Abstract
Cytomegalovirus infections are an important cause of disease for which no licensed vaccine exists. Recent studies have focused on the gH/gL/UL128-131 complex as antibodies to gH/gL/UL128-131 neutralize viral entry into epithelial cells. Prior studies have used cells from the retinal pigment epithelium, while to prevent transmission, vaccine-induced antibodies may need to block viral infection of epithelial cells of the oral or genital mucosa. We found that gH/gL/UL128-131 is necessary for efficient viral entry into epithelial cells derived from oral and genital mucosa, that short peptides from UL130 and UL131 elicit high titer neutralizing antibodies in rabbits, and that such antibodies neutralize viral entry into epithelial cells derived from these relevant tissues. These results suggest that single subunits or peptides may be sufficient to elicit potent epithelial entry neutralizing responses and that secretory antibodies to such neutralizing epitopes have the potential to provide sterilizing immunity by blocking initial mucosal infection.
Keywords: Cytomegalovirus, Vaccine, Mucosal infection, Neutralizing antibody
1. Introduction
Congenital cytomegalovirus (CMV) infections are a frequent cause of birth defects and illness in transplant patients. Studies evaluating active or passive immunization to prevent or treat these infections have been completed. CMV hyperimmune globulin, which contains CMV-reactive antibodies induced by natural infection, appears effective for treating and preventing both congenital and transplant-associated infections [1,2]. Active immunization with either a live attenuated virus or a glycoprotein B subunit vaccine prevents CMV disease associated with renal transplantation [3] and reduces the risk of primary maternal CMV infection [4]. For both active and passive immunization, neutralizing activity is probably essential.
In the past CMV neutralizing activity was measured using fibroblasts as target cells. However, recent experiments demonstrate that antibodies to epitopes within a pentameric complex of gH, gL, UL128, UL130, and UL131 (gH/gL/UL128-131) potently neutralize entry into endothelial, epithelial, and other cell types but have no effect on fibroblast entry [5–8]. This is because the gH/gL/UL128-131 complex is essential for entry into endothelial and epithelial cells but fully dispensable for fibroblast entry [5,9,10]. Indeed, mutations causing loss of UL128, UL130, or UL131 expression are sufficient to eliminate endothelial tropism [9] and occur within relatively few passages in fibroblasts [11]. Natural infection elicits very high titer neutralizing antibodies specific for epithelial cell entry and it has been proposed that antibodies against gH/gL/UL128-131 epitopes may comprise a significant component of this activity [7,8,12]. In contrast, epithelial entry neutralizing titers induced by the Towne live attenuated vaccine or the gB subunit vaccine were 28- and 15-fold lower, respectively, than those induced by natural infection [12]. These results suggest that vaccine efficacy may be improved using antigens that elicit high titer epithelial entry neutralizing antibodies.
In this report we investigate two questions relevant to the design of effective vaccine strategies. First, do antibodies targeting gH/gL/UL128-131 complex neutralize viral entry into tissues relevant to vaccine protection? For example, mucosal and secretory antibodies that neutralize viral entry into epithelial cells of the oral or genital epithelium may prevent or reduce viral transmission. Second, will individual subunits suffice or will more than one subunit, perhaps the entire complex, be required for a vaccine? We observed that a functional gH/gL/UL128-131 complex is essential for efficient CMV entry into epithelial cells derived from both airway and genital mucosa, demonstrated that immunization of rabbits with short peptides derived from UL130 or UL131 is sufficient to achieve high epithelial entry neutralizing titers, and showed that these sera are effective at blocking CMV entry into mucosal epithelial cells. Our results indicate that subunit or peptide immunogens will elicit epithelial entry neutralizing responses and that successful active immunization may provide sterilizing immunity.
2. Materials and methods
2.1. Viruses
Virus HB15-t178b was derived from bacterial artificial chromosome (BAC) clone HB15Tn7Δk [13], which contains the CMV strain AD169 genome [14], by transposition of a green fluorescent protein (GFP) reporter cassette into the attTn7 site, as described [15]. Virus HB15-t178b retains a UL131 frame shift mutation intrinsic to strain AD169. Virus BADrUL131-Y4 (a gift from Thomas Shenk and Dai Wang) was derived from a different BAC clone of the CMV strain AD169 genome [16] that was first modified to express GFP [17] and then, by repair of the UL131 mutation, to express a functional UL131 protein [18]. Viral stocks were prepared from cell culture media that was clarified by centrifugation, adjusted to 0.2 M sucrose, aliquoted, stored at −80 °C, and titered on MRC-5 cells by limiting-dilution in 96-well plates as described [19].
2.2. Cells
Table 1 summarizes the cell lines used. MRC-5 (ATCC CCL-171), ARPE-19 (ATCC CRL-2302), and HBE4-E6/E7 (ATCC CRL-2078) cells were obtained from ATCC. HFK-2, Cx, V428, and HTE 21505 were derived and immortalized by retroviral transduction of human papilloma virus-16 E6E7 as previously described [20]. MRC-5 and ARPE-19 cells were propagated in high glucose Dulbecco’s modified Eagle medium (Gibco-BRL) supplemented with 10% fetal calf serum (HyClone Laboratories), 10,000 IU/L penicillin, 10 mg/L streptomycin (Gibco-BRL) (DMEM). HFK-2, Cx, V428, and HTE 21505 cells were propagated in keratinocyte serum free medium (KSFM, GIBCO 17005042) supplemented with 5 ng/ml human recombinant epidermal growth factor 1-53 (Invitrogen) and 0.05 mg/ml bovine pituitary extract (Invitrogen). HBE4-E6/E7 cells were propagated with KSFM supplemented with 5 ng/ml human recombinant epidermal growth factor 1-53, 0.05 mg/ml bovine pituitary extract, and 10 ng/ml cholera toxin (Sigma). All cell cultures were maintained at 37 °C in a 5% CO2 atmosphere.
Table 1.
Cell lines.
| Cell line | Tissue (cell type) | |
|---|---|---|
| MRC-5 | Fibroblast | Fetal lung |
|
| ||
| ARPE-19 | Epithelial | Retinal pigment epithelium |
| HFK-2 | Foreskin (keratinocyte) | |
| Cx | Cervix (keratinocyte) | |
| V428 | Vagina | |
| HTE 21505 | Tonsil | |
| HBE4-E6/E7 | Bronchus | |
2.3. Entry assay
Virus stocks were carefully titered using MCR-5 fibroblast cells, then matching amounts of HB15-t178b and BADrUL131-Y4 were used to infect replicate cultures of confluent cells prepared in 24-well plates. After 24 h the cultures were washed three times with PBS and fresh medium was added. Photomicrographs were taken daily post infection using an Olympus LX70 Inverted UV microscope.
2.4. Rabbit immunizations
Antisera were initially produced for the purpose of antigen detection and immunoprecipitation. The amino acid sequence of each protein was evaluated using computer algorithms that predict hydrophilic, antigenic, and surface exposed domains. From these results one peptide from each protein was selected based on empirical experience that N- or C-terminal positions, charged residues, and prolines are desirable. Peptides DQYLESVKKIHKRLDV (UL128 residues 147–162), SWSTLTANQNPSPPWSKLTY (UL130 residues 27–46), and SDFRRQNRRGGTNKRTT (UL131 residues 90–106) were synthesized with C-terminal cysteines by PeptidoGenics (Berkley, CA) and coupled to maleimide activated keyhole limpet hemocyanin (KLH) under conditions that produce conjugates in which the peptides comprise 15–30% of the mass. For each peptide one New Zealand White rabbit was immunized with 500–1000 μg of KLH-conjugated peptide mixed with Freund’s adjuvant, then boosted three times at 4–6 week intervals with decreasing doses of KLH-conjugated peptides (250 μg, 100 μg, and 50 μg) in Titer-Max Gold adjuvant (Sigma, St. Louis, MO). An isoleucine at position 10 of the UL128 peptide was unintentionally inserted. However, this does not prevent recognition of native UL128 (which lacks the isoleucine) by the UL128 antiserum. Indeed, all three antisera have been extensively characterized elsewhere and shown to react specifically with UL128, UL130, or UL131 by immunoprecipitation and immunoblotting [21,22].
2.5. Neutralization assays
Neutralizing activities were determined by preparing 1:10 dilutions of each serum followed by additional 2-fold serial dilutions in ARPE-19 culture medium. Each dilution was mixed with an equal volume of ARPE-19 culture medium containing 4000 pfu of BADrUL131-Y4, incubated for 1 h at 37 °C, then added to the wells of 384-well plates containing confluent ARPE-19 monolayers. Each serum was assayed in triplicate and representative photomicrographs were taken using a Nikon Eclipse TS100 inverted UV microscope at four days post infection. GFP fluorescence was measured seven days post infection using a PerkinElmer Victor3 V 1420 Multilable Counter. Fifty percent inhibitory concentration (IC50) values and standard errors of the means were calculated using Prism software (GraphPad Software, Inc.) by plotting the means of triplicate GFP values for each serum dilution against log2 serum concentration, calculating the best fit four-parameter equation for the data, and interpolating the serum dilution at the mid-point of the curve as the IC50 neutralizing titer. To evaluate neutralization of viral entry into mucosal epithelial cells rabbit anti-peptide sera were used at a 1:20 dilution and photomicrographs were taken seven days post infection.
3. Results
3.1. A functional gH/gL/UL128-131 complex is required for efficient CMV entry into epithelial cells from mucosal tissues
To determine the role of gH/gL/UL128-131 in CMV entry into epithelial cells from mucosal tissues, we compared the entry efficiencies of two GFP-tagged viruses (one expressing and one lacking the gH/gL/UL128-131 complex) by measuring the number of GFP+ cells observed at different times after infection. Strain AD169 is the standard laboratory/reference strain of CMV. It has a frame shift mutation in the UL131 gene that disrupts expression of the UL131 protein [9] and prevents formation and virion incorporation of the gH/gL/UL128-131 complex [5]. The two viruses used here, HB15-t178b and BADrUL131-Y4, are both AD169-derived, but while HB15-t178b retains the UL131 mutation and hence fails to express a virion-associated gH/gL/UL128-131 complex, repair of the UL131 gene in BADrUL131-Y4 restores UL131 expression and virion-incorporation of the gH/gL/UL128-131 complex [8].
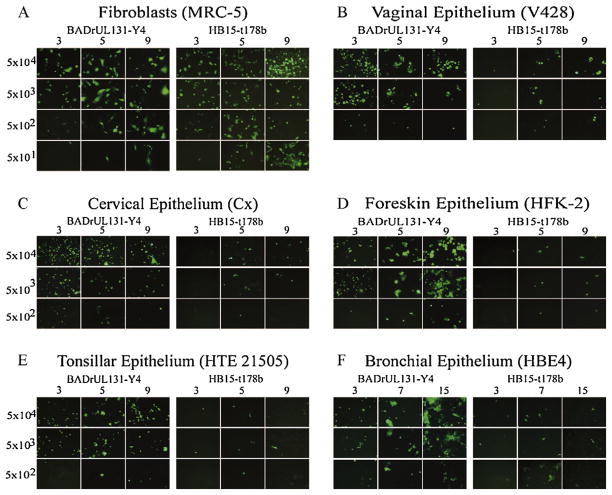
As shown in Fig. 1A, the two viral inocula were well matched for entry into MRC-5 fibroblasts even as the inocula were serially diluted down to low levels. Cells originating from genital mucosal tissues, including vagina, cervix, and foreskin, all displayed a pronounced requirement for gH/gL/UL128-131, as evidenced by high levels of GFP+ cells on day 3 following BADrUL131-Y4 infection and a virtual absence of GFP+ cells from cultures that received matching inocula of HB15-t178b (Fig. 1, panels B–D). Similar data were obtained with airway epithelial cells from tonsil and bronchus (Fig. 1, panels E and F). Foreskin and bronchial epithelial cells appeared to support the full replication cycle of BADrUL131-Y4, resulting in viral spread, as suggested by increased GFP expression in BADrUL131-Y4-infected cell cultures over time (Fig. 1, panels D and F). In contrast, the number of GFP+ cells remained stable over time in BADrUL131-Y4-infected vaginal, cervical, and tonsillar epithelial cells (Fig. 1, panels B, C, and E), suggesting a possible post-entry block to BADrUL131-Y4 replication in these cells.
Fig. 1.
Matching inocula of HB15-t178b and BADrUL131-Y4 were 10-fold serial diluted and added to wells of 24-well plates containing confluent cultures of the indicated cells. Cultures were monitored by fluorescence microscopy and photographed on the days indicated after infection. Numbers on the left indicate infectious viral dose (pfu/well).
3.2. Peptide immunogens elicit potent neutralizing activities in rabbits
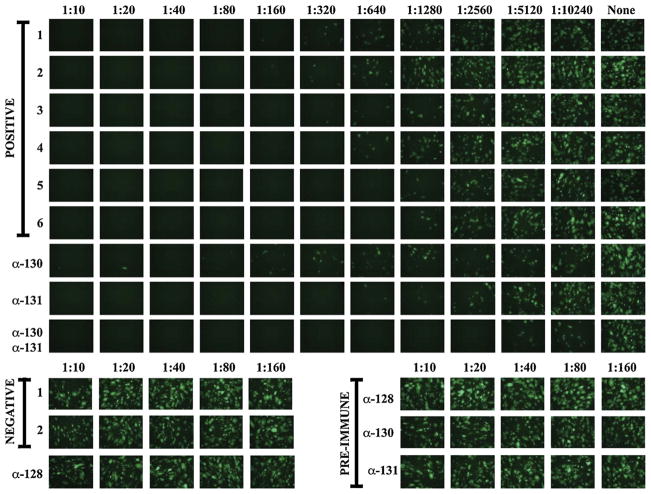
We determined if rabbit sera raised against peptides from UL128, UL130, or UL131 neutralized epithelial cell entry. The rabbit sera were evaluated using a GFP-based neutralizing assay similar to one developed to study sera from naturally infected or experimentally vaccinated humans [12]. Consistent with our previous report [12], sera from two CMV seronegative donors had no effect on epithelial entry, whereas seropositive sera from six naturally infected donors blocked epithelial entry even out to dilutions of 1:640 (Fig. 2). Sera obtained from all three rabbits prior to immunization as well as antiserum to the UL128 peptide failed to neutralize epithelial cell entry at any concentration (Fig. 2). Rabbit antisera to UL130 or UL131 peptides neutralized epithelial entry with activities within the range defined by the seropositive sera; however, a 50:50 mixture of the anti-UL130 and anti-UL131 sera retained neutralizing activity when diluted four-fold higher than the strongest seropositive human serum (Fig. 2). All three rabbit sera failed to neutralize fibroblast entry at any concentration (Fig. 4 and data not shown).
Fig. 2.
The indicated dilutions of sera from six CMV seropositive and two CMV seronegative human subjects, postimmune rabbit anti-peptide sera, and corresponding preimmune rabbit sera were incubated with 4000 pfu of virus BADrUL131-Y4 for 1 h, then used to infect ARPE-19 epithelial cells. In the bottom row (top panel) equal amounts of rabbit anti-UL130 and anti-UL131 were mixed before being assayed as for the other sera. No serum was added to the wells in the right-most column (top panel). Representative micrographs were taken with a fixed exposure four days post infection.
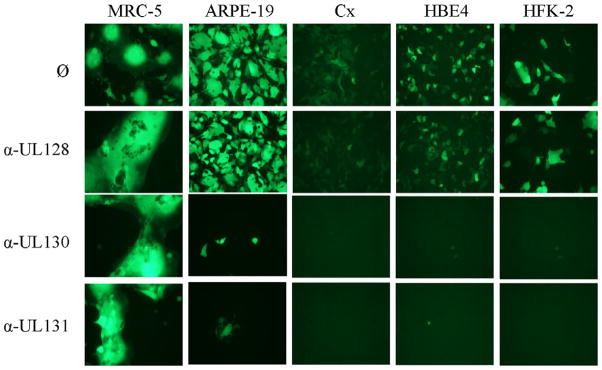
Fig. 4.
Replicate amounts of BADrUL131-Y4 were mixed with no serum (ø) or 1:20 dilutions of the indicated rabbit anti-peptide antisera. After 1 h incubation the mixtures were added to confluent cultures containing the indicated cells and the cultures were monitored daily by fluorescence microscopy. Photographs shown are from day seven post infection.
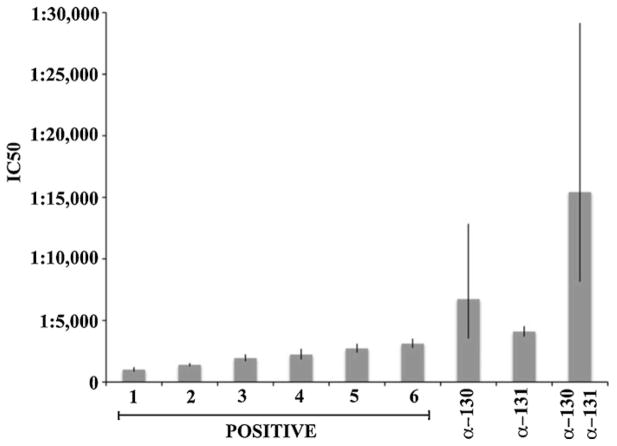
GFP fluorescence was used to calculate neutralizing titers, assessed as IC50 values, for each serum or serum combination (see Section 2). Titers for the six seropositive sera ranged from 1:1007 to 1:3118. Titers for the antiserum to UL130 (1:6732) or UL131 (1:4096) were slightly above the range defined by the seropositive sera, while that of the UL130 + UL131 combination (1:15421) was considerably higher (Fig. 3).
Fig. 3.
IC50 values for the same six seropositive human sera shown in Fig. 2, the postimmune rabbit anti-UL130 and -UL131 sera, and the mixture of anti-UL130/UL131 sera were calculated using GFP fluorescence values measured from triplicate assays seven days post infection. Error bars indicate standard errors of the means.
3.3. Antibodies to UL130 and UL131 peptides neutralize CMV entry into epithelial cells from mucosal tissues
To directly confirm that proteins comprising the gH/gL/UL128-131 complex must be physically present on the virion surface to facilitate viral entry into these cells, we determined the ability of rabbit anti-peptide sera to block viral entry. As before, the three antisera had no effect on BADrUL131-Y4 entry into fibroblasts and the anti-UL130 and anti-UL131 sera potently inhibited entry to ARPE-19 epithelial cells while the anti-UL128 serum did not (Fig. 4). That entry into epithelial cells from cervix, foreskin, and bronchus was highly sensitive to neutralization by both the anti-UL130 and the anti-UL131 sera (Fig. 4) physically confirmed that entry into these cell types involves UL130 as well as UL131.
3.4. The UL128 and UL131 peptides are highly conserved among CMV isolates
Antigenic variation is important for any potential vaccine immunogen. The UL128-131 proteins are known to be highly conserved between CMV strains [23], but to specifically determine amino acid variability within the UL128, UL130, and UL131 peptides, DNA sequences from 29 distinct strains available from GenBank were translated and aligned using ClustalW. Nine amino acid positions in UL128 and three in UL131 were polymorphic, but within the UL128 and UL131 peptide regions the amino acid sequences were 100% identical. UL130 was more variable with 19 polymorphic positions resulting in five variants within the UL130 peptide region, as shown in Table 2. These results suggest that antibodies to the UL131 peptide should cross neutralize the majority of CMV strains, whereas antisera raised against the UL130 peptide might be less effective at neutralizing strains expressing different UL130 variants.
Table 2.
Polymorphisms within the UL130 peptide.
| Variant | UL130 peptide sequencea | Number of strains |
|---|---|---|
| 1 | SWSTLTANQNPSPPWSKLTYb | 2 |
| 2 | PWSTLTANQNPSPPWSKLTY | 11 |
| 3 | PWFTLTANQNPSPPWSKLTY | 1 |
| 4 | PWSTLTANKNPSPPWSKLTY | 6 |
| 5 | PWSTLTANQNPSPLWSKLTY | 9 |
Amino acid changes relative to the reference strain are shown in bold.
Reference strain TR (from which the sequence for the UL130 peptide was derived).
4. Discussion
In previous studies we and others observed that sera from CMV-infected humans have two neutralizing activities; one is a moderate activity, comprised mostly of antibodies to gB, that neutralizes viral entry into fibroblasts. The other is more potent and neutralizes viral entry into epithelial cells [7,12]. The antigen specificities of the latter are unknown, but because both the gH/gL/UL128-131 complex and this neutralizing activity are specific to epithelial cell entry, the antibodies that comprise the epithelial entry neutralizing activity presumably target gH/gL/UL128-131.
Our results further suggest that a vaccine that incorporates gH/gL/UL128-131 epitopes to induce epithelial entry neutralizing activities might be effective at preventing viral acquisition through mucosal epithelia. This presumes, however, that infection of mucosal epithelial cells is gH/gL/UL128-131-mediated and hence neutralizable with gH/gL/UL128-131-specific antibodies. To date, the majority of work on the mechanism and neutralizing activities against epithelial entry have used ARPE-19 cells, which are derived from the retinal pigment epithelium of the eye. The importance of gH/gL/UL128-131 for viral entry has also been confirmed for tumor cells of epithelial origin derived from breast, cervix, lung, and colon [18]. Here, we evaluated CMV entry into cells derived from tissues believed to be most relevant to CMV acquisition –airway and genital mucosa – and in all cases found that entry is gH/gL/UL128-131-dependent. We further observed using a subset of cell lines that entry can be blocked by antibodies to epitopes within the gH/gL/UL128-131 complex. These results support the hypothesis that a vaccine that elicits epithelial entry-specific neutralizing responses in mucosal secretions may provide sterilizing immunity.
Little is known about the neutralizing epitopes within the gH/gL/UL128-131 complex, and of central importance for vaccine design, it remains uncertain whether conformational epitopes unique to the full gH/gL/UL128-131 complex will be required, or whether subunits or even peptides will be sufficient to elicit neutralizing activities comparable to natural infection. Some evidence suggests that neutralizing epitopes may often require multisubunit complexes. A recently described panel of 17 human monoclonals having potent neutralizing activities against epithelial entry predominantly recognize epitopes that require two or more subunits – only one of the 17 antibodies reacted with an individual subunit [8]. In addition, the Towne virus expresses UL128 and UL131, but expression of UL130 is impaired by a C-terminal frame shift that alters the protein’s stability and steady-state levels [24]. Yet, despite the presumed ability to express UL128 and UL131 in vivo, the Towne virus does not elicit high titer neutralizing antibodies specific for epithelial entry [12]. This may be because the absence of UL130 results in retention of the remainder of the complex (gH/gL/UL128/UL131) in the endoplasmic reticulum and subsequent failure of this complex to traffic to the cell surface or become incorporated into virions [21]. Thus, for a live attenuated vaccine, UL128 and UL131 are not sufficient.
Alternatively, animal antibodies raised against individual UL128, UL130, or UL131 peptides or recombinant proteins do neutralize epithelial or endothelial cell entry, indicating that each subunit contains neutralizing epitopes [5–7]. However, potency of animal antisera relative to human immune sera has not been reported. We observed that peptide epitopes within UL130 or UL131 can elicit epithelial entry neutralizing activities comparable to those induced by natural infection when administered to rabbits using optimal adjuvants. This indicates that the gH/gL/UL128-131 complex contains at least two potent neutralizing epitopes that do not require multisubunit complexes. While the anti-UL128 peptide serum did not neutralize, the peptide used to raise this serum contained an inadvertent isoleucine insertion, and although it retains epitopes sufficient for the antiserum to recognize the native protein [21], the possibility remains that the isoleucine disrupts a neutralizing epitope. Moreover, as UL128, UL130, and UL131 are respectively 171, 235, and 129 amino acids long, significant regions of these proteins have not been evaluated and may contain additional neutralizing epitopes. Indeed, that at least two of the three peptides studied contain neutralizing epitopes suggests that there may be many more.
Our data do not necessarily imply that a vaccine based on the peptide epitopes described here would be effective in humans. Development of such a vaccine would face several hurdles. First, the rabbit immunization protocol used here was designed to elicit maximal antibody responses and cannot be recapitulated in humans. To achieve comparable antibody responses in humans it may be necessary to utilize alternative adjuvants, carriers, or vector systems that are being developed specifically to elicit robust responses to peptide epitopes. Second, it is not known whether these particular epitopes are immunogenic in humans. Indeed, peptide-based ELISAs failed to detect antibodies reactive to these peptides in a small panel of seropositive human sera (Cui and McVoy, unpublished results). However, vaccination may be more effective than infection at eliciting anti-peptide antibody responses, and, given that monoclonals that neutralize this entry pathway are exceedingly potent [8], it is possible that low antibody levels could confer significant neutralizing activities. Third, the UL130 peptide exhibits strain heterogeneity and thus antibodies to this epitope may not cross-neutralize all CMV strains. Thus, while it may be possible to overcome these obstacles using novel immunization strategies and inclusion of additional or alternative epitopes, the more instructive implication of our results for vaccine development is that peptide or single subunit immunogens have the potential to produce high titer epithelial entry neutralizing responses, and hence, representation of complex conformational epitopes may not be necessary.
Although theoretically compelling, the premise that epithelial entry neutralizing antibodies can protect against infection is supported mainly by evidence that naturally acquired humoral immunity, which has high epithelial entry neutralizing activity, provides clinical benefits [2,25,26], whereas experimental vaccines that induce weak epithelial entry neutralizing responses (compared to natural infection) [12] provide either partial [4] or no protection against primary infection [27]. Thus, our use of seropositive sera as a benchmark for evaluating immunogens is somewhat arbitrary; neutralizing activities comparable to those found in seropositive sera may not provide adequate protection, and while higher levels may be achievable and might enhance protection, other factors, such as cellular immunity or antibodies that neutralize fibroblast entry, may also be important. Ultimately, the importance of epithelial entry neutralizing antibodies for CMV vaccine protection may only be resolved through clinical trials of candidate vaccines that elicit neutralizing activities equivalent or superior to natural infection. The data presented here may aid in development of such candidate vaccines.
Acknowledgments
We thank Ronzo Lee for technical assistance, Dai Wang and Thomas Shenk for providing BADrUL131-Y4 BAC, David Gewirtz for use of the LX70 Inverted UV microscope, and Huiping Zhou for use of the Victor3 V 1420 Multilable Counter. This work was supported in part by NIH/NIAID grant R21AI073615 (to MM), NIH/NIAID fellowship 1F31A073209 (to AS), and a Summer Research Fellowship Award from the Virginia Commonwealth University School of Medicine (to AA).
References
- 1.Snydman DR. Historical overview of the use of cytomegalovirus hyperimmune globulin in organ transplantation. Transpl Infect Dis. 2001;3(Suppl 2):6–13. doi: 10.1034/j.1399-3062.2001.00002.x. [DOI] [PubMed] [Google Scholar]
- 2.Nigro G, Adler SP, La Torre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353(13):1350–62. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 3.Plotkin SA, Starr SE, Friedman HM, Gonczol E, Weibel RE. Protective effects of Towne cytomegalovirus vaccine against low-passage cytomegalovirus administered as a challenge. J Infect Dis. 1989;159(5):860–5. doi: 10.1093/infdis/159.5.860. [DOI] [PubMed] [Google Scholar]
- 4.Plotkin SA, Higgins R, Kurtz JB, Morris PJ, Campbell DA, Jr, Shope TC, Spector SA, Dankner WM. Multicenter trial of Towne strain attenuated virus vaccine in seronegative renal transplant recipients. Transplantation. 1994 December;58(11):1176–8. [PubMed] [Google Scholar]
- 5.Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci USA. 2005 December;102(50):18153–8. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adler B, Scrivano L, Ruzcics Z, Rupp B, Sinzger C, Koszinowski U. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J Gen Virol. 2006 September;87(Pt 9):2451–60. doi: 10.1099/vir.0.81921-0. [DOI] [PubMed] [Google Scholar]
- 7.Gerna G, Sarasini A, Patrone M, Percivalle E, Fiorina L, Campanini G, et al. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J Gen Virol. 2008 April;89(Pt 4):853–65. doi: 10.1099/vir.0.83523-0. [DOI] [PubMed] [Google Scholar]
- 8.Macagno A, Bernasconi NL, Vanzetta F, Dander E, Sarasini A, Revello MG, et al. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol. 2010 January;84(2):1005–13. doi: 10.1128/JVI.01809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahn G, Revello MG, Patrone M, Percivalle E, Campanini G, Sarasini A, et al. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol. 2004 September;78(18):10023–33. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryckman BJ, Jarvis MA, Drummond DD, Nelson JA, Johnson DC. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J Virol. 2006 January;80(2):710–22. doi: 10.1128/JVI.80.2.710-722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dargan DJ, Douglas E, Cunningham C, Jamieson F, Stanton RJ, Baluchova K, et al. Sequential mutations associated with adaptation of human cytomegalovirus to growth in cell culture. J Gen Virol. 2010 June;91(Pt 6):1535–46. doi: 10.1099/vir.0.018994-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui X, Meza BP, Adler SP, McVoy MA. Cytomegalovirus vaccines fail to induce epithelial entry neutralizing antibodies comparable to natural infection. Vaccine. 2008 August;26:5760–6. doi: 10.1016/j.vaccine.2008.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sauer A, Wang JB, Hahn G, McVoy MA. A human cytomegalovirus deleted of internal repeats replicates with near wild type efficiency but fails to undergo genome isomerization. Virology. 2010 May;401(1):90–5. doi: 10.1016/j.virol.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hobom U, Brune W, Messerle M, Hahn G, Koszinowski UH. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J Virol. 2000;74(17):7720–9. doi: 10.1128/jvi.74.17.7720-7729.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn G, Jarosch M, Wang JB, Berbes C, McVoy MA. Tn7-mediated introduction of DNA sequences into bacmid-cloned cytomegalovirus genomes for rapid recombinant virus construction. J Virol Methods. 2003 February;107(2):185–94. doi: 10.1016/s0166-0934(02)00232-x. [DOI] [PubMed] [Google Scholar]
- 16.Yu D, Smith GA, Enquist LW, Shenk T. Construction of a self-excisable bacterial artificial chromosome containing the human cytomegalovirus genome and mutagenesis of the diploid TRL/IRL13 gene. J Virol. 2002;76(5):2316–28. doi: 10.1128/jvi.76.5.2316-2328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Bresnahan W, Shenk T. Human cytomegalovirus encodes a highly specific RANTES decoy receptor. Proc Natl Acad Sci USA. 2004 November 47;101:16642–7. doi: 10.1073/pnas.0407233101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Shenk T. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol. 2005 August;79(16):10330–8. doi: 10.1128/JVI.79.16.10330-10338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui X, McGregor A, Schleiss MR, McVoy MA. Cloning the complete guinea pig cytomegalovirus genome as an infectious bacterial artificial chromosome with excisable origin of replication. J Virol Methods. 2008 May 2;149:231–9. doi: 10.1016/j.jviromet.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zabner J, Karp P, Seiler M, Phillips SL, Mitchell CJ, Saavedra M, et al. Development of cystic fibrosis and noncystic fibrosis airway cell lines. Am J Physiol Lung Cell Mol Physiol. 2003 May;284(5):L844–54. doi: 10.1152/ajplung.00355.2002. [DOI] [PubMed] [Google Scholar]
- 21.Ryckman BJ, Rainish BL, Chase MC, Borton JA, Nelson JA, Jarvis MA, et al. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J Virol. 2008 January;82(1):60–70. doi: 10.1128/JVI.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryckman BJ, Chase MC, Johnson DC. Human cytomegalovirus TR strain glycoprotein O acts as a chaperone promoting gH/gL incorporation into virions but is not present in virions. J Virol. 2010 March;84(5):2597–609. doi: 10.1128/JVI.02256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldanti F, Paolucci S, Campanini G, Sarasini A, Percivalle E, Revello MG, et al. Human cytomegalovirus UL131A, UL130 and UL128 genes are highly conserved among field isolates. Arch Virol. 2006 June;151(6):1225–33. doi: 10.1007/s00705-005-0696-5. [DOI] [PubMed] [Google Scholar]
- 24.Patrone M, Secchi M, Fiorina L, Ierardi M, Milanesi G, Gallina A. Human cytomegalovirus UL130 protein promotes endothelial cell infection through a producer cell modification of the virion. J Virol. 2005 July 13;79:8361–73. doi: 10.1128/JVI.79.13.8361-8373.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adler SP, Chandrika T, Lawrence L, Baggett J. Cytomegalovirus infections in neonates acquired by blood transfusions. Pediatr Infect Dis. 1983 March–April;2(2):114–8. doi: 10.1097/00006454-198303000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Yeager AS, Grumet FC, Hafleigh EB, Arvin AM, Bradley JS, Prober CG. Prevention of transfusion-acquired cytomegalovirus infections in newborn infants. J Pediatr. 1981 February;98(2):281–7. doi: 10.1016/s0022-3476(81)80662-2. [DOI] [PubMed] [Google Scholar]
- 27.Adler SP, Starr SE, Plotkin SA, Hempfling SH, Buis J, Manning ML, et al. Immunity induced by primary human cytomegalovirus infection protects against secondary infection among women of childbearing age. J Infect Dis. 1995;171(1):26–32. doi: 10.1093/infdis/171.1.26. [published erratum appears in J Infect Dis 1995 Apr;171(4):1080] [DOI] [PubMed] [Google Scholar]