Abstract
Summary
To advance our understanding of the burden of fractures among men, we studied a group of men at high risk for low bone strength due to lung disease. We found high rates of fractures but low rates of bone density testing that could predict fracture before it occurs.
Introduction
To advance understanding of the burden of fragility fractures and attention to bone health among men with chronic obstructive lung disease (COPD), we quantified rates of fragility fracture, bone density testing, and anti-resorptive treatment and calculated the number needed to screen (NNS) to prevent one hip fracture in a cohort of men with COPD.
Methods
Veterans Administration (VA) and VA–Medicare administrative data permitted a retrospective cohort study of 87,360 men aged 50 and older, newly diagnosed with COPD between 1999 and 2003. Logistic regression models including patient characteristics, morbidities, and medication use assessed the effect of covariates on fracture and probability of testing or treatment.
Results
Mean age was 66.8. Hip and wrist fracture rates were 3.99 and 1.31 per 1,000 person years, respectively. Mean follow-up was 2.67 years; 4.4% underwent bone densitometry; 2.8% filled anti-resorptive prescriptions. Age, white race/ethnicity, more COPD exacerbations, barbiturate use, and anti-Parkinson’s drug use were significantly associated with fracture. Age, and systemic corticosteroids were most significantly associated with testing or treatment. Based on published adherence and treatment effects, the cohort’s calculated NNS to prevent one hip fracture is 432.
Conclusions
Fracture rate was high and testing and treatment uncommon. The NNS of 432 to prevent one hip fracture is smaller than 731, the NNS for women aged 65–69 for whom universal screening is recommended. Attention to the bone health of this population is warranted. Future research must determine how testing and treatment impact overall quality of life and mortality of men with COPD.
Keywords: Osteoporosis, Prevention, Pulmonary disease
Introduction
Osteoporosis is increasingly recognized as an important health concern for men. Fragility fractures confer significant morbidity, loss of independence, mortality, and medical costs [1–5]. Identified risk factors for male osteoporosis include many behaviors, exposures, and conditions common to men with chronic obstructive pulmonary disease (COPD) such as smoking, systemic glucocorticoid use, inactivity, underweight, and hypogonadism [6, 7]. Additionally, COPD itself may be an independent risk factor for osteoporosis [8–11]. Prevalence studies have found osteoporosis in 27% to 68% of men with COPD [7, 12–17]. Researchers have called for intensified education, testing, and fracture prevention efforts in this population. Despite growing awareness, the rate of fragility fracture, bone densitometry, and anti-resorptive treatment among men with COPD remains largely unknown [6, 17, 18].
In addition to being at high risk for osteoporosis and consequent fragility fractures, men with COPD may suffer particularly high mortality following a hip fracture. Male gender is associated with an 18–32% 12-month mortality rate following hip fracture. This compares to a mortality rate of 12–18% for women in the same studies [1, 19]. COPD is also independently associated with higher mortality after hip fracture. Compared to patients without COPD, the hazard ratio of death within 12 months of hip fracture in published studies ranges from 1.3 to 1.7 [1, 19–21]. In addition to higher mortality, patients with COPD may suffer greater morbidity from osteoporosis as vertebral compression fractures may diminish lung volume and compromise already limited respiratory function [6, 22].
In response to (1) the increasing recognition of diminished bone density as a health risk for men, (2) growing evidence that men with COPD may be at particularly high risk for osteoporosis, and (3) evidence that those with COPD may be especially susceptible to poor outcomes associated with fragility fracture, we aimed to assess fracture rate, bone density testing, and anti-resorptive treatment in a group of older men with COPD. We hypothesized that the rate of fragility fracture is relatively high and that bone density testing and osteoporosis treatment may be low relative to the burden of disease in this population.
Methods
We conducted a retrospective cohort study of male Veterans Administration (VA) patients over 50 years of age with COPD. VA inpatient, outpatient, pharmacy benefits, and linked VA–Medicare data were used. The Institutional Review Board of the Hines VA Hospital approved this study.
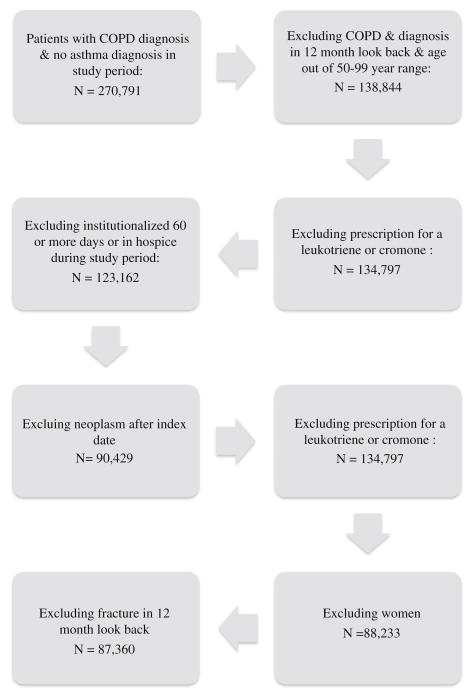
From a national database of VA health systems users, we identified men newly diagnosed with COPD in fiscal years (FY) 1999 through 2003. For inclusion, patients must have had at least one inpatient primary diagnosis or two outpatient primary or secondary diagnoses of COPD (ICD-9-CM 491, 492, 496) within one FY during the enrollment period. Patients were defined as newly diagnosed if COPD diagnosis appeared in a FY and, in the preceding FY, at least one encounter but no COPD diagnosis and no drug dispensing suggestive of COPD (beta-2 agonist, inhaled anticholinergic, methylxanthines, and inhaled glucorticoids). Patients between 50 and 100 years of age at cohort entry (date of hospital discharge or second outpatient encounter) were included. To create a homogeneous COPD cohort, we excluded patients with a diagnosis of asthma (ICD-9 codes 493, 493.0, 493.1, 493.9) or dispensing of an asthma medication not approved for COPD (cromones or leukotriene modifiers), at any time in the FY before cohort entry or any time after cohort entry [23]. To minimize misclassification of prevalent fractures as incident, we excluded all patients with a fracture claim during the 12 months preceding enrollment. Patients were also excluded if they were institutionalized for 60 or more consecutive days in a long-term care facility or nursing home (identified by claim facility code) during the study period. To reduce the risk of attributing to osteoporosis a pathologic fracture due to metastatic cancer, patients with a neoplasm diagnosis (ICD-9 140-239.9) were conservatively excluded as well.
Patients were followed from the date of study entry until censored for: (1) death, (2) end of the study period (September 30, 2003), or (3) lost to follow-up, defined as: 270 days with neither healthcare contact nor a prescription medication dispensing. Patients were censored 270 days from the date of last healthcare contact or end of their last days supply of medication, whichever was more recent.
Fracture outcomes measured were: (1) fracture at the distal forearm (ICD-9, 813; CPT, 25500–25605, 25605, 25606, 25607, 25609, 25650; ICD 9 procedure codes, 79.02, 79.12, 79.22, 79.32), (2) fracture at the hip (ICD-9 820.xx; CPT, 27245, 27244, 27236, 27235; CPT, 27240, 27238, 27232, 27230; ICD-9 procedures 79.05, 79.15, 79.25, 79.35). ICD-9 codes for pathologic fracture (733.1, 733.10, 733.13, 733.95) were excluded from fracture counts and patients with these diagnoses were excluded from the cohort. Because ascertainment of incident vertebral fractures in claims data is complex, due to the indefinite persistence of these fractures on imaging studies, imprecise use of diagnostic codes, and historically low diagnosis rates, we conservatively did not include vertebral fractures as events [24]. Fracture events were included if a diagnosis or procedure appeared once in inpatient or outpatient claims data. A sensitivity analysis included only fractures defined by one inpatient diagnosis or two outpatient diagnoses and/or procedures. Analyses included only first fractures [25].
Bone testing outcomes included: dual energy X-ray studies (CPT 76075, 76076), ultrasound for bone density measure (CPT 76977), CT scan bone density (CPT 76070), and bone mineral, single or dual photon (CPT 78350, 78351). Bone density codes introduced in 2007 were not included. Treatment measures included receipt of one or more anti-resorptive prescription dispensing (alendronate, etidronate, pamidronate, risendronate, zolondronic acid, or calcitonin).
Multivariable logistic regression modeled odds of a first fragility fracture and testing or treatment (combined for clinical relevance as one outcome), which we defined as receipt of bone density testing or anti-resorptive prescription fill. Models included patient characteristics of race/ethnicity (categorized as white, black, Hispanic, and other), age at time of cohort entry (categorized as 50–59, 60–69, 70–79, and ≥80 years), and marital status (dichotomized as married or not married). We also included variables to indicate use of medications associated with fracture: oral corticosteroids, inhaled corticosteroids, proton pump inhibitors, selective seratonin reuptake inhibitors (SSRI), anticonvulsants, tricyclic antidepressants, barbiturates, benzodiazepines, and anti-Parkinson’s agents. Use was defined as more than one prescription fill, because a second fill has been shown to correlate highly with persistent use [26]. Comorbidities (based on one or more ICD-9 diagnosis appearing during the 12-month cohort run in period) included as dummy variables were: diabetes, depression, alcohol abuse, obesity, malnutrition/weight loss, osteoarthritis, and (for fracture models only) osteoporosis. Annual rate of COPD exacerbation was included as a proxy measure of COPD severity. An exacerbation was defined as either (1) a hospitalization with a primary diagnosis of COPD or respiratory failure or (2) an emergency department or outpatient visit with a COPD diagnosis followed, within 5 days, by a new short-course prescription for oral corticosteroids or antibiotics. To avoid double counting, oral corticosteroids prescription fills contributing to a COPD exacerbation event were excluded from the glucocorticoid prescription fill count. Service utilization variables included: annual number of outpatient visits and annual number of non-psychiatric hospital days. Analyses were clustered on VA Integrated Service Network, a geographic organization of VA hospitals and clinics.
We used observed fracture rates and evidence from published studies to determine the number needed to screen (NNS) to prevent one hip fracture for this cohort. We followed the model outlined by Nelson et al. in preparing the US Preventive Health Services Task Force (USPSTF) recommendations for women, but used actual hip fracture numbers for this cohort. In this calculation, we assumed a relative risk (RR) of 0.60 for non-vertebral fracture in treated men based on a meta-analysis of two published studies of bisphosphonate efficacy in men [27, 28]. We likewise substituted a bisphosphonate adherence rate of 0.54 based on one published study of this topic in a male, veteran population [29].
Results
Our cohort of 87,360 male COPD patients reflects the general VA population: older, predominantly white, and with multiple morbidities (Table 1). The mean follow-up time was 2.67 years (SD 1.25). Average count of key comorbidities was 2.8 (SD 1.65). Annually, cohort members had an average of 5.2 (SD 27.1) non-psychiatric inpatient days, 1.3 (SD 3.4) Medicare outpatient visits, 6.8 (SD 8.4) VA outpatient visits, and 0.7 (SD 5.9) COPD exacerbations. Inhaled and systemic corticosteroids were used by 13.8% and 4.1% of all patients, respectively. Considering other medications associated with fracture in previously published studies: 24% of cohort members used SSRIs and 21% used proton pump inhibitors. Other medications were used to a lesser extent (Table 1).
Table 1.
Descriptive characteristics: cohort of older, male, active users of the Veterans Administration Health Aystem, newly diagnosed with chronic obstructive lung disease 1999–2003
| Total cohort | 87,360 |
| Age, mean (SD) | 66.8 (9.74) |
| Age, median | 67.18 |
| Married, N (%) | 48,486 (55.5) |
| Race/ethnicity, N (%) | |
| White | 68,968 (79.0) |
| Black | 10,085 (11.5) |
| Hispanic | 2,741 (3.1) |
| Other | 5,566 (6.4) |
| Mean follow-up time (SD) | 2.67 years (1.25) |
| Median follow-up time | 2.60 years |
| Diagnosed comorbid conditions, N (%) | |
| Diabetes | 20,932 (23.8) |
| Depression | 21,379 (14.2) |
| Osteoarthritis | 21,883 (25.1) |
| Alcohol abuse | 7,473 (8.6) |
| Obesity | 11,215 (12.8) |
| Osteoporosis diagnosis | 1,023 (1.17) |
| Malnutrition/underweight | 883 (1.0) |
| Number of comorbid conditions, mean (SD)a | 0.87 (0.91) |
| Annual non-psychiatric inpatient days, mean (SD) | 5.2 (27.1) |
| Annual COPD exacerbations,b mean (SD) | 0.7 (5.9) |
| Annual COPD exacerbations, median | 0.0 |
| Medication use, N (%)c | |
| Anti-resorptive | 2476 (2.8) |
| Inhaled steroid | 12,033 (13.8) |
| Systemic steroidd | 3,543 (4.1) |
| Proton pump inhibitor | 18,227 (20.9) |
| Selective serotonin reuptake inhibitor | 21,067 (24.1) |
| Tricyclic antidepressant | 5,702 (6.5) |
| Benzodiazepine | 10,310 (11.8) |
| Anti-Parkinson’s | 970 (1.1) |
| Barbiturates | 280 (0.3) |
| Anticonvulsants | 10,577 (12.7) |
Comorbidities are based on one or more ICD-9 diagnosis appearing during a 12-month period preceding index diagnosis of chronic obstructive pulmonary disease
Number of comorbid conditions from above list of conditions
COPD is chronic obstructive pulmonary disease. COPD exacerbations = either (1) a hospitalization for COPD with a primary diagnosis of COPD or respiratory failure or (2) an emergency department or outpatient visit with a COPD diagnosis followed with 5 days a new short-course prescription for either oral corticosteroids or antibiotics
Medication use = two or more prescription dispensing during follow-up
Systemic corticosteroids included in a COPD exacerbation event were excluded from systemic steroid prescription count to avoid double counting
The wrist and hip fracture rates in this population were 1.31 and 3.99 per 1,000 person years, respectively. Overall, 4.4% of the cohort underwent bone density testing during our follow-up period, and 2.8% filled more than one prescription for an anti-resorptive medication.
In the multivariable logistic regression model of first fracture, relative to those age 50–59 years, older age was strongly associated with fracture: OR 2.74 (95% CI 2.25–3.34), age 60–69; OR 5.01 (95% CI 4.08–6.17), age 70–79; and OR 8.2 (95% CI 6.59–10.20), age 80 and higher. Other significant predictors of fracture included: barbiturate use, OR 2.79 (95% CI 1.80–4.30) and anti-Parkinson’s drug use, OR 2.03 (95% CI 1.52–2.71). Greater number of annual COPD exacerbations was strongly associated with fracture, OR 2.13 (95% CI 1.81–2.51) for those with one exacerbation, OR 11.69 (95% CI 10.06–13.58) for those with two or more. Diabetes and obesity were protective against fracture, OR 0.76 (95% CI 0.67–0.86) and OR 0.57 (95% CI 0.49–0.66), respectively (Table 2).
Table 2.
Multivariable logistic regression: probability of first fracture at hip or wrist in a cohort of older, male, active users of the Veterans Administration Health System, newly diagnosed with chronic obstructive lung disease 1999–2003
| Odd ratio | 95% CI | P value | ||
|---|---|---|---|---|
| Age | 50–59 years | 1.0 (reference) | ||
| 60–69 years | 2.74 | 2.25–3.34 | <0.001 | |
| 70–79 years | 5.01 | 4.08–6.17 | <0.001 | |
| 80+ years | 8.20 | 6.59–10.20 | <0.001 | |
| Married (reference not married) | 0.86 | 0.76–0.96 | 0.011 | |
| Race/ethnicity (reference white) | Black | 0.64 | 0.50–0.81 | <0.001 |
| Hispanic | 0.87 | 0.55–1.37 | 0.549 | |
| Other | 0.27 | 0.18–0.40 | <0.001 | |
| Annual non-psychiatric hospital daysa | 1.00 | 1.00–1.00 | <0.001 | |
| Annual COPD exacerbations = 1b | 2.13 | 1.81–2.51 | <0.001 | |
| Annual COPD exacerbation = 2 or moreb | 2.95 | 2.50–3.48 | <0.001 | |
| Comorbidities (1 or more diagnosis) | Diabetes | 0.87 | 0.75–1.01 | 0.077 |
| Obesity | 0.73 | 0.57–0.91 | 0.006 | |
| Depression | 1.11 | 0.97–1.26 | 0.126 | |
| Alcohol abuse | 1.52 | 1.21–1.85 | <0.001 | |
| Osteoarthritis | 1.07 | 0.97–1.18 | 0.198 | |
| Osteoporosis | 1.52 | 1.08–2.12 | 0.015 | |
| Malnutritionc | 1.35 | 0.77–2.34 | 0.283 | |
| Medication used | Inhaled corticosteroid | 1.20 | 1.02–1.40 | 0.025 |
| Systemic corticosteroide | 0.92 | 0.68–1.26 | 0.619 | |
| Proton pump inhibitor | 0.80 | 0.68–0.94 | 0.009 | |
| SSRI | 1.32 | 1.16–1.49 | <0.001 | |
| Tricyclic antidepressant | 1.46 | 1.14–1.88 | 0.003 | |
| Benzodiazepine | 1.18 | 1.03–1.34 | 0.017 | |
| Anti-Parkinson’s disease | 2.03 | 1.52–2.71 | <0.001 | |
| Anti-resorptive | 1.05 | 0.80–1.38 | 0.703 | |
| Anticonvulsants | 1.50 | 1.22–1.84 | <0.001 | |
| Barbiturates | 2.79 | 1.80–4.30 | <0.001 |
COPD exacerbation either a hospitalization for COPD with a primary diagnosis of COPD or respiratory failure or an emergency department or outpatient visit with a COPD diagnosis where a new short-course prescription for either oral corticosteroids or antibiotics was filled within 5 days of the visit
SSRI selective serotonin reuptake inhibitor
Annual non-psychiatric hospital days includes those in Veterans Administration and Medicare data, odds ratio associated with an increase of one
COPD is chronic obstructive pulmonary disease. Annual COPD exacerbation reference is 0 exacerbations
Includes ICD-9 diagnoses for malnutrition and underweight
Medication use = two or more prescription dispensing during follow-up
Systemic corticosteroids included in a COPD exacerbation event were excluded from systemic steroid prescription count to avoid double counting
In logistic regression assessing factors associated with bone mineral density (BMD) testing or treatment, osteoporosis diagnosis was most predictive, OR 30.11 (95% CI 24.71–36.71). Older age categories (compared to age 50–59) were also predictive of this outcome: OR 1.42 (95% CI 1.30–1.55), age 60–69; OR 1.74 (95% CI 1.56–1.94), age 70–79; and OR 1.74 (95% CI 1.45–2.10), age 80 years and greater. Use of systemic corticosteroids predicted testing or treatment as well: OR 7.91 (95% CI 6.92–9.04). Significantly lower likelihood of receiving BMD testing or treatment was associated with two or more annual COPD exacerbations OR 0.51 (95% CI 0.42–0.61) (Table 3).
Table 3.
Multivariable logistic regression: probability of bone density testing or anti-resorptive treatment in a cohort of older, male, active users of the Veterans Administration Health System, newly diagnosed with chronic obstructive lung disease 1999–2003
| Odds ratio | 95% CI | P value | ||
|---|---|---|---|---|
| Age | 50–59 years | 1.0 (reference) | ||
| 60–69 years | 1.42 | 1.30–1.55 | <0.001 | |
| 70–79 years | 1.74 | 1.56–1.94 | <0.001 | |
| 80+ years | 1.74 | 1.45–2.10 | <0.001 | |
| Married (reference is not married) | 1.06 | 0.98–1.14 | 0.167 | |
| Race/ethnicity (reference is white) | Black | 0.88 | 0.74–1.03 | 0.112 |
| Hispanic | 1.02 | 0.89–1.17 | 0.797 | |
| Other | 0.80 | 0.68–0.92 | 0.003 | |
| Annual non-psychiatric hospital daysa | 0.99 | 0.99–1.00 | <0.001 | |
| Annual COPD exacerbations = 1b | 0.97 | 0.86–1.09 | 0.621 | |
| Annual COPD exacerbation = 2 or moreb | 0.51 | 0.42–0.61 | <0.001 | |
| Comorbidities | Diabetes | 0.82 | 0.76–0.88 | 0.007 |
| Obesity | 0.79 | 0.65–0.95 | 0.013 | |
| Depression | 1.02 | 0.95–1.11 | 0.561 | |
| Alcohol abuse | 0.85 | 0.74–0.98 | 0.027 | |
| Osteoarthritis | 1.18 | 1.05–1.33 | 0.005 | |
| Malnutritionc | 1.42 | 1.10–1.84 | 0.007 | |
| Medication used | Inhaled corticosteroid | 1.16 | 1.04–1.30 | 0.007 |
| Systemic corticosteroide | 7.91 | 6.92–9.04 | <0.001 | |
| Proton pump inhibitor | 1.28 | 1.18–1.40 | <0.001 | |
| SSRI | 1.10 | 1.01–1.20 | 0.023 | |
| Tricyclic antidepressant | 1.20 | 1.09–1.32 | <0.001 | |
| Benzodiazepine | 0.88 | 0.78–0.99 | 0.040 | |
| Anti-Parkinson disease | 1.21 | 0.92–1.61 | 0.176 | |
| Anticonvulsant | 1.11 | 1.00–1.22 | 0.041 | |
| Barbiturates | 0.94 | 0.48–1.82 | 0.863 |
COPD exacerbation either a hospitalization for COPD with a primary diagnosis of COPD or respiratory failure or an emergency department or outpatient visit with a COPD diagnosis where a new short-course prescription for either oral corticosteroids or antibiotics was filled within 5 days of the visit
SSRI selective serotonin reuptake inhibitor
Annual non-psychiatric hospital days includes those in Veterans Administration and Medicare data, odds ratio associated with an increase of one
COPD is chronic obstructive pulmonary disease. Annual COPD exacerbation reference is 0 exacerbations
Includes ICD-9 diagnoses for malnutrition and underweight
Medication use = two or more prescription dispensing during follow-up
Systemic corticosteroids included in a COPD exacerbation event were excluded from systemic steroid prescription count to avoid double counting
For this cohort, the NNS to prevent one hip fracture was calculated to be 432, assuming a treatment adherence rate of 0.54, and a RR of hip fracture of 0.60 with treatment, based on prior studies of men [27, 29].
A sensitivity analysis excluded fractures identified with only a single outpatient diagnosis. This exclusion resulted in 237 fewer fractures (121 hip and 116 wrist). Compared to the models including all fractures identified by initial criteria, logistic models including only fractures meeting this more conservative definition resulted in trivial changes of coefficients. The NNS to prevent one hip fracture in this population using this lower hip fracture rate was 507 [28].
Discussion
The fracture rate in this population was much higher than that reported in the literature for men in the general population between the ages of 65 and 69, who experienced 1.12 hip fractures per 1,000 person years in 2006 and 0.95 distal forearm fractures per 1,000 person years in 1990 (the most recent year for which such data are published) [30, 31]. The high fracture rate likely results from a confluence of risks that include medication exposure, inactivity, smoking, hypogonadism, underweight or weight loss as well as inadequate nutrition and effects of the chronic inflammation that characterizes COPD [6, 8, 17, 32].
Juxtaposed to the high rate of fragility fracture, the low rate of bone densitometry and anti-resporptive treatment in this population is striking. The association between osteoporosis and COPD was recognized as early as 1972, and confirmation of this association has repeatedly appeared in the literature since then [33]. The low rate of treatment observed in this cohort likely reflects the low rate of bone density testing which commonly prompts treatment. Of particular note is the fact that more COPD exacerbations were strongly associated with both higher probability of fracture and lower probability of testing or treatment. This may reflect physician and patient distraction as lung disease is prioritized over bone health or simply a lack of understanding of the association between COPD severity (and related treatments) and fracture [8, 10, 11]. The discordant disease burden and testing rate may arise from the fact that recognition of osteoporosis as a health concern for men has been slow to develop and has gained meaningful attention only recently. The small number of existing osteoporosis screening guidelines specific to men did not emerge from US scientific groups until 2007 [34–37]. An outpatient COPD management guideline published by the VA in 1999 and updated in 2007 recommends patients using glucocorticoids be evaluated for bone loss and considered for prevention or treatment of osteoporosis, based on admittedly “insufficient” evidence [38].
The USPSTF recommends universal bone density testing for women at age 65 based on a NNS of 731 to prevent one hip fracture over a 5-year period. By comparison, The NNS of 432 to prevent one hip fracture in this population is much smaller. Our NNS is based on an average of 2.76 years of follow-up and would likely be even smaller were we counted fractures for five full years (the time frame used for USPSTF calculations for women).
Our retrospective, observational study has some important limitations. Using administrative data, we did not have access to important patient factors associated with fracture including: current smoking status, nutritional status, calcium intake, vitamin D intake, activity level, and unmeasured risks for fall such as visual disturbance, limb abnormalities, gait disturbance, and living environment. We did not have prescription drug dispensing data for non-VA pharmacies. The use of outside pharmacies is likely low for this population, as VA prescription benefits are relatively generous and our study precedes implementation of a VA cost-sharing policy [39]. Our data likewise predate the Medicare Part D prescription program (January 1, 2006) that could be used by dually eligible veterans. Our population of veterans has health benefits that include full coverage of preventive health care and screening [40]. The observed rate of bone testing may not match that of COPD patients with financial barriers to bone densitometry or COPD patients with greater or lesser personal interest in bone density testing. Our analysis includes osteporosis/osteopenia diagnosis prior to enrollment (1.17%) but does not include data on testing or treatment prior to cohort entry. It is thus possible that we underestimate the rate of bone health management. We believe our creation of a newly diagnosed COPD cohort results in a study population with relatively mild COPD and an average age younger than the general COPD population. For such men, the strongest indication for bone density testing is likely COPD diagnosis and use of COPD medications such as inhaled and oral corticosteroids. Thus, while it is possible that some men underwent testing and treatment prior to enrollment without incurring a diagnosis of osteoporosis/osteopenia, we expect such cases to be uncommon. Additionally, the young age and early COPD stage of our cohort limits the generalizability of our findings.
We used conservative definitions to ascertain fracture cases, but our findings are still limited by the accuracy and completeness of the administrative data employed. The generalizability of our study findings will depend on the extent to which other populations are similar to this cohort. While it is not likely that the fracture rate for men with COPD has changed substantially in recent years, our study period of 1999 to 2003 predates the emergence of formal risk factor screening and bone density testing guidelines in the US; current testing and treatment rates may be higher than those we observed. More recent fracture, testing and treatment patterns for this population will be possible when more recent VA–Medicare data become available to researchers.
In conclusion, between 1999 and 2003, these 87,360 men with newly diagnosed COPD had a high rate of fragility fracture and a low rate of testing and treatment. The NNS to prevent one hip fracture in these men with COPD is smaller than that for women aged 65 or older for whom universal bone density testing is recommended. The fracture rate is likely even higher in the general population of male COPD patients with more advanced age and lung disease. Our findings highlight the need for further study of bone health in this population. Appropriate testing and treatment recommendations will be strengthened by further studies that assess treatment adherence and effect in the setting of widely diffused weekly, monthly, and yearly bisphosphonate dosing regimens. In this highly comorbid population, such studies, in addition to fracture outcomes, should consider quality of life and all cause mortality associated with osteoporosis testing and treatment.
While awaiting further research, clinicians, clinic administrators, and policy makers should assess their attention to bone health in men with COPD and assure that ongoing care, screening guidelines, testing reimbursement requirements, and shared patient decision making reflect our current understandings of fragility fracture risks in this population.
Acknowledgments
Nancy E. Morden received funding from VA ORH Rural Health Resource Center—Eastern Region and Todd A. Lee from A HSR&D IIR 03-307.
Appendix A
Fig. 1.
Flow chart for creation of a cohort of older, male, active users of the Veterans Administration Health System, newly diagnosed with chronic obstructive lung disease (COPD) in fiscal years 1999–2003
Footnotes
Conflicts of interest None.
Contributor Information
N. E. Morden, Email: Nancy.e.morden@dartmouth.edu, The Department of Community and Family Medicine, Dartmouth Medical School, Hanover, NH, USA. The Dartmouth Institute for Health Policy and Clinical Practice, 35 Centerra Parkway, Suite 300, Lebanon, NH 03766, USA. VA White River Junction Rural Health Research Center—Eastern Region, White River Junction VT, Lebanon, NH, USA
S. D. Sullivan, Department of Pharmacy, Pharmaceutical Outcomes Research and Policy Program, University of Washington, Seattle, Washington, USA
B. Bartle, Hines VA Medical Center, 2100 S 5TH Ave Ste 111L, Hines, IL 60141, USA
T. A. Lee, Hines VA Hospital, Center for Management of Complex Chronic, Institute for Health Research and Policy, University of Illinois at Chicago, Chicago, IL, USA
References
- 1.Bass E, French DD, Bradham DD, Rubenstein LZ. Risk-adjusted mortality rates of elderly veterans with hip fractures. Ann Epidemiol. 2007;17(7):514–519. doi: 10.1016/j.annepidem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003;51(3):364–370. doi: 10.1046/j.1532-5415.2003.51110.x. [DOI] [PubMed] [Google Scholar]
- 3.Haentjens P, Lamraski G, Boonen S. Costs and consequences of hip fracture occurrence in old age: an economic perspective. Disabil Rehabil. 2005;27(18–19):1129–1141. doi: 10.1080/09638280500055529. [DOI] [PubMed] [Google Scholar]
- 4.Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int. 2004;15(11):897–902. doi: 10.1007/s00198-004-1627-0. [DOI] [PubMed] [Google Scholar]
- 5.Khasraghi FA, Lee EJ, Christmas C, Wenz JF. The economic impact of medical complications in geriatric patients with hip fracture. Orthopedics. 2003;26(1):49–53. doi: 10.3928/0147-7447-20030101-14. discussion 53. [DOI] [PubMed] [Google Scholar]
- 6.Biskobing DM. COPD and osteoporosis. Chest. 2002;121 (2):609–620. doi: 10.1378/chest.121.2.609. [DOI] [PubMed] [Google Scholar]
- 7.Iqbal F, Michaelson J, Thaler L, Rubin J, Roman J, Nanes MS. Declining bone mass in men with chronic pulmonary disease: contribution of glucocorticoid treatment, body mass index, and gonadal function. Chest. 1999;116(6):1616–1624. doi: 10.1378/chest.116.6.1616. [DOI] [PubMed] [Google Scholar]
- 8.Dam TT, Harrison S, Fink HA, Ramsdell J, Barrett-Connor E. Bone mineral density and fractures in older men with chronic obstructive pulmonary disease or asthma. Osteoporos Int. 2009;21:1341–1349. doi: 10.1007/s00198-009-1076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk in patients with chronic lung diseases treated with bronchodilator drugs and inhaled and oral corticosteroids. Chest. 2007;132(5):1599–1607. doi: 10.1378/chest.07-1092. [DOI] [PubMed] [Google Scholar]
- 10.Sin DD, Man JP, Man SF. The risk of osteoporosis in Caucasian men and women with obstructive airways disease. Am J Med. 2003;114(1):10–14. doi: 10.1016/s0002-9343(02)01297-4. [DOI] [PubMed] [Google Scholar]
- 11.Lekamwasam S, Trivedi DP, Khaw KT. An association between respiratory function and hip bone mineral density in older men: a cross-sectional study. Osteoporos Int. 2005;16(2):204–207. doi: 10.1007/s00198-004-1673-7. [DOI] [PubMed] [Google Scholar]
- 12.Jorgensen NR, Schwarz P, Holme I, Henriksen BM, Petersen LJ, Backer V. The prevalence of osteoporosis in patients with chronic obstructive pulmonary disease: a cross sectional study. Respir Med. 2007;101(1):177–185. doi: 10.1016/j.rmed.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Shane E, Silverberg SJ, Donovan D, et al. Osteoporosis in lung transplantation candidates with end-stage pulmonary disease. Am J Med. 1996;101(3):262–269. doi: 10.1016/S0002-9343(96)00155-6. [DOI] [PubMed] [Google Scholar]
- 14.Papaioannou A, Parkinson W, Ferko N, et al. Prevalence of vertebral fractures among patients with chronic obstructive pulmonary disease in Canada. Osteoporos Int. 2003;14(11):913–917. doi: 10.1007/s00198-003-1449-5. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Brennan KJ, Gaughan JP, Ciccolella DE, Kuzma AM, Criner GJ. African Americans and men with severe COPD have a high prevalence of osteoporosis. COPD. 2008;5(5):291–297. doi: 10.1080/15412550802363329. [DOI] [PubMed] [Google Scholar]
- 16.Barr RG, Celli BR, Mannino DM, et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med. 2009;122(4):348–355. doi: 10.1016/j.amjmed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franco CB, Paz-Filho G, Gomes PE, et al. Chronic obstructive pulmonary disease is associated with osteoporosis and low levels of vitamin D. Osteoporos Int. 2009;20:1881–1887. doi: 10.1007/s00198-009-0890-5. [DOI] [PubMed] [Google Scholar]
- 18.Carter JD, Patel S, Sultan FL, et al. The recognition and treatment of vertebral fractures in males with chronic obstructive pulmonary disease. Respir Med. 2008;102(8):1165–1172. doi: 10.1016/j.rmed.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Penrod JD, Litke A, Hawkes WG, et al. The association of race, gender, and comorbidity with mortality and function after hip fracture. J Gerontol A Biol Sci Med Sci. 2008;63(8):867–872. doi: 10.1093/gerona/63.8.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vestergaard P, Rejnmark L, Mosekilde L. Increased mortality in patients with a hip fracture-effect of pre-morbid conditions and post-fracture complications. Osteoporos Int. 2007;18 (12):1583–1593. doi: 10.1007/s00198-007-0403-3. [DOI] [PubMed] [Google Scholar]
- 21.de Luise C, Brimacombe M, Pedersen L, Sorensen HT. Chronic obstructive pulmonary disease and mortality following hip fracture: a population-based cohort study. Eur J Epidemiol. 2008;23 (2):115–122. doi: 10.1007/s10654-007-9211-5. [DOI] [PubMed] [Google Scholar]
- 22.Leech JA, Dulberg C, Kellie S, Pattee L, Gay J. Relationship of lung function to severity of osteoporosis in women. Am Rev Respir Dis. 1990;141(1):68–71. doi: 10.1164/ajrccm/141.1.68. [DOI] [PubMed] [Google Scholar]
- 23.McKnight J, Scott A, Menzies D, Bourbeau J, Blais L, Lemiere C. A cohort study showed that health insurance databases were accurate to distinguish chronic obstructive pulmonary disease from asthma and classify disease severity. J Clin Epidemiol. 2005;58(2):206–208. doi: 10.1016/j.jclinepi.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Curtis JR, Mudano AS, Solomon DH, Xi J, Melton ME, Saag KG. Identification and validation of vertebral compression fractures using administrative claims data. Med Care. 2009;47(1):69–72. doi: 10.1097/MLR.0b013e3181808c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curtis JR, Taylor AJ, Matthews RS, et al. “Pathologic” fractures: should these be included in epidemiologic studies of osteoporotic fractures? Osteoporos Int. 2009;20:1969–1972. doi: 10.1007/s00198-009-0840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karter AJ, Parker MM, Moffet HH, Ahmed AT, Schmittdiel JA, Selby JV. New prescription medication gaps: a comprehensive measure of adherence to new prescriptions. Health Serv Res. 2009;44(5 Pt 1):1640–1661. doi: 10.1111/j.1475-6773.2009.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawka AM, Papaioannou A, Adachi JD, Gafni A, Hanley DA, Thabane L. Does alendronate reduce the risk of fracture in men? A meta-analysis incorporating prior knowledge of anti-fracture efficacy in women. BMC Musculoskelet Disord. 2005;6:39. doi: 10.1186/1471-2474-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson HD, Helfand M, Woolf SH, Allan JD. Screening for postmenopausal osteoporosis: a review of the evidence for the U. S. Preventive Services Task Force. Ann Intern Med. 2002;137(6):529–541. doi: 10.7326/0003-4819-137-6-200209170-00015. [DOI] [PubMed] [Google Scholar]
- 29.Hansen KE, Swenson ED, Baltz B, Schuna AA, Jones AN, Elliott ME. Adherence to alendronate in male veterans. Osteoporos Int. 2008;19(3):349–356. doi: 10.1007/s00198-007-0471-4. [DOI] [PubMed] [Google Scholar]
- 30.Melton LJ, 3rd, Crowson CS, O’Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos Int. 1999;9(1):29–37. doi: 10.1007/s001980050113. [DOI] [PubMed] [Google Scholar]
- 31.Ettinger B, Black D, Dawson-Hughes B, Pressman A, Melton JL. Updated fracture incidence rates for US version of FRAX. Osteoporos Int. 2010;21:25–33. doi: 10.1007/s00198-009-1032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jorgensen NR, Schwarz P. Osteoporosis in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2008;14 (2):122–127. doi: 10.1097/MCP.0b013e3282f4efb6. [DOI] [PubMed] [Google Scholar]
- 33.Daniell HW. Osteoporosis and smoking. JAMA. 1972;221(5):509. [PubMed] [Google Scholar]
- 34.Lewiecki EM, Gordon CM, Baim S, et al. International society for clinical densitometry 2007 adult and pediatric official positions. Bone. 2008;43(6):1115–1121. doi: 10.1016/j.bone.2008.08.106. [DOI] [PubMed] [Google Scholar]
- 35.Lim LS, Hoeksema LJ, Sherin K. Screening for osteoporosis in the adult U.S. population: ACPM position statement on preventive practice. Am J Prev Med. 2009;36(4):366–375. doi: 10.1016/j.amepre.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 36.National Osteoporosis Foundation. [Accessed June 17, 2009];The clinician’s guide to prevention and treatment of osteoporosis. 2008 http://www.nof.org/professionals/clinicians_guide.htm.
- 37.Qaseem A, Snow V, Shekelle P, Hopkins R, Jr, Forciea MA, Owens DK. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148(9):680–684. doi: 10.7326/0003-4819-148-9-200805060-00008. [DOI] [PubMed] [Google Scholar]
- 38.Affairs VDCPGUSDoV. [Accessed August 27, 2010];Management of Chronic Obstructive Pulmonary Disease (COPD) 2007 http://www.healthquality.va.gov/Chronic_Obstructive_Pulmonary_Disease_COPD.asp.
- 39.Piette JD, Heisler M. Problems due to medication costs among VA and non-VA patients with chronic illnesses. Am J Manage Care. 2004;10(11 Pt 2):861–868. [PubMed] [Google Scholar]
- 40.Department of Veterans Affairs. [Accessed August 26];Federal benefits for veterans, dependents & survivors. 2010 http://www1.va.gov/opa/publications/benefits_book/federal_benefits.pdf.