Abstract
Srs2 is a 3’ to 5’ DNA helicase that regulates many aspects of DNA metabolism in Saccharomyces cerevisiae. It is best known for its ability to counteract homologous recombination by dismantling Rad51 filaments, but is also involved in checkpoint activation, adaptation and recovery, and in resolution of late recombination intermediates. To further address its biological roles and uncover new genetic interactions, we examined the consequences of overexpressing SRS2 as well as two helicase-dead mutants, srs2-K41A and srs2-K41R, in the collection of 4827 yeast haploid deletion mutants. We identified 274 genes affecting a large variety of cellular functions that are required for cell growth when SRS2 or its mutants are overexpressed. Further analysis of these interactions reveals that Srs2 acts independently of its helicase function at replication forks likely through its recruitment by the sumoylated PCNA replication clamp. This helicase-independent function is responsible for the negative interactions with DNA metabolism genes and for the toxicity of SRS2 overexpression in many of the diverse cellular pathways revealed in our screens.
Keywords: Replication, Repair, Recombination, Srs2, Overexpression, Genetic interactions
1. Introduction
Maintaining genome integrity is crucial to all living cells. The genome is constantly exposed to different DNA damaging agents that can be endogenous, such as side products of normal metabolism, or exogenous, such as UV irradiation. DNA damage can also arise as a consequence of physiological processes such as DNA replication or transcription, leading to accumulation of single-stranded DNA and eventually to DNA double-strand breaks (DSBs) [1, 2]. Several mechanisms are known to repair different types of lesions. In Saccharomyces cerevisiae, homologous recombination (HR) is a major pathway for the repair of DSBs and depends on the RAD52 epistasis group (for reviews see [3–5]). HR is also important for the restart of stalled replication forks (reviewed in [6, 7]), although damage at the fork can also be overcome by post-replicational repair mechanisms (PRR) dependent on the RAD6 pathway. Repair mechanisms therefore compete at sites of DNA damage, particularly in the context of a damaged replication fork.
The Srs2 helicase likely plays a role in the regulation between PRR and HR at replication forks. The SRS2 gene encodes a 3’ to 5’ DNA helicase for which mutants were originally identified as suppressors of the ultraviolet sensitivity of rad6 and rad18 mutations, which are defective in PRR [8, 9]. SRS2 was also independently identified as a hyper-recombination mutant, hpr5 [10]. As the suppressive effect of srs2 on rad6 and rad18 repair defects requires a functional recombination machinery [11], these studies lead to the notion that Srs2 acts as an “anti-recombinase”. Biochemically, Srs2 has been shown to translocate on single-stranded DNA to disrupt Rad51 nucleoprotein filaments, which are required for homologous recombination to occur [12, 13]. More recently, the Srs2 protein was also shown to be recruited to replication forks by the replication clamp PCNA (Proliferating Cell Nuclear Antigen), preferentially when the clamp is SUMOylated [14–16]. These observations suggest the current model in which Srs2 is recruited to replication forks by SUMOylated PCNA where it removes Rad51 filaments thus preventing homologous recombination during replication and favoring PRR [14, 15, 17–19]. However, Srs2 acts as an anti-recombinase also independently of its recruitment to replication forks by PCNA [20, 21], suggesting there are additional roles for the anti-recombinase function outside of DNA replication.
This important regulator of DNA metabolism appears to have multiple roles in different aspects of DNA metabolism (reviewed in [22, 23]) but how these roles are achieved and coordinated is not clearly understood. For example, SRS2 participates in the checkpoint response upon detection of DNA damage. Cdk1 phosphorylates the Srs2 protein in a checkpoint-dependent manner and in turn likely contributes to the proper activation of the checkpoint [24]. It may further modulate the response to DNA damage together with the Mre11 and Sgs1 proteins [24]. Srs2 has also been proposed to participate in the resolution of late recombination intermediates, promoting synthesis dependent strand annealing (SDSA) rather than crossover formation [25–27]. This SDSA promoting activity was recently linked to its phosphorylation by Cdk1 suggesting functional crosstalk between the checkpoint and repair functions of Srs2 [28]. Thus, Srs2 is at the crossroads of DNA replication and repair processes and the understanding of its complex functions in each remains incomplete. Since the expression of SRS2 is tightly regulated - both mRNA and protein levels reach a maximum in early S-phase [29, 30] - part of the complexity of its function is likely due to its roles at various stages of the cell cycle in different cellular contexts. In addition, overexpression of Srs2 is known to impair cell growth both in Saccharomyces cerevisiae and in Schizosaccharomyces pombe, although the basis of this toxicity remains unknown [12, 31, 32]. Therefore, we sought to investigate the regulatory roles of Srs2 by affecting its levels in the cell.
Here, we present three synthetic dosage lethality (SDL) screens designed to gain better insight into the multiple roles of Srs2. In the past, SDL screens have proven extremely powerful in identifying functional relationships between genes involved in the same processes or protein complexes [33–35]. For example, new components of the kinetochore complex were discovered by searching for mutations that are lethal in combination with the overexpression of one kinetochore component [36]. Importantly, SDL interactions do not necessarily overlap with classical synthetic lethal interactions and potentially reveal new genetic and/or physical associations ([37] and present study). SDL thus provides a complementary and global view of the complex genetic interactions in which a gene is involved. The study of SDL interactions of mutant versions of a gene should also provide additional functional relevance to the genetic interactions with the wild-type gene.
Thus, we have performed screens for genes required for cell growth in conditions of controlled overexpression of either wild-type SRS2, or two helicase-dead mutants, srs2-K41A, which is unable to bind ATP, and srs2-K41R, which can bind ATP but cannot hydrolyze it [38]. Upon screening the haploid yeast disruption library [39], we found 274 SDL interaction with genes involved in very diverse cellular functions. Then, we show that overexpression of SRS2 specifically perturbs replication progression independently of its helicase function. This effect is not mediated by Rad51, one of the known Srs2-interacting partners. Instead it relies on the interaction of Srs2 with the sumoylated PCNA replication clamp, as overexpression of an srs2 mutant that cannot interact with the clamp, srs2-R1, abolishes the replication defects. Importantly, overexpression of srs2-R1 also abolishes the sensitivity of many mutants found in the screens that affect diverse cellular functions.
2. Material and methods
2.1. Yeast strains and growth media
Strains deleted for genes in the BY4742 background were obtained from the Saccharomyces Genome Deletion Project [39]. The plasmids were transformed in these strains by lithium acetate transformations [40]. The genotypes of other strains used in this study are listed in Table 1. Yeast strains carrying an overexpression plasmid were grown in synthetic complete medium lacking leucine (SC-LEU): 20 µg/ml adenine, 20 µg/ml L-arginine, 20 µg/ml L-histidine, 30 µg/ml L-isoleucine, 30 µg/ml L-lysine, 20 µg/ml L-methionine, 50 µg/ml L-phenylalanine, 20 µg/ml L-tryptophan, 30 µg/ml L-tyrosine, 17 Ug/ml uracil, 150 µg/ml L-valine, 0.17% Yeast Nitrogen Base, 0.5% (NH4)2SO4, 2% D-glucose. To allow a proper cell cycle progression after alpha-factor arrest in G1, cells were released in SC-5X medium (5-fold enriched in the above aminoacid, adenine and uracil).
Table 1.
Strains
| Strain | Background | Genotype | Reference |
|---|---|---|---|
| BY4742 | S288C | MAT alpha his3Δ0 leu2Δ0 lys2Δ0 ura3Δ0 | [39] |
| ORD9833-3B | S288C |
MAT alpha tel1∷KanMX his3Δ0 leu2Δ0 lys2Δ0 ura3Δ0 |
This study |
| ORD9834-15B | S288C |
MAT alpha mec1∷URA3-kanR sml1∷KanMX his3Δ0 leu2Δ0 lys2Δ0 ura3Δ0 |
This study |
| ORT7009 | S288C |
MAT alpha mec1∷URA3-kanR tel1 ∷KanMX sml1∷KanMX his3Δ0 leu2Δ0 ura3Δ0 met15Δ0 |
This study |
| MK11241 | W303 |
ELG1 ∷13Myc ∷KanMX lys2∷Ty1Sup ade2-1(o) can1-100(o) ura3-52 leu2 3,112 his3del200 trp1del901 HIS3∷lys2∷ura3 his4∷TRP1∷his4 |
[52] |
| W8164-2C | W303 |
MATa CEN1-16∷Gal-K.lactis-URA3 trp1-1 his3-11,15 leu2-3,112 ura3-1 RAD5+ MET17 ADE2 LYS2 |
Reid RJD et al., submitted |
2.2. Plasmids
The CUP1 promoter was fused to SRS2, srs2-K41A or srs2-K41R by PCR using adaptamers [41]. The resulting PCR products were cloned into a centromeric plasmid pRS415 [42] carrying the selection marker LEU2, generating plasmids pWJ1509 (pCUP1-SRS2), pWJ1510 (pCUP1-srs2-K41A) and pWJ1511 (pCUP1-srs2-K41R). The srs2-R1 C-terminal mutation was amplified by PCR from pRS406-srs2-R1 (kind gift from Xavier Veaute) and introduced by cloning into pRS415 to generate pAM19 (pCUP1-srs2-R1), pAM20 (pCUP1-srs2-K41A-R1) and pAM21 (pCUP1-srs2-K41R-R1). ELG1-13 MYC was amplified by PCR from strain MK11241 (kind gift from Martin Kupiec) and cloned by gap-repair downstream of the CUP1 promoter of pWJ1512 [43], generating pAM53.
2.3. Synthetic Dosage Lethality screen
The SDL screen was done by selective ploidy ablation as previously reported [43]. Briefly, the universal donor strain W8164-2C was transformed with the LEU2-marked overexpression plasmids. This haploid strain carries sixteen GAL1 promoters that induce transcription across its 16 centromeres upon induction, perturbing correct chromosome segregation. It contains the K. lactis URA3 gene upstream of each of the GAL1 promoters, allowing for counter-selection of all of its chromosomes upon plating onto 5-fluoroorotic acid (5-FOA). The plasmid carrying donor strains were crossed to the 4827 MATα strains of the yeast disruption library, previously rearranged in plates containing 384 strains in quadruplicate. The diploids were plated onto galactose to allow missegregation of the chromosomes of the donor, and on 5-FOA plates lacking leucine to counter-select for cells that have kept chromosomes of the donor and select for the overexpression plasmid. Plating onto medium without or with 100 µM CuSO4 induces overexpression driven by the CUP1 promoter on the plasmid. The plates obtained were scanned and the images analyzed using ScreenMill [44]. We retained as SDL interactors the strains for which the colonies were more than 2-fold smaller with at least one of the overexpression plasmids than with the empty vector in the same growth conditions. To verify individual SDL interactions, the overexpression plasmids were transformed into the library strains. We verified by protein dot-blots that each of the transformants effectively overexpressed SRS2, srs2-K41A and srs2-K41R. Growth assessment by spot assays were performed upon overnight cultures in SC-LEU and dilution to obtain cultures at 107 cells/ml. Five serial dilutions to one tenth were done from this first dilution, and 10 µl of each of the five dilutions was spotted onto SC-LEU plates containing increasing amounts of copper sulfate (0, 50, 100 and 200 µM CuSO4). The plates were incubated for 3 days at 30°C and scanned. Among, the 73 SDL interactions with DNA metabolism-related genes originally found in the screens, eleven could not be tested either because no transformants could be obtained or because no transformants were found to effectively overexpress the SRS2 plasmids. Among the remaining 62 strains, 51 SDL interactions were definitively validated. Nine additional deletion strains not found in the screen but functionally related to the strains found were tested, 6 of which were found to be SDL with SRS2 or its mutants (CTF8, HHF2, RAD50, RAD55, RAD57, RAD59). Thereafter, we will refer to the SDL interactions of 57 genes related to DNA metabolism.
2.4. Protein analyses
For denaturing protein extractions, overnight cell cultures in SC-LEU were diluted and allowed to grow for 2 hours. Upon addition of 200 µM CuSO4 the cells were incubated for 4 hours to induce CUP1-promoter driven gene expression. The cultures were centrifuged, the pellets resuspended in 100 µl of cold 1.85 N NaOH, 7.5% 2-mercaptoethanol and incubated for 10 minutes in ice. Protein was precipitated upon addition of 30 µl of cold 50% Trichloroacetic acid (Sigma, T0699). After vigorous vortexing, the samples were incubated for another 10 minutes in ice, and the precipitates were recovered upon a 5-min centrifugation at 4°C, maximum speed. The precipitates were resuspended in 40 mM Tris pH6.8, 8 M Urea, 5% SDS, 0.1 mM EDTA, 7.5% 2-mercaptoethanol, 10 µg/ml Bromophenol Blue. The supernatants were recovered upon a 5 minute centrifugation at room temperature, maximum speed, frozen in liquid nitrogen and stored at −80°C. For native protein extractions, overnight cell cultures in SC-LEU were diluted and allowed to grow for 2 hours. Upon addition of CuSO4 the cells were incubated for 4 hours to induce CUP1-promoter driven gene expression. The extractions were done in ice. The pellets were resuspended in 50 mM Tris pH 8, 1% NP-40, 150 mM NaCl, 1 mM DTT, 30 mM NaF, 1mM PMSF, 0.75 µg/ml Pepstatin A, supplemented with Roche Complete protease inhibitors (Roche, 11836153001). For each phosphatase treatment, three samples were generated. First, an “untreated” sample, corresponding to the native extracts described above immediately denatured upon addition of 6X sample buffer (35 mM Tris pH 6.8, 30% Glycerol, 10% SDS, 0.6 M DTT, 0.012% bromophenol blue). Second and third, a “mock” sample and a “phosphatase-treated” sample. These were incubated for 1 hour at 30°C with 10X phosphatase buffer, 10X MnCl2, 20X Roche Complete phosphatase inhibitors (Roche, 11836153001) and without or with lambda-phosphatase (New England Biolabs, P0753S) respectively. The reactions were stopped by addition of 6X sample buffer. For protein blotting, samples were loaded on SDS-PAGE gels (6% for visualizing Srs2, 8% for visualizing Rad53 and 10% for visualizing alpha-tubulin). Migration was carried out at 150–200V in running buffer (25 mM Tris, 190 mM Glycine, 0.1% SDS), and the proteins were transferred at 25V in transfer buffer (12 mM Tris, 100 mM Glycine, 20% EtOH). The membranes were blocked in 5% milk – PBS Tween for 1 hour at room temperature, incubated with primary antibodies overnight at 4°C in 1% milk – PBS-Tween, and finally incubated with the secondary antibodies for 1 hour at room temperature. The anti-Srs2 antibody (Santa Cruz Biotechnology, sc-11989) was used as a 1:200 dilution. The anti-Rad53 antibody (Santa Cruz Biotechnology, sc-6749) was used as a 1:750 dilution. The anti-Myc antibody (Santa Cruz Biotechnology, sc-40) was used as a 1:200 dilution. The anti-alpha-Tubulin antibody (AbD Serotec, MCA77G) was used as a 1:2000 dilution. The secondary antibodies used were an HRP-coupled anti-goat antibody (Promega, V8051) as a 1:5,000 dilution, an HRP-coupled anti-rat antibody (Jackson ImmunoResearch Laboratories, 112035062) as a 1:10,000 dilution and an HRP-coupled anti-mouse antibody (Jackson ImmunoResearch Laboratories, 115035146) as a 1:10,000 dilution. Samples from time-courses after alpha-factor arrest were quantified using the RC DC Protein Assay (BioRad, 500-0120) to load the same amount of protein in each well.
2.5. Cell synchronization
MATa bar1Δ cells were grown at 30°C to exponential phase (OD600~0.5) in SC-LEU medium. To induce G1 arrest, cells were incubated for 2 hours with 50 ng/ml alpha-factor (GenWay, 06–271–83056). Thirty minutes prior to release, CuSO4 was added to 200 µM to induce CUP1 promoter-driven gene expression, and leucine was added to 300 µg/ml for a proper release. To release cells from the alpha-factor arrest, cells were washed twice with one volume of SC-5X medium containing 200 µM CuSO4 and 50 µg/ml pronase (Sigma, P6911). They were finally resuspended in one volume of the latter medium. After release, samples were collected every 15 minutes for FACS analysis and protein extraction.
2.6. Flow cytometry
Cells were prepared for FACS analysis as previously reported [45]. Briefly, cell pellets were fixed on a rotator at 4°C overnight in 70% ethanol. In 50 mM sodium citrate pH 7.0, cells were sonicated for 15 seconds at 30%, incubated with 0.25 mg/ml RNase A for 1 hour at 50°C, washed and finally stained with 16 µg/ml propidium iodide (Sigma, 81845). Samples were then analyzed on a Becton Dickinson FACScalibur, and the resulting data was compiled using WinMDI 2.9.
3. Results
3.1. Genes required for cell viability upon overexpression of SRS2 and/or its helicase mutants srs2-K41A and srs2-K41R
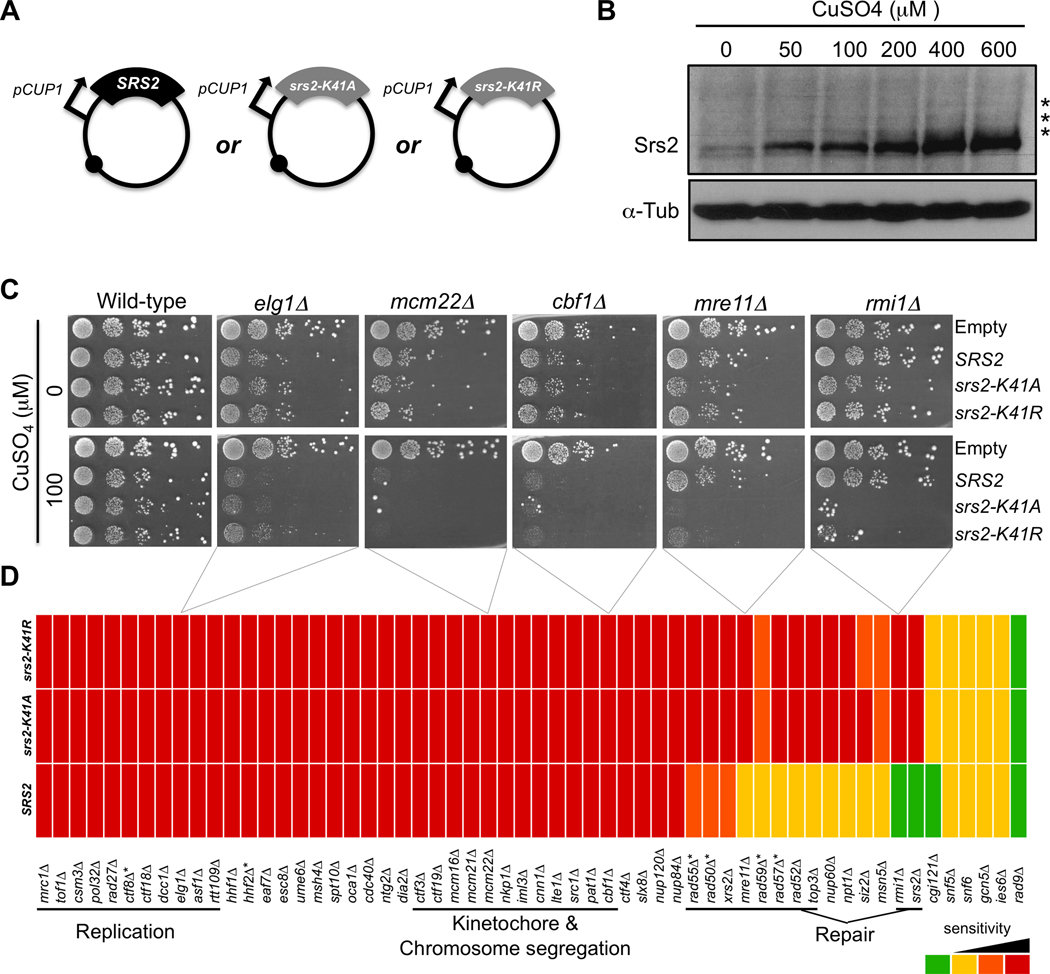
To identify the biological processes dependent on the dosage of the Srs2 protein, we carried out three synthetic dosage lethal screens. Plasmids overexpressing SRS2, the helicase mutant srs2-K41A, which is unable to bind ATP, and the helicase mutant srs2-K41R, which is capable of binding ATP but unable to hydrolyze it [38], were analyzed. To accomplish overexpression, the three genes were cloned into centromeric plasmids under control of the CUP1 promoter [46] (Fig. 1A). Expression levels of the proteins increase as a function of the amount of CuSO4 in the medium, allowing controlled overexpression (Fig. 1B). Individual plasmids were introduced into the 4827 haploid MATα strains deleted for non-essential open-reading frames [39]. With the exception of the srs2Δ strain from this library, all of the other strains contain the endogenous wild-type SRS2 so cells maintain an unperturbed level of the wild-type protein before overexpression is induced. The three overexpression plasmids and an empty vector control were transferred by selective ploidy ablation (SPA) into an arrayed version of the deletion library in which each strain is quadruplicated ([43] and Materials and methods). The plasmid-carrying strains were then replica-plated onto solid medium containing 100 µM CuSO4 to induce overexpression and were grown for three days. Cell growth was measured using ScreenMill, a colony size measurement and analysis tool [44]. We chose to screen for SDL interactions at 100 µM CuSO4 because SRS2 or the helicase-dead mutants did not affect the growth of wild-type cells when expressed at this level (Fig. 1C). We identified 274 SDL interactions corresponding to genes required for cell growth in the context of overproduction of at least one of the forms of the Srs2 protein.
Fig. 1.
Synthetic dosage lethal screen by overexpression of SRS2, srs2-K41A and srs2-K41R in the strains of the yeast disruption library. (A) Centromeric overexpression plasmids containing SRS2, srs2-K41A or srs2-K41R under control of the CUP1 promoter. (B) Denaturing protein extracts from wild-type cells overexpressing Srs2 upon addition of 0, 50, 100, 200, 400 or 600 µM CuSO4 to the growth medium. The extracts were migrated and revealed with an anti-Srs2 antibody and an anti-alpha-Tubulin antibody for a loading control. Asterisks mark post-translational modifications of the protein. (C) Manual verification of the SDL interactions with DNA metabolism genes. The interactions were found in the high-throughput screen and were verified by transforming the overexpression plasmids and an empty vector control into the deletion strains. The transformants were spotted in 10-fold serial dilutions onto medium without or with 100 µM CuSO4. Overexpression in wild-type cells is presented as a control. (D) Heat-map representing the sensitivity of the DNA metabolism strains verified manually. For each strain, the three colour boxes correspond to the degree of its sensitivity to each of the three versions of SRS2: wild-type SRS2 or helicase-dead mutants srs2-K41A and srs2-K41R. Red: severe growth defect. Orange and Yellow: intermediate growth defects. The differences between these two classes are subtle and attributed according to growth of the each strain with the empty control vector. Green: same growth profile as with the empty control vector. * Genes that were not found in the high throughput screen.
Table 2 lists the genes found in the screen sorted by cellular function. As expected for a regulator of homologous recombination, 68 genes involved or related to different aspects of DNA metabolism were found. A large majority was individually confirmed providing a final list of 57 genes (Material and methods). Additionally, genes involved in other diverse cellular functions were found. The major classes are vesicular traffic (40 genes), mitochondrial functions (38 genes), RNA metabolism (26 genes) and ribosome functions (19 genes). To check if these genetic interactions resulted from drastic change in Srs2 overexpression, we examined the level of Srs2 in the DNA metabolism genes further analysed below and in a random subset of 21 strains representing the other Gene Ontology categories. Compared to the wild-type cells, the maximal variation was 1.6-fold overexpression in bro1Δ cells (Supplementary Fig. S1). Therefore, the SDL interactions cannot be simply explained by a difference in the Srs2 overexpression levels. Rather, it reveals that, although SRS2 is mostly known for its role in DNA metabolism, tolerance to changes in its global level requires many cellular functions.
Table 2.
Genes required for viability upon overexpression of SRS2, srs2-K41A or srs2-K41R.
| Function DNA metabolism-related genes |
|
|---|---|
| DNA replication |
MRC1* TOF1* CSM3* CDC40* CTF4* CTF8* CTF18* CTF8 DCC1* DIA2 ELG1 POL32 RAD27 RNR4(a) |
| DNA Repair | MMS22(a) MRE11 NTG2 RAD50 RMI1 SRS2 TOF1* TOP3 XRS2 YLR235C(a) |
| Recombination | MSH4 RAD52 RAD55 RAD57 RAD59 |
| Checkpoint | MRC1* TOF1* CSM3* |
| Chromatin | HHF1 HHF2 |
| Chromatin Remodeling |
ASF1 CBF1 EAF7 ESC8 GCN5 IES6 NPT1 RTT109 SNF12(a) SNF5 SNF6 SPT10 UME6 |
| Telomere maintenance | CGI121 BUD32*(a) |
| Chromosome Segregation | CSM3* PAT1* SRC1 |
| Kinetochore | CHL4(a) CNN1 CTF3 CTF19 IML3 KRE28(a) LTE1* MCM16 MCM21 MCM22 NKP1 |
| Sister Chromatid Cohesion | CTF4* CTF8* CTF18* DCC1* |
| Cell Cycle | CLB2(a) CLN3(a) DOC1(a) LTE1* OCA1 |
| SUMO and Ubiquitin | SIZ2 SLX8 |
| Nuclear Pore | MSN5 NUP60 NUP84 NUP120 |
| Genes unrelated to DNA metabolism | |
| Budding | BUD21* BUD22 BUD31 BUD32* |
| Cell Wall | DFG5 SAC1 |
| Cytoskeleton | MSB3 SAC6 SAC7 SLA1 SLM6 |
| Lipid Metabolism | FAA1 |
| Mitochondria |
ACO1 ATP14 ATP15 ATP7 FMP33 FZO1 GGC1 GEP5 ISA2 ISM1 MDM12 MRPL11 MRPL15 MRPL22 MRPL23 MRPL27 MRPL28 MRPL37 MRPL38 MRPL7 MRPS5 MRPS9 MSM1 OMA1 POR1 QCR10 QCR2 RRF1 RSM22 RSM24 SAM37(b) SLS1 SSQ1 TOM5 TSR2 TUF1 YBL100C YKL169C YDR115W |
| Peroxisome | PEX15 |
| Ribosome |
ARX1(b) BUD21* JJJ1 KAP120 RPL16B RPL19A RPL20B RPL24A RPL31B RPL40A RPP2B RPS0B RPS10A RPS19B RPS1B RPS25A RPS9B RSA1 SSF1 |
| RNA Metabolism |
CDC40*(b) CKB2 DBP3 DUS4 LSM1 LSM6# MED1 MSL1 NOP12 NSR1 PAT1*(b) RNP1 RPA34 RRP6 RRP8(b) RTC3 SKI2(b) SKI3 SKI7 SKI8 SPT3 STB5 THP1 TRM10 YGL214W |
| Transcription | CCR4(b) NUT1# POP2(b) SPT2 SPT8 THO2 |
| Translation | EAP1(b) TIF3 |
| Vacuole | BSD2 KCS1(b) PPA1 TFP1 VAM10 VAM3# VAM6 VMA6(b) VAM7 VPH2 |
| Vesicular Traffic |
BRO1(b) CDC50 COG1 DOA4(b) EDE1 ERP2 FAB1 IMH1 PEP8 RAV2 RVS167(b) SNF8 SRN2 STP22(b) TLG2 VPS1 VPS13 VPS17 VPS20 VPS21 VPS24(b) VPS25(b) VPS27(b) VPS28 VPS29 VPS30 VPS36 VPS38 VPS4(b) VPS41 VPS45 VPS5 VPS51 VPS60 VPS61(b) VPS8 VPS9 YPT6 OPI8 YOR331C |
| Other |
AIM45 APQ13 CCH1 CIN5 CKA2 CLA4 CPR1 CSG2 CYS4 ELM1 EMC2 FET3# FTR1 FYV1 FYV5 GRE2 HEK2 HMG2 HSP31 HUR1 KEX1 LCB5 LTV1 MET7 OPI9 PDX3 PMP1 PMP3 PMT2(b) PRS3 PUF6 RCY1 RRT8 SEC66 SHE4 SRV2 SUR1 TDA1(b) TDA2(b) TDA4(b) TDA6(b) TDA11(b) THI20 TNA1(b) VRP1 YBP1 YVH1 YAR047C YCR051W YGL242C YGR160W YML094C-A YML122C YNR005C# YNR068C YOR364W |
All SDL interactions with DNA metabolism-related genes were individually verified, except for the genes marked with an.
Of the SDL interactions with genes unrelated to DNA metabolism, only the ones marked with a were verified.
The SDL interaction with wild-type SRS2 was verified
Genes involved in more than one function.
3.2. Cells overexpressing SRS2 or its helicase mutants impact DNA replication-related functions
We first examined the effects of overexpression of SRS2 on DNA metabolism genes (Table 2). We individually verified each of the SDL interactions related to DNA metabolic processes by transforming the overexpression plasmids into each strain and assessing the growth of the transformants by spot-assays on plates with or without CuSO4 (Materials and methods, Fig. 1C). After validation, 57 strains deleted for genes involved in DNA metabolism show sensitivity to overexpression of wild-type or mutant SRS2. These deletion strains behave differently with regard to the three overexpression plasmids. For example, like elg1Δ, mcm22Δ and cbf1Δ (Fig. 1C), a subset of strains is equally sensitive to all three alleles of SRS2. In contrast, strains like mre11Δ and rmi1Δ (Fig. 1C) are very sensitive to overexpression of the helicase mutants srs2-K41A and srs2-K41R, but are less or not at all sensitive to wild-type SRS2. Fig. 1D compiles, in a heat-map, the sensitivity profiles of all the strains that were independently tested. Overall, overexpression of wild-type and helicase-mutant SRS2 is very toxic when DNA metabolism is compromised.
The sensitivity profiles of the strains shown in Fig. 1D show that functionally-related genes exhibit a similar phenotype. For example, cells deleted for genes involved in DNA replication or in kinetochore structure and chromosome segregation are equally sensitive to wild-type or helicase-dead SRS2, whereas cells deficient in DNA repair are more sensitive to the helicase mutants than to wild-type SRS2. Moreover, among the mutants deficient in homologous recombination, only a subset (rad52Δ, rad55Δ, rad57Δ, rad59Δ, mre11Δ, rad50Δ and xrs2Δ) is sensitive to overexpression of helicase-dead srs2, while rad51Δ and rad54Δ are not.
Surprisingly, no case was found in which sensitivity to wild-type SRS2 was suppressed by inactivating the helicase function of the gene, suggesting that its helicase function is not responsible for the SDL interactions. Instead, several strains are more sensitive to the helicase mutants than to wild-type SRS2. Therefore, the lack of helicase function makes the mutant protein more toxic, further compromising DNA metabolism and requiring an extra set of genes that are involved in DNA repair processes. To decipher the complex effect of overexpression of SRS2 and its helicase mutants, we more closely examined the molecular and cellular consequences of overproduction of these proteins.
3.3. Overexpression of wild-type and helicase-dead SRS2 requires the DNA replication checkpoint
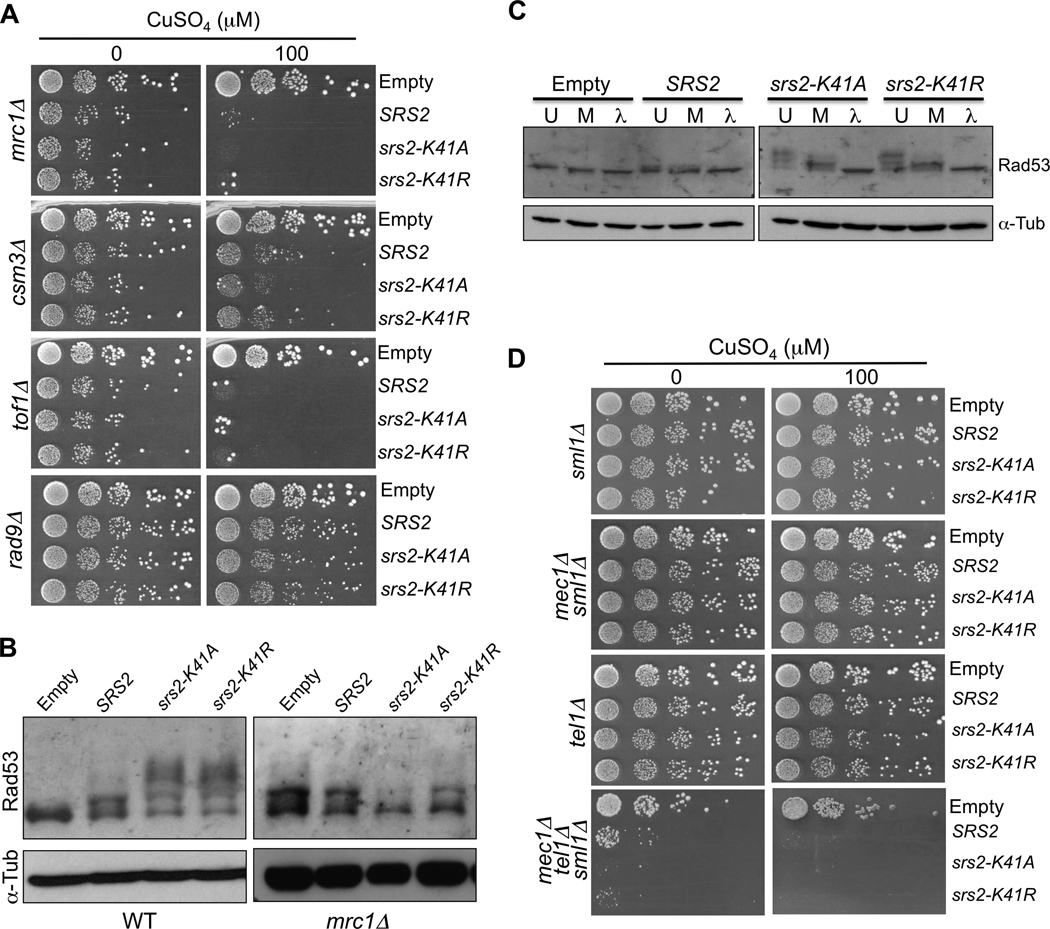
Deletion of genes involved in DNA replication, including the three checkpoint mediators MRC1, TOF1 and CSM3, causes sensitivity to overexpression of all three versions of SRS2 (Figs. 1D and 2A). However, deletion of another checkpoint mediator, RAD9, does not affect sensitivity to overexpression of any of the alleles of SRS2 (Fig. 2A). While MRC1, TOF1 and CSM3 mediate the replication checkpoint [47–50], RAD9 mainly mediates the DNA damage checkpoint [48, 49, 51], suggesting that overexpression of wild-type and helicase-dead SRS2 affects replication but does not directly cause DNA lesions.
Fig. 2.
Overexpression of wild-type and helicase-dead SRS2 triggers the activation of the replication checkpoint. (A) mrc1Δ, csm3A, tof1Δ or rad9Δ mutants containing the overexpression plasmids were diluted in 10-fold serial dilutions and spotted onto medium without or with copper (100 µM CuSO4). (B) Denaturing protein extracts of wild-type or mrc1Δ cells overexpressing wild-type or mutant SRS2 at 200 µM CuSO4. The proteins were migrated on an acrylamide gel and revealed with antibodies against Rad53 and alpha-Tubulin for a loading control. (C) Rad53 phosphorylation. Native extracts of wild-type cells overexpressing wild-type or mutant SRS2. The extracts were either directly denatured after extraction (U: untreated), or incubated for at 1 hour at 30°C with phosphatase buffer, MnCl2 and protease inhibitors (M: mock), or finally treated with λ-phosphatase for 1 hour at 30°C with phosphatase buffer, MnCl2 and protease inhibitors (λ: λ-phosphatase treated). Note that the Rad53 is partially unphosphorylated in “Mock” samples suggesting the presence of phosphatases in the cellular extracts. (D) sml1Δ, mec1Δ sml1Δ, tel1Δ or mec1Δ tel1Δ sml1Δ mutants containing the overexpression plasmids were diluted in 10-fold serial dilutions and spotted onto medium without or with copper (100 µM CuSO4).
Next, we examined the status of the Rad53 checkpoint protein. Slow migrating forms of the Rad53 protein appear upon overexpression of SRS2 and even more extensively with the helicase-dead mutants (Fig. 2B, left panel). Noticeably, the helicase-dead mutants cause a more extensive shift of Rad53 (Fig. 2B and Fig. 2C), consistent with the observation that these mutants are often more toxic than wild-type SRS2. These forms correspond to phosphorylated versions of Rad53, as they are sensitive to a phosphatase treatment (Fig. 2C). These results, together with the requirement of the replication checkpoint mediators MRC1, TOF1 and CSM3 argue that overexpression of SRS2 or the helicase-dead mutants activates the DNA replication checkpoint.
To establish a link between the replication checkpoint mediators and the activation of Rad53, we investigated the phosphorylation state of Rad53 in mrc1Δ cells. In the absence of MRC1, Rad53 is constitutively phosphorylated in control cells that do not overexpress Srs2 (Fig. 2B, right panel), as previously reported [48], and no additional phosphorylation (upper smear bands) occurs upon overexpression of SRS2 or the helicase-dead mutants. Finally, we also examined overexpression in mec1Δ, tel1Δ and mec1Δ tel1Δ strains (where deletion of MEC1 was combined with deletion of SML1 to suppress lethality). As illustrated in Fig. 2D, Srs2 overexpression is tolerated in single mutants but is very toxic in the absence of both Mec1 and Tel1. Altogether, these results directly link checkpoint activation, as measured by Rad53 phosphorylation, with the requirement of the Mec1/Tel1 kinases and the Mrc1 mediator for cell growth upon SRS2 overexpression.
3.4. Overexpression of wild-type and mutant SRS2 delays S-phase progression
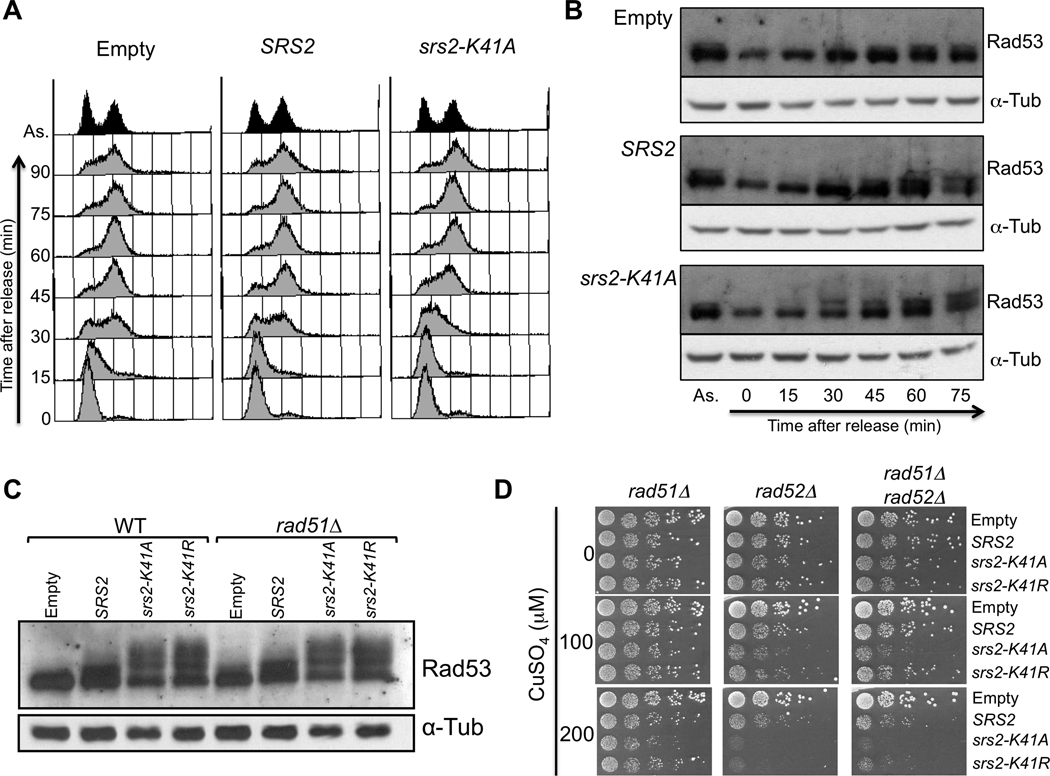
Next, we investigated cell cycle progression. Cells overexpressing wild-type or helicase-dead SRS2 were arrested in G1 using alpha-factor and overexpression was induced by addition of CuSO4 prior to release from the G1 arrest. Progression of the resulting synchronous cultures was assessed by flow cytometry to evaluate DNA content (Fig. 3A). Upon release, control cells containing an empty vector begin entering S-phase by 15 minutes, and complete replication between 45 and 60 minutes. Cells overexpressing SRS2 show a similar progression through the cell cycle, although a slight delay is detected at 45 minutes post-release. This delay is aggravated in cells overexpressing the helicase mutant srs2-K41A as seen by FACS at 30 and 45 minutes post-release. However, the cells manage to fully complete replication between 60 and 75 minutes (Fig. 3A).
Fig. 3.
Overexpression of wild-type or helicase-mutant SRS2 delays S-phase progression and acts independently of RAD51. (A) MATa bar1Δ cells containing an empty vector control or the plasmids for overexpression of SRS2 or srs2-K41A were synchronized in G1 with alpha factor. To induce overexpression, 200 µM CuSO4 were added 30 minutes prior to release, and the induction was kept upon release. Samples were collected every 15 minutes for FACS analysis. (B) Cells were synchronized as described in (A) and samples were collected for protein extractions. The proteins were migrated on an acrylamide gel and revealed with antibodies againt Rad53 and alpha-Tubulin for a loading control. (C) Denaturing protein extracts of wild-type or rad51Δ cells overexpressing wild-type or helicase-dead SRS2. The extracts were revealed with antibodies against Rad53 and alpha-tubulin as a loading control. (B) rad51Δ, rad52Δ or rad51Δ rad52Δ mutants containing the overexpression plasmids were diluted in 10-fold serial dilutions and spotted onto medium without or with copper (100 µM and 200 µM CuSO4).
Total protein extracts from the same synchronous cultures shown in Fig. 3A reveal the status of Rad53 through the cell cycle (Fig. 3B). In asynchronous populations, before CuSO4 induction, Rad53 is phosphorylated at a low level in cells containing an empty vector, SRS2 or srs2-K41A (as indicated by the slow migrating band in Fig. 3B, lanes labeled “As.”). In the cells containing the empty vector, G1 cells (0 time-point) show no detectable phosphorylation of Rad53. A low level of phosphorylation then appears at 45 minutes, when replication is almost complete. Like the control cells, synchronous cells overexpressing SRS2 begin to show Rad53 phosphorylation at 45 minutes post-release, with Rad53 becoming more extensively phosphorylated at 60 and 75 minutes. Cells overexpressing srs2-K41A show an increased phosphorylation of Rad53 as early as 30 minutes post-release, which correlates with the time at which the S-phase progression defect is detected by flow cytometry. Thus, overexpression of SRS2 and to a larger extent, overexpression of srs2-K41A, causes an S-phase progression delay associated with the induction of Rad53 phosphorylation (Fig. 2E), i.e. the activation of the replication checkpoint.
3.5. The toxicity of SRS2 and the helicase-dead mutants on DNA replication is independent of RAD51
Since a well characterized role of Srs2 is its anti-recombinase function and the dismantling of Rad51 presynaptic filaments [12, 13, 21], we hypothesized that the inability to regulate Rad51 filaments could explain at least part of the toxicity induced by overexpression of helicase-dead SRS2. This view would account for the fact that rad51Δ cells are not sensitive to overexpression of these mutants while other recombination mutants are sensitive and predicts that deleting RAD51 would abolish the toxic effects of the helicase-dead mutants on DNA integrity and specifically on DNA replication. However, Rad53 is still strongly phosphorylated in a rad51Δ strain upon overexpression of wild-type or helicase-dead SRS2 (Fig. 3C). Furthermore, in a rad52Δ strain, deleting RAD51 does not abolish sensitivity to the helicase mutants (Fig. 3D). These results show that the toxicity of the overexpressed SRS2 genes is not mediated by the Rad51 protein, but rather the excess Srs2 protein is causing its toxic effects directly. Additionally, Rad51-dependent homologous recombination is not important for overcoming the toxic effects inflicted by the srs2 helicase mutants or by wild-type SRS2, since rad51Δ cells are not sensitive to their overexpression.
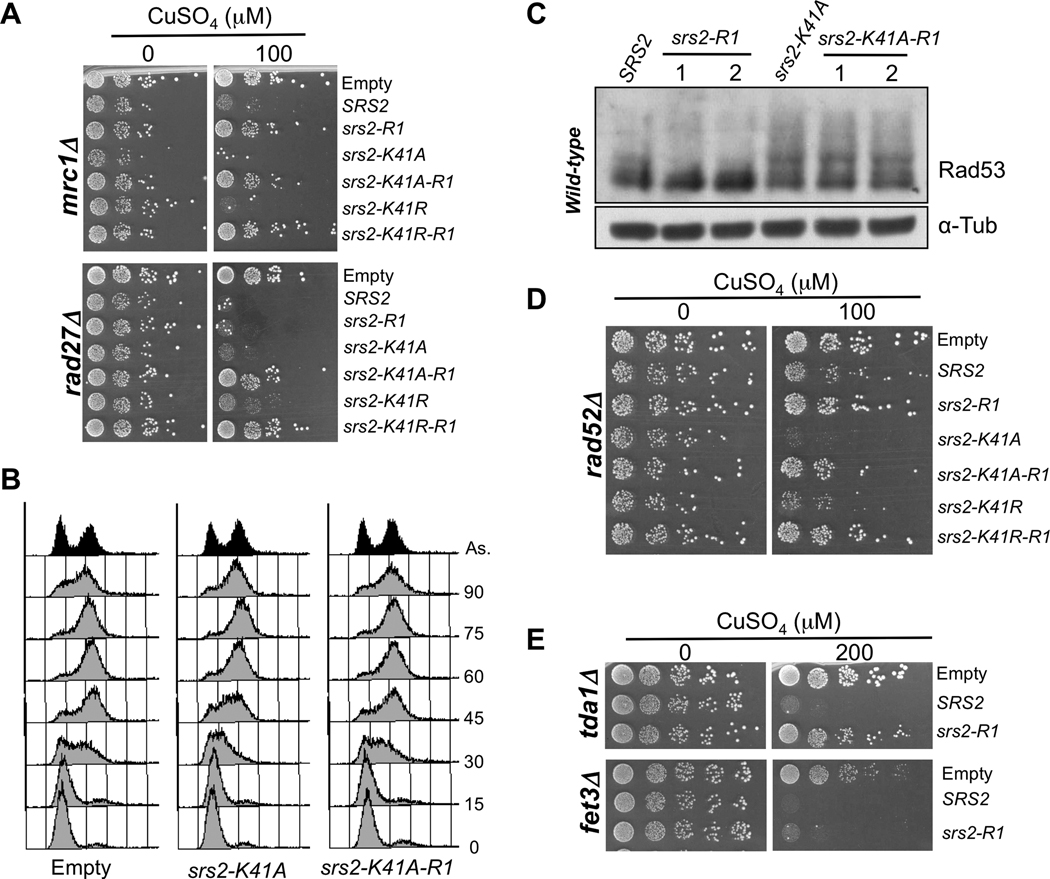
3.6. The effect on replication depends on the domain of Srs2 responsible for its interaction with SUMOylated PCNA
Srs2 interacts with the PCNA replication clamp when the clamp is SUMOylated during S-phase [14–16]. To test whether this interaction is involved in the effect of overexpressed Srs2 on SDL interactions, DNA replication and Rad53 phosphorylation, we examined the behavior of a mutated version of SRS2, srs2-R1, unable to interact with PCNA [20], and also combined -R1 with the helicase mutations. As illustrated in Fig. 4A, overexpression of srs2-R1 no longer sensitizes mrc1Δ cells and -R1 suppresses the sensitivity of mrc1Δ cells to helicase-dead srs2. Globally, srs2-R1 overexpression suppresses the SDL interactions with several replication mutants (cnn1Δ, csm3Δ, ctf3Δ, ctf8Δ, ctf18Δ, dcc1Δ, elg1Δ, hhf1Δ, hhf2Δ, nkp1Δ, pol32Δ, tof1Δ) and partially suppresses the sensitivity of the mec1Δ tel1Δ double mutant, in both the presence or absence of helicase function. This is not due to a decrease in protein since the -R1 mutant protein levels are comparable to Srs2, Srs2-K41A and Srs2-K41R levels at the same CuSO4 concentration (Supplementary Fig. S1C). Surprisingly, in rad27Δ cells, srs2-K41A-R1 or srs2-K41R-R1 mutations abolish the growth defect associated with srs2-K41A or srs2-K41R overexpression, but the -R1 mutation does not abolish the toxicity of SRS2 overexpression (Fig. 4A). Therefore, our results suggest that the -R1 mutant proteins have lost the ability to perturb DNA replication. Accordingly, the S-phase progression delay induced by srs-K41A is no longer seen in srs2-K41A-R1 cells (Fig. 4B). Finally, phosphorylation of Rad53 is greatly diminished upon overexpression of srs2-R1 compared to SRS2 (Fig. 4C). However, loss of the PCNA interaction did not abolish the Rad53 phosphorylation induced by the srs2-K41A mutation, suggesting that part of the checkpoint activation induced by the helicase mutants does not depend on their recruitment to replication forks.
Fig. 4.
The toxicity of Srs2 during replication depends on the domain that interacts with sumoylated PCNA. (A) mrc1Δ or rad27Δ cells transformed with an empty vector or with overexpression plasmids for SRS2, srs2-R1, srs2-K41A, srs2-K41A-R1, srs2-K41R and srs2-K41R-R1. 10-fold serial dilutions of the transformants were spotted on medium without or with copper (100 µM CuSO4). (B) MATa bar1Δ cells containing an empty control vector or the plasmids for overexpression of srs2-K41A or srs2-K41A-R1 were synchronized in G1 with alpha factor. To induce overexpression, 200 µM CuSO4 were added 30 minutes prior to release, and the induction was kept upon release. Samples were collected every 15 minutes for FACS analysis. (C) Protein extracts from wild-type cells overexpressing plasmids for SRS2, srs2-R1, srs2-K41A or srs2-K41A-R1, revealed with antibodies against Rad53 and alpha-Tubulin. 1 and 2 correspond to two independent transformants for srs2-R1 and srs2-K41A-R1. (D) Same as in (A) but with rad52Δ cells. (E) tda1Δ and fet3Δ cells transformed with an empty vector or with overexpression plasmids for SRS2 and srs2-R1. 10-fold serial dilutions of the transformants were spotted on medium without or with copper (200 µM CuSO4).
Then we tested whether the −R1 mutation affects the sensitivity of those mutants that are only sensitive to helicase-dead srs2-K41A and srs2-K41R. Interestingly, rad52A cells are no longer sensitive to srs2-K41A-R1 or srs2-K41R-R1 (Fig. 4D). The same suppression is seen in other repair deficient strains, ccr4Δ, mre11Δ, rmi1Δ, top3Δ, suggesting that the helicase defect lead to DNA replication-dependent accumulation of structures that are resolved by recombination and repair.
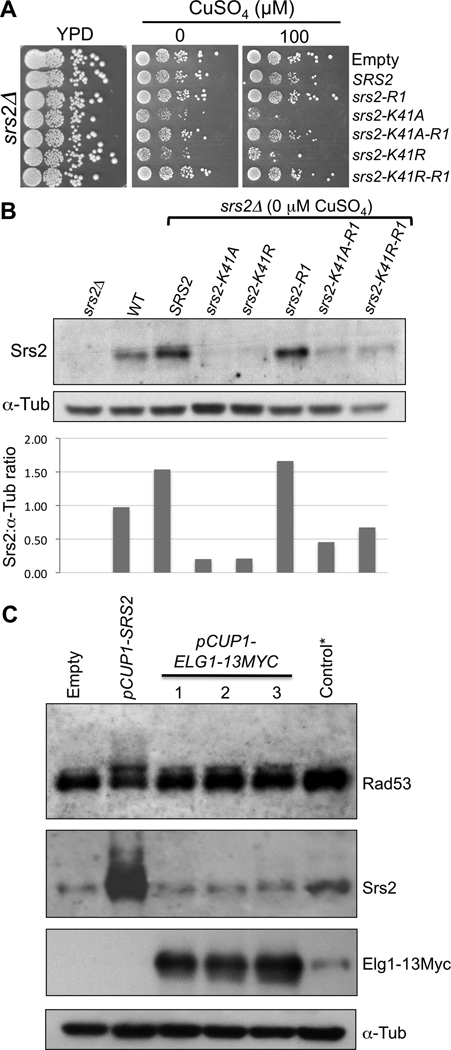
Moreover, we noticed that srs2Δ cells are sensitive to the helicase mutants even in the absence of induction (0 µM CuSO4) and this sensitivity is suppressed by the −R1 mutation (Fig. 5A). Indeed, in the absence of CuSO4, helicase-mutant protein expression in srs2Δ cells is even lower than that of endogenous Srs2 (Fig. 5B).
Fig. 5.
Specificity and relevance of Srs2 overexpression. (A) srs2Δ cells transformed with an empty vector or with overexpression plasmids for SRS2, srs2-R1, srs2-K41A, srs2-K41A-R1, srs2-K41R and srs2-K41R-R1. 10-fold serial dilutions of the transformants were spotted on YPD as a spotting control and on medium without or with 100 µM CuSO4. (B) Denaturing protein extracts of srs2Δ cells expressing SRS2, srs2-K41A, srs2-K41R, srs2-R1, srs2-K41A-R1 or srs2-K41R-R1 at 0 µM CuSO4. Control extracts from srs2Δ cells with an empty vector and wild-type cells with an empty vector were used. The proteins were revealed with an anti-Srs2 antibody and anti-alpha-Tubulin antibody as a loading control. Total Srs2 protein was quantified relative to the amount of alpha-tubulin in each extract. (C) Overexpression of Elg1 does not induce a high Rad53 phosphorylation. Denaturing protein extracts of wild-type cells overexpressing SRS2 or ELG1-13MYC (three different clones) at 200 µM CuSO4. *Control cells with endogenous tagged ELG1-13MYC. The proteins were revealed with ntibodies against Rad53, Srs2, Myc and alpha-Tubulin.
Finally, unlike overexpression of Srs2, overexpression of Elg1, another protein known to preferentially interact with sumoylated PCNA [52] does not induce a strong Rad53 mobility shift (Fig. 5C). Together, these results demonstrates that even low levels of the helicase mutant proteins can perturb the replication fork, and that their overexpression is not simply clogging the PCNA clamp, nor a general property of PCNA-interacting proteins.
3.7. Srs2-R1 also suppresses the SDL interactions with deletions affecting various cellular functions
As listed in Table 2, overexpression of Srs2 impairs growth not only in mutants deficient in DNA metabolism processes, but also in mutants of RNA metabolism, ribosomal functions, mitochondrial functions, vesicular traffic, etc. To test whether or not the SDL interactions with these various classes of functions depended on the interaction of Srs2 with sumoylated PCNA, we examined the effect on cell growth of overexpressing Srs2-R1 in 18 randomly chosen mutants from these various cellular processes. Remarkably, 17 SDL interactions are suppressed by the srs2-R1 mutation: tda1Δ, tda2Δ, tda4Δ, tda6Δ, tda11Δ, bro1Δ, doa4Δ, stp22Δ, vps4Δ, vps24Δ, vps25Δ, vps27Δ, vam3Δ, ynr005cΔ, eap1Δ, nut1Δ, lsm6Δ. Thus, the sensitivity of these strains to Srs2 overexpression is likely due to replication defects. In contrast, fet3Δ cells, deficient in iron ion transport, are very sensitive to both SRS2 and srs2-R1 overexpression (Fig. 4E), uncovering a novel type of genetic interaction that will warrant further investigation.
4. Discussion
Srs2 is a 3’-5’ DNA helicase with multiple roles at the crossroads of DNA metabolism processes. To gain better insight into the complexity of these roles, we performed three synthetic dosage lethal screens by overexpression of SRS2 and two helicase-dead mutants, srs2-K41A and srs2-K41R, in the 4827 strains of the haploid yeast disruption library. We find that overexpression is toxic in a diverse set of deletion mutant strains showing that many cellular functions are involved in resistance to the activities of SRS2. We demonstrate that SRS2 and the helicase-dead mutants cause toxicity associated with DNA replication defects and depends on the interaction with sumoylated PCNA. In addition, the physiological sensitivity of yeast cells to the overexpression of Srs2 may explain the long-standing observation that it is toxic in some genetic backgrounds [12, 31, 32].
4.1. A variety of cellular functions are required for cell growth upon overexpression of SRS2
From our large-scale SDL screens, we find 274 genes required for cell growth upon overexpression of SRS2, srs2-K41A and/or srs2-K41R. Remarkably, these genes are involved in a variety of cellular processes (Table 2). A large number clearly relate to DNA metabolism functions, as expected for a gene that plays an important role in regulating many aspects of DNA recombination and repair. Consistent with the known functions of Srs2, thirty of these genes show a synthetic growth defect with RAD51 (Supplementary Table S1). More surprisingly, other well-represented Gene Ontology categories, e.g., vesicular trafficking, mitochondrial function, RNA metabolism and ribosomal function, are also needed for cell growth upon overexpression of SRS2 or the helicase mutants. It is important to note that most of these unexpected functions are not systematically uncovered in other SDL screens (([33–37], RJDR, JCD and RR, unpublished observations), indicating that the corresponding processes are not affected by the overexpression of just any protein in the cell. The conclusion that these SDL interactions are specific to a function of Srs2 is reinforced by the observation that cell growth is not impaired in the vast majority of mutants upon overexpression of the srs2-R1 separation-of-function mutant protein.
Upon literature data mining, we noted that 57 of the 206 genes, which at first approximation are not known to be associated with DNA metabolism, actually have reported phenotypes related to DNA metabolic processes (e.g., sensitivity to hydroxyurea, camptothecin, methylmethane sulfonate, ionizing radiation, UV or abnormal telomere length or increased recombination centers) (Supplementary Table S2). Thus, for these 57 genes, the SDL interactions with SRS2 and its mutants are likely due to an interference with DNA metabolism. Furthermore, although the data from the previously cited screens may contain false positives, these same genes are also SDL with SRS2 suggesting that they should be classified in DNA metabolism-related gene ontology processes. One of the unexpected classes, vesicular trafficking genes, was also enriched in an SDL screen with a mutant version of TOP1, top1-TA, which mimics camptothecin-induced damage [43]. The Top1-TA protein nicks double-stranded DNA, but remains covalently attached to it, thus generating DNA damage [53]. It will be interesting to investigate how Top1-TA and Srs2 overexpression, which both affect genome integrity, require genes involved in vesicular trafficking.
4.2. The anti-recombinase function of Srs2 is not responsible for its overexpression effects
Srs2 is an important anti-recombinase that displaces Rad51 presynaptic filaments from single-stranded DNA in a helicase-dependent manner [12, 13, 21, 38]. By overexpressing Srs2, we expected to uncover deletion mutants sensitive to a low cellular level of RAD51-dependent recombination. If this view were correct, we would expect the SDL genes from overexpression of SRS2 to be known synthetic lethal with RAD51. However, only 30 of the 274 SDL interacting genes found in our screens are synthetic lethal with RAD51 (Supplementary Table S1). Moreover, for these 30 genes, most are SDL not only with SRS2, but also with the helicase-dead mutants srs2-K41A and srs2-K41R. Therefore, the SDL interactions do not rely on the helicase function of Srs2 and the lethality is not due to an excessive Srs2 anti-recombinase activity.
4.3. Overproduced Srs2 affects DNA replication through its domain that interacts with sumoylated PCNA
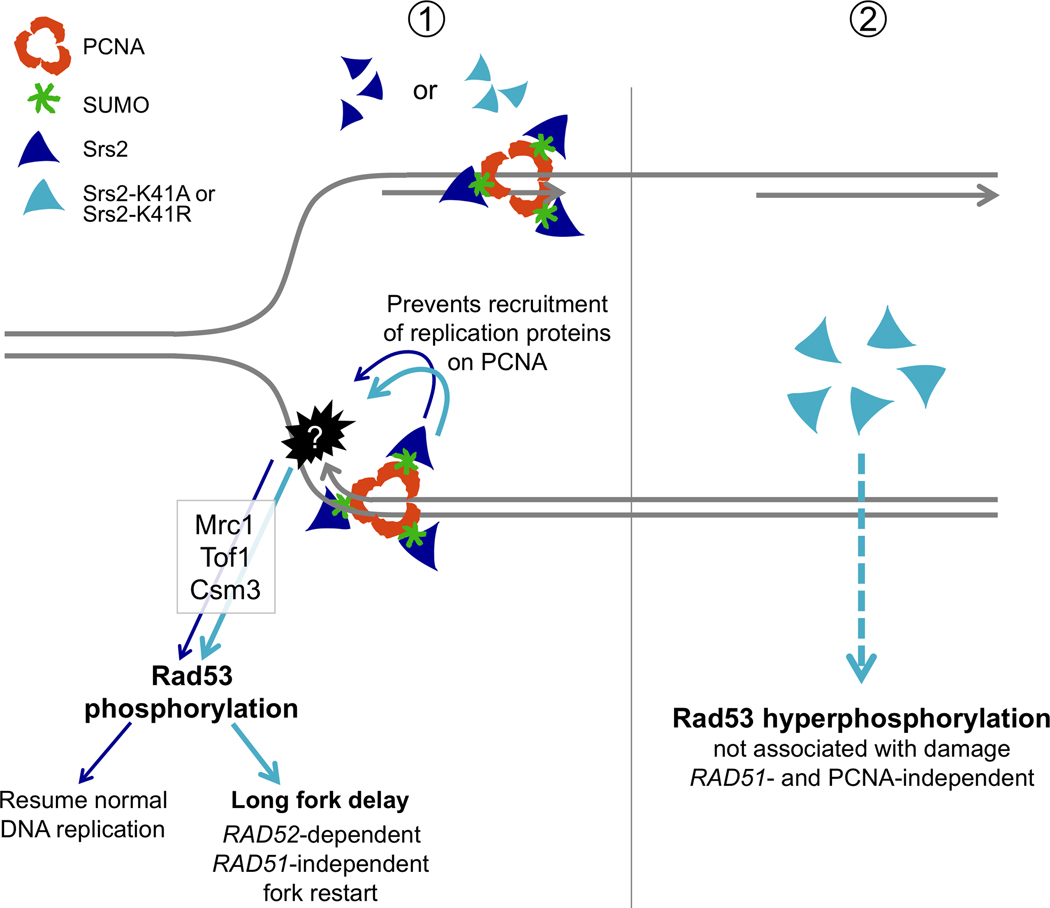
By investigating the cellular consequences of Srs2 overexpression, we find that wild-type or helicase-mutated Srs2 primarily affect progression of DNA replication. First, a number of key non-essential genes involved in DNA replication are required for cell growth when SRS2, srs2-K41A or srs2-K41R are overexpressed (Table 2). Second, SRS2 and its mutants cause replication checkpoint activation dependent on the Mec1/Tel1 kinases, mediated by Mrc1, Tof1 and Csm3 and resulting in the phosphorylation of Rad53 (Figs. 1 and 2). Third, SRS2 and to a larger extent the helicase-dead mutants induce an S-phase progression delay, which is temporally associated with the activation of the checkpoint (Fig. 3). Interestingly, most of these defects are abolished by mutating the PCNA interaction domain on SRS2 or the helicase-dead mutants (srs2-R1, srs2-K41A-R1 or srs2-K41R-R1) [20] (Fig. 4). These separation-of-function mutants suggest that Srs2 wild-type or helicase-dead proteins are recruited to replication forks through their interaction with PCNA, possibly generating fork progression defects that trigger checkpoint activation. Since the srs2-R1 mutation abolishes the SUMO interacting motif of Srs2 (SIM) [16,20], we assume that it is the interaction with sumoylated PCNA that is important, more than the interaction with the unmodified clamp. Formally, if Srs2 interacts with other sumoylated proteins through the same binding site, other targets cannot be excluded. Alternatively, Srs2 may directly activate the replication checkpoint upon its recruitment by PCNA without affecting or compromising fork stability. However, the growth of mrc1Δ, tof1Δ and csm3Δ cells would not be affected in this case. Since their viability is compromised upon overexpression of SRS2 and its mutants, we favor a model in which the excessive presence of Srs2, regardless of the status of its helicase activity, perturbs fork stability, which in turn activates the replication checkpoint controlled by these proteins. Our studies also show that overexpression of the srs2-K41A and srs2-K41R helicase mutants is more toxic than SRS2 and requires additional genes involved in DNA repair (RAD52, MRE11, RAD50, XRS2, NTG2, etc., Fig. 1D). Interestingly, this toxicity is also abolished with the separation-of-function mutants, srs2-K41A-R1 or srs2-K41R-R1, again suggesting that it is through DNA replication that the helicase-dead mutants generate DNA damage or an aberrant intermediate that requires repair genes for resolution. Thus, as depicted in the model presented in Fig. 6, we propose that excess Srs2 recruited to the PCNA replication clamp affects normal replication progression (Panel 1). Perhaps Srs2 prevents other DNA replication factors from easily accessing the fork. Since Srs2 protein levels are highest at the beginning of S-phase [29, 30], we suggest a helicase-independent role for Srs2 during S-phase, which differs from its anti-recombinase function, to prevent the recruitment of proteins that might compromise replication. Our findings likely reflect this role for the endogenous protein, since even very low levels of Srs2-K41A or Srs2-K41R are toxic to srs2Δ and this toxicity is suppressed by mutating the −R1 domain responsible for the interaction with sumoylated PCNA (Fig. 5 A and 5B).
Fig. 6.
Model representing the effects of overexpression of SRS2 and its helicase-dead mutants on DNA replication. ① Wild-type or helicase-dead SRS2 are recruited to replication forks by SUMOylated PCNA. This interaction likely prevents the recruitment of other factors required for replication, leading to fork progression delay, which activates the replication checkpoint (Mrc1, Tof1 and Csm3-mediated phosphorylation of Rad53). Cells overexpressing SRS2 eventually overcome this defect. The helicase-dead mutants srs2-K41A or srs2-K41R show a greater replication delay that likely leads to the accumulation of aberrant intermediates that require RAD52-dependent, RAD51-independent recombination to resume replication. ②The helicase-dead mutants induce activation of the DNA integrity checkpoint, resulting in hyperphosphorylation of Rad53. This effect is independent of DNA replication, is not associated with any damage and likely results from the accumulation of an Srs2 protein capable of triggering checkpoint activation.
rad27Δ cells are unique in that Srs2 toxicity is independent of its interaction with the PCNA clamp, but toxicity depends on the helicase function of the protein (Fig. 4A). As rad27Δ cells require homologous recombination for survival [54–56], SDL interaction of SRS2 with RAD27 may be due to an increased inhibition of recombination by srs2-R1 that does not occur upon overexpression of the helicase-deficient mutants srs2-K41A-R1 and srs2-K41R-R1. Alternatively, since Rad27 is a flap endonuclease important for Okazaki fragment maturation, this particular SDL may reflect a helicase-dependent role of Srs2 in processing Okazaki fragments.
In contrast to overexpression of the srs2-R1 mutant, phosphorylation of Rad53 is not abolished or diminished by overexpression of srs2-K41A-R1 or srs2-K41R-R1 (Fig. 4C). Therefore, the helicase mutants induce Rad53 phosphorylation independently of their PCNA-dependent effect on DNA replication. It is possible that the overproduced mutant protein itself interacts with checkpoint proteins inducing their activation, but not inducing DNA damage per se. This view is supported by the fact that rad9Δ cells are not sensitive to overexpression of any of the SRS2 constructs (Fig. 2B) and that repair deficient strains, rad52Δ, mre11Δ, rmi1Δ, srs2Δ, top3Δ and ccr4Δ, are not sensitive to the srs2-K41A-R1 and srs2-K41R-R1 separation-of-function mutants. Since the helicase-dead mutants do not induce massive Rad53 phosphorylation in mrc1Δ (Fig. 2D), it is likely that the artificial checkpoint activation still depends on the Mrc1 mediator.
Together, the observations that deletion of SRS2 leads to insufficient Rad53 activation [24], while overproduction leads to its hyperactivation highlights the important contribution of Srs2 in the DNA damage response. As illustrated in Fig. 6 (Panel 2), we hypothesize that the overexpressed helicase mutants of SRS2 trigger checkpoint activation that is not associated with DNA damage or with the interaction of Srs2 with the PCNA replication clamp.
In conclusion, we find that the growth defect that can be induced by Srs2 overexpression [12, 31, 32] is primarily due to its toxicity on DNA replication and depends on its capability to interact with the PCNA replication clamp. In addition, our data suggest a new function for endogenous Srs2 at replication forks that does not depend on its helicase activity. Remarkably, this role is responsible for many of the SDL interactions reported here, suggesting that a wide variety of cellular pathways are affected when the integrity of the replication fork is compromised.
Supplementary Material
Levels of overexpression in different SDL interacting strains. (A) Denaturing protein extracts of different strains from the SDL screen that overexpress SRS2 at 200 µM CuSO4. The proteins were migrated on an acrylamide gel and revealed with anti-Srs2 antibody and anti-alpha-Tubulin antibody as a loading control. (B) Quantification of the overexpression in A for each strain. The quantification was done using the open access software ImageJ and the levels of Srs2 protein were normalized to those detected upon overexpression of SRS2 in wild-type cells. (C) srs2-R1 expression. Denaturing protein extracts of wild-type cells overexpressing SRS2, srs2-R1, srs2-K41A or srs2-K41A-R1 at 200 µM CuSO4.
Acknowledgments
We thank the members of our laboratories for constructive discussions; Rebecca C. Burgess, Jean-Baptiste Boulé and Lumir Krejci for critical reading of the manuscript; Valérie Borde for constructive discussions on this work; Xavier Veaute for providing the srs2-R1 mutant; Martin Kupiec for providing the ELG1-13MYC construct. This work was supported by grants awarded to AN laboratory from Institut Curie, The Centre National de la Recherche Scientifique and La Ligue Nationale contre le Cancer “Equipe labélisée LIGUE 2007 and 2010”. AMLO received graduate student fellowships from the Ministère de l’Education Nationale, de la Recherche et de la Technologie and from the Fondation pour la Recherche Médicale. Work was also supported in part by National Institutes of Health grants CA125520, GM50237, and HG01620 awarded to R.R., CA009503 and GM008798 to J.C.D. and HG00193 to R.J.D.R. AMLO performed the experiments; AMLO and JCD analyzed the data; AMLO, RJDR, RR and AN designed the experiments; AMLO, RR and AN wrote the manuscript.
Abbreviations
- SDL
Synthetic Dosage Lethality
- SPA
Selective Ploidy Ablation
Contributor Information
Ana María León Ortiz, Email: Ana-Maria.Leon@curie.fr.
Robert J. D. Reid, Email: rr381@columbia.edu.
John C. Dittmar, Email: jcd2133@columbia.edu.
Rodney Rothstein, Email: rothstein@cancercenter.columbia.edu.
REFERENCES
- 1.Kuzminov A. Single-strand interruptions in replicating chromosomes cause double-strand breaks. Proc Natl Acad Sci U S A. 2001;98:8241–8246. doi: 10.1073/pnas.131009198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michel B, Ehrlich SD, Uzest M. DNA double-strand breaks caused by replication arrest. Embo J. 1997;16:430–438. doi: 10.1093/emboj/16.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Symington LS. Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol Mol Biol Rev. 2002;66:630–670. doi: 10.1128/MMBR.66.4.630-670.2002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krogh BO, Symington LS. Recombination proteins in yeast. Annu Rev Genet. 2004;38:233–271. doi: 10.1146/annurev.genet.38.072902.091500. [DOI] [PubMed] [Google Scholar]
- 5.Aguilera A, Rothstein R. Topics in Current Genetics: Molecular Genetics of Recombination. Berlin/Heidelberg: Springer; 2007. [Google Scholar]
- 6.Michel B. Replication fork arrest and DNA recombination. Trends Biochem Sci. 2000;25:173–178. doi: 10.1016/s0968-0004(00)01560-7. [DOI] [PubMed] [Google Scholar]
- 7.Rothstein R, Michel B, Gangloff S. Replication fork pausing and recombination or "gimme a break". Genes Dev. 2000;14:1–10. [PubMed] [Google Scholar]
- 8.Lawrence CW, Christensen RB. Metabolic suppressors of trimethoprim and ultraviolet light sensitivities of Saccharomyces cerevisiae rad6 mutants. J Bacteriol. 1979;139:866–876. doi: 10.1128/jb.139.3.866-876.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aboussekhra A, Chanet R, Zgaga Z, Cassier-Chauvat C, Heude M, Fabre F. RADH, a gene of Saccharomyces cerevisiae encoding a putative DNA helicase involved in DNA repair. Characteristics of radH mutants and sequence of the gene. Nucleic Acids Res. 1989;17:7211–7219. doi: 10.1093/nar/17.18.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rong L, Palladino F, Aguilera A, Klein HL. The hyper-gene conversion hpr5-1 mutation of Saccharomyces cerevisiae is an allele of the SRS2/RADH gene. Genetics. 1991;127:75–85. doi: 10.1093/genetics/127.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiestl RH, Prakash S, Prakash L. The SRS2 suppressor of rad6 mutations of Saccharomyces cerevisiae acts by channeling DNA lesions into the RAD52 DNA repair pathway. Genetics. 1990;124:817–831. doi: 10.1093/genetics/124.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krejci L, Van Komen S, Li Y, Villemain J, Reddy MS, Klein H, Ellenberger T, Sung P. DNA helicase Srs2 disrupts the Rad51 presynaptic filament. Nature. 2003;423:305–309. doi: 10.1038/nature01577. [DOI] [PubMed] [Google Scholar]
- 13.Veaute X, Jeusset J, Soustelle C, Kowalczykowski SC, Le Cam E, Fabre F. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature. 2003;423:309–312. doi: 10.1038/nature01585. [DOI] [PubMed] [Google Scholar]
- 14.Pfander B, Moldovan GL, Sacher M, Hoege C, Jentsch S. SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase. Nature. 2005;436:428–433. doi: 10.1038/nature03665. [DOI] [PubMed] [Google Scholar]
- 15.Papouli E, Chen S, Davies AA, Huttner D, Krejci L, Sung P, Ulrich HD. Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol Cell. 2005;19:123–133. doi: 10.1016/j.molcel.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Ulrich HD. PCNASUMO and Srs2: a model SUMO substrate-effector pair. Biochem Soc Trans. 2007;35:1385–1388. doi: 10.1042/BST0351385. [DOI] [PubMed] [Google Scholar]
- 17.Chanet R, Heude M, Adjiri A, Maloisel L, Fabre F. Semidominant mutations in the yeast Rad51 protein and their relationships with the Srs2 helicase. Mol Cell Biol. 1996;16:4782–4789. doi: 10.1128/mcb.16.9.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schild D. Suppression of a new allele of the yeast RAD52 gene by overexpression of RAD51, mutations in srs2 and ccr4, or mating-type heterozygosity. Genetics. 1995;140:115–127. doi: 10.1093/genetics/140.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broomfield S, Xiao W. Suppression of genetic defects within the RAD6 pathway by srs2 is specific for error-free post-replication repair but not for damage-induced mutagenesis. Nucleic Acids Res. 2002;30:732–739. doi: 10.1093/nar/30.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Breton C, Dupaigne P, Robert T, Le Cam E, Gangloff S, Fabre F, Veaute X. Srs2 removes deadly recombination intermediates independently of its interaction with SUMO-modified PCNA. Nucleic Acids Res. 2008;36:4964–4974. doi: 10.1093/nar/gkn441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess RC, Lisby M, Altmannova V, Krejci L, Sung P, Rothstein R. Localization of recombination proteins and Srs2 reveals anti-recombinase function in vivo. J Cell Biol. 2009;185:969–981. doi: 10.1083/jcb.200810055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macris MA, Sung P. Multifaceted role of the Saccharomyces cerevisiae Srs2 helicase in homologous recombination regulation. Biochem Soc Trans. 2005;33:1447–1450. doi: 10.1042/BST0331447. [DOI] [PubMed] [Google Scholar]
- 23.Marini V, Krejci L. Srs2: The "Odd-Job Man" in DNA repair. DNA Repair (Amst) 2010;9:268–275. doi: 10.1016/j.dnarep.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liberi G, Chiolo I, Pellicioli A, Lopes M, Plevani P, Muzi-Falconi M, Foiani M. Srs2 DNA helicase is involved in checkpoint response and its regulation requires a functional Mec1-dependent pathway and Cdk1 activity. Embo J. 2000;19:5027–5038. doi: 10.1093/emboj/19.18.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ira G, Malkova A, Liberi G, Foiani M, Haber JE. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell. 2003;115:401–411. doi: 10.1016/s0092-8674(03)00886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robert T, Dervins D, Fabre F, Gangloff S. Mrc1 and Srs2 are major actors in the regulation of spontaneous crossover. Embo J. 2006;25:2837–2846. doi: 10.1038/sj.emboj.7601158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dupaigne P, Le Breton C, Fabre F, Gangloff S, Le Cam E, Veaute X. The Srs2 helicase activity is stimulated by Rad51 filaments on dsDNA: implications for crossover incidence during mitotic recombination. Mol Cell. 2008;29:243–254. doi: 10.1016/j.molcel.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 28.Saponaro M, Callahan D, Zheng X, Krejci L, Haber JE, Klein HL, Liberi G. Cdk1 targets Srs2 to complete synthesis-dependent strand annealing and to promote recombinational repair. PLoS Genet. 2010;6:e1000858. doi: 10.1371/journal.pgen.1000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spellman PT, Sherlock G, Zhang MQ, Iyer VR, Anders K, Eisen MB, Brown PO, Botstein D, Futcher B. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol Biol Cell. 1998;9:3273–3297. doi: 10.1091/mbc.9.12.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heude M, Chanet R, Fabre F. Regulation of the Saccharomyces cerevisiae Srs2 helicase during the mitotic cell cycle, meiosis and after irradiation. Mol Gen Genet. 1995;248:59–68. doi: 10.1007/BF02456614. [DOI] [PubMed] [Google Scholar]
- 31.Mankouri HW, Craig TJ, Morgan A. SGS1 is a multicopy suppressor of srs2: functional overlap between DNA helicases. Nucleic Acids Res. 2002;30:1103–1113. doi: 10.1093/nar/30.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenz A, Osman F, Folkyte V, Sofueva S, Whitby MC. Fbh1 limits Rad51-dependent recombination at blocked replication forks. Mol Cell Biol. 2009;29:4742–4756. doi: 10.1128/MCB.00471-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C, van Dyk D, Li Y, Andrews B, Rao H. A genome-wide synthetic dosage lethality screen reveals multiple pathways that require the functioning of ubiquitin-binding proteins Rad23 and Dsk2. BMC Biol. 2009;7:75. doi: 10.1186/1741-7007-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroll ES, Hyland KM, Hieter P, Li JJ. Establishing genetic interactions by a synthetic dosage lethality phenotype. Genetics. 1996;143:95–102. doi: 10.1093/genetics/143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang M, Luke B, Kraft C, Li Z, Peter M, Lingner J, Rothstein R. Telomerase is essential to alleviate pif1-induced replication stress at telomeres. Genetics. 2009;183:779–791. doi: 10.1534/genetics.109.107631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baetz K, Measday V, Andrews B. Revealing hidden relationships among yeast genes involved in chromosome segregation using systematic synthetic lethal and synthetic dosage lethal screens. Cell Cycle. 2006;5:592–595. doi: 10.4161/cc.5.6.2583. [DOI] [PubMed] [Google Scholar]
- 37.Measday V, Baetz K, Guzzo J, Yuen K, Kwok T, Sheikh B, Ding H, Ueta R, Hoac T, Cheng B, Pot I, Tong A, Yamaguchi-Iwai Y, Boone C, Hieter P, Andrews B. Systematic yeast synthetic lethal and synthetic dosage lethal screens identify genes required for chromosome segregation. Proc Natl Acad Sci U S A. 2005;102:13956–13961. doi: 10.1073/pnas.0503504102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krejci L, Macris M, Li Y, Van Komen S, Villemain J, Ellenberger T, Klein H, Sung P. Role of ATP hydrolysis in the antirecombinase function of Saccharomyces cerevisiae Srs2 protein. J Biol Chem. 2004;279:23193–23199. doi: 10.1074/jbc.M402586200. [DOI] [PubMed] [Google Scholar]
- 39.Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, Chu AM, Connelly C, Davis K, Dietrich F, Dow SW, El Bakkoury M, Foury F, Friend SH, Gentalen E, Giaever G, Hegemann JH, Jones T, Laub M, Liao H, Liebundguth N, Lockhart DJ, Lucau-Danila A, Lussier M, M’Rabet N, Menard P, Mittmann M, Pai C, Rebischung C, Revuelta JL, Riles L, Roberts CJ, Ross-MacDonald P, Scherens B, Snyder M, Sookhai-Mahadeo S, Storms RK, Veronneau S, Voet M, Volckaert G, Ward TR, Wysocki R, Yen GS, Yu K, Zimmermann K, Philippsen P, Johnston M, Davis RW. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 40.Becker DM, Lundblad V. Introduction of DNA into yeast cells. Curr Protoc Mol Biol. 2003;27:13.17.11–13.17.12. doi: 10.1002/0471142727.mb1307s27. [DOI] [PubMed] [Google Scholar]
- 41.Reid RJ, Lisby M, Rothstein R. Cloning-free genome alterations in Saccharomyces cerevisiae using adaptamer-mediated PCR. Methods Enzymol. 2002;350:258–277. doi: 10.1016/s0076-6879(02)50968-x. [DOI] [PubMed] [Google Scholar]
- 42.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reid RJ, Gonzalez-Barrera S, Sunjevaric I, Alvaro D, Ciccone S, Wagner M, Rothstein R. Selective ploidy ablation, a high-throughput plasmid transfer protocol, identifies new genes affecting topoisomerase I-induced DNA damage. Genome Res. 2011 doi: 10.1101/gr.109033.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dittmar JC, Reid RJ, Rothstein R. ScreenMill: a freely available software suite for growth measurement, analysis and visualization of high-throughput screen data. BMC Bioinformatics. 2010;11:353. doi: 10.1186/1471-2105-11-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nash R, Tokiwa G, Anand S, Erickson K, Futcher AB. The WHI1+ gene of Saccharomyces cerevisiae tethers cell division to cell size and is a cyclin homolog. Embo J. 1988;7:4335–4346. doi: 10.1002/j.1460-2075.1988.tb03332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mascorro-Gallardo JO, Covarrubias AA, Gaxiola R. Construction of a CUP1 promoter-based vector to modulate gene expression in Saccharomyces cerevisiae. Gene. 1996;172:169–170. doi: 10.1016/0378-1119(96)00059-5. [DOI] [PubMed] [Google Scholar]
- 47.Alcasabas AA, Osborn AJ, Bachant J, Hu F, Werler PJ, Bousset K, Furuya K, Diffley JF, Carr AM, Elledge SJ. Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat Cell Biol. 2001;3:958–965. doi: 10.1038/ncb1101-958. [DOI] [PubMed] [Google Scholar]
- 48.Osborn AJ, Elledge SJ. Mrc1 is a replication fork component whose phosphorylation in response to DNA replication stress activates Rad53. Genes Dev. 2003;17:1755–1767. doi: 10.1101/gad.1098303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katou Y, Kanoh Y, Bando M, Noguchi H, Tanaka H, Ashikari T, Sugimoto K, Shirahige K. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature. 2003;424:1078–1083. doi: 10.1038/nature01900. [DOI] [PubMed] [Google Scholar]
- 50.Bando M, Katou Y, Komata M, Tanaka H, Itoh T, Sutani T, Shirahige K, Csm3 Tof1. and Mrc1 form a heterotrimeric mediator complex that associates with DNA replication forks. J Biol Chem. 2009;284:34355–34365. doi: 10.1074/jbc.M109.065730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melo J, Toczyski D. A unified view of the DNA-damage checkpoint. Curr Opin Cell Biol. 2002;14:237–245. doi: 10.1016/s0955-0674(02)00312-5. [DOI] [PubMed] [Google Scholar]
- 52.Parnas O, Zipin-Roitman A, Pfander B, Liefshitz B, Mazor Y, Ben-Aroya S, Jentsch S, Kupiec M. Elg1, an alternative subunit of the RFC clamp loader, preferentially interacts with SUMOylated PCNA. EMBO J. 2010;29:2611–2622. doi: 10.1038/emboj.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reid RJ, Fiorani P, Sugawara M, Bjornsti MA. CDC45 and DPB11 are required for processive DNA replication and resistance to DNA topoisomerase I-mediated DNA damage. Proc Natl Acad Sci U S A. 1999;96:11440–11445. doi: 10.1073/pnas.96.20.11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tishkoff DX, Filosi N, Gaida GM, Kolodner RD. A novel mutation avoidance mechanism dependent on S. cerevisiae RAD27 is distinct from DNA mismatch repair. Cell. 1997;88:253–263. doi: 10.1016/s0092-8674(00)81846-2. [DOI] [PubMed] [Google Scholar]
- 55.Symington LS. Homologous recombination is required for the viability of rad27 mutants. Nucleic Acids Res. 1998;26:5589–5595. doi: 10.1093/nar/26.24.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Debrauwere H, Loeillet S, Lin W, Lopes J, Nicolas A. Links between replication and recombination in Saccharomyces cerevisiae: a hypersensitive requirement for homologous recombination in the absence of Rad27 activity. Proc Natl Acad Sci U S A. 2001;98:8263–8269. doi: 10.1073/pnas.121075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Levels of overexpression in different SDL interacting strains. (A) Denaturing protein extracts of different strains from the SDL screen that overexpress SRS2 at 200 µM CuSO4. The proteins were migrated on an acrylamide gel and revealed with anti-Srs2 antibody and anti-alpha-Tubulin antibody as a loading control. (B) Quantification of the overexpression in A for each strain. The quantification was done using the open access software ImageJ and the levels of Srs2 protein were normalized to those detected upon overexpression of SRS2 in wild-type cells. (C) srs2-R1 expression. Denaturing protein extracts of wild-type cells overexpressing SRS2, srs2-R1, srs2-K41A or srs2-K41A-R1 at 200 µM CuSO4.