Abstract
Bone is a composite material, in which collagen fibrils form a scaffold for a highly organized arrangement of uniaxially oriented apatite crystals1,2. In the periodic 67 nm cross-striated pattern of the collagen fibril3–5, the less dense 40-nm-long gap zone has been implicated as the place where apatite crystals nucleate from an amorphous phase, and subsequently grow6–9. This process is believed to be directed by highly acidic non-collagenous proteins6,7,9–11; however, the role of the collagen matrix12–14 during bone apatite mineralization remains unknown. Here, combining nanometre-scale resolution cryogenic transmission electron microscopy and cryogenic electron tomography15 with molecular modelling, we show that collagen functions in synergy with inhibitors of hydroxyapatite nucleation to actively control mineralization. The positive net charge close to the C-terminal end of the collagen molecules promotes the infiltration of the fibrils with amorphous calcium phosphate (ACP). Furthermore, the clusters of charged amino acids, both in gap and overlap regions, form nucleation sites controlling the conversion of ACP into a parallel array of oriented apatite crystals. We developed a model describing the mechanisms through which the structure, supramolecular assembly and charge distribution of collagen can control mineralization in the presence of inhibitors of hydroxyapatite nucleation.
The role of the collagen matrix12–14 during the infiltration of the fibrils with amorphous calcium phosphate (ACP) and its subsequent transformation into oriented crystals of apatite is still unknown. Although crystal nucleation is believed to be directed by non-collagenous proteins6,7,9–11 (NCPs), collagen has also been proposed to nucleate apatite13,14. So far, however, this hypothesis could not be experimentally substantiated, and collagen is generally considered a passive scaffold and template for mineral formation16,17. Providing experimental evidence for the role of collagen in guiding mineral formation requires monitoring the mineralization of the collagen matrix at the molecular level. Investigating details of collagen mineralization using in vivo models has been proved very challenging owing to the complexity of the biological systems6,7,9. Recently, in vitro collagen mineralization was achieved by substituting the NCPs with either polyaspartic acid (pAsp) or fetuin, both inhibitors of hydroxyapatite crystallization18–20. These additives were shown to be instrumental in the intrafibrillar formation of oriented apatite crystals, exhibiting X-ray and electron diffraction patterns similar to those of bone apatite. Combining these in vitro systems with cryogenic transmission electron microscopy (cryoTEM), cryogenic electron tomography and low-dose selected-area electron diffraction (LDSAED) we studied collagen mineralization with nanometre-scale resolution, applying plunge-freeze vitrification to ensure the close-to-native preservation of the molecular structures15.
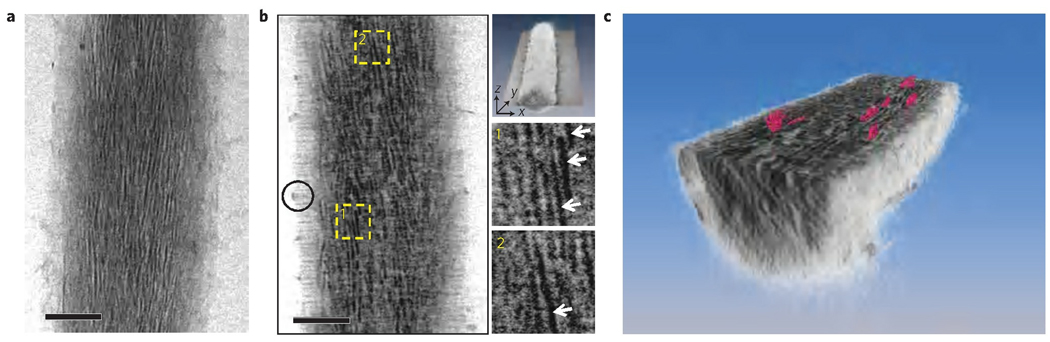
Type I collagen from horse tendon was reconstituted into isolated fibrils on TEM grids and incubated in buffered mineralization solutions containing CaCl2, K2HPO4 and pAsp, as described previously19 (Supplementary S1 and S2, Fig. S1). After 72 h, cryogenic electron tomography of mineralized collagen showed the presence of plate-shaped crystals (2–5 nm thick, 15–55 nm long and 5–25 nm wide) inside the collagen fibril (Fig. 1 and Supplementary S3 and S4, Fig. S2), consistent with what is found in bone19. Control experiments without additives resulted in apatite crystals randomly formed in solution and on the surface of the fibrils (Supplementary S5, Fig. S3).
Figure 1. Cryo-electron tomography of a collagen fibril mineralized in the presence of 10 µg ml−1 of pAsp for 72 h and stained with uranyl acetate.
a, Two-dimensional cryoTEM image. b, Slice from a section of the three-dimensional volume along the xy plane (top-most inset), where crystals are visible edge-on (insets 1 and 2, white arrows). Black circle: ACP infiltrating the fibril (see below). c, Computer-generated three-dimensional visualization of mineralized collagen. The fibril is sectioned through the xy plane, revealing plate-shaped apatite crystals (coloured in pink) embedded in the collagen matrix. Scale bars: 100 nm.
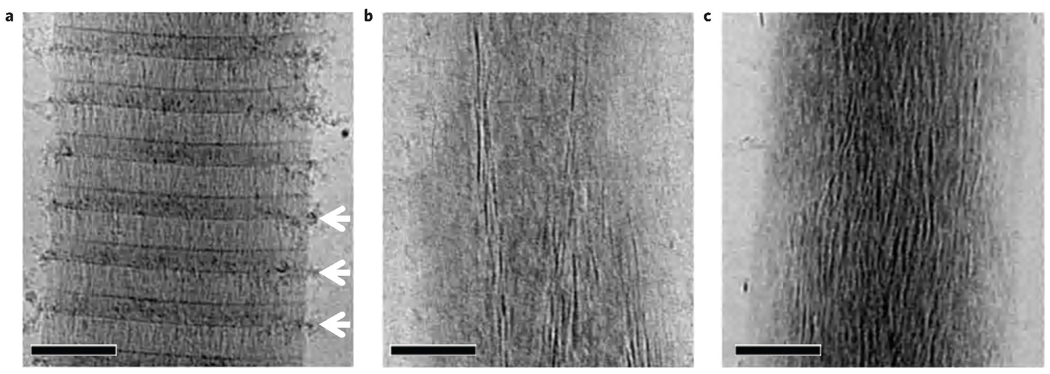
To investigate how the apatite crystals form inside the fibril, we carried out a time-resolved study starting from the earliest stages of mineral formation. After 24 h of mineralization calcium phosphate particles were found outside the fibril, associated with the overlap region, in close proximity to the gap zone (Fig. 2a). Cryogenic energy-dispersive X-ray spectroscopy confirmed that these precipitates are indeed composed of calcium phosphate, and LDSAED showed a diffuse band characteristic of ACP (Supplementary S6, Fig. S4). After 48 h, apatite crystals started to develop within a bed of ACP (Fig. 2b, Supplementary S6, Fig. S5) and after 72 h, elongated electron-dense crystals were abundant within the fibril, in many cases still embedded within a less dense matrix (Fig. 2c). LDSAED demonstrated that the mineral phase consisted of both ACP and oriented apatite, the latter identical to bone apatite19 (Supplementary S6, Fig. S5). As observed previously21, the collagen fibrils expanded in the direction perpendicular to its long axis, becoming deformed by the developing mineral (Supplementary S7, Fig. S6).
Figure 2. CryoTEM images of collagen at different stages of mineralization in the presence of 10 µg ml−1 of pAsp.
a, Mineralization for 24 h. b, Mineralization for 48 h. c, Mineralization for 72 h. Scale bars: 100 nm.
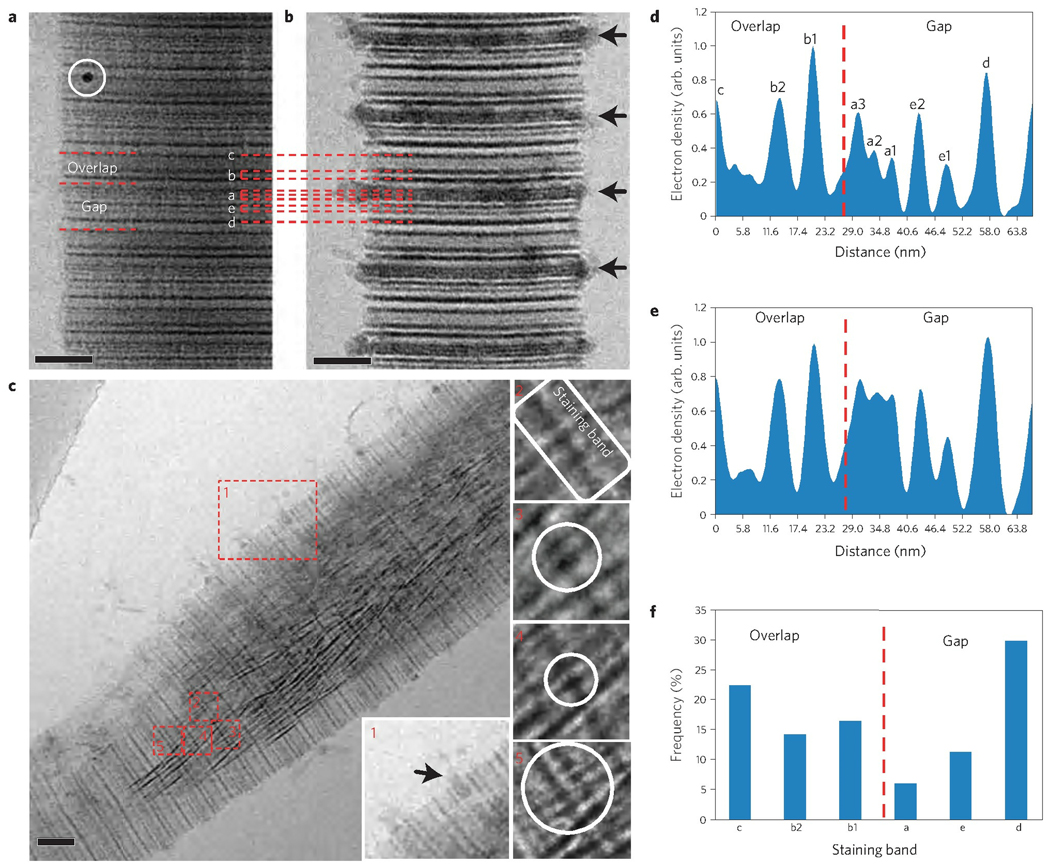
To understand the role of collagen in controlling mineral formation at the molecular level, we correlated our observations with the ultrastructure of the collagen fibril. For this we combined cryoTEM with uranyl acetate staining22, where uranyl acetate binds to both the negatively and positively charged amino acids in the fibrils, thus significantly increasing the local mass density, and hence the image contrast23,24. The resulting staining pattern was consistent with previous studies using conventional TEM; that is, the banding pattern along the 67 nm repeat matched with the positions of the charged amino acids in the crystal structure of collagen5,24 (Fig. 3a,b, Supplementary S8, Fig. S7). Furthermore, the staining did not affect the mineral phase, because the same degree of mineralization was observed as for unstained samples (Fig. 2).
Figure 3. Uranyl acetate map of the different stages of collagen mineralization in the presence of 10 µg ml−1 of pAsp.
a, CryoTEM image of stained, non-mineralized collagen. Staining bands are labelled according to ref. 24. White circle: 10 nm gold marker for electron tomography. b, CryoTEM image of stained collagen, mineralized for 24 h. Calcium phosphate is associated to the fibril in a regular pattern, following the staining bands (black arrows). Peaks are labelled corresponding to their respective staining band. c, CryoTEM image of a stained fibril mineralized for 48 h. Apatite crystals are found within an amorphous calcium phosphate bed, which can still be seen infiltrating into the fibril through the a-band (inset 1, black arrow). Insets 2–5 show crystals nucleating on the staining bands. d, Intensity profile of a, non-mineralized collagen. e, Intensity profile of b, collagen mineralized for 24 h. f, Histogram of the distribution of the number of nucleating crystals per staining band. Scale bars: 50 nm.
After 24 h of mineralization, ACP was observed surrounding and entering the fibril, associated to the a-bands (Fig. 1b, black circle and Fig. 3b–e). These bands are ~9 nm wide and span both the overlap and gap zones at the C-terminal region of the collagen molecules14. Analysis of the intensity profile shows the increase and the broadening of the peaks corresponding to the a-bands, such that the a1 to a3 bands fused and became almost indistinguishable (Fig. 3e).
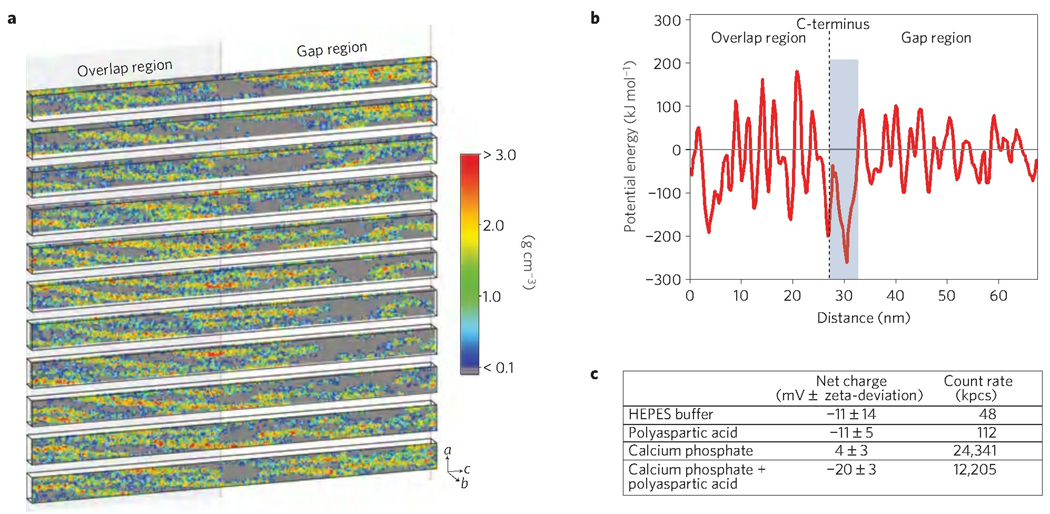
The infiltration of mineral into the fibril through the a-band region is not dependent on the availability of space. Gaps within the microfibril are present throughout the whole 67 nm repeat, both in the gap and overlap regions13 (Fig. 4a), and could provide entry sites for the mineral phase into the fibril. Therefore, the site-specific localization of mineral infiltration must result from a specific interaction between the amorphous mineral phase and the collagen at this location. Indeed, whereas ACP-polyasp forms a negatively charged complex (Fig. 4c), the gaps within the a-band region that serve as entry sites for the ACP into the fibril are located within a 6 nm domain of high positive net charge (Supplementary S8, Fig. S8). This site possesses the lowest electrostatic potential energy in the microfibril for interaction with the negatively charged complex (Fig. 4b) and is therefore the most favourable region for an attractive interaction with the negatively charged complex. This suggests that the attraction between these positively charged sites and the negatively charged calcium phosphate–pAsp complex plays a critical role in mediating the entry of the ACP into the collagen.
Figure 4. Analysis of the mass density and electrostatic potential energy of a microfibril, based on the crystal structure5.
a, Mass density map of a microfibril, depicting slices through the b axis of the crystal unit cell. Grey areas demonstrate the space within the microfibril through which the mineral phase could potentially diffuse. b, Electrostatic potential energy of the empty voxels along the microfibril. The blue-shaded area indicates the region where the potential energy is lowest, meaning that it is the most favourable for interaction with negative charges. This region is close to the C-terminus (dashed line) and corresponds to the mineral infiltration site, that is, the a-bands c, Zeta-potential measurements of the mineral phase and the other solution components, illustrating the negative charge of the pAsp–mineral complex. The high zeta-deviation and low count rate of pure HEPES buffer and pure pAsp show that they are not contributing to the measurements of the calcium phosphate and calcium phosphate–pAsp complexes.
Uranyl acetate staining was also used to identify the crystal nucleation sites within the collagen fibril. Apatite nanocrystals in their early stages were always observed on a staining band (Fig. 3c). Analysis of cryoTEM images showed that these nanocrystals were distributed evenly between the gap and overlap regions, with a small preference for the d-band in the gap zone (Fig. 3f). These results show that once ACP enters the fibril, the collagen is controlling nucleation either directly, with the charged amino acids acting as nucleation sites for apatite formation, or indirectly. As it has been shown that pAsp infiltrates the fibril25, it is possible that the bands of charged amino acids provide locations to which the polymer–ACP complex binds and subsequently induce nucleation.
To investigate this, collagen was mineralized in the presence of fetuin. This protein induces collagen mineralization by inhibiting calcium phosphate precipitation in solution and thus allowing the mineral phase to penetrate into the fibril (Supplementary S9, Fig. S9; refs 20,26). The protein itself cannot diffuse into the fibril owing to its large molecular weight (48 kDa; ref. 27); however, zeta-potential measurements show that it forms a negatively charged complex with calcium phosphate, with a net charge of −14.5 ± 11.2 mV. In the presence of fetuin, oriented apatite crystals were formed inside the fibril with morphologies and orientation similar to the ones observed in the presence of pAsp (Supplementary S9, Fig. S10) and the nucleation of the intrafibrillar crystals again occurred exclusively on the staining bands, although no ACP formation could be detected. Our observations now show that collagen indeed induces the oriented nucleation of apatite, without the involvement of NCPs or other control agents, as previously proposed13,14. This further experimentally confirms computer simulations that showed how specific regions in collagen induce the formation of oriented ion aggregates with motifs corresponding to the apatite structure28. Our results therefore support the notion that the spatial arrangement of the charged groups in the collagen fibril provides a structural template that induces oriented apatite nucleation14, with the c axis of the crystals aligned parallel to the long axis of the fibril (for a more detailed discussion see Supplementary S10). This also implies that in the present system, the main function of pAsp and fetuin is to form a stable complex with calcium phosphate, allowing it to enter the collagen fibril.
It seems, however, unlikely that in biology the function of the complex mixture of NCPs would be limited to just inhibiting extrafibrillar mineralization. This point was further investigated by carrying out the mineralization in the presence of the C-terminal fragment of the dentin matrix protein 1 (C-DMP1) which has been demonstrated to promote apatite nucleation in the presence of collagen29 (Supplementary S11, Fig. S11). In the absence of pAsp this protein fragment induced formation of crystals only in solution and on the surface of the fibrils (Supplementary S11, Figs. S12a,b). When combined with pAsp, mineralization occurred exclusively inside the fibrils, with apatite crystals forming from an ACP precursor phase, similar to what was observed for pAsp alone (Supplementary S11, Figs. S12c–f). However, the addition of C-DMP1 significantly accelerated mineralization, such that after 24 h the fibril already contained large amounts of apatite crystals, which again nucleated exclusively on the staining bands, equally in the gap and overlap regions (Supplementary S11, Figs S12 and S13). The absence of site-specific nucleation is surprising, as C-DMP1 also contains a site that specifically binds to the N-terminal end of collagen29. Hence, even in the presence of C-DMP1 crystal nucleation is controlled by collagen itself. We tentatively attribute this to the reported formation of large assemblies of this 17 kDa protein fragment in the presence of Ca2+ ions30, which would be too large to penetrate the collagen fibril. How the nucleation of apatite is promoted by C-DMP1 in the present system remains unexplained for the moment.
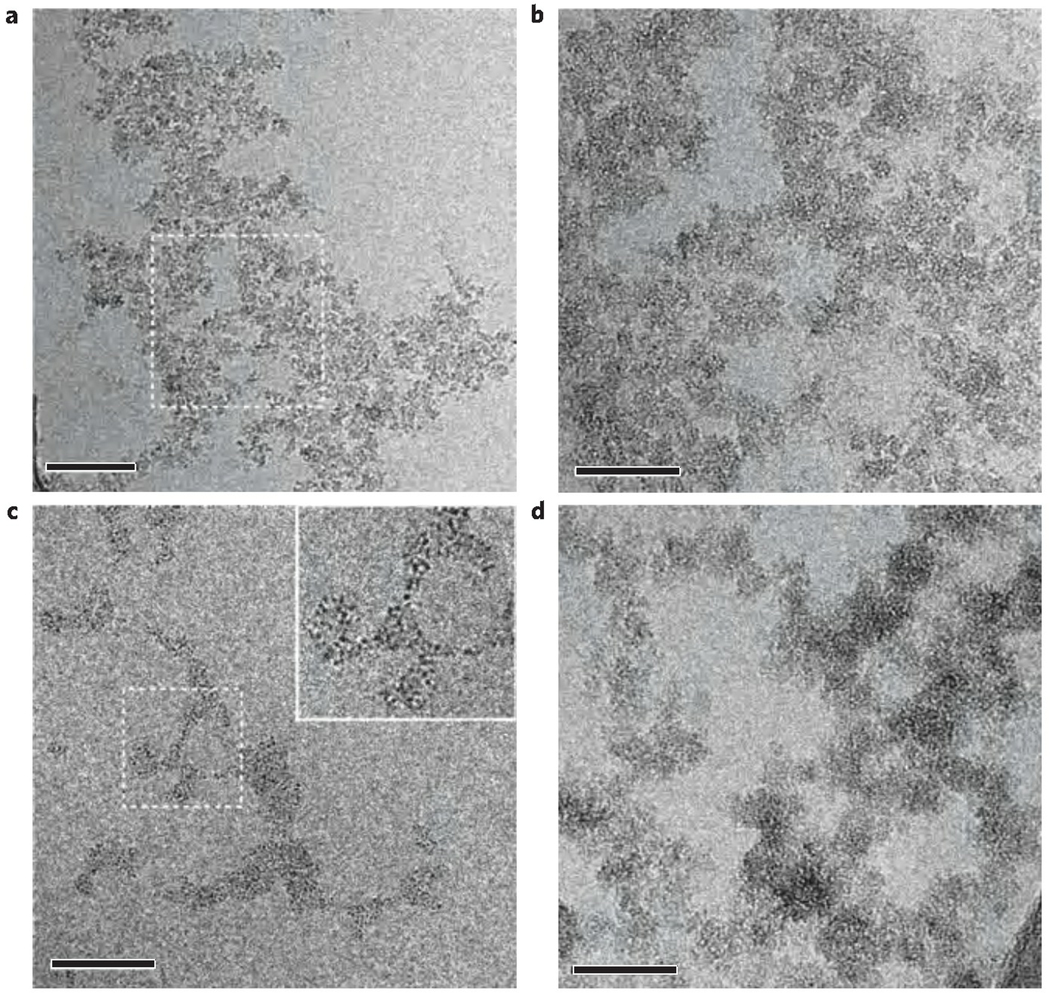
As the formation of ACP has been proposed to proceed through the assembly of nanometre-sized clusters31, and polymer-induced liquid-precursor phases have been described as during the precipitation of calcium phosphate in the presence of pAsp (ref. 19), we investigated the effect of this polymer on mineral formation in more detail. In control experiments without the addition of pAsp, dynamic light scattering measurements showed the rapid formation and growth of calcium phosphate particles, reaching hydrodynamic diameters (Dh) of 1,000 nm after 30 min and 5,000 nm after 2 h (Supplementary S12, Fig. S15a). At this time point, sedimentation occurred quickly, as evidenced by the decrease in count rate. CryoTEM images of samples collected after 10 min of reaction showed the presence of calcium phosphate aggregates 500 nm in size that consisted of densely packed clusters (Fig. 5a,b) of about 1 nm in size. Samples at longer reaction times could not be observed because the particles were too large and not suitable for cryoTEM analysis. In contrast, when pAsp was present in the solution, stable complexes with a Dh of 30–70 nm were formed (Supplementary S12, Fig. S15b). CryoTEM analysis revealed the presence of loosely packed assemblies of calcium phosphate clusters also of approximately 1 nm after 10 min of reaction (Fig. 5c). After 6 h, larger and denser structures were present (Fig. 5d), similar to the ones formed after 10 min without pAsp (Fig. 5b). These results show that at the early stages of mineralization, pAsp binds to and stabilizes the pre-nucleation clusters, forming loosely packed, diffuse structures that slowly aggregate and densify. The aggregates present after 6 h are similar to the ones we observed infiltrating the collagen fibril after 24 h (Fig. 2a), suggesting that these are the structures that enter the fibril.
Figure 5. Analysis of calcium phosphate precipitation in the absence and presence of pAsp.
a, CryoTEM image of calcium phosphate aggregates formed after 10 min of reaction without pAsp. Scale bar: 100 nm. b, Higher magnification of the area marked in a. Scale bar: 50 nm. c, CryoTEM image of calcium phosphate aggregates formed after 10 min of reaction in the presence of pAsp. Scale bar: 50 nm. d, CryoTEM image of calcium phosphate aggregates formed after 6 h of reaction in the presence of pAsp. Scale bar: 50 nm.
The net negative surface charge of the pAsp–ACP complex together with the presence of positively charged regions in the collagen fibril may be essential for the mineral infiltration (Fig. 4b). Hence, in vivo, negatively charged NCPs may not only stabilize the amorphous phase, but may also be needed to form similar negatively charged mineral complexes, that allow the mineral to enter the collagen (for a more detailed discussion see Supplementary S13). Moreover, our studies, carried out using soft-tissue collagen, imply that the ability to mediate mineralization is not related to bone collagen but is intrinsic to type I collagen fibrils. Our results will have implications for the understanding of the process of bone biomineralization, in particular the interplay between collagen, the NCPs and the developing mineral.
Supplementary Material
Acknowledgements
We thank G. Falini (University of Bologna, Italy) for kindly providing the horse tendon collagen; L. B. Gower (University of Florida, Florida, USA) for a critical review of the manuscript; S. Weiner (Weizmann Institute of Science, Israel) and J. P. R. O. Orgel (Illinois Institute of Technology, Illinois, US) for helpful discussions; and J. J. van Roosmalen (Eindhoven University of Technology, The Netherlands) for his help with the tomography reconstructions. Supported by the Dutch Science Foundation, NWO, The Netherlands and by the European Community (FP6, project code NMP4-CT-2006-033277 TEM-PLANT).
Footnotes
Author contributions
F.N. carried out most experiments and co-wrote the manuscript. K.P. and P.A.J.H. carried out the molecular modelling. A.G. provided the C-DMP1 and the expertise in the work with the protein. L.J.B. contributed to the development of the mineralization experiments for cryoTEM. P.H.H.B. provided support with the cryoTEM. H.F. provided support with the tomographic reconstructions. G.W. and N.A.J.M.S. supervised the project and N.A.J.M.S. co-wrote the manuscript. All authors discussed the results and revised the manuscript.
Additional information
The authors declare no competing financial interests. Supplementary information accompanies this paper on www.nature.com/naturematerials. Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions.
References
- 1.Hulmes DJS, Wess TJ, Prockop DJ, Fratzl P. Radial packing, order, and disorder in collagen fibrils. Biophys. J. 1995;68:1661–1670. doi: 10.1016/S0006-3495(95)80391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Traub W, Arad T, Weiner S. 3-dimensional ordered distribution of crystals in turkey tendon collagen-fibers. Proc. Natl Acad. Sci. USA. 1989;86:9822–9826. doi: 10.1073/pnas.86.24.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodge AJ, Petruska JA. In: Aspects of Protein Structure. Ramachandran GN, editor. Academic; 1963. pp. 289–300. [Google Scholar]
- 4.Miller A. Collagen: The organic matrix of bone. Phil. Trans. R. Soc. B. 1984;304:455–477. doi: 10.1098/rstb.1984.0040. [DOI] [PubMed] [Google Scholar]
- 5.Orgel JPRO, Irving TC, Miller A, Wess TJ. Microfibrillar structure of type I collagen in situ. Proc. Natl Acad. Sci. USA. 2006;103:9001–9005. doi: 10.1073/pnas.0502718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glimcher MJ, Muir H. Recent studies of the mineral phase in bone and its possible linkage to the organic matrix by protein-bound phosphate bonds. Phil. Trans. R. Soc. B. 1984;304:479–508. doi: 10.1098/rstb.1984.0041. [DOI] [PubMed] [Google Scholar]
- 7.Landis WJ, Song MJ, Leith A, Mcewen L, Mcewen BF. Mineral and organic matrix interaction in normally calcifying tendon visualized in 3 dimensions by high-voltage electron-microscopic tomography and graphic image-reconstruction. J. Struct. Biol. 1993;110:39–54. doi: 10.1006/jsbi.1993.1003. [DOI] [PubMed] [Google Scholar]
- 8.Mahamid J, et al. Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to bone in zebrafish fin rays. Proc. Natl Acad. Sci. USA. 2010;107:6316–6321. doi: 10.1073/pnas.0914218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Traub W, Arad T, Weiner S. Origin of mineral crystal-growth in collagen fibrils. Matrix. 1992;12:251–255. doi: 10.1016/s0934-8832(11)80076-4. [DOI] [PubMed] [Google Scholar]
- 10.George A, Veis A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem. Rev. 2008;108:4670–4693. doi: 10.1021/cr0782729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maitland ME, Arsenault AL. A correlation between the distribution of biological apatite and amino-acid-sequence of type-I collagen. Calcif. Tissue Int. 1991;48:341–352. doi: 10.1007/BF02556154. [DOI] [PubMed] [Google Scholar]
- 12.Berthet-Colominas C, Miller A, White SW. Structural study of the calcifying collagen in turkey leg tendons. J. Mol. Biol. 1979;134:431–445. doi: 10.1016/0022-2836(79)90362-0. [DOI] [PubMed] [Google Scholar]
- 13.Katz EP, Li S. Structure and function of bone collagen fibrils. J. Mol. Biol. 1973;80:1–15. doi: 10.1016/0022-2836(73)90230-1. [DOI] [PubMed] [Google Scholar]
- 14.Landis WJ, Silver FH. Mineral deposition in the extracellular matrices of vertebrate tissues: Identification of possible apatite nucleation sites on type I collagen. Cells Tissues Organs. 2009;189:20–24. doi: 10.1159/000151454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pouget EM, et al. The initial stages of template-controlled CaCO3 formation revealed by cryo-TEM. Science. 2009;323:1455–1458. doi: 10.1126/science.1169434. [DOI] [PubMed] [Google Scholar]
- 16.Stetlerstevenson WG, Veis A. Type-I collagen shows a specific binding-affinity for bovine dentin phosphophoryn. Calcif. Tissue Int. 1986;38:135–141. doi: 10.1007/BF02556873. [DOI] [PubMed] [Google Scholar]
- 17.Stetlerstevenson WG, Veis A. Bovine dentin phosphophoryn—calcium-ion binding-properties of a high-molecular-weight preparation. Calcif. Tissue Int. 1987;40:97–102. doi: 10.1007/BF02555712. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande AS, Beniash E. Bioinspired synthesis of mineralized collagen fibrils. Cryst. Growth Des. 2008;8:3084–3090. doi: 10.1021/cg800252f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olszta MJ, et al. Bone structure and formation: A new perspective. Mater. Sci. Eng. R. 2007;58:77–116. [Google Scholar]
- 20.Price PA, Toroian D, Lim JE. Mineralization by inhibitor exclusion: The calcification of collagen with fetuin. J. Biol. Chem. 2009;284:17092–17101. doi: 10.1074/jbc.M109.007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fratzl P, Fratzl-Zelman N, Klaushofer K. Collagen packing and mineralization—an X-ray-scattering investigation of turkey leg tendon. Biophys. J. 1993;64:260–266. doi: 10.1016/S0006-3495(93)81362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beniash E, Traub W, Veis A, Weiner S. A transmission electron microscope study using vitrified ice sections of predentin: Structural changes in the dentin collagenous matrix prior to mineralization. J. Struct. Biol. 2000;132:212–225. doi: 10.1006/jsbi.2000.4320. [DOI] [PubMed] [Google Scholar]
- 23.Hodge AJ, Schmitt FO. The charge profile of the tropocollagen macromolecule and the packing arrangement in native-type collagen fibrils. Proc. Natl Acad. Sci. USA. 1960;46:186–197. doi: 10.1073/pnas.46.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chapman JA, Tzaphlidou M, Meek KM, Kadler KE. The collagen fibril—a model system for studying the staining and fixation of a protein. Electron Microsc. Rev. 1990;3:143–182. doi: 10.1016/0892-0354(90)90018-n. [DOI] [PubMed] [Google Scholar]
- 25.Jee SS, Culver L, Li YP, Douglas EP, Gower LB. Biomimetic mineralization of collagen via an enzyme-aided PILP process. J. Cryst. Growth. 2010;312:1249–1256. [Google Scholar]
- 26.Rochette CN, et al. A shielding topology stabilizes the early stage protein-mineral complexes of fetuin-a and calcium phosphate: A time-resolved small-angle X-ray study. Chembiochem. 2009;10:735–740. doi: 10.1002/cbic.200800719. [DOI] [PubMed] [Google Scholar]
- 27.Toroian D, Lim JE, Price PA. The size exclusion characteristics of type I collagen—implications for the role of noncollagenous bone constituents in mineralization. J. Biol. Chem. 2007;282:22437–22447. doi: 10.1074/jbc.M700591200. [DOI] [PubMed] [Google Scholar]
- 28.Kawska A, Hochrein O, Brickmann A, Kniep R, Zahn D. The nucleation mechanism of fluorapatite-collagen composites: Ion association and motif control by collagen proteins. Angew. Chem. Int. Ed. 2008;47:4982–4985. doi: 10.1002/anie.200800908. [DOI] [PubMed] [Google Scholar]
- 29.He G, George A. Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J. Biol. Chem. 2004;279:11649–11656. doi: 10.1074/jbc.M309296200. [DOI] [PubMed] [Google Scholar]
- 30.He G, et al. Spatially and temporally controlled biomineralization is facilitated by interaction between self-assembled dentin matrix protein 1 and calcium phosphate nuclei in solution. Biochemistry. 2005;44:16140–16148. doi: 10.1021/bi051045l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Posner AS, Betts F. Synthetic amorphous calcium phosphate and its relation to bone mineral structure. Acc. Chem. Res. 1975;8:273–281. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.