Abstract
Mortality associated with influenza virus super-infections is frequently due to secondary bacterial complications. To date, super-infections with Streptococcus pyogenes have been studied less extensively than those associated with S. pneumoniae. This is significant because a vaccine for S. pyogenes is not clinically available, leaving vaccination against influenza virus as our only means for preventing these super-infections. In this study, we directly compared immunity induced by two types of influenza vaccine, either inactivated influenza virus (IIV) or live, attenuated influenza virus (LAIV), for the ability to prevent super-infections. Our data demonstrate that both IIV and LAIV vaccines induce similar levels of serum antibodies, and that LAIV alone induces IgA expression at mucosal surfaces. Upon super-infection, both vaccines have the ability to limit the induction of pro-inflammatory cytokines within the lung, including IFN-γ which has been shown to contribute to mortality in previous models of super-infection. Limiting expression of these pro-inflammatory cytokines within the lungs subsequently limits recruitment of macrophages and neutrophils to pulmonary surfaces, and ultimately protects both IIV- and LAIV-vaccinated mice from mortality. Despite their overall survival, both IIV- and LAIV-vaccinated mice demonstrated levels of bacteria within the lung tissue to levels that are similar to those seen in unvaccinated mice. Thus, influenza virus:bacteria super-infections can be limited by vaccine-induced immunity against influenza virus, but the ability to prevent morbidity is not complete.
Keywords: Influenza virus, Streptococcus pyogenes, super-infections, vaccination
1. Introduction
Influenza A virus infections that are complicated by secondary bacterial pneumonia make a significant contribution to deaths during both influenza virus epidemics [1] and pandemics [2]. During typical influenza seasons, Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, and Streptococcus pyogenes [3–5] are all known contributors to the “excess mortality” that results from influenza virus super-infections. In fact, in the 1918–19 influenza pandemic, S. pneumoniae and S. pyogenes (Group A Streptococcus) were the most frequently observed bacterial species in the lungs of infected soldiers [6], and together they likely contributed to as many as 90% of deaths attributed to this pandemic [2]. More recently, findings from the H1N1 swine-origin influenza virus pandemic demonstrate that 29% of deaths were due to secondary bacterial pneumonia in an autopsy series, with 27% of these fatalities being associated with S. pyogenes super-infection [7]. Furthermore, S. pneumoniae and S. pyogenes were the most frequent species associated with increased parapneumonic empyema in a study conducted during the 2009 H1N1 pandemic in Utah [8]. The incidence of invasive diseases caused by S. pyogenes in England increased significantly (26%) in December 2010 and January 2011 in all age groups, due, in part, to widespread influenza infections. Interestingly, the greatest percentage of invasive disease episodes associated with laboratory confirmed influenza infection during this period were caused by S. pyogenes [9].
Several studies have assessed the efficacy and effectiveness of influenza vaccines to prevent influenza-like illness [10–13], but less information is available regarding the ability of influenza vaccines to limit secondary bacterial complications [14–17]. Since secondary bacterial infections are the primary cause of mortality associated with influenza virus, methods to limit these complications are currently being sought [18]. The purpose of this study was to directly compare the contributions of IIV and LAIV toward protection in a murine model of influenza virus:S. pyogenes super-infection. We report that both IIV and LAIV vaccines induced systemic (serum) antibody responses, with LAIV also eliciting local (mucosal) IgA antibodies. Subsequently, mice vaccinated against influenza virus demonstrated reduced inflammatory cytokines within BALF, decreased recruitment of inflammatory cells to the lungs, and increased survival, compared to unvaccinated control mice. Despite limiting mortality associated with these super-infections, similar levels of viable bacteria were detected within the lungs of both vaccinated and unvaccinated mice, an outcome that was not observed after sub-lethal inoculation with S. pyogenes alone. Thus, immunity induced after vaccination against influenza virus (either IIV or LAIV) prevented super-infections within mice, albeit incompletely. Overall, protection against super-infection was similar for recipients of either IIV or LAIV.
2. Methods and Material
2.1. Mice
Adult (6–8-week-old) female BALB/cJ mice were obtained from Harlan Laboratories (Indianapolis, IN) and housed in groups of four, with 24-hour access to food and water. All animal experiments were performed following the guidelines established and approved by the Animal Care and Use committee at the University of South Dakota (Vermillion, SD).
2.2. Super-infection model
Viruses expressing the hemagglutinin (HA) and neuraminidase (NA) from A/Hong Kong/1/68-H3N2 were created as described previously [19,20], and this influenza virus was kindly provided by Jonathan A. McCullers (St. Jude Children’s Research Hospital, Memphis, TN). Throughout this manuscript, this virus will be referred to as “HK68 virus.” This virus had a tissue culture ID50 (TCID50) of 107.5 and a mouse LD50 (MLD50) of 105.75 TCID50. The S. pyogenes strain MGAS315 (serotype M3) was obtained from the American Type Culture Collection (Manassas, VA), and will be referred to as “MGAS315 bacteria.” MGAS315 bacteria were grown in Todd-Hewitt broth supplemented with 0.2% yeast extract until the mid-exponential phase of growth at which time the bacteria were washed and suspended in phosphate-buffered saline (PBS). MGAS315 bacteria had an MLD50 of 107 CFU.
Lightly anesthetized (isoflurane) mice were inoculated intranasally (i.n.) with HK68 virus and/or MGAS315 bacteria. Each pathogen was administered at a sub-lethal dose (0.1 MLD50). When administered sequentially (HK68 virus + MGAS315 bacteria), the interval between inoculations was 7 days [5,15,21]. Controls were similarly inoculated with HK68 virus + PBS (HK68 virus), allantoic fluid + MGAS315 (MGAS315 bacteria), or allantoic fluid + PBS (control). Weight loss (morbidity) and survival (mortality) were monitored daily as described [20].
Influenza viruses of the H3N2 subtype have circulated within the human population since 1968 [22], and have been the dominant subtype circulating during many recent influenza seasons [3]. The choice of MGAS315 for bacterial challenge was primarily due to its genetic similarity to invasive serotype M3 isolates currently circulating among humans [23]. In addition, the complete genome sequence of this isolate is publically available [23] and it can be manipulated genetically [24], which will facilitate future experiments designed to identify bacterial components contributing to influenza:S. pyogenes super-infection.
2.3. Vaccination
IIV was prepared through propagation of HK68 virus in 10-day-old embryonated chicken eggs, followed by concentration, inactivation with 0.025% formalin, purification, and HA content quantitation as described previously [15,20,25]. Mice were vaccinated with IIV (3 μg HA content without adjuvant) in a 100 μL volume delivered intramuscularly (i.m.) into the right rear quadriceps [15]. Two inoculations were administered at 4-week intervals. As a vaccine control, mice were similarly inoculated with PBS. The monovalent vaccine preparations for IIV and LAIV that were used focus immunity solely on the H3N2 subtype, and do not provide immunity toward either H1N1 or influenza B viruses, which trivalent, seasonal vaccines have been designed to do [26].
Jonathan A. McCullers kindly provided LAIV expressing the HK68 virus HA and NA on a PR8 background mutated to confer an LAIV phenotype [27]. This virus was created, propagated, and characterized as described previously [19]. Mice were inoculated intranasally (i.n.) with 106 TCID50 (33 C) of HK68 LAIV in a 50 μL volume [19], and two inoculations were administered at 4-week intervals. As a vaccine control, mice were similarly inoculated with allantoic fluid.
2.4. Immune Assays
Sera were collected from the orbital plexus of isoflurane-anesthetized mice three weeks after secondary vaccination [20,25]. Prior to analyses, sera were treated with receptor-destroying enzyme as described [19,20], and were analyzed for influenza-reactive antibodies using microneutralization and ELISA as described previously [19,28]. For microneutralization assays, MDCK monolayers were incubated with serum:virus mixtures, and a pool of mouse monoclonal antibodies specific for influenza A virus nucleoprotein (Millipore, Billerica, MA) was used to detect infectivity of MDCK cells. Titers were determined as described [28], after measuring the OD at 492 nm using a BioTek EL808 plate reader (BioTek Instruments, Inc., Winooski, VT).
Analysis of sera by ELISA was performed as described previously [20]. Briefly, 96-well plates (Becton Dickinson and Company, Franklin Lakes, NJ) were coated with concentrated HK68 virus (1 μg HA mL−1). RDE-treated sera were serially diluted in 10% FBS (Atlanta Biologicals, Lawrenceville, GA) in PBS containing 0.05% (v/v) Tween-20 (Sigma, St. Louis, MO) (FBS-PBST). Alkaline phosphatase-conjugated goat anti-mouse IgG (H + L), IgG (γ-specific), IgG1, IgG2a, and IgA (Southern Biotechnology, Inc., Birmingham, AL) antibodies diluted in FBS-PBST were added to the plates. Plates were washed, and 1 mg mL−1 p-nitrophenyl phosphate substrate (Sigma, St. Louis, MO) in diethanolamine buffer was added. One hour after substrate addition, OD was read at 405 nm using a BioTek EL808 plate reader (BioTek Instruments, Inc.). Reciprocal serum antibody titers for individual sera are reported at 50% maximal binding on the individual titration curves as described [19].
2.5. Lung viral and bacterial titers
Twenty-four hours after challenge with MGAS315 bacteria (day 8 after HK68 virus inoculation), mice were euthanized, lungs were collected, and both viral and bacterial titers were quantitated. For viral titers, confluent MDCK monolayers were inoculated with log10 serial dilutions of lung homogenates as described [19,20], and titers are reported (limit of detection: 103–108 TCID50/mL). For bacterial titers, log10 serial dilutions of lung homogenates were inoculated onto tryptic soy agar plates supplemented with10% defibrinated sheep blood (Becton Dickinson, Sparks, MD), and CFU were determined after 24 h incubation at 37°C, 5% CO2 (limit of detection: 103–108 CFU/mL).
2.6. Detection of antibodies and cytokines in bronchoalveolar lavage fluid (BALF)
BALF was collected 24 h after MGAS315 bacteria inoculation (day 8 after HK68 virus inoculation). The trachea of euthanized mice was cannulated using a 24-gauge BD Insyte catheter (Becton Dickinson Infusion Therapy Systems Inc., Sandy, UT), and lungs were flushed with 1 mL PBS supplemented with 0.05% EDTA [29]. HK68 virus-reactive antibodies of the IgG1, IgG2a, and IgA isotypes were detected in BALF using the ELISA method detailed above. Data are reported as the reciprocal serum dilution corresponding to 50% maximal binding on the titration curve.
Cytokines present within the BALF were measured using a Th1/Th2/Th17 cytometric bead array kit (BD Biosciences, San Diego, CA), following the manufacturer’s instructions. Samples were analyzed on C6 Flow Cytometer (Accuri Cytometers, Ltd., Ann Arbor, MI), and analyzed using CFlow Plus software (Accuri). Levels of IL-2, IL-4, IL-6, IL-10, IL-17A, TNF-α, and IFN-γ are reported in pg/mL based on standards included with the kit.
2.7. Histopathology
Lungs from euthanized mice were infused intratracheally with 4% paraformaldehyde in PBS at 25 cm water pressure. Superior lobes [30] of the lungs were post-fixed in 4% paraformaldehyde in PBS for 24 h. After paraffin embedding, representative lateral and medial lung areas were sectioned at 7 μm, deparaffinized, and stained with Hematoxylin and Eosin. Tissues were analyzed using a modified system based upon Histopathological Scoring Criteria adapted from previous studies [31,32]. Pathology was conducted in a double blind manner, and scores were assigned using the following criteria: Peribronchiolar Infiltrates of white blood cells (percentage of sites): 0-None, 1-Few (<25%), 2-Many (25–75%), 3-All (>75%); Severity of Peribronchiolar Infiltrates (thickness of lymphoid mass): 0-None, 1-Mild (interrupted collar), 2-Moderate (semi-collar <5 cells), 3-Severe (complete collar >5 cells); Perivascular Infiltrates (percentage of sites): 0-None, 1-Few (<10%), 2-Many (10–50%), 3-All (>50%); Severity of Perivascular Infiltrates (thickness of lymphoid mass): 0-None, 1-Mild (interrupted collar), 2-Moderate (semi-collar <5 cells), 3-Severe (complete collar >5 cells); Parenchymal Pneumonia (inflamed pulmonary interstitium): 0-None, 3-Minimal (intermittent infiltrates), 5-Heavy (confluent infiltrates). Composite scores (with the highest possible score of 34) for medial and lateral sections from each animal were used for data analysis.
2.8. Statistical analyses
Data consisting of three or more groups were analyzed with a one way analysis of variance (ANOVA) and post hoc Student –Newman-Keuls test. A two way repeated ANOVA was utilized to compare effects of treatment and time followed by post hoc Student t tests. To compare the proportion of animals alive at the conclusion of each experiment following various treatments the z test was used to compare the treatment (vaccination) to the control (PBS or other vehicle). Values were accepted as significant if P<0.05. Data were analyzed using Statmost programs (Dataxiom Software Inc. Los Angeles).
3. RESULTS
3.1. Influenza virus:S. pyogenes super-infections can be modeled using an H3N2 HA-expressing virus and MGAS315 bacteria
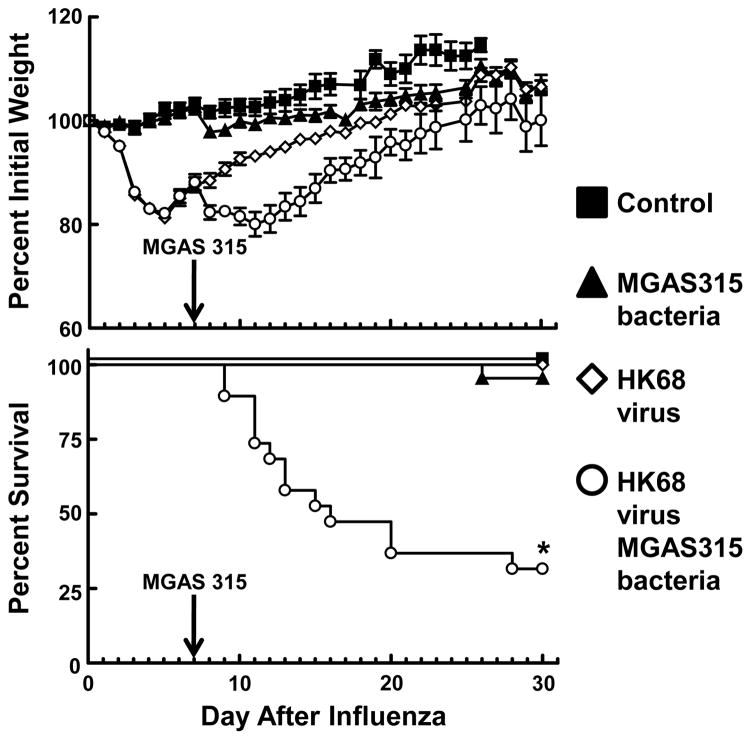
We first established a murine model of influenza virus:S. pyogenes super-infection using a previously characterized strain of HK68 virus [19] and MGAS315 bacteria [23] for super-infection. Mice were divided into groups that received either HK68 virus alone, MGAS315 bacteria alone, or HK68 virus + MGAS315 bacteria. A control group that received neither virus nor bacteria was also included (Fig. 1). The 3 groups inoculated with either HK68 virus alone, MGAS315 bacteria alone, or HK68 virus + MGAS315 bacteria demonstrated morbidity (weight loss) after inoculation with the respective pathogens. However, only mice in the group that were inoculated sequentially with HK68 virus + MGAS315 bacteria demonstrated significant mortality (P<0.05).
Figure 1.
Morbidity and mortality of mice after influenza:S. pyogenes super-infection. Mice were divided into four groups and inoculated intranasally (i.n.) with either allantoic fluid + PBS (control, n = 6), HK68 virus + PBS (HK68 virus, n = 18), allantoic fluid + MGAS315 bacteria (MGAS315 bacteria, n = 22), or HK68 virus + MGAS315 bacteria (HK68 virus + MGAS315 bacteria, n = 19) and monitored daily for weight loss (morbidity) and survival (mortality). *Indicates a significant difference (P<0.01) compared to all other groups, using a z test.
3.2. Serum antibodies are induced by both IIV and LAIV vaccines, but mucosal IgA is preferentially induced by LAIV
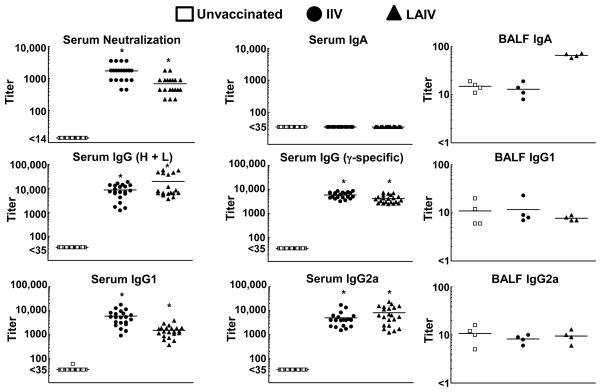
Titers of HK68 virus-neutralizing antibodies and HK68 virus-reactive antibody isotypes were quantitated in all three vaccine groups three weeks after secondary vaccination with either IIV or LAIV (Fig. 2). Significant increases in the titers of virus neutralizing antibodies, and antibodies of the IgG (H + L), IgG (γ-specific), IgG1, and IgG2a isotypes were detected in both IIV and LAIV vaccinated groups compared to unvaccinated mice (P<0.05). Of note, HK68 virus-reactive IgA antibodies were not detected within the serum of mice in any of the three vaccine groups. Analysis of serum antibodies by standard HAI demonstrated titers that correlate with protective immunity (titer ≥ 1:40) [33] within both IIV- and LAIV-vaccinated mice three weeks after secondary inoculation (data not shown). Thus, vaccination with either IIV or LAIV yielded an influenza virus-specific serum antibody response that would predict protection against subsequent influenza virus challenge [20,33], especially with the sub-lethal doses used in our super-infection model (Fig. 1).
Figure 2.
Neutralization and ELISA antibody titers after vaccination with either inactivated influenza virus (IIV) or live, attenuated influenza virus (LAIV). Mice were divided into three groups that received either allantoic fluid + PBS (unvaccinated, n = 33), IIV delivered i.m. (IIV, n = 21), or LAIV delivered i.n. (LAIV, n = 21). Mice were inoculated on day 0 (primary inoculation) and boosted on day 28 (secondary inoculation). Sera were collected and analyzed 21 days after secondary inoculation. *Indicates a significant difference (P<0.05) compared to unvaccinated mice using one-way ANOVA. Bronchoalveolar lavage fluid (BALF) was collected from a subset of super-infected mice (n = 4 per vaccine group) 24 hours after inoculation with bacteria (day 8 after HK68 influenza virus inoculation), and antibodies of the IgG1, IgG2a, and IgA isotypes were measured by ELISA.
Four mice from each vaccine group (unvaccinated, IIV, and LAIV) that were subsequently super-infected were euthanized 24 h after inoculation with MGAS315 bacteria (Day 8 after HK68 virus challenge) to evaluate HK68 virus-reactive IgA, IgG1, and IgG2a antibodies within BALF by ELISA (Fig. 2). The levels of IgA antibodies were increased in LAIV recipients compared to mice in the groups that received either no vaccine or IIV. In contrast, IgG1 and IgG2a antibody titers were similar among all three vaccine groups. An increase in HK68 virus-reactive IgA in BALF after LAIV inoculation was predicted based on previous studies in mice [34], but we were surprised that similar increases in IgG1 and IgG2a were not demonstrated within the BALF of vaccine recipients [34,35].
3.3. Vaccination limits expression of pro-inflammatory cytokines that are induced after super-infection
Four mice from each vaccine group (vaccine control, IIV, and LAIV) were euthanized 24 h after inoculation with MGAS315 bacteria (Day 8 after HK68 influenza virus), and BALF collected from these super-infected mice was analyzed for protein levels of IL-2, IL-4, IL-6, IL-10, IL-17A, TNF-α, and IFN-γ (Fig. 3). Levels of the pro-inflammatory cytokines IL-6, TNF-α, IFN-γ were elevated in the vaccine control (unvaccinated) group compared to either the IIV or the LAIV group, while differences in IL-2, IL-4, IL-10, and IL-17A expression were not observed when all three vaccine groups were compared. The elevated cytokine levels in unvaccinated mice did not achieve statistical significance, but they demonstrate a trend toward a pro-inflammatory response within these unvaccinated control mice at 24 hours post-inoculation with MGAS315 bacteria that has been reported previously in super-infection models [4,36].
Figure 3.
Cytokine levels within BALF after super-infection. Mice were divided into three groups that received either allantoic fluid + PBS (unvaccinated, n = 4), IIV delivered i.m. (IIV, n = 4), or LAIV delivered i.n. (LAIV, n = 4). Mice were inoculated on day 0 (primary inoculation) and boosted on day 28 (secondary inoculation). BALF was collected from super-infected mice 24 hours after inoculation with bacteria (day 8 after HK68 influenza virus inoculation), and cytokines were detected using cytometric bead array (Th1/Th2/Th17).
3.4. Cellular infiltrates within the lungs of influenza vaccine recipients (both IIV and LAIV) are limited in comparison to the cells recruited to the lungs of unvaccinated mice
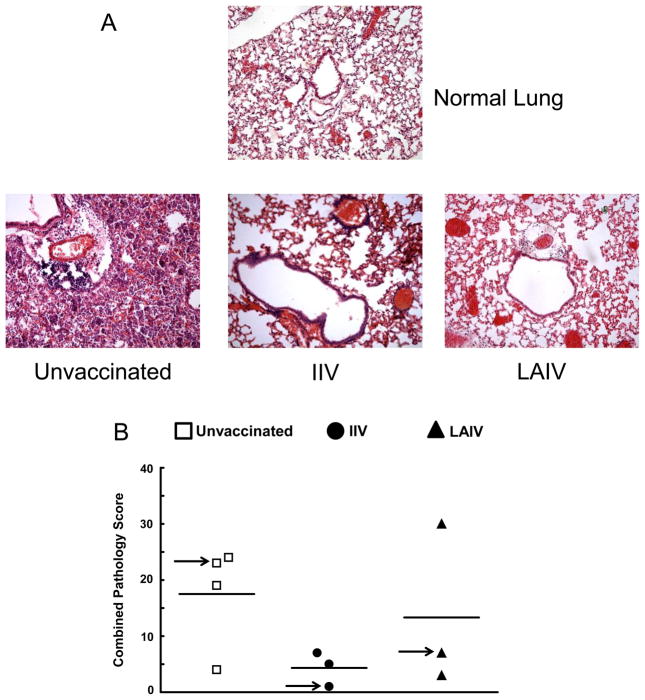
The pathology of lungs was visualized using H&E staining, and samples were scored for inflammation (Fig. 4). Histological examination demonstrated fewer cellular infiltrates in the lungs of vaccinated mice (regardless of whether vaccination was with IIV or LAIV), compared to lungs taken from unvaccinated mice (Fig. 4A). These differences were reflected in pathology scores reported for the individual mice (Fig. 4B). Specifically, in the unvaccinated group, 3 out of 4 mice (75%) received overall pathology scores >15, while only 1 out of 6 vaccinated mice (17%) (IIV and LAIV combined) demonstrated a pathology score >15. Interestingly, this single mouse was vaccinated with LAIV. Minimal signs of inflammation were observed within the lungs of control mice that received either HK68 virus alone or MGAS315 bacteria alone (data not shown). Thus, histological analysis of lungs demonstrated cellular infiltrates in the unvaccinated group, but not in mice that received either IIV or LAIV. The majority of the cells populating the lungs were visually identified as macrophages and neutrophils, with subsequent confirmation of these populations by immunohistochemistry (data not shown).
Figure 4.
Lung histopathology after super-infection. Mice were divided into three groups that received either allantoic fluid + PBS (unvaccinated, n = 4), IIV delivered i.m. (IIV, n = 3), or LAIV delivered i.n. (n = 3). Lungs were collected 24 hours after inoculation of super-infected mice with MGAS315 bacteria (day 8 after HK68 influenza virus infection). Lung sections (A) are shown for a representative mouse in each group. Composite lung scores are reported (B), and the arrow indicates the mouse associated with the individual section in panel A.
3.5. Both IIV and LAIV vaccines limited morbidity and mortality associated with influenza:S. pyogenes super-infection
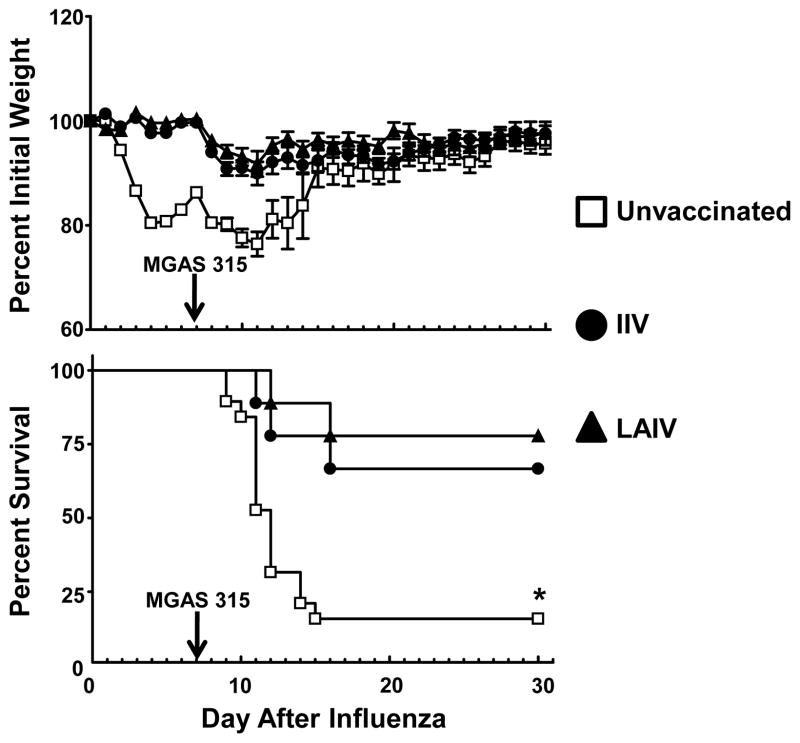
Mice in all three vaccine groups (unvaccinated, IIV, and LAIV) were inoculated i.n. with 0.1 MLD50 of HK68 virus followed 7 d later with 0.1 MLD50 of MGAS315 bacteria to determine the contribution of vaccination against influenza virus toward limiting these super-infections. Mice vaccinated with either IIV or LAIV, and subsequently challenged with HK68 virus, did not demonstrate significant weight loss during the 7 d post-infection period (Fig. 5). In contrast, unvaccinated mice lost a significant amount of weight following inoculation with HK68 virus, and began to recover as demonstrated previously (Fig. 1). Following challenge with 0.1 MLD50 MGAS315 bacteria, weight loss was observed in all mice, regardless of prior vaccination against influenza virus. However, only 3 of the 19 unvaccinated mice (16%) inoculated with HK68 virus and MGAS315 bacteria survived this super-infection (Fig. 5). In contrast, 6 of 9 IIV recipients (67%) and 7 of the 9 mice vaccinated with LAIV (78%) survived the super-infection. Thus, both IIV and LAIV vaccines protected against lethality associated with influenza virus:S. pyogenes super-infection.
Figure 5.
Influenza:S. pyogenes super-infection in mice after vaccination. Mice were divided into three groups that received either allantoic fluid + PBS (unvaccinated, n = 19), IIV delivered i.m. (IIV, n = 9), or LAIV delivered i.n. (n = 9). Four weeks after a secondary inoculation with the appropriate vaccine, mice in all three vaccine groups were inoculated with 0.1 MLD50 HK68 virus on day 0 and 0.1 MLD50 MGAS315 bacteria on day 7. Mice were monitored daily for morbidity (weight loss) and mortality (survival). *Indicates a significant difference (P<0.05) compared to vaccinated mice, using a Z test.
3.6. Viable bacteria are reduced within the BALF of both IIV and LAIV groups, but persisted within the whole lungs of all mice 24 h after inoculation with S. pyogenes
BALF collected from individual super-infected mice were both plated on blood agar plates and examined microscopically for the presence of bacteria 24 h after inoculation with MGAS315 bacteria (8 days after HK68 virus inoculation). Within the BALF of unvaccinated mice, viable S. pyogenes was recovered (from 4 out of 4 mice) and numerous Gram-positive cocci in chains were observed microscopically (data not shown). Similarly, Gram-positive cocci were observed microscopically within the BALF from both the IIV and the LAIV groups, but viable bacteria were only recovered within the BALF collected from a single mouse that received IIV (1 out of 4 mice) and none of the mice that received LAIV (0 out of 4 mice).
At the same time after MGAS315 bacteria inoculation, whole lung homogenates from a separate group of mice were analyzed for the presence of viable virus and bacteria. Influenza virus was not detected in the lung homogenates from any of the super-infected mice within the three vaccine groups (<103 TCID50/mL, n = 4 per group), demonstrating that the virus had been cleared from the host by day 8 post-inoculation. Alternatively, viable S. pyogenes was detected in the lungs of super-infected mice inoculated with both HK68 virus and MGAS315 bacteria, including all of the unvaccinated mice (1.1 × 106 ± 5.3 × 105 CFU), all of the mice vaccinated with IIV (4.5 × 105 ± 2.2 × 105 CFU), and 3 of the mice vaccinated with LAIV (1.3 × 105 ± 4.7 × 104 CFU). Data are reported as the mean ± SEM for groups of 4 mice. This finding is in contrast to mice that were solely inoculated with 0.1 MLD50 MGAS315 bacteria (n = 4), that did not contain viable bacteria within their lung homogenates 24 h after inoculation (<103 CFU). Thus, 24 h after MGAS315 super-infection, in mice vaccinated against influenza virus that ultimately survive this super-infection, influenza virus is no longer detected within the lungs, while viable bacteria appear to be exclusively associated with lung tissue, and not the BALF.
4. DISCUSSION
The mortality associated with influenza is often due to secondary bacterial complications, including those caused by S. pyogenes [2,7,8]. In this study, we established a model of influenza:S. pyogenes super-infection and compared the independent contributions of IIV and LAIV vaccines toward preventing morbidity and mortality associated with this super-infection. Our results demonstrate that despite differences in vaccine-induced immunity, both vaccines provided significant, albeit incomplete, protection against subsequent super-infection with S. pyogenes.
Invasive diseases caused by S. pyogenes, including streptococcal toxic shock, necrotizing fasciitis, and pneumonia can be life-threatening [37], and on an annual basis, approximately 500,000 deaths are attributed to S. pyogenes [38]. During the devastating pandemic of 1918–19, which occurred during the pre-influenza vaccine era, up to 90% of the 40–50 million deaths worldwide [39] were associated with super-infections [2], most of which were due to either S. pneumoniae or S. pyogenes [6]. Current estimates indicate that 11–12% of invasive S. pyogenes infections have lower respiratory complications [37], and that the case fatality rate is approximately 38% [40]. In addition, the incidence of invasive S. pyogenes infections increases during influenza epidemics [41–43] and pandemics [2,44,45], including the 2009 H1N1 pandemic [7,8]. The model used here was designed to study influenza:S. pyogenes synergy specifically in the lower respiratory tract. Such a model was deemed particularly relevant to determine the ability of influenza vaccines, particularly LAIV, to minimize secondary bacterial pneumonia, because it focuses immunity within the lung. Although it was not the goal of this study, it would be of interest to test the efficacy of influenza vaccines on decreasing super-infection when animals are inoculated with bacteria prior to, simultaneously, or shortly after inoculation with influenza virus. Such studies would be relevant to situations in which S. pyogenes is present asymptomatically in the respiratory tract of individuals who are then infected with influenza virus. We would predict that vaccination against influenza virus would provide similar protection as described in the current study; however, this remains to be determined experimentally.
Unlike S. pneumoniae [46–48], there are no FDA-approved vaccines for S. pyogenes [49,50], which severely limits our ability to prevent S. pyogenes super-infections. Currently, our best approach toward limiting influenza virus: S. pyogenes super-infections is to vaccinate against influenza virus. To date, a handful of reports using either population-based [17,51] or laboratory-controlled [14,15] studies suggest that vaccination against influenza virus may reduce the incidence of disease caused by S. pneumoniae [15,52,53] and S. pyogenes [14,17]. However, the study demonstrating that vaccination against influenza virus limits the incidence of S. pyogenes in military recruits [17] was conducted in a population that is treated prophylactically with antibiotics [54], which complicates the interpretation of these findings.
To date, the one laboratory-controlled study that used a murine model of influenza virus: S. pyogenes super-infection incorporated IIV that was delivered either subcutaneously or intranasally (using cholera toxin subunit B as an adjuvant), and demonstrated that vaccination can reduce mortality associated with these super-infections [14]. This super-infection model was tested following a protocol of inoculation with S. pyogenes at Day 2, when influenza virus is still present at high levels within the lung [41], but IFN-γ has not been induced [4]. This is an approach that may not evaluate the macrophage responses, which have been reported to be dysfunctional due to the effects of IFN-γ on alveolar macrophages [4]. In addition, the concept of vaccination using IIV to limit super-infections was recently reported by Huber et al. using an influenza virus:S. pneumoniae super-infection model [15], but to date, no study has directly compared unadjuvanted IIV- and LAIV-induced immunity toward preventing super-infections.
Compared to IIV, a reported advantage to LAIV is its ability to stimulate mucosal immunity [55]. Previous reports using murine [34], ferret [28], and human subjects [56,57] provide inconsistent data regarding IgA induction by LAIV. Here we demonstrate that both IIV and LAIV vaccines similarly protected mice from mortality following S. pyogenes super-infection compared to unvaccinated controls. While serum antibodies, detected using microneutralization and ELISA, did not demonstrate significant differences between the IIV and LAIV vaccine groups, we demonstrate here that mucosal IgA is increased in mice only after vaccination with LAIV, which would be predicted to complement the immunity detected within serum [56]. The finding that 1 of the 3 mice (33%) within the LAIV group received our highest score from lung pathology analysis (Fig. 4B) correlates with our survival data (Fig. 5), further demonstrating that protection within vaccine recipients, including those that received LAIV, is not complete.
The paradoxical finding that lung homogenates from both vaccinated and unvaccinated mice contained viable S. pyogenes 24 h after super-infection, despite the fact that these mice ultimately survive this super-infection, demonstrates that presence of vaccine-induced immunity against influenza virus at the time of influenza virus exposure does not guarantee prevention of S. pyogenes infection. This finding is even more surprising when it is considered along with the fact that no bacteria are recovered from the lungs 24 h after inoculation when delivered without previous influenza virus exposure, indicating that a host exposed solely to the bacterial pathogen can clear this pathogen. We interpret this finding to demonstrate that cells within the lungs of mice vaccinated against influenza virus are better prepared to respond to a super-infection. Specifically, we posit that the antibodies induced by vaccination interact with influenza virus at the time of inoculation [29] in a manner that limits the macrophage defect that occurs in the absence of these antibodies [4,58–60]. Furthermore, these effects on the macrophage population likely have global effects on other pulmonary cell populations [61–65]. Here we present data that demonstrate a lack of induction of pro-inflammatory cytokines within the lungs of vaccinated animals, specifically IFN-γ, which would limit bacterial clearance by macrophages if it were highly expressed [4]. The increased IFN-γ expression within the lungs of unvaccinated mice correlates with increased lung inflammation, increased viable bacteria within BALF, and increased mortality compared to vaccinated mice that do not demonstrate this increase in IFN-γ expression. These findings demonstrate that our model does not provide “sterilizing immunity” against influenza virus, even though signs of morbidity are not observed in vaccine recipients after challenge with influenza virus (Fig. 5), but suggest that immune cells within the lung are ultimately able to limit the bacteria that persist (Fig. 6) from causing death in these mice. A recent study by Sun et al. [66] demonstrated that reductions in IFN-γ expression in LAIV recipients that are subsequently super-infected with S. pneumoniae yielded protection from this super-infection. Our data demonstrate that both IIV and LAIV have the ability to limit expression of IFN-γ, suggesting a common mechanism for this protection against super-infection that does not depend on IgA induced by LAIV. We have plans to further evaluate the mechanism of S. pyogenes clearance within the lungs of vaccinated animals as it pertains to both viral and bacterial components of the super-infection.
In summary, we established a model to study influenza:S. pyogenes super-infections of the lower respiratory tract. The model was used to compare the contribution of the two types of influenza vaccines currently in use clinically, IIV and LAIV, toward prevention of super-infections. These vaccines did not differ in their ability to provide significant, albeit incomplete protection against super-infection. The results presented also demonstrate that vaccination against influenza virus cannot be considered as the sole preventative method for limiting super-infections, even when morbidity associated with influenza virus alone is severely limited by vaccination. Future studies will determine the host:pathogen interactions that contribute to influenza virus:S. pyogenes super-infections, as well as the contribution of immunity against S. pyogenes toward limiting secondary complications [67].
Acknowledgments
The authors recognize the technical contributions of Kara Pitchford and Kevin Cwach. We also thank Melanee Clark and the Animal Resource Center at USD and acknowledge the contributions of the Flow Cytometry Core Facility at Sanford Research, in particular Satoshi Nagata and John Lee. Finally, we thank Jonathan A. McCullers for providing HK68 viruses for use in these studies and for critically reading the manuscript.
Financial support: New Faculty Development Award from the Office of Research and Sponsored Programs, USD (VCH); U. Discover Undergraduate Research Award, USD (MJS and FPD); SD-BRIN Undergraduate Fellows Program: NIH Grant Number 2 P20 RR016479 from the INBRE Program of the National Center for Research Resources (LAA); Division of Basic Biomedical Sciences, USD
Abbreviations
- IIV
inactivated influenza virus
- LAIV
live, attenuated influenza virus
- TCID50
tissue culture 50% infectious dose
- MLD50
mouse 50% lethal dose
- PBS
phosphate-buffered saline
- i.n
intranasally
- i.m
intramuscularly
- FBS-PBST
10% fetal bovine serum in phosphate-buffered saline supplemented with 0.05% (v/v) Tween-20
Footnotes
Potential conflicts of interest: The authors have no conflicts of interest to disclose.
Portions of this manuscript were presented at the Options for the Control of Influenza VII (September, 2010) in Hong Kong SAR (Abstract Number P-569) and the International Conference on Gram-Positive Pathogens (October, 2010) in Omaha, NE (Poster Number 40).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, Miller MA. Influenza and the winter increase in mortality in the United States, 1959–1999. Am J Epidemiol. 2004;160(5):492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- 2.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198(7):962–70. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peltola VT, McCullers JA. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J. 2004;23(1 Suppl):S87–S97. doi: 10.1097/01.inf.0000108197.81270.35. [DOI] [PubMed] [Google Scholar]
- 4.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med. 2008;14(5):558–64. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 5.McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis. 2002;186(3):341–50. doi: 10.1086/341462. [DOI] [PubMed] [Google Scholar]
- 6.Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis. 2006;6(5):303–12. doi: 10.1016/S1473-3099(06)70466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) - United States, May–August 2009. MMWR Morb Mortal Wkly Rep. 2009;58(38):1071–4. [PubMed] [Google Scholar]
- 8.Ampofo K, Herbener A, Blaschke AJ, Heyrend C, Poritz M, Korgenski K, et al. Association of 2009 pandemic influenza A (H1N1) infection and increased hospitalization with parapneumonic empyema in children in Utah. Pediatr Infect Dis J. 2010;29(10):905–9. doi: 10.1097/INF.0b013e3181df2c70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zakikhany K, Degail M, Lamagni T, Waight P, Guy R, Zhao H, et al. Increase in invasive Streptococcus pyogenes and Streptococcus pneumoniae infections in England, December 2010 to January 2011. Euro Surveill. 2011;16(5) [PubMed] [Google Scholar]
- 10.Nichol KL. Efficacy and effectiveness of influenza vaccination. Vaccine. 2008;26 (Suppl 4):D17–D22. doi: 10.1016/j.vaccine.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 11.Ambrose CS, Yi T, Walker RE, Connor EM. Duration of protection provided by live attenuated influenza vaccine in children. Pediatr Infect Dis J. 2008;27(8):744–8. doi: 10.1097/INF.0b013e318174e0f8. [DOI] [PubMed] [Google Scholar]
- 12.Ohmit SE, Victor JC, Teich ER, Truscon RK, Rotthoff JR, Newton DW, et al. Prevention of symptomatic seasonal influenza in 2005–2006 by inactivated and live attenuated vaccines. J Infect Dis. 2008;198(3):312–7. doi: 10.1086/589885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohmit SE, Victor JC, Rotthoff JR, Teich ER, Truscon RK, Baum LL, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006;355(24):2513–22. doi: 10.1056/NEJMoa061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamoto S, Kawabata S, Fujitaka H, Uehira T, Okuno Y, Hamada S. Vaccination with formalin-inactivated influenza vaccine protects mice against lethal influenza Streptococcus pyogenes superinfection. Vaccine. 2004;22(21–22):2887–93. doi: 10.1016/j.vaccine.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 15.Huber VC, Peltola V, Iverson AR, McCullers JA. Contribution of Vaccine-Induced Immunity toward either the HA or the NA Component of Influenza Viruses Limits Secondary Bacterial Complications. J Virol. 2010;84(8):4105–8. doi: 10.1128/JVI.02621-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belshe RB, Gruber WC. Prevention of otitis media in children with live attenuated influenza vaccine given intranasally. Pediatr Infect Dis J. 2000;19(5 Suppl):S66–S71. doi: 10.1097/00006454-200005001-00010. [DOI] [PubMed] [Google Scholar]
- 17.Lee SE, Eick A, Bloom MS, Brundage JF. Influenza immunization and subsequent diagnoses of group A streptococcus-illnesses among U.S. Army trainees, 2002–2006. Vaccine. 2008;26(27–28):3383–6. doi: 10.1016/j.vaccine.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 18.McCullers JA. Planning for an influenza pandemic: thinking beyond the virus. J Infect Dis. 2008;198(7):945–7. doi: 10.1086/592165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber VC, Thomas PG, McCullers JA. A multi-valent vaccine approach that elicits broad immunity within an influenza subtype. Vaccine. 2009;27(8):1192–200. doi: 10.1016/j.vaccine.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber VC, McKeon RM, Brackin MN, Miller LA, Keating R, Brown SA, et al. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vaccine Immunol. 2006;13(9):981–90. doi: 10.1128/CVI.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis. 2003;187(6):1000–9. doi: 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- 22.Cockburn WC, Delon PJ, Ferreira W. Origin and progress of the 1968–69 Hong Kong influenza epidemic. Bull World Health Organ. 1969;41(3):345–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Beres SB, Sylva GL, Barbian KD, Lei B, Hoff JS, Mammarella ND, et al. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc Natl Acad Sci U S A. 2002;99(15):10078–83. doi: 10.1073/pnas.152298499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kappeler KV, Anbalagan S, Dmitriev AV, McDowell EJ, Neely MN, Chaussee MS. A naturally occurring Rgg variant in serotype M3 Streptococcus pyogenes does not activate speB expression due to altered specificity of DNA binding. Infect Immun. 2009;77(12):5411–7. doi: 10.1128/IAI.00373-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber VC, Kleimeyer LH, McCullers JA. Live, attenuated influenza virus (LAIV) vehicles are strong inducers of immunity toward influenza B virus. Vaccine. 2008;26(42):5381–8. doi: 10.1016/j.vaccine.2008.07.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-8):1–52. [PubMed] [Google Scholar]
- 27.Jin H, Zhou H, Lu B, Kemble G. Imparting temperature sensitivity and attenuation in ferrets to A/Puerto Rico/8/34 influenza virus by transferring the genetic signature for temperature sensitivity from cold-adapted A/Ann Arbor/6/60. J Virol. 2004;78(2):995–8. doi: 10.1128/JVI.78.2.995-998.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huber VC, McCullers JA. Live attenuated influenza vaccine is safe and immunogenic in immunocompromised ferrets. J Infect Dis. 2006;193(5):677–84. doi: 10.1086/500247. [DOI] [PubMed] [Google Scholar]
- 29.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166(12):7381–8. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 30.Regal JF. Immunologic effector mechanisms in animal models of occupational asthma. J Immunotoxicol. 2004;1(1):25–37. doi: 10.1080/15476910490438351. [DOI] [PubMed] [Google Scholar]
- 31.Cimolai N, Taylor GP, Mah D, Morrison BJ. Definition and application of a histopathological scoring scheme for an animal model of acute Mycoplasma pneumoniae pulmonary infection. Microbiol Immunol. 1992;36(5):465–78. doi: 10.1111/j.1348-0421.1992.tb02045.x. [DOI] [PubMed] [Google Scholar]
- 32.Takao Y, Mikawa K, Nishina K, Obara H. Attenuation of acute lung injury with propofol in endotoxemia. Anesth Analg. 2005;100(3):810–6. doi: 10.1213/01.ANE.0000144775.19385.8C. table. [DOI] [PubMed] [Google Scholar]
- 33.Eichelberger M, Golding H, Hess M, Weir J, Subbarao K, Luke CJ, et al. FDA/NIH/WHO public workshop on immune correlates of protection against influenza A viruses in support of pandemic vaccine development, Bethesda, Maryland, US, December 10–11, 2007. Vaccine. 2008;26(34):4299–303. doi: 10.1016/j.vaccine.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Wareing MD, Tannock GA. Route of administration is the prime determinant of IgA and IgG2a responses in the respiratory tract of mice to the cold-adapted live attenuated influenza A donor strain A/Leningrad/134/17/57. Vaccine. 2003;21(23):3097–100. doi: 10.1016/s0264-410x(03)00262-7. [DOI] [PubMed] [Google Scholar]
- 35.Arulanandam BP, O’Toole M, Metzger DW. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. J Infect Dis. 1999;180(4):940–9. doi: 10.1086/314996. [DOI] [PubMed] [Google Scholar]
- 36.Karlstrom A, Boyd KL, English BK, McCullers JA. Treatment with protein synthesis inhibitors improves outcomes of secondary bacterial pneumonia after influenza. J Infect Dis. 2009;199(3):311–9. doi: 10.1086/596051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olsen RJ, Ashraf M, Gonulal VE, Ayeras AA, Cantu C, Shea PR, et al. Lower respiratory tract infection in cynomolgus macaques (Macaca fascicularis) infected with group A Streptococcus. Microb Pathog. 2010 doi: 10.1016/j.micpath.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5(11):685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed R, Oldstone MB, Palese P. Protective immunity and susceptibility to infectious diseases: lessons from the 1918 influenza pandemic. Nat Immunol. 2007;8(11):1188–93. doi: 10.1038/ni1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller MP, Low DE, Green KA, Simor AE, Loeb M, Gregson D, et al. Clinical and epidemiologic features of group a streptococcal pneumonia in Ontario, Canada. Arch Intern Med. 2003;163(4):467–72. doi: 10.1001/archinte.163.4.467. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto S, Kawabata S, Nakagawa I, Okuno Y, Goto T, Sano K, et al. Influenza A virus-infected hosts boost an invasive type of Streptococcus pyogenes infection in mice. J Virol. 2003;77(7):4104–12. doi: 10.1128/JVI.77.7.4104-4112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okamoto S, Kawabata S, Terao Y, Fujitaka H, Okuno Y, Hamada S. The Streptococcus pyogenes capsule is required for adhesion of bacteria to virus-infected alveolar epithelial cells and lethal bacterial-viral superinfection. Infect Immun. 2004;72(10):6068–75. doi: 10.1128/IAI.72.10.6068-6075.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Speshock JL, Doyon-Reale N, Rabah R, Neely MN, Roberts PC. Filamentous influenza A virus infection predisposes mice to fatal septicemia following superinfection with Streptococcus pneumoniae serotype 3. Infect Immun. 2007;75(6):3102–11. doi: 10.1128/IAI.01943-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195(7):1018–28. doi: 10.1086/511989. [DOI] [PubMed] [Google Scholar]
- 45.Chien YW, Klugman KP, Morens DM. Bacterial pathogens and death during the 1918 influenza pandemic. N Engl J Med. 2009;361(26):2582–3. doi: 10.1056/NEJMc0908216. [DOI] [PubMed] [Google Scholar]
- 46.Christenson B, Lundbergh P, Hedlund J, Ortqvist A. Effects of a large-scale intervention with influenza and 23-valent pneumococcal vaccines in adults aged 65 years or older: a prospective study. Lancet. 2001;357(9261):1008–11. doi: 10.1016/S0140-6736(00)04237-9. [DOI] [PubMed] [Google Scholar]
- 47.Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348(18):1747–55. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 48.Aaberge IS. Experience with pneumococcal conjugate vaccine in Norway. Expert Rev Vaccines. 2009;8(2):159–65. doi: 10.1586/14760584.8.2.159. [DOI] [PubMed] [Google Scholar]
- 49.Hu MC, Walls MA, Stroop SD, Reddish MA, Beall B, Dale JB. Immunogenicity of a 26-valent group A streptococcal vaccine. Infect Immun. 2002;70(4):2171–7. doi: 10.1128/IAI.70.4.2171-2177.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dale JB. Current status of group A streptococcal vaccine development. Adv Exp Med Biol. 2008;609:53–63. doi: 10.1007/978-0-387-73960-1_5. [DOI] [PubMed] [Google Scholar]
- 51.Grabenstein JD, Pittman PR, Greenwood JT, Engler RJ. Immunization to protect the US Armed Forces: heritage, current practice, and prospects. Epidemiol Rev. 2006;28:3–26. doi: 10.1093/epirev/mxj003. [DOI] [PubMed] [Google Scholar]
- 52.Ozgur SK, Beyazova U, Kemaloglu YK, Maral I, Sahin F, Camurdan AD, et al. Effectiveness of inactivated influenza vaccine for prevention of otitis media in children. Pediatr Infect Dis J. 2006;25(5):401–4. doi: 10.1097/01.inf.0000217370.83948.51. [DOI] [PubMed] [Google Scholar]
- 53.Clements DA, Langdon L, Bland C, Walter E. Influenza A vaccine decreases the incidence of otitis media in 6- to 30-month-old children in day care. Arch Pediatr Adolesc Med. 1995;149(10):1113–7. doi: 10.1001/archpedi.1995.02170230067009. [DOI] [PubMed] [Google Scholar]
- 54.Gray GC, Callahan JD, Hawksworth AW, Fisher CA, Gaydos JC. Respiratory diseases among U.S. military personnel: countering emerging threats. Emerg Infect Dis. 1999;5(3):379–85. doi: 10.3201/eid0503.990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belshe RB. Current status of live attenuated influenza virus vaccine in the US. Virus Res. 2004;103(1–2):177–85. doi: 10.1016/j.virusres.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 56.Belshe RB, Gruber WC, Mendelman PM, Mehta HB, Mahmood K, Reisinger K, et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis. 2000;181(3):1133–7. doi: 10.1086/315323. [DOI] [PubMed] [Google Scholar]
- 57.Gruber WC, Belshe RB, King JC, Treanor JJ, Piedra PA, Wright PF, et al. Evaluation of live attenuated influenza vaccines in children 6–18 months of age: safety, immunogenicity, and efficacy. National Institute of Allergy and Infectious Diseases, Vaccine and Treatment Evaluation Program and the Wyeth-Ayerst ca Influenza Vaccine Investigators Group. J Infect Dis. 1996;173(6):1313–9. doi: 10.1093/infdis/173.6.1313. [DOI] [PubMed] [Google Scholar]
- 58.Kleinerman ES, Daniels CA, Polisson RP, Snyderman R. Effect of virus infection on the inflammatory response. Depression of macrophage accumulation in influenza-infected mice. Am J Pathol. 1976;85(2):373–82. [PMC free article] [PubMed] [Google Scholar]
- 59.Nickerson CL, Jakab GJ. Pulmonary antibacterial defenses during mild and severe influenza virus infection. Infect Immun. 1990;58(9):2809–14. doi: 10.1128/iai.58.9.2809-2814.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lowy RJ, Dimitrov DS. Characterization of influenza virus-induced death of J774.1 macrophages. Exp Cell Res. 1997;234(2):249–58. doi: 10.1006/excr.1997.3602. [DOI] [PubMed] [Google Scholar]
- 61.Kleinerman ES, Snyderman R, Daniels CA. Depression of human monocyte chemotaxis by herpes simplex and influenza viruses. J Immunol. 1974;113(5):1562–7. [PubMed] [Google Scholar]
- 62.Sawyer WD. Interaction of influenza virus with leukocytes and its effect on phagocytosis. J Infect Dis. 1969;119(6):541–56. doi: 10.1093/infdis/119.6.541. [DOI] [PubMed] [Google Scholar]
- 63.Abramson JS, Mills EL. Depression of neutrophil function induced by viruses and its role in secondary microbial infections. Rev Infect Dis. 1988;10(2):326–41. doi: 10.1093/clinids/10.2.326. [DOI] [PubMed] [Google Scholar]
- 64.Colamussi ML, White MR, Crouch E, Hartshorn KL. Influenza A virus accelerates neutrophil apoptosis and markedly potentiates apoptotic effects of bacteria. Blood. 1999;93(7):2395–403. [PubMed] [Google Scholar]
- 65.Fesq H, Bacher M, Nain M, Gemsa D. Programmed cell death (apoptosis) in human monocytes infected by influenza A virus. Immunobiology. 1994;190(1–2):175–82. doi: 10.1016/S0171-2985(11)80292-5. [DOI] [PubMed] [Google Scholar]
- 66.Sun K, Ye J, Perez DR, Metzger DW. Seasonal FluMist Vaccination Induces Cross-Reactive T Cell Immunity against H1N1 (2009) Influenza and Secondary Bacterial Infections. J Immunol. 2011;186(2):987–93. doi: 10.4049/jimmunol.1002664. [DOI] [PubMed] [Google Scholar]
- 67.Chien YW, Klugman KP, Morens DM. Efficacy of Whole-Cell Killed Bacterial Vaccines in Preventing Pneumonia and Death during the 1918 Influenza Pandemic. J Infect Dis. 2010;202(11):1639–48. doi: 10.1086/657144. [DOI] [PMC free article] [PubMed] [Google Scholar]