Abstract
Adult hippocampal neurogenesis is a unique form of neural circuit plasticity that results in the generation of new neurons in the dentate gyrus (DG) throughout life 1, 2. Adult-born neurons exhibit heightened synaptic plasticity during their maturation 3 and can account for up to ten percent of the entire granule cell population 4. Moreover, levels of adult hippocampal neurogenesis are elevated by interventions associated with beneficial effects on cognition and mood such as learning 5, environmental enrichment 6, exercise 6 and chronic antidepressant treatment 7–10. Together, these properties of adult neurogenesis suggest that it may be harnessed to improve hippocampal functions. However, despite a substantial number of studies demonstrating that adult-born neurons are necessary for mediating specific cognitive functions 11 and some of the behavioural effects of antidepressants 8–10, 12, 13, it is unknown whether increasing adult hippocampal neurogenesis is sufficient to improve cognition and mood. Here we show that inducible genetic expansion of the population of adult-born neurons by enhancing their survival improves performance in a specific cognitive task in which an animal must distinguish between two similar contexts. Mice with increased adult hippocampal neurogenesis show normal object recognition, spatial learning, contextual fear conditioning and extinction learning but are more efficient in differentiating between overlapping contextual representations, suggestive of enhanced pattern separation. Furthermore, stimulation of adult hippocampal neurogenesis, when combined with an intervention such as voluntary exercise, produces a robust increase in exploratory behaviour. In contrast, increasing adult hippocampal neurogenesis, on its own, does not produce an anxiolytic or antidepressant-like behavioural response. Together, our findings suggest that strategies designed to specifically increase adult hippocampal neurogenesis, by targeting cell death of adult-born neurons or other means, may have therapeutic potential for reversing impairments in pattern separation such as that seen during normal aging 14, 15.
The DG subregion of the hippocampus is a substrate for both cognition and mood regulation. Convergent lines of evidence from neuroanatomical, computational, electrophysiological, behavioural and human brain imaging studies suggest a crucial role for the DG in the formation of new episodic memories by transforming similar experiences or events into discrete non-overlapping representations, a process known as “pattern separation”16. In addition, overexpression of neurotrophins or transcription factors in the DG elicits antidepressant-like behavioural effects 17, 18. Consistent with these DG functions, ablation of adult hippocampal neurogenesis impairs pattern separation 11, 19 and blocks some of the behavioural effects of antidepressants 8–10, 12, 13. In sharp contrast, the impact of selectively increasing adult hippocampal neurogenesis on cognition and mood is not known. Addressing these questions has proven difficult owing to a lack of strategies that selectively increase adult neurogenesis.
Here, we developed a genetic gain of function strategy to inducibly augment the survival of adult-born neurons in a cell autonomous manner (Fig. 1a). Since 60–80 percent of young adult-born neurons undergo programmed cell death and the pro-apoptotic gene Bax is required for this process 20, we used a transgenic mouse line in which the tamoxifen (TAM) regulatable recombinase CreERT2 is expressed under the control of a rat 5.26 Kb nestin promoter fragment (Dranovsky et al., manuscript in preparation) and a Bax conditional mouse line 21 to ablate Bax selectively in neural stem cells in the adult brain (Supplementary Fig. 1). Using an inducible reporter, enhanced yellow fluorescent protein (YFP), as a surrogate marker for Bax recombination, we found that 57.5 ± 3.3% of Doublecortin (Dcx) expressing neurons expressed YFP (Supplementary Fig. 1 and 2). Analysis of adult hippocampal neurogenesis in mice carrying one Nestin CreERT2 and two Bax floxed alleles (“NCff” mice) that had been injected with TAM (referred to as “iBaxnestin” mice) or Vehicle (Fig. 1b) showed comparable levels of stem cell proliferation in the DG (Supplementary Fig. 3). In contrast, we found a marked upregulation in survival of adult-born neurons 8 weeks following TAM injection. Analysis of the population of 1–3 week old adult-born neurons by Dcx immunohistochemistry revealed a significant 1.8 and 2 fold increase in the total number of Dcx+ neurons and Dcx+ neurons that have at least tertiary dendrites, respectively (Fig. 1c). Quantification of long-term survival by BrdU (5-bromo-2'-deoxyuridine) pulse-chase labeling revealed a 3.6 fold increase in BrdU+ cells in the dentate granule cell layer of iBaxnestin mice without a change in the proportion of adult-born neurons (BrdU+ NeuN+, Supplementary Fig. 5). Paralleling these findings, there was a 3 fold increase in the population of YFP-labelled adult-born neurons 6 weeks post TAM injection (Fig. 1d). The larger increase in the number of Dcx+ cells in iBaxnestin mice at 8 weeks compared to 4 weeks following TAM injection (Supplementary Fig. 4) is consistent with the fact that CreERT2 mediated recombination occurs in both the slow dividing self-renewing type I neural stem cells as well as the type II transit amplifying cells (Supplementary Fig. 1). The expansion of the reservoir of adult-born neurons along the hippocampal septotemporal axis in iBaxnestin mice is comparable to, if not greater than, that observed following chronic antidepressant treatment 7–10, environmental enrichment 6, and exercise 6. iBaxnestin mice also show an increase in adult-born cell survival in the olfactory bulb (Supplementary Fig. 5). iBaxnestin mice and controls have similar body weight, brain architecture and expression of DG markers such as calbindin (Supplementary Fig. 6). Surprisingly, both groups have comparable DG granule cell layer volumes suggesting that neuronal packing density may be increased in iBaxnestin mice (Supplementary Fig. 6). In the absence of tamoxifen, no recombination was observed at the ROSA26 floxed stopYFP conditional locus in NCffY (Nestin CreERT2; Bax f/f; ROSA26 f STOP YFP/+) mice (Fig. 1d).
Figure 1.
Bax ablation in neural stem cells in the adult brain increases hippocampal neurogenesis and neurogenesis-dependent LTP. a, Schematic illustrating genetic gain of function strategy to increase adult hippocampal neurogenesis. (i) In the adult DG, substantial fraction of adult-born neurons undergo Bax-dependent programmed cell death (shown in light red). (ii) Nestin CreERT2 mediated ablation of Bax in type I and type II cells results in generation of adult-born neurons lacking Bax thereby preventing their cell death. b, Experimental design. c, Representative Dcx immunostained coronal hippocampal sections of Veh and TAM treated NCff mice. Arrows in insets indicate Dcx neurons with at least tertiary dendrites. Quantification of Dcx population. Total Dcx+ neurons: 6974 ± 600 (NCff+Veh), 12636 ± 1764 (NCff+TAM), *P=0.038. Dcx+ neurons with at least tertiary dendrites: 1800 ± 340 (NCff+Veh), 4090 ± 285 (NCff+TAM), ** P=0.006, n=3 mice per group. d, Representative coronal hippocampal sections of TAM treated NCY and NCffY mice and Veh treated NCffY mice immunostained for YFP and NeuN. Quantification of YFP+ neuronal population. Average number of YFP+ neurons per section, Whole hippocampus: 29.3± 2.1 (NCY+TAM), 87.6± 14.3 (NCffY+TAM), *P=0.015. Septal: 28.76 ± 2.3 (NCY+TAM), 76.3± 15.4 (NCffY+TAM), *P=0.03. Temporal: 30.66 ± 5.7 (NCY+TAM), 103.6± 20 (NCffY+TAM), *P=0.02. n=3 mice per group. e, NCff+TAM mice show enhanced medial perforant path-dentate gyrus LTP compared to NCff+Veh mice. Repeated measures ANOVA (50 minutes) shows significant effect of treatment, F(1, 17)=5, P=0.039. Post-tetanic potentiation was significantly different between the two groups P=0.003. f, Medial perforant path-DG LTP evoked in presence of bicuculline was similar between the groups, repeated measures ANOVA, last 30 minutes: F(1, 8)<1. (e) n=8 slices, 6 mice (Veh), n=11 slices,7 mice (TAM); (f) n=4 slices, 3 mice (Veh), n=6 slices, 3 mice (TAM). Results are mean ± SEM. Scale bar: 100μm.
We next examined the morphological maturation of adult-born neurons following Bax ablation in neural stem cells in the adult brain. Sholl analysis of genetically labelled 6 week old adult-born neurons in NCffY and NCY (Nestin CreERT2; ROSA26 f STOP YFP/+) mice indicated normal apical dendrite maturation and retraction of basal dendrites (Supplementary Fig. 7). Analysis of genetically labelled (POMC-τ-eGFP) mossy fibers of young adult-born neurons in iBaxnestin mice and controls suggested normal axonal extension and targeting in CA3 (Supplementary Fig. 8). To assess functional integration of adult-born neurons in iBaxnestin mice, we examined a form of long-term potentiation (LTP) at medial perforant path-granule cell synapses that is dependent on young adult-born neurons 22, 23 and is enhanced by chronic fluoxetine treatment 24. iBaxnestin mice showed a significant enhancement in neurogenesis-dependent LTP at 4–6 weeks (Supplementary Fig. 9) and 8 weeks (Fig. 1e) following Vehicle/TAM injection. In contrast, LTP of mature dentate granule neurons recorded in the presence of bicuculline (Fig. 1f) and basal synaptic transmission (Supplementary Fig. 9) were similar in both groups. These findings suggest that increasing the number of adult-born neurons is sufficient to enhance neurogenesis-dependent LTP and that additional adult-born neurons in iBaxnestin mice functionally integrate into the hippocampal network.
To investigate the causal relationship between increased hippocampal neurogenesis and hippocampal dependent learning and memory, we tested iBaxnestin mice and controls in object recognition and spatial learning and memory paradigms. Both iBaxnestin mice and controls showed comparable levels of exploration of a novel or a similar object (Supplementary Fig. 10). Increasing adult hippocampal neurogenesis did not impact spatial learning and memory in the reference version of the morris water maze nor during reversal learning or in the active place avoidance task (Supplementary Fig. 11 and 12). To test if increased adult hippocampal neurogenesis influences rapid contextual encoding, we subjected iBaxnestin mice and controls to a single trial contextual fear conditioning paradigm (Fig. 2a). iBaxnestin mice and controls showed elevated and indistinguishable levels of freezing in the training context “A” 24 hours following training in A, suggesting that both groups acquired and retained contextual fear conditioning equally well [(ANOVA F(1, 26) <1, Average freezing: 34.74 ± 7.18% (NCff+Veh), 35.26 ± 6.6% (NCff+TAM)](Fig. 2b and Supplementary Fig. 13). iBaxnestin mice, like controls, showed negligible levels of freezing behaviour in a distinct context C which shared few features with the training context A [(ANOVA (context C) F(1, 26) <1, Average freezing: 4.89 ± 0.78% (NCff+Veh), 4.03 ± 0.82% (NCff+TAM); comparison of freezing in context A and C, Two-way ANOVA (context) F(1, 52) =38.3, P<0.0001] (Fig. 2b). These results indicate that fear conditioning in both groups was specific to the training context and that increasing adult hippocampal neurogenesis does not impact an animal’s ability to distinguish between two very different contexts. Control experiments using mice only homozygous for the Bax floxed allele (Bax f/f) showed that TAM treatment on its own does not affect contextual fear conditioning [(ANOVA F(1, 29)<1, Average Freezing (context A): 33.8 ± 4.9% (ff+Veh), 28.9 ± 4.7% (ff+TAM); comparison of freezing in context A and C, Two-way ANOVA (context) F(1, 58)=55.23, P<0.0001](Fig. 2b and Supplementary Fig. 13).
Figure 2.
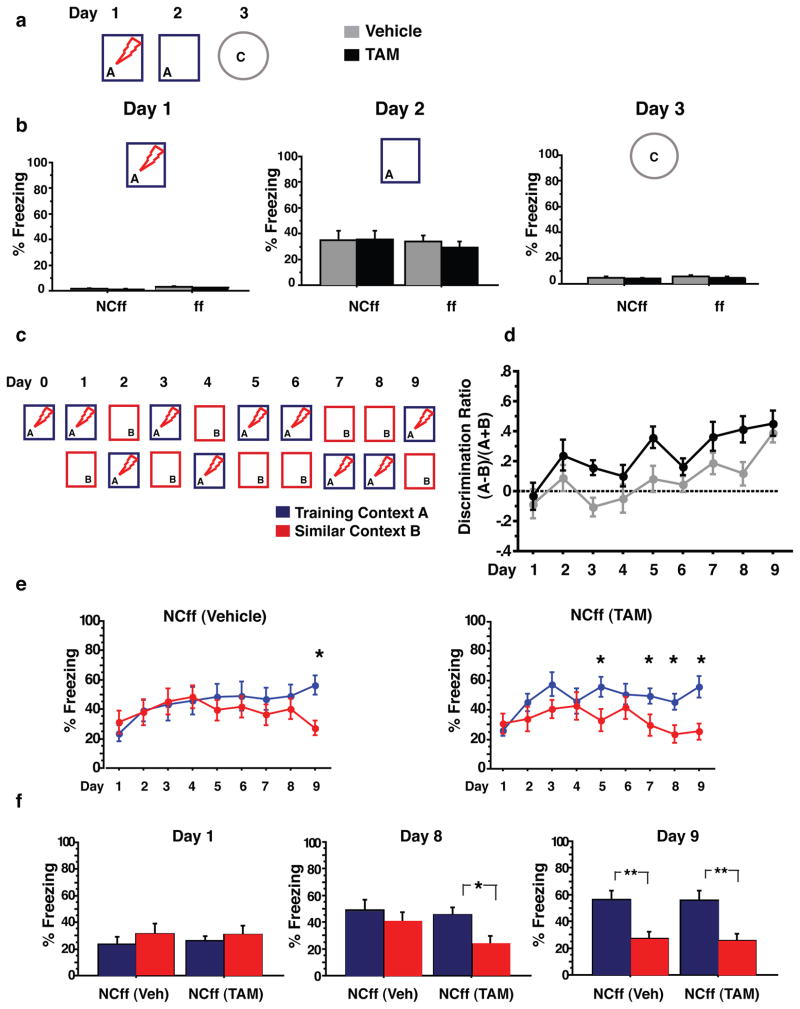
Increasing adult hippocampal neurogenesis is sufficient to improve discrimination between similar contexts. a, Experimental design to test rapid one trial contextual encoding. b, On day 1, both groups show negligible levels of freezing in context A prior to a single 2 second 0.75mA foot-shock delivery. Controls (NCff+Veh)(n=14) and mice with more adult-born neurons (NCff+TAM)(n=14) showed comparable levels of conditioning to the training context A and negligible levels of freezing in a distinct context C. TAM treatment on its own does not affect contextual encoding as reflected in similar levels of freezing of ff+Veh (n=15) and ff+TAM (n=16) mice in contexts A and C. c, Experimental paradigm to test discrimination between two similar contexts A and B. d, Analysis of discrimination ratios. NCff+TAM mice show significantly higher levels of discrimination between the two contexts than NCff+Veh mice. e, Freezing behaviour of mice with increased adult hippocampal neurogenesis and controls over duration of experiment. Although both groups show comparable and extensive generalization between the two contexts at the beginning of the experiment, NCff+TAM mice (n=11) distinguished between context A and B more rapidly than NCff+Veh mice (n=9). f, NCff+Veh mice are able to discriminate between the two contexts by day 9 of testing. *P<0.05, **P<0.01. Results are mean ± SEM.
We next investigated if increasing the number of adult-born neurons impacts a form of learning that requires an animal to distinguish between similar contexts. We chose a contextual fear discrimination learning paradigm as it has been proposed to require pattern separation in the DG-CA3 circuit 25. We first established that this learning paradigm is dependent on adult hippocampal neurogenesis by testing Bax f/f mice in which adult hippocampal, but not subventricular zone neurogenesis, was abolished using hippocampal x-irradiation. Mice lacking adult-born dentate granule neurons were impaired in their ability to distinguish between two similar contexts (Supplementary Fig.14). Next, we examined whether increasing adult hippocampal neurogenesis is sufficient to improve contextual fear discrimination learning (Fig. 2c). On day 1, both iBaxnestin mice and controls showed comparable levels of freezing in contexts A and B suggesting that context B shared enough features with context A to evoke generalization of contextual fear in both groups [(Two-way ANOVA of Context and Treatment, (context) F(1, 18) =1, P=0.3, (treatment) F(1, 18) <1, P=0.85, (context X treatment) F(1, 18) <1, P=0.82 )](Fig 2e, f). However, analysis of discrimination ratios for each animal in both groups over the nine days of testing (see Methods) revealed significantly higher levels of discrimination between the two contexts for iBaxnestin mice compared to controls [(Two-way repeated measures ANOVA of Treatment over days, (treatment) F(1, 18)=6.15, P=0.023, (day) F(1, 18)=9.89, P<0.0001)] (Fig. 2d). Analysis of freezing behaviour of each group in both contexts over days showed that NCff+TAM mice exhibited significantly lower levels of freezing in context B relative to context A four days earlier than the NCff+Veh group [(Two-way repeated measures ANOVA of Context and Day followed by Fisher’s PLSD post hoc tests, NCff+Veh: (context) F(1, 16) <1, P=0.46, (day) F(7, 112) <1, P=0.53, (context X day) F(7, 112) =3.1, P=0.004 and NCff+TAM: (context) F(1, 20) =3.4, P=0.07, (day) F(7, 140) =4.3, P=0.0002, (context X day) F(7, 140) =3.8, P=0.0008)] (Fig.2e,f). Furthermore, iBaxnestin mice exhibited better discrimination than controls in two other versions of the contextual fear discrimination learning paradigm (Supplementary Fig. 15). The enhanced contextual fear discrimination learning of iBaxnestin mice was not accompanied by changes in extinction learning (Supplementary Fig. 16) and increasing adult hippocampal neurogenesis did not facilitate erasure of previously encoded memories 16 (Supplementary Fig. 17). Together, these gain of function studies demonstrate that increasing the number of adult-born neurons is sufficient to enhance contextual fear discrimination learning, suggestive of improved pattern separation.
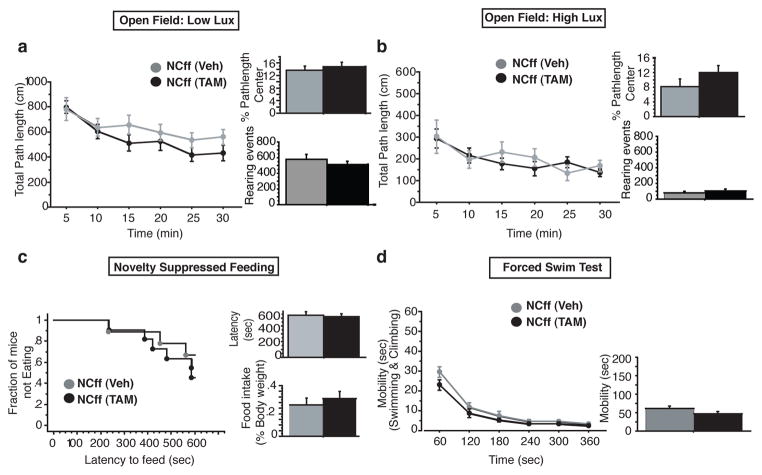
Next, we tested a separate cohort of iBaxnestin and control mice in a range of paradigms that test anxiety-like and depression-like behaviours and which are used to screen for antidepressants. Increasing adult hippocampal neurogenesis did not affect exploratory behaviour (locomotor activity and rearing events) or anxiety-like behaviour in the Open Field, Light-Dark, Elevated-Plus Maze and Novelty Suppressed feeding tests (Fig. 3a–c and Supplementary Fig. 18). No difference in depression-like behaviour between iBaxnestin and control mice was found in the Forced Swim Test (Fig. 3d and Supplementary Fig. 18). As a control, TAM treatment of Bax f/f mice had no effect on anxiety-like and depression-like behaviours (Supplementary Fig. 19). Together with previous studies 8–10, 12, 13, these results suggest that stimulation of adult hippocampal neurogenesis may be necessary, but is not sufficient, to produce the behavioural effects of antidepressants.
Figure 3.
Increasing adult hippocampal neurogenesis does not produce anxiolytic or antidepressant-like behavioural effects. a–b, NCff+Veh and NCff+TAM mice showed comparable locomotor activity, rearing events and anxiety-like behaviour in the Open Field test under two different lighting conditions. c, NCff+Veh and NCff+TAM mice show similar anxiety-like behaviour in the Novelty Suppressed Feeding Paradigm. d, Total mobility of NCff+Veh and NCff+TAM mice did not differ significantly in the Forced Swim Test. n=9–14 mice per group. Results are mean ± SEM.
To determine whether changes in mood may be observed after combining the genetic expansion of adult hippocampal neurogenesis with an environmental intervention known to stimulate network activity and hippocampal neurogenesis, we subjected both iBaxnestin and control mice to a voluntary exercise regimen (Fig. 4a). As expected, voluntary exercise increased adult hippocampal neurogenesis in both groups (compare Fig. 4b with Fig.1c). Following voluntary exercise, iBaxnestin mice, compared to controls, showed a modest increase in the Dcx population and a 4.4 fold increase in the number of surviving adult-born neurons but similar neuronal and glial ratios (Fig. 4b, Supplementary Fig. 20). Surprisingly, iBaxnestin mice displayed a profound increase in exploratory behaviours and decreased anxiety-like behaviour in the Open Field test [(Total Path length: Two-way repeated measures ANOVA, (treatment) F(1, 19)=11.23, P=0.003, (treatment X minute) F(11, 209)=1.9, P=0.03, Rearing events: ANOVA, F(1, 19)=7.54, P=0.01, Percent Path length Center: ANOVA, F(1, 19)=4.5, P=0.04, Time in Center: ANOVA, F(1, 19)=5.01, P=0.037)] (Fig. 4c) but similar levels of locomotor activity in the home cage (Supplementary Fig. 21). The reduction in anxiety-like behaviour exhibited by iBaxnestin mice in the Open Field test may be due to changes in exploratory behaviour rather than anxiety per se since iBaxnestin mice showed normal anxiety-like behaviour in the Light-Dark and Novelty Suppressed Feeding paradigms (Supplementary Fig. 21). Both iBaxnestin and control mice showed similar antidepressant-like behaviour in the Forced Swim Test following voluntary exercise (Supplementary Fig. 21). A change in number of adult-born neurons is unlikely to be solely responsible for the increased exploratory behaviour of iBaxnestin mice since the increase in survival of adult-born neurons in iBaxnestin mice relative to controls following exercise is not much greater than that observed without exercise (Supplementary Fig. 5). Instead, it may be that exercise modifies the properties of the already expanded reservoir of young excitable adult-born neurons in iBaxnestin mice.
Figure 4.
Mice with more adult-born neurons display increased exploratory behaviour and decreased anxiety-like behaviour in the open field test following a voluntary exercise regimen. a, Experimental design. Mice were transferred to cages with running wheels five weeks following Veh/TAM treatment. b, Representative images of Dcx and BrdU (red)/NeuN (green) immunostained coronal hippocampal sections of Veh and TAM treated NCff mice. Quantification of Dcx population. Total Dcx+ neurons: 14527 ± 987 (NCff+Veh), 19893 ± 2022 (NCff+TAM), P=0.05. Number of BrdU+ cells in the GCL: 2119 ± 204 (NCff+Veh), 9324 ± 463 (NCff+TAM), ** P<0.0001. n=4–5 mice per group. c, NCff+TAM mice show significantly greater locomotor activity, reduced anxiety-like behaviour and a significant increase in rearing events in the Open Field test compared to NCff+Veh mice. n=10 (Veh) and n=11 (TAM) mice. *P<0.05, **P<0.01. Results are mean ± SEM. Scale bar 100μm.
Determining the impact of increasing adult hippocampal neurogenesis on cognition and mood is pivotal to defining the therapeutic potential of strategies aimed at stimulating the production of new dentate granule neurons in the adult brain. Here we have shown that selectively increasing the survival of adult-born neurons improves cognitive performance when an animal must distinguish between two similar contexts. In contrast, cognitive gains are not produced when the contexts are very different and in other forms of learning such as object recognition or spatial and reversal learning. Our findings are consistent with the proposed role of the DG in pattern separation 16.
Our studies in the domain of mood regulation reveal a previously unexpected dissociation between the effects of increasing adult hippocampal neurogenesis on learning and mood. Although iBaxnestin mice show enhanced pattern separation, they do not show anxiolytic or antidepressant-like behaviours. Since iBaxnestin mice show only a subset of modifications induced by chronic antidepressant treatment such as enhanced survival of adult-born neurons and increased neurogenesis-dependent LTP 7, 8, 24, it is likely that other antidepressant-dependent modifications of neural circuitry 24, 26, 27 act in concert with increased adult hippocampal neurogenesis to produce the behavioural effects of antidepressants. Consistent with this logic, increasing adult hippocampal neurogenesis enhances exploratory behaviour only when combined with voluntary exercise, but not under baseline conditions. This result may reflect a role for the DG in modulation of exploratory behaviour 28.
Recent studies have found pattern separation-impairments during normal aging in humans14, 15. Deficits in pattern separation may impact not only learning but also anxiety-related behaviours. In fact, impaired contextual fear discrimination (pattern separation) may result in a bias to encode ambiguous cues as threatening and may underlie the excessive generalization observed in post traumatic stress disorder and panic disorder 29, 30. Stimulating adult hippocampal neurogenesis may therefore represent a novel therapeutic strategy for treating these anxiety disorders as well as age related memory impairments.
Methods Summary
Transgenic and conditional mouse lines were used to recombine Bax in stem cells in the adult brain (Dranovsky et al., manuscript in preparation)21. The impact of Bax ablation in stem cell on adult hippocampal neurogenesis and morphological maturation of adult-born neurons was characterized using various genetic reporter lines in combination with BrdU pulse-chase labeling and standard immunohistochemistry techniques and details can be found in Methods. Assessment of LTP at medial perforant path-granule cell synapses was performed as described previously 23. Focal hippocampal x-irradiation was performed 23 using sodium pentobarbital as the anaesthetic agent. Behavioural testing included hippocampal dependent learning paradigms (contextual fear conditioning, contextual fear discrimination learning 25, object recognition, spatial and reversal learning) and tests for anxiety-like and depression-like behaviour. Experimental protocols were approved by the Institutional Animal Care and Use Committee at Columbia University and the New York State Psychiatric Institute. Details for all experimental techniques used in this study are available in Methods.
Methods
Generation of mouse lines
The generation and characterization of the Nestin CreERT2 transgenic mouse line is described in detail elsewhere (Dranovsky, A., et al., manuscript in preparation). NCff or Nestin CreERT2; Bax f/f mice were generated by interbreeding Nestin CreERT2; Bax f/f and Bax f/f mice. Bax f/f mice generated from these crosses were used to assess CreERT2 -independent effects of tamoxifen (TAM) on behaviour. An enhanced yellow fluorescent protein (YFP) reporter line (ROSA26 floxed stopYFP)31 was used in all experiments in which adult-born neurons were inducibly genetically labelled. Specifically, NCffY and NCY mice were generated as littermates from interbreeding Nestin CreERT2; Bax f/+; ROSA26 f STOP YFP/+ and Bax f/+; ROSA26 f STOP YFP/+ mice. NCff mice were maintained on a mixed (C57BL/6 -129/SvEv) genetic background. The POMC-τ-eGFP transgenic mouse line was obtained from GENSAT (http://www.gensat.org) and used to generate NCff; POMC-τ-eGFP mice. To induce CreERT2 mediated recombination of Bax and/or YFP in neural stem cells in the adult brain, mice (NCff/NCY/NCffY/NCff;POMC-τ-eGFP, at least 8 weeks of age) were given 2 mg of TAM, once a day, intraperitoneally for 5 consecutive days. 10 mg/ml Tamoxifen (Sigma, T-5648) solution was prepared in corn oil containing ten percent ethanol. For Vehicle, an identical volume of corn oil with ten percent ethanol was injected intraperitoneally, once a day for 5 consecutive days. Mice were housed four to five per cage in a 12 hour (06:00–18:00) light-dark colony room at 22 Celsius and had free access to food and water. For the voluntary exercise regimen, four to five mice were housed per cage (29.2×19×12.7 cm), each equipped with two running wheels. Experimental protocols were approved by the Institutional Animal Care and Use Committee at Columbia University and the New York State Psychiatric Institute.
Electrophysiological recordings
Electrophysiological recordings in the DG were performed as previously described 32, 33. Brains were collected from animals after deep anesthesia with halothane and decapitation, and transverse hippocampal slices (400 μm) were prepared using a vibratome. The slices were incubated in an interface chamber at 32°C and perfused with oxygenated artificial cerebrospinal fluid (ACSF) (in mM: 119 NaCl, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 26.2 NaHCO3, 1 NaH2PO4, and 11 glucose). Slices were allowed to equilibrate for 2 h before positioning the electrodes and beginning stimulation. To record from the DG, the medial perforant path (MPP) was stimulated using a World Precision Instruments (Sarasota, FL) stimulation isolation unit and a bipolar tungsten electrode. Evoked potentials were recorded in the molecular layer above the upper blade of the DG using a glass capillary microelectrode filled with artificial CSF (tip resistance of 1–3 MΩ). Isolation of the MPP was confirmed by assessing paired-pulse depression (PPD) of the MPP/DG synaptic connection at 50 ms, which generated the highest level of depression. Input output curves were obtained after 10 min of stable recordings. The stimulation intensity that produced one-third of the maximal response was used for the test pulses and tetanus. After 15 min of stable baseline response to test stimulation (once every 20 s), the ability to elicit LTP was assessed. LTP was induced with a weak stimulation paradigm consisting of four trains of 1 s each, 100 Hz within the train, repeated every 15 s 32. Responses were recorded every 20 s for 60 min after LTP induction. A similar protocol was used to elicit and record LTP of mature dentate granule neurons except that 10 μM bicuculline (Bicuculline methobromide, Sigma B7561) was added to the ACSF to block GABAA receptor-mediated inhibition.
Immunohistochemistry and confocal microscopy
For assessment of survival of adult-born neurons in the DG, BrdU was administered once a day intraperitoneally in 0.9% NaCl for 2 or 10 days at 150mg/Kg body weight. Mice were anesthetized with ketamine/xylazine (100 and 7 mg/kg, respectively) and transcardially perfused (cold saline, followed by 4% cold paraformaldehyde in PBS). Brains were postfixed overnight in 4% paraformaldehyde at 4°C, then cryoprotected in 30% sucrose, and stored at 4°C. 40 μm coronal serial sections of the entire hippocampus and 40μm sagittal sections of the olfactory bulb were obtained using a cryostat and stored in PBS. For BrdU/NeuN immunohistochemistry, sections were mounted onto charged glass slides (Superfrost Plus). Following pretreatment with 10mM citrate buffer, sections were subjected to antigen retrieval in 10mM citrate buffer using a boiling protocol. After cooling to room temperature (RT), sections were rinsed three times in PBS and blocked in PBS with 0.3% Triton and 10% normal donkey serum (NDS) for 2 hours at RT. Incubation with primary antibodies was carried out at 4° C overnight (BrdU, rat; 1:100; Serotec, Oxford, UK; neuronal-specific nuclear protein NeuN, mouse; 1:500; Chemicon, Temecula, CA). Fluorescence coupled secondary antibodies (Jackson ImmunoResearch,West Grove, PA) were used at a final concentration of 1:400 in PBS. For GFAP, NeuN and YFP triple immunohistochemistry, floating sections were used. Briefly, sections were washed 3 times in PBS, blocked in PBS buffer containing 0.3% triton and 10% NDS and incubated in primary antibodies overnight shaking at 4° C (GFAP, rabbit 1:2000, DAKO, Denmark; NeuN, mouse; 1:500; Chemicon, Temecula, CA; GFP, chicken 1:500, Abcam, Cambridge, MA). The next day sections were washed 3 times in PBS and incubated with fluorescence coupled secondary antibodies (Jackson ImmunoResearch ,West Grove, PA) for two hours at RT. For GFP immunohistochemistry alone (Rabbit 1:500, Invitrogen, Eugene, OR) was used. For Calbindin immunohistochemistry, a similar protocol was used (mouse anti-Calbindin (1:5000, Swant, Bellinzona, Switzerland). For Doublecortin and Ki67 immunohistochemistry, floating sections were first quenched to remove endogenous peroxidase activity (1% H202 in PBS:Methanol). Sections were then washed in PBS, blocked (PBS containing 0.3% triton and 10% NDS) and incubated with primary antibody overnight at 4° C (DCx, goat, 1:500, SantaCruz, CA; Ki67, rabbit, 1:100, Vector, Burlingame, CA). Following washes in PBS, sections were incubated with horse radish peroxidase coupled biotinylated secondaries. Following incubation with ABC solution (Vector, Burlingame, CA), the color reaction was carried out using a DAB kit (Vector, Burlingame, CA). An unbiased and blinded quantification protocol was used to quantify Doublecortin and BrdU positive cells in the granule cell layer of the DG along the septo-temporal axis 33. For quantification of survival of adult-born cells in the main olfactory bulb, two high magnification (20X) images of randomly selected regions in the granule cell layer were obtained from six matched sagittal sections for each mouse. BrdU positive cells were quantified using a cell counter plugin in NIH ImageJ and surface density was computed. Bright-field images were obtained using a Zeiss (Oberkochen, Germany) Axioplan-2 upright microscope. For quantification of YFP+ neurons in NCY and NCffY mice, 5–6 (dorsal) and 3 (ventral) matched sections were selected and the average number of YFP+ neurons per section was computed. Type I neural stem cells expressing YFP were not included in the analysis. All analyses of mice with the inducible genetic reporter ROSA26 floxed stopYFP were performed at six weeks post Veh/TAM injection. Phenotyping of BrdU expressing cells in the granule cell layer of the DG entailed scanning of at least 80 cells from the dorsal and ventral hippocampus of each mouse using a Zeiss LSM 510 META scanning confocal microscope. Z-stack analysis was performed using LSM510 image browser to determine the number of BrdU positive cells expressing GFAP or NeuN. To compute the percentage of Dcx expressing cells that also expressed YFP in NCffY mice, approximately 120 Dcx+ neurons per mouse DG were scanned using an Olympus Fluoview FV1000 (40X (N.A=1.3) confocal microscope. Z-stack analysis was performed using Fluoview 1000 v1.5 software to determine the number of Dcx +neurons expressing YFP. 3 mice were used for the analysis. A one in six series of adjacent sections stained with nuclear Fast Red (Vector) was used to measure dentate gyrus granule cell layer volume.
Quantification of dentate gyrus granule cell layer volume and mossy fiber length
Surface area of the granule cell layer from 10X images of hippocampal sections spanning the septo-temporal axis were traced in ImageJ and the volume was determined by multiplying the surface area of the granule cell layer by the distance between sections sampled (240 μm). 4 mice per group were used for this analysis. To measure the length of axons of young adult-born neurons, we used NCff; POMC-τ-eGFP mice in which axons are genetically labeled with eGFP. Mossy fiber length was determined by tracing the stratum lucidum along the inner edge of the stratum pyramidale. For measurements, the starting point was the intersection of the trace and a line between the tip of the inner and outer blades of the dentate gyrus 34. 4 dorsal sections from each mouse were used for these measurements.
Sholl analysis
5–6 YFP+ neurons with complex dendritic trees were chosen from each mouse (dorsal and ventral DG) and scanned using an Olympus Fluoview FV1000 (40X (N.A=1.3) or 60X (N.A 1.42). Images of collapsed z-stacks were imported into Adobe Illustrator CS3 and dendritic trees were reconstructed using the tracing tool. Dendritic complexity was analyzed from 8 bit images using the ImageJ Sholl Analysis Plugin (http://www-biology.ucsd.edu/labs/ghosh/software/). The centre of all concentric circles defined as the centre of cell soma. Parameters used were: starting radius (10μm), ending radius (300 μm from the centre), Interval between consecutive radii (10μm). 3–4 mice per group were used.
Focal x-irradiation of hippocampus
10 weeks old “Bax ff” mice were anesthetized with sodium pentobarbital (administered intraperitoneally at 42mg/Kg body weight, once a day for three days, each injection spaced apart by 3–4 days), placed in a stereotaxic frame and exposed to cranial irradiation using a Siemens Stabilopan X-ray system operated at 300 kVp and 20 mA. Animals were protected with a lead shield that covered the entire body, but left unshielded a 3.22 X 11-mm treatment field above the hippocampus (interaural 3.00 to 0.00) exposed to X- Ray. Dosimetry was done using a Capintec Model PR06G electrometer ionization chamber and Kodak Readypack Radiographic XV films. The corrected dose rate was approximately 1.8 Gy per min at a source to skin distance of 30 cm. The procedure lasted 2 min and 47 sec, delivering a total of 5 Gy. Three 5 Gy doses were delivered on days 1, 4 and 8. Behavioural testing was carried out 4 months following hippocampal x-irradiation.
Behavioural testing
Behavioural testing was performed using male and female mice that were 14–18 weeks of age at time of testing, unless otherwise specified. All experiments and analyses were performed blind to genotype or treatment.
Tests for anxiety-like and depression-like behaviors
Testing in the open field test, light-dark test, elevated plus maze, novelty-suppressed feeding, and forced swim test paradigms was carried out at eight or ten weeks following Veh/TAM treatment. The open field is a standard test of both anxiety and locomotor behavior. It consists of a simple square enclosure that is equipped with infrared detectors to track animal movement in the horizontal and vertical planes. Measures of total distance travelled and rearing events are used as an index of exploratory activity 35 while the proportion of time or distance spent in the center is construed as a measure of anxiety-like behavior. Mice were placed into the corner of the open field and activity was recorded for 30 or 60 minutes. Testing took place either under low (200 lux) or bright lighting conditions (1000–1200 lux). The novelty suppressed feeding test has been validated as a paradigm that is sensitive to chronic, but not acute antidepressant treatment 36. Mice were food deprived in their home cages 24h–26h prior to testing. The testing apparatus consisted of a plastic arena (45cm-l X 15cm-h X 30cm-w) whose floor was covered with approximately 2 cm of wood chip bedding. A single food pellet (familiar laboratory mouse chow) was placed on a circular piece of white filter paper (12 cm diameter) positioned in the center of the arena. The test begins with a mouse being placed in a corner of the arena and the latency to approach the pellet and begin feeding is recorded (maximum time 10 min). Testing was carried out under bright light conditions (1000–1200 Lux). Each mouse was weighed prior to food deprivation and just prior to testing to assess change in body weight. Immediately after the test, each mouse was transferred to its home cage and the amount of food consumed in 5 min was measured. When appropriate, survival analysis was performed and statistical differences between latencies were determined using the Kaplan-Meier product-limit method. The elevated plus maze 32 and the Light-Dark test 37 was done as performed previously. For the Forced swim test, mice were placed in transparent plastic buckets (19 cm diameter, 23 cm deep) filled with 23°– 25°C water for 6 minutes and the animal's behaviour was recorded using an automated video-tracking system. Testing was carried out over two consecutive days with the first day serving the purpose of pre-exposure. Mobility (swimming and climbing behaviors) on the second day was analyzed using ViewPoint Life Sciences Software (Montreal, Canada). No differences in swimming and climbing behaviours were observed between treatment groups on day 1 (NCff: Veh vs TAM or ff:Veh vs TAM).
Object Recognition Paradigm
Separate cohorts of mice were tested eight weeks following Bax ablation in neural stem cells for similar/novel object recognition behaviors. NCff mice were tested in the novel/similar object recognition paradigm eight weeks following TAM/Veh treatment. Testing entailed placing mice in an arena (45cm-l X 15cm-h X 30cm-w) with two distinct objects in seven sessions (each seven minutes) spaced apart by a three minute inter trial interval. Mice habituated to the objects during sessions 1–6 and one of the objects was replaced with a novel or a similar object in session 7. Objects and object positions were counterbalanced during testing. Objects selected for testing elicited comparable levels of exploration and were categorized as novel or similar based on exploration levels evoked in NCff mice in pilot experiments. Sessions were video recorded and videos were manually scored for locomotion (grid crossings) and object exploration (when animal’s snout was 2cm or less from object).
Spatial and reversal learning
A cohort of mice was tested eight weeks following Bax ablation in neural stem cells. The reference version of the morris water maze was performed as described 38. The task was performed with three training phases executed in succession: Visible platform (2 days), Acquisition phase (4 days) with a hidden platform in the training quadrant (Q3), Transfer/Reversal Phase (reversal learning, 4 days) with the hidden platform in the opposite quadrant (Q1). Each phase comprised of four trials (120 s maximum, 15-min Inter trial interval) per day. The start location was in a different quadrant in each trial such that no single start location was used on consecutive trials. Shaping was carried out before the first trial of the Visible platform and the Acquisition phases. A Probe trial (60 s, no platform) was performed 24 hours after the last trial of the acquisition and transfer phase. The animals' trajectories were recorded with a videotracking system (HVS Image Analyzing VP-118).
Active Place Avoidance
Spatial learning was also tested using an active place avoidance task, which is sensitive to hippocampal dysfunction 39. The place avoidance training apparatus consists of a slowly rotating (clockwise, 1rpm) circular platform (40cm diameter) within which a non rotating 60° region of the room is a shock zone (delineated in red, Supplementary Fig. 12). Visual cues are located on the walls of the room. Mice walk freely on the rotating platform and learn to avoid the shock zone based on the visual cues. When the mouse enters the shock zone, it receives a brief constant current footshock (500ms, 60Hz, 0.2mA) that is scrambled across pairs of parallel rods located on the platform floor. Additional shocks of the same intensity and duration are administered every 1.5 seconds until the mouse leaves the shock zone. The position of the mouse is tracked by PC-based software that analyzes images from an overhead camera and delivers shocks appropriately (Tracker, Bio-Signal Group Corp., Brooklyn, NY). Track Analysis software is used to compute the number of times each animal enters the shock zone and the number of shocks administered. On the first day of the experiment, mice walked freely on the rotating platform for 10 minutes while the shock was turned off (Pretraining). Then the shock was turned on and mice were given three 10-minute training trials with an inter-trial interval of 50 minutes, for two days (Trials 1–6).
One-trial contextual fear conditioning
Mice were tested eight weeks following Bax ablation in neural stem cells. Conditioning was conducted on one side of a Med-Associates shuttle box (ENV-010MC; 20.3cm X 15.9 cm X 21.3cm high) with a clear plexiglass wall, 3 aluminium walls and a stainless steel grid as a floor. The chamber was lit from above with a houselight (CM1820 bulb), ventilated with a house fan and encased by a sound-dampening cubicle. On the days of testing, mice were brought out of the vivarium and allowed to habituate for an hour outside the testing room prior to starting the experiment. Mouse behaviour was recorded by digital video cameras mounted above the conditioning chamber. Freezeframe and Freezeview software (Actimetrics, Evanston, IL) were used for recording and analyzing freezing behaviour, respectively. The one-trial contextual fear conditioning protocol entailed delivery of a single 2 second foot shock of 0.75mA, 185 seconds following placement of the mouse in the training context. The mouse was taken out 15 seconds following termination of the foot shock and returned to its home cage. For the training context A, the house fan and lights were switched on, stainless steel grids were exposed and a mild lemon scent was used as an olfactory cue. 70% ethanol was used to clean grids in between runs. For the distinct context C, the stainless steel grid floor was covered with a plastic panel and cage bedding. The chamber walls were covered using plastic inserts and the house fan and lights were turned off. The chamber door was left ajar during testing. A mild anise scent was used as an olfactory cue and a non-alcoholic antiseptic was used to clean the chamber in between runs. Mice were brought into the testing room in cardboard buckets by a different handler and the testing room was dimly lit prior to placement of the mice in the testing chambers. The one-trial contextual fear conditioning protocol was used for extinction learning and memory-clearance experiments. Only males were used for these studies.
Contextual fear discrimination learning
Mice were tested eight weeks following Bax ablation in neural stem cells. This paradigm tests the animal's ability to distinguish between two similar contexts, conditions that are most likely to recruit the DG 40. The shock associated training context A and the similar (no-shock) context B shared many features including an exposed stainless steel grid floor (a salient feature of the context) and roof. The similar context differed from the training context in that two plastic inserts were used to cover the walls, the house fan and lights were turned off and the chamber door was left ajar during testing. A mild mint scent was used as an olfactory cue and a non-alcoholic antiseptic was used to clean the grids in between runs. Mice were brought into the testing room in buckets by the same experimenter who handled the mice for the training context. In pilot experiments, the similar context was found to evoke comparable levels of freezing behavior as that observed in the training context, indicative of extensive generalization (pattern completion) between the two contexts. For discrimination learning, mice were exposed to the training context in which they received a single 2 second foot shock of 0.75mA, 185 seconds following placement in the chamber. Mice were taken out of the chamber 15 seconds following termination of the foot shock and returned to their home cage. One hour later, mice were placed in the similar context in which they were left for 180 seconds and were never shocked. Measurement of freezing levels in both training context (three minutes pre shock) and in the similar context (three minutes) each day allowed assessment of discrimination between the two contexts and was computed as a Discrimination ratio:(Freezing Training context - Freezing similar context)/(Freezing Training context + Freezing similar context). A score of 0 indicates complete lack of discrimination i.e. freezing levels are the same in the similar and training contexts (Freezing similar context = Freezing Training context), whereas a score of 1 indicates perfect discrimination i.e. freezing-level in the similar context is zero (Freezing similar context =0). Only males were used for these experiments.
Home cage activity
Animal behavior was recorded for 15 minutes in the home cage and videos were manually scored for locomotion (grid crossings).
Statistical analysis
Statistical analysis was carried out using Statview software or Microsoft Excel. Statistical significance was assessed by unpaired two-tailed student's t-tests or analysis of variance (ANOVA). Significant main effects or interactions were followed up with Fisher’s predicted least-square difference (PLSD) post hoc tests, where appropriate. * p<0.05, **p<0.01.
Supplementary Material
Acknowledgments
We thank Joshua Gordon, Stefano Fusi, Christoph Kellendonk, Helen Scharfman and members of the Hen laboratory for comments on the manuscript and insightful discussions on the project. We thank Elias Pavlopoulos for consultation on spatial learning and Michael Drew and Christine Ann Denny for input on the novel object recognition paradigm. A.S was supported by a 2009 NIMH Grant 1K99MH86615-01, 2006 and 2008 NARSAD Young Investigator Award and 2008 Sackler Institute of Columbia University Award. K.S was supported by Ruth L. Kirschstein National Research Service Awards (NRSA) for Individual Predoctoral Fellows (F31). R.H was supported by NARSAD, New York Stem Cell Initiative (NYSTEM) and R01 MH068542 grants.
Footnotes
The authors declare competing financial interests. R.H is a consultant to Brain Cells Inc. and Astra Zeneca.
Supplementary information accompanies this manuscript and is comprised of 21 supplementary figures.
Author contributions. A.S conceived and designed the experiments, performed the circuitry analysis and behaviour experiments, analyzed the data and wrote the manuscript. K.N.S and A.S.H contributed to behavioural testing and circuitry analysis. C.M.O'C and M.A.K performed the electrophysiological experiments. A.D contributed the Nestin CreERT2 transgenic mouse line used in this study. N.S.B assisted with focal x-irradiation of the mice and performed the active place avoidance experiment in the laboratory of Andre Fenton. R.H oversaw the overall execution of the project, contributed to experimental design and interpretation of the data, provided financial support and helped write the manuscript. All authors discussed the results and commented on the manuscript.
References
- 1.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 3.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imayoshi I, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 5.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 6.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 7.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.David DJ, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 13.Bessa JM, et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14:764–773. 739. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- 14.Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learning & memory. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- 15.Yassa M, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2010 doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treves A, Tashiro A, Witter ME, Moser EI. What is the mammalian dentate gyrus good for? Neuroscience. 2008;154:1155–1172. doi: 10.1016/j.neuroscience.2008.04.073. [DOI] [PubMed] [Google Scholar]
- 17.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen AC, Shirayama Y, Shin KH, Neve RL, Duman RS. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol Psychiatry. 2001;49:753–762. doi: 10.1016/s0006-3223(00)01114-8. [DOI] [PubMed] [Google Scholar]
- 19.Tronel S, et al. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2010 doi: 10.1002/hipo.20895. [DOI] [PubMed] [Google Scholar]
- 20.Sun W, et al. Programmed cell death of adult-generated hippocampal neurons is mediated by the proapoptotic gene Bax. J Neurosci. 2004;24:11205–11213. doi: 10.1523/JNEUROSCI.1436-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeuchi O, et al. Essential role of BAX,BAK in B cell homeostasis and prevention of autoimmune disease. Proc Natl Acad Sci U S A. 2005;102:11272–11277. doi: 10.1073/pnas.0504783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 23.Saxe MD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McHugh TJ, et al. Dentate Gyrus NMDA Receptors Mediate Rapid Pattern Separation in the Hippocampal Network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 27.Maya Vetencourt JF, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 28.Saab BJ, et al. NCS-1 in the dentate gyrus promotes exploration, synaptic plasticity, and rapid acquisition of spatial memory. Neuron. 2009;63:643–656. doi: 10.1016/j.neuron.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- 30.Lissek S, et al. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am J Psychiatry. 2010;167:47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saxe MD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lever C, Burton S, O'Keefe J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev Neurosci. 2006;17:111–133. doi: 10.1515/revneuro.2006.17.1-2.111. [DOI] [PubMed] [Google Scholar]
- 36.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 37.Scobie KN, et al. Kruppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis. J Neurosci. 2009;29:9875–9887. doi: 10.1523/JNEUROSCI.2260-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholls RE, et al. Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron. 2008;58:104–117. doi: 10.1016/j.neuron.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 39.Cimadevilla JM, Wesierska M, Fenton AA, Bures J. Inactivating one hippocampus impairs avoidance of a stable room-defined place during dissociation of arena cues from room cues by rotation of the arena. Proc Natl Acad Sci U S A. 2001;98:3531–3536. doi: 10.1073/pnas.051628398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McHugh TJ, et al. Dentate Gyrus NMDA Receptors Mediate Rapid Pattern Separation in the Hippocampal Network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.