Abstract
Objective:
The influence of alcohol use on opioid dependence is a major problem that warrants a search for more effective treatment strategies. The addition of very-low-dose naltrexone (VLNTX) to methadone taper was recently associated with reduced withdrawal intensity during detoxification. In a secondary analysis of these data, we sought to determine whether problem drinking affects detoxification outcomes and whether symptoms are influenced by VLNTX use.
Method:
Opioid-dependent patients (N = 174) received naltrexone (0.125 or 0.250 mg/day) or placebo in a double-blind, randomized design during methadone-based, 6-day inpatient detoxification. Alcohol consumption was assessed at admission using the Addiction Severity Index and self-report. Outcome measures were opioid withdrawal intensity, craving, and retention in treatment.
Results:
Problem drinking—opioid dependent patients (n = 79) showed episodic heavy alcohol use and reported increased subjective opioid withdrawal intensity (p = .001), craving (p = .001), and significantly lower rate of retention in treatment (p = .02). Individuals with problem drinking and opioid dependence who were treated with VLNTX (n = 55) showed reduced withdrawal (p = .05) and a lower rate of treatment discontinuation (p = .03), resuming alcohol intake in smaller numbers the day following discharge (p = .03). Treatment effects were more pronounced on anxiety, perspiration, shakiness, nausea, stomach cramps, and craving. There were no group differences in use of adjuvant medications and no treatment-related adverse events.
Conclusions:
Heavy drinking is associated with worse opioid detoxification outcomes. The addition of VLNTX is safe and is associated with reduced withdrawal symptoms and better completion rate in these patients. Further studies should explore the use of VLNTX in detoxification and long-term treatment of combined alcohol—opioid dependence and alcohol dependence alone.
Alcohol use is common among opioid-dependent (OD) individuals and often is associated with negative outcomes. Of about 5 million self-reported past-month users of illicit opioids in the United States, 73% consume alcohol and 57% are problem drinkers (Substance Abuse and Mental Health Services Administration, 2009a). One in five OD patients admitted to treatment presents with alcohol problems (Substance Abuse and Mental Health Services Administration, 2009b), and one of four emergency department visits associated with additional drug use in OD individuals involves alcohol use (Substance Abuse and Mental Health Services Administration, 2006).
With the exception of the HIV/AIDS epidemic in the 1980s and 1990s, alcohol use disorders have been a leading cause of morbidity, mortality, and increased health care costs for OD patients in treatment (Appel et al., 2000; Nyamathi et al., 2009; Schuckit, 2009). Thus, it represents a relevant clinical management issue that requires specific interventions if the existing pharmacological approaches are not sufficient.
There is a positive association between alcohol use and relapse into drug use during or after opioid-agonist-replacement treatment (el-Bassel et al., 1993; Stenbacka et al., 2007), but contradictory results exist that methadone or buprenorphine helps reduce alcohol use (Maremmani et al., 2007; Nava et al., 2008) or leads to increased use (Back-mund et al., 2003; Srivastava et al., 2008). The addition of the aversive alcohol medication disulfiram to methadone is efficacious in reducing drinking only when methadone maintenance is contingent on disulfiram ingestion (Bickel et al., 1987), and a suggested greater ability of buprenorphine to suppress drinking in OD patients needs confirmation by randomized trials (Nava et al., 2008). Baclofen, which has shown promise in the treatment of alcohol and cocaine dependence (Kenna et al., 2007), has been evaluated as an alternative to opioid-agonist medications, showing superiority to placebo in treatment retention (Assadi et al. 2003). However, there was no significant reduction in craving and opioid or alcohol use.
Little is known of the effects of alcohol consumption on opioid withdrawal and on the efficacy of specific detoxification approaches. The opioid antagonist naltrexone is used to treat opioid and alcohol dependence and has been safely administered to dual-use-disorder patients (Kampman et al., 2008). Unfortunately, its use during opioid detoxification is limited by the risk of inducing significant withdrawal discomfort (Collins et al., 2005).
Previously, we reported the results of a double-blind, placebo-controlled, randomized study showing that the addition of very-low-dose naltrexone (VLNTX) to methadone attenuates opioid withdrawal severity and craving during inpatient detoxification (Mannelli et al., 2009). Recent alcohol use in this sample was associated with reduced effects of VLNTX treatment on objective measures of opioid withdrawal. However, we found no significant influence of “any” alcohol use on opioid withdrawal severity or VLNTX treatment effects (unpublished data).
In this article, we present the results of secondary analyses to determine whether problem drinking (PD) before detoxification affects opioid withdrawal severity and treatment completion and whether VLNTX addition is safe and improves treatment outcomes in opioid addicts who also use alcohol.
Method
Study design
This was a 6-day, double-blind, randomized trial evaluating the safety and efficacy of two different oral naltrexone regimens (0.125 and 0.250 mg/day) for the treatment of withdrawal in OD subjects during inpatient methadone-based detoxification.
Subjects and procedures
The screening process, treatment, and evaluation procedures are described in detail elsewhere (Mannelli et al., 2009). Participants were non-methadone-treated OD patients age 18 years or older who were seeking detoxification at community treatment programs. Potential subjects were excluded for any of the following criteria: hypersensitivity to naltrexone, pregnancy, psychiatric or medical conditions rendering participation hazardous, current dependence on alcohol, or current dependence on substances other than opioids or nicotine.
Psychiatric and medical examination, with routine laboratory tests including urine testing for drugs of abuse, were performed at screening. The Structured Clinical Interview for the DSM-IV (First et al., 2002) was used to formulate psychiatric diagnoses. The Addiction Severity Index (McLel-lan et al., 1992) was used to evaluate drug use, social status, and psychological status. The Timeline Followback method (Sobell et al., 1996) was adopted to gather information on use of psychotropic medications and pattern of use of illicit drugs. Opiate withdrawal was evaluated using the Subjective Opiate Withdrawal Scale (SOWS) and the Objective Opiate Withdrawal Scale (OOWS) (Handelsman et al., 1987). One item of the SOWS was used to rate craving (Kanof et al., 1992).
Treatment
Participants (N = 174) were randomly assigned to one of three inpatient conditions: (a) placebo and methadone taper (n = 57), (b) naltrexone 0.125 mg per day and methadone taper (n = 59), or (c) naltrexone 0.250 mg per day and methadone taper (n = 58).
Subjects received methadone 30 mg on Day 1, tapered by 5 mg per day, and were discharged on Day 6. VLNTX was administered together with methadone.
The list of ancillary medications is reported elsewhere (Mannelli et al., 2009). Use as needed (PRN) was decided by the medical staff, based on (a) clinical judgment and/or (b) patient's request.
Subjects received psychosocial support, which consisted of daily group activity and one individual session to discuss treatment placement following discharge.
In-study assessments
Daily evaluation was conducted before administration of methadone and naltrexone, using the SOWS and OOWS. Requests for ancillary drugs were rated on a 3-point scale (PRN-S): 0 = no request made, 1 = request made, and 2 = medication ordered. Adverse events were noted as reported by patients and observed during treatment.
Alcohol use
Alcohol use was evaluated at admission using the Addiction Severity Index. Patterns of use in the last 30 days were investigated using the Timeline Followback method. The reliability of this approach has been demonstrated (Rice, 2007; Tucker et al., 2007). The definition of PD (or at-risk alcohol use) was based on the description of more than seven drinks per week or more than three drinks per occasion for women and more than 14 drinks per week or more than four drinks per occasion for men (Enoch and Goldman, 2002).
Statistical analysis
All analyses were carried out on the intent-to-treat sample. Demographic and clinical characteristics were compared using analyses of variance for continuous variables and chi-square tests for categorical variables. Changes from baseline of SOWS and OOWS scores were analyzed using one-way repeated-measure analysis of variance. The same analyses were used in comparisons of individual SOWS symptoms, with Bonferroni test to control for experiment-wise errors. The number of subjects discontinuing treatment were examined using Kaplan-Meier survival analysis.
Results
Subjects
A total of 140 of the 174 OD participants used alcohol: 79 (45.4%) reported PD (PD-OD) and 61 (35%) did not show at-risk alcohol use (AU-OD). Of the latter, 39 (22.4%) used alcohol less than weekly. All PD-OD patients displayed a pattern of episodic heavy alcohol use, defined as four or more drinks for women and five or more drinks for men in about 2 hours (National Institute on Alcohol Abuse and Alcoholism, 2004). They showed at least one episode of heavy alcohol use a week and the last episode occurred in the 48 hours before admission. No use of psychotropic medication was recorded at baseline. Demographic, clinical, and drug use characteristics of participants were compared between groups. No significant differences were noted among PD-OD, AU-OD, and non-alcohol-using patients (N-AU-OD), except for alcohol use (Table 1). In the following comparisons, AU-OD and N-AU-OD individuals were combined into one group of patients without PD (N-PD-OD).
Table 1.
Sociodemographic characteristics, substance use characteristics, and detoxification treatment status of 79 problem drinking-opioid dependent (PD-OD) patients, 61 non-problem-drinking OD (AU-OD) patients, and 34 non-alcohol-using OD (N-AU-OD) patients undergoing 6-day metha-done detoxification
| PD-OD | AU-OD | N-AU-OD | |
| (n = 79) | (n = 61) | (n = 34) | |
| Variable | % or M(SD) | % or M(SD) | % or M(SD) |
| Demographics | |||
| Age | 32.2 (8.2) | 29.8 (7.6) | 33.9(5.1) |
| Male | 67.1 | 68.8 | 64.7 |
| African American | 24.9 | 19.7 | 20.6 |
| Years of education | 10.3 (4.2) | 9.8 (2.2) | 9.8 (1.7) |
| Married or cohabitant | 15.6 | 14.7 | 17.6 |
| Unemployed | 62.0 | 59.0 | 64.7 |
| Substance use | |||
| Days of use in last month | |||
| Opioids | 21.6(2.9) | 19.9 (2.5) | 22.1 (1.5) |
| Alcohol** | 9.8 (2.5) | 2.9 (2.8) | N.A. |
| Cocaine | 6.5 (4.5) | 6.9 (3.5) | 7.2(1.5) |
| Cannabis | 4.7 (6.6) | 6.1 (4.1) | 6.1 (4.1) |
| Years of use | |||
| Opioids | 9.7 (2.7) | 7.7 (3.7) | 8.1 (1.7) |
| Alcohol | 8.9 (4.8) | 7.8 (3.9) | N.A. |
| Cocaine | 5.6 (4.2) | 6.6 (3.5) | 4.6(3.1) |
| Cannabis | 7.8 (2.6) | 6.7 (3.2) | 7.7(1.2) |
| Addiction Severity Index | |||
| Drug composite score | .27 (.16) | .28 (.17) | .21 (.17) |
| Alcohol composite score | .22 (.19) | N.A. | N.A. |
| Psychiatric composite score | .26 (.13) | .22 (.14) | .24 (.10) |
| Other drug tests at admission | |||
| Positive cocaine | 41.8 | 44.3 | 41.2 |
| Positive cannabis | 38.0 | 40.1 | 44.1 |
| Positive amphetamine | 3.8 | 4.9 | 5.9 |
| Opioid withdrawal scores at admission | |||
| SOWS (0–64) | 34.2(11.8) | 35.1 (12.1) | 31.4(8.9) |
| OOWS (0–13) | 4.4 (2.9) | 4.8 (2.6) | 3.9 (1.6) |
| Craving (0–3) | 2.9(0.1) | 2.7 (0.3) | 2.4 (0.3) |
| Treatment randomization | |||
| Placebo | 30.4 | 34.4 | 35.3 |
| Naltrexone 0.125 mg/day | 36.7 | 32.8 | 29.4 |
| Naltrexone 0.250 mg/day | 32.9 | 32.8 | 35.3 |
Notes: n.a. = not applicable; SOWS = Subjective Opiate Withdrawal Scale; OOWS = Objective Opiate Withdrawal Scale.
F(1, 140) = 3.1,p = .01. All other comparisons are nonsignificant.
Opioid withdrawal and craving
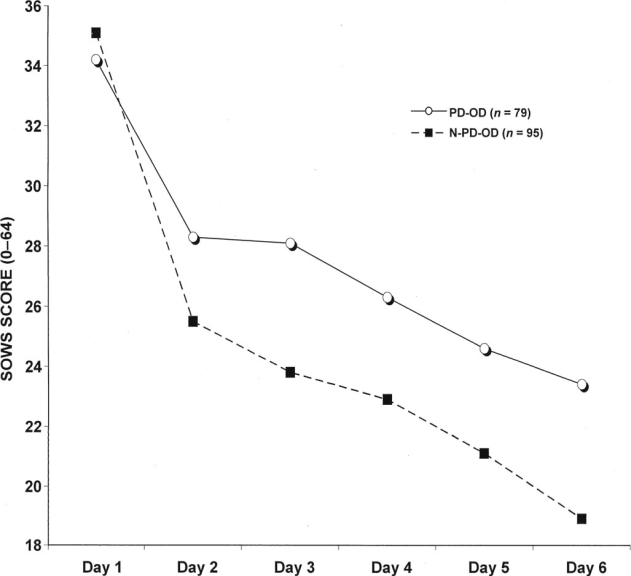
PD-OD participants reported significantly higher SOWS scores and craving compared with N-PD-OD patients, SOWS: F(1, 171) = 11.1,p = .001 (Figure 1); craving: F(1, 171) = 7.3,p = .001. Objective withdrawal symptoms were not significantly different between the two groups, OOWS: F(1, 173) = 2.9, p = .09.
Figure 1.
Subjective Opiate Withdrawal Scale (SOWS) scores in 174 opioid-dependent inpatients undergoing 6-day methadone detoxification. Compared with non-problem drinking, problem drinking was associated with significantly greater withdrawal: repeated-measures analysis of variance, change of scores, F(1, 171) = 11.1, p = .001. PD-OD = problem drinking-opioid dependent patients; N-PD-OD = non-problem-drinking-opioid dependent patients.
Very-low-dose naltrexone and problem drinking
PD-OD patients treated with VLNTX (0.125 mg: n = 29 [36.7%]; 0.25 mg: n = 26 [32.9%]; Table 1) showed a significant reduction of subjective opioid withdrawal severity compared with placebo, F(2, 79) = 4.8, p = .05; for 0.125 mg versus placebo: p = .07; for 0.25 mg versus placebo: p = .03. No significant difference was found in objective withdrawal scores, F(2, 79) = 1.6,p = .14; for 0.125 mg versus placebo: p = .12; for 0.25 mg versus placebo: p = .25. Individual SOWS symptoms were also compared across medication groups. VLNTX (0.25 mg) was significantly more effective than placebo in decreasing anxiety, perspiration, shakiness, nausea, stomach cramps, and craving, F(1, 50) = 6.9–12.1 p = .003-.0001.
Treatment retention
A total of 120 subjects (69%) completed treatment. The number of dropouts was significantly lower among subjects without PD (PD-OD = 58.2%; N-PD-OD = 77.9%), χ2(1) (Breslow) = 5.1, p = .02. PD-OD patients who received VLNTX showed significantly better completion rates (naltrexone 0.125 mg = 65.6%; naltrexone 0.25 mg = 65.4%; placebo = 50%), χ2(2) (Breslow) = 5.9, p = .03.
Use of ancillary medications and adverse events
All subjects received ancillary medications: in 58.6% of cases the medication was administered following a request. There were no significant differences in requests of ancillary medications between PD-OD and N-PD-OD patients (57.9% vs. 59.5%, respectively), PRN-S score: χ2(1) for Days 1–6 range = 0.32–1.62, p = .10-.75, or between PD-OD patients treated with VLNTX and those receiving placebo (naltrexone 0.125 mg = 58.6%; naltrexone 0.25 mg = 62.5%; placebo = 61.5%), F(2,79) = 0.7, p = .55; PRN-S score χ2(2) for Days 1–6 range = 0.61–2.52, p = .16—.57. The daily amount of ancillary medication did not significantly differ, depending on the existence of at-risk alcohol use or on the treatment received by problem drinkers (data not shown).
There were no serious adverse events and no medication-related adverse events. None of the adverse events previously described in this sample (Mannelli et al., 2009) were recorded among PD-OD patients. The only other adverse events were mild somatic and behavioral complaints indistinguishable from signs of opiate withdrawal that were documented by withdrawal scores. There were no episodes of medication-precipitated withdrawal.
Alcohol use at follow-up
Breath alcohol and urine drug tests performed on the first day following treatment completion and discharge showed reduced rates of alcohol and opioid use in PD-OD patients treated with VLNTX (0.125 mg: n = 14; 0.25 mg: n = 13) compared with those who had received placebo (n = 12), alcohol: χ2(2) = 15.9, p = .03; opioids: χ2(2) = 26.3 p = .001. One week later, positivity for alcohol was not significantly different between groups, χ2(2) = 3.7,p = .7, whereas VLNTX-treated individuals were still using opioids less, χ2(2) = 18.7, p = .01. No significant differences were found in use of other drugs (data not shown).
Discussion
In this secondary analysis of previously presented data, we found that opioid withdrawal and craving were more severe, and detoxification completion rates were lower among subjects who reported PD at admission. PD-OD patients receiving VLNTX plus methadone-assisted detoxification showed attenuated subjective withdrawal and better treatment retention compared with those randomized to methadone alone. There were no significant differences in the OOWS. Potential modifiers of withdrawal and retention, such as sociodemographic and drug use characteristics, or use of ancillary medications during detoxification did not differ significantly between groups. It is unlikely that such variables could have influenced the findings.
Almost one half of OD individuals reported at-risk alcohol use, a finding consistent with the existing literature (Nyamathi et al., 2009; Senbanjo et al., 2007; Stenbacka et al., 2007). To our knowledge, this is the first report of an adverse influence of alcohol use on the manifestation of opioid withdrawal and on the outcome of opioid detoxification. Opioid discontinuation remains the first step required for longer-term interventions and is offered to 40% of new admissions to treatment (Substance Abuse and Mental Health Services Administration, 2009b). It may also represent the conclusion of a prolonged opioid-replacement therapy (Kleber, 2007). Either way, given the economic implications and the high incidence and severity of dual and polysubstance abuse and dependence among opioid addicts, the importance of identifying specific pharmacotherapies has been repeatedly emphasized (Kenna et al., 2007; Kosten, 1991; Kreek, 1988; Stimmel et al., 1981). Thus, it is surprising that a medication approved for the treatment of both alcohol and opioid dependence, such as naltrexone, has received little attention. In an open-label study, full-dose oral and extended-release naltrexone administration to patients with mixed alcohol and opioid dependence was associated with treatment retention comparable to that observed in opioid-only-dependent or alcohol-only-dependent individuals, although efficacy data were lacking (Kampman et al., 2008). Anecdotal reports describe worsening of depressive symptoms following naltrexone treatment of combined alcohol-opioid misuse (Dervaux, 2005; Schürks et al., 2005).
VLNTX was well tolerated by PD-OD patients. Comparisons of SOWS individual symptoms revealed that, besides craving, there was a significant decrease of five other symptoms that are common to opioid and alcohol withdrawal (West and Gossop, 1994) in patients treated with the higher naltrexone dose compared with those who had received a placebo. Some of these symptoms such as nausea and abdominal cramps are associated with reduced compliance with and early discontinuation of naltrexone treatment for alcohol dependence (O'Malley et al., 2000; Oncken et al., 2001). The use of VLNTX during alcohol detoxification and before administering higher dose naltrexone may help reduce withdrawal discomfort and side effects, improving retention in treatment. Our short-term follow-up data suggest that naltrexone administration should not be discontinued in order to prevent resumption of alcohol use.
The attenuation of opioid withdrawal intensity has been previously explained by the ability of VLNTX to reduce mu-opioid receptor activity and dependence levels during opioid-agonist treatment (Mannelli et al., 2004, 2009). Additional heavy alcohol use would increase opioid activity and withdrawal discomfort following discontinuation, which would be associated with higher likelihood of dropout and a positive response to VLNTX treatment.
Problem drinkers in this sample display a modality of heavy episodic drinking that is clinically significant per se and is associated with serious medical complications (Man-nelli and Pae, 2007), although such drinkers often do not exhibit somatic or motivational signs of alcohol dependence (National Institute on Alcohol Abuse and Alcoholism, 2004). This may explain why no significant differences were detected in objective withdrawal between PD-OD and N-PD-OD patients. Studies of animal models reproducing this drinking pattern report suppression of alcohol intake by VLNTX, suggesting that opioid antagonist medications may be more effective in treating episodic heavy drinking than excessive drinking associated with alcohol dependence (Ji et al., 2008; Sabino et al., 2006).
This study has several limitations. Alcohol use was assessed by subject self-report rather than biological measures. However, self-reports have shown validity and reliability comparable to those of biochemical measures when collecting admission information on alcoholic patients (Babor et al., 2000). Alcohol use was not taken into account in the prospective randomization of subjects and alcohol withdrawal was not assessed. It is possible that the association of problem alcohol use and VLNTX administration with changes in withdrawal discomfort and retention in treatment is accounted for by unmeasured confounds in this population, although such likelihood is reduced by the randomized assignment to treatment groups. Confounding by individual or treatment variables is unlikely because patients did not significantly differ in such characteristics. Finally, the modality of alcohol use described in this population may limit the generalizability of the treatment to other patterns of PD or alcohol dependence.
Despite these limitations, this study provides evidence that heavy episodic alcohol use is associated with negative outcomes in OD patients who discontinue opioid-agonist treatment. The administration of VLNTX was a safe and effective method to reduce withdrawal severity and facilitate treatment retention in this group of patients. Further studies are warranted to test VLNTX for the treatment of combined alcohol and opioid dependence and to investigate its use during alcohol detoxification before induction to naltrexone relapse-prevention treatment.
Footnotes
This research was supported by National Institute on Drug Abuse (NIDA) grant DA 15469 (Paolo Mannelli). The work was carried out at Duke University, Durham, NC, and Thomas Jefferson University, Philadelphia, PA. David A. Gorelick was supported by the Intramural Research Program, NIDA, National Institutes of Health (NIH). ID number of this study in clintrials. gov: NCT00135759. Portions of this research were presented at the American Psychiatric Association annual conference in New Orleans, LA, May 22—26, 2010.
References
- Appel PW, Joseph H, Richman BL. Causes and rates of death among methadone maintenance patients before and after the onset of the HIV/AIDS epidemic. Mount Sinai Journal of Medicine. 2000;67:444–451. [PubMed] [Google Scholar]
- Assadi SM, Radgoodarzi R, Ahmadi-Abhari SA. Baclofen for maintenance treatment of opioid dependence: A randomized double-blind placebo-controlled clinical trial [ISRCTN32121581] BMC Psychiatry. 2003;3 doi: 10.1186/1471-244X-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Steinberg K, Anton R, Del Boca F. Talk is cheap: Measuring drinking outcomes in clinical trials. Journal of Studies on Alcohol. 2000;61:55–63. doi: 10.15288/jsa.2000.61.55. [DOI] [PubMed] [Google Scholar]
- Backmund M, Schütz CG, Meyer K, Eichenlaub D, Soyka M. Alcohol consumption in heroin users, methadone-substituted and codeine-substituted patients—frequency and correlates of use. European Addiction Research. 2003;9:45–50. doi: 10.1159/000067733. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marion I, Lowinson JH. The treatment of alcoholic methadone patients: A review. Journal of Substance Abuse Treatment. 1987;4:15–19. doi: 10.1016/0740-5472(87)90005-5. [DOI] [PubMed] [Google Scholar]
- Collins ED, Kleber HD, Whittington RA, Heitler NE. Anesthesia-assisted vs buprenorphine- or clonidine-assisted heroin detoxification and naltrexone induction: A randomized trial. Journal of the American Medical Association. 2005;294:903–913. doi: 10.1001/jama.294.8.903. [DOI] [PubMed] [Google Scholar]
- Dervaux A. Alcohol dependence and depressive disorders in the course of opiate dependence. Encephale, Pt. 2005;2:S45–46. [PubMed] [Google Scholar]
- el-Bassel N, Schilling RF, Turnbull JE, Su K-H. Correlates of alcohol use among methadone patients. Alcoholism, Clinical and Experimental Research. 1993;17:681–686. doi: 10.1111/j.1530-0277.1993.tb00819.x. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Goldman D. Problem drinking and alcoholism: diagnosis and treatment. American Family Physician. 2002;65:441–448. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York, NY: Biometrics Research; 2002. (SCID-I/P). New York State Psychiatric Institute. [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. American Journal of Drug and Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behavioural Pharmacology. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman K, Gromov I, Sirota A, Silverman B, Qiao X, Gastfriend DR. Long-term safety and tolerability of extended-releasenaltrexone in alcohol- and/or opioid-dependentpatients: A randomized, active-controlled study; Paper presented at the American PsychiatricAssociation Annual Conference; Washington, DC. May, 2008. [Google Scholar]
- Kanof PD, Handelsman L, Aronson MJ, Ness R, Cochrane KJ, Rubinstein KJ. Clinical characteristics of naloxone- precipitated withdrawal in human opioid-dependent subjects. Journal of Pharmacology and Experimental Therapeutics. 1992;260:355–363. [PubMed] [Google Scholar]
- Kenna GA, Nielsen DM, Mello P, Schiesl A, Swift RM. Pharmacotherapy of dual substance abuse and dependence. CNS Drugs. 2007;21:213–237. doi: 10.2165/00023210-200721030-00003. [DOI] [PubMed] [Google Scholar]
- Kleber HD. Pharmacologic treatments for opioid dependence: Detoxification and maintenance options. Dialogues in Clinical Neuro-science. 2007;9:455–470. doi: 10.31887/DCNS.2007.9.2/hkleber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR. Client issues in drug abuse treatment: Addressing multiple drug abuse. NIDA Research Monograph. 1991;106:136–151. [PubMed] [Google Scholar]
- Kreek MJ. Opiate-ethanol interactions: Implications for the biological basis and treatment of combined addictive diseases. NIDA Research Monograph. 1988;81:428–439. [PubMed] [Google Scholar]
- Mannelli P, Gottheil E, Peoples JF, Oropeza VC, Van Bockstaele EJ. Chronic very low dose naltrexone administration attenuates opioid withdrawal expression. Biological Psychiatry. 2004;56:261–268. doi: 10.1016/j.biopsych.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Mannelli P, Pae CU. Medical comorbidity and alcohol dependence. Current Psychiatry Reports. 2007;9:217–224. doi: 10.1007/s11920-007-0022-x. [DOI] [PubMed] [Google Scholar]
- Mannelli P, Patkar AA, Peindl K, Gorelick DA, Wu LT, Gottheil E. Very low dose naltrexone addition in opioid detoxification: A randomized, controlled trial. Addiction Biology. 2009;14:204–213. doi: 10.1111/j.1369-1600.2008.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maremmani I, Pani PP, Mellini A, Pacini M, Marini G, Lovrecic M, Shinderman M. Alcohol and cocaine use and abuse among opioid addicts engaged in a methadone maintenance treatment program. Journal of Addictive Diseases. 2007;26:61–70. doi: 10.1300/J069v26n01_08. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Argeriou M. The fifth edition of the addiction severity index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Bethesda, MD: 2004. NIAAA council approves definition of binge drinking. NIAAA newsletter (NIH Publication No. 04–5346) [Google Scholar]
- Nava F, Manzato E, Leonardi C, Lucchini A. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: Preliminary results of an open randomized study. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32:1867–1872. doi: 10.1016/j.pnpbp.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Nyamathi A, Compton P, Cohen A, Marfisee M, Shoptaw S, Green-gold B, Leake B. Correlates of hospitalization for alcohol-using methadone-maintained persons with physical health problems. Western Journal of Nursing Research. 2009;31:525–543. doi: 10.1177/0193945908328784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley SS, Krishnan-Sarin S, Farren C, O'Connor PG. Naltrexone-induced nausea in patients treated for alcohol dependence: Clinical predictors and evidence for opioid-mediated effects. Journal of Clinical Psychopharmacology. 2000;20:69–76. doi: 10.1097/00004714-200002000-00012. [DOI] [PubMed] [Google Scholar]
- Oncken C, Van Kirk J, Kranzler HR. Adverse effects of oral naltrexone: Analysis of data from two clinical trials. Psychopharmacology. 2001;154:397–402. doi: 10.1007/s002130000666. [DOI] [PubMed] [Google Scholar]
- Rice C. Retest reliability of self-reported daily drinking: Form 90. Journal of Studies on Alcohol and Drugs. 2007;68:615–618. doi: 10.15288/jsad.2007.68.615. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology. 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Alcohol-use disorders. Lancet. 2009;373:492–501. doi: 10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- Schürks M, Overlack M, Bonnet U. Naltrexone treatment of combined alcohol and opioid dependence: Deterioration of co-morbid major depression. Pharmacopsychiatry. 2005;38:100–102. doi: 10.1055/s-2005-837812. [DOI] [PubMed] [Google Scholar]
- Senbanjo R, Wolff K, Marshall J. Excessive alcohol consumption is associated with reduced quality of life among methadone patients. Addiction. 2007;102:257–263. doi: 10.1111/j.1360-0443.2006.01683.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug and Alcohol Dependence. 1996;42:49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: A systematic review. Journal of Substance Abuse Treatment. 2008;34:215–223. doi: 10.1016/j.jsat.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Stenbacka M, Beck O, Leifman A, Romelsjö A, Helander A. Problem drinking in relation to treatment outcome among opiate addicts in methadone maintenance treatment. Drug and Alcohol Review. 2007;26:55–63. doi: 10.1080/09595230601036994. [DOI] [PubMed] [Google Scholar]
- Stimmel B, Korts D, Cohen M, Jackson G, Sturiano V, Hanbury R. Opiate addiction and alcoholism: The feasibility of combined treatment approaches. Annals of the New York Academy of Sciences. 1981;362:50–56. doi: 10.1111/j.1749-6632.1981.tb12790.x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (Office of Applied Studies) Drug abuse warning network report. Rockville, MD: Author; 2006. p. 3. [AU: Please verify this reference and provide the suggested citation or a link to the original document.] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (Office of Applied Studies) Results from the 2008 National Survey on Drug Use and Health: National findings. Rockville, MD: Office of Applied Studies, NSDUH Series H-36, HHS Publication No. SMA 09–4434; 2009a. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (Office of Applied Studies) The TEDS report: Heroin and other opiate admissions to substance abuse treatment. Rockville, MD: 2009b. [Google Scholar]
- Tucker JA, Foushee HR, Black BC, Roth DL. Agreement between prospective interactive voice response self-monitoring and structured retrospective reports of drinking and contextual variables during natural resolution attempts. Journal of Studies on Alcohol and Drugs. 2007;68:538–542. doi: 10.15288/jsad.2007.68.538. [DOI] [PubMed] [Google Scholar]
- West R, Gossop M. Overview: A comparison of withdrawal symptoms from different drug classes. Addiction. 1994;89:1483–1489. doi: 10.1111/j.1360-0443.1994.tb03747.x. [DOI] [PubMed] [Google Scholar]