PubMed] [Google Scholar]
- Ferraye M, Debu B, Fraix V, Goetz L, Ardouin C, Yelnik J, Henry-Lagrange C, Seigneuret E, Piallat B, Krack P, Le Bas J, Benabid A, Chabardes S, Pollak P. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain. 2010;133:205–214. doi: 10.1093/brain/awp229. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. Modulation of rhythmic function in the posterior midbrain. Neuroscience. 1988;27:639–654. doi: 10.1016/0306-4522(88)90295-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. The sleep state-dependent P50 midlatency auditory evoked potential. In: Lee-Chiong TL, Carskadon MA, Sateia MJ, editors. Sleep medicine. Philadelphia: Hanley and Belfus; 2001. pp. 697–704. [Google Scholar]
- Garcia-Rill E, Skinner RD, Fitzgerald JA. Chemical activation of the mesencephalic locomotor region. Brain Res. 1985;330:43–54. doi: 10.1016/0006-8993(85)90006-x. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Houser CR, Skinner RD, Smith W, Woodward DJ. Locomotion-inducing sites in the vicinity of the pedunculopontine nucleus. Brain Res Bull. 1987;18:731–738. doi: 10.1016/0361-9230(87)90208-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD, Clothier J, Dornhoffer J, Uc E, Fann A, et al. The sleep state-dependent midlatency auditory evoked P50 potential in various disorders. Thal Relat Syst. 2002;2:9–19. [Google Scholar]
- Garcia-Rill E, Heister DS, Ye M, Charlesworth A, Hayar A. Electrical coupling: novel mechanism for sleep–wake control. Sleep. 2007;30:1405–1414. doi: 10.1093/sleep/30.11.1405. (subject of an editorial commentary) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Charlesworth A, Heister D, Ye M, Hayar A. The developmental decrease in REM sleep: the role of transmitters and electrical coupling. Sleep. 2008;31:673–690. doi: 10.1093/sleep/31.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heister DS, Hayar A, Charlesworth A, Yates C, Zhou YH, Garcia-Rill E. Evidence for electrical coupling in the subcoeruleus (SubC) nucleus. J Neurophysiol. 2007;97:3142–3147. doi: 10.1152/jn.01316.2006. (subject of an editorial) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamondi A, Williams J, Hutcheon B, Reiner P. Membrane properties of mesopontine cholinergic neurons studied with the whole-cell patch-clamp technique: implications for behavioral state control. J Neurophysiol. 1992;68:1359–1372. doi: 10.1152/jn.1992.68.4.1359. [DOI] [PubMed] [Google Scholar]
- Kevanishvili Z, von Specht H. Human auditory evoked potentials during natural and drug-induced sleep. Electroencephalogr Clin Neurophysiol. 1979;47:280–288. doi: 10.1016/0013-4694(79)90280-3. [DOI] [PubMed] [Google Scholar]
- Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–1813. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- Leonard CS, Llinas R. Electrophysiology of mammalian pedunculopontine and laterodorsal tegmental neurons in vitro: implications for the control of REM sleep. In: Steriade M, Biesold D, editors. Brain cholinergic systems. Oxford: Oxford Science; 1990. pp. 205–223. [Google Scholar]
- Mazzone P, Lozano A, Stanzione P, Galati S, Scarnati E, Peppe A, Stefani A. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson’s disease. Neuroreport. 2005;16:1877–1883. doi: 10.1097/01.wnr.0000187629.38010.12. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Sposato S, Insola A, Dilazzaro V, Scarnati E. Stereotaxic surgery of the nucleus tegmenti pedunculopontinus. Br J Neurosurg. 2008;22:S33–S40. doi: 10.1080/02688690802448327. [DOI] [PubMed] [Google Scholar]
- Metherate R, Cruikshank SJ. Thalamocortical inputs trigger a propagating envelope of gamma-band activity in auditory cortex in vitro. Exp Brain Res. 1999;126:160–174. doi: 10.1007/s002210050726. [DOI] [PubMed] [Google Scholar]
- Middleton SJ, Racca C, Cunningham MO, Traub RD, Monyer H, Knopfel T, Schofield IS, Jenkins A, Whittington MA. High-frequency network oscillations in cerebellar cortex. Neuron. 2008;58:763–774. doi: 10.1016/j.neuron.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazato H, Skinner RD, Reese NB, Boop FA, Garcia-Rill E. A middle-latency auditory-evoked potential in the rat. Brain Res Bull. 1995;37:247–255. doi: 10.1016/0361-9230(95)00003-w. [DOI] [PubMed] [Google Scholar]
- Miyazato H, Skinner RD, Garcia-Rill E. Neurochemical modulation of the P13 midlatency auditory evoked potential in the rat. Neuroscience. 1999a;92:911–920. doi: 10.1016/s0306-4522(98)00762-3. [DOI] [PubMed] [Google Scholar]
- Miyazato H, Skinner RD, Cobb M, Andersen B, Garcia-Rill E. Midlatency auditory evoked potentials in the rat—effects of interventions which modulate arousal. Brain Res Bull. 1999b;48:545–553. doi: 10.1016/s0361-9230(99)00034-9. [DOI] [PubMed] [Google Scholar]
- Moro E, Hamani C, Poon Y, Al-Khairallah T, Dostrovsky J, Hutchison W, Lozano A. Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain. 2010;133:215–224. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- Orieux G, Francois C, Feger J, Yelnik J, Vila M, Ruberg M, Agid Y, Hirsch E. Metabolic activity of excitatory parafascicular and pedunculopontine inputs to the subthalamic nucleus in a rat model of Parkinson’s disease. Neuroscience. 2001;97:79–88. doi: 10.1016/s0306-4522(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Pedroarena C, Llinas R. Dendritic calcium conductance’s generate high-frequency oscillations in thalamocortical neurons. Proc Nat Acad Sci. 1997;94:724–728. doi: 10.1073/pnas.94.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. Neuroreport. 2005;16:1883–1887. doi: 10.1097/01.wnr.0000187637.20771.a0. [DOI] [PubMed] [Google Scholar]
- Reese NB, Garcia-Rill E, Skinner RD. Auditory input to the pedunculopontine nucleus: II. Unit responses. Brain Res Bull. 1995;37:265–273. doi: 10.1016/0361-9230(95)00001-u. [DOI] [PubMed] [Google Scholar]
- Sakai S, El Mansari M, Jouvet M. Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res. 1990;527:213–223. doi: 10.1016/0006-8993(90)91140-c. [DOI] [PubMed] [Google Scholar]
- Scarnati E, Florio T. The pedunculopontine nucleus and related structures. Functional organization. Adv Neurol. 1997;74:97–110. [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovsky GN. Control of walking and running by means of electrical stimulation of the mid-brain. Biophysics. 1966;11:756–765. [PubMed] [Google Scholar]
- Simon C, Kezunovic N, Ye M, Hyde J, Hayar A, Williams DK, Garcia-Rill E. Gamma band unit activity and population responses in the pedunculopontine nucleus. J Neurophysiol. 2010;104:463–474. doi: 10.1152/jn.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnamon HM. Forelimb and hindlimb stepping by the anesthetized rat elicited by electrical stimulation of the diencephalon and mesencephalon. Physiol Behav. 1984;33:191–199. doi: 10.1016/0031-9384(84)90099-4. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Rasco L, Fitzgerald J, Karson CN, Matthew M, Williams DK, et al. Reduced sensory gating of the P1 potential in rape victims and combat veterans with posttraumatic stress disorder. Depress Anxiety. 1999;9:122–130. doi: 10.1002/(sici)1520-6394(1999)9:3<122::aid-da4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Stefani A, Lozano A, Peppe A, Stanzione P, Galati S, Troppei D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130:1596–1607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCarley RW. Brainstem control of wakefulness and sleep. New York: Plenum Press; 1990. [Google Scholar]
- Steriade M, Datta S, Pare D, Oakson G, Curro Dossi R. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990a;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Pare D, Datta S, Oakson G, Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990b;10:2560–2579. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Curro Dossi R, Pare D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Nat Acad Sci. 1991;88:4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukov W, Barth DS. Cellular mechanisms of thalamically evoked gamma oscillations in auditory cortex. J Neurophysiol. 2001;85:1235–1245. doi: 10.1152/jn.2001.85.3.1235. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Kitai ST. Ionic mechanisms involved in the spontaneous firing of tegmental pedunculopontine nucleus neurons of the rat. Neuroscience. 1997;78:771–794. doi: 10.1016/s0306-4522(96)00540-4. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Hagabuchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T. Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience. 2003;119:293–308. doi: 10.1016/s0306-4522(03)00095-2. [DOI] [PubMed] [Google Scholar]
- Teo C, Rasco L, Al-Mefty K, Skinner RD, Garcia-Rill E. Decreased habituation of midlatency auditory evoked responses in Parkinson’s disease. Movement Disord. 1997;12:655–664. doi: 10.1002/mds.870120506. [DOI] [PubMed] [Google Scholar]
- Teo C, Rasco L, Skinner RD, Garcia-Rill E. Disinhibition of the sleep state-dependent P1 potential in Parkinson’s disease-improvement after pallidotomy. Sleep Res Online. 1998;1:62–70. [PubMed] [Google Scholar]
- Uc E, Skinner RD, Rodnitzky L, Garcia-Rill E. The midlatency auditory evoked potential P50 is abnormal in Huntington’s disease. J Neurol Sci. 2003;212:1–5. doi: 10.1016/s0022-510x(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Urbano FJ, Leznik E, Llinas R. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc Natl Acad Sci. 2007;104:12554–12559. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]