Abstract
Background. Iron deficiency is a common cause of anaemia and hyporesponsiveness to erythropoiesis-stimulating agents (ESAs) in non-dialysis-dependent chronic kidney disease (ND-CKD) patients. Current intravenous iron agents cannot be administered in a single high dose because of adverse effects. Ferric carboxymaltose, a non-dextran parenteral iron preparation, can be rapidly administered in high doses.
Methods. This open-label trial randomized 255 subjects with glomerular filtration rates ≤ 45 mL/min/1.73 m2, haemoglobin ≤ 11 g/dL, transferrin saturation ≤ 25%, ferritin ≤ 300 ng/mL, and stable ESA dose to either intravenous ferric carboxymaltose 1000 mg over 15 min (with up to two additional doses of 500 mg at 2-week intervals) or oral ferrous sulphate 325 mg thrice daily for a total of 195 mg elemental iron daily for 56 days.
Results. In the modified intent-to-treat population, the proportion of subjects achieving a haemoglobin increase ≥ 1 g/dL at any time was 60.4% with ferric carboxymaltose and 34.7% with oral iron (P < 0.001). At Day 42, mean increase in haemoglobin was 0.95 ± 1.12 vs 0.50 ± 1.23 g/dL (P = 0.005), mean increase in ferritin was 432 ± 189 ng/mL vs 18 ± 45 ng/mL (P < 0.001) and mean increase in transferrin saturation was 13.6 ± 11.9% vs 6.1 ± 8.1% (P < 0.001). Treatment-related adverse events were significantly fewer with ferric carboxymaltose than with oral iron (2.7% and 26.2%, respectively; P < 0.0001).
Conclusions. We conclude that 1000 mg ferric carboxymaltose can be rapidly administered, is more effective and is better tolerated than oral iron for treatment of iron deficiency in ND-CKD patients.
Keywords: anaemia, CKD, ferritin, intravenous iron, transferrin saturation
Introduction
Iron deficiency is frequently encountered in anaemic non-dialysis-dependent chronic kidney disease (ND-CKD) patients. Indeed, the prevalence of low iron indices such as transferrin saturation (TSAT) levels < 20% and ferritin levels < 100 ng/mL is 20–70%, depending on CKD stage [1–3]. Moreover, iron deficiency is the most common cause of hyporesponsiveness to erythropoiesis-stimulating agents (ESA) [1,4]. On the other hand, iron supplementation improves iron indices and haemoglobin (Hb) concentration, and reduces the required ESA dose [5–11]. Thus, correction of anaemia in CKD patients requires careful balance of the ESA and iron.
The current National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines recommend either oral or intravenous (IV) iron in iron-deficient, anaemic ND-CKD patients [12]. Unfortunately, administration of oral iron is limited by poor gastrointestinal absorption and high rate of adverse events [13–16]. Over 30% of patients may experience adverse events that result in dose reduction and/or non-adherence to treatment [15]. Alternatively, available IV iron formulations are limited by the maximum dose that can be given in a single visit, although higher non-FDA approved doses have been given [17–19]. The administration of the typical 1000-mg course would require at least 10 doses of iron dextran, 8 doses of ferric gluconate, 5 doses of iron sucrose or 2 doses of ferumoxytol, which can be both inconvenient and costly for patients and healthcare providers. Thus, there is a need for an IV iron agent that can be safely administered in large single doses, which require less frequent clinic visits and fewer venipunctures.
Ferric carboxymaltose (FCM, manufactured in the USA by American Regent, Shirley, NY, USA under licence from Vifor Pharma Ltd, Switzerland) is a stable type I polynuclear iron (III)–hydroxide carbohydrate complex. It is a novel, non-dextran iron preparation that was developed for rapid IV administration in high doses for the treatment of iron deficiency anaemia [20–22]. The FCM complex has a nearly neutral pH (5.0–7.0), physiologic osmolarity, and no cross-reactivity with dextran. It is generally less reactive than iron gluconate and iron sucrose, producing slow and controlled delivery of iron into target tissues [20–22]. As a result, rapid infusion of up to 1000 mg of FCM over 15 min is well tolerated [20–22].
This phase 3, open-label, randomized, controlled, multicentre trial was designed to compare the efficacy and safety of large doses of FCM, given intravenously over 15 min, with oral ferrous sulphate to ND-CKD patients.
Materials and methods
This phase 3, open-label, randomized, controlled, multicentre trial was conducted between May 2005 and February 2007 in 47 centres. The study protocol and the informed consent form were approved by the institutional review boards of each participating centre. The trial complied with the Declaration of Helsinki.
Subjects ≥ 12 years of age with estimated glomerular filtration rate (GFR) ≤ 45 mL/min/1.73 m2, Hb level ≤ 11 g/dL, TSAT ≤ 25% and ferritin ≤ 300 ng/mL were enrolled. Those taking ESA had to be on fixed doses for ≥ 8 weeks prior to study enrolment. Exclusion criteria included a history of gastrointestinal intolerance to oral iron, IV iron within 12 weeks prior to the study, significant blood loss or blood transfusion within the last 12 weeks, active infection, concomitant severe liver or cardiovascular diseases, severe psychiatric disorders, pregnant or lactating women, known hepatitis B or hepatitis C infection, HIV, anticipated dialysis or renal transplant in the next 3 months, chronic alcohol or drug abuse within the past 6 months, an investigational drug in the previous 30 days, or any condition which, in the opinion of the investigator, made participation unacceptable.
At randomization, subjects were stratified by severity of CKD based on GFR, baseline Hb and use of ESA. The starting ESA dose was maintained for the duration of the study unless safety concerns dictated a change.
Study design
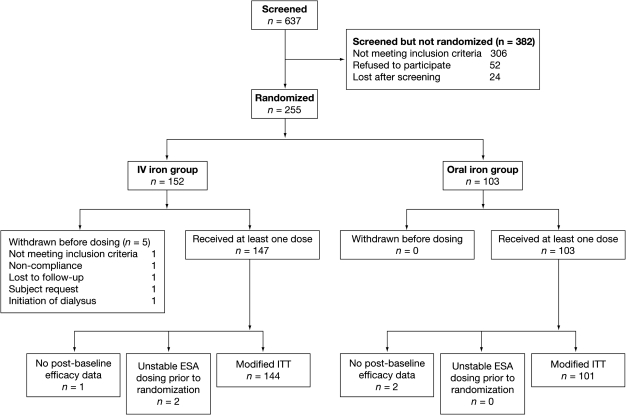
Subjects who provided informed consent entered the 2–9-week screening phase (Figure 1), during which those who met the study criteria underwent history and clinical evaluation, and discontinued any non-study oral iron therapy. Subjects were randomized into the treatment phase at Week 0 by a centralized interactive voice-response system. Initial randomization was in a 2:1 ratio of FCM to oral iron, but because of slow enrolment, the protocol was amended during the trial to a 1:1 ratio. A total of 80 FCM and 40 oral iron subjects were already enrolled at the time of the change in randomization ratio. The remaining 140 were randomized in a 1:1 ratio.
Fig. 1.
Patient disposition.
Study subjects were randomized into two arms. The first arm received up to three IV infusions of FCM (n = 147). The second arm received oral ferrous sulphate 325 mg orally 1 h before meals three times daily (195 mg elemental iron) for 56 days (Figure 1). FCM was infused over 15 min at a maximum dose of 1000 mg (15 mg/kg) diluted in 250 mL normal saline. If TSAT remained < 30% and ferritin remained < 500 ng/mL, two additional doses of FCM, each at a maximum dose of 500 mg in 100 mL normal saline, were infused on approximately Day 17 and/or Day 31. Subjects were evaluated clinically prior to drug administration, immediately after, and 30 and 60 min after FCM infusion.
Laboratory tests were obtained at baseline and all follow-up visits at the end of Week 2, 4, 6 and 8 or early termination visit (End of Study). Samples were analysed by three central laboratories (ICON Laboratories, Farmingdale, NY, USA; SydPath Clinical Trials, Sydney, NSW, Australia; and ALab Clinical Trials Centre, Queen Mary Hospital, Hong Kong, China). All females were tested for pregnancy at baseline and at the end of study.
Statistical analyses
The primary efficacy end point of this study was the percentage of subjects achieving an increase in Hb of ≥ 1.0 g/dL at any study point between baseline and End of Study or introduction or dose increase of ESA, blood transfusion, or use of iron outside of protocol.
The major predefined secondary efficacy end points included percent of subjects achieving a clinical response (defined as increase in Hb ≥ 1.0 g/dL and an increase in ferritin ≥ 160 ng/mL); mean change in Hb from baseline to end of Week 6 (Day 42) and end of Week 8 (Day 56)/End of Study; percentage of subjects achieving an increase from baseline in Hb of ≥ 1.0 g/dL on or before the end of Week 6; and mean change from baseline to highest Hb.
The safety population included all subjects who took at least one dose of study medication. Adverse events were reported from the day of initial treatment to the completion of the study (end of Week 8) or 21 days after the last treatment, whichever was later.
The efficacy analyses were based on the modified intent-to-treat (mITT) population, which included all subjects who received at least one dose of study medication, had results of at least one post-baseline Hb level, had stable ESA dose for at least 8 weeks before randomization and had a GFR ≤ 45 mL/min/1.73 m2. Sample size calculation was based on the assumption that the proportion of subjects with an increase in Hb of ≥ 1.0 g/dL anytime between baseline and end of study or time of intervention would be 45% for FCM and 25% for oral iron.
Treatment differences for proportions were assessed with Fisher’s exact test. The t-test was used for mean changes. The effect of baseline characteristics on the primary efficacy end point was examined by means of logistic regression. Missing data for the summary of percentage of subjects achieving the primary efficacy end point across weeks were estimated with the last observation carried forward. Otherwise, missing data were not imputed.
Results
Of 637 ND-CKD patients screened, 255 were randomly assigned to the study: 152 to FCM and 103 to oral ferrous sulphate. Five subjects in the FCM group and none in the oral iron group were withdrawn before receiving the first dose, leaving 147 subjects in the FCM group and 103 in the oral iron group (Figure 1). In the FCM group, 91.2% of subjects (n = 134) completed the study vs 81.6% (n = 84) in the oral iron group. Three subjects who lacked post-baseline Hb levels were removed from the mITT analysis: one in the FCM group and two in the oral iron group; two subjects in the FCM group were removed because of an unstable dose of ESA before randomization. Thus, a total of 245 subjects were included in the mITT analysis: 144 in the FCM group and 101 in the oral iron group (Figure 1).
Mean subject age was 65.4 years in the FCM group and 66.8 years in the oral iron group (P = 0.39). There were no significant differences between the two groups with respect to demographic or baseline anaemia-related laboratory characteristics (Table 1). The proportion of subjects with baseline Hb ≤ 9.0 g/dL was 10.2% in the FCM group and 13.6% in the oral iron group. The majority of subjects in both treatment groups did not receive an ESA. The percentage of subjects in the mITT safety population who had received previous iron therapy was 44.2% in the FCM group vs 56.3% in the oral iron group (P = 0.07).
Table 1.
Baseline characteristics of subjects who received ≥ 1 dose of the study drug (safety population)
| FCM (n = 147) | Oral iron (n = 103) | P-value | |
|---|---|---|---|
| Age (years) | 65.4 ± 12.6 | 66.8 ± 13.5 | 0.39a |
| Sex (% female) | 63.9 | 70.9 | 0.28b |
| Race (%) | 0.16b | ||
| Caucasian | 49.0 | 58.3 | |
| African American | 27.9 | 26.2 | |
| Hispanic | 15.6 | 9.7 | |
| Asian | 6.1 | 5.8 | |
| Other | 1.4 | 0.0 | |
| Weight (kg) | 84.6 ± 23.0 | 89.5 ± 27.2 | 0.12a |
| Height (cm) | 163.8 ± 11.7 | 164.3 ± 8.6 | 0.73a |
| CKD degree (%) | 0.67b | ||
| 30.1–45.0 mL/min/1.73 m2 | 32.7 | 32.0 | |
| 15.1–30.0 mL/min/1.73 m2 | 53.1 | 49.5 | |
| ≤ 15.0 mL/min/1.73 m2 | 14.3 | 18.4 | |
| Hb (g/dL) | 10.1 ± 0.74 | 10.0 ± 0.86 | 0.38a |
| Hb category (%) | 0.70b | ||
| Hb ≤ 9.0 g/dL | 10.2 | 13.6 | |
| Hb 9.1–10.0 g/dL | 26.5 | 26.2 | |
| Hb 10.1–11.0 g/dL | 63.3 | 60.2 | |
| Ferritin (ng/mL) | 111.8 ± 85.1 | 104.8 ± 75.1 | 0.50a |
| TSAT (%) | 15.4 ± 5.5 | 15.8 ± 5.6 | 0.59a |
| Previous iron therapy (%) | 44.2 | 56.3 | 0.07b |
| History of sensitivity to IV iron (%) | 2.0 | 1.9 | 1.00b |
| ESA use at baseline (%) | 23.8 | 25.2 | 0.88b |
Values are expressed as mean ± standard deviation or percentage.
CKD, chronic kidney disease; ESA, erythropoiesis-stimulating agent; FCM, ferric carboxymaltose; Hb, haemoglobin; IV, intravenous; TSAT, transferrin saturation.
t-test.
Fisher’s exact test.
The mean cumulative dose of iron for the mITT population was 1218 ± 333 mg in the FCM group vs 9322 ± 2638 mg in the oral iron group. Fifty-eight percent of the FCM subjects received only one dose.
Efficacy
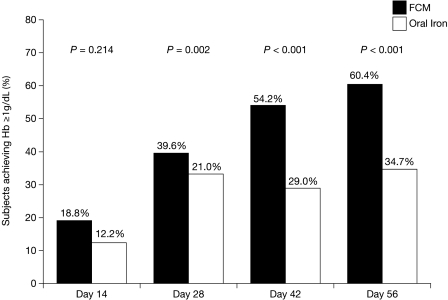
The proportion of subjects who achieved the primary efficacy end point of an increase in Hb of ≥ 1.0 g/dL at any study point between the baseline and End of Study visits was almost twice as great with FCM as with oral iron (60.4% vs 34.7%, P < 0.001) (Figure 2). Greater proportions of FCM subjects compared with oral iron subjects achieved an increase in Hb ≥ 1.0 g/dL on or before each visit (Figure 2). The difference was significant regardless of age, gender, race, or ESA use at baseline. It was also significant in subgroups with baseline ferritin < 100 ng/mL, baseline Hb 10.1–11.0 g/dL and GFR > 15.0 mL/min/1.73 m2 (Table 2). Logistic regression analyses indicated that only higher baseline TSAT values were associated with decreased odds of achieving an increase in Hb ≥ 1.0 g/dL during the study. The superiority of FCM over oral iron was unaffected by baseline TSAT.
Fig. 2.
Percent of subjects (mITT population) achieving Hb ≥ 1 g/dL at any study time point (last observation carried forward). Hb, haemoglobin; mITT, modified intention-to-treat.
Table 2.
Summary of subjects who achieved the primary end point of an increase in Hb ≥ 1.0 g/dL anytime during the study by subgroup (mITT population)
| FCM n/N (%) | Oral iron n/N (%) | P-value | |
|---|---|---|---|
| Baseline Hb | |||
| Hb ≤ 9.0 g/dL | 9/14 (64.3) | 5/14 (35.7) | 0.257 |
| Hb 9.1–10.0 g/dL | 28/38 (73.7) | 14/27 (51.9) | 0.113 |
| Hb 10.1–11.0 g/dL | 50/92 (54.3) | 16/60 (26.7) | < 0.001 |
| CKD degree | |||
| 30.1–45.0 mL/min/1.73 m2 | 33/48 (68.8) | 5/18 (27.8) | 0.192 |
| 15.1–30.0 mL/min/1.73 m2 | 43/75 (57.3) | 18/51 (35.3) | 0.019 |
| ≤ 15.0 mL/min/1.73 m2 | 11/21 (52.4) | 5/18 (37.5) | 0.011 |
| Baseline ferritin | |||
| < 100 ng/mL | 56/78 (71.8) | 21/51 (41.2) | < 0.001 |
| ≥ 100 ng/mL | 31/66 (47.0) | 14/50 (28.0) | 0.054 |
| Baseline TSAT | |||
| < 15% | 47/61 (77.0) | 20/41 (48.8) | 0.005 |
| 15–< 20% | 24/50 (48.0) | 8/35 (22.9) | 0.024 |
| 20–25% | 16/33 (48.5) | 7/25 (28.0) | 0.175 |
| ESA use at baseline | |||
| No | 59/111 (53.2) | 23/77 (29.9) | 0.002 |
| Yes | 28/33 (84.8) | 12/24 (50.0) | 0.008 |
| Race | |||
| Caucasian | 47/71 (66.2) | 22/59 (37.3) | 0.001 |
| Non-Caucasian | 40/73 (54.8) | 13/42 (31.0) | 0.019 |
| Gender | |||
| Female | 54/91 (59.3) | 24/71 (33.8) | 0.002 |
| Male | 33/53 (62.3) | 11/30 (36.7) | 0.039 |
| Age | |||
| < 65 years | 45/64 (70.3) | 16/42 (38.1) | 0.001 |
| ≥ 65 years | 42/80 (52.5) | 19/59 (32.2) | 0.024 |
CKD, chronic kidney disease; ESA, erythropoiesis-stimulating agent; FCM, ferric carboxymaltose; Hb, haemoglobin; mITT, modified intention-to-treat; n/N number of subjects with response/number of subjects in group; TSAT, transferrin saturation.
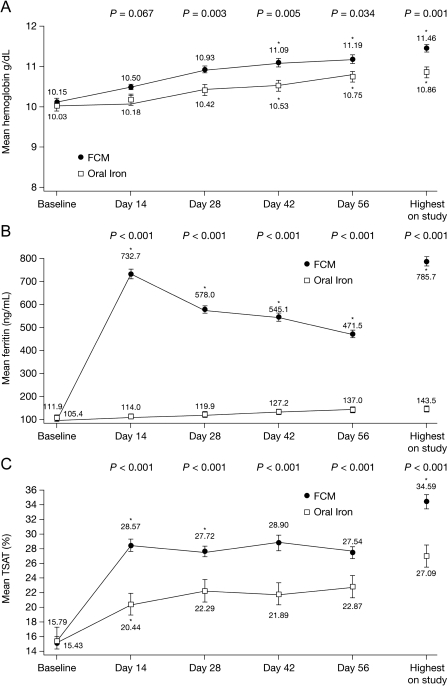
In the mITT population, a statistically significant difference favouring FCM was observed for each of the major secondary efficacy end points. Haemoglobin concentrations increased from baseline in both groups but were higher at every study time point during FCM treatment (Figure 3A). The mean change to highest Hb level was significantly greater throughout the study in the FCM group (1.31 ± 1.11 vs 0.83 ± 1.18 g/dL, P = 0.001), as was the mean increase in Hb by the end of Week 8 (1.05 ± 1.10 vs 0.70 ± 1.25 g/dL; P = 0.034). For subjects receiving < 1 g of FCM, Hb increased from baseline of 10.26 ± 0.66 to 10.97 ± 1.03 g/dL at Day 56 (P < 0.001). For subjects receiving > 1 g of FCM, Hb increased from baseline of 10.0 ± 0.78 to 11.46 ± 1.2 g/dL at Day 56 (P < 0.001). Other Hb-related parameters that were significantly higher in the FCM group were increase from baseline in mean haematocrit, mean corpuscular volume, mean corpuscular Hb, mean reticulocyte haemoglobin and mean red cell distribution width.
Fig. 3.
Mean (SE) for (A) haemoglobin (g/dL), (B) ferritin (ng/mL) and (C) TSAT (%) values by treatment group over time. FCM, ferric carboxymaltose; TSAT, transferrin saturation. *Significantly higher compared with baseline value in the FCM group. P-values are from unpaired two-sample t-tests, assuming equal variances for differences in change from baseline, and are for differences between the FCM and oral iron groups at various study points.
Significant differences favouring FCM were observed for each of the remaining major secondary efficacy end points (proportion of subjects with ferritin change ≥ 160 ng/mL; proportion with Hb change ≥ 1.0 g/dL on or before end of Week 4, 6 and 8; and mean Hb change from baseline to end of Week 4, 6 and 8/End of Study).
The mean increase in serum ferritin values from baseline was significantly greater throughout the treatment period in the FCM group than in the oral iron group. The difference was 620.8 ± 255.2 vs 7.7 ± 34.4 ng/mL at Week 2 and 358.8 ± 178.4 vs 25.8 ± 49.4 ng/mL at Week 8/End of Study in the mITT population (P < 0.001) (Figure 3B). The mean increase to the highest ferritin during the study was also greater with FCM (673.8 ± 232.1 vs 38.1 ± 49.6 ng/mL; P < 0.001) (Figure 3B). The increase from baseline in mean serum ferritin declined slowly from Week 2 to Week 8 in the FCM group. The mean TSAT level also increased significantly more in the FCM-treated subjects at end of Week 8 compared with the oral iron group (12.1 ± 8.8% vs 7.0 ± 10.3%; P < 0.001) (Figure 3C), and greater increases from baseline to the highest TSAT were observed in the FCM-treated subjects (19.2% ± 11.5% vs 11.3% ± 12.7%; P < 0.001). Similarly, the mean increase from baseline in reticulocyte Hb concentration values at end of Week 8 was significantly higher in the FCM group compared with the oral iron group. The increase from baseline to the highest reticulocyte Hb content was also higher in the FCM group (2.46 ± 2.36 vs 1.39 ± 1.98 pg/cell; P < 0.001).
Adherence to therapy during the study was excellent. The proportion of subjects who used at least 67% of the study drug was 97.9% in the FCM treatment group and 82.2% in the oral iron group, and the mean study drug usage based on actual days on study was > 95% for both treatments.
Safety
The most commonly experienced adverse events in the FCM group were peripheral oedema (6.1%), hyperkalaemia (4.1%), urinary tract infection (3.4%), hypotension (3.4%), bronchitis, headache, and infusion site reaction (2.0% each) (Table 3). The corresponding rates in the oral iron were: peripheral oedema (1.9%), hyperkalaemia (1.0%), urinary tract infection (1.0%), hypotension (0.0%), headache (1.9%), bronchitis and infusion site reaction (0.0% each). In the FCM treatment group, four pruritus/skin rash and five hypotensive events occurred. Only one of these events, skin rash, was determined to be drug-related and was rated by the investigator as mild. Each of the hypotensive episodes was determined by the investigator to be mild or moderate in severity and not related to the study drug, and occurred 2–7 weeks after FCM dosing. The most commonly experienced adverse events in the oral iron group were constipation (17.5%), nausea (4.9%), diarrhoea, upper respiratory tract infection (3.9% each), discoloured faeces, and gastrointestinal haemorrhage (2.9% each) (Table 3). Serious adverse events were recorded in 13 (8.8%) subjects in the FCM group, including two subjects who died, and 10 (9.7%) in the oral iron group. One subject who died was an 85-year-old man who had a history of advanced prostate cancer and bladder outlet obstruction, and experienced severe sepsis 33 days following his last and only dose of FCM. The other was a 76-year-old man who died as a result of multiple traumas following a motor vehicle accident. A summary of the subjects who experienced serious adverse events during the study is presented in Supplementary Table 1. Five (3.4%) subjects in the FCM group and seven (6.8%) subjects in the oral iron group were prematurely discontinued from the study drug due to the occurrence of adverse events. All five of the subjects in the FCM group experienced adverse events that were considered to have no relationship with the study drug by the investigator. Three of the seven oral iron subjects who prematurely discontinued the study drug experienced gastrointestinal symptoms that were determined to be probably related to the study drug by the investigator.
Table 3.
Adverse events experienced by ≥ 2% of subjects in either treatment group (safety population)
| MedDRA SOC-preferred term, n (%) | FCM (n = 147) | Oral iron (n = 103) | P-value |
|---|---|---|---|
| ≥ 1 adverse event | 64 (43.5) | 61 (59.2) | 0.02 |
| Gastrointestinal disorders | 12 (8.2) | 40 (38.8) | |
| Constipation | 2 (1.4) | 18 (17.5) | < 0.001 |
| Diarrhoea | 2 (1.4) | 4 (3.9) | 0.23 |
| Faeces discoloured | 0 (0.0) | 3 (2.9) | 0.07 |
| Gastrointestinal haemorrhage | 0 (0.0) | 3 (2.9) | 0.07 |
| Nausea | 2 (1.4) | 5 (4.9) | 0.13 |
| General disorders and administration site conditions | 18 (12.2) | 6 (5.8) | |
| Infusion site reactions | 3 (2.0) | 0 | 0.27 |
| Oedema peripheral | 9 (6.1) | 2 (1.9) | 0.13 |
| Infections and infestations | 20 (13.6) | 8 (7.8) | |
| Bronchitis | 3 (2.0) | 0 | 0.27 |
| Upper respiratory tract infection | 2 (1.4) | 4 (3.9) | 0.23 |
| Urinary tract infection | 5 (3.4) | 1 (1.0) | 0.41 |
| Metabolism and nutrition disorders | 10 (6.8) | 3 (2.9) | |
| Hyperkalaemia | 6 (4.1) | 1 (1.0) | 0.25 |
| Nervous system disorders | 6 (4.1) | 7 (6.8) | |
| Headache | 3 (2.0) | 2 (1.9) | 1.00 |
| Vascular disorders | 9 (6.1) | 2 (1.9) | |
| Hypotension | 3 (2.0) | 0 | 0.08 |
FCM, ferric carboxymaltose; MedDRA, Medical Dictionary for Regulatory Activities; SOC, System Organ Class.
The proportion of subjects who experienced at least one possibly drug-related adverse event was significantly lower in the FCM group compared with the oral iron group: 2.7% in the FCM group and 26.2% in the oral iron group (P = 0.0001) (Table 4). No adverse events that were considered to be drug-related were experienced by more than one subject in the FCM group. In contrast, events experienced by more than one subject in the oral iron group were constipation, discoloured faeces, upper abdominal pain, diarrhoea and nausea. Only one subject in the oral iron group and none in the FCM group experienced severe adverse events that the investigator considered to be study drug-related. That subject had severe upper abdominal pain and severe diarrhoea on study Day 1 and was discontinued from the study.
Table 4.
Possibly or probably drug-related adverse events experienced by ≥ 1 subject in either treatment group
| MedDRA SOC-preferred term, n (%) | FCM (n = 147) | Oral iron (n = 103) | P-value |
|---|---|---|---|
| ≥ 1 adverse event | 4 (2.7) | 27 (26.2) | < 0.0001 |
| Gastrointestinal disorders | 1 (0.7) | 27 (26.2) | < 0.0001 |
| Abdominal distension | 0 | 1 (1.0) | 0.41 |
| Abdominal pain (upper) | 0 | 2 (1.9) | 0.17 |
| Constipation | 0 | 17 (16.5) | < 0.0001 |
| Diarrhoea | 1 (0.7) | 2 (1.9) | 0.57 |
| Eructation | 0 | 1 (1.0) | 0.41 |
| Faeces discoloured | 0 | 3 (2.9) | 0.07 |
| Flatulence | 0 | 1 (1.0) | 0.41 |
| Frequent bowel movements | 0 | 1 (1.0) | 0.41 |
| Nausea | 0 | 2 (1.9) | 0.17 |
| Vomiting | 0 | 1 (1.0) | 0.41 |
| General disorders and administration site conditions | 1 (0.7) | 0 | 1.00 |
| Rigors | 1 (0.7) | 0 | 1.00 |
| Skin and subcutaneous tissue disorders | 2 (1.4) | 0 | 0.51 |
| Rash | 1 (0.7) | 0 | 1.00 |
| Skin discolouration | 1 (0.7) | 0 | 1.00 |
FCM, ferric carboxymaltose; MedDRA, Medical Dictionary for Regulatory Activities; SOC, System Organ Class.
A significantly greater but not clinically relevant increase in gamma glutamyl transferase (GGT) from baseline to end of Week 8 was observed in the FCM group compared with the oral iron group (9.1 vs 0.6 IU/L). However, there was no relationship between the FCM dose and the changes in GGT.
Four (3.8%) subjects in the FCM group had low serum phosphorus levels during the study, but none in the oral iron group. The low values were transient and did not result in any clinical adverse event. The lowest serum phosphorus level during the study was 1.7 mg/dL.
Discussion
Treatment of iron deficiency in ND-CKD patients can be challenging. Oral iron is associated with significant adverse events and high degree of non-compliance [13–16,23,24]. Unfortunately, currently available IV iron agents have their own limitations and have not been consistently shown to be superior to oral iron in randomized controlled trials [6,14–16,24,25]. A few studies suggest that oral iron may be as effective as IV iron [15,24]; however, a greater number of studies show that IV iron is more effective in increasing Hb levels and in addition replenishing iron stores [14,25].
This randomized multicentre study in ND-CKD patients showed that FCM can overcome many of these challenges as it can be rapidly administered in 1000-mg IV dose in a single clinic visit. Moreover, the study showed that FCM is significantly more effective in treating iron deficiency anaemia than daily administration of oral ferrous sulphate over an 8-week treatment period. The superior efficacy of FCM compared with oral iron in increasing Hb level, replenishing iron stores and improving the availability of iron for erythropoiesis was clearly demonstrated. Subjects in the FCM group were almost twice as likely as those in the oral iron group to achieve the primary end point of an increase in Hb ≥ 1.0 g/dL. This was true regardless of whether the subjects were receiving ESA therapy at baseline and regardless of their baseline ferritin level. Similarly, achievement of other anaemia-related efficacy end points, such as mean increase in Hb, haematocrit and reticulocyte Hb content, was also greater in the FCM group. Also, FCM was more effective than oral iron in increasing body iron supply for erythropoiesis. The mean increase in ferritin levels indicated greater repletion of body iron stores with FCM than with oral iron. Similarly, the mean level of TSAT, another indicator of iron supply for erythropoiesis, increased significantly more with FCM treatment than with oral iron. In addition, iron repletion was significantly faster with FCM than with oral iron; the difference could be clearly identified by 2 weeks after the first dose of FCM.
In contrast, administration of large doses of other IV iron agents has been associated with high rates of adverse events. Iron dextran can be administered as high-dose infusion but may be associated with life-threatening anaphylaxis or death [18,19,26–28]. Although second-generation parenteral iron products are much less likely to cause anaphylactic reactions, doses of iron sucrose > 300 mg per infusion or ferric gluconate > 250 mg are not recommended, as they have been associated with higher rates of adverse reactions such as hypotension [2,14,16,29–33]. In a study of iron sucrose, adverse events occurred in 36% of subjects who received 500-mg doses [29]. Another study reported that an infusion of 500 mg of iron sucrose over 3.5–4 h was associated with hypotension in 6.7% of subjects [14]. Similarly, administration of 500 mg of sodium ferric gluconate over 5 h resulted in a 30% rate of adverse events including hypotension [30]. Thus, safe administration of a typical 1000-mg course of IV iron sucrose or ferric gluconate will require either long infusion times [14,30] or from five to eight clinic visits with repeated venipuncture. This is clearly inconvenient for outpatient administration and adds to the expense for both patients and healthcare providers. In contrast, 1000 mg of FCM can be administered in a single dose in only 15 min, which would require fewer clinic visits and venipunctures. Thus, FCM appears to be better suited for outpatient use in ND-CKD patients. Only one other IV iron preparation, ferumoxytol, which can be quickly administered in 500-mg doses, has been shown to be superior to oral iron, but it still requires two separate clinic visits for a 1000-mg course [25].
Besides its efficacy, FCM administration was well tolerated and was associated with fewer adverse events than oral iron. No adverse event that was considered drug-related occurred in more than one FCM-treated subject, and no anaphylactoid reactions were reported. In subjects receiving oral iron, however, adverse events included constipation, discoloured faeces, upper abdominal pain, diarrhoea and nausea. Thus, while oral iron is a convenient and less expensive option of treating iron deficiency in patients with CKD, its efficacy in replenishing iron stores may be limited by its ineffective absorption, potential for gastrointestinal adverse events and non-compliance [14,15,22].
Transient hypophosphataemia that was observed in four subjects (2.7%) in the FCM group was not associated with any clinically relevant adverse event and was much less frequent than previously reported in women with heavy uterine bleeding who received IV FCM therapy [22]. The cause of hypophosphataemia in these subjects is not clear, but this adverse effect has been previously reported in other haematopoietic disorders [34–36]. The decrease in serum phosphorus in these clinical conditions suggests increased cellular uptake of phosphorus during stimulated erythropoiesis [37,38]. However, renal tubular leak of phosphorus cannot be excluded. A recent study by Schouten et al. implicated increased FGF-23 levels in the development of hypophosphataemia after parenteral iron administration [39]. There is an obvious need for further evaluation of this issue in FCM-treated patients in order to ascertain its mechanism and clinical consequences, if any.
Among the multiple developmental clinical trials with FCM in various clinical conditions, including CKD, there was a numerical imbalance in deaths (5/1206 with FCM and 1/994 with iron sucrose in controlled trials, and 5/925 with FCM in uncontrolled trials) including the two deaths in the subjects who were part of the current study. None of these two deaths was determined by the investigator to be related to the study medication. Although the FDA issued a non-approval letter, pending further studies to assess the safety of FCM, the drug has been registered by the public health authorities in Europe since 2007 and has been used to treat iron deficiency anaemia in over 20 countries. Current studies are underway in the USA to evaluate more rigorously this issue [40].
There are a number of limitations to our study. First, the duration of the study was relatively short; this does not allow conclusions about long-term efficacy and safety of FCM compared with oral iron. Second, the relative cost of FCM compared with oral iron and other IV iron preparations is not currently available. These comparisons will have to be inclusive of the cost of supplies such as infusion sets, nursing time and, most importantly, patient convenience.
In conclusion, IV administration of FCM was more effective than oral iron for increasing Hb level and replenishing iron stores in ND-CKD patients with iron deficiency anaemia and was associated with fewer adverse events. Importantly, because it can be given in large single doses, ferric carboxymaltose is more convenient than oral iron therapy for outpatient administration in ND-CKD patients.
Supplementary data
Supplementary data is available online at http://ndt.oxfordjournals.org.
Acknowledgments
The study was presented in part at the annual meeting of the American Society of Nephrology in San Francisco, CA, USA, November 2007. All authors were involved in the recruitment of patients for the study. This study was supported by American Regent, Shirley, NY, USA. The authors gratefully acknowledge the contributions of patients who participated in the study, study coordinators for their help with patients’ recruitment and conduct of the study, and the staff of the renal clinics. We also thank Todd Koch (Luitpold Pharmaceuticals Inc, Norristown, PA, USA), and William C. Fridrich, RPh (American Regent, Inc) for editorial support in preparation of this manuscript.
Conflict of interest statement. W.Y.Q. has received research grants from American Regent and is on the speaker bureau of American Regent. S.D.R. has received commercial assistance, speaker’s fees and financial sponsorship to attend conferences, advisory boards and clinical trials from Amgen, Janssen Cilag, Roche, Sandoz and Vifor Pharma. J.B. has received consulting fees and speaker’s fees from American Regent.
References
- 1.Hsu CY, McCulloch CE, Curhan GC. Iron status and hemoglobin level in chronic renal insufficiency. J Am Soc Nephrol. 2002;13:2783–2786. doi: 10.1097/01.asn.0000034200.82278.dc. [DOI] [PubMed] [Google Scholar]
- 2.Gotloib L, Silverberg D, Fudin R, et al. Iron deficiency is a common cause of anemia in chronic kidney disease and can often be corrected with intravenous iron. J Nephrol. 2006;19:161–167. [PubMed] [Google Scholar]
- 3.Post JB, Wilkes BM, Michelis MF. Iron deficiency in patients with chronic kidney disease: potential role for intravenous iron therapy independent of erythropoietin. Int Urol Nephrol. 2006;38:719–723. doi: 10.1007/s11255-006-0035-0. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg DS, Iaina A, Peer G, et al. Intravenous iron supplementation for the treatment of the anemia of moderate to severe chronic renal failure patients not receiving dialysis. Am J Kidney Dis. 1996;27:234–238. doi: 10.1016/s0272-6386(96)90546-6. [DOI] [PubMed] [Google Scholar]
- 5.Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008;74:791–798. doi: 10.1038/ki.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal HK, Nand N, Singh S, et al. Comparison of oral versus intravenous iron therapy in predialysis patients of chronic renal failure receiving recombinant human erythropoietin. J Assoc Physicians India. 2003;51:170–174. [PubMed] [Google Scholar]
- 7.Besarab A, Amin N, Ahsan M, et al. Optimization of epoetin therapy with intravenous iron therapy in hemodialysis patients. J Am Soc Nephrol. 2000;11:530–538. doi: 10.1681/ASN.V113530. [DOI] [PubMed] [Google Scholar]
- 8.DeVita MV, Frumkin D, Mittal S, et al. Targeting higher ferritin concentrations with intravenous iron dextran lowers erythropoietin requirement in hemodialysis patients. Clin Nephrol. 2003;60:335–340. doi: 10.5414/cnp60335. [DOI] [PubMed] [Google Scholar]
- 9.Macdougall IC, Tucker B, Thompson J, et al. A randomized controlled study of iron supplementation in patients treated with erythropoietin. Kidney Int. 1996;50:1694–1699. doi: 10.1038/ki.1996.487. [DOI] [PubMed] [Google Scholar]
- 10.Taylor JE, Peat N, Porter C, et al. Regular low-dose intravenous iron therapy improves response to erythropoietin in haemodialysis patients. Nephrol Dial Transplant. 1996;11:1079–1083. [PubMed] [Google Scholar]
- 11.Sunder-Plassmann G, Hörl WH. Importance of iron supply for erythropoietin therapy. Nephrol Dial Transplant. 1995;10:2070–2076. [PubMed] [Google Scholar]
- 12.K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Part 6. Association of Level of GFR with Complications in Adults. Guideline 12. Association of Level of GFR with Indices of Functioning and Well-Being. http://www.kidney.org/Professionals/Kdoqi/guidelines_ckd/p6_comp_g12.htm (21 May 2010, last accessed date) [Google Scholar]
- 13.Macdougall IC. Strategies for iron supplementation: oral versus intravenous. Kidney Int Suppl. 1999;69:S61–S66. doi: 10.1046/j.1523-1755.1999.055suppl.69061.x. [DOI] [PubMed] [Google Scholar]
- 14.Van Wyck DB, Roppolo M, Martinez CO, et al. For the United States Iron Sucrose (Venofer) Clinical Trials Group. A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int. 2005;68:2846–2856. doi: 10.1111/j.1523-1755.2005.00758.x. [DOI] [PubMed] [Google Scholar]
- 15.Charytan C, Qunibi W, Bailie GR. Comparison of intravenous iron sucrose to oral iron in the treatment of anemic patients with chronic kidney disease not on dialysis. Nephron Clin Pract. 2005;100:c55–c62. doi: 10.1159/000085049. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R, Rizkala AR, Bastani B, et al. A randomized controlled trial of oral versus intravenous iron in chronic kidney disease. Am J Nephrol. 2006;26:445–454. doi: 10.1159/000096174. [DOI] [PubMed] [Google Scholar]
- 17.Folkert VW, Michael B, Agarwal R, et al. Chronic use of sodium ferric gluconate complex in hemodialysis patients: safety of higher-dose (≥250 mg) administration. Am J Kidney Dis. 2003;41:651–657. doi: 10.1053/ajkd.2003.50141. [DOI] [PubMed] [Google Scholar]
- 18.Fishbane S. Safety in iron management. Am J Kidney Dis. 2003;41:S18–S26. doi: 10.1016/s0272-6386(03)00373-1. [DOI] [PubMed] [Google Scholar]
- 19.Nissenson AR, Charytan C. Controversies in iron management. Kidney Int. 2003;64:S64–S71. doi: 10.1046/j.1523-1755.64.s87.10.x. [DOI] [PubMed] [Google Scholar]
- 20.Geisser P. The pharmacology and safety profile of ferric carboxymaltose (Ferinject(r)): structure/reactivity relationships of iron preparations. Port J Nephrol Hypert. 2009;23:11–16. [Google Scholar]
- 21.Kulnigg S, Stoinov S, Simanenkov V, et al. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103:1182–1192. doi: 10.1111/j.1572-0241.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- 22.van Wyck DB, Martens MG, Seid MH, et al. Intravenous ferric carboxymaltose compared with oral iron in the treatment of postpartum anemia: a randomized controlled trial. Obstet Gynecol. 2007;110:267–278. doi: 10.1097/01.AOG.0000275286.03283.18. [DOI] [PubMed] [Google Scholar]
- 23.Srigiridhar K, Nair KM, Subramanian R, et al. Oral repletion of iron induces free radical mediated alterations in the gastrointestinal tract of rat. Mol Cell Biochem. 2001;219:91–98. doi: 10.1023/a:1011023111048. [DOI] [PubMed] [Google Scholar]
- 24.Stoves J, Inglis H, Newstead CG. A randomized study of oral vs intravenous iron supplementation in patients with progressive renal insufficiency treated with erythropoietin. Nephrol Dial Transplant. 2001;16:967–974. doi: 10.1093/ndt/16.5.967. [DOI] [PubMed] [Google Scholar]
- 25.Spinowitz BS, Kausz AT, Baptista J, et al. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol. 2008;19:1599–1605. doi: 10.1681/ASN.2007101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailie GR, Clark JA, Lane CE, et al. Hypersensitivity reactions and deaths associated with intravenous iron preparations. Nephrol Dial Transplant. 2005;20:1443–1449. doi: 10.1093/ndt/gfh820. [DOI] [PubMed] [Google Scholar]
- 27.Chertow GM, Mason PD, Vaage-Nilsen O, et al. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21:378–382. doi: 10.1093/ndt/gfi253. [DOI] [PubMed] [Google Scholar]
- 28.Faich G, Strobos J. Sodium ferric gluconate complex in sucrose: safer intravenous iron therapy than iron dextrans. Am J Kidney Dis. 1999;33:464–470. doi: 10.1016/s0272-6386(99)70183-6. [DOI] [PubMed] [Google Scholar]
- 29.Chandler G, Harchowal J, Macdougall IC. Intravenous iron sucrose: establishing a safe dose. Am J Kidney Dis. 2001;38:988–991. doi: 10.1053/ajkd.2001.28587. [DOI] [PubMed] [Google Scholar]
- 30.Bastani B, Jain A, Pandurangan G. Incidence of side-effects associated with high-dose ferric gluconate in patients with severe chronic renal failure. Nephrology (Carlton) 2003;8:8–10. doi: 10.1046/j.1440-1797.2003.00118.x. [DOI] [PubMed] [Google Scholar]
- 31.Schroder O, Mickisch O, Seidler U, et al. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease—a randomized, controlled, open-label, multicenter study. Am J Gastroenterol. 2005;100:2503–2509. doi: 10.1111/j.1572-0241.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 32.Ferrlecit® [package insert] Corona, CA: Watson Pharma, Inc.; 2001. [Google Scholar]
- 33.Venofer® [package insert] Shirley, NY: American Regent Laboratories, Inc.; 2000. [Google Scholar]
- 34.Mohammed S, Knoll S, van AA III, et al. Cefotetan-induced hemolytic anemia causing severe hypophosphatemia. Am J Hematol. 1994;46:369–370. doi: 10.1002/ajh.2830460422. [DOI] [PubMed] [Google Scholar]
- 35.Sahara N, Tamashima S, Ihara M. Hereditary spherocytosis associated with severe hypophosphatemia in patients recovering from aplastic crisis. Rinsho Ketsueki. 1998;39:386–391. [PubMed] [Google Scholar]
- 36.Steiner M, Steiner B, Wilhelm S, et al. Severe hypophosphatemia during hematopoietic reconstitution after allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2000;25:1015–1016. doi: 10.1038/sj.bmt.1702407. [DOI] [PubMed] [Google Scholar]
- 37.Campos MS, Barrionuevo M, Alferez MJ, et al. Interactions among iron, calcium, phosphorus and magnesium in the nutritionally iron-deficient rat. Exp Physiol. 1998;83:771–781. doi: 10.1113/expphysiol.1998.sp004158. [DOI] [PubMed] [Google Scholar]
- 38.McLane JA, Fell RD, McKay RH, et al. Physiological and biochemical effects of iron deficiency on rat skeletal muscle. Am J Physiol. 1981;241:C47–C54. doi: 10.1152/ajpcell.1981.241.1.C47. [DOI] [PubMed] [Google Scholar]
- 39.Schouten BJ, Hunt PJ, Livesey JH, et al. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab. 2009;94:2332–2337. doi: 10.1210/jc.2008-2396. [DOI] [PubMed] [Google Scholar]
- 40.Szczech LA, Bregman DB, Harrington RA, et al. Randomized evaluation of efficacy and safety of ferric carboxymaltose in patients with iron deficiency anaemia and impaired renal function (REPAIR-IDA): rationale and study design. Nephrol Dial Transplant. 2010;25:2368–2375. doi: 10.1093/ndt/gfq218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.