Abstract
Curcumin is a natural polyphenol derived from the plant Curcuma longa, commonly called turmeric. Extensive research over past 50 years has indicated that this polyphenol is highly pleiotropic molecule capable of preventing and treating various cancers. The anticancer potential of Curcumin is severely affected by its limited systemic and target tissue bioavailability and rapid metabolism. In the present review article, we provide a summarized account of different drug delivery systems employed for tackling the problem of curcumin's bioavailability such as liposomes, phospholipid complexes and nanoparticles. Concomitantly we have reviewed the large volume of literature reports describing structural modifications of Curcumin and the anticancer potential of its analogs. Some of the difluorocurcumin analogs allowing longer circulation times and preferential accumulation in the pancreas seem to offer promising leads for conducting first in-depth animal studies and subsequently clinical trials for the use of these analogs for prevention of tumor progression and/or treatments of human malignancies.
Keywords: Curcumin, chemoprevention, therapy, synthetic analog
1. INTRODUCTION
Prior to our discussion on Curcumin and its analogs, we would like to provide a brief survey on phytochemicals in general because phytochemicals are becoming the novel backbone of medicinal chemistry, and thus it is the subject of this “Mini Reviews in Medicinal Chemistry”. The term “phytochemicals” refers to a broad variety of biologically active compounds produced by plants such as β-carotene, ascorbic acid (vitamin C), folic acid, vitamin E and many others that possess either antioxidant or hormone-like actions. They are found in fruits, vegetables, beans, grains, and exudates of plants. Diets rich in fruits, vegetables or whole grains have been shown to reduce the risk of certain cancers, heart diseases, diabetes and other health disorders and hence researchers are attempting to identify specific compounds in these foods that may account for their beneficial effects in humans. Since phytochemicals help prevent formation of potential cancer-causing moieties, and by blocking their action, they are viewed as chemopreventive agents against cancers [1]. Phytochemicals consist of several classes of chemical compounds having different structural scaffolds as discussed later in this article.
One of the important classes of phytochemicals consists of polyphenols which are represented by a large subgroup of chemicals called flavonoids. These are found in a broad range of fruits, grains, and vegetables especially in soybeans and soy products, garbanzo beans, chickpeas, and licorice which are capable of mimicking the actions of female estrogenic hormones. Estrogen-like substances derived from these plant sources are called phytoestrogens. They can compete with endogenous estrogenic compounds and can block development of hormone-dependent breast and prostate cancers. Whereas non-estrogenic polyphenols found in broccoli, Brussels sprouts, cabbage, cauliflower and in tea leaves have been shown to scavenge harmful free radicals arising out of the metabolic activity, which can damage DNA, RNA and several important proteins, resulting in the triggering of some forms of cancers and other diseases. Similarly grapes, eggplant, red cabbage and radishes also contain a group of compounds called anthocyanidins which act as antioxidants and may provide protection against some cancers and heart diseases. Examples of this class of compounds are provided by Quercetin found in apples, onion skin, tea, red wine, and Ellagic acid found in raspberries, blackberries, cranberries, strawberries and walnuts.
Another class of compounds possessing chemopreventive potential against cancer includes Carotenoids which are the coloring compounds present in carrots, yams, cantaloupe, squash and apricots. Similarly tomatoes, red peppers, and pink grapefruit contain a compound called Lycopene which is a powerful antioxidant capable of scavenging out certain reactive oxygen radicals. The phytochemicals lutein and zeaxanthin found in spinach, kale, and turnip greens have also been shown to reduce the risk of gastrointestinal cancers. Allyl sulfides which are found in garlic and onions are known to stimulate intracellular antioxidant enzymes providing natural defense to the cells from oxidative damages and help strengthen the immune system [2]. In summary, many of these phytochemicals are now available as single dietary supplements, although no one knows if a single supplement is as beneficial as the foods from which they are derived. In the United States, dietary supplements are regulated differently than drugs. Epidemiologically it has been well-established that populations consuming diets rich in specific phytochemicals have lower rates of certain cancers and heart diseases. For example, relatively low rates of breast and endometrial cancers in some Asian countries are specific, at least, in part due to dietary habits. This has provided impetus to examine the effects of specific phytochemicals contained in foods on human health and diseases [3].
2. MAJOR BIOACTIVITIES OF PHYTOCHEMICALS
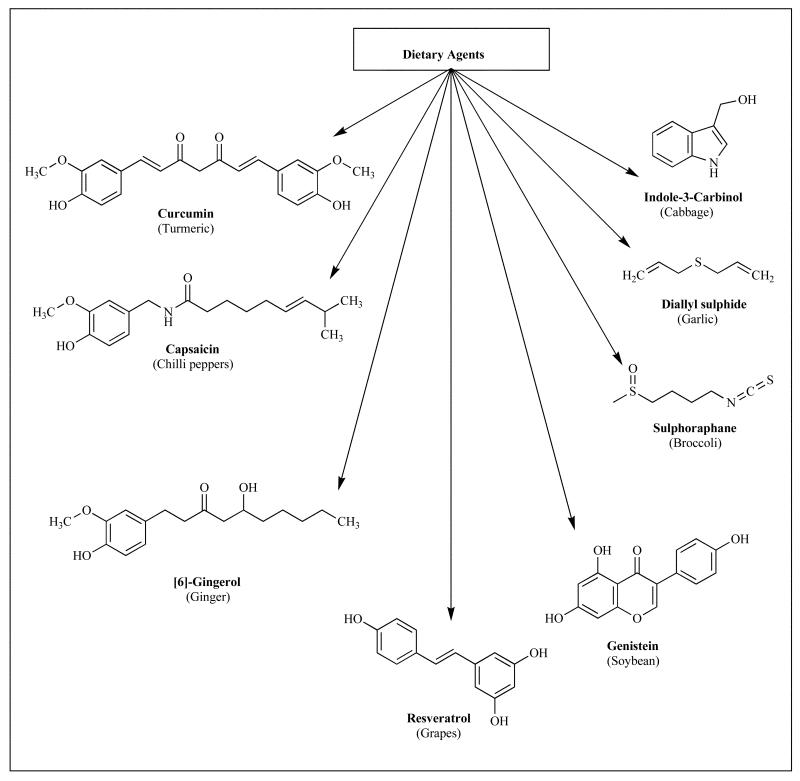
Phytochemicals are associated with the prevention and treatment of at least four leading causes of health disorders in the world, viz. cancer, diabetes, cardiovascular diseases and hypertension [4]. They also influence various cellular processes that help prevent cellular damages. The chemical structures of phytochemicals are often used as ‘privileged structures’ for creating their synthetic analogs which have improved pharmacological activities through optimized bioavailability and pharmacokinetic profiles. Some of the common biological activities associated with certain groups of phytochemicals are discussed in the following paragraphs prior to our main discussion on Curcumin and its analog, and the chemical structures of some of the major active phyto-chemicals are shown in Fig. (1).
Fig. (1).
Chemical structures of active phytochemicals and their dietary sources.
a. Anti-Diabetic Activity
Tea polyphenolics apart from their much cited antioxidant activities have been reported to inhibit α-amylase, sucrose and glucose transport across the intestine by inhibiting sodium glucose co-transporter-1 (S-GLUT-1) [5, 6]. The manipulation of S-GLUT-1-mediated transport along with amylase and α-glycosidase inhibitory activity by catechin products from soybeans and tannic acid as well as chlorgenic acid from saponin make them interesting candidates in the control and management of hyperglycemia [7].
b. Cardiovascular Activity
Naturally occurring sulfur-containing compounds of the allium family have been shown to modulate plasma cholesterol and atherosclerosis and thereby lowering the risk of cardiovascular diseases. These substances are found especially in garlic, onions and leeks respectively. Garlic contains several biologically active compounds, the most active ones being Diallyl disulfide and Allicin. Garlic has also been reported to inhibit platelet aggregation, decrease coagulation time, and lower blood pressure. In large quantities, however, garlic can cause certain side effects, such as anemia or some allergic manifestations [8].
c. Anticancer Activity
While several dietary phytochemicals are recommended for prevention of carcinogenesis, their mechanisms of actions are as yet not well understood. Aggarwal et al. have recently reviewed the cell signaling pathways in cancers that are disrupted by agents isolated from natural origins including Curcumin (Turmeric), Resveratrol (Red Grapes), Genistein (Soybean), Capsaicin (Red Chili) and many others [9]. Consequently there is a resurgence of interest in plant-derived medications based on new screening methodologies and understanding of indigenous healing practices. Examples of drug molecules finding place in clinics has recently emerged, which are based on natural products including anti-tumor agents like Taxol from Yew, Campothecin from leaves of Camptotheca acuminata and Etoposide from podophyllotoxin [9].
The dietary agents are excellent sources of fibers, vita-mins, and minerals as well as suppliers of structure building components to polyphenols, terpenes, alkaloids, and steroids that provide substantial health benefits beyond basic nutrition. The active principles of these dietary phytochemicals are believed to act on numerous pathways and molecular targets that lead to prevent transformation, hyperproliferation, and initiation of the processes of carcinogenesis including angiogenesis and metastasis. However, due to multitude of processes affected by these phytochemicals, researchers are still facing a challenging task in trying to determine which phytochemicals are responsible for preventing a specific disease or disorder. Some of the links between individual phytochemicals and cancer risk as revealed from the in vitro studies are very compelling and make a very strong case for further research [10].
Presently individual phytochemicals are being evaluated for their safety and effectiveness in regard to disease prevention. Obviously, like any other newly discovered chemical there is a need for further investigations for potential health benefits and possible health risks. Optimal levels of phyto-chemicals useful for beneficial effects have yet to be fully determined. In addition, individual recommendations in terms of requirements for different genders, age groups and body types also require further study. Among various dietary chemopreventive agents, turmeric powder or its extract are broadly used as therapeutic preparations in Indian System of medicine, viz. Ayurved [11]. A paste made from the powdered turmeric rhizomes mixed with slaked lime and applied locally is an ancient household remedy for sprains, muscular pain and inflamed joints. Turmeric powder is the most common constituent of Indian spices used for food coloring as well as aroma while the main active principle responsible for its potent biological activity has been found to be Curcumin [1].
In the present review we now provide an account of chemical and biological properties of Curcumin analogs along with the major limitations in using Curcumin as therapeutic agent which led to the preparation of new synthetic analogs. This was done with the help of their structure-activity correlation, structural chemistry of all synthetic analogs described in the literature up to august 2009 and molecular modeling of these analogs in the COX-2 cavity, for the first time, as documented by our laboratory because Curcumin has previously been documented to target Cox-2 and thereby inhibiting the enzymatic production of PGE2 as discussed below.
3. CHEMICAL PROPERTIES OF CURCUMINOIDS
Curcumin (diferuloylmethane, 1; Scheme 1) is the active phenolic compound extracted from the rhizome of the plant Curcuma longa Linn (Family:Zingiberaceae) grown in tropical Southeast Asia [12-14]. It is a tropical plant native to southern and southeastern tropical Asia and is a perennial herb belonging to the ginger family. The plant is recognized by various names in different countries like Kurkum Uqdah safra (in Arabia), Toormerik or Turmerig (in Armenia), Curcuma or Safran des Indes (in France), Kurkuma (in Germany) and kurkumy (in Russia), respectively [15], The compound has been used as a spice and coloring agent in Indian cuisine. The medicinal use of this plant has been documented in Ayurvedic medicine, a traditional indian system of medicine, for over 6000 years. Broad investigations over the last five decades have indicated that Curcumin has potential utility in the prevention and therapeutic treatment of various diseases which includes respiratory conditions, inflammation, liver disorders, diabetic wounds, cough and certain tumors, Recent investigations have provided evidence that Curcumin can also prevent a variety of carcinogen-induced cancers in rodents in addition to suppressing the mutagenic effects of various chemical carcinogens such as tobacco, cigarette smoke condensates, benzo (α) pyrene, 1, 2-dimethyl-benz(α)anthracene(DMBA) and aflatoxin B1 respectively [16-35].
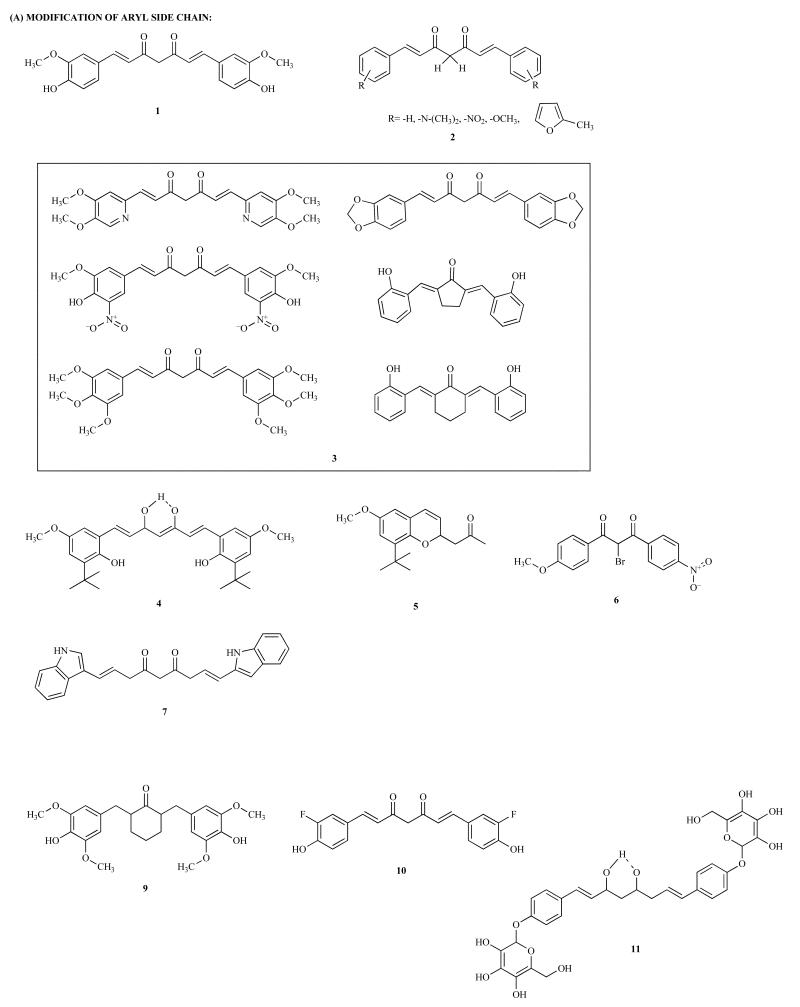
Scheme 1. Chemical structures of Curcumin analogs referred within the current review.
(A) Modification of aryl side chain 1-18; (B) Modification of conjugated double bond 19; (C) Modification of active methylene group 20-26; (D) Modification of diketo functionality 27-33; (E) Modification of adjacent carbon 34.
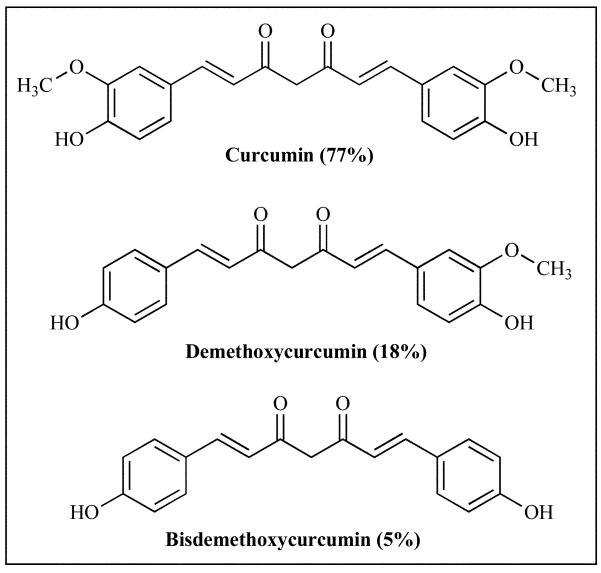
The compound was isolated first in 1815 and its chemical structure was determined by Rougghley and Whitting in 1973 [15]. It is obtained through extraction of the rhizomes and isolation through chromatographic separations [15]. Chemically its molecular formula is C21H20O6 with a molecular weight of 368.37 g/mol. It is hydrophobic in nature, practically insoluble in water and ether but soluble in ethanol, dimethylsulfoxide, and acetone and has a melting point of 183 °C. Spectrophotometrically, the maximum absorption (λmax) of Curcumin in methanol occurs at 430 nm and in acetone at 415–420 nm [15]. Curcuma spp. contains Turmerin, essential oils, and curcuminoids, including Curcumin. Commercial product available in the market contains three major components, viz. 77% Curcumin (diferuloylmethane), 18% demethoxycurcumin and 5% bismethoxycurcumin which together are referred as curcuminoids as shown in Fig. (2); but some product may contain higher percentage of Curcumin in different parts of the world.
Fig. (2).
Chemical constituents of Curcuma longa.
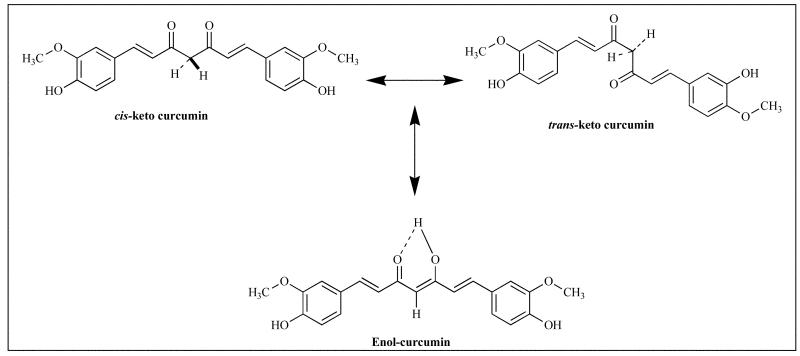
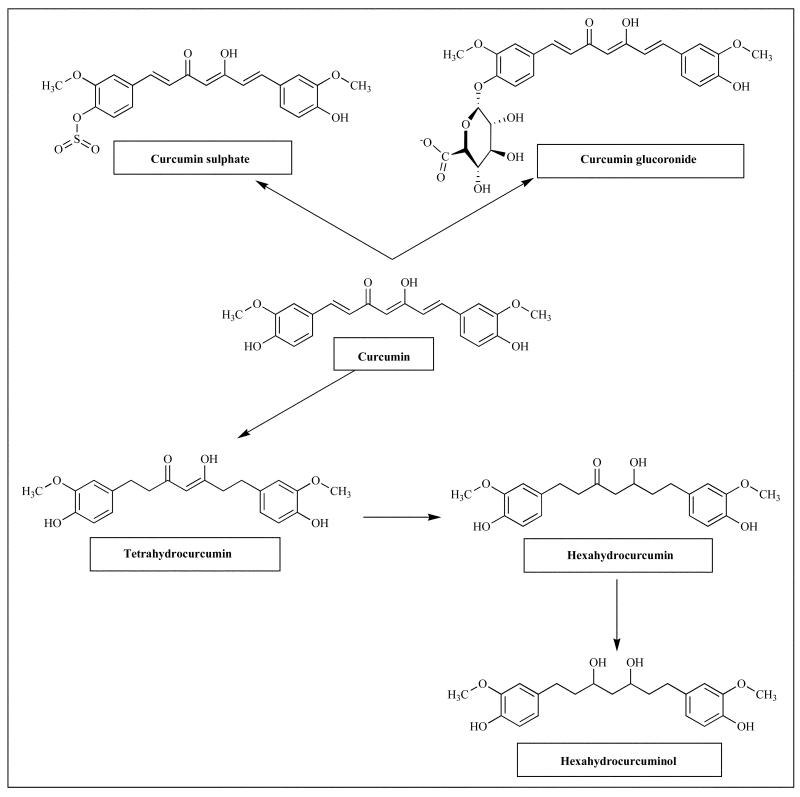
Curcumin is a bis-α,β-unsaturated β-diketone and exists in equilibrium with its enol tautomer (Fig. 3). The bis-keto form predominates in acidic and neutral aqueous solutions as well as in the cell membrane due to the heptadienone linkage between two methoxyphenol rings containing a highly activated carbon atom [36]. The C–H bonds on this carbon are very weak due to delocalization of the unpaired electron on the adjacent oxygens. Between pH 3–7 Curcumin acts as a potent H-atom donor [36] while above pH 8 the enolate form of the heptadienone chain predominates and the compound acts mainly as an electron donor similar to many phenolic antioxidants [36]. The major metabolic product of Curcumin has been shown to be trans-6-(4′-hydroxy-3′-methoxyphenyl) 2,4-dioxo-5-hexenal while vanillin, ferulic acid and feruloyl methane were identified as minor degradation products. The pharmacokinetic data has shown that Curcumin undergoes efficient first-pass and some degree of intestinal metabolism leading to glucuronidation and sulfation as shown in Fig. (4) which may explain its poor systemic bioavailability upon oral administration. Clinically it has been shown that a daily oral dose of 3.6 g of Curcumin results in pharmacologically efficacious levels in colorectal tissue, with negligible distribution of the parent drug in hepatic tissue or other tissues of the gastrointestinal tract [37].
Fig. (3).
Various isoforms of Curcumin.
Fig. (4).
Major metabolites of curcumin in rodents and humans.
4. STRUCTURE OF CURCUMIN ANALOGS AND DRUG DELIVERY SYSTEMS
As discussed earlier that the motivation for building Curcumin analogs is based upon three main considerations. First, animal studies have shown that Curcumin is rapidly metabolized and conjugated in the liver and excreted in the feces. As a result its systemic bioavailability is limited. For example, when an intravenous dose of 40 mg/kg Curcumin is administered to rats the compound undergoes complete plasma clearance at the end of one hour post-dosing. An oral dose of 500 mg/kg to rats shows peak plasma concentration of only 1.8 ng/mL with the major metabolites identified being Curcumin sulfate and Curcumin glucuronide, respectively [38]. These reports indicate two problems that need to be addressed for making the scientific rationale for the synthesis of novel Curcumin analog, which include its limited bioavailability and rapid metabolism. The former problem is generally tackled through formulation of Curcumin using different drug delivery systems such as liposomes, micelles, phospholipid complexes and nanoparticles. However, the problem of slowing down the metabolism of Curcumin has been addressed marginally. For example, concomitant administration of (20 mg) Piperine, which is the alkaloid of black pepper with Curcumin (2gm) has shown to increase serum concentration of Curcumin 20-fold which is attributed to inhibition of glucuronidation and intestinal metabolism by the alkaloid [28]. Many attempts in improving the systemic as well as tissue bioavailability of Curcumin with improved retention time have largely been unsuccessful.
There is another aspect which has not been paid much attention. In case of Curcumin, numerous studies have established that Curcumin is a highly pleiotropic molecule capable of interacting with various signaling intermediates involved in cancer cascades. However, there have been no attempts to tailor the application of Curcumin to some specific cancer types through structural modification which may be a desirable goal in the future. In the following discussion, we first provide the account of drug formulations of Curcumin aimed at increasing its bioavailability before embarking upon the detailed description on various structural analogs of Curcumin that have been reported in the literature including our attempts, which can be claimed as success in improving the systemic and tissue kinetics and bioavailability of a potent analog of Curcumin [39, 40].
5. DRUG DELIVERY SYSTEMS
In an animal experiment using Sprague-Dawley (SD) rats, pharmacokinetic parameters showed that liposome-encapsulated Curcumin possessed high bioavailability demonstrating a faster rate and better absorption of Curcumin but the retention time was not improved substantially [37]. Oral liposome-encapsulated Curcumin gave higher Cmax and shorter Tmax values, indicating enhanced gastrointestinal absorption. Moreover, the plasma antioxidant activity after oral administration of liposome-encapsulated Curcumin was significantly higher than that of controls and there was a good correlation between plasma Curcumin concentration and plasma antioxidant activities [37]. The minimum effective dose and optimal dosing schedule of liposomal Curcumin in a xenograft mouse model of human pancreatic cancer have been investigated by Smith and co-workers [38]. The effects of liposome-encapsulated Curcumin were also examined in other cancers. The liposomal Curcumin has been found to inhibit the growth of head and neck squamous cell carcinoma in a dose-dependent manner through down-regulation of NF-κB and targeting cyclin D1, COX-2, MMP-9, Bcl-2, Bcl-xL and Mcl-1 respectively [41]. Liposomal Curcumin also suppressed pancreatic cancer growth in vivo in murine xenograft models and inhibited tumor angiogenesis with down-regulation of NF-κB signaling [28]. These results demonstrate that liposomal Curcumin could be a more potent agent for the treatment of various cancers due to its improved bioavailability. However, the target tissue bioavail-ability or toxicity of such preparations needs to be carried out prior to any clinical investigation.
Nanoparticulate formulation of Curcumin is another approach for improving the bioavailability of Curcumin for enhancing treatment efficacy. A polymer-based nanoparticle approach to improve bioavailability of Curcumin has been designed and tested where the authors have found that nanoparticle Curcumin exhibited very rapid and more efficient cellular uptake than Curcumin [38]. Nanoparticle Curcumin was more potent than Curcumin in inducing apoptosis and suppressing proliferation of various cancer cells concomitant with down-regulation of NF-κB, cyclin D1, MMP-9, and VEGF. Another report also showed that nanoparticle encapsulation improved oral bioavailability of Curcumin by at least 9 fold when compared to Curcumin [38]. Several other forms of nanoparticle Curcumin including lipid-based and polymer-based nanoparticle-encapsulated Curcumin have shown enhanced bioavailability and improved anti-cancer activities although none has shown substantial retention in the target tissues [28, 38, 42].
Although these formulations improve the bioavailability of Curcumin, they fail to slow down the metabolism of Curcumin processing and as a result they exhibited rapid plasma clearance after one hour, which appears to limit the application of these advances. Moreover, the toxicity profiles of these formulations need to be investigated prior to assessing their anticancer efficacy against human malignancies. Ambike and co-workers [43] have prepared solid dispersions of Curcumin in different ratios with polyvinyl pyrrolidine (PVP) by spray drying. Physical characterization by SEM, IR, DSC, and XRPD studies, in comparison with corresponding physical mixtures revealed the changes in solid state during the formation of these dispersions and justified the formation of high-energy amorphous phase. Dissolution studies on Curcumin and its physical mixtures in 0.1N HCl showed negligible release even after 90 min while solid dispersions showed complete dissolution within 30 min indicating improved bioavailability. However, the dispersions failed to inhibit the activation of NF-κB by TNF-α limiting its therapeutic utility.
Wang et al. have successfully prepared high-speed and high-pressure homogenized O/W emulsions using medium chain triacylglycerols (MCT) as oil and Tween 20 as emulsifier, with mean droplet sizes ranging from 618.6 nm to 79.5 nm. The anti-inflammatory activity of Curcumin encapsulated in such O/W emulsions was found to be enhanced in the mouse ear inflammation model [44]. Shau et al. have synthesized a novel polymeric amphiphile, mPEG–PA, with methoxy poly (ethylene glycol) (mPEG) as the hydrophilic and palmitic acid (PA) as the hydrophobic segment. Fourier transform infrared spectroscopy revealed that the conjugation was through an ester linkage, which is biodegradable. Enzymes having esterase activity, such as lipase, can degrade the conjugate easily, as observed by in vitro studies. This micellar formulation can be used as an enzyme-triggered drug release carrier, as suggested by in vitro enzyme-catalyzed drug release studies using pure lipase and HeLa cell lysate. The IC50 values of free Curcumin vs. encapsulated Curcumin were found to be 14.32 and 15.58 μM respectively [45].
6. STRUCTURAL ANALOGS OF CURCUMIN
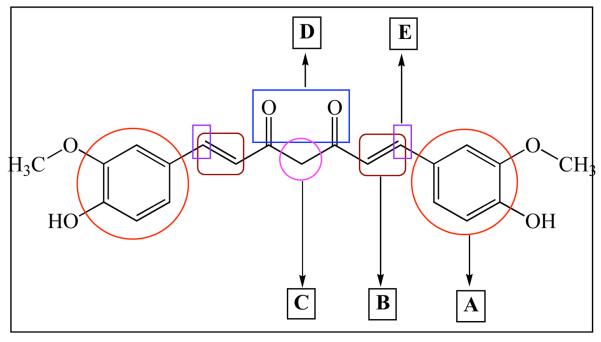
Curcumin is a simple symmetrical β-diketone and incorporates several functional groups. The two aromatic rings containing phenolic groups are connected by two α, β-unsaturated carbonyl groups. These carbonyl groups form a diketone which exists in keto- and enol- tautomeric forms where energetically more stable enol form exists in the solid phase and in acidic solutions. It can be easily deprotonated under mild alkaline condition to yield enolate moiety. Such facile tautomeric conversions are suspected to contribute to the rapid metabolism of Curcumin. The mechanism can be blocked through Knoevenagel condensation of the active methylenic group in Curcumin as suggested by Padhye and co-workers [46]. In its unmodified form the α, β-unsaturated carbonyls in Curcumin play the role of a good Michael acceptor and can undergo nucleophilic additions under biological conditions which may enhance its bioavailability. Exploiting this strategy has yielded limited success in terms of modulating Curcumin's metabolism resulting in ill-defined and unstable products. In Fig. (5), we have summarized all possible sites used by various workers for structural modifications reported in the literature and have discussed their results in the following sections.
Fig. (5).
Possible sties for modification in Curcumin.
a. Modification of Aryl Side Chain
Several Curcumin analogues 2 (Scheme 1) were synthesized by Qui et al. with substitutions in side aryl rings with furan motif. It has been reported that analogs with furan moiety have excellent inhibitory effect on thioredoxin reductase (TrxR) enzyme, responsible for reduction of thioredoxin in the presence of NADPH giving rise to free radicals. The analogs with methoxy substitution on the furan ring showed higher inhibitory activity with an IC50 value of 1.6 μM [47]. Jankun and co-workers have studied Curcumin analogs belonging to four different types: monophenyl analogs, hetero-cyclic analogs, analogs bearing various substituents in the side aryl rings and analogs with various linkers 3 (Scheme 1). When tested for cytotoxicity against androgen-dependent LNCaP and androgen-independent PC-3 cells, ten analogs were found to possess potent cytotoxicity against LNCaP and PC-3 cells; seven were inhibitory against LNCaP, and one solely against PC-3 [48]. These results clearly indicate structural influences on the biological activity in case of Curcumin analogs.
Since the presence of hydroxyl groups at ortho-position on the aromatic rings and β-diketone motif were considered as crucial functionalities for the antioxidant potential of Curcumin, Venketshwarlu and co-workers have introduced butylated hydroxyanisole (BHA) groups 4 (Scheme 1) in the side chains which, however, did not improve metabolic profiles yielding monoarylated compound 5 (Scheme 1) which was slightly better antioxidant compound in comparison to Curcumin [49]. Ligeret et al. have studied effects of Curcumin analogs 6 and 7 (Scheme 1) on mitochondrial Permeability Transition Pore (PTP) [50]. It was found that Curcumin induces apoptosis in tumor cells by increasing the permeability of the mitochondrial membrane where phenol and methoxy groups were essential to promote PTP opening. Compound 10 (Scheme 1) was able to induce apoptosis through reduction of Fe3+ to Fe2+ leading to pore opening which was confirmed by the release of cytochrome C as assessed by Western blot analysis. It was found to be a potent antioxidant as determined by DPPH assay with IC50 value of 18μM. The crystal structure of Curcumin analog 8 (Scheme 1) which is 4-[7-(4-acetoxy-3-methoxyphenyl) 3, 5-dioxoheptyl-2-methoxy phenyl ester has been reported by Lozada et al. [51].
The derivative has monoclinic unit cell dimensions. The two terminal groups are identical but the inherent symmetry of the molecule is distorted by a rotation of −101.0(5)0 about the C2-C3 bond. The molecule is stabilized by the formation of intramolecular hydrogen bonding between the enolate hydroxyl group and the ketonic carbonyl. The molecules in the crystal are packed at the normal Van der Waals distances. Gafner has carried out the biological evaluation of two Curcumin analogs 9 and 10 (Scheme 1) in the chemoprevention model system consisting of LPS-induced COX-2 and iNOS gene expression-dependent skin cancers revealing that substitution pattern on aromatic moiety is crucial for activity and cyclization, which leads to decrease in the activity drastically as compared to Curcumin wherein these compounds exhibit IC50 values of 5.5 and 23.5 respectively [52]. Ferrari et al. have carried out glycosylation of the aromatic rings in Curcumin which provides more water-soluble compounds with greater kinetic stability which is a desired feature for drug bioavailability 11 (Scheme 1). NMR data show that the ability of Curcumin to coordinate metal ion (in particular Ga (III)) is maintained in the synthesized products. Although the binding of glucose to Curcumin in this analog reduces the cytotoxicity of these derivatives towards cisplatin (cDDP)-sensitive and -resistant human ovarian carcinoma cell lines, although the compounds displayed good selectivity and they were much less toxic against non-tumorigenic Vero cells [53].
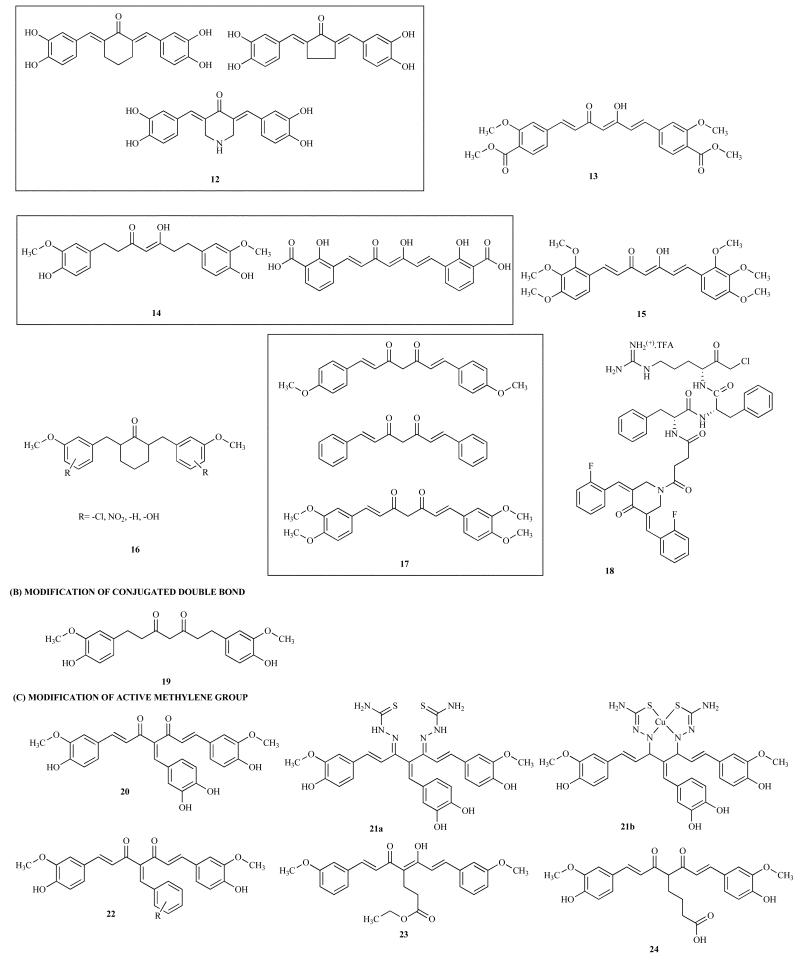
Du and co-workers have synthesized tetrahydroxycurcumin compounds where the central diketo function was replaced by cyclohexanone, cyclopentanone and piperidine motif 12 (Scheme 1) which show potent inhibitory effects on aldose reductase enzyme leading to PPAR activation which, in turn, inhibited cell proliferation with IC50 values in the range 2.6 to 4.9μM, respectively [54]. Mizushina have found the analog, monoacetylcurcumin ([1E, 4Z, 6E]-7-(4″-acetoxy-3″-methoxyphenyl)-5-hydroxy-1-(4′-hydroxy-3′-methoxyphenyl)hepta-1,4,6-trien-3-on), 13 (Scheme 1) to be more potent inhibitor than Curcumin for meiosis with an IC50 value 3.9μM [55].
Another synthetic Curcumin analog, viz. dimethoxycurcumin, was found to be more potent than Curcumin in inhibiting cell proliferation and inducing apoptosis in human HCT116 colon cancer cells [56]. Other Curcumin analogs such as tetrahydrocurcumin (THC) and salicylic Curcumin (SC) 14 (Scheme 1) also showed anti-angiogenic activity upon intraperitoneal administration against B16F10 melanoma cells by reducing the number of tumor directed capillaries on the ventral side of C57BL/6 mice. They also down-regulated the expression of angiogenesis-associated genes, VEGF and MMP-9 [57]. However, none of these analogs have been reported to be better in terms of systemic or tissue bioavailability.
In order to find more selective COX-1 inhibitors, a series of novel curcumin analogs were synthesized and evaluated for their ability to inhibit COX-1 by measuring COX-1, COX-2, and PGE2. It was found that the most potent Curcumin analog was (1E,6E)-1,7-di-(2,3,4-trimethoxyphenyl)-1,6-heptadien-3,5-dione 15 (Scheme 1) with IC50 value of 0.06 μM [58]. Davis and coworkers have synthesized fifteen 2,6-Bis(benzylidene)-4-phenylcyclohexanones 16 (Scheme 1) class of Curcumin analogs which were tested for in vitro cytotoxicity towards B16 and L1210 murine cancer cell lines using MTT assay. Significant activity was observed for two analogs with IC50 value in the range of 0.35 to 1.16μM. Structure-activity correlations suggested that large electron-withdrawing substituents placed at the meta-position of the arylidene aryl rings enhance antitumor potencies. The most potent compounds had virtually no effects on microtubules at concentrations up to 40 μM suggesting that tubulin inhibition is not the principal mechanism for their activities [59]. Chen and co workers have studied the antioxidant properties of several Curcumin analogs of 1,6-heptadiene-3,5-dione class 17 (Scheme 1) against free radical initiated peroxidation of human low density lipoprotein (LDL) [60]. Youssef has synthesized four new piperidine derivatives of Curcumin 18 (Scheme 1) which exhibited more potent activities than Curcumin against breast and skin cancers possibly due to piperidine's modulation of liver metabolism as pointed out earlier [61]; however none has reported any systemic or tissue bioavailability results.
b. Modification of Conjugated Double Bond
Arbiser et al. have reported Curcumin analogs without the conjugated double bond 19 (Scheme 1) which do not exhibit any cytotoxic effects against breast cancer cells indicating the importance of conjugated double bond for anti-cancer activity of the analogs [71].
c. Modification of Active Methylene Group
In order to modulate metabolism of Curcumin, Padhye and coworkers initially synthesized arylhydroxy Knoevenagel condensates of Curcumin and their thiosemicarbazone derivatives along with corresponding copper (II) complexes [62]. When these were evaluated for their NF-κB inhibitory activities, only dihydroxyaryl Knoevenagel condensate 20 (Scheme 1) showed moderate inhibition at 10μM concentration. Interestingly, while the thiosemicarbazone derivatives 21a (Scheme 1) of these Knoevenagel condensates were inactive, their corresponding copper complexes showed potent NF-κB inhibitory activities 21b (Scheme 1) [46]. This observation resembles Ping Dou's results on the polyhydroxy flavonoid ligands and their copper conjugates [63]. The copper complexes had 1:1 metal to ligand stoichiometric, distorted square planar geometries and reversible redox couple. In further studies Padhye and Sarkar developed the bioisosteric Difluoro Knoevenagel condensates 22 (Scheme 1) of Curcumin which showed potent growth inhibition and apoptosis induction in pancreatic cancer cell lines [40]. The compounds also inhibited activities of rabbit 20S proteasome and cellular 26S proteasome, leading to inhibition of cell proliferation and the induction of apoptosis. The most active compound from this series was CDF which was evaluated for pharmacokinetics and tissue distribution studies in mice compared to Curcumin with equal oral dosing. Pharmacokinetic parameters revealed that CDF had better retention through modulation of metabolism and that the concentration of CDF in the pancreas tissue was 10-fold higher compared to Curcumin [40]. These observations suggested that the bioavailability of CDF was much superior compared to Curcumin indicating that CDF could be a clinically useful agent, and thus further research on CDF is an active area in our laboratory.
Another Curcumin analog with modification at the active methylene group 23 (Scheme 1) is also found to be significantly effective as a free radical scavenger. This compound exhibited comparable anti-androgenic activity on androgen-dependent prostate cancer cells. Curcumin has been known to show inhibitory effects in both androgen-dependent and independent prostate cancers. It was found to down-regulate the transactivation and expression of AR and AR-related molecules such as AP-1 and NF-κB, respectively, and inhibited colony formation of prostate cancer cells [64]. Moreover, some analogs 24 and 25 (Scheme 1) also possess potent anti-androgenic activities, and have been found to be superior to hydroxyflutamide 26 (Scheme 1) which is the currently available anti-androgenic agent for the treatment of prostate cancer [65].
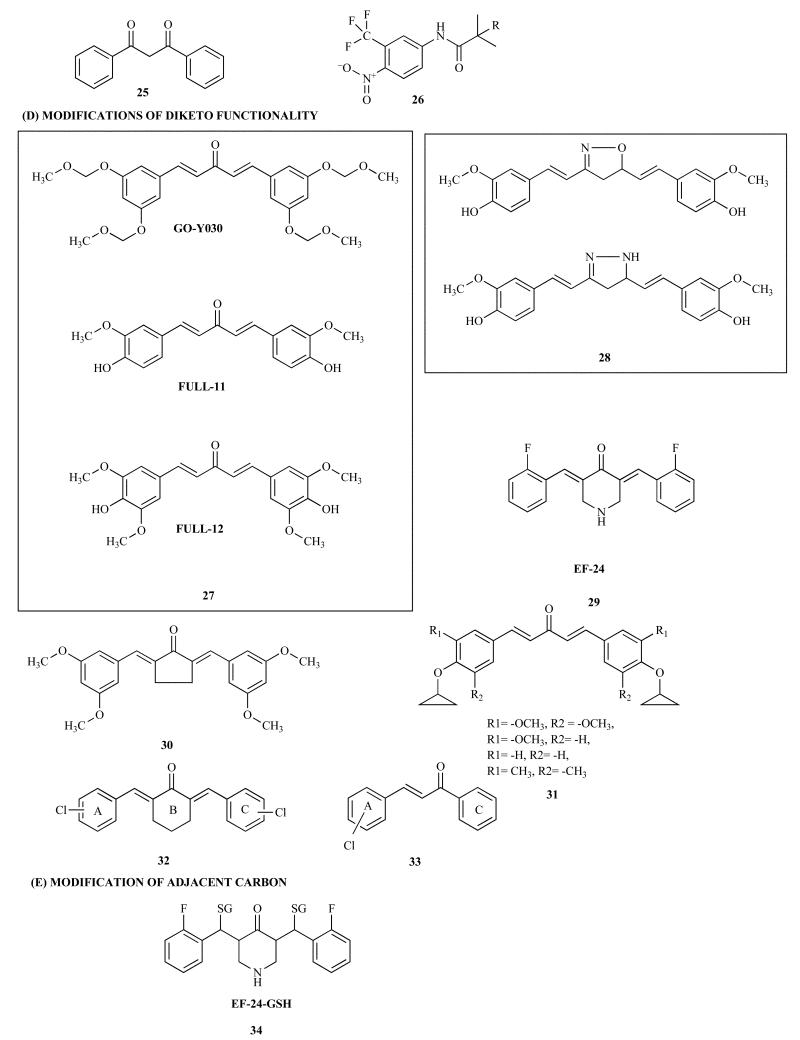
d. Modifications of Diketo Functionality
Shibata et al. have synthesized new analog GO-Y030 [(1E, 4E)-1,5-bis-(3,5(-bismethoxymethoxyphenyl) penta-1,4-dien-3-one] 27 (Scheme 1) which showed 30-fold greater growth suppression in vitro for colorectal cancer cell lines SW480, HT-29 and HCT116 via molecular mechanisms similar to Curcumin [66]. The bioavailability of this analog was examined by in vivo studies using a mouse model harboring the germ-line mutation of Apc, Apc (580D/+). The Apc (580D/+) mice had a very limited survival time with an intestinal obstruction due to polyps. The average tumor number in mice fed with GO-Y030 was reduced to 61.2% compared to those fed on basal diet with significantly prolonged lifespan (213 days) against those fed with the basal diet (166.5 days). The chemopreventive effect of GO-Y030 was also better than that of Curcumin (median survival time =191 days). Degradation of accumulated β-catenin was found to be one of the major mechanisms of chemoprevention in colorectal carcinogenesis in case of Curcumin. It was demonstrated that the number of β-catenin-positive adenoma cells in Apc (580D/+) mice fed GO-Y030 was also reduced [66].
Other Curcumin analogs, FLLL11 and FLLL12, also inhibited phosphorylation of STAT3 in breast and prostate cancer cells [67]. In addition, these analogs exhibited more potent activities than Curcumin on the down-regulation of STAT3, Akt, and HER-2/neu, as well as the inhibition of cancer cell growth and migration [68]. GO-Y030, FLLL11, and FLLL12 27 also induced apoptosis through increased cleavage of PARP and caspase-3 in pancreatic and colorectal cancer cells, suggesting their potential use as chemopreventive and therapeutic agents for various cancers [69, 70]. However, further pre-clinical animal experiments and toxicity studies are needed in order to understand whether these analogs could have any value for the treatment of human malignancies.
The question of whether the activity of Curcumin is based upon its scaffold or whether it results from the Michael acceptor properties due to α, β-unsaturated diketone moiety central to its structure remains unsolved and there are experimental evidences supporting both. Recently, Curcumin analogs such as pyrazole and isoxazole 28 (Scheme 1) has been synthesized and evaluated for their anticancer activity. Both the analogs were found to possess enhanced anti-tumor potency against MCF-7 and MDR-transfected MCF-7 breast cancer cells [71, 72]. Furthermore, they reduced the expression levels of Bcl-2, Bcl-XL, and COX-2 in the breast cancer cells. These results suggest that these analogs could be effective anticancer agents for the treatment of hormone-independent MDR breast cancer [72]. In another report, electron-rich pyrazole and isoxazole analogs were synthesized and evaluated against two hormone-dependent and independent breast cancer cell lines [71]. All these analogs showed lower IC50 values in the sub-micromolar range, which were ten to fifty times lower than those for Curcumin [71, 73]; however none was reported for their systemic or tissue bioavailability.
A monoketo compound EF24, 29 (Scheme 1) developed from Curcumin, showed potent anticancer activity through down-regulation of NF-κB signaling [74]. The compound induced apoptotic cell death in lung, breast, ovarian, and cervical cancer cells with ten time higher potency than that of Curcumin. It inhibited phosphorylation and degradation of IκB and blocked the nuclear translocation of NF-κB, leading to inhibition of NF-κB activation. It also induced cell cycle arrest and apoptosis by means of a redox-dependent mechanism in MDA-MB-231 human breast cancer cells and DU-145 human prostate cancer cells [74]. Treatment of MDA-MB231 breast and PC3 prostate cancer cells with EF24 led to inhibition of HIF-1alpha protein levels and, consequently, inhibition of HIF transcriptional activity. This inhibition occurred in a VHL-dependent but proteasome-independent manner. While Curcumin inhibited HIF-1alpha gene transcription, EF24 exerted its activity by inhibiting HIF-1alpha post-transcriptionally which suggests that although two compounds are structurally similar but they exert their effects through mechanistically through distinct pathways. Another cellular effect that further differentiates the two compounds was the ability of EF24 but not Curcumin to induce microtubule stabilization in cells. EF24 had no stabilizing effect on tubulin polymerization as judged from in vitro assay using purified bovine brain tubulin, suggesting that the EF24-induced cytoskeletal disruption in cells may be the result of upstream signaling events rather than EF24's ability for direct binding to tubulin [75, 76].
Monoketo analogs of Curcumin with a 3, 5, or 7-carbon spacers have been compared with Curcumin for their abilities to inhibit the TNF-α-induced activation of NF-κB wherein the analogs with the 5-carbon spacer have been especially found to be active for the inhibition of NF-κB [77]. Ohori et al. have synthesized symmetrical 1, 5-diarylpentadienone as Curcumin analogs whose aromatic rings possess an alkoxy substitution at each of the C-3 and C-5 positions 30 (Scheme 1). After examining their effects on the cancer-related genes usually affected by Curcumin, it was found that some of these analogs can induce down-regulation of β-catenin, Ki-ras, cyclin D1, c-Myc, and ErbB-2, respectively [78]. The dienone cyclopropoxy Curcumin analogs 31 when tested in mice bearing Ehrlich Ascites Tumor (EAT) in vivo have revealed that these analogs increase the life span of mice bearing EAT with significant reduction in the micro vessel density in the peritoneum walls of mice with concomitant reduction in the ascites volume in this animal model using the synthesized analog 31 (1a and 4a); (Scheme 1) among many, which was found to be especially more potent for angiogenesis assay [79].
In order to determine which part of Curcumin molecule is critical for its chemoprotective potential, Donkova-Kostova and coworkers have examined its ability for inducing Phase 2 detoxification enzymes in murine hepatoma cells. For this purpose two groups of compounds were studied including classical Michael acceptors (such as Curcumin) and related ß-diketones such as dibenzoylmethane which lack direct Michael reactivity. It was concluded that the presence of hydroxyl groups at ortho-position on the aromatic rings and ß-diketone functionality are crucial for the induction of Phase 2 enzymes, which inhibit production of highly reactive carcinogens in the body leading to tumor initiation [80].
Robinson et al. have reported evaluation of aromatic enone and dienone analogs 32 and 33 (Scheme 1) of Curcumin. These compounds were screened for anti-angiogenic properties in vitro by Sluzhba Vneshney Razvedki (SVR) assay and were found to inhibit cell proliferation. Aromatic enone and aromatic dienone analogs of curcumin are excellent anti-angiogenic compounds, having inhibition patterns equivalent or better than the parent Curcumin molecule [81]. Substitution of electronegative groups at either or both of the rings seems to enhance their activity through stereo electronic effects and conformational flexibility. Shim, et al. have found that isoxazole and pyrazole derivatives 28 (Scheme 1) also interfere with the cell cycle progression of colon cancer cells by antagonizing the Ca2+/Calmodulin function which was confirmed by IR, NMR and Mass spectrometric analysis [82]. Selvam et al. have examined anti-oxidant and cyclooxygenase inhibitory activities of these analogs 28 (Scheme 1) [83] but none has been investigated for their systemic and tissue bioavailability.
e. Modification of Adjacent Carbon
Snyder and co-workers have prepared glutathione conjugates 34 (Scheme 1) of curcumin named as EF-24-GSH with enhanced water solubility. However, anticancer potential of the new analog remains more or less similar to that of parent molecule [33].
7. MOLECULAR MODELING OF CURCUMIN ANALOGS IN COX-2
Numerous studies have established that Curcumin is a pleiotropic molecule capable of interacting with numerous molecular targets involved in inflammation. It has also been proven that pro-inflammatory states are linked to tumor promotion [84, 85]. Consequently, Curcumin's strong anti-inflammatory effects can be better understood through its modulatory response by down-regulation of the enzymes, viz. cyclooxygenase-2 (COX-2), lipooxygenase (LOX) and inducible nitric oxide synthase (iNOS), respectively, and all of these enzymes give rise to generation of several inflammatory factors such as cytokines, leukotrienes, prostaglandins, tumor necrosis factor-alpha (TNF-alpha) and many others. The COX-2 and iNOS inhibition are likely accomplished via Curcumin's suppression of NF-κB, which is a ubiquitous transcription factor involved in the regulation of inflammation, cellular proliferation, transformation and tumorigenesis. Curcumin is thought to suppress NF-κB activation and pro-inflammatory gene expression by blocking phosphorylation of inhibitory factor I-kappa B kinase (IκB). Suppression of NF-κB activation subsequently down-regulates COX-2 and iNOS expression thereby inhibiting the inflammatory processes and tumorigenesis.
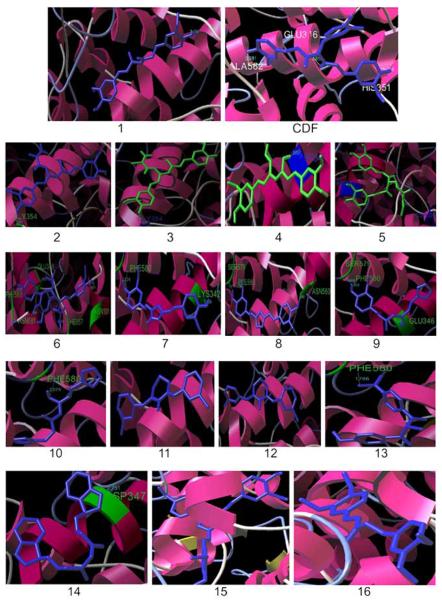
In order to evaluate the efficacy of various Curcumin analogs described earlier we have docked 15 of them into COX-2 cavity using Auto Dock 3.05 software for the first time. The crystal structure of COX-2 protein was obtained from PDB ID (6COX). The active site of the enzyme was defined to include residues ALA562, GLU 346, GLN 350 within 6.5 A° radius to any of the inhibitor atoms. The Auto Dock 3.05 program is an automated docking program, which was used to dock all fifteen Curcumin analogs as well as parent Curcumin molecule in the active site of the COX-2 enzyme. For each compound, the most stable docking model was selected based upon conformation of best score predicted by the Auto Dock scoring function. The compounds were energy minimized with a MMFF94 force field till the gradient convergence value of 0.05 kcal/mol was reached using distance dependence dielectric function (ε = 4r). Various Curcumin analogs were found to dock into the active site of COX-2 enzyme. In case of difluorocurcumin analog, our novel compound CDF, did not introduce any major steric changes compared to the parent Curcumin molecule except allowing more hydrogen bonding interactions (Fig. 6 and Scheme 1). The binding energies of the Curcumin analogs were in the range of −5.69 to −9.79 kcal/mol compared to the binding energy of parent Curcumin being −5.71 kcal /mol.
Fig. (6).
Binding of Curcumin analogs into the active site of COX-2.
The lower interaction energy observed for the difluorocurcumin analog CDF rationalizes the tighter binding of this compound in the active site of COX-2 than other analogs. In our docking experiments, Curcumin showed only one H-bonding interaction with ALA582. On the other hand the most potent difluoro-curcumin analog CDF exhibits 4 H-bonding interactions involving residues GLU346, ALA582, SER353 and HIS351, respectively. Curcumin analogs 10, 11, 14 and 15 (Scheme 1) did not exhibit H-bond interactions [40]. Favorable van der Waals interactions between styryl carbon atoms and the hydrophobic residues such as GLU 346(3.01A°), SER 353(3.13A°) and between methoxy group of CDF and HIS 351, ALA 582 residues contribute to stabilization of the ligand-enzyme complexes of this analog. Obviously detailed QSAR analysis is required to understand the structural features that may be playing crucial role in the anti-inflammatory characteristics of Curcumin analogs, and their subsequent influence on the anti-tumor properties. However, our recent results are very encouraging together with our data on the systemic and target tissue bioavailability especially in the pancreas, which could indeed be greatly useful for the prevention and/or treatment of human pancreatic cancer for which there is no curative therapy at the present time. We are hopeful that our Curcumin analog CDF could be a novel agent for the treatment of human malignancies in the near future.
8. PERSPECTIVES ON CURCUMIN ANALOGS
Due to limited bioavailability and retention in the target tissue, which is primarily due to rapid plasma clearance and conjugation, the therapeutic usefulness of Curcumin has been somewhat limited especially for tumors other than gastrointestinal tissues. Consequently a large number of studies have been carried out employing a variety of drug delivery systems for Curcumin, which although somewhat successful in improving its aqueous solubility but these emerging evidence did not address the problem of its rapid metabolism. On the other hand, a large number of studies dealing with structural modifications of Curcumin have provided many insights into the structural peculiarities of Curcumin required for its antioxidant, anti-inflammatory, anticancer and anti-angiogenic activities but failed to document target tissue bioavailability. The key structural positions for the anticancer activities include the side aryl rings, conjugated double bonds, the central diketo function and modification of active methylene group. Among these, the substituents on active methylene group seem to hold the key for modulating the metabolic rates of Curcumin. The difluorocurcumin Knoevenagel condensate reported recently by our group [40] has shown that CDF was metabolically stable allowing longer circulation times in the biological system. Since these newly synthesized analogs especially CDF as documented in our recent studies show preferential target tissue accumulation in pancreas, it may pave the way for specific targeting of CDF in pancreatic tumors, which of course need further in-depth investigations. This finding clearly opens the possibility of using pleiotropic agent such as Curcumin-derived synthetic analogs for specific targeting; although as stated above further-in-depth experimental investigation along with animal studies, and eventual clinical trials are needed in order to fully evaluate these new Curcumin analogs and their novel formulations for the prevention and/or treatment of human malignancies.
ACKNOWLEDGEMENTS
SP would like to acknowledge encouragement and support received from authorities of Dr. D. Y. Patil University, Pune. The work of FHS cited in this review was funded by grants from the National Cancer Institute, NIH (R01CA083695, R01CA131151, and R01CA132794 awarded to FHS), and a sub-contract award to FHS from the University of Texas MD Anderson Cancer Center through a SPORE grant (5P20-CA101936) on pancreatic cancer awarded to James Abbruzzese. We also thank the contribution of Guido and Puschelberg Foundation for their generous contribution in supporting our research.
REFERENCES
- 1.Craig WJ. Health-promoting properties of common herbs. Am. J. Clin. Nutr. 1999;70:491S–9S. doi: 10.1093/ajcn/70.3.491s. [DOI] [PubMed] [Google Scholar]
- 2.PDR for Herbal Medicines. 3rd ed. Montvale, NJ: 2004. [Google Scholar]
- 3.Setchell K, Cassidy A. Absorption and Metabolism of Soy Isoflavones - from Food to Dietary Supplements and Adults to Infants. J. Nutr. 1999;129:758S–67S. doi: 10.1093/jn/130.3.654S. [DOI] [PubMed] [Google Scholar]
- 4.Bloch A. Should a mild to moderate ischemic mitral valve regurgitation in patients with poor left ventricular function be repaired or not? JADA. 1995;95:493–496. [PubMed] [Google Scholar]
- 5.Hara Y, Honda M. alpha-glucosidase Inhibitory Action of Natural Acylated Anthocyanins. Agric. Biochem. 2009;54:1939–1945. [Google Scholar]
- 6.Matsumato M, Ishigaki F, Ishigaki A, Iwashina H, Hara Y. Green Tea Polyphenols Inhibit the Sodium-Dependent Glucose Transporter of Intestinal Epithelial Cells by a Competitive Mechanism. Biosci. Biotechnol. Biochem. 1993;57:525–527. [Google Scholar]
- 7.Tiwari AK, Rao JM. Diabetes mellitus and multiple therapeutic approaches of phytochemicals: Present status and future prospects. Curr. Sci. 2002;83:30–38. [Google Scholar]
- 8.Howard BV, Kritchevsky D. Phytochemicals and cardiovascular disease. A statement for healthcare professionals from the American Heart Association. Circulation. 1997;95:2591–2593. doi: 10.1161/01.cir.95.11.2591. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar FH, Li Y. Harnessing the fruits of nature for the development of multi-targeted cancer therapeutics. Cancer Treat. Rev. 2009;35:597–607. doi: 10.1016/j.ctrv.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadkarni A., Dr. K.M. Nadkarni's Indian Materia Medica, with Ayurvedic, Unani-Tibbi, Siddha, Allopathic, Homeopathic, Naturopathic and Home Remedies, Appendices and Indexes. Indian Mater. Med. 1954 [Google Scholar]
- 12.Babu PS, Srinivasan K. Hypolipidemic action of curcumin, the active principle of turmeric (Curcuma longa) in streptozotocin induced diabetic rats. Mol. Cell Biochem. 1997;166:169–175. doi: 10.1023/a:1006819605211. [DOI] [PubMed] [Google Scholar]
- 13.Gilani AH, Shah AJ, Ghayur MN, Majeed K. Pharmacological basis for the use of turmeric in gastrointestinal and respiratory disorders. Life Sci. 2005;76:3089–3105. doi: 10.1016/j.lfs.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Goel A, Jhurani S, Aggarwal BB. Multi-targeted therapy by curcumin: how spicy is it? Mol. Nutr. Food Res. 2008;52:1010–1030. doi: 10.1002/mnfr.200700354. [DOI] [PubMed] [Google Scholar]
- 15.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Jang EM, Choi MS, Jung UJ, Kim MJ, Kim HJ, Jeon SM, Shin SK, Seong CN, Lee MK. Beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat-fed hamsters. Metabolism. 2008;57:1576–1583. doi: 10.1016/j.metabol.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 18.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, Krishnan S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin. Cancer Res. 2008;14:2128–2136. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 20.Shishodia S, Chaturvedi MM, Aggarwal BB. Role of curcumin in cancer therapy. Curr. Probl. Cancer. 2007;31:243–305. doi: 10.1016/j.currproblcancer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Sidhu GS, Mani H, Gaddipati JP, Singh AK, Seth P, Banaudha KK, Patnaik GK, Maheshwari RK. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound. Repair Regen. 1999;7:362–374. doi: 10.1046/j.1524-475x.1999.00362.x. [DOI] [PubMed] [Google Scholar]
- 22.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) J. Biol. Chem. 1995;270:24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 23.Singh SV, Hu X, Srivastava SK, Singh M, Xia H, Orchard JL, Zaren HA. Mechanism of inhibition of benzo[a]pyrene-induced forestomach cancer in mice by dietary curcumin. Carcinogenesis. 1998;19:1357–1360. doi: 10.1093/carcin/19.8.1357. [DOI] [PubMed] [Google Scholar]
- 24.Xu YX, Pindolia KR, Janakiraman N, Noth CJ, Chapman RA, Gautam SC. Curcumin, a compound with anti-inflammatory and anti-oxidant properties, down-regulates chemokine expression in bone marrow stromal cells. Exp. Hematol. 1997;25:413–422. [PubMed] [Google Scholar]
- 25.Verma SP, Salamone E, Goldin B. Curcumin and genistein, plant natural products, show synergistic inhibitory effects on the growth of human breast cancer MCF-7 cells induced by estrogenic pesticides. Biochem. Biophys. Res. Commun. 1997;233:692–696. doi: 10.1006/bbrc.1997.6527. [DOI] [PubMed] [Google Scholar]
- 26.Wahlstrom B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. (Copenh) 1978;43:86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 27.Bhutani MK, Bishnoi M, Kulkarni SK. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neuro-chemical changes. Pharmacol. Biochem. Behav. 2009;92:39–43. doi: 10.1016/j.pbb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 29.Sahu A, Kasoju N, Bora U. Fluorescence study of the curcumincasein micelle complexation and its application as a drug nanocarrier to cancer cells. Biomacromolecules. 2008;9:2905–2912. doi: 10.1021/bm800683f. [DOI] [PubMed] [Google Scholar]
- 30.Shen L, Ji HF. Theoretical study on physicochemical properties of curcumin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007;67:619–623. doi: 10.1016/j.saa.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Liang G, Yang S, Jiang L, Zhao Y, Shao L, Xiao J, Ye F, Li Y, Li X. Synthesis and anti-bacterial properties of monocarbonyl analogues of curcumin. Chem. Pharm. Bull. (Tokyo) 2008;56:162–167. doi: 10.1248/cpb.56.162. [DOI] [PubMed] [Google Scholar]
- 32.Liang G, Yang S, Zhou H, Shao L, Huang K, Xiao J, Huang Z, Li X. Synthesis, crystal structure and anti-inflammatory properties of curcumin analogues. Eur. J. Med. Chem. 2009;44:915–919. doi: 10.1016/j.ejmech.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 33.Mosley CA, Liotta DC, Snyder JP. Highly active anticancer curcumin analogues. Adv. Exp. Med. Biol. 2007;595:77–103. doi: 10.1007/978-0-387-46401-5_2. [DOI] [PubMed] [Google Scholar]
- 34.Poma P, Notarbartolo M, Labbozzetta M, Maurici A, Carina V, Aliamo A, Rizzi M, Simoni D, D'Alessandro N. The anti-tumor activities of curcumin and of its isoxazole analogue are not affected by multiple gene expression changes in an MDR model of the MCF-7 breast cancer cell line: analysis of the possible molecular basis. Int. J. Mol. Med. 2007;20:329–335. [PubMed] [Google Scholar]
- 35.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 36.Bandyopadhyay U, Das D, Banerjee RK. Reactive oxygen species: Oxidative damage and pathogenesis. Curr. Sci. 1999;77:658–666. [Google Scholar]
- 37.Krishnankutty K, Venugopalan P. Unsaturated beta-Keto esters and their Ni(II), Cu(II), Zn(II) complexes. Synth. Pract. Inorg. Met. Org. Chem. 1998;28:1313. [Google Scholar]
- 38.Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, Steward WP, Gescher A. Characterization of metabolites of the chemo-preventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61:1058–1064. [PubMed] [Google Scholar]
- 39.Padhye S, Yang H, Jamadar A, Cui QC, Chavan D, Dominiak K, McKinney J, Banerjee S, Dou QP, Sarkar FH. New difluoro Knoevenagel condensates of curcumin, their Schiff bases and copper complexes as proteasome inhibitors and apoptosis inducers in cancer cells. Pharm. Res. 2009;26:1874–1880. doi: 10.1007/s11095-009-9900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padhye S, Banerjee S, Chavan D, Pandye S, Swamy KV, Ali S, Li J, Dou QP, Sarkar FH. Fluorocurcumins as Cyclooxygenase-2 Inhibitor: Molecular Docking, Pharmacokinetics and Tissue Distribution in Mice. Pharm. Res. 2009;26(11):2438–2445. doi: 10.1007/s11095-009-9955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D, Veena MS, Stevenson K, Tang C, Ho B, Suh JD, Duarte VM, Faull KF, Mehta K, Srivatsan ES, Wang MB. Liposome-encapsulated curcumin suppresses growth of head and neck squamous cell carcinoma in vitro and in xenografts through the inhibition of nuclear factor kappaB by an AKT-independent pathway. Clin Cancer Res. 2008;14:6228–6236. doi: 10.1158/1078-0432.CCR-07-5177. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi M, Uechi S, Takara K, Asikin Y, Wada K. Evaluation of an oral carrier system in rats: bioavailability and antioxidant properties of liposome-encapsulated curcumin. J. Agric. Food Chem. 2009;57:9141–9146. doi: 10.1021/jf9013923. [DOI] [PubMed] [Google Scholar]
- 43.Paradkar A, Ambike AA, Jadhav BK, Mahadik KR. Characterization of curcumin-PVP solid dispersion obtained by spray drying. Int. J. Pharm. 2004;271:281–286. doi: 10.1016/j.ijpharm.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Jiang Y, Huang Q, Ho Q, Huang M. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008;108:419–424. doi: 10.1016/j.foodchem.2007.10.086. [DOI] [PubMed] [Google Scholar]
- 45.Sahu A, Bora U, Kasoju N, Goswami P. Synthesis of novel biodegradable and self-assembling methoxy poly(ethylene glycol)-palmitate nanocarrier for curcumin delivery to cancer cells. Acta Biomater. 2008;4:1752–1761. doi: 10.1016/j.actbio.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 46.Zambre AP, Kulkarni VM, Padhye S, Sandur SK, Aggarwal BB. Novel curcumin analogs targeting TNF-induced NFkappaB activation and proliferation in human leukemic KBM-5 cells. Bioorg. Med. Chem. 2006;14:7196–7204. doi: 10.1016/j.bmc.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 47.Qiu X, Liu Z, Shao WY, Liu X, Jing DP, Yu YJ, An LK, Huang SL, Bu XZ, Huang ZS, Gu LQ. Synthesis and evaluation of curcumin analogues as potential thioredoxin reductase inhibitors. Bioorg. Med. Chem. 2008;16:8035–8041. doi: 10.1016/j.bmc.2008.07.054. [DOI] [PubMed] [Google Scholar]
- 48.Jankun J, Aleem AM, Malgorzewicz S, Szkudlarek M, Zavodszky MI, Dewitt DL, Feig M, Selman SH, Skrzypczak-Jankun E. Synthetic curcuminoids modulate the arachidonic acid metabolism of human platelet 12-lipoxygenase and reduce sprout formation of human endothelial cells. Mol. Cancer Ther. 2006;5:1371–1382. doi: 10.1158/1535-7163.MCT-06-0021. [DOI] [PubMed] [Google Scholar]
- 49.Venkataswarlu S. PolyhydroxyCinnamic acid ester derivative as antioxidant and antimicrobial agents. J. Chem. Res. 2000;138 [Google Scholar]
- 50.Ligeret H, Barthelemy S, Zini R, Tillement JP, Labidalle S, Morin D. Effects of curcumin and curcumin derivatives on mitochondrial permeability transition pore. Free Radic. Biol. Med. 2004;36:919–929. doi: 10.1016/j.freeradbiomed.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 51.Hallgrimsson B, Donnabhain BO, Blom DE, Lozada MC, Willmore KT. Why are rare traits unilaterally expressed?: trait frequency and unilateral expression for cranial nonmetric traits in humans. Am. J. Phys. Anthropol. 2005;128:14–25. doi: 10.1002/ajpa.20187. [DOI] [PubMed] [Google Scholar]
- 52.Gafner S, Lee SK, Cuendet M, Barthelemy S, Vergnes L, Labidalle S, Mehta RG, Boone CW, Pezzuto JM. Biologic evaluation of curcumin and structural derivatives in cancer chemo-prevention model systems. Phytochemistry. 2004;65:2849–2859. doi: 10.1016/j.phytochem.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Ferrari E, Lazzari S, Marverti G, Pignedoli F, Spagnolo F, Saladini M. Synthesis, cytotoxic and combined cDDP activity of new stable curcumin derivatives. Bioorg. Med. Chem. 2009;17:3043–3052. doi: 10.1016/j.bmc.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 54.Du ZY, Bao YD, Liu Z, Qiao W, Ma L, Huang ZS, Gu LQ, Chan AS. Curcumin analogs as potent aldose reductase inhibitors. Arch. Pharm. (Weinheim) 2006;339:123–128. doi: 10.1002/ardp.200500205. [DOI] [PubMed] [Google Scholar]
- 55.Mizushina Y, Ishidoh T, Takeuchi T, Shimazaki N, Koiwai O, Kuramochi K, Kobayashi S, Sugawara F, Sakaguchi K, Yoshida H. Monoacetylcurcumin: a new inhibitor of eukaryotic DNA polymerase lambda and a new ligand for inhibitor-affinity chromatography. Biochem. Biophys. Res. Commun. 2005;337:1288–1295. doi: 10.1016/j.bbrc.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Tamvakopoulos C, Dimas K, Sofianos ZD, Hatziantoniou S, Han Z, Liu ZL, Wyche JH, Pantazis P. Metabolism and anti-cancer activity of the curcumin analogue, dimethoxycurcumin. Clin. Cancer Res. 2007;13:1269–1277. doi: 10.1158/1078-0432.CCR-06-1839. [DOI] [PubMed] [Google Scholar]
- 57.Hahm ER, Gho YS, Park S, Park C, Kim KW, Yang CH. Synthetic curcumin analogs inhibit activator protein-1 transcription and tumor-induced angiogenesis. Biochem. Biophys. Res. Commun. 2004;321:337–344. doi: 10.1016/j.bbrc.2004.06.119. [DOI] [PubMed] [Google Scholar]
- 58.Handler N, Jaeger W, Puschacher H, Leisser K, Erker T. Synthesis of novel curcumin analogues and their evaluation as selective cyclooxygenase-1 (COX-1) inhibitors. Chem. Pharm. Bull. (Tokyo) 2007;55:64–71. doi: 10.1248/cpb.55.64. [DOI] [PubMed] [Google Scholar]
- 59.Davis R, Das U, Mackay H, Brown T, Mooberry SL, Dimmock JR, Lee M, Pati H. Syntheses and cytotoxic properties of the curcumin analogs 2,6-bis(benzylidene)-4-phenylcyclohexanones. Arch. Pharm. (Weinheim) 2008;341:440–445. doi: 10.1002/ardp.200800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen WF, Deng SL, Zhou B, Yang L, Liu ZL. Curcumin and its analogues as potent inhibitors of low density lipoprotein oxidation: H-atom abstraction from the phenolic groups and possible involvement of the 4-hydroxy-3-methoxyphenyl groups. Free Radic. Biol. Med. 2006;40:526–535. doi: 10.1016/j.freeradbiomed.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 61.Youssef KM, El-Sherbeny MA. Synthesis and antitumor activity of some curcumin analogs. Arch. Pharm. (Weinheim) 2005;338:181–189. doi: 10.1002/ardp.200400939. [DOI] [PubMed] [Google Scholar]
- 62.Padhye S, Zambre AP, Jamadar A, Kulkarni VM. Copper conjugates of Knoevenagel condensates of curcumin and their Schiff base derivatives: Synthesis, spectroscopy, magnetism, ESR, and electrochemistry Synthesis and Reactivity in Inorganic, Metal-Organic and Nano-Metal Chemistry. Acta Biomater. 2007;37:19–27. [Google Scholar]
- 63.Daniel KG, Gupta P, Harbach RH, Guida WC, Dou QP. Organic copper complexes as a new class of proteasome inhibitors and apoptosis inducers in human cancer cells. Biochem. Pharmacol. 2004;67:1139–1151. doi: 10.1016/j.bcp.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 64.Nakamura K, Yasunaga Y, Segawa T, Ko D, Moul JW, Srivastava S, Rhim JS. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int. J. Oncol. 2002;21:825–830. [PubMed] [Google Scholar]
- 65.Ohtsu H, Xiao Z, Ishida J, Nagai M, Wang HK, Itokawa H, Su CY, Shih C, Chiang T, Chang E, Lee Y, Tsai MY, Chang C, Lee KH. Antitumor agents. 217. Curcumin analogues as novel androgen receptor antagonists with potential as anti-prostate cancer agents. J. Med. Chem. 2002;45:5037–5042. doi: 10.1021/jm020200g. [DOI] [PubMed] [Google Scholar]
- 66.Shibata H, Yamakoshi H, Sato A, Ohori H, Kakudo Y, Kudo C, Takahashi Y, Watanabe M, Takano H, Ishioka C, Noda T, Iwabuchi Y. Newly synthesized curcumin analog has improved potential to prevent colorectal carcinogenesis in vivo. Cancer Sci. 2009;100:956–960. doi: 10.1111/j.1349-7006.2009.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hutzen B, Friedman L, Sobo M, Lin L, Cen L, De AS, Yamakoshi H, Shibata H, Iwabuchi Y, Lin J. Curcumin analogue GO-Y030 inhibits STAT3 activity and cell growth in breast and pancreatic carcinomas. Int. J. Oncol. 2009;35:867–872. doi: 10.3892/ijo_00000401. [DOI] [PubMed] [Google Scholar]
- 68.Lin L, Hutzen B, Ball S, Foust E, Sobo M, Deangelis S, Pandit B, Friedman L, Li C, Li PK, Fuchs J, Lin J. New curcumin analogues exhibit enhanced growth-suppressive activity and inhibit AKT and signal transducer and activator of transcription 3 phosphorylation in breast and prostate cancer cells. Cancer Sci. 2009;100:1719–1727. doi: 10.1111/j.1349-7006.2009.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Friedman L, Lin L, Ball S, Bekaii-Saab T, Fuchs J, Li PK, Li C, Lin J. Curcumin analogues exhibit enhanced growth suppressive activity in human pancreatic cancer cells. Anticancer Drugs. 2009;20:444–449. doi: 10.1097/CAD.0b013e32832afc04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cen L, Hutzen B, Ball S, Deangelis S, Chen CL, Fuchs JR, Li C, Li PK, Lin J. New structural analogues of curcumin exhibit potent growth suppressive activity in human colorectal carcinoma cells. BMC. Cancer. 2009;9:99. doi: 10.1186/1471-2407-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amolins MW, Peterson LB, Blagg BS. Synthesis and evaluation of electron-rich curcumin analogues. Bioorg. Med. Chem. 2009;17:360–367. doi: 10.1016/j.bmc.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Labbozzetta M, Notarbartolo M, Poma P, Maurici A, Inguglia L, Marchetti P, Rizzi M, Baruchello R, Simoni D, D'Alessandro N. Curcumin as a possible lead compound against hormone-independent, multidrug-resistant breast cancer. Ann. N. Y. Acad. Sci. 2009;1155:278–283. doi: 10.1111/j.1749-6632.2009.03699.x. [DOI] [PubMed] [Google Scholar]
- 73.Fuchs JR, Pandit B, Bhasin D, Etter JP, Regan N, Abdelhamid D, Li C, Lin J, Li PK. Structure-activity relationship studies of curcumin analogues. Bioorg. Med. Chem. Lett. 2009;19:2065–2069. doi: 10.1016/j.bmcl.2009.01.104. [DOI] [PubMed] [Google Scholar]
- 74.Kasinski AL, Du Y, Thomas SL, Zhao J, Sun SY, Khuri FR, Wang CY, Shoji M, Sun A, Snyder JP, Liotta D, Fu H. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol. Pharmacol. 2008;74:654–661. doi: 10.1124/mol.108.046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adams BK, Cai J, Armstrong J, Herold M, Lu YJ, Sun A, Snyder JP, Liotta DC, Jones DP, Shoji M. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer Drugs. 2005;16:263–275. doi: 10.1097/00001813-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 76.Thomas SL, Zhong D, Zhou W, Malik S, Liotta D, Snyder JP, Hamel E, Giannakakou P. EF24, a novel curcumin analog, disrupts the microtubule cytoskeleton and inhibits HIF-1. Cell Cycle. 2008;7:2409–2417. doi: 10.4161/cc.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weber WM, Hunsaker LA, Roybal CN, BobrovnikovaMarjon EV, Abcouwer SF, Royer RE, Deck LM, Vander Jagt DL. Activation of NFkappaB is inhibited by curcumin and related enones. Bioorg. Med. Chem. 2006;14:2450–2461. doi: 10.1016/j.bmc.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 78.Ohori H, Yamakoshi H, Tomizawa M, Shibuya M, Kakudo Y, Takahashi A, Takahashi S, Kato S, Suzuki T, Ishioka C, Iwabuchi Y, Shibata H. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol. Cancer Ther. 2006;5:2563–2571. doi: 10.1158/1535-7163.MCT-06-0174. [DOI] [PubMed] [Google Scholar]
- 79.Chandru H, Sharada AC, Bettadaiah BK, Kumar CS, Rangappa KS, Sunila, Jayashree K. In vivo growth inhibitory and anti-angiogenic effects of synthetic novel dienone cyclopropoxy curcumin analogs on mouse Ehrlich ascites tumor. Bioorg. Med. Chem. 2007;15:7696–703. doi: 10.1016/j.bmc.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 80.nkova-Kostova AT, Talalay P. Relation of structure of curcumin analogs to their potencies as inducers of Phase 2 detoxification enzymes. Carcinogenesis. 1999;20:911–914. doi: 10.1093/carcin/20.5.911. [DOI] [PubMed] [Google Scholar]
- 81.Robinson TP, Ehlers T, Hubbard IV RB, Bai X, Arbiser JL, Goldsmith DJ, Bowen JP. Design, synthesis, and biological evaluation of angiogenesis inhibitors: aromatic enone and dienone analogues of curcumin. Bioorg. Med. Chem. Lett. 2003;13:115–117. doi: 10.1016/s0960-894x(02)00832-6. [DOI] [PubMed] [Google Scholar]
- 82.Shim JS, Kim DH, Jung HJ, Kim JH, Lim D, Lee SK, Kim KW, Ahn JW, Yoo JS, Rho JR, Shin J, Kwon HJ. Hydrazinocurcumin, a novel synthetic curcumin derivative, is a potent inhibitor of endothelial cell proliferation. Bioorg. Med. Chem. 2002;10:2439–2444. doi: 10.1016/s0968-0896(02)00116-5. [DOI] [PubMed] [Google Scholar]
- 83.Selvam C, Jachak SM, Thilagavathi R, Chakraborti AK. Design, synthesis, biological evaluation and molecular docking of curcumin analogues as antioxidant, cyclooxygenase inhibitory and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2005;15:1793–1797. doi: 10.1016/j.bmcl.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 84.Bennett A. The production of prostanoids in human cancers, and their implications for tumor progression. Prog. Lipid Res. 1986;25:539–542. doi: 10.1016/0163-7827(86)90109-8. [DOI] [PubMed] [Google Scholar]
- 85.Qiao L, Kozoni V, Tsioulias GJ, Koutsos MI, Hanif R, Shiff SJ, Rigas B. Selected eicosanoids increase the proliferation rate of human colon carcinoma cell lines and mouse colonocytes in vivo. Biochim. Biophys. Acta. 1995;1258:215–223. doi: 10.1016/0005-2760(95)00100-q. [DOI] [PubMed] [Google Scholar]