Abstract
Importance of the field
Bcl-2 family proteins are a component of the anti-apoptotic machinery and are overexpressed in different malignancies. Accordingly, their enhanced expression has been attributed to the observed chemoresistance in most of the cancers. Therefore, targeting Bcl-2 family members becomes an important and attractive approach towards cancer therapy and is currently a very rapidly evolving area of research. This article highlights the numerous advancements that have been made in the design and synthesis of small molecule inhibitors (SMI) of pro-survival Bcl-2 proteins.
Areas covered in this review
This review comprehensively describes the progress made over the last 2 decades on this subject including the clinical status of SMIs of Bcl-2 family proteins.
What the reader will gain
Newer insights will be gained on the status of our knowledge on SMIs of Bcl-2 family proteins, their most beneficial application as well as current and future directions in this field.
Take home message
Targeting Bcl-2 family proteins using SMI strategies is gaining momentum with emergence of certain new classes of inhibitors in Phase I and II clinical setting. In view of the tremendous progress toward the development of such inhibitors, this innovative approach certainly holds promise and has the potential to become a future mainstay for cancer therapy.
Keywords: apoptosis, Bcl-2, cancer therapy, small molecule inhibitor
1. Background and introduction
Apoptosis is a natural and well-studied process through which multi-cellular organisms shed ageing or damaged cells. This is an intricately complex yet coordinated mechanism that is governed by a set of proteins that either guides a cell to death or survival [1]. These proteins have been characterized into two groups: i) the anti-apoptotic or survival proteins such as Bcl-2, Bcl-XL, BclW and Mcl-1 [2,3] and ii) the pro-apoptotic death signaling proteins such as Bax and Bak [4,5]. The regulation of life and death of cells is also regulated by related proteins that include Bim [6], Bad [7], puma [8] and noxa [9]. Evasion of cell death is a characteristic of cancer cells that is one of the major causes for treatment failure [10]. The lack of efficacy of established therapeutic regimens is due, at least in part, to the oncogenic blockade of cell death pathways along with the development of multi-drug resistance [11,12]. Thus, utilizing agents designed to activate the cell death or apoptotic machinery becomes an attractive approach and is believed to be a more clinically effective therapeutic option [13,14]. Selective modulation of the apoptotic machinery has been the goal of synthetic chemist for > 2 decades; however, the field continues to face enormous hurdles and challenges. This is mainly due to the fact that many of these targets are mechanistically linked with intracellular protein–protein interactions, which are difficult to modulate.
The two major apoptosis pathways of interest are the extrinsic [15] and intrinsic [16] pathways that are mutually exclusive yet intricately linked to each other. The extrinsic pathway operates through cell-surface death receptors [17], while the intrinsic pathway involves Bcl-2 family of proteins-mediated alterations in mitochondrial membrane integrity and potential in response to cellular insults or other stress signals. Both pathways converge at the level of the effector caspases that are proteolytic enzymes responsible for carrying out the effector cell death response. Furthermore, the intrinsic and extrinsic apoptosis pathways crosstalk with major signaling networks that includes the two master transcriptional regulators p53 and NF-κB. There are other smaller and independent players in the apoptotic machinery that include the important yet underappreciated pro-apoptotic protein ‘prostate apoptosis response-4 (Par-4)’ that is under the negative regulation of Bcl-2 through the NF-κB axis. Par-4 can either induce apoptosis intracellularly through its nuclear localization signal [18] or extracellularly where secreted Par-4 can interact with cell-surface death receptor DR5 and induce extrinsic apoptotic signaling [19]. The importance of targeting Bcl-2 family members is increasingly being realized as an effective therapeutic strategy and this review summarizes the recent advances in this field including the development of small molecule inhibitors (SMIs) that target different family members of Bcl-2.
1.1 The Bcl-2 family proteins and mitochondrial apoptosis
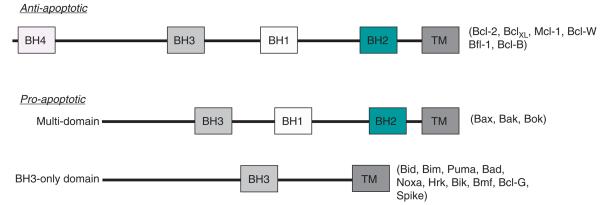
The Bcl-2 family of proteins governs whether a cell continues to live or instead commits to death through the mitochondrial apoptotic pathway. According to the widely accepted Krosmeyer rheostat model, a balance between proand anti-apoptotic Bcl-2 family proteins controls the decision-making process at the mitochondrion, modulating cell sensitivity to death [20,21]. The survival signal is activated if the rheostat balance is towards the Bcl-2, BclXL, Mcl-1, Bcl-W and Bfl-1 that are the anti-apoptotic members of the family. They share sequence homology in four α-helical Bcl-2 homology domains (BH) BH1 – 4, whereas the apoptosis signaling is activated if the balance shifts towards multi-domain pro-apoptotic proteins Bax and Bak (sharing homology in the domains BH1 – 3) [22]. Additionally, lesser studied players include Bok that is also projected as a proapoptotic protein working in similar fashion as Bax and Bak (summarized in Table 1) [23,24]. Lastly, the group sharing the least homology is the BH3-only pro-death group, which includes Puma, Noxa, Bim, Bid, Bad and Bik [25]. The BH3 domain, along with the presence of Bax and Bak, is absolutely essential for the death function of the BH3-only proteins (Figure 1).
Table 1.
Classification of Bcl-2-family proteins.
| Apoptotic role | Subclass | Protein nomenclature | Domains |
|---|---|---|---|
| Anti-apoptotic | Multi-domain | Bcl-2, Bcl-XL, Mcl-1, A1, Bcl-w, Bcl-B | BH1 - 4 |
| Pro-apoptotic | Multi-domain | Bax, Bak, Bok | BH1 - 3 |
| BH3 only (direct activators) | Bid, Bim | BH3 | |
| BH3 (de-repressors) | Bad, Bik, Bnip3, Puma, Noxa, Bmf, Hrk, others | BH3 |
Figure 1.
Schematic domain structures of different anti- and pro-apoptotic family members of Bcl-2.
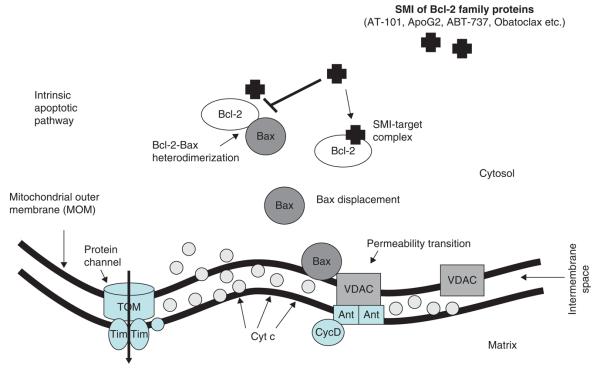
The role of both anti-apoptotic and pro-apoptotic family members in the regulation of apoptosis has become clearer in recent years. Researchers have successfully identified two competing models of activation of Bax by BH3-only proteins, namely, i) direct and ii) indirect activation models. In the direct activation model, the BH3-only activator proteins such as Bim and Bid directly bind to Bax and Bak leading to their activation. In the indirect mode of activation, the sensitizers bind to Bcl-2 like proteins, thus reducing their interaction with Bax and Bak and thereby inducing activation. It is this interaction of Bcl-2 to the Bax and Bak that gave a window of opportunity as a druggable target in the apoptotic machinery. Death signals emanating from diverse cellular stimuli caused by stresses such DNA damage, nutrient deprivation and oncogenic signaling are directed through the mitochondrial pathway by transcriptional activation and post-translational modifications of BH3-only proteins. In viable cells, Bax and Bak exist as monomers; however, on receiving death signals from the activated BH3-only proteins, they homo-oligomerize and insert into the mitochondrial membrane [3]. The insertion of homo-oligomerized Bak and Bax causes mitochondrial outer membrane permeabilization, which is a key step in apoptosis and is followed by the release of cytochrome c and other pro-apoptotic factors from the mitochondria (intrinsic apoptotic pathway summarized in Figure 2). It is well established that prior to membrane permeabilization, the activation of both Bax and Bak is required, which is further facilitated by direct binding to Bim, Bid or Puma [26]. After its release, cytochrome c binds in a complex with APAF-1, caspase-9 and dATP/dADP to form the apoptosome that ultimately triggers the downstream effector caspases resulting in cell death [27].
Figure 2. The inter membrane space of the mitochondrion in healthy cells is a rich storage of apoptotic regulators, including Cyt C (circles).
On stress signaling, mitochondrial membrane potential dramatically decreases with the formation of a PTPC (associated with VDAC channel), compromising the integrity of the MOM, and spillage of Cyt C into the cytosol. Small molecule inhibitors such as ApoG2, TW-37, AT-101 and ABT-263 bind to the hydrophobic groove of the pro-survival Bcl-2 family members thereby releasing the pro-apoptotic proteins such as Bax. This leads to the formation of mitochondrial membrane channel with concurrent release of Cyt C, resulting in apoptotic cell death.
Cyt C: Cytochrome c; MOM: Mitochondrial outer membrane; PTPC: Permeability transition pore complex.
Over the last 2 decades, numerous important inroads have been made towards understanding of the apoptotic machinery. Once the components of the apoptotic machinery were defined at the molecular detail, researchers turned their focus towards approaches on targeting the key members of this crucial pathway with an ultimate goal towards shifting the pro-survival cancer cell pathways into pro-death mechanisms. A number of potential targets were identified and numerous strategies have been developed over the years in this direction, some of which are very promising and others not as successful as discussed below in this article.
2. Medical need
In recent years, it is increasingly being realized that over-expression of Bcl-2, Mcl-1, BclXL and other members contributes to cancer progression and confers resistance to apoptosis induced by standard anticancer therapies [28,29]. This is more common in genetically complex and inherently resistant cancers such as pancreatic, ovarian, prostate and metastatic breast cancers that are known to overexpress at least one family member of anti-apoptotic proteins. Currently available anticancer therapies are based on targeting cancer cell DNA integrity or replication, which indirectly trigger apoptosis in tumor cells [30]. Tumors expressing high levels of Bcl-2, Mcl-1 or BclXL are often found to be resistant to chemotherapeutic agents or radiation therapy [31]. There is an urgent medical need for the development of novel strategies that can circumvent the observed resistance developed towards currently available chemotherapeutics. Therefore, inhibition of the function of the anti-apoptotic members represents a novel and promising strategy for designing new classes of anticancer drugs that can overcome the resistance of cancer cells to chemotherapy or radiation. Various different approaches have been made towards the development of targeted therapies that involve Bcl-2 proteins or components of the apoptotic machinery and are discussed in this review.
3. Existing treatments and advancements in the development of Bcl-2 targeted therapy
Initial advancements in this area were based on silencing strategies using antisense technology that in principle could inhibit Bcl-2 expression levels, which formed the basis of the concept behind Bcl-2 antisense therapy [32-37]. A promising Bcl-2 antisense oligonucleotide G3139 was in several clinical trials for treatment of different cancers [38-42]. Phase III studies suggest that this approach is reasonably successful, at least, in myeloid leukemia patients in whom robust intracellular concentrations of G3139 could be achieved in vivo in bone marrow (range, 3.4 – 40.6 pmol/mg protein) and PBMCs (range, 0.47 – 19.4 pmol/mg protein) that were directly related to Bcl-2 mRNA downregulation [43]. Among such Bcl-2 antisense, oblimersen is already in Phase III clinical trials. However, antisense oligonucleotides have short half-life and are prone to rapid enzymatic degradation and turnover. This is certainly a hindrance in the success of antisense therapy and, therefore, researchers are focusing on the development of better chemical modifications of such antisense oligonucleotides to increase resistance to nuclease digestion, prolong tissue half-lifes and improve scheduling [44].
Another attractive approach to block the activity of Bcl-2 is the use of antibody directed against Bcl-2. The concept that antibodies might be effective for the treatment of cancers originated more than a century ago with Paul Ehrlich's hypothesis that it would someday be feasible to develop a ‘magic bullet’ that has an affinity for ‘parasites’ sparing normal tissues. However, since then, a hundred years have elapsed before antibodies could actually be developed as effective agents for the treatment of cancer. An intracellular anti-Bcl-2 single-chain antibody has been shown to increase drug-induced cytotoxicity in the MCF-7 breast cancer cell lines as well as other cancers [45]. Other approaches include the use of a ribozyme against Bcl-2 and, more recently, a synthetic, cell permeable Bak BH3 peptide that binds to Bcl-2 has been shown to be partially successful both in vitro and in vivo against myeloid leukemia growth [46]. Like antisense therapy, the use of antibody, ribozymes or peptides as therapeutic strategy is hindered by the lack of stability and effective delivery. To overcome this issue, a chemical strategy has also been pursued by some researchers using hydrocarbon stabling to generate stapled BH3 peptide with increased pharmacological properties [47,48]. The stapled peptides, called ‘stabilized α-helix of Bcl-2 domains’ (SAHBs), are helical, protease-resistant and cell-permeable molecules that bind with increased affinity to multi-domain Bcl-2 member pockets. Such a SAHB of the BH3 domain from the Bid protein was shown to specifically activate the apoptotic pathway to kill leukemia cells. Furthermore, other stapled Bid-BH3 peptides have also been synthesized that have shown to have better apoptotic potential than parent peptide alone.
4. Current research goal
The last 2 decades have witnessed numerous advancements in our understanding of the apoptotic machinery and many approaches have been designed towards targeting the Bcl-2 family members. Even though partially successful, none of these approaches has been proven to be useful in the clinic, and thus attention has been focused on newer agents with better clinical outcome such as non-peptidic SMI. Researchers over the years have realized that peptide and enzyme based approaches may not be successful due to stability issues. Therefore, the current goal of researchers is to devise newer approaches that could be more stable and also overcome the membrane barrier. To this end, an important step has been taken, that is, the development of SMIs and is the theme of this review.
5. Scientific rationale
Due to limited success of antisense, oligonucleotide and antibody-based approaches, the researchers changed their center of attention towards a different strategy that was focused on antagonizing the function of Bcl-2 rather than to reduce its levels. This was approached mechanistically following the delineation of the crystal structure of BclXL, which revealed that the BH1 – 3 domains formed a hydrophobic groove [49], where the α-helix of the BH3-only proteins bind [50]. The structural analysis of BclXL bound to the Bak BH3 peptide was a proof-of-concept experiment indicating that it could be possible to create small molecules that bind to the hydrophobic groove of BclXL, thereby, inhibiting its anti-apoptotic function.
SMIs are organic molecules of low molecular mass (usually < 750 Dalton). Their small size makes their use in vivo even more practical, and possibly more cost-efficient, compared to oligonucleotides or other small peptides. The anti-apoptotic function of Bcl-2 is attributed, at least in part, to the ability to hetero-dimerize with pro-apoptotic members such as Bim, and it has been hypothesized that SMIs that bind to this BH3-binding site could in theory be capable of blocking the hetero-dimerization of Bcl-2 with pro-apoptotic members of the Bcl-2 protein family, such as Bid and Bim. Drug occupation of the hydrophobic groove is thus thought to abrogate the anti-apoptotic function of Bcl-2 (and others) and induce apoptosis. This decade has witnessed a tremendous enthusiasm in the area of SMI design and development initiated by half a dozen groups of scientists who have taken up the challenge to develop SMIs targeting the ‘elongated hydrophobic cleft’ (Table 2).
Table 2.
Small molecule inhibitors of Bcl-2 in the clinic.
| Agent | Source | Preclinical studies | Clinical trial status |
Molecular targets |
|---|---|---|---|---|
| Gossypol | Natural | Head and neck, pancreatic cancer |
Phase II/III | Bcl-2 family members, other non specific targets |
| AT-101 (BL-193) | Ascenta Therapeutics | Pancreatic cancer, head and neck, malignant glioma |
Phase II/III | Mcl-1, Bcl-2, BclXL |
| TW-37 | University of Michigan | Pancreatic cancer, non-Hodgkin's lymphoma, lung |
Phase I/II | BclW, BclXL, A1, Mcl-1, Bcl-2 |
| ApoG2 | Ascenta Therapeutics | Pancreatic cancer, non-Hodgkin's and Hodgkin's lymphoma |
Preclinical | Bcl-2, BclW, Mcl-1 |
| ABT-737 | IDUN & Abbott | Multiple myeloma, small cell lung cancer, acute leukemia, lymphoma |
Phase I/II | Bcl-2, BclXL |
| ABT-263 | Abbott | Multiple myeloma, small cell lung cancer, acute leukemia, chronic lymphocytic leukemia |
Phase I/II | BclXL, Bcl2, BclW |
| Obatoclax | Gemin X | Myeloma and mantle cell lymphoma | Phase I/II | Bcl-2, BclXL, BclW Mcl-1 |
In order to understand the structural basis of (−)-gossypol binding to Bcl-2, Wang et al. have performed computational docking of (−)-gossypol into the BH3-binding groove in Bcl-2 [51]. This group has also modeled the binding of a Bim BH3 peptide in a complex with Bcl-2 as this Bim BH3 peptide binds to Bcl-2 with a high affinity. Based on their predicted binding model, (−)-gossypol forms a hydrogen bonding network with residues Arg146 and Asn143 in Bcl-2 through the aldehyde group and the adjacent hydroxyl group on the right naphthalene ring. This mimics the hydrogen bonding network formed by Asp99 and Asn102 in Bim, and Arg146 and Asn143 in Bcl-2. The isopropyl group on the same naphthalene ring inserts into a hydrophobic pocket in Bcl-2, in part mimicking the Phe101 in the Bim peptide. The left half of the (−)-gossypol molecule interacts primarily with Bcl-2 through hydrophobic contacts, mimicking Ile97 in the Bim peptide. This binding model suggests that the two halves of (−)-gossypol interact differently with Bcl-2 and provided clues as well as structural basis for the design of novel SMIs of Bcl-2 such as ApoG2 and TW-37.
The following paragraphs summarize the currently studied SMIs, their mode of action and provide a brief description of their success in the clinic.
6. Competitive environment (currently available SMIs of Bcl-2)
Over the last 10 years, the field has witnessed emergence of numerous SMIs of Bcl-2. These inhibitors belong to different class of compounds as well as source or origin. The early inhibitors were originally discovered from natural sources and then later modified for optimal activity and reduced toxi-city. The second generation witnessed rapid development of synthetic SMIs each designed to target individual or the entire Bcl-2 family of proteins. The field has progressed quickly and competitively and some of the latest discoveries on individual inhibitors are described below.
6.1 Gossypol and related small molecule analogs
Prior to chemically synthesizing SMIs against Bcl-2, researchers extensively searched for natural compounds that could function as cell permeable, small molecule mimetic of the crucial Bcl-2 BH3 domain. A number of natural agents were discovered using a library-screening process that includes tetrocarcin A [52], antimycin [53] and gossypol [54]. Gossypol, also known as BL-193, is a natural compound isolated from cottonseeds and roots [55], which was the first compound to reach the clinic, but its mechanism of action at the time was not known [56]. It has been studied since the 1980s for its contraceptive properties [57], DNA damaging capacity [58,59], ability to generate ROS and cytochrome c release [60] and many other modulatory properties that cumulatively were believed to be responsible for its anticancer effects. There are two iso-forms of gossypol, which include (−)-BL-193, (+)-BL-193, while (±)-BL-193 is a racemic mixture of the two. The (−)-BL-193 has been shown to be more potent than its other isoforms as inhibitors of cell growth. Multidimensional NMR methods have shown that (−)-BL-193 binds the hydro-phobic groove of Bcl-2 and BclXL [51], and is currently in Phase III testing as a Bcl-2 inhibitor. Apart from apoptotic cell death, other mechanisms of growth inhibition have been attributed to gossypol. Recently, Voss et al. have shown that in malignant gliobalstoma, (−)-gossypol triggers authophagic cell death through neutralization of Mcl-1 [61]. Therefore, in view of the multiple non-apoptotic effects of gossypol, it is suggested that further studies are needed to completely decipher its anticancer potential. Studies have indicated that in addition to apoptosis, gossypol can also induce autophagy by disrupting the interaction between beclin-1 and Bcl-2 proteins [62]. In this elegant study, Gao et al. have shown that gossypol induces both beclin-1-dependent and -independent autophagic response in breast cancer cells. However, the same study concluded that the observed autophagy was cytoprotective rather than aiding the apoptotic pathway.
6.2 The role of TW37
The second generation SMI ‘TW37’ is a benzenesulfonyl derivative and was derived from gossypol [63,64]. The drug has both pro-apoptotic [65] and anti-angiogenic effects [66]. It was originally designed to target the BH3-binding groove in the BclXL and has shown to have high affinities for Bcl-2 as well as BclXL, and unlike most SMIs, also targets Mcl-1. Our laboratory has extensively studied this SMI for its apoptotic action in leukemia, lymphoma and pancreatic cancers [67]. The third generation SMI ‘apogossypolone’ (ApoG2) is a derivative of gossypol that was designed by Ascenta in order to reduce the nonspecific reactivity and toxicity of gossypol and is currently in the preclinical phase of testing. This modification involved the removal of two reactive aldehyde groups on the polyphenolic rings of gossypol. Studies from our laboratory have shown that ApoG2 blocks binding of Bim and Bcl-2 and induces apoptosis in lymphoma cell lines with minimal toxicity. Furthermore, it has also been shown that ApoG2 induces apoptosis in follicular small cleaved cell lymphoma model, pre-B-acute lymphoblastic leukemia, mantle cell lymphoma, marginal zone lymphoma, as well as chronic lymphocytic leukemia. Therefore, ApoG2 could potentially be a more effective drug in the lymphoma clinic spanning a greater array of patients [68-71]. An important feature of ApoG2 is that it can effectively target the crucial Mcl-1 that is also emerging to be a key player in the pro-survival machinery.
6.3 Further development of AT-101
As mentioned earlier, natural gossypol is a racemic mixture of (−)-gossypol and (+)-gossypol. The (−)-gossypol is the most clinically acceptable form of gossypol and has been brand named as AT-101 (Ascenta). AT-101 is in Phase III clinical trials for chronic lymphocytic leukemia (in combination with rituximab) and in hormone refractory prostate cancer (in combination with docetaxel). AT-101 exhibits submicromolar binding affinity for Bcl-2 and Mcl-1. This SMI has partial gastrointestinal toxicity that was a dose-limiting factor in a Phase I–II clinical trial in prostate cancer patients. Based on its toxicity, previously discussed gossypol analog, apogossypol, may be a better alternate but is still in preclinical development.
6.4 The ABT series of Bcl-2 inhibitors
Of the compounds discovered to date, one of the most promising candidates that selectively kills cancer cells through direct interaction with the Bcl-2 family is the BH3 mimetic ABT-737 developed at the Abbott Laboratories, Abbott Park, IL, USA. This is a Bcl-2 specific SMI, which was confirmed by the observation that ABT-737 has been effective at activating apoptosis in cells doubly deficient of Bax and Bak [72]. ABT-737 was discovered by Abbott using structure–activity relationship (SAR) by NMR strategy that combines nuclear magnetic screening coupled with structure-based design and combinatorial synthesis [73]. The SAR by NMR strategy led to the discovery of lead compound ABT-737 that mimicked the BH3 domain of BAD and bound selectively to Bcl-2, BclXL and Bcl-W. Most interesting was the observation that the binding affinity of ABT-737 to different anti-apoptotic members was in the nanomolar range making this an ideal clinical candidate. ABT-737 is fairly specific to Bcl-2, BclXL and Bcl-w as it binds with poor affinity to Mcl-1 and Bfl-1 with a dissociation constant in the micro-molar range [73]. ABT-263 is the orally applicable version of ABT-263 that has been extensively studied, which inhibits the anti-apoptotic proteins Bcl-2, BclXL and Bcl-w, and has shown single-agent efficacy in numerous small cell lung carcinoma (SCLC) and leukemia/lymphoma cell lines in vitro and in vivo [74-76]. It is currently in clinical trials for treating patients with SCLC and various leukemia/lymphomas.
A very recent study by Tahir et al. has utilized a systems approach and identified the expression of Bcl-2 family genes that correlated best with sensitivity to ABT-263 in a panel of 36 SCLC and 31 leukemia/lymphoma cell lines [77]. The group has examined global expression differences to identify gene signature sets that correlated with sensitivity to ABT-263 to generate optimal signature sets predictive of sensitivity to ABT-263. In their study, independent cell lines were used to verify the predictive power of the gene sets and to refine the optimal gene signatures. When comparing normal lung tissue and SCLC primary tumors, the expression pattern of these genes in the tumor tissue was found to be similar to sensitive SCLC lines, whereas normal tissue response was similar to resistant SCLC lines. Most of the genes identified using global expression patterns were related to the apoptotic pathway. This study leverages global expression data to identify key gene expression patterns for sensitivity to Bcl-2 targeted drugs, which may provide guidance in the selection of patients in future clinical trials using different cancer patients.
6.5 The role of obatoclax
Obatoclax is a synthetic derivative of prodiginines (GX015 – 070) from Gemin X Biotech (Avenue du Parc, Montreal, Quebec, Canada) and is a pan-Bcl-2 inhibitor with lower binding affinity to Bcl-2 family members than ABT-737 [78,79]. Recently, it has been shown that in cholangiocarcinoma, obatoclax induces Bax-mediated apoptosis [80]. However, similar to many other putative BH3 mimetics, obatoclax is not entirely dependent on Bax and Bak and can induce cancer cell death independently [81,82]. In this regard, it has been shown earlier that obatoclax can also antagonize Mcl-1 and overcome the resistance observed through Mcl-1. Unfortunately, this compound may need to be redesigned for pharmacokinetic reasons especially because a recent study has shown that the treatment of mice with bolus injection of GX-015 – 070 failed to reach pharmacologically effective levels in the blood; and dose escalation was limited by significant neurologic toxicity.
6.6 The role of HA14 – 1
The new compound ethyl-2-amino-6-cyclopentyl-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-chromone-3-carboxylate (HA14 – 1) is the first reported small molecule antagonist for Bcl-2 protein and was identified by Wang et al. [83]. This simple chemical structure is a putative Bcl-2 inhibitor, which was identified from in silico screens. HA14 – 1 disrupts the binding interaction of the Bak BH3-domain peptide with Bcl-2 and Bcl-xL proteins, and strongly inhibits the Bcl-2–Bax interaction [84], and also inhibits the interaction between Bcl-2 and BH3-only protein Bim [85]. Since its discovery, HA14 – 1 has been shown to enhance the cytotoxic effects of a variety of anticancer agents. HA14 – 1 has also been shown to act as a chemosensitizer with selectivity to enhance the apoptotic effect of cisplatin by modulating Bcl-2 family members in MDA-MB-231 breast cancer cells. However, HA14 – 1 has shown redox reactivity and instability for which newer stable versions of sHA14 – 1 have been developed that are less redox active [86].
6.7 Bcl-2 family inhibitors as sensitizers to chemotherapy
The BH3 mimetic are often not very effective as single agents especially against some of the epithelial and genetically complex cancers, such as the pancreatic, ovarian and breast cancers [87,88]. The observed resistance to Bcl-2 family targeted drugs is due to multiple factors that include redundancies in crosstalk between major signaling pathways, lack of target validation and acquired resistance to chemotherapy. In the last few years, results from our laboratory and those of others have proven that an important niche for Bcl-2 family SMIs is in combination therapy with standard chemotherapeutics such as cisplatin, gemcitabine [67] or other therapies such as TRAIL [89]. This is especially exemplified in pancreatic cancer where chemotherapy has been shown to induce prosurvival molecule NF-κB and Bcl-2, and the SMI serves to inhibit Bcl-2-mediated resistance, enabling killing by conventional chemotherapy. Numerous examples exist in the literature in support of the enhanced apoptotic response when the BH3 mimetics are combined with traditional therapies to treat various cancers such as melanoma. Thus, it seems that the SMIs are capable of priming the cancer cells to make them vulnerable to be killed by conventional chemotherapeutics especially those cancers that are drug resistant due to genetic complexity. Indeed, recent reports have shown the use of the BH3 mimetic ABT-737 for overcoming both de novo and acquired resistance for the killing of cancer cells [90]. It was effective at re-sensitizing drug-resistant breast cancer cell lines to apoptosis induced by paclitaxel emphasizing the potential effectiveness of the use of BH3 mimetics in combination therapy [91].
6.8 Mcl-1: an emerging potential target for SMI-based therapy
As our understanding of the pathophysiology of different cancers increases, so does the knowledge of the mechanism of drug action. Microarray-based molecular network modeling addresses previously unanswered questions such as the mechanism behind the development of de novo and acquired resistance as observed in different cancer cell types. Preliminary studies from our laboratory have suggested that the observed resistance to Bcl-2 family SMIs in some cell lines could be attributed to the variable status of basal Mcl-1. This apoptotic protein is emerging to be an important player in the observed resistance towards both standard chemotherapy and SMIs of the apoptotic machinery. The human Mcl-1 gene is located on chromosome 1q21 and comprises three exons with alternative splicing giving rise to two distinct Mcl-1 mRNAs either containing or lacking exon 2 and encoding the Mcl-1L and Mcl-1S iso-forms, respectively [92]. Mcl-1 is primarily localized to the outer mitochondrial membrane and promotes cell survival by suppressing cytochrome c release from mitochondria via heterodimerization and neutralization of effector proapoptotic Bcl-2 family members including Bak [93]. Mcl-1 also selectively interacts with BH3-only proteins, Bim, tBid, Bik, Puma and Noxa [94]. It has been suggested that Mcl-1 may function as an anti-apoptotic factor by sequestering Bak on the outer mitochondrial membrane, preventing Bak oligomerization and cytochrome c release from the mitochondria [95]. Our earlier work has shown that the ratio of Bcl-2:Mcl-1 is a decisive factor in the drug response using certain Bcl-2 family inhibitors [96]. Currently, our group is developing SMI against Mcl-1, and preliminary studies revealed that Mcl-1 targeting could become a mainstay therapy in complex tumors such as pancreas that overexpress Mcl-1 and are resistant to standard chemotherapy or Bcl-2 family inhibitors. Other researchers have also demonstrated the significance of Mcl-1 in the observed differential sensitivities to Bcl-2 targeted drugs. High levels of Mcl-1 or Bfl-1 have been shown to correlate with resistance to ABT-737. The resistance caused by Mcl-1 could be attenuated by addition of agents that could decrease Mcl-1, such as seliciclib [97,98]. Based on the available information, Mcl-1 certainly becomes an important SMI target, and thus researchers are in pursuit of developing a highly specific small molecule drug against this important pro-survival protein.
7. Potential developmental issues and hindrances in clinical progress of Bcl-2 family inhibitors
Although a number of different classes of SMIs have been developed over the last decade, only five are at present in clinical trials: genasense (G3139), TW-37, AT-101, obatoclax and ABT-263 (Table 2). The progress in clinical trials has been hindered by the observed toxicity with most of these inhibitors. In preclinical in vitro experiments, ABT-737 as a single agent showed toxicity to leukemia and lymphoma, while at higher doses it induces apoptosis in multiple cancer cell lines [73,99]. Numerous studies have shown that primary cells from patients with chronic lymphocytic leukemia [100], acute myeloid leukemia [82] and acute lymphocytic leukemia [101] are extremely responsive to ABT-737, with widespread apoptosis at nano-molar concentrations. To enhance its clinical potential, ABT-737 has been modified (ABT-263) to make the drug orally available. ABT-263 binds to serum proteins resulting in a longer oral half-life. The tumoricidal action of ABT-263 was shown in xenograft models of small cell lung cancer, where ABT-263 caused complete regression of the tumors and is now in clinical trials. Similarly, obatoclax, the pan-Bcl-2 inhibitor, shows single agent efficacy in the killing of multiple myeloma, acute myeloid leukemia cell lines and primary tumor cells. However, it also causes cell cycle arrest independent of apoptosis. Our laboratory has shown that TW-37 causes a reduction in cell proliferation of pancreatic and B-cell lymphomas, and is capable of reducing the tumor size in xenograft models [102]. TW-37 shows efficacy in cell lines and patient derived cells and is effective in a spectrum of B-cell tumors, irrespective of their proliferative and differentiation status. Finally, AT-101 from Ascenta is in multiple clinical trials including an open-label, multi-center Phase I/II single agent study in castrate resistant prostate cancer patients [103] (clinical status of different SMIs of Bcl-2 family members is summarized in Table 2).
This brings up the issue of specificity of these SMIs. For a drug to be approved clinically, it has to be specific with lesser side effects or beneficial off target effects. Even though SMIs were originally designed to specifically hit a single target, researchers have constantly observed that these Bcl-2 family SMIs also modulate other key molecules that are considered secondary or off targets. Studies from our laboratory and others have revealed that different Bcl-2 family SMIs modulate numerous key molecules such as NF-κB, Notch and Par-4 [67,104]. Whether modulation of secondary targets is beneficial for the overall activity of these Bcl-2 family targeting agents or the off targets mask the SMI potential is a very important question that needs to be addressed for SMIs to achieve success as novel drugs in the clinical setting.
8. Conclusions
Previous attempts to target Bcl-2 family members therapeutically using antisense technology to inhibit protein translation so far have not significantly improved outcomes for cancer patients, although improved oligonucleotide design may enhance efficacy. The development of orally bioavailable Bcl-2 family inhibitors, such as AT-101, ApoG2, TW-37 and ABT-263, with the ability to inhibit specifically BH3-Bcl-2 protein–protein interactions at low nanomolar concentrations potentially marks a significant development in cancer therapy. Clinical studies on ABT-263 and AT-101 are currently ongoing and whether these compounds can find their way into routine clinical use will depend not only on efficacy but also on manageable, acceptable toxicities. Thrombocytopenia, for example, may limit the value of ABT-263 in patients with heavy bone marrow infiltration; the long-term toxicities of prolonged Bcl-2 suppression remain to be determined. Identification of proteins that mediate resistance to ABT-263 may also allow the development of rational combinations. After many years of development, it now seems likely that the rational design of specific compounds that inhibit specific protein–protein interactions may lead to a significant therapeutic advance for the treatment of most human malignancies.
9. Expert opinion
As our understanding of the regulation of Bcl-2 family members and their roles in tissue dynamics has greatly expanded in recent years, so did the progress in the field of development of targeted agents against these pro-survival family members. In the last decade, researchers have been able to decipher how BH3-only proteinssense signalstoinduce apoptosis and relay this information through the core Bcl-2 family members to initiate cell death. This is followed by Bax and Bak conformational change that results in permeabilization of the outer mitochondrial membrane. Nevertheless, many important questions remained unanswered such as how Bax and Bak function in this process. The structure of these proteins after conformational change, dynamics of their oligomerization, localizationaswellasdetermination of their intermolecular-binding partners in membranes are important issues that have eluded researchers for years and even to-date the molecular trigger that induces Bax translocation and Bak activation is not fully understood. The difference between anti- and pro-apoptotic Bcl-2 family proteins needs to be defined in greater detail both on a structural and functional basis. Moreover, the emerging and important functional effect of Mcl-1 deserves more study. Recent advances in understanding the intermolecular interactions among the family members corroborated at the level of animal studies, along with cell biology advances, offer abundant clues for deciphering the remaining mysteries of cellular commitment to apoptosis. In our opinion, in the next 5 – 10 years more focus will be on the development of SMIs against underappreciated yet crucial players such as Mcl-1. Other emerging molecules in the apoptotic machinery will also be explored and targeted. However, this would require high-throughput screening coupled with omics technology to validate and target newer entities in the apoptotic machinery. Interestingly, using systems approach, some inroads have been made in delineating the SMI drug target gene signatures in apoptotic pathway, especially the discovery of selective protein–protein interactions among the Bcl-2 family of proteins that controls apoptosis. Simultaneously, an understanding of how cancer cells evade death at the molecular level has been achieved in which the Bcl-2 family appears to play a starring role in avoiding death. Based on the progress in this area, in our expert opinion, the stage is set for the next generation of SMIs for not only Bcl-2 proteins but also for Mcl-1, which is being currently exploited both preclinically and clinically in order to appreciate the value of these SMIs as a single agent or in combination with conventional therapeutics. In our opinion, the future looks brighter than the last several decades, which would probably change the practice of personalized medicine for improving the survival of patients diagnosed with malignancies.
Acknowledgments
National Cancer Institute, NIH grant R01CA109389 (RM Mohammad) and NIH grants 5R01CA131151 and 5R01CA132794 (FH Sarkar) are gratefully acknowledged. We thank the Guido Foundation for their generous support.
Footnotes
Declaration of interest
Bibliography
- 1.Moffitt KL, Martin SL, Walker B. From sentencing to execution – the processes of apoptosis. J Pharm Pharmacol. 2010;62:547–62. doi: 10.1211/jpp.62.05.0001. [DOI] [PubMed] [Google Scholar]
- 2.Cosulich SC, Worrall V, Hedge PJ, et al. Regulation of apoptosis by BH3 domains in a cell-free system. Curr Biol. 1997;7:913–20. doi: 10.1016/s0960-9822(06)00410-6. [DOI] [PubMed] [Google Scholar]
- 3.Ottilie S, Diaz JL, Horne W, et al. Dimerization properties of human BAD. Identification of a BH-3 domain and analysis of its binding to mutant BCL-2 and BCL-XL proteins. J Biol Chem. 1997;272:30866–72. doi: 10.1074/jbc.272.49.30866. [DOI] [PubMed] [Google Scholar]
- 4.Billen LP, Shamas-Din A, Andrews DW. Bid: a Bax-like BH3 protein. Oncogene. 2008;27(Suppl 1):S93–104. doi: 10.1038/onc.2009.47. [DOI] [PubMed] [Google Scholar]
- 5.Ghiotto F, Fais F, Bruno S. BH3-only proteins: the death-puppeteer's wires. Cytometry A. 2010;77:11–21. doi: 10.1002/cyto.a.20819. [DOI] [PubMed] [Google Scholar]
- 6.Akiyama T, Dass CR, Choong PF. Bim-targeted cancer therapy: a link between drug action and underlying molecular changes. Mol Cancer Ther. 2009;8:3173–80. doi: 10.1158/1535-7163.MCT-09-0685. [DOI] [PubMed] [Google Scholar]
- 7.Danial NN. BAD: undertaker by night, candyman by day. Oncogene. 2008;27(Suppl 1):S53–70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- 8.Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27(Suppl 1):S71–83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ploner C, Kofler R, Villunger A. Noxa: at the tip of the balance between life and death. Oncogene. 2008;27(Suppl 1):S84–92. doi: 10.1038/onc.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fulda S. Tumor resistance to apoptosis. Int J Cancer. 2009;124:511–15. doi: 10.1002/ijc.24064. [DOI] [PubMed] [Google Scholar]
- 11.Fulda S. Evasion of apoptosis as a cellular stress response in cancer. Int J Cell Biol. 2010;2010:370835. doi: 10.1155/2010/370835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baguley BC. Multidrug resistance in cancer. Methods Mol Biol. 2010;596:1–14. doi: 10.1007/978-1-60761-416-6_1. [DOI] [PubMed] [Google Scholar]
- 13.Patel MP, Masood A, Patel PS, Chanan-Khan AA. Targeting the Bcl-2. Curr Opin Oncol. 2009;21:516–23. doi: 10.1097/CCO.0b013e328331a7a4. [DOI] [PubMed] [Google Scholar]
- 14.Vogler M, Dinsdale D, Dyer MJ, Cohen GM. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 2009;16:360–7. doi: 10.1038/cdd.2008.137. [DOI] [PubMed] [Google Scholar]
- 15.Ashkenazi A. Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev. 2008;19:325–31. doi: 10.1016/j.cytogfr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Parsons MJ, Green DR. Mitochondria in cell death. Essays Biochem. 2010;47:99–114. doi: 10.1042/bse0470099. [DOI] [PubMed] [Google Scholar]
- 17.Russo M, Mupo A, Spagnuolo C, Russo GL. Exploring death receptor pathways as selective targets in cancer therapy. Biochem Pharmacol. 2010;80:674–82. doi: 10.1016/j.bcp.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Shrestha-Bhattarai T, Rangnekar VM. Cancer-selective apoptotic effects of extracellular and intracellular Par-4. Oncogene. 2010;29:3873–80. doi: 10.1038/onc.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burikhanov R, Zhao Y, Goswami A, et al. The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis. Cell. 2009;138:377–88. doi: 10.1016/j.cell.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korsmeyer SJ, Gross A, Harada H, et al. Death and survival signals determine active/inactive conformations of pro-apoptotic BAX, BAD, and BID molecules. Cold Spring Harb Symp Quant Biol. 1999;64:343–50. doi: 10.1101/sqb.1999.64.343. [DOI] [PubMed] [Google Scholar]
- 21.Korsmeyer SJ, Shutter JR, Veis DJ, et al. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4:327–32. [PubMed] [Google Scholar]
- 22.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–19. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 23.Bartholomeusz G, Wu Y, Ali SM, et al. Nuclear translocation of the pro-apoptotic Bcl-2 family member Bok induces apoptosis. Mol Carcinog. 2006;45:73–83. doi: 10.1002/mc.20156. [DOI] [PubMed] [Google Scholar]
- 24.Inohara N, Ekhterae D, Garcia I, et al. Mtd, a novel Bcl-2 family member activates apoptosis in the absence of heterodimerization with Bcl-2 and Bcl-XL. J Biol Chem. 1998;273:8705–10. doi: 10.1074/jbc.273.15.8705. [DOI] [PubMed] [Google Scholar]
- 25.Reed CJ. Apoptosis and cancer: strategies for integrating programmed cell death. Semin Hematol. 2000;37:9–16. doi: 10.1016/s0037-1963(00)90055-6. [DOI] [PubMed] [Google Scholar]
- 26.Korsmeyer SJ, Wei MC, Saito M, et al. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell DeathDiffer. 2000;7:1166–73. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 27.Acehan D, Jiang X, Morgan DG, et al. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell. 2002;9:423–32. doi: 10.1016/s1097-2765(02)00442-2. [DOI] [PubMed] [Google Scholar]
- 28.Chao DT, Linette GP, Boise LH, et al. Bcl-XL and Bcl-2 repress a common pathway of cell death. J Exp Med. 1995;182:821–8. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 30.Borner C. The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol. 2003;39:615–47. doi: 10.1016/s0161-5890(02)00252-3. [DOI] [PubMed] [Google Scholar]
- 31.Reed JC. Bcl-2 family proteins: strategies for overcoming chemoresistance in cancer. Adv Pharmacol. 1997;41:501–32. doi: 10.1016/s1054-3589(08)61070-4. [DOI] [PubMed] [Google Scholar]
- 32.Cotter FE. Antisense therapy for B cell lymphomas. Cancer Surv. 1997;30:311–25. [PubMed] [Google Scholar]
- 33.Cotter FE. Antisense therapy for lymphomas. Hematol Oncol. 1997;15:3–11. doi: 10.1002/(sici)1099-1069(199702)15:1<3::aid-hon583>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 34.Cotter FE, Waters J, Cunningham D. Human Bcl-2 antisense therapy for lymphomas. Biochim Biophys Acta. 1999;1489:97–106. doi: 10.1016/s0167-4781(99)00139-6. [DOI] [PubMed] [Google Scholar]
- 35.Morris MJ, Tong WP, Cordon-Cardo C, et al. Phase I trial of BCL-2 antisense oligonucleotide (G3139) administered by continuous intravenous infusion in patients with advanced cancer. Clin Cancer Res. 2002;8:679–83. [PubMed] [Google Scholar]
- 36.Morris MJ, Cordon-Cardo C, Kelly WK, et al. Safety and biologic activity of intravenous BCL-2 antisense oligonucleotide (G3139) and taxane chemotherapy in patients with advanced cancer. Appl Immunohistochem Mol Morphol. 2005;13:6–13. doi: 10.1097/00129039-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Mohammad R, Abubakr Y, Dan M, et al. Bcl-2 antisense oligonucleotides are effective against systemic but not central nervous system disease in severe combined immunodeficient mice bearing human t(14;18) follicular lymphoma. Clin Cancer Res. 2002;8:1277–83. [PubMed] [Google Scholar]
- 38.Di CC, Koropatnick J. Antisense treatment in human prostate cancer and melanoma. Curr Cancer Drug Targets. 2010 doi: 10.2174/156800910791859452. [DOI] [PubMed] [Google Scholar]
- 39.Shah MH, Varker KA, Collamore M, et al. G3139 (Genasense) in patients with advanced merkel cell carcinoma. Am J Clin Oncol. 2009;32:174–9. doi: 10.1097/COC.0b013e31817eebf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moulder SL, Symmans WF, Booser DJ, et al. Phase I/II study of G3139 (Bcl-2 antisense oligonucleotide) in combination with doxorubicin and docetaxel in breast cancer. Clin Cancer Res. 2008;14:7909–16. doi: 10.1158/1078-0432.CCR-08-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu G, Kolesar J, McNeel DG, et al. A phase I pharmacokinetic and pharmacodynamic correlative study of the antisense Bcl-2 oligonucleotide g3139, in combination with carboplatin and paclitaxel, in patients with advanced solid tumors. Clin Cancer Res. 2008;14:2732–9. doi: 10.1158/1078-0432.CCR-07-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudin CM, Salgia R, Wang X, et al. Randomized phase II Study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. J Clin Oncol. 2008;26:870–6. doi: 10.1200/JCO.2007.14.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai G, Wei X, Liu Z, et al. Characterization and quantification of Bcl-2 antisense G3139 and metabolites in plasma and urine by ion-pair reversed phase HPLC coupled with electrospray ion-trap mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;825:201–13. doi: 10.1016/j.jchromb.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 44.Gleave ME, Monia BP. Antisense therapy for cancer. Nat Rev Cancer. 2005;5:468–79. doi: 10.1038/nrc1631. [DOI] [PubMed] [Google Scholar]
- 45.Piche A, Grim J, Rancourt C, et al. Modulation of Bcl-2 protein levels by an intracellular anti-Bcl-2 single-chain antibody increases drug-induced cytotoxicity in the breast cancer cell line MCF-7. Cancer Res. 1998;58:2134–40. [PubMed] [Google Scholar]
- 46.Wang JL, Zhang ZJ, Choksi S, et al. Cell permeable Bcl-2 binding peptides: a chemical approach to apoptosis induction in tumor cells. Cancer Res. 2000;60:1498–502. [PubMed] [Google Scholar]
- 47.Walensky LD, Kung AL, Escher I, et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305:1466–70. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walensky LD, Pitter K, Morash J, et al. A stapled BID BH3 helix directly binds and activates BAX. Mol Cell. 2006;24:199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 49.Muchmore SW, Sattler M, Liang H, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–41. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 50.Sattler M, Liang H, Nettesheim D, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–6. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 51.Wang G, Nikolovska-Coleska Z, Yang CY, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem. 2006;49:6139–42. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 52.Nakashima T, Miura M, Hara M. Tetrocarcin A inhibits mitochondrial functions of Bcl-2 and suppresses its anti-apoptotic activity. Cancer Res. 2000;60:1229–35. [PubMed] [Google Scholar]
- 53.Tzung SP, Kim KM, Basanez G, et al. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nat Cell Biol. 2001;3:183–91. doi: 10.1038/35055095. [DOI] [PubMed] [Google Scholar]
- 54.Kitada S, Leone M, Sareth S, et al. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem. 2003;46:4259–64. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Howell CP, Chen F, et al. Gossypol – polyphenolic compound from cotton plant. Adv Food Nutr Res. 2009;58:215–63. doi: 10.1016/S1043-4526(09)58006-0. [DOI] [PubMed] [Google Scholar]
- 56.Bushunow P, Reidenberg MM, Wasenko J, et al. Gossypol treatment of recurrent adult malignant gliomas. J Neurooncol. 1999;43:79–86. doi: 10.1023/a:1006267902186. [DOI] [PubMed] [Google Scholar]
- 57.Costantino A, Cerpolini S, Perrone AM, et al. Current status and future perspectives in male contraception. Minerva Ginecol. 2007;59:299–310. [PubMed] [Google Scholar]
- 58.Zaidi R, Hadi SM. Complexes involving gossypol, DNA and Cu(II) Biochem Int. 1992;28:1135–43. [PubMed] [Google Scholar]
- 59.Zaidi R, Hadi SM. Strand scission in DNA by gossypol and Cu(II): role of Cu(I) and oxygen-free radicals. J Biochem Toxicol. 1992;7:213–17. doi: 10.1002/jbt.2570070404. [DOI] [PubMed] [Google Scholar]
- 60.Ko CH, Shen SC, Yang LY, et al. Gossypol reduction of tumor growth through ROS-dependent mitochondria pathway in human colorectal carcinoma cells. Int J Cancer. 2007;121:1670–9. doi: 10.1002/ijc.22910. [DOI] [PubMed] [Google Scholar]
- 61.Voss V, Senft C, Lang V, et al. The pan-Bcl-2 inhibitor (−)-gossypol triggers autophagic cell death in malignant glioma. Mol Cancer Res. 2010;7:1002–16. doi: 10.1158/1541-7786.MCR-09-0562. [DOI] [PubMed] [Google Scholar]
- 62.Gao P, Bauvy C, Souquere S, et al. The BH3-mimetic gossypol induces both beclin 1-dependent and beclin 1-independent cytoprotective autophagy in cancer cells. J Biol Chem. 2010;285:25570–81. doi: 10.1074/jbc.M110.118125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azmi AS, Mohammad RM. Non-peptidic small molecule inhibitors against Bcl-2 for cancer therapy. J Cell Physiol. 2009;218:13–21. doi: 10.1002/jcp.21567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Z, Song W, Aboukameel A, et al. TW-37, a small-molecule inhibitor of Bcl-2, inhibits cell growth and invasion in pancreatic cancer. Int J Cancer. 2008;123:958–66. doi: 10.1002/ijc.23610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Mohammad RM, Goustin AS, Aboukameel A, et al. Preclinical studies of TW-37, a new nonpeptidic small-molecule inhibitor of Bcl-2, in diffuse large cell lymphoma xenograft model reveal drug action on both Bcl-2 and Mcl-1. Clin Cancer Res. 2007;13:2226–35. doi: 10.1158/1078-0432.CCR-06-1574. [DOI] [PubMed] [Google Scholar]
- 66.Zeitlin BD, Joo E, Dong Z, et al. Antiangiogenic effect of TW37, a small-molecule inhibitor of Bcl-2. Cancer Res. 2006;66:8698–706. doi: 10.1158/0008-5472.CAN-05-3691. [DOI] [PubMed] [Google Scholar]
- 67.Azmi AS, Wang Z, Burikhanov R, et al. Critical role of prostate apoptosis response-4 in determining the sensitivity of pancreatic cancer cells to small-molecule inhibitor-induced apoptosis. Mol Cancer Ther. 2008;7:2884–93. doi: 10.1158/1535-7163.MCT-08-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu ZY, Sun J, Zhu XF, et al. ApoG2 induces cell cycle arrest of nasopharyngeal carcinoma cells by suppressing the c-Myc signaling pathway. J Transl Med. 2009;7:74. doi: 10.1186/1479-5876-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun J, Li ZM, Hu ZY, et al. Apogossypolone inhibits cell growth by inducing cell cycle arrest in U937 cells. Oncol Rep. 2009;22:193–8. doi: 10.3892/or_00000424. [DOI] [PubMed] [Google Scholar]
- 70.Hu ZY, Zhu XF, Zhong ZD, et al. ApoG2, a novel inhibitor of antiapoptotic Bcl-2 family proteins, induces apoptosis and suppresses tumor growth in nasopharyngeal carcinoma xenografts. Int J Cancer. 2008;123:2418–29. doi: 10.1002/ijc.23752. [DOI] [PubMed] [Google Scholar]
- 71.Arnold AA, Aboukameel A, Chen J, et al. Preclinical studies of Apogossypolone: a new nonpeptidic pan small-molecule inhibitor of Bcl-2, Bcl-XL and Mcl-1 proteins in Follicular Small Cleaved Cell Lymphoma model. Mol Cancer. 2008;7:20. doi: 10.1186/1476-4598-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 74.Ackler S, Xiao Y, Mitten MJ, et al. ABT-263 and rapamycin act cooperatively to kill lymphoma cells in vitro and in vivo. Mol Cancer Ther. 2008;7:3265–74. doi: 10.1158/1535-7163.MCT-08-0268. [DOI] [PubMed] [Google Scholar]
- 75.Park CM, Bruncko M, Adickes J, et al. Discovery of an orally bioavailable small molecule inhibitor of prosurvival B-cell lymphoma 2 proteins. J Med Chem. 2008;51:6902–15. doi: 10.1021/jm800669s. [DOI] [PubMed] [Google Scholar]
- 76.Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–8. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 77.Tahir SK, Wass J, Joseph MK, et al. Identification of expression signatures predictive of sensitivity to the Bcl-2 family member inhibitor ABT-263 in small cell lung carcinoma and leukemia/lymphoma cell lines. Mol Cancer Ther. 2010;9:545–57. doi: 10.1158/1535-7163.MCT-09-0651. [DOI] [PubMed] [Google Scholar]
- 78.Li J, Viallet J, Haura EB. A small molecule pan-Bcl-2 family inhibitor, GX15-070, induces apoptosis and enhances cisplatin-induced apoptosis in non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2008;61:525–34. doi: 10.1007/s00280-007-0499-3. [DOI] [PubMed] [Google Scholar]
- 79.Trudel S, Li ZH, Rauw J, et al. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007;109:5430–8. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 80.Smoot RL, Blechacz BR, Werneburg NW, et al. A Bax-mediated mechanism for obatoclax-induced apoptosis of cholangiocarcinoma cells. Cancer Res. 2010;70:1960–9. doi: 10.1158/0008-5472.CAN-09-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pan J, Cheng C, Verstovsek S, et al. The BH3-mimetic GX15-070 induces autophagy, potentiates the cytotoxicity of carboplatin and 5-fluorouracil in esophageal carcinoma cells. Cancer Lett. 2010;293:167–74. doi: 10.1016/j.canlet.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 82.Konopleva M, Watt J, Contractor R, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax) Cancer Res. 2008;68:3413–20. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang JL, Liu D, Zhang ZJ, et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA. 2000;97:7124–9. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manero F, Gautier F, Gallenne T, et al. The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res. 2006;66:2757–64. doi: 10.1158/0008-5472.CAN-05-2097. [DOI] [PubMed] [Google Scholar]
- 85.Zimmermann AK, Loucks FA, Le SS, et al. Distinct mechanisms of neuronal apoptosis are triggered by antagonism of Bcl-2/Bcl-x(L) versus induction of the BH3-only protein Bim. J Neurochem. 2005;94:22–36. doi: 10.1111/j.1471-4159.2005.03156.x. [DOI] [PubMed] [Google Scholar]
- 86.Tian D, Das SG, Doshi JM, et al. sHA 14-1, a stable and ROS-free antagonist against anti-apoptotic Bcl-2 proteins, bypasses drug resistances and synergizes cancer therapies in human leukemia cell. Cancer Lett. 2008;259:198–208. doi: 10.1016/j.canlet.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Witham J, Valenti MR, De-Haven-Brandon AK, et al. The Bcl-2/Bcl-XL family inhibitor ABT-737 sensitizes ovarian cancer cells to carboplatin. Clin Cancer Res. 2007;13:7191–8. doi: 10.1158/1078-0432.CCR-07-0362. [DOI] [PubMed] [Google Scholar]
- 88.Huang S, Okumura K, Sinicrope FA. BH3 mimetic obatoclax enhances TRAIL-mediated apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2009;15:150–9. doi: 10.1158/1078-0432.CCR-08-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song JH, Kandasamy K, Kraft AS. ABT-737 induces expression of the death receptor 5 and sensitizes human cancer cells to TRAIL-induced apoptosis. J Biol Chem. 2008;283:25003–13. doi: 10.1074/jbc.M802511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Traini R, Ben-Josef G, Pastrana DV, et al. ABT-737 overcomes resistance to immunotoxin-mediated apoptosis and enhances the delivery of Pseudomonas exotoxin-based proteins to the cell cytosol. Mol Cancer Ther. 2010;7:2007–15. doi: 10.1158/1535-7163.MCT-10-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kutuk O, Letai A. Alteration of the mitochondrial apoptotic pathway is key to acquired paclitaxel resistance and can be reversed by ABT-737. Cancer Res. 2008;68:7985–94. doi: 10.1158/0008-5472.CAN-08-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bingle CD, Craig RW, Swales BM, et al. Exon skipping in Mcl-1 results in a bcl-2 homology domain 3 only gene product that promotes cell death. J Biol Chem. 2000;275:22136–46. doi: 10.1074/jbc.M909572199. [DOI] [PubMed] [Google Scholar]
- 93.Shimazu T, Degenhardt K, Nur-E-Kamal, et al. NBK/BIK antagonizes MCL-1 and BCL-XL and activates BAK-mediated apoptosis in response to protein synthesis inhibition. Genes Dev. 2007;21:929–41. doi: 10.1101/gad.1522007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clohessy JG, Zhuang J, de BJ, et al. Mcl-1 interacts with truncated Bid and inhibits its induction of cytochrome c release and its role in receptor-mediated apoptosis. J Biol Chem. 2006;281:5750–9. doi: 10.1074/jbc.M505688200. [DOI] [PubMed] [Google Scholar]
- 95.Willis SN, Chen L, Dewson G, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Al-Katib AM, Sun Y, Goustin AS, et al. SMI of Bcl-2 TW-37 is active across a spectrum of B-cell tumors irrespective of their proliferative and differentiation status. J Hematol Oncol. 2009;2:8. doi: 10.1186/1756-8722-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen S, Dai Y, Harada H, et al. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–91. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 98.Lin X, Morgan-Lappe S, Huang X, et al. ‘Seed’ analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene. 2007;26:3972–9. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 99.Tagscherer KE, Fassl A, Campos B, et al. Apoptosis-based treatment of glioblastomas with ABT-737, a novel small molecule inhibitor of Bcl-2 family proteins. Oncogene. 2008;27:6646–56. doi: 10.1038/onc.2008.259. [DOI] [PubMed] [Google Scholar]
- 100.Del GMV, Schlis KD, Sallan SE, et al. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood. 2008;111:2300–9. doi: 10.1182/blood-2007-06-098012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Del GMV, Brown JR, Certo M, et al. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–21. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mohammad RM, Goustin AS, Aboukameel A, et al. Preclinical studies of TW-37, a new nonpeptidic small-molecule inhibitor of Bcl-2, in diffuse large cell lymphoma xenograft model reveal drug action on both Bcl-2 and Mcl-1. Clin Cancer Res. 2007;13:2226–35. doi: 10.1158/1078-0432.CCR-06-1574. [DOI] [PubMed] [Google Scholar]
- 103.Liu G, Kelly WK, Wilding G, et al. An open-label, multicenter, phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin Cancer Res. 2009;15:3172–6. doi: 10.1158/1078-0432.CCR-08-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Z, Song W, Aboukameel A, et al. TW-37, a small-molecule inhibitor of Bcl-2, inhibits cell growth and invasion in pancreatic cancer. Int J Cancer. 2008;123:958–66. doi: 10.1002/ijc.23610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]