Abstract
This study examined patterns of neural response to feedback received during simulated interpersonal interactions in adolescents with anxiety disorders and healthy peers. To this aim, behavioral and neural responses during the Prisoner’s Dilemma (PD) game, an economic exchange task, were compared between adolescents with anxiety disorders (N=12) and healthy controls (n=17). Participants were deceived to believe that their co-player (a pre-programmed computer algorithm) was another study participant. Anxious participants and controls differed significantly in patterns of neural activation in the medial prefrontal cortex (mPFC), ACC, precuneus, insula, and temporoparietal junction (TPJ) when receiving feedback about co-player defection or cooperation. Groups also differed significantly in post-feedback behavior; specifically anxious adolescents were more likely than controls to cooperate following trials when the co- player betrayed them. Our findings provide preliminary evidence that, in social situations, anxious adolescents may not only behave differently than healthy peers, but they may also engage neural resources in different ways. These findings constitute a first step toward elucidating mechanisms underlying social impairment in youth with internalizing disorders.
Keywords: fMRI, anxiety, depression, cooperation, betrayal, interpersonal interaction, Prisoner’s Dilemma, medial prefrontal cortex, precuneus, temporoparietal junction
Individuals with social fears show atypical cognitive and emotional responses to social signals, particularly when they interpret those signals as cues of negative or ambiguous interpersonal feedback. For example, they exhibit both a bias to over-attend to interpersonal threat cues such as angry faces and a hypersensitivity to criticism or rejection (Harb, Heimberg, Fresco, Schneier, & Liebowitz, 2002; Mogg, Bradley, Millar, & White, 1995; Mogg, Philippot, & Bradley, 2004). Such cognitive/emotional manifestations of anxiety, which increase risk for maladaptive behaviors including withdrawal, passivity, and avoidance (London, Downey, Bonica, & Paltin, 2007; Schneider, 2009), may relate to aberrant patterns of neural function (Cannistraro & Rauch, 2003).
A sizable literature consistently demonstrates that neural responses to self-irrelevant social signals, such as negative emotional face photographs presented outside of a social context, differ between anxious and healthy individuals, with anxious individuals showing exaggerated amygdala and attenuated prefrontal cortex (PFC) responsiveness (Goldin, Manber, Hakimi, Canli, & Gross, 2009; Killgore & Yurgelun-Todd, 2005; McClure, Monk, et al., 2007; Monk, et al., 2008; Shin, et al., 2005; Stein, Goldin, Sareen, Zorrilla, & Brown, 2002; Stein, Simmons, Feinstein, & Paulus, 2007). Findings from at least two recent studies of anxiety-disordered adults and adolescents also document perturbations in these regions when negative social cues appear in more complex, self-relevant contexts (e.g., faces or words explicitly conveying critical or rejecting feedback to the participant) (Blair, et al., 2008; Guyer, et al., 2008).
Notably, although the two studies that compared neural responses to self-relevant negative social cues between anxious individuals and controls focused primarily on the amygdala and prefrontal cortical regions (Blair, et al., 2008; Guyer, et al., 2008), both yielded evidence that group differences extend to a broader network of neural structures. Specifically, Blair and colleagues (2008) found that, while reading negative self-referential statements (e.g., “you are ugly”), adults with generalized social phobia showed significantly more activation than controls not only in the medial PFC and amygdala, but also in the ACC and bilateral precuneus. In Guyer et al.’s (2008) study, which focused on amygdala activation during receipt of negative evaluations from valued peers, amygdala hyperactivity was evident in anxiety-disordered adolescents with prominent social concerns relative to healthy peers. Additionally, however, the authors found group differences in patterns of coactivation between the amygdala and several regions. Specifically, patients showed increased amygdala-ventrolateral PFC (vlPFC) coactivation relative to controls and controls showed evidence of stronger amygdala connectivity with bilateral precuneus, left insula, bilateral parahippocampal gyrus, and cerebellum.
Taken together, these findings provide suggestive evidence that anxious individuals, particularly those with social fears, may respond atypically to self-relevant negative social cues in a broad, loosely integrated network of brain regions thought to be engaged during reflection about self and others. These regions extend beyond the amygdala and PFC to include the ACC; the precuneus; the temporoparietal junction (TPJ); the insula, and the temporal poles (Amodio & Frith, 2006; Craig, 2009; Frith & Frith, 2003; Legrand & Ruby, 2009; Lombardo, et al., 2009; Mitchell, Banaji, & Macrae, 2005; Wolf, Dziobek, & Heekeren, 2010).
To elicit activation in these regions, some researchers have used dynamic, interactive tasks that have the advantage of closely approximating real-life social interactions. Reciprocal economic exchange games [e.g., the Prisoner’s Dilemma (PD) game, the Trust game], for example, have been shown to activate neural regions implicated in self/other reflection, including medial PFC, ACC, right TPJ, and insula, although specific findings vary depending on the precise task and contrast conditions examined (Kircher, et al., 2009; Rilling, Goldsmith, et al., 2008; Rilling, et al., 2002; Rilling, Sanfey, Aronson, Nystrom, & Cohen, 2004; van den Bos, van Dijk, Westenberg, Rombouts, & Crone, 2009) These tasks consist of simple, but real and consequential, social interactions that require sophisticated interpersonal reasoning and decisions. Therefore, they permit investigation of complex interpersonal processes such as betrayal and cooperation and their behavioral and neural correlates (Sanfey, 2007).
Only recently have researchers begun to measure associations among economic exchange game play, neural activation, and emotional/personality characteristics. For example, findings link atypical anterior insula, ACC, and amygdala activation with poor cooperation during the Trust Game in adults with borderline personality disorder (King-Casas, et al., 2008; Seres, Unoka, & Kéri, 2009). Research using the PD game in healthy adults has also shown that individuals who report high levels of psychopathy show attenuated right amygdala activation in response to unreciprocated cooperation, and diminished ACC and dorsolateral PFC activity when choosing to defect (Rilling, et al., 2007). In the one published study to examine neural activation in anxiety-disordered adults during a reciprocal exchange task, Sripada and colleagues (2009) found attenuated activation in the medial PFC in adults with social anxiety disorder relative to controls when participants imagined that they were playing the Trust Game with a real person (versus with a computer).
Neuroimaging studies of economic exchange game play in adolescents, particularly those with psychopathology, are uncommon, which leaves open questions about whether and how patterns of behavior and associated neural activation manifest before adulthood. Additionally, regardless of participant age, studies have varied in the economic exchange games used and the contrast conditions selected, with many studies averaging activation across the course of entire games. Although this is a logical approach for some comparisons, such as neural responses during play with humans versus computers (Kircher, et al., 2009), it does not permit examination of more finely-grained differences in brain activity during specific trial types. Studies of healthy adults that have compared activation during different types of trials have also yielded interesting findings that can be more tightly linked to specific patterns of social behavior (Rilling, Dagenais, Goldsmith, Glenn, & Pagnoni, 2008; Rilling, et al., 2007; Rilling, Goldsmith, et al., 2008; Rilling, et al., 2002), which suggests that this approach holds merit as well.
The present study takes a first step toward characterizing anxious adolescents’ neural, behavioral, and emotional responses during the context of PD game play. Given evidence that anxious adolescents respond distinctively to unwelcome interpersonal feedback (Guyer, et al., 2008), we focused explicitly on neural activation during feedback regarding co-player responses. We hypothesized that anxious adolescents would differ from healthy adolescents in their neural responses to co-player defection but not cooperation in regions associated with self/other reflection: medial PFC, ACC, insula, TPJ, and precuneus; as well as the amygdala. Additionally, we predicted that anxious participants’ behavioral and emotional responses to negative feedback would differ from those of controls. To test this prediction, we compared participants’ patterns of cooperation and defection after they received different kinds of feedback, as well as their emotional responses to the other player over the course of play.
Method
Participants
Using methods described previously (McClure, Monk, et al., 2007), we recruited 26 adolescents with anxiety disorders and 39 psychiatrically healthy youth. Participants for whom a) post-task debriefing indicated that they had not believed they were playing a real co-player (anxious: n = 7; controls: n = 11) or b) fMRI data were problematic (e.g., motion artifact, equipment malfunction, or incomplete data acquisition; anxious: n = 6; controls: n = 12) were excluded. Thus, data were analyzed from 12 anxious and 17 healthy adolescents. All patients were enrolled in an ongoing treatment study of anxious youth that was approved by the National Institute of Mental Health Institutional Review Board. Prior to participation, parents provided written informed consent and youths granted written assent. Participants and parents were informed at consent that they would receive misinformation during the study; debriefing at the end of the study indicated no adverse reactions due to deception or other aspects of the study.
Each participant and a parent was administered the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS-PL) (Kaufman, et al., 1997) to determine psychiatric diagnoses. All K-SADS-PL interviews were conducted by clinicians with excellent inter-rater reliability (all kappa values > .90). To be included in the anxious group, participants had to meet DSM-IV criteria for an anxiety disorder; exhibit a high level of symptoms, as indicated by a score > 9 on the Pediatric Anxiety Rating Scale (PARS) (RUPP, 2001) that persisted over a three-week trial of supportive therapy; and show impaired global function as indicated by a score < 60 on the Children’s Global Assessment Scale (CGAS; (Shaffer, et al., 1983). Exclusion criteria specific to the anxious group consisted of: use of any psychotropic medication; primary DSM-IV psychiatric diagnoses other than anxiety disorders, MDD, attention deficit hyperactivity disorder (ADHD) or oppositional defiant disorder. Controls were required to be free of psychiatric diagnoses and psychotropic medication. Exclusion criteria for both groups consisted of medical illness; pregnancy; substance abuse; history of head injury involving loss of consciousness, or IQ<70.
All 12 anxious participants met DSM-IV criteria for at least one of the following three anxiety disorders: generalized anxiety disorder (GAD; n = 7), separation anxiety disorder (SAD; n = 5), social phobia (n = 3). Of these participants, seven were diagnosed with two or more of these anxiety disorders; five of these seven also had comorbid diagnoses of specific phobia. Whether or not they met full diagnostic criteria for social phobia, all participants reported clinically significant social/performance fears on the PARS and/or the K-SADS-PL.
In addition to primary anxiety disorders, five anxious patients had secondary comorbid diagnoses of MDD (n=2), ADHD (n=3), and/or ODD (n=1). All controls were free of current or past psychiatric disorders. As detailed in an earlier publication (McClure, Parrish, et al., 2007) and consistent with prior research (Birmaher, et al., 2003; Kendall, et al., 1997; RUPP, 2001), we combined participants with three disorders, GAD, social phobia, and SAD, into a single anxiety-disorders group. We elected to do so because of the high rates of comorbidity among patients, the limited number of participants meeting criteria for only one disorder (n=2), small numbers of participants within each specific diagnostic category, and to maintain consistency with the approach used in our prior study with the PD (McClure, Parrish, et al., 2007).
Groups did not differ according to age, t(27)=1.29, p> .05, sex, χ2(1)=0.36, p> .05, or IQ, t(26)= 0.48, p> .05 (See Table 1 for all demographic information and behavioral data).
Table 1.
Demographic/Clinical Characteristics and Task Performance of Anxious Patients and Healthy Controls.
| Patients (n=12) | Controls (n=17) | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age | 12.8 (2.5) | 13.9 (1.9) |
| IQ Score | 112.8 (15.8) | 112.9 (10.0) |
| Baseline PARS score | 13.7 (1.9) | -- |
| Baseline CDRS raw score | 26.7 (5.6) | -- |
| N(%_ | N(%) | |
| Sex | ||
| Race/Ethnicity | ||
| Caucasian (non-Latino) | 9 (69) | 13 (76) |
| African American | 1 (8) | 1 (6) |
| Latino | 0 (0) | 0 (0) |
| Mixed/Other | 3 (23) | 3 (18) |
| DSM-IV Diagnoses | ||
| Generalized Anxiety Disorder |
6 (46) | -- |
| Separation Anxiety Disorder |
5 (38) | -- |
| Social Phobia | 4 (31) | -- |
| Specific Phobia | 5 (38) | |
| Major Depressive Disorder | 3 (23) | -- |
| Mean (SD) | Mean (SD) | |
| Games Won | 2.67 (1.07) | 2.82 (1.29) |
| Average Earnings | ||
| Game 1 | 29.25 (4.37) | 29.77 (4.02) |
| Game 2 | 31.17 (4.82) | 28.88 (4.82) |
| Game 3 | 32.25 (5.94) | 31.71 (4.50) |
| Game 4 | 27.83 (6.19) | 31.00 (6.87) |
| All Games | 30.13 (2.79) | 30.34 (2.48) |
| Percent of Trials1 | ||
| Mutual Cooperation (CC) | 18 (10) | 15 (13) |
| Co-player Betrayal (CD) | 19 (7) | 12 (8) |
| Player Betrayal (DC) | 26 (5) | 26 (8) |
| Mutual Defection (DD) | 18 (10) | 15 (13) |
| Cooperate following Co-player | 20 (11) | 18 (15) |
| Cooperation | ||
| Cooperate following Co-player Defection | 17 (8) | 9 (7) |
| Feeling Toward Co-player Ratings (0–100 Scale) |
||
| Following First 5 Trials | 63.04 (18.95) | 66.49 (13.41) |
| Following Final 5 Trials | 49.75 (15.46) | 66.15 (22.80) |
| Overall mean ratings | 56.74 (14.42) | 65.91 (14.64) |
Note that percents are means across participants for each trial type; consequently the percent values do not sum to 100.
Procedures
While undergoing an MRI scan, participants played a version of the iterated PD game (Rilling, et al., 2002). Each participant played the game four times with a computerized confederate, whom they had been deceived to believe was a human co-player at a remote location. In the interest of clarity, we identify the four PD games that each participant played as “runs” in the rest of the manuscript. An examiner told each participant at the beginning of the session that during the fMRI scan he or she would play a game with a study participant at another research site via a wireless computer network. Participants received no further information about the co-player. The examiner then trained participants, explaining that they must decide whether or not to cooperate with their co-player. Depending on the conjunction of players’ choices, each would receive payments: mutual cooperation yielded two dollars for both players, mutual non-cooperation (defection) yielded one dollar for both players, and trials in which one player cooperated and the other defected yielded nothing for the cooperator and three dollars for the defector. During training each participant completed 10 practice trials. Participants were informed during training that after completing the scan they would be paid the amount that they earned during one of the four runs (selected randomly at the end of the fMRI scan).
We have described the PD game in detail in a prior publication (McClure, Parrish, et al., 2007); briefly, during each of 20 trials within a run, two players independently and simultaneously cooperate with or defect from each other. There are thus four possible trial outcomes: mutual cooperation (CC trials), mutual defection (DD trials), participant defects/co-player cooperates (DC trials), and participant cooperates/co-player defects (CD trials). The computerized co-player uses an algorithm (Rilling, et al., 2002) to generate its “choice.” This “choice” varies according to the human participant’s choices in the prior two trials; to ensure that each participant experiences periodic defection by the co-player there is also a 50% likelihood that the computer will defect after four consecutive rounds of mutual cooperation. To provide some consistency across players, the computer always cooperates during the first trial and defects during the final two trials. After both players submit their choices, the computer screen displays the trial outcome and running totals of both players’ cumulative earnings. As the extensive literature on the iterated PD task shows, different strategies of play yield different short and long-term results (Axelrod & Hamilton, 1981; Imhof, Fudenberg, & Nowak, 2007). For example, in the present game version, players obtain the highest long-term payoff by both cooperating across all trials. However, the short-term (single trial) payoff for an individual player is highest if he/she defects and the co-player cooperates.
After every five trials, participants rated (on 100-point scales 1--most negative, 100--most positive) how they felt toward the co-player, generating a set of peer-rating variables. For each participant, ratings at each point (after trials 5, 10, 15, and 20) were averaged across all four runs. Mean initial rating and mean final rating served as dependent variables in the present study to facilitate examination of changes in co-player perceptions over time. After the MRI scan, an examiner asked participants if they had believed that they were playing a real peer; participants were then debriefed about the deception involved in the task and the why it was used, following guidelines for ethically appropriate authorized deception (Wendler & Miller, 2004). In the present study, no significant differences on demographic or behavioral variables were evident between deceived and non-deceived adolescents (all p.’s > .05).
Measures
The PD game was presented in four nine-minute runs, each of which consisted of 20 game trials and 4 fixation trials. The game trials (see Figure 1) varied in duration from 11.5 to 16.1 seconds and each included three components: a) a 4600 ms selection component, b) an interval that varied from 2300 to 9600 ms, and c) a 4600 ms feedback component. Four fixation trials varying in duration from 11.5 to 16.1 seconds appeared randomly during each run to provide a baseline. The inter-trial interval was 1 second.
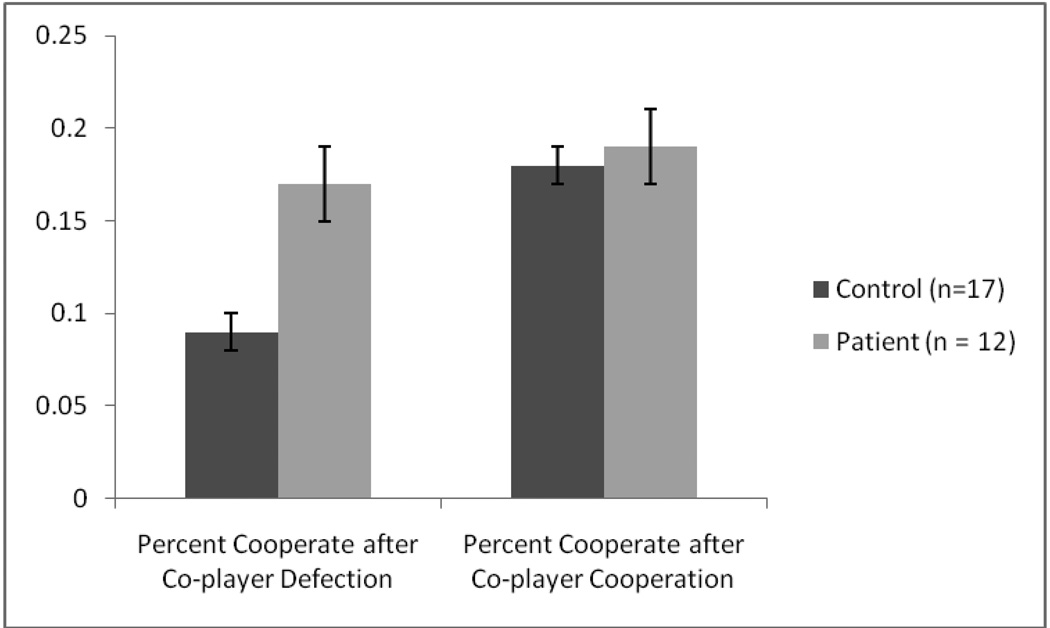
Figure 1.
Group differences in cooperative responses following co-player cooperation and co-player defection.
To address our primary hypotheses, we analyzed patterns of BOLD activity associated with the feedback components of trials, when participants learned whether or not the co-player had cooperated. For each participant, the SPM model included eight regressors of interest, with the first four representing BOLD activity during the feedback component of CC, CD, DC, and DD trials. To examine general responses to feedback regarding defection, we collapsed CD and DD trials; to examine general responses to feedback regarding cooperation, we collapsed CC and DC trials. We labeled these two composite conditions “Co-player defected” and “Co-player cooperated”, respectively. Because of our hypotheses that emphasize distinct responses to negative feedback in anxious individuals, we also examined CD and DD trials separately. Additional nuisance regressors represented activation during the selection component of each trial type (n=4) and described residual motion (n=6).
fMRI Data Acquisition and Preprocessing
A localizer and a manual shim procedure preceded each functional scan. We used T2* imaging (axial plane, 29 contiguous slices parallel to the AC-PC line, taken in an odd-first interleaved ascending order) on a General Electric (Waukesha, WI) Signa 3 Tesla magnet (64×64 matrix; TR=2300 ms, TE=23 ms, FOV=240 mm; 3.75 × 3.75 × 2.9 mm voxels). Stimuli were projected onto a screen at the foot of the scanner bed that participants viewed via a head coil-mounted mirror. During scanning, foam padding was used to limit head movement. Participants used a hand-held, two-button response box (Research Services Branch, NIMH, Bethesda, MD) to indicate whether they had decided to cooperate or defect and to rate their feelings toward the other player. High-resolution T1 weighted anatomical images were acquired to aid with spatial normalization (MPRAGE acquisition of 128 1.2-mm axial slices, FOV=22, NEX=1, TR=7.8 ms, TE=1.9 ms, matrix=256×192, bandwidth=31.25 kHz/256 pixels).
Using SPM software (SPM2, Wellcome Department of Neurology), we corrected functional data for slice timing and motion, co-registered them to the anatomical data, spatially normalized to the T1 template of SPM and resliced the data into isotropic 2 mm voxels, and smoothed using a 8 mm FWHM Gaussian kernel. After completing these pre-processing steps, we evaluated the quality of the co-registration procedure by visually inspecting fMRI images.
Data Analysis
Between group differences in behavioral data collected during scans were analyzed using t-tests and repeated measure ANOVAs conducted in SPSS 16.0 (Chicago, IL).
To analyze fMRI data, we used SPM2 (Wellcome Department of Neurology) to estimate event-related response amplitudes at the individual subject level for the feedback component of each event type of interest (CC, DC, CD, DD) using the General Linear Model (GLM). To examine general responses to co-player cooperation we combined CC and DC trials (co-player Cooperation); to examine responses to co-player defection we combined CD and DD trials (co-player Defection). The waveform for each event-related response was a rectangular pulse (4.6 second duration) convolved with the HRF specified by SPM2.
We employed a random effects model focused on regions of interest (ROIs) (Holmes & Friston, 1998) to detect group differences in activation in select regions thought to implement responses to self-relevant feedback. To adjust for multiple comparisons, we applied the small volume correction within each region as implemented in SPM2 (Worsley, et al., 1996). ROIs were defined for ACC, bilateral insula, precuneus, temporal poles, and amygdala, according to the Automated Anatomical Labeling (AAL) system (Tzourio-Mazoyer, et al., 2002). The ROI for right TPJ, which does not have boundaries defined in the AAL system, was instead defined using an approach derived from prior research (Rilling, Dagenais, et al., 2008; Rilling, et al., 2004; Van Boven, Ingeholm, Beauchamp, Bikle, & Ungerleider, 2005). Specifically, this ROI consisted of a sphere with a radius of 15 mm, centered at coordinates 48, −54, 27, the peak that corresponded best to those used in prior PD game studies (Rilling, Dagenais, et al., 2008; Rilling, et al., 2004). We used a similar approach to define our mPFC ROI, which we based on prior economic exchange task research (Rilling et al., 2004; Kircher et al., 2009). This ROI also consisted of a 15 mm sphere, which was centered at coordinates 3, 44, 20. All ROIs were applied at the group level, allowing us to compare activation during events of interest [co-player Cooperation (CC + DC); co-player Defection (CD + DD); co-player Betrayal (CD trials); Mutual Defection (DD trials)] using between-group t-tests.
To examine group differences in correlations between neural activation and both patterns of game play and peer ratings, we extracted contrast values for the peak voxel in each ROI for each subject. We then conducted Pearson product moment correlations between contrast values and both frequency of cooperation (overall [CC + CD] and following co-player cooperation [Player C after CC + DC trials] and defection [Player C after CD + DD trials]) and overall mean peer ratings. Correlations were converted to Z scores to permit between-group comparisons.
Results
Behavioral Findings
Summary statistics for all dependent variables are presented in Table 2. Groups did not differ significantly in the number of games won (defined as games in which the participant won more money than did the computer), t(27) = 0.35, p > .05, or the average amount of money earned, t(27) = 0.22, p > .05. We conducted a 2 (Group) × 2 (Feedback type) Greenhouse-Geisser-corrected repeated-measures ANCOVA to examine group differences in percent of player cooperation responses following feedback about co-player cooperation versus defection. To control for potential group differences in overall cooperation (regardless of prior trial feedback), we covaried total percent of player responses that were cooperative (CC + CD). Results indicated a significant interaction between group and feedback type, F(1, 26) = 6.87, p < .05, with anxious participants more likely than controls to cooperate following co-player defection, but no significant group differences following co-player cooperation (see Figure 1).
Table 2.
Group differences in Region of Interest (ROI) activation for feedback components of trials (initial map thresholded at p < .05, only clusters >20 voxels reported).
| Contrast | Region | Cluster Size |
Voxel t | Voxel p (unc) |
MNI Coordinates x,y,z {mm} |
||
|---|---|---|---|---|---|---|---|
| Co-player Defection vs. Baseline1 | |||||||
| Patients vs. Controls | Precuneus | 272 | 2.99 | .003 | 10 | −54 | 38 |
| 2.33 | .01 | 26 | −54 | 24 | |||
| 2.10 | .02 | 14 | −42 | 48 | |||
| 2.06 | .02 | 14 | −48 | 24 | |||
| 1.99 | .03 | 20 | −52 | 24 | |||
| 1.92 | .03 | 12 | −54 | 24 | |||
| TPJ | 262 | 2.97 | .003 | 40 | −46 | 30 | |
| 2.36 | .01 | 50 | −40 | 28 | |||
| Controls vs. Patients | mPFC/ACC | 278 | 2.94 | .003 | −2 | 54 | 28 |
| 2.77 | .005 | 0 | 56 | 24 | |||
| 1.81 | .04 | −2 | 54 | 10 | |||
| 1.80 | .04 | 12 | 44 | 32 | |||
| 1.80 | .04 | 0 | 52 | 4 | |||
| 1.77 | .04 | 16 | 50 | 24 | |||
| 2.94 | .003 | −2 | 54 | 28 | |||
| Co-player Betrayal vs. Baseline2 | |||||||
| Patients vs. Controls | Precuneus | 117 | 2.78 | .005 | 10 | −54 | 38 |
| TPJ | 136 | 2.69 | .006 | 40 | −46 | 30 | |
| 2.47 | .01 | 34 | −52 | 28 | |||
| Controls vs. Patients | mPFC/ACC | 386 | 2.69 | .006 | 0 | 56 | 24 |
| 2.64 | .007 | −2 | 54 | 28 | |||
| 2.48 | .01 | 2 | 52 | 30 | |||
| 1.93 | .03 | −4 | 54 | 12 | |||
| 1.88 | .04 | 14 | 46 | 30 | |||
| 1.83 | .04 | 14 | 50 | 28 | |||
| 1.83 | .04 | 10 | 48 | 32 | |||
| 1.79 | .04 | 0 | 50 | 4 | |||
| ACC | 43 | 2.07 | .02 | 2 | 36 | −2 | |
| 2.01 | .03 | 2 | 42 | −2 | |||
| 2.00 | .03 | 8 | 32 | −2 | |||
| 1.84 | .04 | −4 | 40 | −4 | |||
| Mutual Defection vs. Baseline3 | |||||||
| Patients vs. Controls | Precuneus | 30 | 2.61 | .007 | 20 | −40 | 2 |
| 2.41 | .01 | 16 | −38 | 4 | |||
| 2.13 | .02 | 14 | −42 | 6 | |||
| 37 | 2.46 | .01 | 14 | −42 | 48 | ||
| 1.77 | .04 | 4 | −42 | 48 | |||
| 123 | 2.39 | .01 | 10 | −54 | 40 | ||
| 32 | 2.11 | .02 | −10 | −40 | 46 | ||
| Insula | 73 | 2.53 | .009 | −36 | −32 | 22 | |
| 2.36 | .01 | −32 | −24 | 8 | |||
| 2.20 | .02 | −38 | −22 | 14 | |||
| 2.20 | .02 | −30 | −26 | 14 | |||
| 2.02 | .03 | −32 | −20 | 4 | |||
| TPJ | 363 | 2.49 | .01 | 40 | −46 | 32 | |
| 2.06 | .02 | 56 | −42 | 26 | |||
| 2.05 | .03 | 52 | −40 | 26 | |||
| Controls vs. Patients | mPFC/ACC | 116 | 2.60 | .007 | −2 | 54 | 28 |
| Co-Player Cooperation vs. Baseline4 | |||||||
| Patients vs. Controls | Insula | 26 | 2.40 | .01 | −32 | −20 | 4 |
| Precuneus | 30 | 2.40 | .01 | 20 | −40 | 2 | |
| 2.08 | .02 | 28 | −48 | 2 | |||
| 2.05 | .03 | 24 | −46 | 0 | |||
| 1.86 | .04 | 24 | −44 | 4 | |||
| TPJ | 107 | 1.98 | .03 | 52 | −52 | 22 | |
| 1.94 | .03 | 38 | −50 | 32 | |||
| 1.90 | .03 | 36 | −58 | 32 | |||
| Controls vs. Patients | L Temporal Pole |
45 | 2.23 | .02 | −36 | 8 | −22 |
Co-player defection vs. baseline: all trials in which the co-player defected (CD + DD);
Co-player betrayal vs. baseline: trials in which the co-player defected and the player cooperated (CD only);
Mutual defection vs. baseline: trials in which both players defected (DD);
Co-player cooperation vs. baseline: all trials in which the co-player cooperated (CC + DC).
A Greenhouse-Geisser-corrected repeated measures ANCOVA, with total earnings in each run covaried, yielded evidence of group differences in mean peer ratings between the beginning and the end of a game, F(1, 23) = 4.72, p < .05. Decomposition of these results using univariate ANCOVAs, with average earnings covaried, showed that although initial peer ratings (averaged across all four games) did not differ between groups, F(1,23)=0.32, p >.05, participant feelings toward co-players were more negative for the anxious than the control group at the end of each run. Specifically, there were significant group differences in average final feeling ratings (averaged across all four runs), F(1, 23) = 6.21, p < .05. Taken together, these data suggest that anxious youths were more likely than controls to respond cooperatively after co-player defection; at the same time, over the course of a run, they felt more negatively towards their co-players.
fMRI Findings
Responses to co-player defection
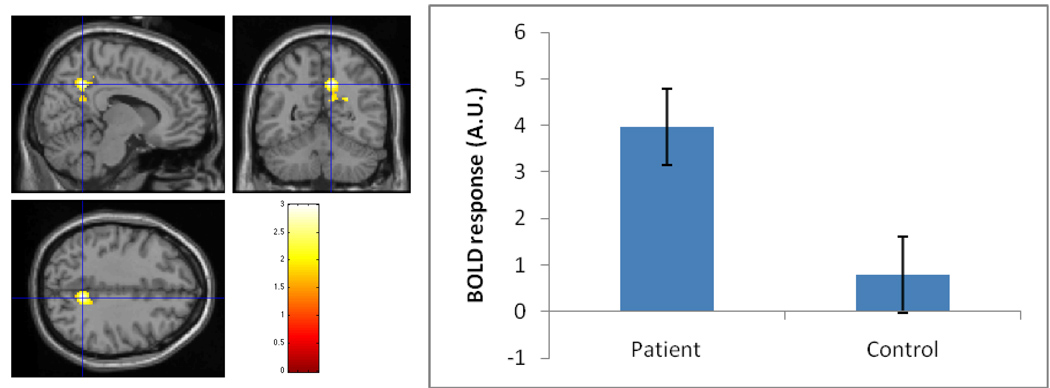
We first tested the hypothesis that the co-player Defection (CD + DD) vs. Baseline contrast would elicit differential activity in patients versus controls. Small volume corrected (SVC) analyses focused on ROIs yielded evidence of significantly greater activation in patients than controls in the precuneus and right TPJ (see Table 2 and Figure 2) but not in other ROIs. Controls, in contrast, showed significantly greater mPFC activation than patients (see Table 2).
Figure 2.
Peak precuneus (10, −54, 38) activation during feedback regarding co-player defection in patients vs. controls (image masked to show only precuneus Region of Interest), p <.05. (A.U. = arbitrary units.)
Correlations between peer ratings and right TPJ peak voxel contrast values differed significantly, z=−1.97, p < .05. Whereas a significant negative association emerged in patients r(12)=−.63, p < .05, a nonsignificant positive association appeared in controls, r(17)=.10, p >.05. Groups did not differ significantly in correlations between contrast values for peak voxels in the precuneus or mPFC and either frequency of cooperation or peer ratings (all p’s > .05).
To examine if group differences in activation were specific to trials when the co-player betrayed the participant (CD) or if they extended to mutual defection (DD) trials as well, we conducted further analyses focused on each of these conditions separately.
Response to Co-player Betrayal
Notably, betrayal (CD) trials were relatively uncommon, and controls had significantly fewer of these trials on average (M = 9.76) than did patients (M = 14.92), t(27) = −2.29, p < .05, which likely decreased our power to find significant group differences. For this contrast, small volume corrected analyses focused on ROIs yielded evidence of significant group differences in right precuneus and right TPJ activation, with greater activation in patients than controls. Additionally, controls showed stronger mPFC/ACC activation than patients (see Table 2). There were no group differences in other ROIs, nor did precuneus, mPFC/ACC, or TPJ activation correlate with behavioral/emotional data for either group (p’s > .05).
Response to mutual defection
Small volume corrected analyses focused on ROIs yielded evidence of significantly greater activation in patients than controls during DD trials in the precuneus, insula, and TPJ (see Table 2) but not the amygdala. Controls showed greater activation than patients in the mPFC/ACC. The only group differences in correlations between neural activity and behavioral/emotional data appeared in the right TPJ, where peak contrast values correlated differently with ratings, z=−2.15, p < .05, for patients, r(12)=−.64, p < .05, and controls, r(17)=.16, p >.05.
Response to Co-player Cooperation
We next examined patterns of neural response to feedback that the co-player had cooperated versus baseline. Small volume corrected analyses indicated significantly greater activation in patients than controls in the precuneus, insula, and right TPJ. Controls showed greater activation than patients in the mPFC and ACC (see Table 2). Group differences emerged in correlations between overall peer ratings and peak contrast values in the precuneus, z = 2.97, p < .05, (patients: r(12)=−.81, p < .05; controls: r(17)=.14, p >.05) and insula, z = 2.60, p < .05, (patients: r(12)=−.61, p < .05; controls: r(17)=.38, p >.05).
Discussion
Findings yielded mixed support for our hypotheses regarding between-group differences in behavior, emotion ratings, and neural activation in the context of negative interpersonal feedback during the PD game. Consistent with prior behavioral research (McClure, Parrish, et al., 2007), anxious adolescents were more likely than healthy peers to try to sustain positive interactions with their co-players by cooperating. In the present study, however, this pattern was particularly pronounced in the aftermath of co-player defection, whereas in our prior study, it was more pronounced after co-player cooperation.
This inconsistency is puzzling; one possible explanation is that the higher rate of comorbid depressive disorders in the anxious sample in our earlier study (43%) than in the sample for the present study (23%) influenced patterns of response. Attention bias findings in depressed and anxious populations suggest that depressed youths might be less responsive to socially rewarding cues, such as signals of cooperation, than are anxious youths without prominent depressive symptoms, whereas exclusively anxious youths may show heightened sensitivity to punitive social signals (Dalgleish, et al., 2003; Joormann, Talbot, & Gotlib, 2007). The small sample sizes across the two anxious groups in the present study precludes tests of this hypothesis; further research is needed to tease apart patterns of game play behavior that are specific to different types of psychopathology. Indeed, given the surprisingly limited array of published research on associations between PD game play and either personality features or psychological disorders, research that examines PD game play in individuals who vary on such characteristics as impulsivity, agreeableness, or reward sensitivity, as well in individuals representing different clinical groups, would provide important information about variables that may influence patterns of response.
Over the course of all four runs in the current study, as in our prior research, anxious youths reported increasingly negative feelings about the other player. Moreover, consistent with our earlier findings, although healthy adolescents were no more likely to win or to earn more money than their anxious peers, they reported more stable, positive perceptions of the co-player over the course of each game. Although we lacked trial-by-trial ratings of co-players, which would have allowed us to examine whether changes in emotional response related specifically to the experience of different kinds of feedback, our findings suggest broadly that anxious and healthy adolescents may experience periodic aversive responses from others in different ways.
Data regarding neural activation also provide some support for our hypotheses. Our most consistent findings were a) elevated activation within the anterior precuneus and right TPJ in anxious youths relative to healthy peers, and b) elevated mPFC and ACC activation in controls relative to patients. Notably, these group differences were not limited, as had been predicted, to trials in which participants received feedback that their co-player had defected. Instead, with the exception of the elevated mPFC and ACC activation in controls relative to patients, they extended as well to trials involving feedback that the co-player had cooperated.
These findings are broadly consistent with patterns of group differences obtained in prior research. Taken together, they suggest that anxious and healthy youths respond in strikingly different ways, particularly in midline structures implicated in self-referential and other-referential processing (Northoff & Bermpohl, 2004; Uddin, Iacoboni, Lange, & Keenan, 2007), to social feedback cues. Specifically, in response to feedback about co-player defection, controls show greater activation in mPFC and ACC; patients, in contrast, show greater precuneus and TPJ activation.
These findings raise questions about interaction among frontoparietal structures during evaluation of self-relevant feedback. One possibility is that controls more effectively engage prefrontal structures to monitor and integrate their responses across the broader frontoparietal network. Additionally, the greater rostral ACC activation observed in controls than patients could reflect group differences in expectations about co-player behavior. Specifically, consistent with research on neural responses to social pain (Eisenberger & Lieberman, 2004), rostral ACC activation may reflect a tendency among healthy controls to more effectively anticipate or expect co-player defection than do anxious youths. Experiencing an expected negative response, in contrast to experiencing an unexpected one, could trigger different networks of neural structures, thus leading to group differences at behavioral and emotional levels as well. Research examining patterns of co-activation among these structures in both groups during self-referential and other types of feedback, as well as research that better ties neural activation to thought and emotion will be necessary to test these hypotheses.
Regardless of their interpretation, our findings of stronger mPFC activation in controls than in patients are consistent with results from the one published study to compare neural activation during an economic exchange task between anxiety-disordered adults and controls (Sripada, et al., 2009). Notably, similar findings emerged even though our studies differed in both the economic exchange tasks selected and the specific contrasts examined. Our finding that this difference was evident only during feedback about co-player defection suggests that anxious individuals may be particularly derailed by undesirable feedback, as we expected. Replication of both studies in different age and anxious patient groups would be helpful in clarifying the circumstances under which differential mPFC activation emerges.
Our findings regarding group differences in precuneus and TPJ activation serve to extend Sripada and colleagues’ (2009) findings. Some evidence shows associations between activation in anterior regions of the precuneus and, among other things, self-reflection (Cavanna & Trimble, 2006) and retrieval of information about oneself (Lou, et al., 2004). Additional research suggests that these regions of the precuneus, along with the right TPJ, are preferentially activated when healthy adults assume another person’s perspective (Decety & Ruby, 2001; Lombardo, et al., 2009). Our results would thus be consistent with several mutually compatible interpretations. First, anxious youths exhibit a heightened tendency to focus on their own behavior in the general context of interpersonal interactions (Mor & Winquist, 2002). Social anxiety, in particular, appears to be associated with intensified self-focus; in a widely cited and empirically supported model of social anxiety, Wells and Clark (1995) postulate that when socially anxious adults and youths enter feared situations, they are primed to over-attend to their own thoughts, feelings, and somatic sensations. Consequently they are at risk for distorted self-perceptions, given their tendency to over-rely on internal, as opposed to environmental cues (Clark & Wells, 1995; Hodson, McManus, Clark, & Doll, 2008).
Second, our findings could also reflect a bias among anxious youths to ruminate about what others might be thinking. Caution is warranted in interpreting such results, as it remains unclear how elevated BOLD activity translates into changes in implementation of correlated cognitive processes. Additionally, our findings do not map cleanly onto precuneus coordinates reported in prior research on self- and other-processing (Cavanna & Trimble, 2006), which raises questions about how confidently we can link observed group difference in activation to functions previously shown to relate to activation in the precuneus. Further research replicating our study in larger samples and extending it to include overt measurement of cognitive processes that occur during the receipt of social feedback would be helpful in clarifying group differences in activation in both the precuneus and right TPJ.
Notably, although other researchers have found atypical precuneus activation in anxious individuals in the context of social stress (Kilts, et al., 2006), their findings were inconsistent with ours. Specifically, Kilts and colleagues found that adults with social anxiety disorder showed attenuated, rather than amplified, responses relative to healthy controls. This discrepancy between studies could reflect several factors, including group composition (mixed anxious versus social anxiety disorder), task differences, and developmental differences, which have been observed previously in studies of healthy adolescents and adults (McClure, et al., 2004; Monk, et al., 2003; van Duijvenvoorde, Zanolie, Rombouts, Raijmakers, & Crone, 2008).
Of note, we failed to replicate other studies’ findings of group differences in activation in the insula and amygdala. Although feedback regarding co-player cooperation was associated with a stronger response in the insula in patients than in controls (and stronger temporal pole activation in controls than patients), the spatial extents of these group differences were small. The absence of significant group differences in insula activation specific to co-player betrayal is inconsistent with Rilling et al.’s (2008) findings in healthy adults; task and/or sample differences may account, at least in part, for this discrepancy. Further, the relatively limited number of trials involving co-player betrayal may have decreased our power to detect group differences, which were evident in other, more frequently observed conditions. Our failure to find group differences in amygdala activation, in light of earlier findings linking amygdala activity with negative self-referential feedback (Blair, et al., 2008; Guyer, et al., 2008), is surprising. However, considerable evidence suggests that amygdala activation can be attenuated by cognitive evaluation of salient stimuli (Hariri, Bookheimer, & Mazziotta, 2000; Hariri, Tessitore, Mattay, Fera, & Weinberger, 2002), which could have influenced the current findings, which were obtained in the context of a cognitively complex task.
We observed group differences in correlations between neural activation in the right TPJ during feedback about co-player defection and peer ratings. Whereas patients showed significant, negative associations between right TPJ activation in these contrasts and peer ratings, controls showed largely non-significant associations. Thus, during receipt of feedback about co-player defection, anxious youths who showed a stronger neural response in a region associated with self/other reflection were also more likely to evaluate their co-player negatively. Notably, although groups differed in the direction of correlations in comparable ways (negative correlations for patients, positive correlations for controls) across trial types (CD, DD, and CD + DD), statistically significant differences were only evident in the DD and CD+DD conditions. This likely primarily reflects power constraints posed by the smaller number of CD than DD trials; future studies might further explore the possibility of substantive differences by gathering trial-by-trial ratings of reactions to the co-player and examining correlations between these data and activation during feedback about different types of interaction (CD vs. DD, for instance).
Potentially, anxious and healthy youths may differ in the aspects of social settings that they find emotionally salient, which may in turn drive different patterns of neural response. A comparable pattern of group differences was found for associations between peer ratings and activation in the precuneus and insula during feedback about co-player cooperation.
Collectively, these findings suggest that brain activity in regions broadly implicated in self/other reflection processes relates in specific ways to the receipt of negative interpersonal feedback in anxious versus healthy youths. In the absence of information regarding group differences in thought content during the game (e.g., thoughts of retaliation, unfairness, or competition) these data are difficult to interpret. However, they could be consistent with perception-action models of empathy (Preston & de Waal, 2002), which are thought to be mediated by an array of neural structures including those examined in this study. Such models postulate that observing or imagining another person in an emotional state elicits a comparable emotional state in the observer.
Similarly, cognitive models of anxiety suggest that biases to attend preferentially to perceived negative social content may influence stimulus processing and associated neural activity (Mathews & MacLeod, 1994; Mogg & Bradley, 1998; Wilson, Macleod, Mathews, & Rutherford, 2006). In the context of such models, our findings could indicate that anxious adolescents imagine that their co-players are responding negatively to them in a personalized way; this negative social-emotional attribution then elicits both elevated neural activation in regions of the brain involved in self/other reflection and heightened negative affect toward the co-player. To further investigate this possibility, future research might include participant verbalizations of thoughts during the game.
The present study has several limitations that merit attention. First, the sample of anxious patients is relatively small and diagnostically heterogeneous, although all participants reported high levels of social fears/concerns. Given high rates of comorbidity among anxious/depressive disorders in youths, pooling such mixed groups is a common strategy in clinical research on mood and anxiety disorders (RUPP, 2001). Indeed, such high comorbidity strongly suggests shared underlying mechanisms among these disorders. However, the inclusion of youths with a range of internalizing psychopathologies may introduce variability in the data, obscuring some group differences. To address these issues, studies with adequate data in distinct diagnostic groups to permit cross-diagnosis comparisons are warranted.
Second, because both individuals and groups differed in patterns of play, they also differed in number of interactions of each type (CC, CD, DC, DD) available for analysis. Participants were more likely to defect than to cooperate in general; this pattern of behavior, combined with the probabilities within the computer algorithm dictating co-player response patterns, yielded particularly few CD trials for most participants. In addition, controls were also more likely than patients to defect following computer co-player cooperation or defection, which given the increased likelihood that the computer would defect in response to player defection, frequently initiated sequences of repeated mutual defection. Controls thus had fewer CC and CD trials, on average, than patients. This imbalance in trial types within and across groups may have affected our power to detect salient group differences. Future research might rig the PD game so as to manipulate the ratio of cooperate to defect responses comparably in both groups, although such an approach has inherent problems as well. In the present study, we elected against such manipulation, due to concerns that it could interfere with successful deception (i.e., make it less likely that participants believe that they are playing a real person) or alter group-specific patterns of play. Studies comparing patterns of response to different versions of the PD game may be helpful in identifying the approach that will yield the most data for relevant comparisons.
Third, we did not gather data regarding participants’ mood states during the task. Data regarding affective experience during play would be helpful in understanding temporal changes in patterns of both play and neural response. Indeed, research that incorporates both regular mood ratings and thought listing techniques into tasks such as the PD game could provide data about the ways in which a negative social interaction can lead to different cascades of emotion and cognition in different individuals.
Fourth, we stringently excluded participants who had not been successfully deceived to believe that they were playing another adolescent, which led to exclusion of 25–30% of each recruited group. This rate is sizable; however, “suspicion rates” of 40–60% have been reported in other types of research involving deception (Oortman & Hertwig, 2002; Stang, 1976), which suggests that it is not atypical. Further, although we identified no significant differences between deceived and non-deceived participants on demographic or behavioral variables, these groups could have differed on variables that we failed to measure (e.g., personality characteristics, etc.). We suggest that future studies better characterize potential differences between those who do and do not endorse having been deceived, which is potentially a variable of interest in and of itself.
Finally, because of the exploratory nature of our study, as well as the relatively small sample size and the varying amount of data across trial types, we use lenient thresholds in reporting fMRI findings. On the one hand, this increases the possibility of Type I error. Accordingly, results need to be interpreted with caution until replicated. On the other hand, however, because this is among the first studies of anxious and healthy adolescents to examine neural activity associated with feedback about cooperation and defection, we wanted to provide researchers with data on which to base hypotheses for future studies in this area.
Despite these limitations, our findings add to the literature on neural responses to social experiences in the context of anxiety disorders by providing preliminary evidence of atypical activation in regions implicated in reflection on self and others in anxious adolescents, as has been found in anxious adults (Sripada, et al., 2009). Findings in adults also suggest that these brain-based differences may be malleable and responsive to treatment; studies, for example, have shown activation changes in right precuneus (Carey, et al., 2004; Kilts, et al., 2006) and mPFC (Harmer, Mackay, Reid, Cowen, & Goodwin, 2006) following a course of psychotropic medication. How such changes in neural activity relate to concomitant changes in social behavior remains unclear—research is needed that examines in tandem the effects of treatment on brain function and on behavioral response to negative cues in the context of anxiety.
Given our intriguing findings of differential associations in patients and controls between neural activation in regions implicated in self-other processing and peer ratings, future research might also explore participants’ thoughts during the course of the games. It would be interesting, for example, for participants to describe their interpretations of other players’ actions and perceived intentions, given evidence of group differences during the PD game in brain regions implicated in theory of mind processes. Developmental research, comparing anxious adults and adolescents with same-age peers might also help reconcile inconsistencies in the literature regarding the nature of atypical brain activity during the task.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIMH.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Axelrod R, Hamilton WD. The evolution of cooperation. Science. 1981;211:1390–1396. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson DA, Monk K, Kalas C, Clark DB, Ehmann M, et al. Fluoxetine for the treatment of childhood anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:415–423. doi: 10.1097/01.CHI.0000037049.04952.9F. [DOI] [PubMed] [Google Scholar]
- Blair K, Geraci M, Devido J, McCaffrey D, Chen G, Vythilingam M, et al. Neural response to self- and other referential praise and criticism in Generalized Social Phobia. Archives of General Psychiatry. 2008;65:1176–1184. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannistraro P, Rauch S. Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharmacology Bulletin. 2003;37:8–25. [PubMed] [Google Scholar]
- Carey P, Warwick J, Niehaus D, van der Linden G, van Heerden B, Harvey B, et al. Single photon emission computed tomography (SPECT) of anxiety disorders before and after treatment with citalopram. BMC Psychiatry. 2004;4:30. doi: 10.1186/1471-244X-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, editors. Social phobia: Diagnosis, assessment, and treatment. New York: Guilford Press; 1995. pp. 69–93. [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Taghavi R, Neshat-Doost H, Moradi A, Canterbury R, Yule W. Patterns of processing bias for emotional information across clinical disorders: a comparison of attention, memory, and prospective cognition in children and adolescents with depression, generalized anxiety, and posttraumatic stress disorder. Journal of Clinical Child and Adolescent Psychology. 2003;32:10–21. doi: 10.1207/S15374424JCCP3201_02. [DOI] [PubMed] [Google Scholar]
- Decety J, Ruby P. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nature Neuroscience. 2001;4:546–550. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends in Cognitive Sciences. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of Social Anxiety Disorder: Emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry. 2009;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JYF, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry. 2008;65:1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harb GC, Heimberg RG, Fresco DM, Schneier FR, Liebowitz MR. The psychometric properties of the Interpersonal Sensitivity Measure in social anxiety disorder. Behaviour Research and Therapy. 2002;40:961–979. doi: 10.1016/s0005-7967(01)00125-5. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biological Psychiatry. 2006;59:816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Hodson KJ, McManus FV, Clark DM, Doll H. Can Clark and Wells' (1995) cognitive model of social phobia be applied to young people. Behavioural and Cognitive Psychotherapy. 2008;36:449–461. [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, random effects and population inference. Neuroimage. 1998;7:s754. [Google Scholar]
- Imhof LA, Fudenberg D, Nowak MA. Tit-for-tat or win-stay, lose-shift? Journal of Theoretical Biology. 2007;247:574–580. doi: 10.1016/j.jtbi.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Flannery-Schroeder E, Panichelli-Mindel SM, Southam-Gerow M, Henin A, Warman M. Therapy for youths with anxiety disorders: a second randomized clinical trial. Journal of Consulting & Clinical Psychology. 1997;65:366–380. doi: 10.1037//0022-006x.65.3.366. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16:1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Kelsey JE, Knight B, Ely TD, Bowman FD, Gross RE, et al. The neural correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology. 2006;31:2243–2253. doi: 10.1038/sj.npp.1301053. [DOI] [PubMed] [Google Scholar]
- King-Casas B, Sharp C, Lomax-Bream L, Lohrenz T, Fonagy P, Montague PR. The rupture and repair of cooperation in Borderline Personality Disorder. Science. 2008;321:806–810. doi: 10.1126/science.1156902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher T, Blümel I, Marjoram D, Lataster T, Krabbendam L, Weber J, et al. Online mentalising investigated with functional MRI. Neuroscience Letters. 2009;454:176–181. doi: 10.1016/j.neulet.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Legrand D, Ruby P. What is self-specific? Theoretical investigation and critical review of neuroimaging results. Psychological Review. 2009;116:252–282. doi: 10.1037/a0014172. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, et al. Shared neural circuits for mentalizing about the self and others. Journal of Cognitive Neuroscience, Epub ahead of print. 2009 doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- London B, Downey G, Bonica C, Paltin I. Social causes and consequences of rejection sensitivity. Journal of Research on Adolescence. 2007;17:481–506. [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, et al. Parietal cortex and representation of the mental Self. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive approaches to emotion and emotional disorders. Annual Review of Psychology. 1994;45:25–50. doi: 10.1146/annurev.ps.45.020194.000325. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJR, et al. Abnormal attention modulation of fear circuit activation in pediatric Generalized Anxiety Disorder. Archives of General Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Zarahn E, Leibenluft E, Bilder RM, et al. A developmental examination of gender differences in brain engagement during evaluation of threat. Biological Psychiatry. 2004;55:1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- McClure EB, Parrish JM, Nelson EE, Easter J, Thorne JF, Rilling JK, et al. Responses to conflict and cooperation in adolescents with anxiety and mood disorders. Journal of Abnormal Child Psychology. 2007;35:567–577. doi: 10.1007/s10802-007-9113-8. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. General and specific contributions of the medial prefrontal cortex to knowledge about mental states. Neuroimage. 2005;28:757–762. doi: 10.1016/j.neuroimage.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Millar N, White J. A follow-up study of cognitive bias in generalized anxiety disorder. Behaviour Research and Therapy. 1995;33:927–935. doi: 10.1016/0005-7967(95)00031-r. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113:160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HMC, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with Generalized Anxiety Disorder. Archives of General Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor N, Winquist J. Self-focused attention and negative affect: A meta-analysis. Psychological Bulletin. 2002;128:638–662. doi: 10.1037/0033-2909.128.4.638. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Oortman A, Hertwig R. The costs of deception: Evidence from psychology. Experimental Economics. 2002;5:111–131. [Google Scholar]
- Preston S, de Waal FBM. Empathy: Its ultimate and proximate bases. Behavioral and Brain Sciences. 2002;25:1–20. doi: 10.1017/s0140525x02000018. discussion 20. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Dagenais JE, Goldsmith DR, Glenn AL, Pagnoni G. Social cognitive neural networks during in-group and out-group interactions. Neuroimage. 2008;41:1447–1461. doi: 10.1016/j.neuroimage.2008.03.044. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glenn AL, Jairam MR, Pagnoni G, Goldsmith DR, Elfenbein HA, et al. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biological Psychiatry. 2007;61:1260–1271. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Goldsmith DR, Glenn AL, Jairam MR, Elfenbein HA, Dagenais JE, et al. The neural correlates of the affective response to unreciprocated cooperation. Neuropsychologia. 2008;46:1256–1266. doi: 10.1016/j.neuropsychologia.2007.11.033. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Sanfey AG, Aronson JA, Nystrom LE, Cohen JD. The neural correlates of theory of mind within interpersonal interactions. Neuroimage. 2004;22:1694–1703. doi: 10.1016/j.neuroimage.2004.04.015. [DOI] [PubMed] [Google Scholar]
- RUPP. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. The Research Unit on Pediatric Psychopharmacology Anxiety Study Group. New England Journal of Medicine. 2001;344:1279–1285. doi: 10.1056/NEJM200104263441703. [DOI] [PubMed] [Google Scholar]
- Sanfey AG. Social decision-making: Insights from Game Theory and neuroscience. Science. 2007;318:598–602. doi: 10.1126/science.1142996. [DOI] [PubMed] [Google Scholar]
- Schneider BH. An observational study of the interactions of socially withdrawn/anxious early adolescents and their friends. Journal of Child Psychology and Psychiatry. 2009;50:799–806. doi: 10.1111/j.1469-7610.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- Seres I, Unoka Z, Kéri S. The broken trust and cooperation in borderline personality disorder. Neuroreport. 2009;20:388–392. doi: 10.1097/WNR.0b013e328324eb4d. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould M, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A children's global assessment scale (CGAS) Archives of General Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in Posttraumatic Stress Disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Sripada CS, Angstadt M, Banks S, Nathan PJ, Liberzon I, Phan KL. Functional neuroimaging of mentalizing during the trust game in social anxiety disorder. NeuroReport: For Rapid Communication of Neuroscience Research. 2009;20:984–989. doi: 10.1097/WNR.0b013e32832d0a67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang DJ. “Ineffective deception in conformity research: Some causes and consequences”. European Journal of Social Psychology. 1976;6:353–367. [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LTE, Brown GG. Increased amygdala activation to angry and contemptuous faces in Generalized Social Phobia. Archives of General Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. American Journal of Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends in Cognitive Sciences. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Van Boven RW, Ingeholm JE, Beauchamp MS, Bikle PC, Ungerleider LG. Tactile form and location processing in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12601–12605. doi: 10.1073/pnas.0505907102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, van Dijk E, Westenberg M, Rombouts SARB, Crone EA. What motivates repayment? Neural correlates of reciprocity in the Trust Game. Social cognitive and affective neuroscience. 2009;4:294–304. doi: 10.1093/scan/nsp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Zanolie K, Rombouts SARB, Raijmakers MEJ, Crone EA. Evaluating the negative or valuing the positive? Neural mechanisms supporting feedback-based learning across development. Journal of Neuroscience. 2008;28:9495–9503. doi: 10.1523/JNEUROSCI.1485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler D, Miller FG. Deception in the pursuit of science. Archives of Internal Medicine. 2004;164:597–600. doi: 10.1001/archinte.164.6.597. [DOI] [PubMed] [Google Scholar]
- Wilson EJ, Macleod C, Mathews A, Rutherford EM. The causal role of interpretive bias in anxiety reactivity. Journal of Abnormal Psychology. 2006;115:103. doi: 10.1037/0021-843X.115.1.103. [DOI] [PubMed] [Google Scholar]
- Wolf I, Dziobek I, Heekeren HR. Neural correlates of social cognition in naturalistic settings: A model-free analysis approach. Neuroimage. 2010;49:894–904. doi: 10.1016/j.neuroimage.2009.08.060. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]