Abstract
The efficiency of somatic energy metabolism is correlated with cognitive change over the lifespan. This relationship is bidirectional, with improved overall fitness associated with enhanced synaptic function and neuroprotection, and synaptic endangerment occurring in the context of impaired energy metabolism. In this review, we discuss recent advancements in the fields of exercise, dietary energy intake and diabetes, as they relate to neuronal function in the hippocampus. Because hippocampal neurons have energy requirements that are relatively higher than those of other brain regions, they are uniquely poised to benefit from exercise, and to be harmed by diabetes. We view exercise and dietary energy restriction as being associated with enhanced hippocampal plasticity at one end of a continuum, with obesity and diabetes accompanied by cognitive impairment at the other end of the continuum. Understanding the mechanisms for this continuum may yield novel therapeutic targets for the prevention and treatment of cognitive decline following aging, disease, or injury.
Keywords: diabetes, exercise, caloric restriction, cognition, hippocampus
The global obesity epidemic has been associated with the development of a novel human phenotype. Over the course of human evolution, somatic metabolism developed in relationship to significant energy investments associated with the process of obtaining sustenance. Over the past century, humans have progressed from a state of widespread food insecurity, to the ready availability of inexpensive, calorically dense, highly palatable food products. Concomitantly, humans have reduced energy expenditure during activities of daily life. We have maximized energy influx, while minimizing expenditure, leading to an increase in the proportion of the population that is obese across multiple regions of the world (World Health Organization, 2000). In this regard, we are a species that evolved a ‘thrifty metabolism’ that is no longer adaptive under current circumstances.
Insulin resistant diabetes arises from obesity, sedentary lifestyle, and genetic predisposition (Lazar, 2005). Age is also a risk factor for insulin resistance, with older adults developing type 2 diabetes at a higher rate than middle-aged or younger populations (Defronzo, 1981). Aging is often accompanied by cognitive decline, specifically on tasks that recruit the hippocampus and associated cortical structures (Burke & Barnes, 2010). Susceptibility to cognitive decline is greater among individuals with type 2 diabetes (Velayudhan, Poppe, Archer, Proitsi, Brown, & Lovestone, 2010; Ott, Stolk, Hofman, van Harskamp, Grobbee, & Breteler, 1996). Insulin resistance is associated with cognitive impairment among middle aged (Sandeep, Yau, MacLullich, Noble, Deary, Walker, & Seckl, 2004) and aged individuals without dementia (Convit, Wolf, Tarshish, & de Leon, 2003; Bruehl, Wolf, Sweat, Tirsi, Richardson, & Convit, 2009). Even in non-diabetic individuals, more efficient glycemic control is correlated with improved cognitive function (Rolandsson, Backeström, Eriksson, Hallmans, & Nilsson, 2009). Based on these observations, it is likely that neurocognitive function is related to overall somatic metabolism, such that metabolic compromise impairs neuronal plasticity.
Changes in brain structure in insulin resistant diabetes occur within temporal lobe circuits that are also sensitive to aging and Alzheimer’s disease (AD). Individuals with insulin resistant diabetes exhibit hippocampal atrophy (Rasgon, Kenna, Wroolie, Kelley, Silverman, Brooks, Williams, Powers, Hallmayer, & Reiss, in press), particularly in the dentate gyrus subfield (Wu, Brickman, Luchsinger, Ferrazzano, Pichiule, Yoshita, Brown, DeCarli, Barnes, Mayeux, Vannucci, & Small, 2008). The entorhinal cortex, which sends the primary afferent input to the hippocampus (van Strien, Cappaert, & Witter, 2009) and exhibits early vulnerability during aging and AD (Mattson & Magnus, 2006), also displays a reduction in cerebral blood flow in aged individuals with insulin resistant diabetes (Wu et al., 2008). The extent of hippocampal shrinkage is correlated with body composition, such that type 2 diabetics with greater visceral adiposity have more hippocampal atrophy relative to type 2 diabetics with less abdominal fat (Anan, Masaki, Shimomura, Fujiki, Umeno, Eshima, Saikawa, & Yoshimatsu, 2010). Resting-state connectivity among the hippocampus and neocortical regions implicated in cognitive function is also impaired in insulin resistant diabetes (Zhou, Lu, Shi, Bai, Chang, Yuan, Teng, & Zhang, 2010). Taken together with the behavioral observations suggesting that cognition is impaired with insulin resistance, the neuroimaging findings provide additional support for a metabolic continuum with consequences for neuronal function.
Diabetes has a negative impact on neurocognitive function in individuals without dementia. Moreover, insulin resistance exacerbates the symptoms of dementia when they are present. Within a population of individuals with both mild cognitive impairment and diabetes, poor glycemic control was a significant predictor of earlier progression to dementia (Velayudhan et al., 2010). Both cross-sectional (Ott et al., 1996) and longitudinal (Yen, Yeh, Wang, Liao, Chen, Chen, Liang, Lai, Lin, Lee & Lee, 2010; Velayudhan et al., 2010) studies have identified insulin resistance as a risk factor for dementia, although this is not the case in all studies (Sanz, Andrieu, Sinclair, Hanaire, & Vellas, 2009). Because the number of aged individuals is increasing, with expanded prevalence of insulin resistance among the elderly, the possibility that insulin resistance presages AD would predict a greater-than-expected increase in the number of cases of age-related dementia.
While diabetes and the metabolic syndrome impair cognition, energy expenditure within the normal physiological range (i.e. not to exhaustion) improves cognition in humans (Winter, Breitenstein, Mooren, Voelker, Fobker, Lechtermann, Krueger, Fromme, Korsukewitz, Floel, & Knecht, 2007). Exercise exists within a constellation of self-care behaviors that are correlated with maintenance of cognitive function during aging in humans (Hillman, Erickson, & Kramer, 2008). At the level of regional morphology, there appears to be a relationship between cardiovascular fitness and hippocampal structure, both in young individuals (Pereira, Huddleston, Brickman, Sosunov, Hen, McKhann, Sloan, Gage, Brown, & Small, 2007) in normal aging (Erickson, Prakash, Voss, Chaddock, Hu, Morris, White, Wójcicki, McAuley, & Kramer, 2009) and in AD (Honea, Thomas, Harsha, Anderson, Donnelly, Brooks, & Burns, 2009). Just as functional connectivity in temporal lobe regions declines in diabetes (Zhou et al., 2010), those same regions show preserved functional connectivity in aged, non-diabetic individuals with greater cardiorespiratory fitness (Voss, Erickson, Prakash, Chaddock, Malkowski, Alves, Kim, Morris, White, Wójcicki, Hu, Szabo, Klamm, McAuley, & Kramer, 2010). The behavioral data are broadly consistent with the structural findings, such that both memory and attention improve following aerobic exercise training in older adults (Erickson & Kramer, 2009). Understanding basic mechanisms underlying the increased susceptibility of diabetics and reduced vulnerability of physically active individuals to cognitive decline may help to identify potential therapeutic targets.
Cognitive deficits in animal models of diabetes and obesity
Animal models are essential to achieve the goal of identifying targets for pharmacological intervention based on the opposing effects of diabetes and physical activity on cognitive function. While a number of animal models exist to recapitulate the symptoms of human insulin resistance, rodents that lack functional leptin receptors (db/db mice and Zucker rats) are the best-characterized in terms of their cognitive phenotype. Both db/db mice and Zucker rats exhibit impaired hippocampus-dependent memory in the water maze (Li, Aou, Oomura, Hori, Fukunaga, & Hori, 2002; Stranahan, Arumugam, Lee, Cutler, Egan, & Mattson, 2008a), although in the Zucker rat, impairments were not reported in studies using training trials that were spaced over longer periods of time (Bélanger, Lavoie, Trudeau, Massicotte, Gagnon, 2004). db/db mice also perform poorly in the novel object preference task, relative to wild type mice (Stranahan et al., 2008a), suggesting that multiple memory systems are compromised in this model. Zucker rats learned a variable-interval delayed alternation task less efficiently than lean control rats (Winocur, Greenwood, Piroli, Grillo, Reznikov, Reagan, & McEwen, 2005), which is also indicative of medial temporal lobe dysfunction. The observation that leptin-deficient rodents are cognitively impaired could arise not from their diabetes symptoms, but instead from a direct role of leptin in learning and memory (Harvey, 2007). However, leptin resistance can be considered a component of diabetes, or of the metabolic syndrome more generally. In this respect, the etiology of endocrine alterations in diabetes is conducive to neuronal dysfunction in the hippocampus.
The diet-induced obesity model in mice and rats has been used as an ethologically relevant system to assess cognitive alterations in the context of energy excess. Diets high in fat and sugar impair learning in the hippocampus-dependent water maze in rats (Molteni, Barnard, Ying, Roberts, & Gómez-Pinilla, 2002) and also elevate fasting blood glucose levels. Prolonged maintenance on a high-fat, high-sugar diet leads to endocrine alterations resembling diabetes and impairs spatial memory in the water maze in rats (Stranahan, Norman, Lee, Cutler, Telljohann, Egan, & Mattson, 2008b). In rats, fructose feeding elevates plasma triglycerides and impairs cognition, with deficits in water maze performance that are correlated with the extent of hyperlipidemia (Ross, Bartness, Mielke, & Parent, 2009). However, in mice, diets high in fat failed to influence water maze performance, despite peripheral endocrine adaptations suggestive of metabolic dysfunction (Mielke, Nicolitch, Avellaneda, Earlam, Ahuja, Mealing, & Messier, 2006). This disparity could arise from a true species difference, with rats being more susceptible to diet-induced cognitive deficits than mice; alternatively, because mice learn the water maze less effectively than rats under identical training conditions (Frick, Stillner, & Berger-Sweeney, 2000), it could be more difficult to detect a deficit in mice on this task. Despite differences in the cognitive sequelae following diets high in saturated fat and/or sugar in rats and mice, the rat data suggest that metabolic alterations contribute to deficits in hippocampus-dependent spatial memory.
Cognitive deficits following maintenance on a high-energy diet are not specific to spatial memory performance, as impairment of spatial working memory has also been reported in the radial arm maze (Murray, Knight, Cochlin, McAleese, Deacon, Rawlins, & Clarke, 2009). Diets high in saturated fat have a negative impact on performance in a variable-interval delayed alternation task, specifically during longer delays, which recruit the hippocampus (Greenwood & Winocur, 2001). Diet-induced obesity blocks the facilitation of cognition following intrahippocampal (McNay, Ong, McCrimmon, Cresswell, Bogan, & Sherwin, 2010) or intranasal insulin (Marks, Tucker, Cavallin, Mast, & Fadool, 2009). However, because diet-induced obesity models exhibit alterations across a number of metabolic and endocrine factors that could contribute to cognitive deficits, it is difficult to say whether changes in one specific factor could account for their memory phenotype. It is possible to conceive of a model in which diet-induced hyperlipidemia contributes to neuronal endangerment during aging (Cutler, Kelly, Storie, Pedersen, Tammara, Hatanpaa, Troncoso, & Mattson, 2004), and also antagonizes the positive effects of insulin on learning and memory over the lifespan (McNay et al., 2010; Marks et al., 2009).
Voluntary running enhances cognition
The most widespread physical activity paradigm in rodents involves access to a running wheel. Mice and rats housed with a wheel will voluntarily run long distances (Stranahan, Lee, Martin, Maudsley, Golden, Cutler, & Mattson, 2009; Stranahan, Khalil, & Gould, 2006), even in old age (Stranahan, Lee, Becker, Zhang, Maudsley, Martin, Cutler, & Mattson, in press; van Praag, Shubert, Zhao, & Gage, 2005). Wheel running is a hedonic behavior; rats will bar press for wheel access (Pierce, Epling, & Boer, 1986; Iversen, 1993) and develop conditioned place preference for environments previously paired with a running wheel (Belke & Wagner, 2005). The natural territorial range for male rats in the wild is much larger than standard laboratory conditions (Calhoun, 1963). Rodents appear to be predisposed to exhibit a certain level of physical activity, and standard laboratory housing precludes this behavior by restricting movement. In this respect, the divergence between evolutionary predilection for physical activity and modern constraints on energy expenditure are similar between laboratory rodents and humans, as recently reviewed by Martin, Maudsley, & Mattson (2010).
Wheel running improves cognition in young animals across a variety of learning paradigms, including the hippocampus-dependent water maze (van Praag, Christie, Sejnowski, & Gage, 1999; Clark, Brzezinska, Thomas, Ryzhenko, Toshkov, & Rhodes, 2008), contextual fear conditioning (Baruch, Swain, Helmstetter, 2004; Clark et al., 2008; Greenwood, Strong, Foley, & Fleshner, 2009), radial arm maze (Lambert, Fernandez, & Frick, 2005; Berchtold, Castello, & Cotman, 2010), and object recognition tasks (García-Capdevila, Portell-Cortés, Torras-Garcia, Coll-Andreu, & Costa-Miserachs, 2009). During normal aging, brief (van Praag et al., 2005, Harburger, Nzerem, & Frick, 2007) or lifelong (Stranahan et al., in press) wheel running also improves water maze performance. Whether this is a true improvement, or deprivation reversal, remains to be determined. However, it is clear that wheel running protects against cognitive deficits following a variety of neurological insults, including stroke (Stummer, Baethmann, Murr, Schürer, & Kempski, 1995), kainate seizures (Reiss, Dishman, Boyd, Robinson, & Holmes, 2009), and in animal models of AD (Adlard, Perreau, Pop, & Cotman, 2005). Wheel running also attenuates the negative impact of a high-fat diet on cognition (Molteni, Wu, Vaynman, Ying, Barnard, & Gómez-Pinilla, 2004).
A large body of work has now demonstrated that physical activity enhances hippocampal function and protects against age-associated deficits in rodents (van Praag et al., 2005; Harburger et al., 2007; Stranahan et al., in press) and humans (Hillman et al., 2008), but the extent to which early-life exercise may attenuate cognitive decline during old age remains unexplored. Because early life experience influences the likelihood of cognitive decline (Meaney, Aitken, van Berkel, Bhatnagar, & Sapolsky, 1988), it is entirely possible that exercise during brain development might also exert enduring effects that last through old age. Indeed, gestational exposure to maternal use of a running wheel promotes neural stem cell proliferation in pups (Bick-Sander, Steiner, Wolf, Babu, & Kempermann, 2006), but the effects are transient, and levels of neurogenesis return to baseline by early adulthood. The question of whether exercise enhances cognition after cessation of activity has recently been explored following short periods of exercise discontinuance. The pro-cognitive effects of adult-onset exercise on hippocampus-dependent radial arm maze performance last for at least one week after removal of the running wheel (Berchtold et al., 2010). Still, the issue of whether a transient period of running wheel activity during brain development might alter the trajectory of brain aging has not yet been addressed.
Variations in glucose and insulin metabolism influence cognition
Impairment of insulin sensitivity is the hallmark of type 2 diabetes. Aging is also associated with reduced insulin sensitivity in humans (Defronzo, 1981) and rodents (Wang, Perfetti, Greig, Holloway, DeOre, Montrose-Rafizadeh, Elahi, & Egan, 1997). Lifelong exercise lowers circulating insulin levels (Stranahan et al., in press), indicative of improved insulin sensitivity in mice. Acute exercise (van Praag et al., 2005; Harburger et al., 2007) or regular long-term exercise (Stranahan et al., in press) also improves spatial memory in aged mice. Is there a role for improved insulin sensitivity following exercise in the protective effects of exercise on cognition in the aged brain?
The effects of acute and chronic exposure to elevated insulin levels on cognition are distinct. While chronic exposure to elevated insulin levels reduces neuronal insulin sensitivity, acute exposure to intranasal insulin improves cognition on tasks associated with hippocampal function in rodents (Marks et al., 2009) and humans (Reger, Watson, Frey, Baker, Cholerton, Keeling, Belongia, Fishel, Plymate, Schellenberg, Cherrier, & Craft, 2006). This effect is also detected following acute administration of insulin to the hippocampus (McNay et al., 2010). The extent to which insulin acts directly on neurons or indirectly through local modulation of glucose metabolism remains to be determined. In favor of the direct hypothesis, coadministration of insulin-blocking antibodies and phosphoinositol-3 kinase (PI3K) inhibitors blocks the facilitation of spatial recognition memory by insulin, suggesting that activation of known insulin signaling targets is necessary for the effects of insulin on cognition (McNay et al., 2010). However, intrahippocampal insulin treatment lowers extracellular glucose levels and elevates extracellular lactate (McNay et al., 2010); this opens the possibility that either an increase in intracellular glucose or improved bioavailability of lactate as a substrate for neuronal energy metabolism could mediate improvements in cognition. The direct and indirect hypotheses are by no means mutually exclusive; it is possible to conceive of a scenario in which exposure to insulin both activates pro-survival signaling pathways (such as PI3K) and facilitates neuronal energy metabolism, thereby recruiting both mechanisms for cognitive enhancement.
The likelihood of a dual-process mechanism for the effects of insulin on cognition is supported by the extensive literature surrounding the effects of glucose on hippocampal function. In young rodents and humans, acute peripheral administration of glucose improves memory (for review, see Gold, 2005). Likewise, intrahippocampal, but not extrahippocampal, injections of glucose improve performance on spatial alternation tasks (Ragozzino, Pal, Unick, Stefani, & Gold, 1998). Spontaneous alternation learning depletes extracellular glucose (McNay, Fries, & Gold, 2000) in a manner similar to intrahippocampal insulin administration (McNay et al., 2010). Spontaneous alternation is compromised and the learning-induced reduction in hippocampal extracellular glucose concentrations is prolonged in aged rats (Gold, 2005). Learning deficits in aged rats are reversible following peripheral or intrahippocampal administration of glucose. Similarly, aged humans respond to acute peripheral glucose administration with enhanced memory (Hall, Gonder-Frederick, Chewning, Silveira, & Gold, 1989). These findings point to synergistic actions for glucose and insulin in the regulation of hippocampal function. Furthermore, they reinforce the essential role of glucose and insulin sensitivity in synaptic function underlying cognitive performance. One question remains unanswered: does the hippocampus itself produce insulin?
Neuronal synthesis of insulin, or the lack thereof, has been debated for over twenty years (Baskin, Wilcox, Figlewicz, & Dorsa, 1988). Conventional immunohistochemical antibodies frequently show cross-reactivity between insulin and insulin-like growth factors, and immunoreactivity for insulin (when detected) could reflect insulin that is synthesized peripherally and internalized by cells in the central nervous system. At the level of messenger RNA, different investigators have reported conflicting results, with some in situ hybridization experiments showing robust hippocampal insulin mRNA expression in rabbits (Devaskar, Giddings, Rajakumar, Carnaghi, Menon, & Zahm, 1994), but others showing no signal in the hippocampus of rats, and minimal signal in the hypothalamus (Young, 1986). Northern blot experiments showed no evidence of insulin mRNA expression in brain tissue (Giddings, Chirgwin, & Permutt, 1985). The general consensus among these lines of research is that insulin is probably not synthesized in the hippocampus of rats.
Peripherally synthesized insulin crosses the blood-brain barrier, is taken up by neurons, and eventually gets degraded by insulin-degrading enzyme (IDE; Baskin et al., 1988; Shii, Baba, Yokono, Roth, 1985). IDE also degrades amyloid-β (Vekrellis, Ye, Qiu, Walsh, Hartley, Chesneau, Rosner, & Selkoe, 2000), revealing another point of commonality between diabetes and AD. AD model mice maintained on a high-fat diet exhibit concomitant reductions in IDE and increases in amyloid-β (Ho, Qin, Pompl, Xiang, Wang, Zhao, Peng, Cambareri, Rocher, Mobbs, Hof, & Pasinetti, 2004; Zhao, Teter, Morihara, Lim, Ambegaokar, Ubeda, Frautschy, & Cole, 2004), suggesting that diet-induced obesity might accelerate neuropathology. Insulin treatment upregulates IDE in neurons in a PI3K-dependent fashion, and the insulin-induced upregulation of IDE mediates the facilitation of amyloid-β clearance from the brain following acute insulin treatment (Zhao et al., 2004). This is in line with previous reports of insulin-induced neuroprotection following stress (Moosavi, Naghdi, Maghsoudi, & Zahedi Asl, 2007) and stroke (Voll, Whishaw, & Auer, 1989). Taken together with the work of McNay et al. (2010) demonstrating that the procognitive effects of insulin are diminished in diet-induced insulin resistance, these observations suggest that insulin resistance arising from dietary energy excess exacerbates neuronal damage during AD.
Elevated glucocorticoids contribute to the negative effects of diabetes on cognition, but do not preclude the positive effects of exercise or dietary energy restriction
Both diabetes and voluntary exercise are associated with increased levels of glucocorticoid hormones, but the time course, metabolic context, and consequences for hippocampal plasticity are distinct. Diabetic humans (Bruehl et al., 2009) and rodents (Tokuyama & Himms-Hagen, 1987) have elevated levels of adrenal steroid hormones, with perturbation of normal circadian rhythmicity, leading to greater cumulative glucocorticoid exposure in the hippocampus (Tornello, Coirini, & De Nicola, 2001). In contrast, while physical exercise elevates glucocorticoid concentrations in serum (Droste, Chandramohan, Hill, Linthorst, & Reul, 2007; Stranahan et al., 2006; Droste, Gesing, Ulbricht, Müller, Linthorst, & Reul, 2003), there is some dissociation between peripheral and central elevations in corticosteroids. Normally, corticosteroid levels are tightly correlated in the hippocampus and periphery (McEwen, Weiss, & Schwartz, 1969; Droste, de Groote, Atkinson, Lightman, Reul, & Linthorst, 2008), but in the case of running, new data suggest that serum corticosteroid levels do not always correspond with levels in the hippocampus (Droste, Collins, Lightman, Linthorst, & Reul, 2009). Therefore, dissociations between serum glucocorticoid levels and those in the hippocampus could account for the absence of negative consequences following exercise-induced elevations in corticosteroids. However, the mechanism for this dissociation is likely under the control of other factors in the animal’s environment, as combined running and social isolation stress suppresses hippocampal structural plasticity in a glucocorticoid-dependent manner (Stranahan et al., 2006).
Other compensatory mechanisms exist to buffer the negative consequences of running-induced exposure to elevated glucocorticoids for hippocampal plasticity. Specifically, runners exhibit increased hippocampal serotonin levels (Gomez-Merino, Béquet, Berthelot, Chennaoui, & Guezennec, 2001), which interact with glucocorticoid hormones to modulate synaptic function (Joels & Van Riel, 2004). Similarly, running enhances hippocampal production of brain-derived neurotrophic factor (BDNF; Neeper, Gómez-Pinilla, Choi, & Cotman, 1995; Stranahan et al., 2009), which is typically downregulated following exposure to glucocorticoid hormones (Smith, Makino, Kvetnansky, & Post, 1995). Although both running and diabetes increase production of glucocorticoids, concurrent neuroprotective mechanisms are recruited by running, leading to improved hippocampal function. Likewise, deleterious alterations in the neuronal milieu occur in the diabetic hippocampus, leading to impaired cognition.
Dietary energy restriction improves insulin sensitivity and autonomic function, and slows the aging process and the development of multiple age-related diseases (Anderson & Weindruch, 2010; Wan, Camandola, & Mattson, 2003; Mager, Wan, Brown, Cheng, Wareski, Abernethy, & Mattson, 2006). With regards to brain aging and cognitive impairment, rats maintained on lifelong dietary energy restriction exhibit superior cognitive performance in late life (Goodrick, 1984). Senescence-accelerated mice also exhibit improved learning and memory in response to caloric restriction (Komatsu, Chiba, Yamaza, Yamashita, Shimada, Hoshiyama, Henmi, Ohtani, Higami, de Cabo, Ingram, & Shimokawa, 2008). Alternate day fasting and 40% daily caloric restriction diets ameliorated age-related deficits in spatial memory acquisition and retention in a mouse model of AD (Halagappa, Guo, Pearson, Matsuoka, Cutler, Laferla, & Mattson, 2007). A recent study demonstrated that caloric restriction improves memory in elderly human subjects (Witte, Fobker, Gellner, Knecht, & Floel, 2009). Dietary energy restriction may preserve and enhance cognitive performance by inducing the expression of genes that encode proteins involved in synaptic plasticity and neuronal survival including BDNF, protein chaperones and mitochondrial uncoupling proteins (Figure 1; Martin, Mattson, & Maudsley, 2006; Arumugam, Phillips, Cheng, Morrell, Mattson, & Wan, 2010). Interestingly, as with regular exercise, levels of glucocorticoids are elevated in animals maintained on dietary energy restriction (Patel & Finch, 2002). However, in contrast to chronic stress pathology, which is associated with reduced expression of neuronal mineralocorticoid receptors (López, Chalmers, Little, & Watson, 1998), alternate day fasting results in maintenance of mineralocorticoid receptor levels and a reduction in levels of glucocorticoid receptors (Lee, Herman, & Mattson, 2000). The latter findings suggest that the way in which neurons respond to glucocorticoids is different under conditions of chronic uncontrollable stress and beneficial stressors such as exercise and alternate day fasting.
Figure 1. The metabolic continuum confers resistance or vulnerability to neuronal mechanisms underlying cognitive impairment and AD.
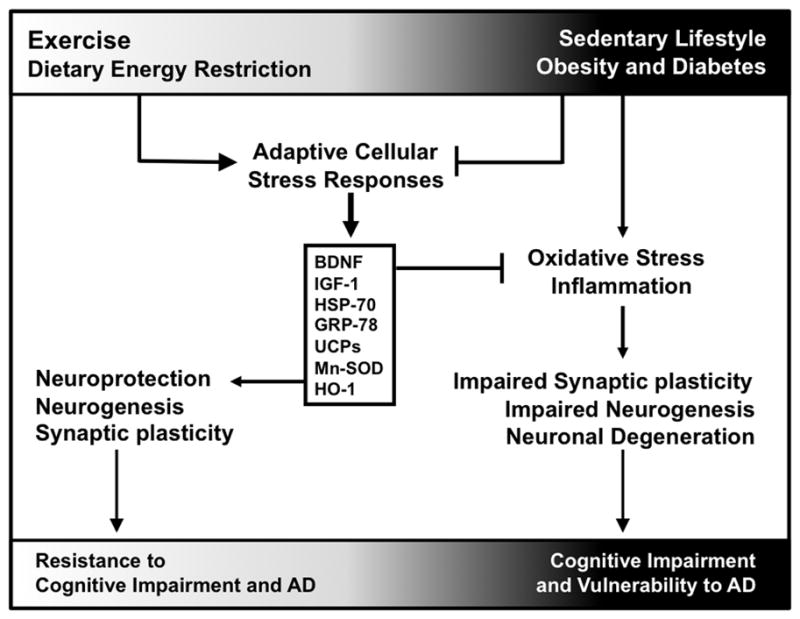
Exercise and dietary energy restrictions induce adaptive cellular stress responses that are associated with neuroprotection, neurogenesis, and synaptic plasticity. These mechanisms both promote neuronal function and suppress deleterious changes that lead to cognitive impairment and AD. On the other hand, sedentary lifestyles, obesity, and diabetes reduce adaptive cellular stress responses and promote negative outcomes such as oxidative stress and inflammation. These factors contribute to impaired synaptic plasticity and neurogenesis, rendering neurons more susceptible to changes that lead to cognitive impairment and AD. Abbreviations: BDNF, brain-derived neurotrophic factor; IGF-1, insulin-like growth factor 1; HSP-70, heat shock protein 70; GRP-78, 78kDa glucose-regulated protein; UCPs, uncoupling proteins; Mn-SOD, manganese superoxide dismutase; HO-1, heme-oxygenase 1.
Insulin signaling contributes to the interface between stress hormones and cognition by counteracting the negative consequences of exposure to elevated glucocorticoids (Figure 2). Chronic exposure to elevated levels of the stress hormone corticosterone impairs hippocampal insulin signaling (Piroli, Grillo, Reznikov, Adams, McEwen, Charron, & Reagan, 2007) and reduces glucose metabolism in hippocampal neurons (Horner, Packan, & Sapolsky, 1990). Intrahippocampal insulin also protects against stress-induced impairments in learning and memory (Moosavi et al., 2007). These observations suggest that in diabetes, deficient insulin signaling combined with HPA axis hyperactivity synergistically renders neurons more susceptible to energetic stress. In contrast, improved insulin sensitivity with exercise and dietary energy restriction might mitigate the effects of stress on learning and memory.
Figure 2. Insulin signaling counterbalances glucocorticoid signaling to maintain neuroplasticity, and impairment of insulin signaling increases neuronal vulnerability to stress.
(A), Under normal physiological circumstances, hippocampal insulin signaling protects against the negative effects of exposure to moderately elevated glucocorticoid hormones. Glucocorticoids act via the hippocampal glucocorticoid receptor (GR) and mineralocorticoid receptor (MR), facilitating HPA axis shutoff. While activation of GR in particular leads to transcription of genes that attenuate plasticity (Morsink et al., 2006), concurrent insulin receptor (IR) activation would counteract this effect by promoting survival signaling. Similarly, neuronal utilization of glucose and lactate would suppress the energetic stress associated with glucocorticoid receptor activation. (B), In the diabetic hippocampus, GR expression is compromised (Campbell et al., in press), leading to impairment of HPA axis shutoff. Moreover, insulin receptor signaling is impaired, and neuronal access to energy substrates is diminished. This creates a scenario in which the effects of exposure to elevated corticosterone levels, in the context of reduced insulin signaling and impaired metabolism, lead to neuronal endangerment. This model is a reductionist view of how insulin signaling and the availability of energy substrates might interact with the stress response. In the interests of space, other factors such as 11β-hydroxysteroid dehydrogenase 1, which locally modulates glucocorticoid actions in the hippocampus, and feeding-related peptides such as ghrelin and leptin, are not included in the diagram but could potentially contribute to the relationship between metabolism and stress.
Gut peptides and adipocyte-derived factors as potential contributors to the relationship between metabolism and cognition
Peptides released from the gut and adipocyte-derived factors are involved in appetite and body weight regulation, and are increasingly implicated in learning and memory. Some gastric peptides (such as ghrelin) signal hunger, while other hormones such as cholecystokinin (CCK), glucose-dependent insulinotropic peptide 1 (GIP-1), and glucagon-like peptide 1 (GLP-1) act as satiety signals to facilitate meal termination. Ghrelin is essential for normal hippocampus-dependent memory, and also positively regulates synapse number and function in normal-weight animals (Diano, Farr, Benoit, McNay, da Silva, Horvath, Gaskin, Nonaka, Jaeger, Banks, Morley, Pinto, Sherwin, Xu, Yamada, Sleeman, Tschöp, & Horvath, 2006). It remains to be seen whether the positive effects of ghrelin on hippocampal function might be blunted in obese, diabetic states. Exercise increases ghrelin receptor expression in the hippocampus (Gomez-Pinilla & Ying, 2010). Because exercise stimulates ghrelin signaling, it is possible that changes in levels of ghrelin contribute to the positive effects of exercise on hippocampal function.
The satiety signal CCK also contributes to hippocampus-dependent memory. Training on a spatial recognition memory tasks increases extracellular CCK in the hippocampus (Sebret, Léna, Crété, Matsui, Roques, & Daugé, 1999). Activation of the CCK-B receptor selectively facilitates spatial recognition memory following hippocampal, but not extrahippocampal drug infusions (Sebret. et al., 1999). In humans, exercise increases circulating CCK levels (Bailey, Davies, Castell, Newsholme, & Calam, 2001), and in rats, treadmill training increases hippocampal CCK mRNA expression (Ni, Li, Tao, & Cen, 2009). The question of whether voluntary exercise might similarly enhance hippocampal CCK expression levels, and the extent to which exercise-induced upregulation of hippocampal CCK might contribute to enhancements in learning and memory, remains to be addressed. Although levels of CCK are reduced in the cerebral cortex of mice that lack leptin (Strauss & Yalow, 1979) few studies have evaluated the potential for changes in hippocampal CCK signaling in diabetes. The functional significance of changes in central CCK levels in relation to cognitive deficits in diabetes remains unexplored.
GIP-1 is an incretin hormone released from the gut, and also expressed locally in the hippocampus, where it positively regulates adult neurogenesis in the dentate gyrus subfield (Nyberg, Anderson, Meister, Alborn, Ström, Brederlau, Illerskog, Nilsson, Kieffer, Hietala, Ricksten, & Eriksson, 2005). Transgenic overexpression of GIP-1 promotes exploratory behavior in mice, suggestive of decreased anxiety (Ding, Zhong, Xie, Chen, Della-Fera, Bollag, Bollag, Gujral, Kang, Sridhar, Baile, Curl, & Isales, 2006). Exercise training improves peripheral GIP-1 metabolism in humans with comorbid insulin resistance and obesity (Kelly, Brooks, Solomon, Kashyap, O’Leary, & Kirwan, 2009). Because GIP-1 agonists enhance long-term potentiation (Gault & Hölscher, 2008), a candidate cellular model of learning and memory that also exhibits bidirectional metabolic regulation following exercise and diabetes (for review, see Stranahan & Mattson, 2008c), there is the possibility that changes in GIP-1 metabolism may contribute to the energetic continuum. However, no studies to date have evaluated the contributions of changes in GIP-1 metabolism to the pro-cognitive effects of exercise, or the impairment of cognition in diabetes models.
GLP-1 is an incretin hormone produced in response to specific macronutrient elements in the gut. Emerging data suggest that GLP-1 analogs, developed for the treatment of diabetes, also confer neuroprotection following a variety of insults. GLP-1 is necessary for hippocampus-dependent memory (During, Cao, Zuzga, Francis, Fitzsimons, Jiao, Bland, Klugmann, Banks, Drucker, & Haile, 2003; Abbas, Faivre, & Hölscher, 2009), and was also protective following excitotoxic lesion (During et al., 2003). GLP-1 metabolism becomes less efficient during age-associated declines in glucose and insulin metabolism (Doyle & Egan, 2001). Exercise training reverses the impairment of GLP-1 metabolism in diabetic rats (Park, Hong, & Ahn, 2010). However, these data remain correlative, as no mechanistic studies have characterized the contributions of changes in GLP-1 metabolism to the opposite effects of running and diabetes on cognition.
Neurons throughout the brain express GLP-1 receptors and recent studies have shown that GLP-1 and the longer-lived GLP-1 analog Exendin-4 can act directly on neurons to promote their resistance to dysfunction and degeneration in animal models of AD, Parkinson’s disease (PD), and Huntington’s disease (HD). Exendin-4 was developed for the treatment of type 2 diabetes and has a longer half-life than GLP-1. Treatment with Exendin-4 increases both insulin production and insulin sensitivity (Lovshin & Drucker, 2009). In one study, Aβ levels were elevated by experimentally-induced diabetes in a mouse model of AD in which Aβ pathology is associated with cognitive deficits. When mice were treated with Exendin-4 continuously for several months, their glucose regulation improved and their Aβ pathology lessened (Li, Duffy, Ottinger, Ray, Bailey, Holloway, Tweedie, Perry, Mattson, Kapogiannis, Sambamurti, Lahiri, & Greig, 2010). GLP-1 and Exendin-4 also exert neuroprotective actions in animal models of PD, HD and stroke, by a mechanism that may involve elevation of cyclic AMP levels and increased production of BDNF in neurons (Li, Perry, Kindy, Harvy, Tweedie, Holloway, Powers, Shen, Egan, Sambamurti, Brossi, Lahiri, Mattson, Hoffer, Wang, & Greig, 2009; Martin, Golden, Carlson, Pistell, Zhou, Kim, Frank, Thomas, Chadwick, Greig, Bates, Sathasivam, Bernier, Maudsley, Mattson, & Egan, 2009).
Neuronal receptivity to gut peptides is influenced by ambient concentrations of adipocyte-derived factors. Signals such as leptin and adiponectin allow neurons to detect the availability of stored energy. Leptin levels are positively correlated with adiposity, while adiponectin levels are inversely correlated with body fat. Just as insulin sensitivity is diminished with chronic exposure to high levels of insulin, leptin sensitivity is also dynamically regulated by the availability of leptin as a ligand (Morrison, 2008). Although leptin’s actions have been best characterized in the hypothalamus, the hippocampus also expresses the leptin receptor (Hâkansson, Brown, Ghilardi, Skoda, & Meister, 1998). There is an inverse relationship between circulating leptin levels and hypothalamic leptin receptor expression (Martin, Perez, He, Dawson, & Millard, 2000), but a similar relationship has not yet been demonstrated in the hippocampus. Leptin signaling in hippocampal neurons may protect them against metabolic and excitotoxic insults (Guo, Jiang, Xu, Duan, & Mattson, 2008). Chronic exercise lowers circulating leptin levels, suggestive of improved leptin sensitivity (Stranahan et al., 2009). Because leptin signaling contributes to hippocampal synaptic mechanisms that support learning and memory (Harvey, 2007), the opposing effects of diabetes and exercise on leptin metabolism could contribute to the cognitive alterations that occur under these conditions.
The possible contributions of adiponectin to hippocampal plasticity are less well characterized than the actions of leptin in the hippocampus. The hippocampus expresses adiponectin receptors (Guillod-Maximin, Roy, Vacher, Aubourg, Bailleux, Lorsignol, Pénicaud, Parquet, & Taouis, 2009), and intracerebroventricular adiponectin reduces hippocampal cell death following kainate seizures in mice (Jeon, Shin, Kim, Kim, Lee, Kim, Kim, Kang, Cho, Choi, & Roh, 2009). In humans, exercise increases circulating adiponectin concentrations (Varady, Bhutani, Church, & Phillips, 2010), and diabetes exerts the opposite effect (Weyer, Funahashi, Tanaka, Hotta, Matsuzawa, Pratley, & Tataranni, 2001). However, because so little is known about the role of hippocampal adiponectin in plasticity under basal, non-diabetic, non-exercise conditions, it is difficult to speculate on a potential contribution of changes in adiponectin signaling to changes in hippocampal plasticity following running or insulin resistance.
Diabetes aggravates and energetic challenges attenuate central nervous system inflammation
In addition to exerting opposite effects on metabolic mediators of cognition and neuroplasticity, exercise or dietary energy restriction and diabetes also oppose one another with regard to the immune response. Peripherally, voluntary exercise increases basal levels of natural IgM in serum (Elphick, Wieseler-Frank, Greenwood, Campisi, & Fleshner, 2003), and enhances expression of serum interleukin-10 following an immune challenge (Kohut, Boehm, Moynihan, 2001; Nickerson, Elphick, Campisi, Greenwood, & Fleshner, 2005). T-cells produced in the thymus contribute to adaptive immunity, and the mobilization of T-cells is enhanced by moderate exercise (Krüger, Lechtermann, Fobker, Völker, & Mooren, 2008). Centrally, wheel running alters the expression of genes associated with the immune response in the hippocampus (Tong, Shen, Perreau, Balazs, & Cotman, 2001), and also increases the number of microglia in the cortex (Ehninger & Kempermann, 2003). Neuroinflammation is a hallmark of AD (Glass, Saijo, Winner, Marchetto, & Gage, 2010), and in AD model mice, wheel running regulates hippocampal expression of genes associated with the immune response (Parachikova, Nichol, & Cotman, 2008).
Overall, the effects of running on peripheral and central immune markers are consistent with the idea of running as a preconditioning mechanism that facilitates adaptive immunity, with neuroprotection secondary to reduced inflammation. In contrast, diabetes is associated with increases in inflammatory marker expression both centrally and peripherally (Wellen & Hotamisligil, 2005). In the case of running, increased microglial cell number and immunoresponsive gene expression occurs in the context of improved cognition, suggesting that the acute immune response to exercise may be protective. In diabetes, chronic activation of the immune system occurs in concert with cognitive impairment (Marioni, Strachan, Reynolds, Lowe, Mitchell, Fowkes, Frier, Lee, Butcher, Rumley, Murray, Deary, & Price, 2010), suggesting that increased susceptibility to inflammation might contribute to neurological deficits. Consistent with a role for inflammation in the adverse effects of overeating and diabetes on cognitive function, dietary energy restriction suppresses the production of pro-inflammatory cytokines in brain cells (Arumugam et al., 2010). These data indicate that one mechanism for the relationship between metabolism and hippocampal function may involve changes in the neuronal response to immune challenges.
Running and dietary energy restriction ameliorate and diabetes exacerbates the disease process in models of Alzheimer’s disease
AD is associated with cognitive impairment accompanied by the accumulation of amyloid-β plaques and phosphorylated tau tangles. Neuropathological alterations in AD occur within specific neural circuits in the medial temporal lobe. Within this circuitry, the connection between entorhinal layer II neurons and dentate gyrus granule cells shows early vulnerability over the course of aging in rats (Smith, Adams, Gallagher, Morrison, & Rapp, 2000) and humans (Scheff, Price, Schmitt, & Mufson, 2006). In parallel with its susceptibility to aging, the entorhinal-dentate projection accumulates amyloid-β plaques before the rest of the hippocampus (Reilly, Games, Rydel, Freedman, Schenk, Young, Morrison, & Bloom, 2003). This same pathway exhibits enhanced long-term potentiation following exercise (van Praag et al., 1999; Farmer, Zhao, van Praag, Wodtke, Gage, & Christie, 2004) and impaired long-term potentiation in animal models of insulin resistant diabetes (Stranahan et al., 2008a). This suggests that the perforant path projection arising from layer II neurons in the entorhinal cortex and terminating on dentate gyrus granule cells may represent a ‘weak link’ that is strengthened following exercise and weakened in diabetes. The strengthening or weakening of perforant path circuitry might then contribute to the reduced risk of AD in physically active individuals (Lautenschlager, Cox, Flicker, Foster, van Bockxmeer, Xiao, Greenop, & Almeida, 2008) and to the increased risk of AD in insulin resistant diabetes (Ott et al., 1996).
At the molecular level, exercise inhibits accumulation of Aβ1–40 and Aβ1–42, and also alters amyloid precursor protein processing in the TgCRND8 mouse model of AD (Adlard et al., 2005). Wheel running also reduced soluble A fibril accumulation in the Tg2576 model (Nichol, Poon, Parachikova, Cribbs, Glabe, & Cotman, 2008). While no studies have yet evaluated the consequences of voluntary exercise on phosphorylated tau accumulation, involuntary treadmill training attenuates neuropathology in mouse models of tauopathy (Leem, Lim, Shim, Cho, Kim, & Han, 2009). Based on these studies, it is apparent that molecular mechanisms central to AD can be offset by energetic stress associated with physical activity.
Diabetes is frequently accompanied by impairment of leptin sensitivity (Segal, Landt, & Klein, 1996). A recent study suggests that in the APP23 mouse model of AD, concurrent loss of leptin signaling accelerates cognitive deficits (Takeda, Sato, Uchio-Yamada, Sawada, Kunieda, Takeuchi, Kurinami, Shinohara, Rakugi, & Morishita, 2010). Hippocampus-dependent memory impairment occurred in the context of increased vascular amyloid deposition and exacerbation of reactive gliosis, accompanied by elevated expression of neuroinflammatory markers (Takeda et al., 2010). The earlier onset of cognitive impairment in AD mice that lack leptin could reflect a protective role for leptin signaling against AD pathology. However, learning deficits were also accelerated in APP23 mice that had been crossed with the Nagoya-Shibata-Yasuda (NSY) model of diabetes, in which insulin resistance arises from genetic selection for glucose intolerance, rather than loss of leptin signaling (Takeda et al., 2010). Therefore, it is more likely that global metabolic impairment (rather than changes in one specific factor) in insulin resistant diabetes worsens cognitive impairment in AD. Taken together with previous reports suggesting that diet-induced obesity contributes to neuropathology in AD model mice (Ho et al., 2004; Zhao et al., 2004), the studies by Takeda and colleagues (2010) support the idea that metabolic compromise arising from lifestyle factors renders neurons more susceptible to degeneration.
Trauma- and ischemia-induced cognitive impairment are modified by energy intake and exercise
In addition to aging and AD, two major causes of cognitive impairment are traumatic brain injury and ischemic stroke. Increasing evidence suggests that similar to age-related cognitive decline and AD, overeating and diabetes exacerbate, whereas exercise and dietary moderation attenuate, cognitive deficits associated with traumatic brain injury (TBI) and stroke. For example, diabetic rats exhibit worsened cognitive impairments following ischemia induced by cortical compression compared to non-diabetic rats (Moriera, Cebers, Pickering, Ostenson, Efendic, & Liljequist, 2007). In another study, poststroke cognitive deficits were exacerbated by diabetes, in association with elevated levels of Aβ in the brain (Zhang, Pan, Zhao, Zhang, Huang, & Sun, 2009). Exercise improved cognitive performance after TBI in a rat model by a mechanism involving exercise-induced production of BDNF in the hippocampus (Griesbach, Hovda, & Gomez-Pinilla, 2009). Dietary energy restriction attenuated learning and memory deficits induced by global cerebral ischemia in an animal model relevant to brain damage caused by cardiac arrest in humans (Roberge, Messier, Staines, & Plamendon, 2008). The mechanism by which dietary energy restriction improves functional outcome in models of cerebral ischemia likely involves activation of adaptive cellular stress response pathways and suppression of inflammation (Arumugam et al., 2010). These studies open the possibility that the relationship between metabolism and cognition generalizes across multiple insults of different etiology and anatomy.
Cognition and metabolism: intertwined and interdependent
Synaptic activity requires energy. Whether in the form of glucose or lactate, neurons have high metabolic requirements and cognitive function will, of necessity, be regulated by the availability of energy substrates. Neuronal sensitivity to homeostatic fluctuations in nutrient bioavailability has a strong mnemonic component, with the organism’s history of energy intake and expenditure reflected in local concentrations of gut peptides and adipocyte-derived factors. The endocrine environment also contributes to neuronal metabolism, with chronic and acute alterations in glucocorticoids, insulin, and other factors driving energy use, and frequently acting in opposition to one another. The complexity of the local microenvironment combined with cell-type-specific differences in energetic requirements limit the utility of a reductionist approach to the question of how cognition can be improved following exercise and impaired in diabetes. While it may not be possible to identify a single mechanism for the opposite effects of running and diabetes on hippocampal function, changes in inflammation, glucocorticoids, insulin sensitivity, and leptin signaling are all possible contributors. Future studies using integrative genomic and metabolomic strategies may help to shed light on the relationship between somatic metabolism and cognition.
Acknowledgments
This work was supported by NIH NRSA AG034818-01 to A.M.S., and by the Intramural Research Program of the National Institute on Aging (M.P.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas T, Faivre E, Hölscher C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: Interaction between type 2 diabetes and Alzheimer’s disease. Behav Brain Res. 2009;205:265–7. doi: 10.1016/j.bbr.2009.06.035. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–21. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anan F, Masaki T, Shimomura T, Fujiki M, Umeno Y, Eshima N, Saikawa T, Yoshimatsu H. Abdominal visceral fat accumulation is associated with hippocampus volume in non-dementia patients with type 2 diabetes mellitus. Neuroimage. 2010;49:57–62. doi: 10.1016/j.neuroimage.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab. 2010;21:134–141. doi: 10.1016/j.tem.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DM, Davies B, Castell LM, Newsholme EA, Calam J. Physical exercise and normobaric hypoxia: independent modulators of peripheral cholecystokinin metabolism in man. J Appl Physiol. 2001;90:105–13. doi: 10.1152/jappl.2001.90.1.105. [DOI] [PubMed] [Google Scholar]
- Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behav Neurosci. 2004;118:1123–7. doi: 10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Wilcox BJ, Figlewicz DP, Dorsa DM. Insulin and insulin-like growth factors in the CNS. Trends Neurosci. 1988;11:107–11. doi: 10.1016/0166-2236(88)90155-5. [DOI] [PubMed] [Google Scholar]
- Bélanger A, Lavoie N, Trudeau F, Massicotte G, Gagnon S. Preserved LTP and water maze learning in hyperglycaemic-hyperinsulinemic ZDF rats. Physiol Behav. 2004;83:483–94. doi: 10.1016/j.physbeh.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav Processes. 2005;68:165–72. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167:588–97. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick-Sander A, Steiner B, Wolf SA, Babu H, Kempermann G. Running in pregnancy transiently increases postnatal hippocampal neurogenesis in the offspring. Proc Natl Acad Sci U S A. 2006;103:3852–7. doi: 10.1073/pnas.0502644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl H, Wolf OT, Sweat V, Tirsi A, Richardson S, Convit A. Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res. 2009;1280:186–94. doi: 10.1016/j.brainres.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci. 2010;33:153–61. doi: 10.1016/j.tins.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun JB. The ecology and sociology of the Norway rat. U.S. Public Health Service Publication No. 1008. Washington, D.C: U.S. Government Printing Office; 1963. [Google Scholar]
- Campbell JE, Kiraly MA, Atkinson DJ, D’souza AM, Vranic M, Riddell MC. Regular exercise prevents the development of hyperglucocorticoidemia via adaptations in the brain and adrenal glands in male Zucker Diabetic Fatty rats. Am J Physiol Regul Integr Comp Physiol. doi: 10.1152/ajpregu.00155.2010. in press. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–58. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Convit A, Wolf OT, Tarshish C, de Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc Natl Acad Sci U S A. 2003;100:2019–22. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:2070–5. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA. Glucose intolerance and aging. Diabetes Care. 1981;4:493–501. doi: 10.2337/diacare.4.4.493. [DOI] [PubMed] [Google Scholar]
- Devaskar SU, Giddings SJ, Rajakumar PA, Carnaghi LR, Menon RK, Zahm DS. Insulin gene expression and insulin synthesis in mammalian neuronal cells. J Biol Chem. 1994;269:8445–54. [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschöp MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–8. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- Ding KH, Zhong Q, Xie D, Chen HX, Della-Fera MA, Bollag RJ, Bollag WB, Gujral R, Kang B, Sridhar S, Baile C, Curl W, Isales CM. Effects of glucose-dependent insulinotropic peptide on behavior. Peptides. 2006;27:2750–5. doi: 10.1016/j.peptides.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Doyle ME, Egan JM. Glucagon-like peptide-1. Recent Prog Horm Res. 2001;56:377–99. doi: 10.1210/rp.56.1.377. [DOI] [PubMed] [Google Scholar]
- Droste SK, Chandramohan Y, Hill LE, Linthorst AC, Reul JM. Voluntary exercise impacts on the rat hypothalamic-pituitary-adrenocortical axis mainly at the adrenal level. Neuroendocrinology. 2007;86:26–37. doi: 10.1159/000104770. [DOI] [PubMed] [Google Scholar]
- Droste SK, Collins A, Lightman SL, Linthorst AC, Reul JM. Distinct, time-dependent effects of voluntary exercise on circadian and ultradian rhythms and stress responses of free corticosterone in the rat hippocampus. Endocrinology. 2009;150:4170–9. doi: 10.1210/en.2009-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste SK, de Groote L, Atkinson HC, Lightman SL, Reul JM, Linthorst AC. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology. 2008;149:3244–53. doi: 10.1210/en.2008-0103. [DOI] [PubMed] [Google Scholar]
- Droste SK, Gesing A, Ulbricht S, Müller MB, Linthorst AC, Reul JM. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 2003;144:3012–23. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–9. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex. 2003;13:845–51. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- Elphick GF, Wieseler-Frank J, Greenwood BN, Campisi J, Fleshner M. B-1 cell (CD5+/CD11b+) numbers and nIgM levels are elevated in physically active vs. sedentary rats. J Appl Physiol. 2003;95:199–206. doi: 10.1152/japplphysiol.01054.2002. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Kramer AF. Aerobic exercise effects on cognitive and neural plasticity in older adults. Br J Sports Med. 2009;43:22–4. doi: 10.1136/bjsm.2008.052498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wójcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–9. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Frick KM, Stillner ET, Berger-Sweeney J. Mice are not little rats: species differences in a one-day water maze task. Neuroreport. 2000;11:3461–3465. doi: 10.1097/00001756-200011090-00013. [DOI] [PubMed] [Google Scholar]
- García-Capdevila S, Portell-Cortés I, Torras-Garcia M, Coll-Andreu M, Costa-Miserachs D. Effects of long-term voluntary exercise on learning and memory processes: dependency of the task and level of exercise. Behav Brain Res. 2009;202:162–170. doi: 10.1016/j.bbr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Gault VA, Hölscher C. Protease-resistant glucose-dependent insulinotropic polypeptide agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta-amyloid. J Neurophysiol. 2008;99:1590–1595. doi: 10.1152/jn.01161.2007. [DOI] [PubMed] [Google Scholar]
- Giddings SJ, Chirgwin J, Permutt MA. Evaluation of rat insulin messenger RNA in pancreatic and extrapancreatic tissues. Diabetologia. 1985;28:343–347. doi: 10.1007/BF00283141. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Fleshner M. A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus. 2009;19:988–1001. doi: 10.1002/hipo.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood CE, Winocur G. Glucose treatment reduces memory deficits in young adult rats fed high-fat diets. Neurobiol Learn Mem. 2001;75:179–189. doi: 10.1006/nlme.2000.3964. [DOI] [PubMed] [Google Scholar]
- Gold PE. Glucose and age-related changes in memory. Neurobiol Aging. 2005;26(Suppl 1):60–64. doi: 10.1016/j.neurobiolaging.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Gomez-Merino D, Béquet F, Berthelot M, Chennaoui M, Guezennec CY. Site-dependent effects of an acute intensive exercise on extracellular 5-HT and 5-HIAA levels in rat brain. Neurosci Lett. 2001;301:143–146. doi: 10.1016/s0304-3940(01)01626-3. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z. Differential effects of exercise and dietary docosahexaenoic acid on molecular systems associated with control of allostasis in the hypothalamus and hippocampus. Neuroscience. 2010;168:130–137. doi: 10.1016/j.neuroscience.2010.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrick CL. Effects of lifelong restricted feeding on complex maze performance in rats. Age. 1984;7:1–2. [Google Scholar]
- Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillod-Maximin E, Roy AF, Vacher CM, Aubourg A, Bailleux V, Lorsignol A, Pénicaud L, Parquet M, Taouis M. Adiponectin receptors are expressed in hypothalamus and colocalized with proopiomelanocortin and neuropeptide Y in rodent arcuate neurons. J Endocrinol. 2009;200:93–105. doi: 10.1677/JOE-08-0348. [DOI] [PubMed] [Google Scholar]
- Guo Z, Jiang H, Xu X, Duan W, Mattson MP. Leptin-mediated cell survival signaling in hippocampal neurons mediated by JAK STAT3 and mitochondrial stabilization. J Biol Chem. 2008;283:1754–1763. doi: 10.1074/jbc.M703753200. [DOI] [PubMed] [Google Scholar]
- Hâkansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci. 1998;18:559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Hall JL, Gonder-Frederick LA, Chewning WW, Silveira J, Gold PE. Glucose enhancement of performance on memory tests in young and aged humans. Neuropsychologia. 1989;27:1129–38. doi: 10.1016/0028-3932(89)90096-1. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Nzerem CK, Frick KM. Single enrichment variables differentially reduce age-related memory decline in female mice. Behav Neurosci. 2007;121:679–88. doi: 10.1037/0735-7044.121.4.679. [DOI] [PubMed] [Google Scholar]
- Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol. 2007;7:643–7. doi: 10.1016/j.coph.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J. 2004;18:902–4. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, Burns JM. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:188–97. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner HC, Packan DR, Sapolsky RM. Glucocorticoids inhibit glucose transport in cultured hippocampal neurons and glia. Neuroendocrinology. 1990;52:57–64. doi: 10.1159/000125539. [DOI] [PubMed] [Google Scholar]
- Iversen IH. Techniques for establishing schedules with wheel running as reinforcement in rats. J Exp Anal Behav. 1993;60:219–38. doi: 10.1901/jeab.1993.60-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon BT, Shin HJ, Kim JB, Kim YK, Lee DH, Kim KH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Adiponectin protects hippocampal neurons against kainic acid-induced excitotoxicity. Brain Res Rev. 2009;61:81–8. doi: 10.1016/j.brainresrev.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Joëls M, Van Riel E. Mineralocorticoid and glucocorticoid receptor-mediated effects on serotonergic transmission in health and disease. Ann N Y Acad Sci. 2004;1032:301–3. doi: 10.1196/annals.1314.046. [DOI] [PubMed] [Google Scholar]
- Kelly KR, Brooks LM, Solomon TP, Kashyap SR, O’Leary VB, Kirwan JP. The glucose-dependent insulinotropic polypeptide and glucose-stimulated insulin response to exercise training and diet in obesity. Am J Physiol Endocrinol Metab. 2009;296:E1269–74. doi: 10.1152/ajpendo.00112.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut ML, Boehm GW, Moynihan JA. Prolonged exercise suppresses antigen-specific cytokine response to upper respiratory infection. J Appl Physiol. 2001;90:678–84. doi: 10.1152/jappl.2001.90.2.678. [DOI] [PubMed] [Google Scholar]
- Komatsu T, Chiba T, Yamaza H, Yamashita K, Shimada A, Hoshiyama Y, Henmi T, Ohtani H, Higami Y, de Cabo R, Ingram DK, Shimokawa I. Manipulation of caloric content but not diet composition, attenuates the deficit in learning and memory of senescence-accelerated mouse strain P8. Exp Gerontol. 2008;43:339–346. doi: 10.1016/j.exger.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Krüger K, Lechtermann A, Fobker M, Völker K, Mooren FC. Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain Behav Immun. 2008;22:324–38. doi: 10.1016/j.bbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Lambert TJ, Fernandez SM, Frick KM. Different types of environmental enrichment have discrepant effects on spatial memory and synaptophysin levels in female mice. Neurobiol Learn Mem. 2005;83:206–16. doi: 10.1016/j.nlm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, Greenop KR, Almeida OP. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–37. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307:373–5. doi: 10.1126/science.1104342. [DOI] [PubMed] [Google Scholar]
- Lee J, Herman JP, Mattson MP. Dietary restriction selectively decreases glucocorticoid receptor expression in the hippocampus and cerebral cortex of rats. Exp Neurol. 2000;166:435–441. doi: 10.1006/exnr.2000.7512. [DOI] [PubMed] [Google Scholar]
- Lee J, Herman JP, Mattson MP. Dietary restriction selectively decreases glucocorticoid receptor expression in the hippocampus and cerebral cortex of rats. Exp Neurol. 2000;166:435–41. doi: 10.1006/exnr.2000.7512. [DOI] [PubMed] [Google Scholar]
- Leem YH, Lim HJ, Shim SB, Cho JY, Kim BS, Han PL. Repression of tau hyperphosphorylation by chronic endurance exercise in aged transgenic mouse model of tauopathies. J Neurosci Res. 2009;87:2561–70. doi: 10.1002/jnr.22075. [DOI] [PubMed] [Google Scholar]
- Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–15. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, Tweedie D, Perry T, Mattson MP, Kapogiannis D, Sambamurti K, Lahiri DK, Greig NH. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J Alzheimers Dis. 2010;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López JF, Chalmers DT, Little KY, Watson SJ. A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry. 1998;43:547–73. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–269. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, Mattson MP. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20:631–637. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- Marioni RE, Strachan MW, Reynolds RM, Lowe GD, Mitchell RJ, Fowkes FG, Frier BM, Lee AJ, Butcher I, Rumley A, Murray GD, Deary IJ, Price JF. Association between raised inflammatory markers and cognitive decline in elderly people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes. 2010;59:710–3. doi: 10.2337/db09-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci. 2009;29:6734–51. doi: 10.1523/JNEUROSCI.1350-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RL, Perez E, He YJ, Dawson R, Jr, Millard WJ. Leptin resistance is associated with hypothalamic leptin receptor mRNA and protein downregulation. Metabolism. 2000;49:1479–84. doi: 10.1053/meta.2000.17695. [DOI] [PubMed] [Google Scholar]
- Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5:332–353. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Golden E, Carlson OD, Pistell P, Zhou J, Kim W, Frank BP, Thomas S, Chadwick WA, Greig NH, Bates GP, Sathasivam K, Bernier M, Maudsley S, Mattson MP, Egan JM. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington’s disease. Diabetes. 2009;58:318–328. doi: 10.2337/db08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Ji S, Maudsley S, Mattson MP. “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci U S A. 2010;107:6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–94. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Weiss JM, Schwartz LS. Uptake of corticosterone by rat brain and its concentration by certain limbic structures. Brain Res. 1969 Nov;16(1):227–41. doi: 10.1016/0006-8993(69)90096-1. [DOI] [PubMed] [Google Scholar]
- McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci U S A. 2000;97:2881–5. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem. 2010;93:546–53. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–8. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Mielke JG, Nicolitch K, Avellaneda V, Earlam K, Ahuja T, Mealing G, Messier C. Longitudinal study of the effects of a high-fat diet on glucose regulation, hippocampal function, and cerebral insulin sensitivity in C57BL/6 mice. Behav Brain Res. 2006;175:374–82. doi: 10.1016/j.bbr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gómez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–14. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gómez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–40. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Moosavi M, Naghdi N, Maghsoudi N, Zahedi Asl S. Insulin protects against stress-induced impairments in water maze performance. Behav Brain Res. 2007;176:230–236. doi: 10.1016/j.bbr.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Moreira T, Cebers G, Pickering C, Ostenson CG, Efendic S, Liljequist S. Diabetic Goto-Kakizaki rats display pronounced hyperglycemia and longer-lasting cognitive impairments following ischemia induced by cortical compression. Neuroscience. 2007;144:1169–1185. doi: 10.1016/j.neuroscience.2006.10.054. [DOI] [PubMed] [Google Scholar]
- Morrison CD. Leptin resistance and the response to positive energy balance. Physiol Behav. 2008;94:660–3. doi: 10.1016/j.physbeh.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsink MC, Steenbergen PJ, Vos JB, Karst H, Joëls M, De Kloet ER, Datson NA. Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. J Neuroendocrinol. 2006;18:239–52. doi: 10.1111/j.1365-2826.2006.01413.x. [DOI] [PubMed] [Google Scholar]
- Murray AJ, Knight NS, Cochlin LE, McAleese S, Deacon RM, Rawlins JN, Clarke K. Deterioration of physical performance and cognitive function in rats with short-term high-fat feeding. FASEB J. 2009;23:4353–60. doi: 10.1096/fj.09-139691. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Ni H, Li C, Tao LY, Cen JN. Physical exercise improves learning by modulating hippocampal mossy fiber sprouting and related gene expression in a developmental rat model of penicillin-induced recurrent epilepticus. Toxicol Lett. 2009;191:26–32. doi: 10.1016/j.toxlet.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation. 2008;5:13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson M, Elphick GF, Campisi J, Greenwood BN, Fleshner M. Physical activity alters the brain Hsp72 and IL-1beta responses to peripheral E. coli challenge. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1665–74. doi: 10.1152/ajpregu.00601.2004. [DOI] [PubMed] [Google Scholar]
- Nyberg J, Anderson MF, Meister B, Alborn AM, Ström AK, Brederlau A, Illerskog AC, Nilsson O, Kieffer TJ, Hietala MA, Ricksten A, Eriksson PS. Glucose-dependent insulinotropic polypeptide is expressed in adult hippocampus and induces progenitor cell proliferation. J Neurosci. 2005;25:1816–25. doi: 10.1523/JNEUROSCI.4920-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, Breteler MM. Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia. 1996;39:1392–1397. doi: 10.1007/s001250050588. [DOI] [PubMed] [Google Scholar]
- Parachikova A, Nichol KE, Cotman CW. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol Dis. 2008;30:121–9. doi: 10.1016/j.nbd.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Hong SM, Ahn IS. Exendin-4 and exercise improve hepatic glucose homeostasis by promoting insulin signaling in diabetic rats. Metabolism. 2010;59I:123–33. doi: 10.1016/j.metabol.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Patel NV, Finch CE. The glucocorticoid paradox of caloric restriction in slowing brain aging. Neurobiol Aging. 2002;23:707–717. doi: 10.1016/s0197-4580(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–43. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH. Protection and reversal of excitotoxic neuronal damage by glucagon–like peptide-1 and exendin-4. J Pharmacol Exp Ther. 2002;302:881–8. doi: 10.1124/jpet.102.037481. [DOI] [PubMed] [Google Scholar]
- Pierce WD, Epling WF, Boer DP. Deprivation and satiation: The interrelations between food and wheel running. J Exp Anal Behav. 1986;46:199–210. doi: 10.1901/jeab.1986.46-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piroli GG, Grillo CA, Reznikov LR, Adams S, McEwen BS, Charron MJ, Reagan LP. Corticosterone impairs insulin-stimulated translocation of GLUT4 in the rat hippocampus. Neuroendocrinology. 2007;85:71–80. doi: 10.1159/000101694. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Pal SN, Unick K, Stefani MR, Gold PE. Modulation of hippocampal acetylcholine release and spontaneous alternation scores by intrahippocampal glucose injections. J Neurosci. 1998;18:1595–601. doi: 10.1523/JNEUROSCI.18-04-01595.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon NL, Kenna HA, Wroolie TE, Kelley R, Silverman D, Brooks J, Williams KE, Powers BN, Hallmayer J, Reiss A. Insulin resistance and hippocampal volume in women at risk for Alzheimer’s disease. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2009.12.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Frey WH, 2nd, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, Cherrier MM, Craft S. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 2006;27:451–8. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Reilly JF, Games D, Rydel RE, Freedman S, Schenk D, Young WG, Morrison JH, Bloom FE. Amyloid deposition in the hippocampus and entorhinal cortex: quantitative analysis of a transgenic mouse model. Proc Natl Acad Sci U S A. 2003;100:4837–42. doi: 10.1073/pnas.0330745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss JI, Dishman RK, Boyd HE, Robinson JK, Holmes PV. Chronic activity wheel running reduces the severity of kainic acid-induced seizures in the rat: possible role of galanin. Brain Res. 2009;1266:54–63. doi: 10.1016/j.brainres.2009.02.030. [DOI] [PubMed] [Google Scholar]
- Roberge MC, Messier C, Staines WA, Plamondon H. Food restriction induces long-lasting recovery of spatial memory deficits following global ischemia in delayed matching and non-matching-to-sample radial arm maze tasks. Neuroscience. 2008;156:11–29. doi: 10.1016/j.neuroscience.2008.05.062. [DOI] [PubMed] [Google Scholar]
- Rolandsson O, Backeström A, Eriksson S, Hallmans G, Nilsson LG. Increased glucose levels are associated with episodic memory in nondiabetic women. Diabetes. 2008;57:440–3. doi: 10.2337/db07-1215. [DOI] [PubMed] [Google Scholar]
- Ross AP, Bartness TJ, Mielke JG, Parent MB. A high fructose diet impairs spatial memory in male rats. Neurobiol Learn Mem. 2009;92:410–6. doi: 10.1016/j.nlm.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeep TC, Yau JL, MacLullich AM, Noble J, Deary IJ, Walker BR, Seckl JR. 11Beta-hydroxysteroid dehydrogenase inhibition improves cognitive function in healthy elderly men and type 2 diabetics. Proc Natl Acad Sci U S A. 2004;101:6734–9. doi: 10.1073/pnas.0306996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz C, Andrieu S, Sinclair A, Hanaire H, Vellas B, REAL FR Study Group. Diabetes is associated with a slower rate of cognitive decline in Alzheimer disease. Neurology. 2009;73:1359–66. doi: 10.1212/WNL.0b013e3181bd80e9. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–84. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Sebret A, Léna I, Crété D, Matsui T, Roques BP, Daugé V. Rat hippocampal neurons are critically involved in physiological improvement of memory processes induced by cholecystokinin-B receptor stimulation. J Neurosci. 1999;19:7230–7. doi: 10.1523/JNEUROSCI.19-16-07230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal KR, Landt M, Klein S. Relationship between insulin sensitivity and plasma leptin concentration in lean and obese men. Diabetes. 1996;45:988–91. doi: 10.2337/diab.45.7.988. [DOI] [PubMed] [Google Scholar]
- Shii K, Baba S, Yokono K, Roth RA. Covalent linkage of 125I-insulin to a cytosolic insulin-degrading enzyme. J Biol Chem. 1985;260:6503–6. [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–77. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–93. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Lee K, Cutler RG, Egan JP, Mattson MP. Diabetes impairs hippocampal function via glucocorticoid-mediated effects on new and mature neurons. Nature Neuroscience. 2008a;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nature Neuroscience. 2006;9:526–33. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Becker KG, Zhang Y, Maudsley S, Martin B, Cutler RG, Mattson MP. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2008.10.016. in press. Epub ahead of print; doi: 10.1016-j.neurobiolaging.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Impact of energy intake and expenditure on neuronal plasticity. Neuromolecular Medicine. 2008c;10:209–18. doi: 10.1007/s12017-008-8043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008b;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus E, Yalow RS. Cholecystokinin in the brains of obese and nonobese mice. Science. 1979;203:68. doi: 10.1126/science.758680. [DOI] [PubMed] [Google Scholar]
- Stummer W, Baethmann A, Murr R, Schürer L, Kempski OS. Cerebral Protection Against Ischemia by Locomotor Activity in Gerbils: Underlying Mechanisms. Stroke. 1995;26:1423–1430. doi: 10.1161/01.str.26.8.1423. [DOI] [PubMed] [Google Scholar]
- Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A. 2010;107:7036–41. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama K, Himms-Hagen J. Increased sensitivity of the genetically obese mouse to corticosterone. Am J Physiol. 1987;252:E202–8. doi: 10.1152/ajpendo.1987.252.2.E202. [DOI] [PubMed] [Google Scholar]
- Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Dis. 2001;8:1046–56. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- Tornello S, Coirini H, De Nicola AF. Effects of experimental diabetes on the concentration of corticosterone in central nervous system, serum and adrenal gland. J Steroid Biochem. 1981;14:1279–84. doi: 10.1016/0022-4731(81)90332-0. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10:272–82. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Church EC, Phillips SA. Adipokine responses to acute resistance exercise in trained and untrained men. Med Sci Sports Exerc. 2010;42:456–62. doi: 10.1249/MSS.0b013e3181ba6dd3. [DOI] [PubMed] [Google Scholar]
- Vekrellis K, Ye Z, Qiu WQ, Walsh D, Hartley D, Chesneau V, Rosner MR, Selkoe DJ. Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J Neurosci. 2000;20:1657–65. doi: 10.1523/JNEUROSCI.20-05-01657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayudhan L, Poppe M, Archer N, Proitsi P, Brown RG, Lovestone S. Risk of developing dementia in people with diabetes and mild cognitive impairment. Br J Psychiatry. 2010;196:36–40. doi: 10.1192/bjp.bp.109.067942. [DOI] [PubMed] [Google Scholar]
- Voll CL, Whishaw IQ, Auer RN. Postischemic insulin reduces spatial learning deficit following transient forebrain ischemia in rats. Stroke. 1989;20:646–51. doi: 10.1161/01.str.20.5.646. [DOI] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, Kim JS, Morris KS, White SM, Wójcicki TR, Hu L, Szabo A, Klamm E, McAuley E, Kramer AF. Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010;48:1394–406. doi: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J Nutr. 2003;133:1921–1929. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- Wang Y, Perfetti R, Greig NH, Holloway HW, DeOre KA, Montrose-Rafizadeh C, Elahi D, Egan JM. Glucagon-like peptide-1 can reverse the age-related decline in glucose tolerance in rats. J Clin Invest. 1997;99:2883–9. doi: 10.1172/JCI119482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, McEwen BS. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005;119:1389–95. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, Krueger K, Fromme A, Korsukewitz C, Floel A, Knecht S. High impact running improves learning. Neurobiol Learn Mem. 2007;87:597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Witte AV, Fobker M, Gellner R, Knecht S, Flöel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO Technical Report Series 894. 2000. Obesity: preventing and managing the global epidemic. [PubMed] [Google Scholar]
- Wu W, Brickman AM, Luchsinger J, Ferrazzano P, Pichiule P, Yoshita M, Brown T, DeCarli C, Barnes CA, Mayeux R, Vannucci SJ, Small SA. The brain in the age of old: the hippocampal formation is targeted differentially by diseases of late life. Ann Neurol. 2008;64:698–706. doi: 10.1002/ana.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CH, Yeh CJ, Wang CC, Liao WC, Chen SC, Chen CC, Liang J, Lai TJ, Lin HS, Lee SH, Lee MC. Determinants of cognitive impairment over time among the elderly in Taiwan: results of the national longitudinal study. Arch Gerontol Geriatr. 2010;50(Suppl 1):S53–7. doi: 10.1016/S0167-4943(10)70014-5. [DOI] [PubMed] [Google Scholar]
- Young WS., 3rd Periventricular hypothalamic cells in the rat brain contain insulin mRNA. Neuropeptides. 1986;8:93–97. doi: 10.1016/0143-4179(86)90035-1. [DOI] [PubMed] [Google Scholar]
- Zhang T, Pan BS, Zhao B, Zhang LM, Huang YL, Sun FY. Exacerbation of poststroke dementia by type 2 diabetes is associated with synergistic increases of beta-secretase activation and beta-amyloid generation in rat brains. Neuroscience. 2009;161:1045–1056. doi: 10.1016/j.neuroscience.2009.04.032. [DOI] [PubMed] [Google Scholar]
- Zhao L, Teter B, Morihara T, Lim GP, Ambegaokar SS, Ubeda OJ, Frautschy SA, Cole GM. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer’s disease intervention. J Neurosci. 2004;24:11120–6. doi: 10.1523/JNEUROSCI.2860-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Lu W, Shi Y, Bai F, Chang J, Yuan Y, Teng G, Zhang Z. Impairments in cognition and resting-state connectivity of the hippocampus in elderly subjects with type 2 diabetes. Neurosci Lett. 2010;473:5–10. doi: 10.1016/j.neulet.2009.12.057. [DOI] [PubMed] [Google Scholar]



