Abstract
Background. Elderly patients with end-stage renal disease and severe extra-renal comorbidity have a poor prognosis on renal replacement therapy (RRT) and may opt to be managed conservatively (CM). Information on the survival of patients on this mode of therapy is limited.
Methods. We studied survival in a large cohort of CM patients in comparison to patients who received RRT.
Results. Over an 18-year period, we studied 844 patients, 689 (82%) of whom had been treated by RRT and 155 (18%) were CM. CM patients were older and a greater proportion had high comorbidity. Median survival from entry into stage 5 chronic kidney disease was less in CM than in RRT (21.2 vs 67.1 months: P < 0.001). However, in patients aged > 75 years when corrected for age, high comorbidity and diabetes, the survival advantage from RRT was ~ 4 months, which was not statistically significant. Increasing age, the presence of high comorbidity and the presence of diabetes were independent determinants of poorer survival in RRT patients. In CM patients, however, age > 75 years and female gender independently predicted better survival.
Conclusions. In patients aged > 75 years with high extra-renal comorbidity, the survival advantage conferred by RRT over CM is likely to be small. Age > 75 years and female gender predicted better survival in CM patients. The reasons for this are unclear.
Keywords: Chronic kidney disease, Comorbidity, Conservative management, Elderly, Survival
Introduction
Twenty-five years ago, a landmark publication drew attention to the rationing of access to renal replacement therapy (RRT) in the UK [1]. In 1981, only 26.7 new patients per million population (pmp) had been accepted into RRT programmes in the UK, in contrast to 42.3 pmp in France and > 60 pmp in the USA. Rationing was most striking in those aged ≥ 45 years. Following these revelations, there was a welcome liberalization of access to RRT, fuelling a dramatic increase in acceptance rates, mirroring those across the developed world. In the early years of the new millennium, the incidence rates in the UK, like those in Northern Europe, stabilized at ~ 110 pmp, though far below those in the USA currently at 361 pmp [2–4]. As a result, RRT populations throughout the developed world have expanded rapidly, the fastest growth occurring in the elderly, many of whom are dependent, and frail.
The benefits of dialysis for elderly, dependent patients with end-stage renal disease (ESRD), who often have multiple extra-renal comorbidities, have been questioned. The prospects for rehabilitation in such patents tend to be slim, and prognosis is often poor [5,6]. Dialysis in such circumstances can pose huge additional burdens for patients and their carers. The practice of withholding dialysis in such circumstances has been common [7–9]. In recent years, the concept of the conservative management programme has gained sway in an attempt to provide a comprehensive package of care to patients who have chosen to forego dialysis. Conservative management of ESRD involves a shift from efforts to prolong life to those which focus on care, quality of life and symptom control. Control of fluid and electrolyte balance, anaemia management by use of erythropoietin if need be, the provision of appropriate end-of-life care and ongoing support for the patient and family/carers are important aspects [10]. The concept has been embodied as a switching focus from curing to caring, though the notion of cure in the context of ESRD may be stretching the point [11].
It is important to distinguish this rational and appropriate use of therapies from the rationing approach referred to above. Rationing in this context refers to the limiting of access to expensive medical interventions in order to control the use of resources. A rational or appropriate approach refers to foregoing therapies in circumstances in which their use is likely to be futile or detrimental to patient well-being.
A number of studies have described the outcome of conservative kidney management [10,12–17]. In the comparative studies, dialysis for elderly patients with high comorbidity did not confer a significant survival advantage over conservative management [10,12], at least in terms of hospital-free days [13]. In all these studies, the numbers of patients treated by conservative kidney management were small and follow-up relatively short. We studied a large series of conservatively managed patients and compared survival to those of a contemporaneous group of RRT patients. We also studied the predictors of survival in both groups.
Materials and methods
Patients
We reviewed the computerized records of all patients who had attended our nephrology clinics with progressive chronic kidney disease (CKD). We selected for further study all patients who had progressed as far as stage 5 CKD based on eGFR estimated by MDRD-4 equation. This was over an 18-year period to August 2008. To meet this criterion, each patient had:
At least one value of eGFR in the range 10–15 mL/min/1.73 m2
All subsequent recorded values of eGFR < 15 mL/min/1.73 m2
We examined detailed computerized records and the case notes for all patients included in the study.
Survival in all patients was calculated from the date of the first recorded value in stage 5 CKD as outlined above. Patients who presented in advanced stage 5 CKD (eGFR < 10) were not included since the unavailability of the date at which they reached stage 5 CKD made it impossible to estimate survival time from entry to stage 5.
Patients were categorized into RRT patients or conservatively managed patients. Patients were designated as RRT patients if they:
Subsequently commenced on dialysis either haemodialysis (HD) or peritoneal dialysis (PD)—OR
Subsequently underwent pre-emptive transplantation—OR
Had made a decision to commence dialysis and had begun preparations for this, often involving the creation of an A-V fistula, but had died before dialysis initiation.
Patients were designated as conservatively managed if they had made a decision to forego dialysis, should their kidney failure continue to progress.
Modality choice
We aimed to offer patients a free choice of modality constrained only by clinical and social imperatives. After the diagnosis of progressive CKD, patients were referred by the nephrologists to a liaison team, led by a senior nurse and a renal counsellor. This referral usually took place when the patient had stage 4 CKD—but for a minority of patients this was sometimes later. The role of the team included assessment, education, counselling and support of the patient and family/carer before, during and after modality choice. This process included a number of interviews with each patient and significant others and at least one visit to the patient’s home. After subsequent discussions within the multidisciplinary team, treatment options were discussed with each patient and significant others and an individualized treatment plan formulated. Some patients with low comorbidity chose CM when the chances of survival could have been higher on RRT. They were specifically counselled on the potential benefits of RRT, but their final decision was respected.
Conservative management programme
Patients opting for conservative management were offered ongoing support by the multidisciplinary team in liaison with community, primary care and hospice services. Full medical treatment was continued, which included the use of erythropoietin as appropriate to treat or prevent anaemia.
Dialysis programme
HD patients were treated exclusively using high-flux membranes, predominantly polysulphone. Around 40% of patients were treated by online haemodiafiltration (HDF). Bicarbonate was used exclusively as the buffer, and ultrapure water was standard. Target total two-pool Kt/V urea (Kt/VTotal) was 1.2 per session for thrice-weekly HD and HDF. PD patients were treated by continuous ambulatory peritoneal dialysis (CAPD) or automated peritoneal dialysis. Disconnect systems were used exclusively for CAPD. Minimum weekly Kt/VTotal target for both PD modes was 2.0.
Data collected
The following information was obtained on all study patients from contemporaneous case notes and computer records:
Date of entry into stage 5 CKD—defined as the date of the first value of eGFR in the range 10–15 mL/min/1.73 m2.
Age—at entry into stage 5 CKD [see (i)]
Gender
Ethnicity—defined as white/non-white
Presence of diabetes mellitus
The presence and severity of extra-renal comorbidity. Patients were scored on the number and severity of the following conditions: cardiac disease, peripheral vascular disease, cerebrovascular disease and respiratory disease. The severity of these diseases was scored as 0 = none, 1 = minimal, 2 = mild, 3 = moderate and 4 = advanced. Cancer was also graded (1–4) according to its activity and nature (medium-term survival). Cirrhosis was scored as a 4. Scores were summed to form a combined comorbidity score [5]. A score of > 4 was graded as ‘high’ and a score ≤ 4 was deemed as ‘low’.
Serum creatinine level and eGFR (calculated by the MDRD-4 equation), at the time of entry into stage 5 CKD [see (i)]
Date of death
Statistical analysis
Groups were compared, using unpaired t-tests and chi-square tests as appropriate, with respect to age, gender ratio, ethnicity (white vs non-white), the proportion with diabetes mellitus, comorbidity severity score and eGFR on entry into stage 5 CKD. The Kaplan–Meier method was used to compare the survival time between different groups. Differences were assessed using the log-rank test. We used Cox proportional hazards model to determine the predictors of survival. We used SPSS version 18 for all statistical analyses.
Results
The total number of patients selected for study was 844, 689 (82%) of whom had been treated by RRT and 155 (18%) had been managed conservatively. The RRT group included 18 patients (2.6% of the RRT group) in whom dialysis had been planned, but died before dialysis initiation. Using intention-to-treat analysis, these 18 patients were included in all analyses. Patients who received conservative treatment were significantly older than those treated by RRT, and a much greater proportion of them had high comorbidity (Table 1). RRT and conservatively managed patients did not differ with respect to the distribution of gender or ethnicity (white vs non-white), nor with respect to the prevalence of diabetes. Estimated GFR at the onset of the study period was also similar in RRT and conservatively managed groups (Table 1). Patients with severe comorbidity started dialysis at a significantly higher mean eGFR compared to those with low comorbidity (8.71, SD 2.58 vs 8.03, SD 2.54; P = 0.017).
Table 1.
Demographic and clinical details of patients treated by dialysis and conservative kidney management
| Conservative | Dialysis | P-value | |
|---|---|---|---|
| Number | 155 (18%) | 689 (82%) | |
| Age at stage 5 (years) | 77.5 ± 7.6 | 58.5 ± 15.0 | < 0.001 |
| % > 75 years | 68.4 | 11.2 | < 0.001 |
| % Male | 59.4 | 66.6 | NS |
| % Non-white | 14.2 | 15.7 | NS |
| % Diabetes | 35.5 | 34.3 | NS |
| % High comorbidity | 49.7 | 17.3 | < 0.001 |
| eGFR at stage 5 (mL/min/1.73 m2) | 13.2 ± 1.4 | 13.2 ± 1.4 | NS |
Of the 155 patients in the CM group, 29 (18.7%) were surviving at last analysis. Of the remaining 126 patients, 57 (45.2%) died with a last recorded eGFR < 8.22 mL/min/1.73 m2 (median eGFR at start of dialysis in elderly patients) and 69 (54.8%) had last recorded eGFR higher than this. However, it is not possible to conclude that renal failure did not contribute to death in the latter group or even that at least some of these patients would not already have started dialysis had they chosen this option.
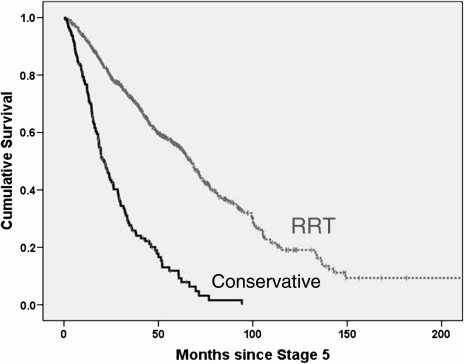
Considering the whole group of 844 patients, median survival in Kaplan–Meier analysis was far superior in RRT patients than in those conservatively managed (67.1 vs 21.2 months: P < 0.001: Figure 1). Median survival was also greater in those aged ≤ 75 years than in older patients (67.0 vs 28.5 months: P < 0.001), in those without high comorbidity compared to those with comorbidity (68.4 vs 25.1 months: P < 0.001) and in those without diabetes than in those with diabetes (63.9 vs 44.7 months: P < 0.001). Gender and ethnicity did not predictably influence survival. In the RRT group, there was improved median survival in patients aged < 75 years (69.6 vs 33.0 months: P < 0.001), in the absence of high comorbidity (72.5 vs 33.0 months: P < 0.001) and in non-diabetics (74.4 vs 52.8 months: P < 0.001). Gender and ethnicity again did not influence survival. In contrast, in the conservatively managed group, patients > 75 years had improved median survival compared with younger patients (25.1 vs 15.5 months: P = 0.001), as did non-diabetics (24.4 vs 18.1 months: P = 0.011). Although median survival was a little higher in patients without high comorbidity in this group (26.0 vs 17.4 months), the difference was not statistically significant (P = 0.124). Women also had a slightly higher median survival than men (24.0 vs 19.5 months: P = 0.084). There were no ethnic differences.
Fig. 1.
Kaplan–Meier survival curves from entry into stage 5 CKD for patients treated by RRT (n = 689) and by conservative kidney management (n = 155).
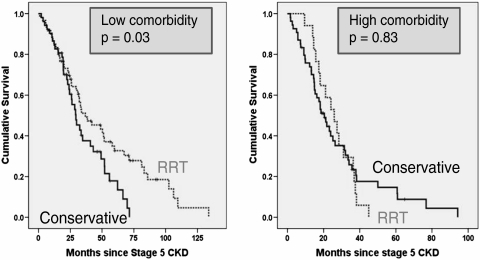
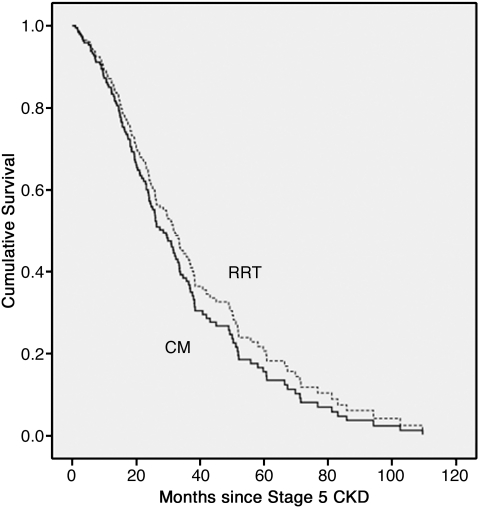
Considering all patients aged > 75 years (Table 2 and Figure 2), those without high comorbidity had better survival when treated by RRT rather than by conservative means (36.8 vs 29.4 months: P = 0.03). However, patients in this age group with high comorbidity, treatment by RRT was associated with a smaller increase in median survival, of around 5 months, which was not statistically significant (Table 2 and Figure 2). Even within this elderly group, CM patients were significantly older than RRT patients (81.8 vs 79.3 years; P < 0.001). When all patients who reached stage 5 CKD aged > 75 years were analysed in a Cox proportional hazards model, after correction for age, diabetes, comorbidity, gender and ethnicity, RRT did not confer a significant survival advantage (Table 3 and Figure 3).
Table 2.
Median survival by Kaplan–Meier analysis of patients aged > 75 treated by conservative means or by dialysis, stratified by comorbidity group
| Number | Median | SE | 95% CI |
P-value | |||
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| Low comorbidity | Dialysis | 60 | 36.8 | 8.4 | 20.4 | 53.2 | 0.03 |
| Conservative | 52 | 29.4 | 3.7 | 22.2 | 36.6 | ||
| Severe comorbidity | Dialysis | 17 | 25.8 | 4.4 | 17.3 | 34.4 | 0.83 |
| Conservative | 54 | 20.4 | 2.4 | 15.7 | 25.2 | ||
Fig. 2.
Comparison of Kaplan–Meier survival curves by modality (RRT vs conservative kidney management) in patients > 75 years. The panel on the left depicts the relationships in those with low comorbidity and that on the right in those with high comorbidity.
Table 3.
Cox proportional hazards model for survival in patients aged > 75. Increasing age, the presence of high comorbidity and male gender but not the modality (CM or RRT) are significant predictors of mortality in this group of patients. Numbers in square brackets indicate the number of patients in each category
| Chi square = 30.91 (P < 0.001) | P-value | Hazard ratio | 95.0% CI for HR |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Modality (CM [77] vs RRT [106]) | 0.428 | 1.177 | 0.787 | 1.759 |
| Age at stage 5 (years) | 0.004 | 1.076 | 1.024 | 1.131 |
| Comorbidity (high [71] vs low [112]) | 0.002 | 1.823 | 1.255 | 2.650 |
| Diabetes (diabetic [48] compared with non-diabetic [135]) | 0.176 | 1.308 | 0.887 | 1.928 |
| Gender (female [59] compared with male [124) | 0.025 | 0.646 | 0.440 | 0.948 |
| Ethnicity (non-white [12] compared with white [171]) | 0.806 | 1.111 | 0.479 | 2.577 |
Fig. 3.
Cox proportional model survival curve of patients aged > 75 years—CM vs RRT—adjusted for age, gender, ethnicity, the presence of diabetes and the presence of high comorbidity. Median survival in RRT patients is better by < 4 months, which is not statistically significant (P = 0.43).
In addition to the differences in age (81.8 ± 3.9 vs 68.4 ± 5.1 years), patients aged > 75 years in the conser@vatively managed group were less likely to have diabetes than younger patients and more likely to be white (Table 4). There were no significant differences between those > 75 years and younger patients with respect to gender distribution, the prevalence of high comorbidity and mean eGFR at the start of the study.
Table 4.
Comparison of demographic and clinical features in conservatively managed patients aged > 75 years and in those who were younger
| Age ≤ 75 | Age > 75 | P-value | |
|---|---|---|---|
| Number | 49 (31.6%) | 106 (68.4%) | |
| Age at stage 5 (years) | 68.4 ± 5.1 | 81.8 ± 3.9 | < 0.001 |
| % Male | 53.1 | 63.5 | NS |
| % Non-white | 26.5 | 8.5 | 0.005 |
| % Diabetic | 51.0 | 28.3 | 0.007 |
| % High comorbidity | 46.9 | 50.9 | NS |
| eGFR at stage 5 (mL/min/1.73 m2) | 13.0 ± 1.4 | 13.3 ± 1.4 | NS |
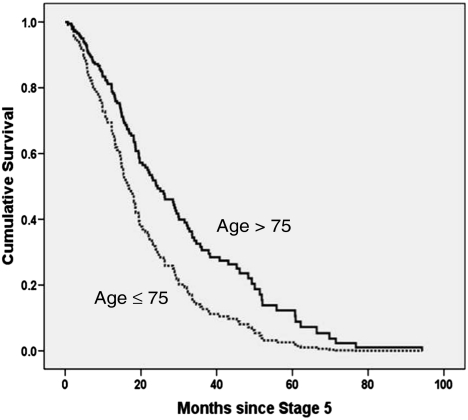
In a Cox proportional hazards model, the significant predictors of mortality in RRT patients were age—each decade increasing mortality risk by > 30%, the presence of high comorbidity—incurring over twice the risk compared to less comorbid patients and the presence of diabetes—associated with a 60% increased risk compared to non-diabetic patients (Table 5). The findings were similar if a dichotomized age covariate (≤ 75 and > 75 years) was substituted for the continuous age covariate in the model, though the model's predictive power was slightly less (model not shown). Applying the same model in conservatively managed patients produced differing results. The significant predictors of mortality were age—mortality being more than double in those aged < 75 years compared to older patients and gender—women having a 35% reduced risk compared to men (Table 6). Presence of high comorbidity and diabetes both conferred higher risk—though this did not reach statistical significance. The better survival of conservatively managed patients aged > 75 yeas, adjusted for gender, ethnicity, the presence of diabetes, the presence of high comorbidity and eGFR at the start of the study, is shown in Figure 4.
Table 5.
Cox proportional hazards model for survival in patients treated by dialysis. Increasing age, the presence of diabetes and the presence of high comorbidity are significant predictors of mortality in this group of patients. Numbers in square brackets indicate the number of patients in each category
| Chi square = 131 (P < 0.001) | P-value | Hazard ratio | 95% CI for HR |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Gender (female [293] compared with male [551) | 0.752 | 0.965 | 0.771 | 1.207 |
| Ethnicity (non-white [132] compared with white [712]) | 0.261 | 1.201 | 0.873 | 1.651 |
| Diabetes (diabetic [291] compared with non-diabetic [553]) | 0.000 | 1.604 | 1.293 | 1.990 |
| Comorbidity (high [196] vs low [648]) | <0.001 | 2.214 | 1.732 | 2.830 |
| eGFR at stage 5 (mL/min/1.73 m2) | 0.258 | 0.959 | 0.891 | 1.031 |
| Age at stage 5 (years) | <0.001 | 1.033 | 1.024 | 1.043 |
Table 6.
Cox proportional hazards model for predictors of survival in patients treated by conservative management. Age ≤ 75 and male gender are significantly associated with increased mortality in this group of patients
| Chi square = 22 (P < 0.001) | P-values | Hazard ratio | 95% CI for HR |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Gender (female [63] vs male [92]) | 0.026 | 0.648 | 0.442 | 0.949 |
| Ethnicity (non-white [22] vs white [133]) | 0.824 | 1.062 | 0.627 | 1.799 |
| Diabetic [55] vs non-diabetic [100] | 0.094 | 1.409 | 0.943 | 2.105 |
| Comorbidity (high [77] vs low [78]) | 0.099 | 1.365 | 0.943 | 1.976 |
| eGFR at stage 5 (mL/min/1.73 m2) | 0.252 | 0.923 | 0.804 | 1.059 |
| Age > 75 (yes vs no) | 0.009 | 0.574 | 0.379 | 0.869 |
Fig. 4.
Cox proportional model survival curve of conservatively managed patients aged > 75 years vs younger patients—adjusted for gender, ethnicity, the presence of diabetes, the presence of high comorbidity and eGFR at the start of the study. Survival of older patients is significantly better than that of younger patients (P = 0.009).
Discussion
For the vast majority of patients with ESRD, RRT provides a huge survival advantage. However, in those > 75 years of age, this advantage may be restricted to those without high comorbidity. We found that elderly patients with high comorbidity treated by dialysis had a median survival that was only 5 months longer from entry into stage 5 CKD than patients who had undergone conservative kidney management—a small proportion of whom outlived all those on RRT by a number of years (Figure 2). In fact, when corrected for age, gender and comorbidity (Table 3), patients aged > 75 years did not gain a significant survival advantage from RRT. This does not mean that dialysis will not be of benefit for all elderly patients with ESRD. Clearly an individualized approach is necessary. RRT is likely to be beneficial in many patients especially those with low comorbidity and in those with rapidly declining renal function. Conversely, CM may have a role in those patients with high comorbidity and slowly declining renal function.
The predictors of survival in RRT and conservatively managed patients were also markedly different. As might be expected, in the RRT population, the independent predictors of increased mortality were increasing age, high comorbidity and the presence of diabetes. In the conservatively managed group, however, age > 75 years and female gender were both independent predictors of improved survival. The presence of high comorbidity and diabetes approached significance.
Why do older patients and females show better survival in the CM group? In this study, older patients were less likely to have diabetes, but the prevalence of high comorbidity was similar to that in younger patients (Table 4). In any case, both these factors were controlled for in the Cox model. We cannot exclude a role for gender difference in the prevalence of primary renal diseases in this population, for instance renovascular disease is more prevalent in men, though the Cox model corrected for diabetes and presence of severe comorbidity including vascular disease. The proportion of non-whites was also significantly lower in older patients (Table 4), but ethnicity was not an independent predictor of survival. It may be that there are factors other than those featuring in our survival model that are involved in the choice of the conservative management option, and which may be overrepresented in men and in younger patients, e.g. rate of decline of renal function, frailty, functional status, social support and depression. Alternatively, it may be that older patients can survive with less renal function than younger patients and women with less than men. Renal function declines progressively with increasing age [18], perhaps associated with decreased metabolic demand [19], but there is conflicting evidence for significant gender differences in energy expenditure. Paul et al. found that total energy expenditure, physical activity expenditure and resting energy expenditure were significantly lower in women [20]. Other authors have suggested that these differences are abolished when corrected for body weight, though the validity of such normalization is debated [20,21]. Notwithstanding this, there are major gender differences in body composition and insulin resistance that may be relevant [21]. Females have also been shown to have slightly higher iothalamate glomerular filtration rates corrected for body surface area than males at all ages, at least up to 60 years [22], but this is unlikely to be relevant to elderly patients with ESRD.
Precisely how long patients survive on conservative kidney management is difficult to gauge since the reference point from which survival was measured has varied from study to study. Some studies have attempted to match conservatively managed patients with a comparable dialysis group whilst others report survival in the conservative group alone. In a previous original study, we assessed survival from a ‘putative dialysis date’ obtained by matching Cockcroft–Gault creatinine clearances in the conservatively managed group with those in the relevant dialysis group at dialysis initiation [10]. In that study, we found median survivals of 6.3 months in conservatively managed patients and 8.3 months in patients recommended for conservative treatment but opting for dialysis. Carson et al. took a similar approach, in ESRD patients aged > 70 years, and found a median survival of 13.9 months in the conservative group and 37.8 months in RRT patients, though hospital-free survival was similar in both groups [13]. The groups were similar in terms of comorbidity, but median age was 8 years greater in the conservative group. In octogenarians, median survival was 28.9 months in patients undergoing dialysis and 8.9 months in those treated conservatively measured from date of the decision not to perform dialysis (Cockcroft–Gault creatinine clearance < 10 mL/min in all cases) [17]. The groups however, differed considerably with respect to social isolation, late referral, Karnofsky performance score and diabetic status. Murtagh et al. found survival from an eGFR of 15 mL/min to be similar at around 22 months in patients > 75 years with high comorbidity in the conservative and dialysed groups [12]. Ellam et al. found that median patient survival on conservative management from the time of first known CKD stage 5 was 21 months [15]. Wong found a median survival of 1.95 years in conservatively managed patients though some had not reached stage 5 at entry into the study [14]. Our finding of a median survival of 21 months for conservatively managed patients following entry into stage 5 CKD is compatible with much of the data outlined above.
There are a number of drawbacks in our study including its retrospective nature and the absence of detail on patient characteristics such as Karnofsky performance score and frailty. This obliges caution in the interpretation of our results. These drawbacks however, are somewhat offset by the size of the study, which is the largest currently available, and the length of follow-up. We also used a non-standard simplified comorbidity assessment derived from our previous work [5]. Our main purpose in this was to identify a high-risk group using a score that reflected both the number and the severity of extra-renal comorbid conditions rather than just a count of affected organs. Assignment to the high comorbidity cluster was a powerful predictor of mortality in the dialysis group. We think, therefore, this approach was justified. It should also be noted that our selection criteria effectively excluded most patients who were referred late. Such patients represent > 25% of new starters on dialysis, and elderly, dependent patients are over-represented in this group [5,23]. The lack of time to counsel and plan in this setting may mean that patients start on dialysis by default, perhaps inappropriately. This is an important issue that is not addressed by this study.
Whilst our results contribute to the information that can guide patients in making treatment decisions, an individualized approach to decision making is mandatory. Thus, an elderly patient with high comorbidity and slow decline of renal function is likely to benefit from conservative management; this may not be an appropriate recommendation for someone with no comorbidity or rapidly declining renal function. Survival figures can only provide generalized information, and each patient should be counselled individually on his or her chances of benefiting from dialysis. Patients are free to change their treatment decisions and these need to be respected when planning care.
Our study has contributed to our understanding of prognosis in patients treated by conservative management. It has also demonstrated a survival advantage for the elderly (> 75 years) and for women on this modality, though the reasons for this are unclear. Conservative kidney management is a valid treatment option in selected patients, and our aversion to rationing should not dissuade us from rational treatment decisions. There is a dearth of prospective work in this area. The little information we have on the quality of life of patients on this pathway is reassuring [16]. For many reasons, we are unlikely to acquire information from randomized studies in this area [24]. High-quality prospective observational studies of outcomes, including quality of life, are urgently required.
Conflict of interest statement. None declared.
References
- 1.Challah S, Wing AJ, Bauer R, et al. Negative selection of patients for dialysis and transplantation in the United Kingdom. Br Med J (Clin Res Ed) 1984;288:1119–1122. doi: 10.1136/bmj.288.6424.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrington K, Udayaraj U, Gilg J, et al. UK Renal Registry 11th Annual Report (December 2008): Chapter 3 ESRD incident rates in 2007 in the UK: national and centre-specific analyses. Nephron Clin Pract. 2009;111:c13–c41. doi: 10.1159/000209992. [DOI] [PubMed] [Google Scholar]
- 3.Zoccali C, Kramer A, Jager K. The databases: renal replacement therapy since 1989—the European Renal Association and European Dialysis and Transplant Association (ERA–EDTA) Clin J Am Soc Nephrol. 2009;4:S18–S22. doi: 10.2215/CJN.05210709. [DOI] [PubMed] [Google Scholar]
- 4.U S Renal Data System. USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2009. [Google Scholar]
- 5.Chandna SM, Schulz J, Lawrence C, et al. Is there a rationale for rationing chronic dialysis? A hospital based cohort study of factors affecting survival and morbidity. BMJ. 1999;318:217–223. doi: 10.1136/bmj.318.7178.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurella TM, Covinsky KE, Chertow GM, et al. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch DJ, West ML, Cohen AD, et al. Experience with not offering dialysis to patients with a poor prognosis. Am J Kidney Dis. 1994;23:463–466. doi: 10.1016/s0272-6386(12)81012-2. [DOI] [PubMed] [Google Scholar]
- 8.Sekkarie M, Cosma M, Mendelssohn D. Nonreferral and nonacceptance to dialysis by primary care physicians and nephrologists in Canada and the United States. Am J Kidney Dis. 2001;38:36–41. doi: 10.1053/ajkd.2001.25179. [DOI] [PubMed] [Google Scholar]
- 9.Singer PA. Nephrologists’ experience with and attitudes towards decisions to forego dialysis. The End-Stage Renal Disease Network of New England. J Am Soc Nephrol. 1992;2:1235–1240. doi: 10.1681/ASN.V271235. [DOI] [PubMed] [Google Scholar]
- 10.Smith C, Da Silva-Gane M, Chandna S, et al. Choosing not to dialyse: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract. 2003;95:c40–c46. doi: 10.1159/000073708. [DOI] [PubMed] [Google Scholar]
- 11.Van BW, Lameire N, Veys N, et al. From curing to caring: one character change makes a world of difference. Issues related to withholding/withdrawing renal replacement therapy (RRT) from patients with important co-morbidities. Nephrol Dial Transplant. 2004;19:536–540. doi: 10.1093/ndt/gfg539. [DOI] [PubMed] [Google Scholar]
- 12.Murtagh FE, Marsh JE, Donohoe P, et al. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant. 2007;22:1955–1962. doi: 10.1093/ndt/gfm153. [DOI] [PubMed] [Google Scholar]
- 13.Carson RC, Juszczak M, Davenport A, et al. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol. 2009;4:1611–1619. doi: 10.2215/CJN.00510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong CF, McCarthy M, Howse ML, et al. Factors affecting survival in advanced chronic kidney disease patients who choose not to receive dialysis. Ren Fail. 2007;29:653–659. doi: 10.1080/08860220701459634. [DOI] [PubMed] [Google Scholar]
- 15.Ellam T, El-Kossi M, Prasanth KC, et al. Conservatively managed patients with stage 5 chronic kidney disease—outcomes from a single center experience. QJM. 2009;102:547–554. doi: 10.1093/qjmed/hcp068. [DOI] [PubMed] [Google Scholar]
- 16.De Biase V, Tobaldini O, Boaretti C, et al. Prolonged conservative treatment for frail elderly patients with end-stage renal disease: the Verona experience. Nephrol Dial Transplant. 2008;23:1313–1317. doi: 10.1093/ndt/gfm772. [DOI] [PubMed] [Google Scholar]
- 17.Joly D, Anglicheau D, Alberti C, et al. Octogenarians reaching end-stage renal disease: cohort study of decision-making and clinical outcomes. J Am Soc Nephrol. 2003;14:1012–1021. doi: 10.1097/01.asn.0000054493.04151.80. [DOI] [PubMed] [Google Scholar]
- 18.Roberts SB, Dallal GE. Effects of age on energy balance. Am J Clin Nutr. 1998;68:975S–979S. doi: 10.1093/ajcn/68.4.975S. [DOI] [PubMed] [Google Scholar]
- 19.Singer MA. Of mice and men and elephants: metabolic rate sets glomerular filtration rate. Am J Kidney Dis. 2001;37:164–178. doi: 10.1016/s0272-6386(01)80073-1. [DOI] [PubMed] [Google Scholar]
- 20.Paul DR, Novotny JA, Rumpler WV. Effects of the interaction of sex and food intake on the relation between energy expenditure and body composition. Am J Clin Nutr. 2004;79:385–389. doi: 10.1093/ajcn/79.3.385. [DOI] [PubMed] [Google Scholar]
- 21.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6:60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poggio ED, Rule AD, Tanchanco R, et al. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int. 2009;75:1079–1087. doi: 10.1038/ki.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lameire N, Wauters JP, Teruel JL, et al. An update on the referral pattern of patients with end-stage renal disease. Kidney Int Suppl. 2002:27–34. doi: 10.1046/j.1523-1755.61.s80.6.x. [DOI] [PubMed] [Google Scholar]
- 24.Dasgupta I, Rayner HC. In good conscience—safely withholding dialysis in the elderly. Semin Dial. 2009;22:476–479. doi: 10.1111/j.1525-139X.2009.00617.x. [DOI] [PubMed] [Google Scholar]