Abstract
Background
The development of a safe, effective, and affordable combination microbicide to prevent the sexual transmission of HIV combination is urgently needed. Our previous studies demonstrated that 3-hydroxyphthalic anhydride-modified chicken ovalbumin (HP-OVA) exhibited potent antiviral activity against a broad spectrum of HIV, simian immunodeficiency virus (SIV) and herpes simplex virus (HSV), making it a promising candidate as a component of combination microbicide. Here we intended to evaluate potential synergistic anti-HIV-1 effect of HP-OVA in combinations with antiretroviral drug (ARV)-based microbicide candidates.
Methods
The antiviral activity of HP-OVA and the ARVs, including HIV-1 entry inhibitors (T20, C52L, NB64, NBD556, AMD3100 and Maraviroc) and reverse transcriptase inhibitors (Tenofovir, UC781 and TMC120), tested alone or in combination, against HIV-1 X4 and R5 viruses, including some drug-resistant strains, was determined in MT-2 and peripheral blood mononuclear cells using p24 assay. The immune responses induced by HP-OVA that was applied in the vaginas of rats were detected by ELISA.
Results
When each of these ARV-based microbicide candidates was combined with HP-OVA, synergistic activity was observed against infection by both X4 and R5 strains, and the degree of synergy differed in each case. HP-OVA was highly effective against several ARV-resistant HIV-1 strains, suggesting that combining HP-OVA with these ARV-based microbicide candidates might work cooperatively against both drug-sensitive and resistant HIV-1 strains. Human body fluids and human proteins had little or no effects on HP-OVA-mediated inhibitory activity against HIV-1 infection. HP-OVA formulated in the universal gel maintained its antiviral activity for at least one month and only induced weak immune responses after its multiple applications in the vaginas of rats.
Conclusions
Synergistic and complementary effects against infection by a broad spectrum of HIV-1 strains were observed by combining HP-OVA with the ARV-based microbicide candidates. These findings provide a sound scientific platform for the development of a safe, effective and affordable combination microbicide to prevent the sexual transmission of HIV and other sexually transmissible viruses.
Keywords: HIV, 3-hydroxyphthalic anhydride-modified chicken ovalbumin, synergism, antiretroviral drug-based microbicides
INTRODUCTION
The development of microbicides is one of the few biologically sound prevention strategies with the potential to greatly empower women to protect themselves from human immunodeficiency virus (HIV) in many developing countries. It is a self-seeking preventive option over which women can take full control, and it does not require any negotiations with their partners. Therefore, topically applied microbicides could be a promising way to slow the global spread of HIV, if proven safe and effective.
Currently, the progress of microbicide candidates in clinical efficacy trials is particularly challenging. Phase III clinical trials of the most promising non-specific anti-HIV polymer-based microbicide candidates, such as Carraguard1,2, cellulose sulfate (Ushercell)3–5 and PRO 20006,7, showed no effectiveness in preventing HIV infection. It is now generally accepted that these microbicides failed because of low efficacy against HIV-1 R5 isolates, the most commonly sexually transmitted viruses8,9. The focus in microbicide development is shifting away from nonspecific anti-HIV microbicides to specific and more effective antiretroviral drug (ARV)-based microbicides, including reverse transcriptase inhibitors (RTIs)10,11 and HIV-1 entry inhibitors10,12. One of the most advanced ARV-based microbicide candidates is Tenofovir13–15, a nucleoside reverse transcriptase inhibitor (NRTI). It was reported that the 1% vaginal gel formulation of Tenofovir that was evaluated in a Phase IIb study in South Africa (CAPRISA 004 trial) showed moderate efficacy in preventing HIV infection16, reducing HIV acquisition by an estimated 39% overall and by 54% in women with high gel adherence17. However, the major limitations in developing these specific ARV-based microbicide candidates include their potential to induce ARV-resistant HIV-1 strains and their ineffectiveness against other sexually transmissible viruses, such as HSV. Therefore, it is essential to develop combination microbicides consisting of specific and non-specific combination microbicides for prevention of sexual transmission of HIV and other sexually transmissible viruses.
We have recently reported that 3-hydroxyphthalic anhydride-modified chicken ovalbumin (HP-OVA) exhibits potent antiviral activity against HIV-1 strains, including those resistant to HIV entry inhibitors, NRTIs and non-nucleoside reverse transcriptase inhibitors (NNRTIs). Furthermore, HP-OVA is also effective against HIV-2 and simian immunodeficiency virus (SIV), as well as other pathogens causing sexually transmitted infections (STIs), such as herpes simplex virus type 2 (HSV-2)18. HP-OVA inhibits HIV-1 entry into the target cell by binding to both gp120 and CD4 and blocking the interaction between gp120 and the target cell-surface receptor CD418. Because chicken ovalbumin is widely available and relatively inexpensive19,20, HP-OVA shows considerable promise for development into a safe, effective and affordable non-specific microbicide for the prevention of sexual transmission of HIV and other STI pathogens. In the present study, we tested whether HP-OVA could be used in combination with some specific ARV-based microbicide candidates with different mechanisms of action to prevent the sexual transmission of HIV.
METHODS
Reagents
MT-2 cells; TZM-bl cells; laboratory-adapted and primary HIV-1 strains, AZT-R (resistant to NRTIs), A17 (resistant to NNRTIs), NL4-3(D36G), NL4-3(D36G)V38E/N42S (resistant to T20); anti-p24 monoclonal antibody (183-12H-5C); HIV immunoglobulin (HIVIG); Tenofovir; T20; AMD3100 and Maraviroc were all obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. Ovalbumin (OVA); human serum albumin (HSA); normal human IgG (NHIgG) and 3-hydroxyphthalic anhydride (HP) were purchased from Sigma (St. Louis, MO). NB64 was purchased from Cambridge (San Diego, CA). NBD556 was provided by Dr. A. K. Debnath at Lindsley F. Kimball Research Institute of the New York Blood Center (New York, NY). UC781 and TMC120 were obtained by custom synthesis from Albany Molecular Research (Albany, NY). C52L21 was kindly provided by Dr. M. Lu at Weill Medical College of Cornell University. Seminal fluid (SF) was purchased from Lee BioSolutions, Inc. (St. Louis, Missouri, MO). Vaginal fluid simulant (VFS) was prepared as described by Owen and Katz 22.
Preparation of HP-OVA
HP-OVA was prepared as previously described18,23,24. Briefly, OVA (final concentration, 20 mg/ml in 0.1 M phosphate, pH 8.5) was treated with HP (1.19 M in DMSO), added into five aliquots (final concentration, 40 mM) at 12 min intervals, while the pH was maintained at 8.5. The mixture was kept for another 1 h at 25 °C, extensively dialyzed against phosphate buffer saline (PBS), and filtered through 0.45 μm syringe filters (Acrodisc, Gelman Sciences, Ann Arbor, MI). Protein concentrations were determined using the BCA Protein Assay Reagent Kit (Pierce, Rockford, IL).
Measurement of the anti-HIV-1 activity of the antiviral agents individually and in combination
The inhibitory activity of HP-OVA alone, the HIV-1 entry inhibitors (e.g., T20, C52L, NB64, NBD556, and AMD3100) alone, the reverse transcriptase inhibitors (e.g., Tenofovir, UC781 and TMC120) alone, and the combinations of HP-OVA with each of these ARVs, on infection caused by HIV-1 X4 strain IIIB was determined as previously described18,25,26. The antiviral activity of Tenofovir, UC781 and T20 against the corresponding drug-resistant HIV-1 strains, AZT-R, A17, and NL4-3(D36G)V38E/N42S, respectively, was also assessed. Briefly, HIV-1 strains at 100 TCID50 (50% tissue culture infective dose) were incubated with the antiviral agents, alone or combinations, at indicated concentration at 37 °C for 30 min, followed by addition of 100 μl of MT-2 cells (1 × 105/ml). After incubation at 37 °C overnight, the culture supernatants were replaced with fresh medium. On the fourth day post-infection, 100 μl of culture supernatants were collected and mixed with equal volumes of 5% Triton X-100 for detection of p24 antigen using an in-house ELISA as previously described18,26. Absorbance at 450 nm was recorded in an ELISA reader (Ultra 384; Tecan, Research Triangle Park, NC).
To detect the inhibitory activity of those anti-HIV-1 agents, including Maraviroc, on infection by HIV-1 R5 strains (BaL and primary isolates), 1 × 104 peripheral blood mononuclear cells (PBMCs), which were isolated from the blood of healthy donors at the New York Blood Center by standard density gradient centrifugation by using Histopaque-1077 (Sigma) and stimulated with phytohemagglutinin (PHA) and interleukin-2 as previously described27 were infected with 100 TCID50 HIV-1 in the presence of the anti-HIV-1 agents, alone or in combination at graded concentrations. Culture media were changed every 3 days. The supernatants were collected 7 days post-infection and tested for p24 antigen by ELISA as described above.
The effective concentration for 50% and 90% inhibition (IC50 and IC90) values were calculated using the Calcusyn software28, kindly provided by Dr. T. C. Chou at Sloan-Kettering Cancer Center (New York, NY).
Synergy analysis
The data were analyzed for cooperative effects by using the Chou-Talalay Method. The combination index (CI) values were calculated by using the CalcuSyn program based on the protocol as described26. Compounds or peptides were tested at a fixed molar ratio individually and in combination. The CI value reflects the nature of the interaction between compounds. CI values of <1, =1 and >1 indicate synergy, additivity and antagonism, respectively. In detail, CI <0.10 means very strong synergism, 0.10~0.30 means strong synergism, 0.30~0.70 means synergism, 0.70~0.85 means moderate synergism and 0.85~0.90 means slight synergism. Dose Reductions were calculated as the ratio between the IC50 of the compound used alone and in combination26.
Determination of the effects of SF, VFS, HSA and NHIgG on the anti-HIV-1 activities of HP-OVA
The effects of human SF or VFS on anti-HIV-1 activities of HP-OVA were determined as previously described 29–31. SF was first centrifuged at 500 g for 30 min to remove spermatozoa. VFS was prepared as previously described22. HP-OVA (lyophilized powder) was reconstituted to 40 mg/ml with SF, VFS, or PBS (control), respectively, followed by an incubation at 37 °C for 60 min. To avoid the toxic effect of SF and VFS on the target cells or viruses, the mixtures were diluted with culture medium 1,000 times (40 μg/ml) for testing anti-HIV-1IIIB activity and 100 times (400 μg/ml) for testing anti-HIV-1BaL activity, respectively, as described above.
The inhibitory activities of HP-OVA on HIV-1 IIIB and HIV-1BaL infection in the presence of HSA or NHIgG were also tested. Briefly, a series of 2-fold diluted HSA or NHIgG was mixed with HP-OVA at a fixed concentration, and then incubated at 37 °C for 60 min. For testing the anti-HIV-1IIIB activity, the final concentrations of HSA or NHIgG ranged from 0.5 to 32 μg/ml and that of HP-OVA was 4 μg/ml. For testing anti-HIV-1BaL activity, the final concentrations of HSA and NHIgG ranged from 2.5 to 160 μg/ml and that of HP-OVA was 20 μg/ml. The inhibitory activities on infection by HIV-1 IIIB and BaL were detected as described above.
Assessment of the stability of HP-OVA in microbicide gel
HP-OVA was added into the universal gel (10 mg/g gel = 1%) and the HP-OVA-containing gel was kept at room temperature for 1 hour, 1 day and 1 week, respectively, before addition of the gel to virus. After incubation at 37 °C for 30 min, the inhibitory activities on HIV-1 IIIB and BaL infection were detected as described above.
Analysis of the immune responses induced by HP-OVA-containing gel that was multiply applied in the vaginas of rats
HP-OVA gels at 1% (10 mg HP-OVA/g gel) and 0.1% (1 mg HP-OVA/g gel) were respectively administrated into the vaginas of rats with a dose of 0.5 ml/rat, once a week for 4 weeks. Sera of the rats were collected for testing anti-HP-OVA antibody responses by ELISA. The universal gel and OVA gel were used as controls. For ELISA, wells of 96-well polystyrene plates were coated with 5 μg/ml HP-OVA in 0.1 M Tris buffer (pH 8.8) at 4 °C overnight, followed by washing with PBS-T buffer (0.01 M PBS containing 0.05% Tween 20). Then the wells were blocked for 1 h at 37 °C with 1% dry fat-free milk. After three washes, sera (1:50 diluted in PBS) of rats with vaginal application of HP-OVA gel, OVA gel or gel only were added to the wells, followed by an incubation for 1 h at 37 °C. After extensive washes, biotin-labeled anti-rat IgE or anti-rat IgG+IgA+IgM (Invitrogen), SA-HRP, TMB and 1N H2SO4 were added, sequentially. The absorbance at 450 nm (A450) was measured by using an ELISA reader. Statistical analysis was performed by student t test using SPSS 13.0 statistical software and a p value of less than 0.05 was considered significant.
RESULTS
Combinations of HP-OVA with HIV-1 fusion/entry inhibitors showed synergistic effects against HIV-1 infection
HIV fusion/entry inhibitors constitute one class of next-generation ARV-based microbicide candidates. They inhibit HIV-1 infection by specifically targeting virus envelope glycoproteins (gp120/gp41) or cellular co-receptors (CXCR4/CCR5) 12,32. Our previous studies have shown that HP-OVA itself could block the interaction between gp120 and target cell surface receptor CD4 by binding to both gp120 and CD4, resulting in the inhibition of HIV-1 entry18. Here we first investigated the potential cooperative effects of combining HP-OVA with several different HIV-1 fusion/entry inhibitors, including those targeting gp41 (NB6426, T20 and C52L21), gp120-CD4 interaction (NBD55633), CXCR4 co-receptor (AMD310034) and the CCR5 co-receptor (Maraviroc35), against HIV-1 X4 (IIIB) and R5 (BaL) strains. The ratios of HP-OVA and each of the above-noted HIV fusion/entry inhibitors in the combinations were estimated based on the pre-determined IC50 values of these agents when they were tested individually under the same conditions by testing the combinations at the equipotent ratio and several ratios at higher or lower than the equipotent ratio. One ratio with the best synergism in the preliminary experiment was selected for the subsequent studies. As shown in the Table 1, the combination of HP-OVA with NB64, T20, C52L or NBD556 exhibited strong synergism with CI50 values (combination index at IC50) from 0.139 to 0.350 and dose reductions of IC50 for each drug in a given combination of about 3- to 23-fold. The synergistic effects of these combinations were also observed intuitively (Figure S1 A–D and F–I). We further determined the anti-HIV-1 activity of HP-OVA in combination with CXCR4 antagonist AMD3100 on HIV-1IIIB and CCR5 antagonist Maraviroc on HIV-1BaL infection. In the former case, synergism was observed with a CI50 value of 0.531 and dose reductions for HP-OVA and AMD3100 of 2.5-fold and 7.4-fold, respectively (Table 1 and Figure S1E). However, the strongest synergism was observed in the latter case with CI50 = 0.066 and the dose reductions of IC50 of HP-OVA and Maraviroc about 40-fold and 32-fold, respectively (Table 1 and Figure S1J).
Table 1.
Combination index (CI) and dose reduction values for inhibition of HIV-1 infection by combining HP-OVA with HIV-1 entry inhibitors a
| Combinations of HP-OVA with HIV-1 entry inhibitors (weight ratio) | CI | HP-OVA | HIV-1 entry inhibitors | ||||
|---|---|---|---|---|---|---|---|
| Conc. (μg/ml) |
Dose reduction | Conc. (μg/ml) |
Dose reduction | ||||
| Alone | Mix | Alone | Mix | ||||
| Inhibition of HIV-1IIIB infection | |||||||
| HP-OVA: NB64 (1:1) | |||||||
| 50% inhibition | 0.159 | 0.774 | 0.090 | 8.600 | 2.094 | 0.090 | 23.27 |
| 90% inhibition | 0.325 | 3.463 | 0.873 | 3.967 | 11.91 | 0.873 | 13.65 |
| HP-OVA: T20 (1:1) | |||||||
| 50% inhibition | 0.229 | 0.906 | 0.067 | 13.52 | 0.437 | 0.067 | 6.522 |
| 90% inhibition | 0.310 | 2.242 | 0.262 | 8.557 | 1.360 | 0.262 | 5.191 |
| HP-OVA: C52L (1:1) | |||||||
| 50% inhibition | 0.223 | 0.906 | 0.111 | 8.162 | 1.115 | 0.111 | 10.05 |
| 90% inhibition | 0.208 | 2.242 | 0.270 | 8.304 | 3.100 | 0.270 | 11.48 |
| HP-OVA: NBD556 (1:5) | |||||||
| 50% inhibition | 0.276 | 0.774 | 0.107 | 7.234 | 3.918 | 0.537 | 7.296 |
| 90% inhibition | 0.396 | 3.463 | 0.526 | 6.584 | 10.78 | 2.630 | 4.099 |
| HP-OVA: AMD3100 (40:1) | |||||||
| 50% inhibition | 0.531 | 0.847 | 0.330 | 2.567 | 0.059 | 0.008 | 7.375 |
| 90% inhibition | 0.422 | 3.050 | 0.828 | 3.684 | 0.137 | 0.021 | 6.524 |
| Inhibition of HIV-1BaL infection | |||||||
| HP-OVA: NB64 (10:1) | |||||||
| 50% inhibition | 0.238 | 4.854 | 0.783 | 6.199 | 1.027 | 0.078 | 13.17 |
| 90% inhibition | 0.426 | 22.09 | 5.338 | 4.138 | 2.892 | 0.534 | 5.416 |
| HP-OVA: T20 (44:1) | |||||||
| 50% inhibition | 0.350 | 10.95 | 1.758 | 6.229 | 0.412 | 0.078 | 5.282 |
| 90% inhibition | 0.509 | 33.00 | 6.359 | 5.189 | 0.892 | 0.283 | 3.152 |
| HP-OVA: C52L (50:1) | |||||||
| 50% inhibition | 0.139 | 4.854 | 0.325 | 14.94 | 0.090 | 0.007 | 12.86 |
| 90% inhibition | 0.420 | 22.09 | 2.585 | 8.545 | 0.171 | 0.052 | 3.288 |
| HP-OVA: NBD556 (2:1) | |||||||
| 50% inhibition | 0.277 | 4.854 | 0.875 | 5.547 | 18.12 | 1.749 | 10.36 |
| 90% inhibition | 0.439 | 22.09 | 4.270 | 5.173 | 34.77 | 8.540 | 4.071 |
| HP-OVA: Maraviroc (5000:1) | |||||||
| 50% inhibition | 0.066 | 4.854 | 0.122 | 39.79 | 0.001 | 0.00003 | 31.54 |
| 90% inhibition | 0.123 | 22.09 | 0.872 | 25.33 | 0.002 | 0.0002 | 9.858 |
Strong synergism were also observed when HP-OVA was combined with Maraviroc against infection by the primary HIV-1 isolates 93IN101 (clade C, R5) and 92US657 (clade B, R5). For inhibiting 93IN101 infection, the CI50 value is 0.162 and dose reductions of IC50 of HP-OVA and Maraviroc were about 11-fold and 16-fold, respectively (Table 3). For inhibiting 92US657 infection, the CI50 is 0.295 and dose reductions of IC50 of HP-OVA and Maraviroc were about 6.3-fold and 7.3-fold, respectively (Table 3).
Table 3.
Combination index (CI) and dose reduction values for inhibition of primary HIV-1 infection by combining HP-OVA with ARV-based microbicides a
| Combinations of HP-OVA with ARV-based microbicides(weight ratio) | CI | HP-OVA | ARV-based microbicides | ||||
|---|---|---|---|---|---|---|---|
| Conc.(μg/ml) |
Dose reduction | Conc.(μg/ml) |
Dose reduction | ||||
| Alone | Mix | Alone | Mix | ||||
| Inhibition of primary HIV-1 R5 infection (93IN101) | |||||||
| HP-OVA: Maraviroc (4000:1) | |||||||
| 50% inhibition | 0.162 | 10.19 | 0.924 | 11.03 | 0.003 | 0.0002 | 16.10 |
| 90% inhibition | 0.223 | 37.58 | 6.299 | 5.97 | 0.028 | 0.002 | 17.96 |
| HP-OVA: Tenofovir (200:1) | |||||||
| 50% inhibition | 0.319 | 10.19 | 1.725 | 5.91 | 0.058 | 0.009 | 6.68 |
| 90% inhibition | 0.453 | 37.58 | 11.42 | 3.29 | 0.384 | 0.057 | 6.73 |
| HP-OVA: TMC120 (200:1) | |||||||
| 50% inhibition | 0.642 | 10.19 | 2.274 | 4.48 | 0.027 | 0.011 | 2.38 |
| 90% inhibition | 0.722 | 37.58 | 9.465 | 3.97 | 0.100 | 0.047 | 2.12 |
| Inhibition of primary HIV-1 R5 infection (92US657) | |||||||
| HP-OVA: Maraviroc (8000:1) | |||||||
| 50% inhibition | 0.295 | 15.09 | 2.415 | 6.25 | 0.002 | 0.0003 | 7.33 |
| 90% inhibition | 0.349 | 51.67 | 14.50 | 3.56 | 0.027 | 0.002 | 14.72 |
| HP-OVA: Tenofovir (400:1) | |||||||
| 50% inhibition | 0.482 | 15.09 | 6.250 | 2.41 | 0.231 | 0.016 | 14.81 |
| 90% inhibition | 0.499 | 51.67 | 23.93 | 2.16 | 1.684 | 0.060 | 28.16 |
| HP-OVA: TMC120 (800:1) | |||||||
| 50% inhibition | 0.296 | 15.09 | 2.214 | 6.81 | 0.014 | 0.003 | 4.89 |
| 90% inhibition | 0.430 | 51.67 | 13.16 | 3.93 | 0.094 | 0.016 | 5.72 |
Data are the means of two independent experiments performed in triplicate.
These results suggest that combining HP-OVA with six different HIV fusion/entry inhibitors, especially with CCR5 antagonist, Maraviroc, results in potent synergistic anti-HIV-1 activity and significant dose reduction of HP-OVA and these HIV-1 fusion/entry inhibitors.
Combinations of HP-OVA with RTIs exhibited synergistic anti-HIV-1 effects
ARV-based microbicide candidates being tested in clinical trials include one NRTI, Tenofovir, and two NNRTI inhibitors, UC781 and TMC12036, which specifically target the construction of viral DNA by inhibiting activity of reverse transcriptase. Here we selected these three microbicide candidates and combined them individually with HP-OVA to investigate the potential cooperative effects against infection by laboratory-adapted and primary HIV-1 strains. As shown in Tables 2 and 3 as well as Figure S2, the observed CI50 values of HP-OVA combined with these RTIs ranged from 0.162 to 0.722. More specifically, the combination of HP-OVA with Tenofovir displayed strong synergism against infection by HIV-1IIIB and HIV-1BaL, with CI50 values of 0.242 and 0.162, respectively, and dose reductions ranging from 11- to 15-fold. The synergism was also observed when combination of HP-OVA with Tenofovir against infection by primary HIV-1 isolates 93IN101 and 92US657, with CI50 values of 0.319 and 0.482, respectively, and dose reductions of HP-OVA ranging from 5.9- to 2.4-fold. The combination of HP-OVA with TMC120 also showed strong synergistic effects against HIV-1 IIIB, BaL, 92US657 and 93IN101 viruses, with CI50 values of 0.284, 0.173, 0.296 and 0.642, respectively. Similar to TMC120, the synergistic effect was achieved when HP-OVA was combined with UC781, with CI50 values of 0.536 on HIV-1IIIB and 0.363 on HIV-1BaL, respectively (Table 2).
Table 2.
Combination index (CI) and dose reduction values for inhibition of HIV-1 infection by combining HP-OVA with RTIs a
| Combinations of HP-OVA with RTIs (weight ratio) | CI | HP-OVA | RTIs | ||||
|---|---|---|---|---|---|---|---|
| Conc.(μg/ml) |
Dose reduction | Conc.(μg/ml) |
Dose reduction | ||||
| Alone | Mix | Alone | Mix | ||||
| Inhibition of HIV-1IIIB infection | |||||||
| HP-OVA: Tenofovir (20:1) | |||||||
| 50% inhibition | 0.242 | 0.687 | 0.061 | 11.26 | 0.020 | 0.003 | 6.667 |
| 90% inhibition | 0.270 | 3.914 | 0.237 | 16.51 | 0.057 | 0.012 | 4.750 |
| HP-OVA: UC781 (100:1) | |||||||
| 50% inhibition | 0.536 | 1.312 | 0.484 | 2.711 | 0.029 | 0.005 | 5.800 |
| 90% inhibition | 0.604 | 3.053 | 1.232 | 2.478 | 0.061 | 0.012 | 5.083 |
| HP-OVA: TMC120 (1000:1) | |||||||
| 50% inhibition | 0.284 | 0.847 | 0.188 | 4.505 | 0.003 | 0.0002 | 15.92 |
| 90% inhibition | 0.490 | 3.050 | 1.043 | 2.924 | 0.007 | 0.001 | 6.836 |
| Inhibition of HIV-1BaL infection | |||||||
| HP-OVA: Tenofovir (1000:1) | |||||||
| 50% inhibition | 0.162 | 4.854 | 0.320 | 15.17 | 0.017 | 0.002 | 8.500 |
| 90% inhibition | 0.343 | 22.09 | 1.900 | 11.63 | 0.037 | 0.010 | 3.700 |
| HP-OVA: UC781 (1250:1) | |||||||
| 50% inhibition | 0.363 | 4.854 | 1.349 | 3.598 | 0.013 | 0.001 | 13.00 |
| 90% inhibition | 0.300 | 22.09 | 4.354 | 5.073 | 0.034 | 0.003 | 11.33 |
| HP-OVA: TMC120 (8000:1) | |||||||
| 50% inhibition | 0.173 | 4.854 | 0.413 | 11.75 | 0.001 | 0.00008 | 12.64 |
| 90% inhibition | 0.124 | 22.09 | 0.952 | 23.20 | 0.002 | 0.0002 | 11.32 |
Data are the means of two independent experiments performed in triplicate.
HP-OVA was effective against infection by NRTI-, NNRTI- and T20-resistant HIV-1 strains
The rapid emergence of drug-resistant viruses is one of the major obstacles for developing clinically used ARVs as microbicides. For example, the HIV-1 strains AZT-R and A17 are highly resistant to NRTIs and NNRTIs, respectively37–39. NL4-3(D36G)V38E/N42S, which contains a double mutation in the principal determinant of T20 resistance (aa 36–45: GIVQQQNNLL) in the gp41 NHR domain, is highly resistant to T2040,41. Here we demonstrate that HIV-1 strains AZT-R, A17, and NL4-3(D36G)V38E/N42S were highly resistant to Tenofovir, UC781 and T20 (ranging from 62- to 190-fold of resistance), respectively, while HP-OVA exhibited similar potency against these drug-resistant variants and the wild-type HIV-1 strains (Table 4). These results suggest that combinations of HP-OVA with the ARVs have not only synergistic but also complementary effects against infection by HIV-1 strains, including those resistant to ARVs.
Table 4.
Inhibitory activity of HP-OVA and ARV-based microbicides on infection by drug-resistant HIV-1 strains
| Inhibitors | IC50 (μg/ml) for inhibition of infection by | Fold of resistance | |
|---|---|---|---|
| HIV-1 IIIB | HIV-1 AZT-R | ||
| HP-OVA | 0.924 ± 0.293 | 7.357 ± 0.848 | 7.96 |
| Tenofovir | 0.023 ± 0.005 | 3.116 ± 0.010 | 135.48 |
| HIV-1 IIIB | HIV-1 A17 | ||
| HP-OVA | 0.924 ± 0.293 | 1.405 ± 0.529 | 1.52 |
| UC781 | 0.021 ± 0.003 | > 4.00 | > 190.48 |
| HIV-1 NL4-3(D36G) | HIV-1 NL4-3(D36G)V38E/N42S | ||
| HP-OVA | 2.835 ± 0.810 | 0.622 ± 0.176 | 0.22 |
| T20 | 0.162 ± 0.090 | > 10.00 | >61.73 |
Data are the means of two independent assay performed in triplicate.
Human body fluids and proteins had no significant effect on the anti-HIV-1 activity of HP-OVA
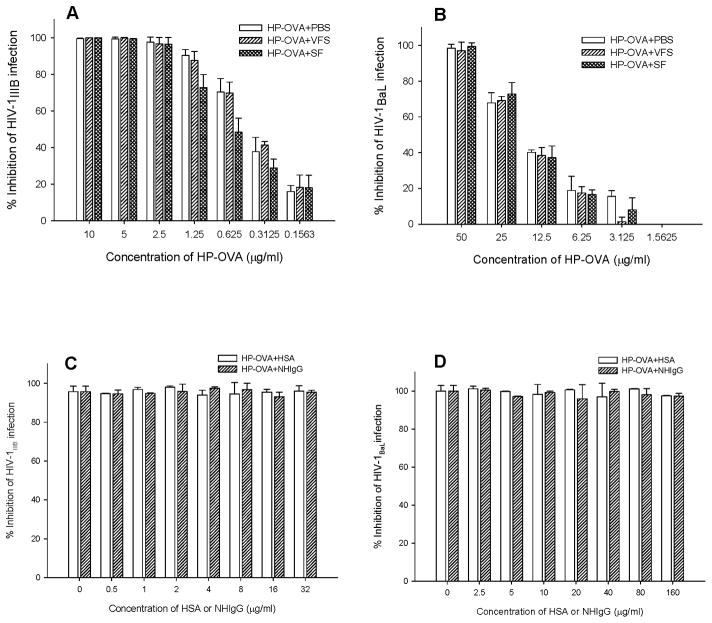
Human body fluids such as seminal and vaginal fluids may have negative effect on the efficacy of the topical microbicides 29–31,42, while sexual transmission of HIV-1 occurs in the presence of those human body fluids. Therefore, it is necessary to determine if the antiviral activity of HP-OVA is stable in the presence of seminal fluids (SF) and vaginal fluids (VF). Since we were unable to collect human cervicovaginal secretions from clinics, we used the vaginal fluid simulant (VFS), instead, for the test. After incubation of HP-OVA with SF, VFS or PBS at 37 °C for 60 min, IC50 values of HP-OVA in inhibiting infection by HIV-1 X4 or R5 virus were assayed by p24 assay. As shown in Figure 1A and 1B, neither SF nor VFS had significant effect on the inhibitory activity of HP-OVA against infection by HIV-1 X4 and R5 strains. The IC50 values of HP-OVA for inhibiting HIV-1IIIB infection in the presence of SF and VFS were 0.480 and 0.380 μg/ml, respectively, while that in the presence of PBS control was 0.395 μg/ml. The IC50 values of HP-OVA for inhibiting HIV-1BaL infection in the presence of SF and VFS were 11.50 and 15.00 μg/ml, respectively, whereas that in the presence of PBS control was 10.82 μg/ml.
Figure 1.
Influence of human body fluids and proteins on anti-HIV-1 activities of HP-OVA. (A) The effect of human SF and VSF on HP-OVA-mediated anti-HIV-1IIIB activity; (B) The effect of human SF and VSF on HP-OVA-mediated anti-HIV-1BaL activity; (C) The effect of HSA and NHIgG on HP-OVA-mediated anti-HIV-1IIIB activity and (D) The effect of HSA and NHIgG on HP-OVA-mediated anti-HIV-1BaL activity. Each sample was tested in triplicate, and the data are presented as means ± SD.
Since HP-OVA is a non-specific microbicide candidate with activities against a broad spectrum of HIV strains18, it is necessary to demonstrate whether it could bind to some host proteins non-specifically with diminished or decreased antiviral activity. In this study, we chose two human proteins, HSA and NHIgG for the test. Results showed that the inhibitory activities of HP-OVA against infection with HIV-1 IIIB (Figure 1C) and HIV-1 BaL (Figure 1D) in the presence of HSA or NHIgG at the concentration as high as 32 μg/ml were not significantly decreased, suggesting that the human proteins may have little effect on HP-OVA-mediated anti-HIV-1 activity.
HP-OVA formulated in microbicide gels is stable for a sufficient period of time
To assess the stability of HP-OVA in the gel formulation, we kept the HP-OVA-containing gels at room temperature for 1 hour, 1 day and 1 week, respectively, before testing their anti-HIV-1 activity. As shown in Figure S3, the IC50 values of HP-OVA in the universal gel stored at room temperature for 1 hour, 1 day and 1 week for inhibiting HIV-1IIIB infection were 5.73, 7.03 and 7.81 μg/ml, respectively, and those for inhibiting HIV-1BaL infection were 20.3, 27.1 and 28.3 μg/ml, respectively. Those results indicated that HP-OVA is stable in microbicide gel for a sufficient period of time.
Intravaginal application of HP-OVA gel induced no significant immune responses in rats
As a foreign protein-based microbicide, the repeated topical use of HP-OVA may induce anti-HP-OVA immune responses that may neutralize its antiviral activity. To test this possibility, we applied the HP-OVA gels into the vaginas of rats once a week for 4 weeks and then tested the serum levels of HP-OVA antibodies, including IgE, one of the important antibodies mediating type I hypersensitivity, and IgG, IgA, and IgM. As shown in Figure S4, the antibody levels in the sera of rats receiving HP-OVA gel or OVA gel were not significantly higher than that in the sera of rats receiving PBS as control (P>0.05). These results suggest that multiple vaginal application of HP-OVA gel may not induce harmful immune responses.
DISCUSSION
With the failure of large-scale clinical trials of the non-specific first generation microbicides (surfactants) and second generation microbicides (polyanionic polymers), interest has grown in developing specific next-generation microbicides (ARVs). The specific ARVs, including RTIs10,11,36 and HIV fusion/entry inhibitors10,12 have been widely and successfully used in clinics for the treatment of HIV/AIDS for many years and are now being tested in clinical or preclinical trials as microbicide candidates. Most recently, it was reported that one of the ARV-based microbicide candidates, a 1% vaginal gel formulation of Tenofovir, could cut HIV infection rates by 50% after one year of use and by 39% after two and a half years in women16,17. This is the first time to show that a microbicide gel is effective in the prevention of HIV sexual transmission.
However, further development of ARV-based microbicides faces a great challenge because of the rapid emergency of ARV-resistant viruses in the ARV-treated HIV/AIDS patients. ARV-based microbicides will become ineffective against these drug-resistant HIV-1 strains circulating in the high-risk populations. Besides, most of ARV-based microbicides are small molecules and can be systemically absorbed after topic application, which may cause systemic toxic effect, while non-specific microbicides usually are big molecules which have no risk of systematic absorption. Therefore, it is essential to develop a combination microbicide containing a non-specific microbicide that is effective against HIV and other sexually transmissible viruses and a specific ARV with high potency against both R5 and X4 HIV-1 strains. This combination microbicide is expected to have synergistic antiviral activity against a broad spectrum of HIV-1 strains, including those ARV-resistant mutants, and other STI pathogens.
The results from the present study suggest that combinations of HP-OVA, a non-specific microbicide candidate with potent antiviral activity against HIV-1, HIV-2, SHIV, SIV and HSV18, with several HIV-1 entry inhibitors or RTIs that are used in clinics or tested in clinical or preclinical studies exhibited potent synergisms against both R5 and X4 HIV-1 strains, suggesting that HP-OVA has a good potential to be included in a combination microbicide for prevention of sexual transmission of HIV and other STI pathogens.
The main advantage of the HP-OVA and ARV-based combination microbicides is their effectiveness against ARV-resistant HIV-1 variants existing in the ARV-experienced HIV/AIDS patients that can be sexually transmitted. Our results have shown that HP-OVA alone is highly effective against infection by NRTI-, NNRTI- and T20-resistant strains, which are AZT-R, A17, and NL4-3(D36G)V38E/N42S, respectively (Table 4), suggesting that combining HP-OVA with ARVs may have synergistic antiviral activity against both drug-sensitive and resistant HIV-1 strains.
Another advantage of the HP-OVA/ARV combination microbicides is their relatively low cost, compared with the ARV only microbicides. Ovalbumin is one of the most abundant food proteins consumed by people all over the world, and it is therefore generally recognized as safe (GRAS) and inexpensive. Moreover, the preparation of HP-OVA is easy, cheap, fast and reproducible. Therefore, the cost of combination HP-OVA/ARV-based microbicide candidates might be greatly decreased, which would make it possible to distribute this type of drug in many developing countries, where about 90% of HIV-infected people are living43. Our previous studies also showed that HP-OVA had very low cytotoxicity to both human immune T-cell lines and human vaginal and cervical epithelial cell lines, even at a concentration of more than 1,000 times of its IC90 against HIV-1IIIB infection18. Because of the synergism and dose-reduction of the antiviral agents in the combinations, the amounts of HP-OVA and ARVs that will be used in the combination microbicide formulations should be much lower than those in the corresponding non-combination microbicide formulations, indicating that HP-OVA/ARV combinations can be further developed as a safe, effective and affordable combination microbicide for prevention of sexual transmission of HIV and other STI pathogens.
An ideal microbicide should be stable in a topical formulation for a sufficient time and its antiviral activity should remain at high level in the presence of human proteins or human body fluids. Our study showed that the anti-HIV-1 activity of HP-OVA in the universal gel stored at room temperature remained unchanged for at least one week. In the presence of human proteins, including HSA and NHIgG, or human body fluids, such as SF, HP-OVA-mediated anti-HIV-1 activity was not significantly decreased. These results suggest that HP-OVA as an anti-HIV-1 microbicide candidate is stable in the topical formulations and in the presence of human proteins or human body fluids. One may raise a concern that HP-OVA, as a repeatedly used foreign protein-based microbicide, may induce anti-HP-OVA responses to neutralize its antiviral activity. Fortunately, our results demonstrated that HP-OVA applied in the vagina of rats once a week for 4 weeks did not induce significantly anti-HP-OVA antibody responses.
In conclusion, the combinations of HP-OVA with an ARV-based microbicide candidate may have the following advantages: (i) maximizing antiviral efficacy and/or expanding antiviral spectrum by synergistic effect, (ii) minimizing toxic effects and/or reducing high cost by combining dose reduction, and (iii) delaying the emergence of HIV-1 drug resistance by using agents with different mechanisms of action. For all these reasons, our study provides a sound scientific platform and, hence, a rational basis for designing and testing microbicide combinations for preventing sexual transmission of HIV and other STI pathogens.
Supplementary Material
Acknowledgments
We thank Jiaying Qiu and Minghui Chen at the Southern Medical University for technical assistance. This study was supported by a U.S. NIH grant (AI 076964) to S.J., and by the Natural Science Foundation of China (U0832001 to S.L and 30801412 to L.L), the National Key Project (2008ZX10001-015) to S.L., the Medical Science and Technology Project of Guangdong Province (A2008381) and the Nursery Project of Guangdong Provincial Department of Education (LYM08032) to L.L.
Footnotes
Conflict of Interest: The authors do not have a commercial or other association that might pose a conflict of interest.
References
- 1.Perotti ME, Pirovano A, Phillips DM. Carrageenan formulation prevents macrophage trafficking from vagina: implications for microbicide development. Biol Reprod. 2003;69:933–939. doi: 10.1095/biolreprod.102.014555. [DOI] [PubMed] [Google Scholar]
- 2.Bollen LJ, Blanchard K, Kilmarx PH, et al. No increase in cervicovaginal proinflammatory cytokines after Carraguard use in a placebo-controlled randomized clinical trial. J Acquir Immune Defic Syndr. 2008;47:253–257. doi: 10.1097/QAI.0b013e31815d2f12. [DOI] [PubMed] [Google Scholar]
- 3.Check E. Scientists rethink approach to HIV gels. Nature. 2007;446:12. doi: 10.1038/446012a. [DOI] [PubMed] [Google Scholar]
- 4.Van DL, Govinden R, Mirembe FM, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359:463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 5.Keller MJ, Herold BC. Understanding basic mechanisms and optimizing assays to evaluate the efficacy of vaginal microbicides. Sex Transm Dis. 2009;36:S92–S95. doi: 10.1097/OLQ.0b013e318199417d. [DOI] [PubMed] [Google Scholar]
- 6.Roehr B. Microbicide offers no protection against HIV infection. BMJ. 2009;339:b5538. doi: 10.1136/bmj.b5538. [DOI] [PubMed] [Google Scholar]
- 7.McGowan I. Microbicides for HIV prevention: reality or hope? Curr Opin Infect Dis. 2010;23:26–31. doi: 10.1097/QCO.0b013e328334fe70. [DOI] [PubMed] [Google Scholar]
- 8.Shattock RJ, Doms RW. AIDS models: microbicides could learn from vaccines. Nat Med. 2002;8:425. doi: 10.1038/nm0502-425. [DOI] [PubMed] [Google Scholar]
- 9.Dezzutti CS, James VN, Ramos A, et al. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob Agents Chemother. 2004;48:3834–3844. doi: 10.1128/AAC.48.10.3834-3844.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cutler B, Justman J. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect Dis. 2008;8:685–697. doi: 10.1016/S1473-3099(08)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz JL, Kovalevsky G, Lai JJ, et al. A randomized six-day safety study of an antiretroviral microbicide candidate UC781, a non-nucleoside reverse transcriptase inhibitor. Sex Transm Dis. 2008;35:414–419. doi: 10.1097/OLQ.0b013e318162c4d8. [DOI] [PubMed] [Google Scholar]
- 12.Ketas TJ, Schader SM, Zurita J, et al. Entry inhibitor-based microbicides are active in vitro against HIV-1 isolates from multiple genetic subtypes. Virology. 2007;364:431–440. doi: 10.1016/j.virol.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Carballo-Dieguez A, Balan IC, Morrow K, et al. Acceptability of tenofovir gel as a vaginal microbicide by US male participants in a Phase I clinical trial (HPTN 050) AIDS Care. 2007;19:1026–1031. doi: 10.1080/09540120701294237. [DOI] [PubMed] [Google Scholar]
- 14.Mayer KH, Maslankowski LA, Gai F, et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006;20:543–551. doi: 10.1097/01.aids.0000210608.70762.c3. [DOI] [PubMed] [Google Scholar]
- 15.Rosen RK, Morrow KM, Carballo-Dieguez A, et al. Acceptability of tenofovir gel as a vaginal microbicide among women in a phase I trial: a mixed-methods study. J Womens Health (Larchmt) 2008;17:383–392. doi: 10.1089/jwh.2006.0325. [DOI] [PubMed] [Google Scholar]
- 16.Global Campaign for Microbicides and PATH. Microbicide Trial Lifts Hope for Women’s HIV Prevention. 2010 Jul 19; www.global-campaign.org/CAPRISA004.htm.
- 17.Karim QA, Karim SS, Frohlich JA, et al. Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women. Science. 2010 doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, He L, Tan S, et al. 3-hydroxyphthalic anhydride-modified chicken ovalbumin exhibits potent and broad anti-HIV-1 activity: a potential microbicide for preventing sexual transmission of HIV-1. Antimicrob Agents Chemother. 2010;54:1700–1711. doi: 10.1128/AAC.01046-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huntington JA, Stein PE. Structure and properties of ovalbumin. J Chromatogr B Biomed Sci Appl. 2001;756:189–198. doi: 10.1016/s0378-4347(01)00108-6. [DOI] [PubMed] [Google Scholar]
- 20.Nisbet AD, Saundry RH, Moir AJ, et al. The complete amino-acid sequence of hen ovalbumin. Eur J Biochem. 1981;115:335–345. doi: 10.1111/j.1432-1033.1981.tb05243.x. [DOI] [PubMed] [Google Scholar]
- 21.Deng Y, Zheng Q, Ketas TJ, et al. Protein design of a bacterially expressed HIV-1 gp41 fusion inhibitor. Biochemistry. 2007;46:4360–4369. doi: 10.1021/bi7001289. [DOI] [PubMed] [Google Scholar]
- 22.Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59:91–95. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 23.Neurath AR, Debnath AK, Strick N, et al. Blocking of CD4 cell receptors for the human immunodeficiency virus type 1 (HIV-1) by chemically modified bovine milk proteins: potential for AIDS prophylaxis. J Mol Recognit. 1995;8:304–316. doi: 10.1002/jmr.300080504. [DOI] [PubMed] [Google Scholar]
- 24.Neurath AR, Jiang S, Strick N, et al. Bovine beta-lactoglobulin modified by 3-hydroxyphthalic anhydride blocks the CD4 cell receptor for HIV. Nat Med. 1996;2:230–234. doi: 10.1038/nm0296-230. [DOI] [PubMed] [Google Scholar]
- 25.Jiang SB, Lin K, Neurath AR. Enhancement of human immunodeficiency virus type 1 infection by antisera to peptides from the envelope glycoproteins gp120/gp41. J Exp Med. 1991;174:1557–1563. doi: 10.1084/jem.174.6.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang S, Lu H, Liu S, et al. N-substituted pyrrole derivatives as novel human immunodeficiency virus type 1 entry inhibitors that interfere with the gp41 six-helix bundle formation and block virus fusion. Antimicrob Agents Chemother. 2004;48:4349–4359. doi: 10.1128/AAC.48.11.4349-4359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H, Zhao Q, Wallace G, et al. Cellulose acetate 1,2-benzenedicarboxylate inhibits infection by cell-free and cell-associated primary HIV-1 isolates. AIDS Res Hum Retroviruses. 2006;22:411–418. doi: 10.1089/aid.2006.22.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou TC, Hayball MP. CalcuSyn: Windows software for dose effect analysis. Ferguson, MO 63135, USA: BIOSOFT; 1991. [Google Scholar]
- 29.Fernandez-Romero JA, Thorn M, Turville SG, et al. Carrageenan/MIV-150 (PC-815), a combination microbicide. Sex Transm Dis. 2007;34:9–14. doi: 10.1097/01.olq.0000223287.46097.4b. [DOI] [PubMed] [Google Scholar]
- 30.Fletcher PS, Harman SJ, Boothe AR, et al. Preclinical evaluation of lime juice as a topical microbicide candidate. Retrovirology. 2008;5:3. doi: 10.1186/1742-4690-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Qiao P, Yang J, et al. Maleic anhydride-modified chicken ovalbumin as an effective and inexpensive anti-HIV microbicide candidate for prevention of HIV sexual transmission. Retrovirology. 2010;7:37. doi: 10.1186/1742-4690-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilby JM, Eron JJ. Novel therapies based on mechanisms of HIV-1 cell entry. N Engl J Med. 2003;348:2228–2238. doi: 10.1056/NEJMra022812. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Q, Ma L, Jiang S, et al. Identification of N-phenyl-N’-(2,2,6,6-tetramethyl-piperidin-4-yl)-oxalamides as a new class of HIV-1 entry inhibitors that prevent gp120 binding to CD4. Virology. 2005;339:213–225. doi: 10.1016/j.virol.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Donzella GA, Schols D, Lin SW, et al. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co- receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 35.Dorr P, Westby M, Dobbs S, et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49:4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nel AM, Smythe SC, Habibi S, et al. Pharmacokinetics of 2 Dapivirine Vaginal Microbicide Gels and Their Safety Vs. Hydroxyethyl Cellulose-Based Universal Placebo Gel. J Acquir Immune Defic Syndr. 2010 doi: 10.1097/QAI.0b013e3181e3293a. [DOI] [PubMed] [Google Scholar]
- 37.Hara H, Fujihashi T, Sakata T, et al. Tetrahydronaphthalene lignan compounds as potent anti-HIV type 1 agents. AIDS Res Hum Retroviruses. 1997;13:695–705. doi: 10.1089/aid.1997.13.695. [DOI] [PubMed] [Google Scholar]
- 38.Larder BA, Darby G, Richman DD. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989;243:1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- 39.Nunberg JH, Schleif WA, Boots EJ, et al. Viral resistance to human immunodeficiency virus type 1-specific pyridinone reverse transcriptase inhibitors. J Virol. 1991;65:4887–4892. doi: 10.1128/jvi.65.9.4887-4892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rimsky LT, Shugars DC, Matthews TJ. Determinants of human immunodeficiency virus type 1 resistance to gp41-derived inhibitory peptides. J Virol. 1998;72:986–993. doi: 10.1128/jvi.72.2.986-993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei X, Decker JM, Liu H, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neurath AR, Strick N, Li YY. Role of seminal plasma in the anti-HIV-1 activity of candidate microbicides. BMC Infect Dis. 2006;6:150. doi: 10.1186/1471-2334-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balzarini J, Van DL. Microbicide drug candidates to prevent HIV infection. Lancet. 2007;369:787–797. doi: 10.1016/S0140-6736(07)60202-5. [DOI] [PubMed] [Google Scholar]
- 44.Roan NR, Sowinski S, Munch J, et al. Aminoquinoline surfen inhibits the action of SEVI (semen-derived enhancer of viral infection) J Biol Chem. 2010;285:1861–1869. doi: 10.1074/jbc.M109.066167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.