Abstract
The Notch signaling pathway maintains a balance between cell proliferation and apoptosis, and thus it is believed that Notch signaling pathway may play an important role in the development and progression of several malignancies. However, the functions of Notch signaling in EMT are largely unknown. This mini review describes the role of Notch signaling pathway in EMT, and cataloging how its deregulation is involved in EMT and tumor aggressiveness. Further attempts have been made to summarize the role of several chemopreventive agents that could be useful for targeted inactivation of Notch signaling, and thus it may cause reversal of EMT, which could become a novel approach for cancer prevention and treatment.
Keywords: Notch, EMT, cancer
THE CONCEPT OF EPITHELIAL-MESENCHYMAL TRANSITION (EMT)
In recent years, we have witnessed the sudden explosion in the literature regarding the biological significance of epithelial-mesenchymal transition (EMT) in tumor aggressiveness [1-4]. It is now widely accepted that epithelial cells can acquire mesenchymal phenotype by a fundamental yet complex processes. EMT is a unique process by which epithelial cells undergo remarkable morphologic changes characterized by a transition from epithelial cobblestone phenotype to elongated fibroblastic phenotype (mesenchymal phenotype) leading to increased motility and invasion [1, 2]. During the acquisition of EMT characteristics, cells lose epithelial cell-cell junction, actin cytoskeleton reorganization and the expression of proteins that promote cell-cell contact such as E-cadherin and γ-catenin, and gains in the expression of mesenchymal markers such as vimentin, fibronectin, α-smooth muscle actin (SMA), fibrillar collagen (type I and III), fibroblast-specific protein-1, N-cadherin as well as increased activity of matrix metalloproteinases (MMPs) like MMP-2, MMP-3 and MMP-9 [3, 4]. Specifically, a disassembly of cell-cell junction, including down-regulation and relocation of E-cadherin and β-catenin from cell membrane to nucleus, results in the induction of EMT [1-4].
The original definition of EMT was reported in different areas of embryos [5]. Although, EMT was originally identified as a crucial differentiation and morphogenetic process during embryogenesis, it has now been well illustrated in many patho-physiological conditions including tumor progression and metastasis. Interestingly, it has been proposed that cancer stem cells (CSCs) may represent the critical contributions of the complex processes of EMT in the context of a growing tumor mass [6]. In addition, recent studies have shown that EMT is associated with drug resistance and cancer cell metastasis [7-9]. EMT has been shown to be important on conferring drug resistance characteristics to cancer cells against conventional therapeutics including taxol, vincristine and oxaliplatin [10]. Therefore, discovery of precise mechanisms that governs the acquisition of EMT phenotype of growing cancer cells would likely be useful for devising targeted therapeutic approaches in combination with conventional therapeutics for the treatment of human malignancies.
A number of factors that transcriptionally repress E-cadherin have emerged as potent EMT drivers during normal development and cancer. These include the zinc finger binding transcription factors Snail homologues (Snail1, Snail2/Slug, and Snail3) and several other basic helix-loop-helix transcription factors such as Twist, ZEB1, ZEB2/SIP1, and TCF3/E47/E12 [1, 2]. One must acknowledge that the processes of EMT is a dynamic process, and it is triggered by the interplay of extracellular signals (such as collagen and hyaluronic acid) and many secreted soluble factors including, at least, transforming growth factor-β (TGF-β), fibroblast growth factor (FGF), epidermal growth factor (EGF), hepatocyte growth factor (HGF), platelet-derived growth factors (PDGF) as well as different isoforms of Wnt proteins, MMPs, bone morphogenic proteins and many others. Among many of these signaling pathways, the Wnt, TGF-β, Hedgehog, and nuclear factor-κB (NF-κB) signaling pathways have been claimed to be critical for the induction of EMT phenotype [3, 11-13]. Emerging evidence also implicated the critical role of several microRNAs (miRNAs) in the processes of EMT [14, 15]. Recent experimental evidence has shown that the presence of hypoxia can also induce EMT [16, 17]. Moreover, Notch signaling pathway was reported to be involved in the acquisition of EMT in both physiological conditions and pathological processes [7, 18-21]. Notch signaling pathway has been believed to play critical roles in CSCs. Recently, we have reviewed the emerging roles of Notch signaling pathway in cancer stem cells [22]. However, the functions of Notch in the processes of EMT are largely unknown. Therefore, in this review article, we will focus our discussion on describing the role of Notch in the acquisition of EMT phenotype. The reader can also be referred to several other excellent reviews that have been published in the field of EMT involving many signaling events [4, 14, 23, 24].
BACKGROUND LITERATURE ON NOTCH SIGNALING PATHWAY
Notch signaling is involved in cell proliferation, survival, apoptosis, and differentiation which affects the development and function of many organs [25-27]. Notch genes encode proteins which can be activated by interacting with a family of its ligands. To date, in mammals, the Notch family of trans-membrane receptors consists of four members: Notch-1-4. Mammals also express Notch ligands for which five members have been found: Dll-1 (Delta-like 1), Dll-3 (Delta-like 3), Dll-4 (Delta-like 4), Jagged-1 and Jagged-2 [27]. Notch signaling is initiated when Notch ligand binds to an adjacent Notch receptor between two neighboring cells. Upon activation, Notch is cleaved, releasing the intracellular domain of the Notch (ICN) through a cascade of proteolytic cleavages by the metalloprotease tumor necrosis factor-α-converting enzyme (TACE) and γ-secretase complex [26, 27]. Therefore, inhibiting γ-secretase function would prevent the cleavage of the Notch receptor, blocking Notch signal transduction [26, 27], and thus γ-secretase inhibitors could be useful for the treatment of human malignancies. Consistent with this rationale, γ-secretase inhibitors are now undergoing clinical trials (see website: clinicaltrials.gov).
In the absence of ICN cleavage, transcription of Notch target genes is inhibited by a repressor complex mediated by the CSL (C protein binding factor 1/Suppressor of Hairless/Lag-1). When ICN enters the nucleus, it binds to CSL and recruits transcription activators to the CSL complex and converts it from a transcriptional repressor into a transcription activator complex [26, 27]. The transcriptionally active Notch-CSL complexes then direct the expression of numerous downstream target genes, including two families of basic helix-loop-helix transcription factors: Hes (Hairy enhance of split) family and Hey (Hairy/enhancer of spit related with YRPW motif). A few other Notch target genes have been identified, some of which are dependent on Notch signaling in multiple tissues, while others are tissue specific. Notch target genes also include nuclear factor-kappa B (NF-κB), cyclin D1, c-myc, p21, p27, Akt, mTOR, VEGF, etc [25-27], all of which have been well documented for their roles in tumor development and progression.
Because Notch signaling has been shown to maintain the balance between cell proliferation, differentiation and apoptosis, alterations in Notch signaling are also associated with tumorigenesis as discussed below. It has been reported that Notch gene is abnormally regulated in many human malignancies [25-27]. These observations suggest that dysfunction of ICN prevents differentiation, ultimately guiding undifferentiated cells toward malignant transformation. Interestingly, it has been shown that the function of Notch signaling in tumorigenesis could be either oncogenic or anti-proliferative, and the function could be context dependent [28-30]. In a limited number of tumor types, including skin cancer, human hepatocellular carcinoma and small cell lung cancer, Notch signaling has been shown to be anti-proliferative rather than oncogenic [29]; however, most of the studies have shown opposite function of Notch in many human carcinomas. Emerging evidence suggest that the Notch signaling network is frequently deregulated in human malignancies with up-regulated expression of Notch receptors and their ligands in cervical, lung, colon, head and neck, renal carcinoma, acute myeloid, Hodgkin and large-cell lymphomas and pancreatic cancer [25-27, 31, 32], thus in these tumors Notch is undoubtedly oncogenic. Moreover, high-level expression of Notch-1 and its ligand Jagged-1 was found to be associated with poor prognosis in breast cancer, bladder cancer, leukemia, and prostate cancer [33-38]. Moreover, Jagged-1 was found to be highly expressed in metastatic prostate cancer compared to localized prostate cancer or benign prostatic tissues [39]. Furthermore, high Jagged-1 expression in a subset of clinically localized tumors was significantly associated with recurrence, suggesting that Jagged-1 may be a useful marker in distinguishing indolent vs. aggressive prostate carcinomas [40, 41]. Multiple oncogenic pathways, such as NF-κB, Akt, Sonic hedgehog (Shh), mammalian target of rapamycin (mTOR), Ras, Wnt, estrogen receptor (ER), androgen receptor (AR), epidermal growth factor receptor (EGFR) and platelet-derived growth factor (PDGF) signaling have been reported to cross-talk with Notch signaling pathway, and thus it is believed that the cross-talk between Notch and other signaling pathways may play critical roles in tumor aggressiveness. The main features of these pathways and cross-talk with Notch signaling have recently been reviewed [42]. Those readers who are interested in learning more on the cross-talk between these pathways and Notch pathway are also referred to other published review articles [25-27, 42-46]. In the following sections, we have attempted to summarize the functional role of Notch during the acquisition of EMT and its biological significance in a succinct manner.
THE ROLE OF NOTCH IN THE PROCESSES OF EMT
It is believed that the processes that govern the acquisition of EMT is stimulated and regulated by many stimuli, signal transduction pathways and transcription factors. Recently, Notch signal pathway has been found to be a key regulator in the induction of EMT [18-21]. Notch activation in endothelial cells results in morphological, phenotypic, and functional changes consistent with mesenchymal transformation. These changes include down-regulation of endothelial markers (VE-cadherin, Tie1, Tie2, platelet-endothelial cell adhesion molecule-1, and endothelial NO synthase), up-regulation of mesenchymal markers (α-SMA, fibronectin, and platelet-derived growth factor receptors), and migration toward PDGF-B driven processes [47]. Therefore it is believed that Notch-induced EMT is restricted to cells expressing activated Notch. Moreover, Jagged-1 stimulation of endothelial cells is known to induce a similar mesenchymal transformation, suggesting that Jagged-1 mediated activation of Notch signaling is important during the induction of EMT [47]. In the following sub-sections, we will describe the role of Notch and other associated molecules that are known to be important in the processes of EMT.
Notch and Snail
During the acquisition of EMT phenotype, the loss of E-cadherin expression appears to be a crucial step which reduces the cell-cell adhesion, and leading to destabilization of the epithelial architecture. Moreover, a central role of E-cadherin gene repression has been attributed to the function of Snail expression, which is activated during the acquisition of EMT. Snail can bind to the two E-boxes of human E-cadherin promoter and function as a repressor of E-cadherin gene expression [48], thus any biological processes that will trigger Snail over-expression is likely to down-regulate E-cadherin expression resulting in the acquisition of EMT. Because Snail-1 is an important regulator of E-cadherin expression, questions have been asked whether the effects of Notch on E-cadherin expression could be mediated through ICN via regulating the expression of Snail-1. The answer to this question is yes because Notch has been shown to promote EMT through the regulation of Snail. For example, Timmerman et al. reported that over-expression of Notch-1 in immortalized endothelial cells in vitro induces Snail, yielding attenuated VE-cadherin expression, loss of contact inhibition, and the acquisition of EMT, which was accompanied by oncogenic transformation [20]. These authors have also found that Notch acts via lateral induction in the endocardium, and is crucial for endocardial EMT. They have also demonstrated the absolute requirement for Notch in the promotion of EMT during cardiac valve development. Cardiac explants of Notch mutants or wild-type embryos treated with γ-secretase inhibitor DAPT show severely impaired EMT. Consistent with this notion, transient ectopic expression of Notch-1 in embryos led to hypertrophic cardiac valves, and DAPT treatment inhibited valve formation, suggesting that Notch plays a crucial role in the promotion of EMT both during development and tumor progression [20].
Recently, Sahlgren et al. found that Notch signaling is required for the induction of EMT mediated by hypoxic stimulus, which was associated with increased cell motility and invasiveness [19]. These authors have proposed that Notch signaling functions by two distinct mechanisms in controlling the expression of Snail-1. First, Notch could directly up-regulate Snail-1 expression by recruitment of ICN to the Snail-1 promoter. Secondly, Notch mediated induction of hypoxia-inducible factor 1α(HIF-1α) could get recruited to the lysyl oxidase (LOX) promoter, resulting in the elevated expression of LOX, which stabilizes the Snail-1 protein [19]. Interestingly, Snail could also regulate the Notch expression. For example, Kuphal et al found down-regulation of Notch-4 by anti-sense Snail cDNA transfection of melanoma cells [49], and the above results are convincing in support of the complex cross-talk between Notch and Snail during the acquisition of EMT.
Notch and Slug
Slug (also called Snail-2) is one of the repressors of E-cadherin promoter similar to Snail-1. Repression of E-cadherin by Slug initiates EMT through permitting loss of cell-cell adhesion, thereby disrupting apical-basal polarity and promoting cell migration. Recently, it has been reported that Slug is a direct Notch target required for initiation of cardiac cushion cellularization [18]. Slug is expressed by a subset of endothelial cells and mesenchymal cells of the atrioventricular canal at E9.5 (embryonic day), at the initiation step of EMT. Slug deficiency results in impaired cellularization of the cardiac cushion at E9.5 but not E10.5, as EMT in Slug-deficient embryos is rescued by an increase in Snail expression by E10.5 [18]. During this EMT process, Notch signaling directly regulates the Slug promoter through CSL interaction, resulting in the up-regulation of Slug in endothelial cells [18]. Slug also can directly binds and represses the VE-cadherin promoter. Furthermore, activation of Notch in the context of TGF-β stimulation results in synergistic up-regulation of Snail in endothelial cells, suggesting that combined expression of Slug and Snail is required for the induction of EMT in cardiac cushion morphogenesis [18].
Recently, Slug has been demonstrated to be important for cancer cells, especially in the down-regulation of epithelial markers and up-regulation of mesenchymal markers in order for cancer cells to become motile and invasive. In addition, several studies have shown Slug over-expression in breast carcinoma, esophageal carcinoma, colorectal carcinoma, which was associated with shorter survival [50-52]. The expression of Slug was significantly associated with Dukes stage and distant metastasis, and the expression of Slug had a significant impact on patient overall survival in human colorectal carcinoma. Moreover, patients with positive expression of Slug and reduced expression of E-cadherin showed the worst prognosis. For example, Jethwa et al. reported that Slug was more abundant in adenocarcinoma and inversely correlated to E-cadherin expression compared to matched Barrett's metaplastic specimens [53]. Over-expression of Slug in human esophageal carcinoma OE33 was linked with E-cadherin repression and over-expression of mesenchymal markers such as vimentin and fibronectin [53]. It has also been reported that Slug up-regulation is intimately linked with the down-regulation of E-cadherin, which was found to be correlated with the presence of distant metastases and with advanced pTNM stages in gastric carcinomas [54]. Moreover, it has been reported that Slug is a downstream target gene of Notch that is up-regulated in Jagged-1 and Notch-1 positive human breast cancers [55], suggesting that Jagged-1-mediated activation of Notch in breast epithelial cells leads to the induction of EMT mediated via induction of Slug and subsequent repression of the Ecadherin. In addition, Slug was found to be essential for Notch-mediated repression of E-cadherin, resulting in β-catenin activation and resistance to anoikis [55]. Notch-1 target gene Hey is also known to be a potential marker of human breast cancers that exhibit activation of the Jagged-Notch-Slug signaling axis [55]. These findings clearly suggest that Jagged-induced activation of Notch and its downstream events is mediated via activation of Slug, thereby promotes tumor growth and metastasis, which are associated with the acquisition of EMT.
Notch and TGF-β
TGF-β signaling is involved in the vast majority of cellular processes including EMT. The functioning of the TGF-β pathway depends on its constitutive and extensive communication with other signaling pathways, resulting in synergistic or antagonistic effects and desirable biological outcomes. Recent studies have documented a cross-talk between TGF-β and Notch signaling. For example, TGF-β can induce the expression of Notch ligands and that Jagged-1 up-regulation contributes to TGF-β-stimulated p21 expression and cytostasis in epithelial cells [56]. TGF-β, a primary driver of cellular changes in the kidney during nephropathy, showed increased Jagged-1 and Hes-1 expression in human kidney epithelial cells. Elevated levels of Jagged-1 and Hes-1 were also detected in extracts from renal biopsies from diabetic nephropathy patients, suggesting that Notch signaling pathway-regulated gene expression is relevant in the pathogenic processes primarily due to activated functioning of TGF-β [57]. Moreover, Zavadil et al. reported that expression of Hey-1 and Jagged-1 was induced by TGF-β at the onset of EMT in epithelial cells from mammary gland, kidney tubules, and epidermis [21]. The Hey-1 expression was found to be biphasic, consisting of immediate-early Smad3-dependent, Jagged-1/Notch-independent activation, followed by delayed, indirect Jagged-1/Notch-dependent activation [21]. TGF-β-induced EMT was found to be blocked by RNA silencing using Hey-1 or Jagged-1, and by chemical inactivation of Notch, and thus these limited yet interesting findings clearly suggest the functional role of Hey-1, Smad3, and Jagged-1/Notch in mediating TGF-β-induced EMT [21]; however further studies in this interesting area would be highly rewarded.
Notch and FGF
FGF signals have been implicated in the control of cell proliferation, differentiation, migration, and survival, which is mediated by the activation of FGF receptors (FGFR). FGF dimers are associated with heparan sulfate proteoglycan, which then binds to FGF receptors in inducing receptor dimerization, activation of its tyrosine kinase activity associated with receptor auto-phosphorylation. The FGFs together with their tyrosine kinase receptors play a critical role in autocrine and paracrine growth control of malignant tumors [58]. Enhanced FGF/FGFR signaling activity has been reported in human cancers [59]. It has been reported that FGF signals are transduced via activation of the PI3KAKT signaling pathway, the MAPK signaling pathway, and the PLCγ signaling pathway [58-60], suggesting a complex cross-talk between several ligand-receptor mediated signaling pathways in transducing FGF-FGFR signaling.
It has now been well accepted that FGF could induce EMT in many cell lines. Rat bladder carcinoma NBT-II cells was found to undergo EMT upon stimulation with FGF [61]. Moreover, non-autocrine cells behave like epithelial parental cells, whereas autocrine cells have a mesenchymal pheno-type, which was correlated with the over-expression of urokinase plasminogen activator receptor (uPAR), the internalization of E-cadherin, and the redistribution of β-catenin from the cell surface to the cytoplasm and nucleus, and the behavior of autocrine cells was consistent with a decrease in tumor-suppressive activity of E-cadherin [61]. Interestingly, epithelial carcinoma NBT-II cells stimulated over time with FGF-1 allowed the identification of immediate, immediate-early and late EMT gene targets [62]. Among those genes Notch-1 was found to be expressed at a basal level in the non-stimulated cells whereas it was up-regulated at 0.5 and 2 hours with FGF-1 treatment, suggesting that Notch-1 is an immediate early target gene that is involved in the acquisition of EMT induced by FGF-1 [62]. The cross-talk between FGF-FGFR signaling with Notch signaling pathways during the acquisition of EMT clearly suggest that further research in this exciting area is urgently needed for the development of targeted therapies for human malignancies.
Notch and PDGF
PDGF signaling has been reported to regulate the expression of the Notch-1 receptor in some cell lines. The PDGFs are composed of four different polypeptide chains encoded by different genes. Four PDGF family members have been identified to date: PDGF A-D. The four PDGF chains assemble into disulphide-bonded dimers via homo- or hetero-dimerization, and five different dimeric isoforms have been described so far; PDGF-AA, PDGF-AB, PDGF-BB, PDGF-CC and PDGF-DD [63-65]. The PDGFs have a common structure with the typical growth factor domain involved in the dimerization of the two subunits, and in receptor binding and activation [64, 65]. Growing body of evidence in the literature strongly suggests the role of PDGF signaling in cancer cell growth, invasion and metastasis [66-69]. The expression of PDGF-B correlates with Notch ligand Dll-4 expression in developing retinal arteries [70]. We also found recently that down-regulation of PDGF-D leads to the inactivation of Notch-1 and NF-κB DNA-binding activity and, in turn, down-regulates the expression of its target genes, such as VEGF and MMP-9 [71]. Therefore, the inactivation of PDGF-D-mediated cell invasion and angiogenesis as reported by our results could in part be due to inactivation of Notch-1 activity, and further supporting a critical cross-talk between PDGF and Notch signaling.
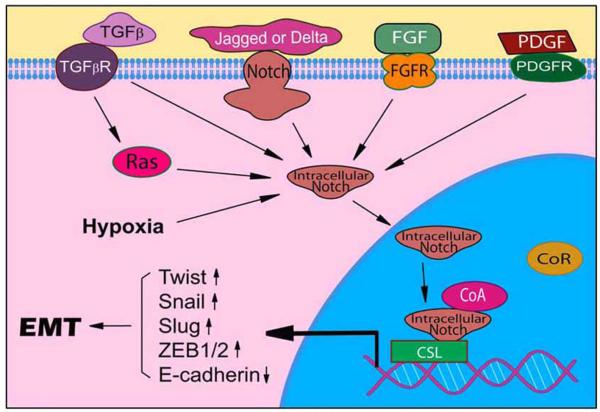
Moreover, we have also reported that PDGF signaling contributes to EMT phenotype, resulting in the aggressiveness of tumor cells such as increased invasion and angiogenesis characteristics [11]. We have also found that over-expression of PDGF-D in prostate cancer cells (PC3 PDGFD cells) resulted in a significant induction of EMT as shown by changes in cellular morphology concomitant with the loss of E-cadherin and zonula occludens-1 (ZO-1), and gain of vimentin [11]. Interestingly, we found that Notch-1 was highly expressed in PC3 PDGF-D cells (unpublished data), suggesting a cross-talk between PDGF-D and Notch signaling in the acquisition of EMT by prostate cancer cells. Moreover, we have recently found down-regulation of miR-200 family in PC3 PDGF-D cells, which was associated with up-regulation of ZEB1, ZEB2 and Slug expression [72]. Similar findings were also observed in PC3 cells chronically treated with purified recombinant active PDGF-D. Interestingly, re-expression of miR-200b in PC3 PDGF-D cells led to the reversal of EMT phenotype, which was associated with the down-regulation of ZEB1, ZEB2 and Slug expression and these results were consistent with increased gene expressions of epithelial markers [72]. Moreover, transfection of PC3 PDGF-D cells with miR-200b also inhibited cell migration and invasion with concomitant repression of cell adhesion to culture surface and cell detachment [72]. We also found that re-expression of miR-200 down-regulated the expression of Notch-1 (unpublished data). From these results, we concluded that PDGF-D-induced acquisition of EMT phenotype of PC3 cells is in part due to repression of miR-200 and activation of Notch-1. Taken together, Notch pathway plays an important role in EMT progression as illustrated in Fig. (1).
Fig. (1).
A mechanistic diagram showing how Notch could promote EMT progress.
NOTCH AS A CANCER THERAPEUTIC TARGET AND OVERALL PERSPECTIVES
The growing body of literature strongly suggests that increased expression of Notch genes and their ligands are detected in many human cancer cells and tissues [22, 26, 27, 45, 73, 74]. These results clearly suggest that inactivation of Notch signaling by novel approaches will have a significant impact in cancer therapy. Interestingly, our recently published data showed that pancreatic cancer cells that are gemcitabine-resistant (GR) acquired epithelial-mesenchymal transition (EMT) phenotype as evidenced by elongated fibroblastoid morphology, lower expression of epithelial marker E-cadherin, and higher expression of mesenchymal markers such as vimentin and ZEB1 [7, 75, 76]. We also found that Notch-2 and its ligand, Jagged-1, are highly up-regulated in GR cells, which is consistent with the role of the Notch signaling pathway in the acquisition of EMT [7]. We also found that down-regulation of Notch signaling by siRNA approach led to partial reversal of the EMT phenotype, resulting in the mesenchymal-epithelial transition, which was associated with decreased expression of vimentin, ZEB1, Slug, Snail, and NF-κB [7]. These results provide molecular evidence showing that the activation of Notch signaling is mechanistically linked with chemoresistance phenotype consistent with the acquisition of EMT phenotype of pancreatic cancer cells, suggesting that the inactivation of Notch signaling by novel strategies could be a potential targeted therapeutic approach for overcoming chemoresistance toward the prevention of tumor progression and/or treatment of pancreatic cancer for which current conventional therapeutic strategies are highly disappointing.
Since Notch signaling is activated via the enzymatic activity of γ-secretase and, thus inhibition of γ-secretase is becoming an exciting new area of drug development for the targeted therapy of many human cancers. Several forms of γ-secretase inhibitors have been tested for their anti-tumor effects. Recently, γ-secretase inhibitor DAPT was reported to suppress medulloblastoma growth and induce G0-G1 cell cycle arrest and apoptosis in a T-ALL animal model [77]. We also found that γ-secretase inhibitor suppress the prostate cancer cell growth [78]. Inhibitors of γ-secretase are being tested in Phase I clinical trials, suggesting that Notch signaling is an important target in cancer therapy (see website: clinicaltrials.gov). However, one of the major challenges is to eliminate unwanted toxicity associated with the γ-secretase inhibitors, especially the cytotoxicity in the gastrointestinal tract [79]. Moreover, γ-secretase inhibitors could have widespread adverse effects in vivo because proteases such as γ-secretase participates in a wide array of cellular functions [80], suggesting that novel targeted agents must be developed for the inactivation of Notch signaling toward the treatment of human malignancies.
Interestingly, our recent studies have shown that chemo-preventive agents that are known to be non-toxic to humans, such as genistein and curcumin (non-toxic dietary agents) inhibited Notch-1 activation in pancreatic cancer cells leading to apoptotic cell death [32, 74]. Whereas other studies have shown that resveratrol, another non-toxic dietary agent, could also induce apoptosis by inhibiting the Notch pathway mediated via inactivation of p53 and PI3K/Akt in T-ALL [81]. Moreover, one Chinese herb anti-tumor B also inhibited Notch expression in a mouse lung tumor model [82]. More recently, we found that the expression of miR-200b, miR-200c, let-7b, let-7c, let-7d, and let-7e was significantly down-regulated in gemcitabine-resistant (GR) cells having EMT characteristics and interestingly re-expression of miR-200 by transfection studies or treatment of GR cells with either 3,3'-diinodolylmethane (DIM) or isoflavone resulted in the down-regulation of ZEB1, slug, and vimentin, which was consistent with morphological reversal of EMT phenotype leading to epithelial morphology. We also found that miR-200 down-regulated the expression of Notch-1 in pancreatic cancer cell lines (unpublished data). These results provide experimental evidence suggesting that DIM and isoflavone could function as miRNA regulators leading to the reversal of EMT phenotype, which is likely to be important for designing novel therapies for pancreatic cancer especially those with EMT phenotype that are typically drug-resistant, the typical characteristics of most pancreatic cancer. Collectively, our findings together with those reported in the literature are becoming an exciting area for further in-depth research for targeted inactivation of Notch signaling, especially by genistein, DIM, resveratrol, curcumin and others, as a novel therapeutic approach for the treatment of human malignancies.
ACKNOWLEDGEMENTS
The authors' work cited in this review article was funded by grants from the National Cancer Institute, NIH (5R01CA101870, 5R01CA131151) to F.H.S. and Department of Defense Postdoctoral Training Award W81XWH-08-1-0196 (Zhiwei Wang), and also partly supported by a subcontract award (F.H.S.) from the University of Texas MD Anderson Cancer Center through a SPORE grant (5P20-CA101936-05) on pancreatic cancer awarded to James Abbruzzese. We also sincerely thank Puschelberg foundation for their generous contribution awarded to F.H.S.
REFERENCES
- 1.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–26. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 2.Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher's conceptual friend and foe. Am J Pathol. 2009;174:1588–93. doi: 10.2353/ajpath.2009.080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min C, Eddy SF, Sherr DH, Sonenshein GE. NF-kappaB and epithelial to mesenchymal transition of cancer. J Cell Biochem. 2008;104:733–44. doi: 10.1002/jcb.21695. [DOI] [PubMed] [Google Scholar]
- 4.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–69. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 5.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 6.Prindull G. Hypothesis: cell plasticity, linking embryonal stem cells to adult stem cell reservoirs and metastatic cancer cells? Exp Hematol. 2005;33:738–46. doi: 10.1016/j.exphem.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Li Y, Kong D, et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400–7. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs BC, Fujii T, Dorfman JD, et al. Epithelial-to-mesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Res. 2008;68:2391–9. doi: 10.1158/0008-5472.CAN-07-2460. [DOI] [PubMed] [Google Scholar]
- 9.Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–87. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- 10.Sabbah M, Emami S, Redeuilh G, et al. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist Updat. 2008;11:123–51. doi: 10.1016/j.drup.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Kong D, Wang Z, Sarkar SH, et al. Platelet-derived growth factor-D overexpression contributes to epithelial-mesenchymal transition of PC3 prostate cancer cells. Stem Cells. 2008;26:1425–35. doi: 10.1634/stemcells.2007-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–20. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–74. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 14.Gregory PA, Bracken CP, Bert AG, Goodall GJ. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008;7:3112–8. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- 15.Peter ME. Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle. 2009;8:843–52. doi: 10.4161/cc.8.6.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannito S, Novo E, Compagnone A, et al. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis. 2008;29:2267–78. doi: 10.1093/carcin/bgn216. [DOI] [PubMed] [Google Scholar]
- 17.Yang MH, Wu MZ, Chiou SH, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 18.Niessen K, Fu Y, Chang L, Hoodless PA, McFadden D, Karsan A. Slug is a direct Notch target required for initiation of cardiac cushion cellularization. J Cell Biol. 2008;182:315–25. doi: 10.1083/jcb.200710067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci U S A. 2008;105:6392–7. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timmerman LA, Grego-Bessa J, Raya A, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23:1155–65. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Li Y, Banerjee S, Sarkar FH. Emerging role of Notch in stem cells and cancer. Cancer Lett. 2009;279:8–12. doi: 10.1016/j.canlet.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39:305–18. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 24.Hugo H, Ackland ML, Blick T, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–83. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 25.Miele L, Osborne B. Arbiter of differentiation and death: Notch signaling meets apoptosis. J Cell Physiol. 1999;181:393–409. doi: 10.1002/(SICI)1097-4652(199912)181:3<393::AID-JCP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Miele L, Miao H, Nickoloff BJ. Notch signaling as a novel cancer therapeutic target. Curr Cancer Drug Targets. 2006;6:313–23. doi: 10.2174/156800906777441771. [DOI] [PubMed] [Google Scholar]
- 27.Miele L. Notch signaling. Clin Cancer Res. 2006;12:1074–9. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- 28.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–33. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dotto GP. Notch tumor suppressor function. Oncogene. 2008;27:5115–23. doi: 10.1038/onc.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dufraine J, Funahashi Y, Kitajewski J. Notch signaling regulates tumor angiogenesis by diverse mechanisms. Oncogene. 2008;27:5132–7. doi: 10.1038/onc.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Banerjee S, Li Y, Rahman KM, Zhang Y, Sarkar FH. Down-regulation of Notch-1 inhibits invasion by inactivation of nuclear factor-{kappa}B, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 2006;66:2778–84. doi: 10.1158/0008-5472.CAN-05-4281. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Inhibition of nuclear factor kappab activity by genistein is mediated via Notch-1 signaling pathway in pancreatic cancer cells. Int J Cancer. 2006;118:1930–6. doi: 10.1002/ijc.21589. [DOI] [PubMed] [Google Scholar]
- 33.Shi TP, Xu H, Wei JF, et al. Association of low expression of notch-1 and jagged-1 in human papillary bladder cancer and shorter survival. J Urol. 2008;180:361–6. doi: 10.1016/j.juro.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 34.Reedijk M, Pinnaduwage D, Dickson BC, et al. JAG1 expression is associated with a basal phenotype and recurrence in lymph node-negative breast cancer. Breast Cancer Res Treat. 2008;111:439–48. doi: 10.1007/s10549-007-9805-3. [DOI] [PubMed] [Google Scholar]
- 35.Dickson BC, Mulligan AM, Zhang H, et al. High-level JAG1 mRNA and protein predict poor outcome in breast cancer. Mod Pathol. 2007;20:685–93. doi: 10.1038/modpathol.3800785. [DOI] [PubMed] [Google Scholar]
- 36.Zhu YM, Zhao WL, Fu JF, et al. NOTCH1 mutations in T-cell acute lymphoblastic leukemia: prognostic significance and implication in multifactorial leukemogenesis. Clin Cancer Res. 2006;12:3043–9. doi: 10.1158/1078-0432.CCR-05-2832. [DOI] [PubMed] [Google Scholar]
- 37.Reedijk M, Odorcic S, Chang L, et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005;65:8530–7. doi: 10.1158/0008-5472.CAN-05-1069. [DOI] [PubMed] [Google Scholar]
- 38.Parr C, Watkins G, Jiang WG. The possible correlation of Notch-1 and Notch-2 with clinical outcome and tumour clinicopathological parameters in human breast cancer. Int J Mol Med. 2004;14:779–86. doi: 10.3892/ijmm.14.5.779. [DOI] [PubMed] [Google Scholar]
- 39.Santagata S, Demichelis F, Riva A, et al. JAGGED1 expression is associated with prostate cancer metastasis and recurrence. Cancer Res. 2004;64:6854–7. doi: 10.1158/0008-5472.CAN-04-2500. [DOI] [PubMed] [Google Scholar]
- 40.Reedijk M, Odorcic S, Chang L, et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005;65:8530–7. doi: 10.1158/0008-5472.CAN-05-1069. [DOI] [PubMed] [Google Scholar]
- 41.Reedijk M, Pinnaduwage D, Dickson BC, et al. JAG1 expression is associated with a basal phenotype and recurrence in lymph node-negative breast cancer. Breast Cancer Res Treat. 2008;111:439–48. doi: 10.1007/s10549-007-9805-3. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Li Y, Banerjee S, Sarkar FH. Exploitation of the Notch signaling pathway as a novel target for cancer therapy. Anticancer Res. 2008;28:3621–30. [PubMed] [Google Scholar]
- 43.Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poellinger L, Lendahl U. Modulating Notch signaling by pathway-intrinsic and pathway-extrinsic mechanisms. Curr Opin Genet Dev. 2008;18:449–54. doi: 10.1016/j.gde.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 45.Rizzo P, Osipo C, Foreman K, Golde T, Osborne B, Miele L. Rational targeting of Notch signaling in cancer. Oncogene. 2008;27:5124–31. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 46.Zardawi SJ, O'Toole SA, Sutherland RL, Musgrove EA. Dysregulation of Hedgehog, Wnt and Notch signalling pathways in breast cancer. Histol Histopathol. 2009;24:385–98. doi: 10.14670/HH-24.385. [DOI] [PubMed] [Google Scholar]
- 47.Noseda M, McLean G, Niessen K, et al. Notch activation results in phenotypic and functional changes consistent with endothelial-tomesenchymal transformation. Circ Res. 2004;94:910–7. doi: 10.1161/01.RES.0000124300.76171.C9. [DOI] [PubMed] [Google Scholar]
- 48.Becker KF, Rosivatz E, Blechschmidt K, Kremmer E, Sarbia M, Hofler H. Analysis of the E-cadherin repressor Snail in primary human cancers. Cells Tissues Organs. 2007;185:204–12. doi: 10.1159/000101321. [DOI] [PubMed] [Google Scholar]
- 49.Kuphal S, Palm HG, Poser I, Bosserhoff AK. Snail-regulated genes in malignant melanoma. Melanoma Res. 2005;15:305–13. doi: 10.1097/00008390-200508000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Shioiri M, Shida T, Koda K, et al. Slug expression is an independent prognostic parameter for poor survival in colorectal carcinoma patients. Br J Cancer. 2006;94:1816–22. doi: 10.1038/sj.bjc.6603193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elloul S, Elstrand MB, Nesland JM, et al. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 2005;103:1631–43. doi: 10.1002/cncr.20946. [DOI] [PubMed] [Google Scholar]
- 52.Uchikado Y, Natsugoe S, Okumura H, et al. Slug Expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11:1174–80. [PubMed] [Google Scholar]
- 53.Jethwa P, Naqvi M, Hardy RG, et al. Overexpression of Slug is associated with malignant progression of esophageal adenocarcinoma. World J Gastroenterol. 2008;14:1044–52. doi: 10.3748/wjg.14.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castro AC, Rosivatz E, Schott C, et al. Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin. J Pathol. 2007;211:507–15. doi: 10.1002/path.2138. [DOI] [PubMed] [Google Scholar]
- 55.Leong KG, Niessen K, Kulic I, et al. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Exp Med. 2007;204:2935–48. doi: 10.1084/jem.20071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niimi H, Pardali K, Vanlandewijck M, Heldin CH, Moustakas A. Notch signaling is necessary for epithelial growth arrest by TGF-beta. J Cell Biol. 2007;176:695–707. doi: 10.1083/jcb.200612129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walsh DW, Roxburgh SA, McGettigan P, et al. Co-regulation of Gremlin and Notch signalling in diabetic nephropathy. Biochim Biophys Acta. 2008;1782:10–21. doi: 10.1016/j.bbadis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Katoh M, Katoh M. FGF signaling network in the gastrointestinal tract (review) Int J Oncol. 2006;29:163–8. [PubMed] [Google Scholar]
- 59.Katoh M. Networking of WNT, FGF, Notch, BMP, and Hedgehog signaling pathways during carcinogenesis. Stem Cell Rev. 2007;3:30–8. doi: 10.1007/s12015-007-0006-6. [DOI] [PubMed] [Google Scholar]
- 60.Katoh M, Katoh M. Cross-talk of WNT and FGF signaling pathways at GSK3beta to regulate beta-catenin and SNAIL signaling cascades. Cancer Biol Ther. 2006;5:1059–64. doi: 10.4161/cbt.5.9.3151. [DOI] [PubMed] [Google Scholar]
- 61.Billottet C, Elkhatib N, Thiery JP, Jouanneau J. Targets of fibroblast growth factor 1 (FGF-1) and FGF-2 signaling involved in the invasive and tumorigenic behavior of carcinoma cells. Mol Biol Cell. 2004;15:4725–34. doi: 10.1091/mbc.E04-04-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Billottet C, Tuefferd M, Gentien D, et al. Modulation of several waves of gene expression during FGF-1 induced epithelial-mesenchymal transition of carcinoma cells. J Cell Biochem. 2008;104:826–39. doi: 10.1002/jcb.21667. [DOI] [PubMed] [Google Scholar]
- 63.Wang Z, Kong D, Li Y, Sarkar FH. PDGF-D signaling: a novel target in cancer therapy. Curr Drug Targets. 2009;10:38–41. doi: 10.2174/138945009787122914. [DOI] [PubMed] [Google Scholar]
- 64.Li X, Eriksson U. Novel PDGF family members: PDGF-C and PDGF-D. Cytokine Growth Factor Rev. 2003;14:91–8. doi: 10.1016/s1359-6101(02)00090-4. [DOI] [PubMed] [Google Scholar]
- 65.Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15:197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 66.Reigstad LJ, Varhaug JE, Lillehaug JR. Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family. FEBS J. 2005;272:5723–41. doi: 10.1111/j.1742-4658.2005.04989.x. [DOI] [PubMed] [Google Scholar]
- 67.Shih AH, Holland EC. Platelet-derived growth factor (PDGF) and glial tumorigenesis. Cancer Lett. 2006;232:139–47. doi: 10.1016/j.canlet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Pietras K, Sjoblom T, Rubin K, Heldin CH, Ostman A. PDGF receptors as cancer drug targets. Cancer Cell. 2003;3:439–43. doi: 10.1016/s1535-6108(03)00089-8. [DOI] [PubMed] [Google Scholar]
- 69.Yu J, Ustach C, Kim HR. Platelet-derived growth factor signaling and human cancer. J Biochem Mol Biol. 2003;36:49–59. doi: 10.5483/bmbrep.2003.36.1.049. [DOI] [PubMed] [Google Scholar]
- 70.Claxton S, Fruttiger M. Periodic Delta-like 4 expression in developing retinal arteries. Gene Expr Patterns. 2004;5:123–7. doi: 10.1016/j.modgep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z, Kong D, Banerjee S, et al. Down-regulation of platelet-derived growth factor-D inhibits cell growth and angiogenesis through inactivation of Notch-1 and nuclear factor-kappaB signaling. Cancer Res. 2007;67:11377–85. doi: 10.1158/0008-5472.CAN-07-2803. [DOI] [PubMed] [Google Scholar]
- 72.Kong D, Li Y, Wang Z, et al. The MiR-200 Regulates PDGF-D Mediated Epithelial-Mesenchymal Transition, Adhesion and Invasion of Prostate Cancer Cells. Stem Cells. 2009;27:1712–21. doi: 10.1002/stem.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Z, Zhang Y, Li Y, Banerjee S, Liao J, Sarkar FH. Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol Cancer Ther. 2006;5:483–93. doi: 10.1158/1535-7163.MCT-05-0299. [DOI] [PubMed] [Google Scholar]
- 74.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106:2503–13. doi: 10.1002/cncr.21904. [DOI] [PubMed] [Google Scholar]
- 75.Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14:3629–37. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 76.Arumugam T, Ramachandran V, Fournier KF, et al. Epithelial to Mesenchymal Transition Contributes to Drug Resistance in Pancreatic Cancer. Cancer Res. 2009;69:5820–8. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O'Neil J, Calvo J, McKenna K, et al. Activating Notch1 mutations in mouse models of T-ALL. Blood. 2006;107:781–5. doi: 10.1182/blood-2005-06-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, Wang Z, Ahmed F, Banerjee S, Li Y, Sarkar FH. Down-regulation of Jagged-1 induces cell growth inhibition and S phase arrest in prostate cancer cells. Int J Cancer. 2006;119:2071–7. doi: 10.1002/ijc.22077. [DOI] [PubMed] [Google Scholar]
- 79.Barten DM, Meredith JE, Jr., Zaczek R, Houston JG, Albright CF. Gamma-secretase inhibitors for Alzheimer's disease: balancing efficacy and toxicity. Drugs R D. 2006;7:87–97. doi: 10.2165/00126839-200607020-00003. [DOI] [PubMed] [Google Scholar]
- 80.Shih I, Wang TL. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res. 2007;67:1879–82. doi: 10.1158/0008-5472.CAN-06-3958. [DOI] [PubMed] [Google Scholar]
- 81.Cecchinato V, Chiaramonte R, Nizzardo M, et al. Resveratrol-induced apoptosis in human T-cell acute lymphoblastic leukaemia MOLT-4 cells. Biochem Pharmacol. 2007;74:1568–74. doi: 10.1016/j.bcp.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Z, Wang Y, Yao R, et al. Cancer chemopreventive activity of a mixture of Chinese herbs (antitumor B) in mouse lung tumor models. Oncogene. 2004;23:3841–50. doi: 10.1038/sj.onc.1207496. [DOI] [PubMed] [Google Scholar]