Abstract
Adolescence is a transitional stage of development characterized by protracted refinements in the neural circuits required for adult level proficiency of working memory. Because impaired working memory is a hallmark feature of several psychiatric disorders that have their onset during adolescence, model systems that can be used to assess the maturation of working memory function, and of disease-related risk factors that disrupt its development, are of particular importance. However, few studies have investigated the maturation of working memory in nonhuman primates. Thus in the present study, we adapted two working memory tests that are among the most widely used in human and adult nonhuman primates, for adolescent rhesus monkeys. Using a touch-screen apparatus, monkeys were trained on a spatial delayed-response task to assess spatial working memory and a delayed match-to-sample task to assess object working memory. The results indicate that adolescent rhesus monkeys readily and efficiently acquire the ability to perform touch-screen based, complex tests of working memory. These data establish that distinct components of adult prefrontal cortex-dependent cognitive functions can be effectively modeled and evaluated in adolescent monkeys. As such, this approach should be useful for assessing the influence of environmental risk factors on the protracted maturation of working memory in adolescent macaques.
Keywords: working memory, adolescence, macaques, prefrontal cortex, motivation, executive function
1. Introduction
Adolescence, a transitional stage of development, is associated with protracted refinements in the circuitry of the prefrontal cortex (PFC) that are temporally correlated with improvements in the maintenance and manipulation of information in spatial working memory (WM) until adult proficiency is achieved. Indeed, performance of spatial delayed-response tasks by humans becomes substantially faster and more accurate during adolescence (Luna et al., 2004). The neural basis for the protracted developmental changes in spatial WM has been attributed to the simultaneous age-related increases in dorsolateral (DL) PFC and posterior parietal cortex activation (Kwon et al., 2002). Performance of object-familiar WM tasks has also been found to improve with age, with concomitant increases in DLPFC and parietal cortex recruitment; however, performance and recruitment differences were observed between children and older participants (adolescents and adults) (Crone et al., 2006). Thus, although the neural bases of spatial and object-familiar WM have some overlap, the developmental trajectories of these distinct constructs differ.
The functional development of spatial and object-familiar WM parallels the protracted (relative to primary sensory and motor areas) structural maturation of the PFC during childhood and adolescence (Huttenlocher, 1979; Huttenlocher et al., 1983). More recent studies suggest that the synaptic pruning and axonal myelination that occurs during adolescence underlies the changes in DLPFC activity and associated functional improvements in WM performance (Sowell et al., 1999; Giedd et al., 1999; Gogtay et al., 2004; Blakemore and Choudhury, 2006; Casey et al. 2008; Rubia et al. 2006). Although these refinements in connectivity appear to be crucial for the normal development of WM, they may also result in periods of increased vulnerability to environmental risk factors that could perturb the shaping of PFC circuitry and ultimately lead to impaired object-familiar and/or spatial WM maturation. In fact, impaired WM has been associated with numerous psychiatric disorders that have their onset during childhood or adolescence, such as depression (Kutcher and Sokolov, 1995) obsessive-compulsive disorder (Samuels, 2009), schizophrenia (Lewis, 1997), attention-deficit/hyperactivity disorder (Barkley, 1997) and drug abuse/addiction (Spear, 2000).
Identifying potential environmental risk factors for mental disorders is an important function of epidemiological studies. However possible confounding factors, such as premorbid differences in cognitive abilities between comparison groups, limit the determination of cause and effect (Taubes and Mann, 1995). Therefore, laboratory animals provide important experimental models to determine the impact of experience on brain development because confounding can be better controlled. Nonhuman primates are particularly well-suited for modeling higher cognitive functions because performance of WM tasks by macaques is qualitatively similar to humans. Indeed, functional inactivation of the PFC in adult macaques, whether permanent (cortical ablation) or reversible (localized hypothermia), impairs performance of spatial and object-familiar delayed-response tasks (Bauer and Fuster, 1976). Moreover, a seminal study by Goldman and Alexander (1977) determined that participation of the DLPFC in the mediation of spatial delayed-response task performance does not begin until after 12 months of age and gradually increases until functional maturity is achieved around 36 months of age. Additionally, an electrophysiological study in macaques has revealed that the population of PFC neurons activated during the delay period of the same spatial WM task increases, almost twofold, between 12 and 36 months of age (Alexander, 1982). These latter two studies suggest that macaques are ideal for modeling the development of higher cognitive functions observed in adolescent humans since macaques also have an extended period of adolescence characterized by protracted refinements in PFC circuits and a gradual increase in the participation of these circuits in the mediation of WM task performance (Bourgeois et al. 1994; Goldman-Rakic, 1987).
The goal of this report was to expand upon these important findings by describing a training method and model system that can be utilized for assessing the long-term influence of environmental risk factors on the protracted maturation of PFC-dependent WM in adolescent macaque monkeys. Thus, we sought to determine whether adolescent monkeys could be trained in a time-efficient manner to perform touch-screen based WM tasks. Two novel delayed-response tasks were employed that are qualitatively similar to those utilized in previous studies, but are more complex and easily manipulated to retain sensitivity for long-term developmental studies. Finally, these tasks were carefully designed to directly address the possibility that changes in other functional domains (e.g., sensorimotor abilities, motivation) could manifest as differences in WM performance.
2. Results
2.1 Touch-Screen Training Sessions
Similar to a previous report concerning touch-screen training of young adult (age undefined) male rhesus monkeys (Moore et al. 2005), the current cohort of monkeys varied widely in the time required to achieve perfect responding to the 2 cm2 stimulus used for training. On average, the cohort required 13.9 ± 1.1 days (range 8–20 days) of touch-screen training. Factors such as age and weight did not affect the length of time required for the monkeys to reliably respond to the touch-screen.
2.2 Basic Training Sessions
Table 1 presents the average number of sessions required for the subjects to reach the criteria for the steps used during the basic training phase. The spatial delayed response (SDR) task required fewer training sessions than the delayed match to sample (DMTS) task, however this may reflect an effect of order; i.e., because the monkeys were trained on the DMTS task first, they presumably had a better understanding of what was necessary to successfully complete a trial on the SDR task. Alternatively, or in addition, this may reflect the higher degree of difficulty associated with the DMTS task since monkeys had to discriminate both the color and the shape of a stimulus (2 dimensions), whereas the SDR task required a monkey to discriminate only the spatial locations (1 dimension). Nonetheless, the number of sessions needed to achieve criteria on both tasks varied widely across the cohort.
Table 1.
Average Number of Basic Training Sessions
| Task | Step | Criteria | Number of Sessions | |||
|---|---|---|---|---|---|---|
| Mean | SD | CV | Range | |||
| DMTS | 1 | 70% | 4.64 | 2.82 | 0.61 | 1 – 10 |
| 2 | 65% | 28.57 | 16.50 | 0.58 | 8 – 65 | |
| 3 | 60% | 14.29 | 8.35 | 0.58 | 5 – 36 | |
| 4 | 60% | 15.79 | 7.07 | 0.45 | 3 – 27 | |
| SDR | 1 | 80% | 1.43 | 0.65 | 0.45 | 1 – 3 |
| 2 | 65% | 6.57 | 4.15 | 0.63 | 2 – 15 | |
| 3 | 65% | 2.50 | 1.22 | 0.49 | 1 – 5 | |
| 4 | 65% | 5.79 | 4.26 | 0.74 | 2 – 17 | |
| SDR/DMTS | 5 | 65%/65% | 7.50 | 5.68 | 0.76 | 2 – 21 |
2.2.1 DMTS Basic Training Sessions
On average, monkeys required 63.3 ± 22.8 sessions (range 41–131) to reach the criteria set for the four DMTS basic training steps. The variability observed for training of the DMTS task was primarily due to monkey #505 who required 252% more sessions for step 2 and 285% more sessions for step 3 than the average across animals to reach criteria. However, #505 required a similar number of sessions to reach criterion for step 4 as the other monkeys.
2.2.2 SDR Basic Training Sessions
The SDR task was acquired with less variability across animals. Notably, monkey #505 required fewer total sessions than four other monkeys, which suggests that he was capable of acquiring similar tasks within the standard numbers of sessions. On average, monkeys required 16.3 ± 6.1 sessions (range 8–26) to reach the criteria set for all four steps. Most of the variability associated with the SDR training was due to monkey #523, who required 345% more sessions than the group average to reach criterion on step 4.
2.2.3 SDR/DMTS Dual Training Sessions
On average, monkeys required 7.5 ± 5.7 sessions (range 2–21) to reach the set criteria of 65% accuracy for the dual training sessions that employed a 1 s delay period. The variability observed on the SDR/DMTS dual training sessions was due mostly to monkey #523, who required 325% more sessions relative to the mean number of sessions. These data collectively suggest that under these task conditions, adolescent monkeys can be efficiently trained within a similar time frame. Moreover, although an occasional animal required additional training, with perseverance it was possible to train all 14 adolescent monkeys to perform both tasks in a relatively short period of time. Finally, the sensitivity of these training tasks to the idiosyncratic abilities/disabilities of the monkeys may make it a valuable resource to identify outliers that could impact measures and confound interpretations of the results.
2.3 Baseline SDR WM Trial Performance
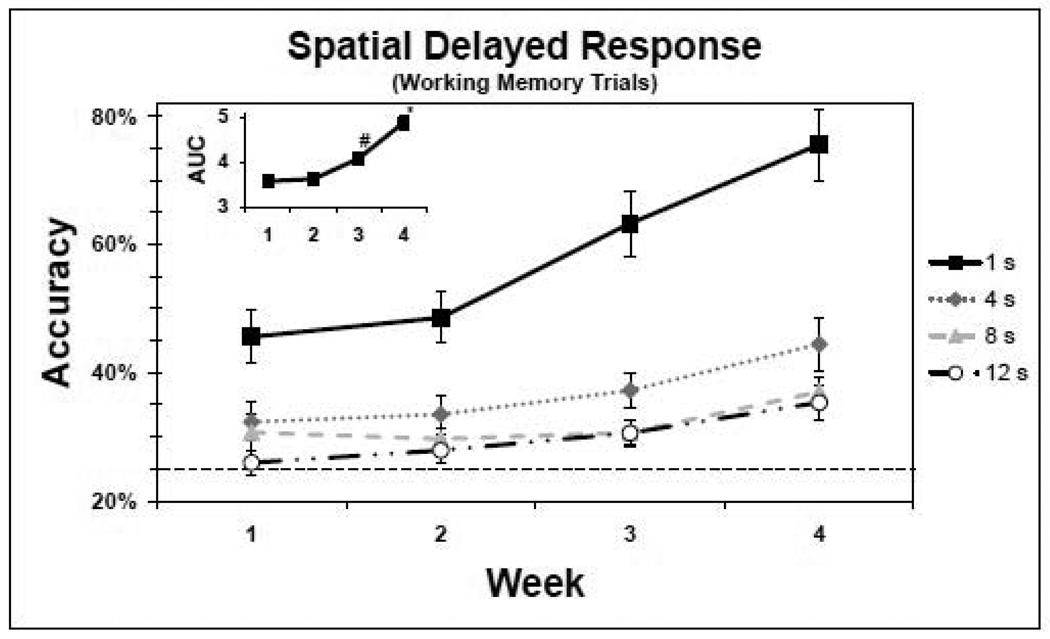
The performance of the adolescent monkeys on the SDR WM trials over the last 4 baseline weeks is provided in Figure 1 and Table 2. When performance was collapsed over days and delays, a significant effect of week (training) on accuracy (F(3,2098) = 66.53, p < 0.0001) was revealed (Figure 1). Post hoc tests revealed that this effect was due to a significant improvement in accuracy during week 4 compared to each of the three previous weeks (p < 0.0001), during week 3 compared to weeks 1 and 2 (p < 0.001), and during week 2 compared to week 1 (p = 0.003). In addition, accuracy was delay-dependent; i.e., accuracy was higher for the shorter delays. When performance was collapsed over days and weeks, a significant effect of delay on accuracy (F(3,2098) = 225.14, p < 0.0001) was revealed, with post hoc tests showing that this was due to significant differences in performance between the 1 second (s) delay and the 4, 8 and 12 s delays (p < 0.0001) and between the 4 s delay and the 8 and 12 s delays (p < 0.001). In addition, there was a significant delay by week interaction (F(9,2110) = 8.48, p < 0.0001), which indicates that the increased accuracy observed across the weeks differed between the delay conditions. Post hoc tests revealed that this was due to the fact that, across weeks, the increase in accuracy of 1 s delay trials was significantly greater than the increase in accuracy of trials with the 4, 8 and 12 s delays. Nonetheless, for each delay period the accuracy rate improved across weeks. In particular, there was a significant increase in accuracy from the first week of baseline to the last week of baseline for each delay condition (p < 0.001). In fact, average accuracy rates improved from 45%, 31%, 29% and 25%, for the 1, 4, 8 and 12 s delays respectively, week 1 to 76%, 44%, 37% and 35% week 4.
Figure 1.
Baseline performance of the spatial delayed response (SDR) task working memory (WM) trials (n=14). The y-axis represents the accuracy means (±SEM) of completed trials, and the x-axis represents the week. The dashed line indicates the chance rate of performance (25%). The solid line and square markers indicate performance for the 1 second (s) delay, the dotted line and diamond markers indicate performance for the 4 s delay, the dashed line and triangle markers indicate performance for the 8 s delay and the dashed and dotted line and circle markers indicate performance for the 12 s delay. The inset represents the corresponding AUC. *accuracy week 4 significantly improved compared to weeks 1, 2 and 3. #accuracy week 3 significantly improved compared to accuracy at weeks 1 and 2.
Table 2.
SDR Baseline Performance
| Effect | Num DF | Den DF | F Value | Pr > F |
|---|---|---|---|---|
| Delay | 3 | 2110 | 225.09 | <0.0001 |
| Week | 3 | 2110 | 66.27 | <0.0001 |
| Delay*Week | 9 | 2110 | 8.48 | <0.0001 |
The effect of week (training) was also assessed in a secondary model in which week was included as a continuous variable rather than a categorical variable, where for each delay period the slope over time was significantly larger than zero (p < 0.001), indicating increased WM trial accuracy over the 4 weeks. This result indicates that accuracy significantly improved across time (weeks of training) for the 1, 4, 8 and 12 s delay conditions.
Finally, performance was also assessed by measuring the area under the curve (AUC) for WM accuracy rate over the 4 delay conditions. When performance was collapsed across days, a significant effect of week was revealed with regards to accuracy as measured by the AUC (Figure 1 inset; F(3,249.1) = 37.76, p < 0.0001). Post hoc tests revealed that this effect was due to a significant improvement in week 4 compared to each of the three previous weeks (p < 0.0001) and in week 3 compared to weeks 1 and 2 (p < 0.01).
Another approach to assessing learning is to view each monkey individually, and to determine over the trials in a given week whether each monkey performs significantly better than chance. For example, the performance of monkey #407 on SDR trials across week 4 with the 12 s delay was 47% correct. A standard statistical test rejects the hypothesis that the monkey achieved this score by chance because the observed score is ≥ 34% (the upper limits of the 95% confidence interval for chance performance of 25%). Thus, this monkey clearly performed significantly above chance for this delay. In fact, none of the monkeys performed significantly above chance on SDR trials with the 12 s delay during week 1; however, 8 of 14 monkeys (57%) performed significantly above chance at this delay by week 4.
Collectively, these analyses corroborate the performance graphs in Figure 1. Specifically, adolescent monkeys were more accurate at the shorter delays and their accuracy significantly improved across all delay conditions over the 4 weeks of training, suggesting that the SDR task is likely to be sensitive to changes in performance over a longer period of development.
2.3.1 SDR Control Trials
Importantly, accuracy on the SDR control trials also improved with training. Average accuracies for the 1, 4, 8 and 12 s delays during week 1 were 89%, 84%, 82% and 83%, respectively, and these improved by week 4 to 95%, 88%, 90% and 86%.
2.4 Baseline DMTS WM Trial Performance
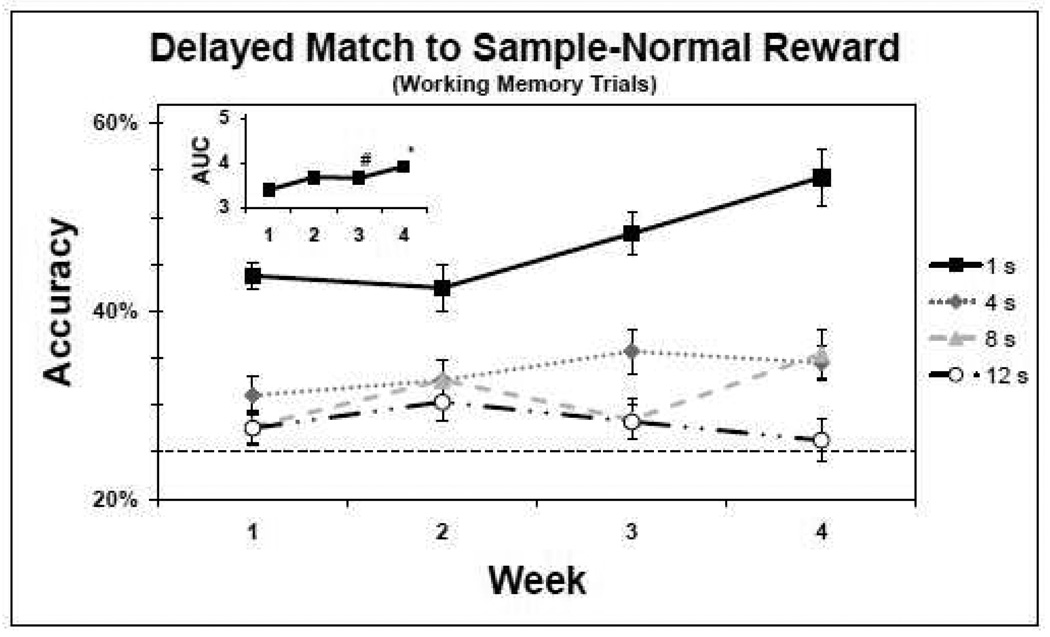
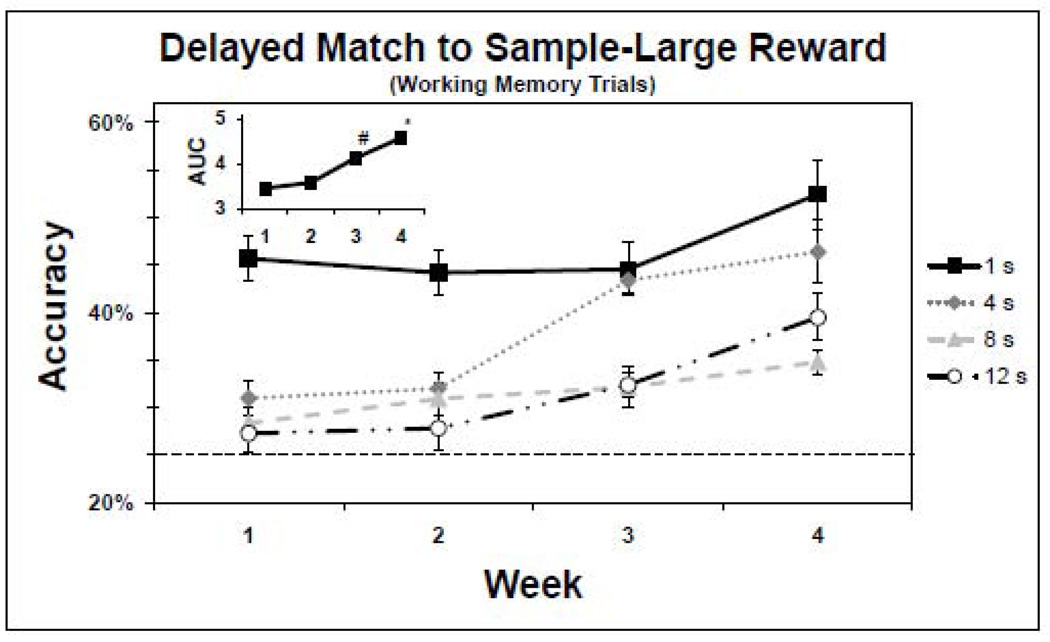
Similar to performance on the SDR task, analysis of performance on the DMTS WM trials indicated that accuracy improved over the 4 weeks and was largely delay-dependent (Figures 2 and 3, and Table 3). In addition, the analyses indicate that the large reward was associated with better performance. When performance was collapsed across delays, days, and rewards a significant effect of week on accuracy (F(3,4208) = 23.51, p < 0.0001) was revealed. Post hoc tests revealed that this effect was due to a significant improvement during week 4 compared to each of the three previous weeks (p < 0.01) and during week 3 compared to week 1 (p < 0.0001). Moreover, accuracy was delay-dependent; collapsing over days and weeks revealed a significant effect of delay on accuracy (F(3,4208) = 126.95, p < 0.0001). Post hoc tests revealed that this effect was due to significant differences in performance between the 1 s delay and the 4, 8 and 12 s delays (p < 0.0001) and between the 4 s delay and the 8 and 12 s delays (p < 0.0001). In addition, collapsing across delays, days, and weeks revealed a significant effect of reward on accuracy (F(1,4208) = 10.78, p = 0.001), indicating that the large reward had a greater appetitive value. The delay by reward interaction (F(3,4208) = 1.99, p = 0.01) was due to a significant difference in performance between the large and normal reward conditions for the 4 s delay compared to the 1 s delay. There was also a significant week by reward interaction (F(3,4208) = 3.12, p = 0.03), indicating that the changes in performance across weeks of training differed between the reward conditions. Post hoc tests revealed that this effect was due to significantly improved performance of trials associated with the large reward during week 4 compared to weeks 1 and 2. There was also a significant delay by week by reward interaction (F(18,4208) = 1.99, p = 0.01), indicating that the effect of delay and reward on changes in accuracy differed across weeks. However, due to the strict significance level used in making multiple comparisons, post hoc tests failed to reveal the significant differences among the interaction levels that contributed to the significance of this three way interaction. Nonetheless, there was a significant increase in WM trial accuracy rate from the first week of baseline to the last week of baseline for the 1 s and 8 s delays in the normal reward condition (p < 0.003), and for each delay period in the large reward condition (p < 0.01). In fact, average accuracy rates for DMTS-large trials improved from 43%, 33%, 28% and 26%, for the 1, 4, 8 and 12 s delays respectively, week 1 to 54%, 47%, 36% and 40%, week 4.
Figure 2.
Baseline performance of the delayed match to sample (DMTS) task working memory (WM) trials with the normal reward (n=14). The y-axis represents the accuracy means (±SEM) of completed trials, and the x-axis represents the week. The dashed line indicates the chance rate of performance (25%). The solid line and square markers indicate performance for the 1 second (s) delay, the dotted line and diamond markers indicate performance for the 4 s delay, the dashed line and triangle markers indicate performance for the 8 s delay and the dashed and dotted line and circle markers indicate performance for the 12 s delay. The inset represents the corresponding AUC. *accuracy week 4 significantly improved compared to weeks 1, 2 and 3. #accuracy week 3 significantly improved compared to accuracy at week 1.
Figure 3.
Baseline performance of the delayed match to sample (DMTS) task working memory (WM) trials with the large reward (n=14). The y-axis represents the accuracy means (±SEM) of completed trials, and the x-axis represents the week. The dashed line indicates the chance rate of performance (25%). The solid line and square markers indicate performance for the 1 second (s) delay, the dotted line and diamond markers indicate performance for the 4 s delay, the dashed line and triangle markers indicate performance for the 8 s delay and the dashed and dotted line and circle markers indicate performance for the 12 s delay. The inset represents the corresponding AUC. *accuracy week 4 significantly improved compared to weeks 1, 2 and 3. #accuracy week 3 significantly improved compared to accuracy at week 1.
Table 3.
DMTS Baseline Performance
| Effect | Num DF | Den DF | F Value | Pr > F |
|---|---|---|---|---|
| Delay | 3 | 4208 | 126.95 | <.0001 |
| Week | 3 | 4208 | 23.51 | <.0001 |
| Reward | 1 | 4208 | 10.78 | 0.001 |
| Delay*Reward | 3 | 4208 | 1.99 | 0.01 |
| Week*Reward | 3 | 4208 | 3.12 | 0.03 |
| Delay*Week*Reward | 18 | 4208 | 1.99 | 0.01 |
Similar to the SDR task, the effect of week was also assessed in a secondary model where week was included as a continuous variable. In the normal reward condition, the slopes for the 1 s, 4 s, and 8 s delays were significantly larger than zero (p < 0.02). Also, the slope for each delay period was significantly larger than zero in the large reward condition (p < 0.0001). In the large reward condition, the slope for the 4 s delay was significantly larger than that for the 1 s delay (p = 0.03), 8 s delay (p = 0.01), and 12 s delay (p = 0.05). These results indicate that accuracy significantly improved across time for each delay period in the large reward condition, and that the improvement in accuracy for each delay period was significantly more pronounced for the large reward condition relative to the normal reward condition.
Finally, collapsing across days and rewards, a significant effect of week was revealed with regards to accuracy as measured by AUC as defined for the SDR task (Figures 2 and 3, insets; F(3,513.6) = 22.42, p < 0.0001). Post hoc tests revealed that this effect was due to significantly improved accuracy during week 4 compared to each of the three previous weeks (p < 0.01) and during week 3 compared to week 1 (p < 0.0001). In addition, a significant main effect of reward was revealed (F(1,513.1) = 11.53, p = 0.001). There was also a significant week by reward interaction (F(3,513.1) = 4.81, p = 0.003), which was due to significantly improved performance in the large reward condition compared to the normal reward condition in week 4 compared to weeks 1 and 2.
Analyses of individual monkeys revealed that 2 of the14 monkeys performed significantly above chance on DMTS-large reward trials with the 12 s delay during week 1; however, 9 of 14 (64%) performed significantly above chance by week 4. These results suggest that the DMTS task will be sensitive to changes in performance over a longer period of development.
The fact that monkeys were more accurate when working for the more valued reward suggests that generalized readiness (e.g., arousal, attention, motor preparedness) was enhanced when the anticipated reward was more valued. This interpretation was further supported by the fact that the large reward significantly decreased initiation (F(1,4167) = 21.00, p < 0.0001) and response latencies (F(1,4170) = 6.71, p = 0.01). Collectively, these findings suggest that the adolescent monkeys were more motivated to perform the large reward trials.
2.4.1 DMTS Control Trials (SMTS)
Accuracy on the control (DMTS) trials also improved with training. Indeed, average accuracies for the large reward during week 1 were 72%, 68%, 69% and 65% for the 1, 4, 8 and 12 s delays, respectively, which improved by week 4 to 81%, 83%, 75% and 77%. Similarly, average normal reward accuracies during week 1 were 72%, 62%, 60% and 65% for the 1, 4, 8 and 12 s delays, respectively, which improved by week 4 to 79%, 77%, 77% and 79%, respectively.
3. Discussion
The results from these studies indicate that adolescent male rhesus monkeys readily and efficiently acquired the ability to perform touch-screen based, complex tests of WM. In fact, this model may ultimately provide a clearer understanding of whether the development of PFC-dependent spatial and object-familiar WM occurs in parallel. In addition, these baseline data will be a valuable resource for studies designed to determine whether long-term exposure to environmental risk factors differentially affect specific aspects of WM performance. Moreover, since performance of neuropsychological tasks requires subjects to perform a sequence of several connected events that engage processes supporting the temporal organization of behavior, such as stimulus processing, internal processing (ability to plan and sequence responses), reward-related properties (motivation), and/or motor output (Chafee and Ashe, 2007), the control trials were carefully designed to assess whether these underlying processes/functions may be affected. Finally, the importance of the present data extends beyond the demonstration of the feasibility of training adolescent rhesus monkeys on these WM tasks in a relatively short period of time; these data provide a set of normative data useful for comparison with subsequent studies in adolescent rhesus monkeys. Indeed, as previously suggested by Weed et al. (1999), future comparisons of baseline performance of other monkeys to the performance of the monkeys in the present study, may prove to be useful for screening and rejecting poor performers prior to experimental manipulations, which should result in more consistent and reliable data generation.
3.1 Rationale for Selecting the Object-Familiar DMTS Task
Before considering the value of evaluating performance of ~28 month old monkeys on the spatial and object-familiar WM tasks utilized in the present study, it is notable that although these and other tasks share some commonalities, there are important distinctions. For example, performance norms have been reported for 3–4 year old rhesus monkeys (Weed et al., 1999) and 3 year old baboons (Zurcher et al., 2010) on the touch-screen based Cambridge neuropsychological test automated battery (CANTAB), which includes an object WM task. However, the object WM associated with the CANTAB is a delayed non-match to sample (DNMTS) task that utilizes novel objects on every trial (trial-unique). The trial-unique DNMTS task is formally quite similar to the trial-familiar DMTS task employed in the current studies; i.e., both tasks have a sample object presentation, followed by a delay before the subject has to choose between the sample stimulus and a non-matching stimulus. However, performance of matching and non-matching tasks is most strongly affected by the number of objects used in the set of sample and comparison stimuli; i.e. whether the objects used between trials were the same (trial-familiar) or unique (Keppel and Underwood, 1962; Jonides and Nee, 2006). In fact, the studies of Mishkin and Delacour (1975) determined that monkeys required more than twice as many trials to reach criterion on trial-familiar tasks (irrespective of matching or non-matching) as they did for the trial-unique matching task, and 8-fold more trials as they did for the trial-unique non-matching task. Thus, trial-unique tasks, which yield quantitative information about whether a stimulus was previously observed (recognition memory), are thought to be easier for monkeys because they have an innate preference for novelty (Mishkin et al., 1962; Gaffan and Harrison, 1984). In contrast, trial-familiar tasks, which yield qualitative information about the context during which a stimulus was encoded (recall memory), are thought to be more difficult because they require subjects to transiently maintain the relevant stimulus-reward association for the current trial and proactively inhibit the irrelevant stimulus-reward associations from previous trials (Jarvick et al., 1969). Additionally, electrophysiological and ablation studies suggest that the task-specific cognitive processes associated with trial-unique and trial-familiar tasks depend on distinct anatomical substrates. In fact, although neurons of the inferior temporal cortex provide object information to the PFC and both of these regions have been shown to be essential for object memory (Van Hoesen et al. 1975; Bachevalier and Mishkin, 1986; Kowalska et al. 1991; Meunier et al. 1997), activity in the inferior temporal cortex (Desimone, 1992; Rodman 1994; Xiang and Brown 1998) and perirhinal cortex (Riches et al., 1991; Fahy et al., 1993; Miller et al., 1993) decreases when stimuli are repeatedly presented (i.e., as they become more familiar). In contrast, trial-familiar tasks have been associated with preferential activation of the PFC, which appears to be necessary for maintaining the temporal order of trial-familiar stimuli and for avoiding interference effects (Courtney et al., 1997; Rao et al., 1997; Postle and D’Esposito, 1999; Postle et al., 2000; Stern et al., 2001). Moreover, medial temporal lobe lesions have been reported to impair performance of DMTS tasks with trial-unique, but not trial-familiar stimuli in adult macaques (Eacott et al., 1994). In contrast, lesions of the inferior prefrontal convexity impair performance of trial-familiar DMTS tasks (Passingham, 1975; Bachevalier and Mishkin, 1986).
Perhaps more relevant to the current report, Weed et al. (2008) employed the frontal cortex-dependent intradimensional/extradimensional set-shifting (ID/ED) task (Robbins et al., 1998) from the CANTAB and found that ~2.3 year old rhesus monkeys had poorer performance than adults. This suggests that similar to the SDR task, adult proficiency on the ID/ED task occurs in a protracted manner. The ID/ED task requires cognitive flexibility and increased PFC-dependent attentional control towards the currently relevant stimulus feature while filtering out the irrelevant stimulus features (Egner and Hirsch, 2005). Similarly, the trial-familiar DMTS task in the current study required cognitive flexibility since the monkeys had to recall both the color and shape dimensions of the sample stimulus because it matched one of the incorrect choice stimuli in color and another in shape. Specifically, the monkeys had to form color- and shape-reward associations during the sample phase, maintain these associations during the delay period, and recall the correct associations during the choice phase to earn a reward. Thus, if successive trials used the same sample stimulus, the dimension-reward associations would theoretically become more indelible. If the ensuing trial required a monkey to switch attention to form at least one new dimension-reward association (e.g., a different shape-reward association), then the monkey would need to proactively inhibit the shape-reward association formed during the two preceding trials, which requires cognitive flexibility. This fact underscores part of the increased complexity associated with the DMTS task used in the current study, relative to the more commonly employed 2-item trial-familiar DMTS tasks, as well as trial-unique tasks. In this regard, the trial-familiar DMTS task is similar to the ID/ED task. However, in contrast to the ID/ED task where the relevant stimulus dimension changes without explicit notice, in this DMTS task the monkey was made aware of a switch in reward contingencies during the sample phase, which undoubtedly reduces the demands on cognitive flexibility. Thus, we elected to use a trial-familiar DMTS task because we are interested in modeling WM functions associated with the PFC; although it will be interesting to see whether performance of the trial-familiar DMTS and SDR tasks differentially develop in the current cohort of young monkeys.
3.2 Spatial Working Memory Task Selection
Although the CANTAB has a spatial WM task (a self-ordered spatial search (SOSS) task) that is similar to the SDR task, to our knowledge, only performance of the SDR task has been reported to become fully dependent on the functional integrity of the DLPFC in macaques around 36 months of age. For example, the percentage of DLPFC neurons that exhibit delay period activity while a monkey performs an SDR task doubles between 12 and 36 months of age (Alexander, 1982). Moreover, before 12 months of age, lesions of the caudate (Goldman and Rosvold, 1972) but not the DLPFC (Goldman and Alexander, 1977) impair performance of an SDR task. In fact, temporarily disrupting DLPFC activity while a monkey simultaneously performs the SDR task does not significantly impair performance until monkeys are ~ 30 months of age (Goldman and Alexander, 1977).
3.3 Comparison between the SDR and Object-Familiar DMTS tasks
The ability to organize and separate temporal task events is thought to increasingly depend on the PFC as primates mature. In fact, tasks that require subjects to hold spatial or object information in mind during a delay and inhibit a response tendency, elicit robust activation of the PFC in adult humans (Cohen et al. 1997) and are impaired when PFC function is perturbed in adult macaques (Bauer and Fuster, 1976). Thus, because the trial-familiar DMTS and SDR tasks used in the present study differ only with regards to whether the location or identity of a stimulus had to be maintained during the delay, both tasks should activate the PFC in adult monkeys and humans. Indeed, numerous studies have indicated that during the delay period of spatial and object-familiar WM task, some PFC neurons exhibit elevated activity, the loss of which was associated with performance errors (Fuster and Alexander, 1971; Funahashi et al., 1989; Bauer and Fuster, 1976). However, although spatial WM and object WM have some overlapping representations in the lateral PFC (Rao et al., 1997; Rainer et al., 1998), there is strong evidence for PFC-dependent process-specificity (Petrides, 1995; Owen et al., 1996, 1998). For example, neurophysiological data from adult macaques suggests spatial WM preferentially activates the DLPFC, whereas object-familiar WM preferentially activates the VLPFC (Funahashi et al., 1989; Wilson et al., 1993). In addition, monkeys with DLPFC lesions exhibit deficits on SDR tasks, while memory for the features of objects is unaffected (Goldman and Rosvold, 1970; Goldman et al., 1971). In contrast, monkeys with lesions of the inferior prefrontal convexity exhibit deficits on tasks requiring WM for the visual features of objects (e.g., color, shape) rather than their location (Mishkin and Manning, 1978; Jones and Mishkin, 1972). Moreover, a recent event-related fMRI study confirmed that this what/where and ventral/dorsal dichotomy of visual information streams exists in adult humans (Schon et al. 2008). Thus, the ability to perform spatial and object-familiar WM tasks appear to depend on distinct areas within the lateral PFC.
In contrast to the studies that have determined SDR task performance becomes increasing dependent on the integrity of the DLPFC, to our knowledge, similar studies have not been reported for object-familiar WM tasks. However, human studies suggest that adult level proficiency of object-familiar WM tasks occurs before spatial WM tasks. For example, Kwon et al. (2002) determined that the protracted developmental changes in spatial WM, which they associated with increasing activity in the PFC, extended well into adulthood. In contrast, Crone et al. (2006) determined that during the delay period of an object WM task, adults and adolescents (13–17 years old) recruited PFC similarly, whereas children (8–12 years old) did not. These findings correspond with the increase in white matter (Reiss et al., 1996) and longitudinal measurements of cortical gray matter volume observed in dorsal but not ventral PFC regions during adolescence in humans (Gogtay et al., 2004). Thus, as others have suggested, there appears to be protracted, yet differential, trajectories of development concerning the areas related to specific forms of WM (Distler et al., 1996). Collectively, these human and monkey studies suggest that performance of DLPFC-dependent tasks (SDR) stabilizes after performance of VLPFC-dependent tasks (trial-familiar DMTS) achieves functional maturity (Conklin et al., 2007).
3.4 Additional Measures
In addition to PFC-dependent WM circuitry, adolescence is also characterized by protracted refinements in mesolimbic incentive-motivational circuits (Castellanos et al. 2002), which are temporally correlated with improved inhibitory control and decreased impulsivity (Bjork et al. 2010). Developmental changes in these incentive-motivational circuits are critically important for attributing incentive salience to environmental factors, such as drugs of abuse (Spear, 2000). In fact, adolescent-onset of alcohol and cannabis use has been associated with shorter times from first exposure to dependence (Estroff et al., 1989). Furthermore, electrophysiology studies in monkeys (Roesch and Olson 2004; Wallis and Miller, 2003) and fMRI studies in humans (Krawczyk, et al. 2007; Szatkowska et al. 2008) have identified subpopulations of PFC neurons that respond to the cognitive aspects of a WM task or the reward associated with correct completion of a WM trial. Thus, since performance of WM tasks is directly affected by motivation, the neural circuits underlying motivation and WM are refined during adolescence, and adolescence is associated with increased sensitivity to environmental factors that strongly influence these circuits, it seems critical that assessments of WM development carefully control for potential effects of altered motivation.
Traditionally, progressive ratio (PR) schedules, as employed in the CANTAB, are used to evaluate the effect of an environmental factor (e.g., an abused drug) on motivation (e.g. see Taffe et al., 2002). However, PR schedules have limited sensitivity because they provide only a single data point per session and performance quickly stabilizes across sessions. In contrast, some electrophysiology studies have evaluated the effect of motivation on WM performance using a single WM task that employs two reinforcement conditions to modulate motivation (Roesch and Olson, 2004; Wallis and Miller, 2003). The latter approach is advantageous not only because it eliminates training on an additional task, but it also provides a continuous assessment of motivation throughout a test session. In fact, consistent with electrophysiological studies (Roesch and Olson, 2004; Wallis and Miller, 2003), the large reward used in the current DMTS task resulted in increased accuracy and decreased latencies (initiation and reaction times). Thus, this strategy should be useful for determining whether any effects of environmental factors on WM performance during adolescence are the result of changes in motivation versus cognitive ability.
3.5 Age Selection
Based on the previous reports that the DLPFC is not involved in the mediation of SDR task performance before 12 months of age and gradually increases until functional maturity is achieved around 36 months of age, the ages of the monkeys in the current report (27.6 ± 1.1 months) were carefully selected. Thus, long-term exposure to an environmental factor known to affect WM in adult humans and monkeys will coincide with the protracted period when spatial WM, as assessed by the SDR task, has been reported to become progressively dependent on the DLPFC. Importantly, and similar to the period of adolescence in humans, the extended period of WM maturation also coincides with the protracted refinements that occur in cortical connectivity required for WM in macaques (Erickson and Lewis, 2002; Freund et al., 2003; Eggan et al., 2010). Although this synaptic reorganization may be crucial for the normal development of WM it may also make this a period of increased vulnerability to environmental risk factors that could disrupt PFC maturation and ultimately impair WM maturation. In fact, examining the developmental curves on two WM tasks that are likely to reach functional maturity at different rates may reveal a sensitive period of maturation during which environmental exposures could alter normal developmental trajectories in DLPFC circuitry.
3.6 Advantages of the Current Paradigm
Relative to manual versions of WM tasks, the use of touch-sensitive screens in the present study had the advantage of reducing human interactions with the test subjects and presenting the tasks in a highly consistent manner, which has been reported to improve reliability and comparability across species (Robbins et al., 1994). Another advantage of the computerized touch-screen tests was the ability to immediately check the accuracy of each response, provide direct feedback when it was appropriate, and ensure that the task demands were correctly understood (see section 5.3 below). In addition, the monkeys in the present study completed over 200 trials per day, which is substantially more than others have reported using a manual version of cognitive assessments with food-reinforcement (Goldman and Alexander, 1978; Verrico et al., 2008). Moreover, although performance of the SDR and DMTS tasks is not likely to quickly reach a ceiling, both of the versions used in this study can be easily manipulated to increase difficulty should performance asymptote near perfection over time. For example, additional spatial locations (SDR) or additional stimuli (DMTS) can be added to increase difficulty. Moreover, delays can be manipulated and/or distracters can be added during the delays to increase difficulty and assess other aspects of executive functioning (e.g., see Prendergast et al. 1998). Finally, although these tasks were intentionally designed to assess PFC-dependent WM, altering the DMTS task to utilize novel objects between trials and sessions would allow an assessment of more hippocampal-dependent functions. This approach may be useful for determining whether an environmental risk factor differentially affects PFC- and hippocampal-dependent WM.
4. Conclusion
Using appropriate animal models, it is possible to minimize or eliminate the impact of extraneous factors (e.g., socioeconomic and nutritional status, age, sex, previous drug history, concurrent drug use, etc.) known or suspected to influence research findings in human studies of cognitive development (Wert and Raulin, 1986). An adolescent model of executive functions is particularly important for elucidating the refinements in neural circuits that underlie adult levels of WM performance. We suggest that this model provides reliable and valid measures of WM function in normal adolescent monkeys. Beyond normal behavior, this paradigm was developed as a means to assess the effects of various environmental factors on WM in adolescent monkeys over extended periods of time. Ultimately, we plan to use this model to assess, in a controlled fashion, the impact of environmental factors that have been implicated in the etiology of obsessive-compulsive disorder, schizophrenia, attention-deficit/hyperactivity disorder and drug abuse/addiction, all of which have their onset during childhood or adolescence.
5. Experimental Procedures
5.1 Subjects
Based on the previous report from Goldman and Alexander (1977) that the DLPFC is not involved in the mediation of SDR task performance until after 12 months of age and gradually increases until functional maturity is achieved around 36 months of age, we sought to establish task comprehension by ~28 months of age. Thus, 14 experimentally-naïve, male, specific pathogen-free, Chinese origin rhesus monkeys (Macaca mulatta), between 15 and 19 months of age were acquired from Covance Research Products (Princeton, NJ). Upon arrival at the University of Pittsburgh Primate Research Laboratory animals were housed in single cages with standard environmental enrichment, grouped together in the same room, where they remained for the duration of the study. Lights were on in the housing room from 6 am to 6 pm. Housing and experimental procedures were conducted in accordance with United States Department of Agriculture and National Institutes of Health guidelines and with approval of the University of Pittsburgh’s Institutional Animal Care and Use Committee.
5.2 Touch-Screen Training
At 23.9 ± 0.6 months of age (range 23.0–25.1 months), monkeys were placed on water regulation for the duration of these studies and rewarded for a very quick and easy response (a hand movement) with a very quick and small water reward. Initial reward levels were ~0.5 ml/kg for each correct response, which allowed 50 correct responses before the daily minimum of 25 ml/kg of body weight was met. This large volume of water ensured that the motivation to work was very high as the monkeys adjusted to working for their daily water allotment at the beginning of the study. As the monkeys acclimated to the procedures, the reward volume was decreased so that the daily number of trials completed could be increased. Thus, reward levels were slowly decreased until the last week of the baseline phase when the reward levels were ~0.1 ml/kg for each correct response. Monkeys normally worked until satiated during the training phase, and those that failed to earn their daily minimum were supplemented to the daily minimum. Moreover, supplemental fruit with high water content (e.g. oranges, grapes, etc.) was used to reward consistent responding immediately following behavioral sessions. In addition, the animals were given access to an additional 25 ml/kg of supplemental water on Fridays (after behavioral sessions) and Saturdays. In fact, total water intake (averaged over a week) was greater than 25cc/kg/day, which agrees with the typical sensible and insensible maintenance fluid losses over a 24 hour period (Rosenberg, 1995). It is important to note that the controlled water access paradigm has been widely used for over 20 years, does not deprive monkeys of their daily fluid requirements, and the daily minimum used in these studies (25ml/kg) is equivalent to 7.4 – 8 oz glasses of water for a 70 kg human. Finally, an essential part of this procedure was careful daily monitoring of the animal's health by the investigators, veterinarians, and/or animal care staff. Body weight, food intake, urine and feces and skin turgor were evaluated on a daily basis. None of the monkeys had any indication of dehydration at any point and all of the monkeys gained ~ 0.1 kg/month over the course of these studies, which was similar to a study that used food-rewards with 24 month old rhesus monkeys as subjects (Rodriguez et al., 2010).
The animals were fed at ~8 am. Monkeys were familiarized with behavioral chairs (Primate Products Incorporated; Immokalee, FL) and touch-screens (World Wide Technology Model Planar PL1510M; Swedesboro, NJ), which were located in sound-attenuating booths (Eckel Model 4240; Morrisburg, Ontario) where background white noise was broadcast over three speakers. The training/familiarization process was based on (Liu et al., 2009) or similar to (Zürcher et al., 2010; Rodriguez et al., 2010) previously described procedures. During the familiarization process, animals were placed in a chamber and an initial stimulus response program was run via E-Prime software (Psychology Software Tools, Incorporated; Pittsburgh, PA). The program displayed a central square nearly filling the monitor’s screen (6.5 cm on edge); touching the square resulted in the delivery of a water reward, which was controlled by a liquid delivery system (Crist Instrument Company Incorporated Model 5-RLD-E1; Hagerstown, MD). The size of the square was gradually reduced until monkeys were reliably responding to a 2 cm square randomly presented at various locations. Responding outside the square resulted in a blanking of the screen without water delivery. The inter-trial interval (ITI) was 3 s and sessions were conducted 2–3x/day, 5–7 days/week and ranged from 5–30 minutes/session. Monkeys were required to correctly respond to all 20 trials of a session before moving on to the next training phase.
5.3 Delayed Match to Sample (DMTS)
The use of computerized touch-screen tests allowed the primary investigator to immediately check the accuracy of each response, provide direct feedback when appropriate, and ensure the task demands were correctly understood (Robbins et al., 1994). The ability to provide direct feedback was particularly useful with these young subjects since some incorrect responses were clearly unrelated to task comprehension (e.g., responding with both hands, occasionally placing a foot on the screen, tapping the screen with one hand, etc.). This allowed adjustments to be made when comprehension of a training step was evident. Importantly, direct feedback was employed only when it would promote task comprehension and motivation. Therefore, based on the knowledge gained from training three similarly aged monkeys and four adult monkeys on similar tasks (pilot data from 7 monkeys not shown), the subjects in the current report had to achieve a criterion that was determined as being sufficient for a subject to successfully progress through the remaining steps.
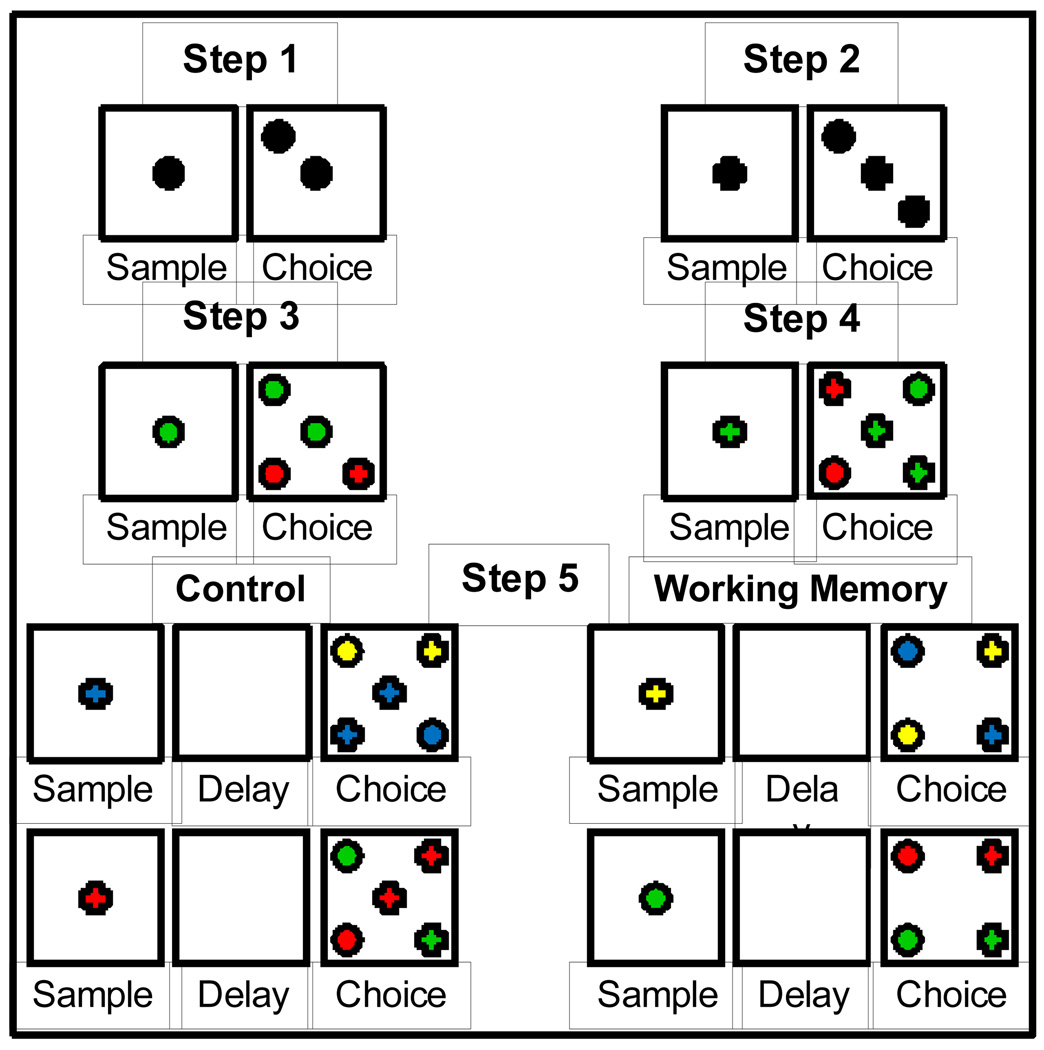
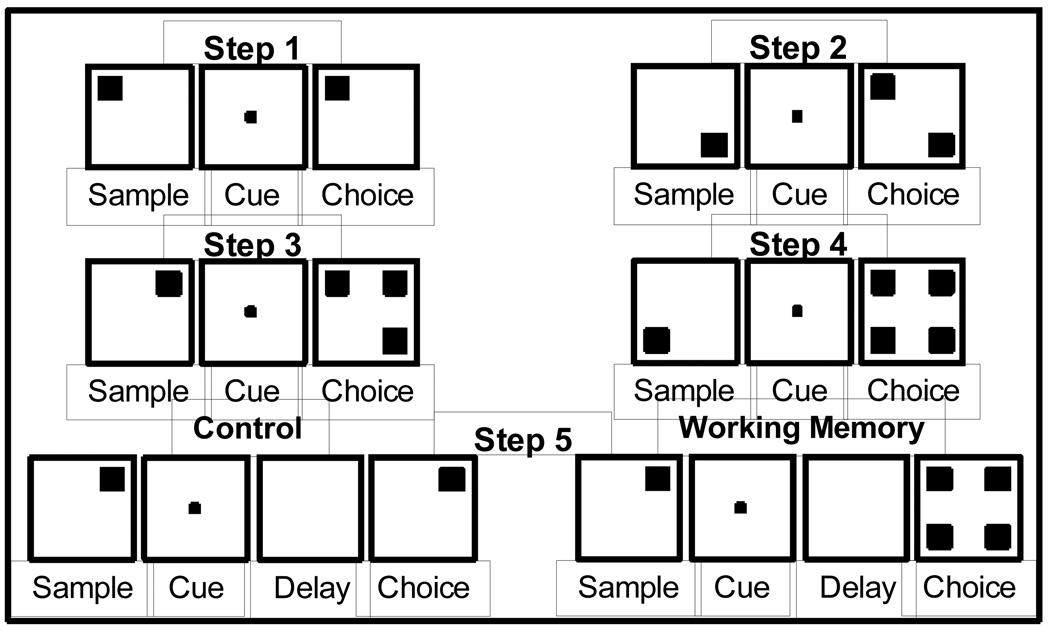
Similar to Rodriguez et al. (2010), training on the DMTS task consisted of five steps (Figure 4). If a subject did not reach the criterion set for a step (see below) in a single 48 trial (steps 1 – 4) or 64 trial (step 5) session, the step was repeated the same day or the next training session. Steps 1 – 4 included a sample phase and choice phase, but did not include a delay period. In addition, for steps 1 – 4, the sample stimulus remained at the center of the screen throughout a trial, which corresponds to simultaneous match-to sample (SMTS) trials used as attentional and perceptual controls in a human version of the DMTS task (Robbins et al., 1994). The following four parameters were consistent across steps 1 – 5. First, the same stimuli were used from trial-to-trial and day-to-day. Second, the sample stimulus was randomly selected from two (steps 1 and 2), four (steps 3 and 4), or eight (step 5) stimuli and centrally located during the sample phase. Third, during the choice phase, responses to the stimulus that remained (steps 1 – 4) or reappeared (step 5) at the center of the screen had no programmed consequences as this was intended to remove the memory requirement and guide the monkey to choose the correct stimulus. Fourth, across all steps, the choice stimuli were randomly positioned at one of four corner locations equidistant from the center (top right, bottom right, top left or bottom left). Finally, responses to somewhere other than the stimuli resulted in the screen going blank, no reward being delivered and a 3 s timeout.
Figure 4.
Schematic illustration of steps and stimuli used for training the DMTS task. Correct completion of step 1 required monkeys to touch the sample stimulus and then to touch the same stimulus during the choice phase at one of four locations. Touching the central stimulus during the choice phase had no programmed consequences. To correctly complete steps 2, 3, and 4 required the same sequence of responses; responding to the non-matching stimuli during the choice phase caused the screen to blank, the inter-trial interval (3 s) to begin and no reinforcer to be delivered. Step 5 added a 1 s delay between the sample phase and choice phase and included trials that did not have the sample stimulus appear at the center of the screen during the choice phase.
For step 1, a randomly selected sample stimulus (black cross or black circle) appeared at the center of the touch-screen. The monkey had to touch it. Immediately following this response, the same stimulus randomly appeared at one of the four corners of the screen. The monkey had to touch this stimulus to earn a reward. After a monkey achieved 70% correct in a session, training began on step 2, which utilized the same stimuli as step 1. Thus, the sample phases for steps 1 and 2 were identical. However, the choice phase for step 2 required the monkeys to visually discriminate between the randomly positioned black cross and black circle; touching the choice stimulus that matched the sample stimulus was reinforced with water delivery while touching the non-matching stimulus was not reinforced. Once animals achieved 65% correct on step 2, training began on step 3. Step 3 introduced one of the two final stimulus sets, which included a red cross, a red circle, a green cross and a green circle. Thus, during the sample phase, one of the four stimuli was randomly selected and appeared at the center of the touch-screen. The monkey had to touch it. This resulted in that sample stimulus, along with 2 of the 3 remaining stimuli (randomly chosen), to be randomly positioned in three of the four corners of the screen. As such, step 3 required subjects to discriminate among 3 stimuli that differed in color and/or shape during the choice phase to earn a reward. Once animals achieved 60% correct on step 3, training began on step 4. Step 4 differed from step 3 only in the addition of the fourth stimulus during the choice phase thereby decreasing the odds of guessing correctly from 33% to 25%. Once animals achieved 60% correct on step 4, training began on step 5. The most apparent change for step 5 was the introduction of the second set of stimuli that differed only in color (blue/yellow) from the red/green stimulus set utilized for steps 3 and 4. Notably, although both sets of stimuli were utilized during a session, there was no overlap between these 2 stimulus sets during a trial; i.e., if a sample stimulus was yellow or blue then all of the choice stimuli were yellow and blue. These red/green and yellow/blue stimulus sets were used trial-to-trial and day-to-day. Similar to the previous steps, step 5 included trials (16 trials/stimulus set) that displayed the sample stimulus at the center of the screen during the choice phase. These SMTS trials were used throughout the remainder of the study as attentional and perceptual controls. However, all step 5 trials had a 1 s delay period between the sample and choice phases, during which the touch-screen was blank (white). In addition, step 5 incorporated trials (16 trials/stimulus set) that did not have the sample stimulus reappear at the center of the screen during the choice phase; thus requiring the monkey to recall which sample stimulus was previously presented (i.e. WM trials). Thus for step 5, a sample was randomly selected from one of the two stimulus sets and appeared at the center of the touch-screen. The monkey had to touch it. A 1 s delay ensued. At the end of the delay period, all four choice probes (same color set as sample stimulus) were randomly positioned at the four corners of the screen. The monkey had to touch the choice stimulus at the location occupied by the sample stimulus presented earlier in the trial to earn a reward.
5.4 Spatial Delayed Response (SDR)
Monkeys were required to complete one 64 trial session of DMTS step 5 prior to training on the SDR task. Similar to DMTS, SDR (48 trials per session) also consisted of five steps (Figure 5). If subjects did not complete a SDR step in a given session, they began the next session at the same step. SDR steps 1 – 4 included trials with a sample phase, a central cue phase, and a choice phase, but did not include a delay period. The following parameters were consistent across SDR steps 1 – 5. The same sample stimulus was utilized and randomly positioned at one of four locations equidistant from the center (top right, bottom right, top left or bottom left), and responses to somewhere other than the stimuli resulted in the screen going blank, no reward being delivered and a 3 s timeout.
Figure 5.
Schematic illustration of the steps and stimuli used for training the SDR task. Correct completion of step 1 required monkeys to touch the sample stimulus at one of the four possible spatial locations, then to touch the central cue and finally to touch the stimulus at the same spatial location were the sample stimulus was presented. To correctly complete steps 2, 3, and 4 required the same sequence of responses; responding to the non-matching stimulus location(s) during the choice phase caused the screen to blank, the inter-trial interval (3 s) to begin and no reinforcer to be delivered. Step 5 added a 1 s delay between the central cue phase and choice phase.
For SDR step 1, a sample stimulus (2 cm black square) randomly appeared at one of the four corners of the touch-screen. The monkey had to touch it. Immediately following this response, a fixation cue stimulus (0.5 cm black square) appeared at the center of the screen. The monkey had to touch it. Immediately following this response, a choice stimulus (2 cm black square) appeared at the same location occupied by the sample earlier in the trial. The monkey had to touch the choice probe to earn a reward; touching any other location on the screen was not reinforced and resulted in the screen going blank and the ITI to begin. Step 2 included an additional choice probe location, but only responding to the initial sample location was rewarded. Steps 3 and 4 differed from step 2 only by the presence of a third and fourth choice stimulus location option, respectively. Step 5 introduced a 1 s delay between the fixation cue and choice phase. The screen was blank during the delay period. Thus, at step 5, the sample stimulus randomly appeared at one of the corners of the touch-screen. The monkey had to touch it. Immediately following this response, the fixation cue stimulus appeared at the center of the screen. The monkey had to touch it. A 1 s delay ensued. At the end of the delay period, four choice probes appeared at the four corners of the screen. The monkey had to touch the choice probe at the location occupied by the sample earlier in the trial to earn a reward.
5.5 Dual Training
Before proceeding to the ‘baseline’ stage that employed all four delay periods, the monkeys were required to perform step 5 of the SDR task with a 1 s delay followed by step 5 of the DMTS task with a 1 s delay until an accuracy of 65% was achieved for each task in a single session. This criterion was based on pilot data from 7 monkeys that indicated a criterion level of 65% was sufficient for monkeys to learn to perform the task under the delay conditions and because it was approximately half-way between chance (25%) and perfect performance.
5.6 Baseline
At an average age of 27.6 ± 1.1 months (range 26.4–30.0 months), the monkeys began training on both tasks in a single session. The difference between SDR and DMTS level 5 training and the SDR and DMTS tasks used for baseline sessions was that the recall delay intervals were 1, 4, 8, or 12 s, which were selected randomly across the trials in a session. The SDR task was presented followed by the DMTS task, which included randomly presented trials with the large and normal reinforcer conditions (described in section 5.6.1).
These monkeys served as subjects in a longitudinal study that included a control monkey paired with a monkey exposed to an environmental perturbation. Thus, because we were targeting a specific age range, each pair was introduced to the baseline phase in a staggered manner (1 pair every 3 weeks). This allowed us to modify task parameters based on the performance of the first 2 monkeys. Indeed, when the first pair began day 1 of baseline training, the SDR task randomly incorporated 64 WM and 16 control trials, while the DMTS task randomly included 112 WM and 16 control trials (56 WM and 8 control trials for each stimulus set). However, both monkeys omitted more than half of the trials on both tasks. This persisted for 2 more days before the number of trials/session was modified (this first week of data for these 2 monkeys was not included in the data analysis and these monkeys were required to repeat baseline week 1 of training). Therefore, in order to increase motivation and decrease omissions we increased the reward volume and decreased the total number of trials/session. However, the monkeys were required to complete two sessions each day (run 1 and run 2) and the number of trials/session increased while the reward volume/correct response decreased on a weekly basis. This approach nearly eliminated omissions (average < 1 omission/task/run) while allowing the collection of data from 80 SDR trials/day and 128 DMTS trials/day during the fourth week of baseline. Thus, baseline weeks 1 and 2 randomly incorporated a total of 48 SDR trials/day (8 WM trials/delay and 4 control trials/delay), which was increased to 80 trials/day for weeks 3 and 4 (16 WM trials/delay and 4 control trials/delay). The delayed and visually guided sensorimotor control trials employed here were similar to step 1 of SDR training with the exception that they were subjected to the delays before the choice stimulus was displayed. Consequently, the SDR control trials, which were a hybrid of the two control tasks utilized by Funahashi et al. (1993), had the same stimulus sequence, cue locations, delay durations and motor requirements as the SDR WM trials; however, only a single choice probe appeared at the location occupied by the sample earlier in the trial. The monkeys were simply required to touch the single choice stimulus to earn a reward. Similarly, baseline weeks 1, 2, and 3 randomly incorporated a total of 96 DMTS trials/day [80 WM (40/stimulus set) and 16 SMTS control trials/day (4/stimulus set)], which was increased to 128 trials/day [112 WM (56/stimulus set) and 16 SMTS control trials/day (8/stimulus set)] for week 4. As such, by the last week of baseline training, the monkeys were completing a total of 80 SDR/control and 128 DMTS/SMTS (64/reinforcer condition) trials each day. SDR sessions were always run prior to DMTS sessions. This approach was previously determined to facilitate learning in young monkeys (unpublished pilot data).
5.6.1 DMTS-Large Reward
An important nuance of the DMTS task was that one of the two stimulus sets was now associated with delivery of a reward that was twice the volume for correct responses (DMTS-large). Thus, for 6 of the monkeys the red/green stimulus set was associated with the delivery of a reward that was twice the volume for correct responses (DMTS-large) while the yellow/blue stimulus set was still associated with the same reward as the SDR task. The remaining 8 monkeys had the reverse stimulus set associations (i.e., red/green stimuli were associated with double the reward). The rationale behind administering a large reward was because in addition to PFC-dependent WM circuitry, adolescence was characterized by protracted refinements in mesolimbic incentive-motivational circuits (Castellanos et al., 2002), which are temporally correlated with improved inhibitory control and decreased impulsivity (Bjork et al., 2010). In addition, the magnitude of a reward (motivational incentive) has been shown to affect WM performance in monkeys (Roesch and Olson, 2004; Wallis and Miller, 2003) and humans (Krawczyk et al., 2007; Szatkowska et al., 2008). Thus, by associating one stimulus set with a larger reward we sought to increase motivation and hence, prime or potentiate the response mechanism that leads to the appetitive behavior (Stellar and Stellar, 1985), which theoretically should allow us to determine whether an experimental manipulation affected motivation and ultimately, WM performance. In fact, since the only difference between control trials was reward values and delay lengths, even subtle changes in motivation may be detectable since the control trials were, in essence, measuring only motivation. Each of the two daily sessions consisted of 24 WM and 8 control for each reward condition. Thus, each session included a total of 64 randomly presented DMTS trials.
5.7 Statistical Analysis
The goal of these analyses was to determine whether WM performance improved over the last 4 weeks of the baseline phase. Thus, performance on both tasks was measured using WM trial accuracy rate (correct/total trials attempted). In addition, the area under the curve (AUC) for WM accuracy rate over the 4 delay periods was also measured for both tasks over the 4 weeks. For each day across runs, WM accuracy rate AUC was computed as: [(4-1)/2]*(difference between WM accuracy rate for 1 s and 4 s delays) + [(8-4)/2]*(difference between WM accuracy rate for 4 s and 8 s delays) + [(12-8)/2]*(difference between WM accuracy rate for 8 s and 12 s delays). For the DMTS tasks, performance was also assessed using mean initiation time (interval between the appearance of the sample stimulus and response from the monkey) and mean reaction time (interval between the appearance of the choice stimuli and response from the monkey) across WM trials. For each of the subsequent models, all statistical tests were two-sided and conducted at the 0.05 significance level.
Conditional on subject, since accuracy was assumed to follow a binomial distribution, a generalized linear mixed model was used to model WM accuracy rate, with the logit as the link function. In all analyses, the correlation among observations within each subject was accounted for by treating subject as a normally distributed random effect. For the SDR task, run, delay, day, and week were treated as fixed effects. In the model for WM accuracy rate, the interaction between delay and week was also included as a significant fixed effect. For the DMTS task, run, delay, day, week, and reward (normal vs. large) were treated as fixed effects. In the model for WM accuracy rate, the interaction between week and reward, as well as the three-way interaction between delay, week, and reward were also included as significant fixed effects. No other two-way or three-way interactions were significant for this model. For the SDR and DMTS tasks, two models were fit for WM accuracy rate. The primary model treated week as a categorical variable and the secondary model treated week as a continuous variable.
A linear mixed model was used to model the AUC for WM accuracy rate. For the SDR task, day and week were treated as fixed effects. For the DMTS task, day, week, and reward were treated as fixed effects. In addition, the interaction between week and reward was included as a fixed effect since it was a significant two-way interaction.
A linear mixed model was used to assess initiation time and WM reaction time. For the DMTS task, run, delay, day, week, and reward (normal vs. large) were treated as fixed effects. In the model for initiation time, the interaction between week and reward was also included as a significant fixed effect. No other two-way or three-way interactions were significant for these two models.
The analyses were implemented in SAS PROC GLIMMIX (Version 9.2, SAS Institute Inc., Cary, NC) using the Kenward-Roger degrees of freedom method. This method was the recommended correction (Littell et al. 2006; Guerin and Stroup, 2000) to obtain the appropriate degrees of freedom for the error term to account for repeated measures and related dependence structures. For fixed effects with more than 2 levels, p-values for pair-wise differences were computed using Holm and Shaffer’s step-down adjustment method for multiple comparisons.
Acknowledgements
This work was supported by NIH grant DA023109 and a NARSAD Distinguished Investigator award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE. Functional development of frontal association cortex in monkeys, behavioural and electrophysiological studies. Neurosci. Res. Program Bull. 1982;20(4):471–479. [PubMed] [Google Scholar]
- Bachevalier J, Mishkin M. Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav. Brain Res. 1986;20(3):249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bauer RH, Fuster JM. Delayed-matching and delayed-response deficit from cooling dorsolateral prefrontal cortex in monkeys. J. Comp. Physiol. Psychol. 1976;90(3):293–302. doi: 10.1037/h0087996. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards, comparing motivational neurocircuitry recruitment using fMRI. P.L.o.S. One. 2010;5(7):e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury S. Development of the adolescent brain, implications for executive function and social cognition. J. Child Psychol. Psychiatry. 2006;47(3–4):296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Bourgeois J-P, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb. Cortex. 1994;4(1):78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann. N. Y. Acad. Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries. NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. J.A.M.A. 2002;288(14):1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Ashe J. Intelligence in action. Nat. Neurosci. 2007;10(2):142–143. doi: 10.1038/nn0207-142. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Luciana M, Hooper CJ, Yarger RS. Working memory performance in typically developing children and adolescents: behavioral evidence of protracted frontal lobe development. Dev. Neuropsychol. 2007;31(1):103–128. doi: 10.1207/s15326942dn3101_6. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proc. Natl. Acad. Sci. U. S. A. 2006;103(24):9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R. The physiology of memory: Recordings of things past. Science. 1992;258:245–246. doi: 10.1126/science.1411523. [DOI] [PubMed] [Google Scholar]
- Distler C, Bachevalier J, Kennedy C, Mishkin M, Ungerleider LG. Functional development of the corticocortical pathway for motion analysis in the macaque monkey: a 14C-2-deoxyglucose study. Cereb. Cortex. 1996;6(2):184–195. doi: 10.1093/cercor/6.2.184. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D, Murray EA. Preserved recognition memory for small sets, and impaired stimulus identification for large sets, following rhinal cortex ablations in monkeys. Eur. J. Neurosci. 1994;6(9):1466–1478. doi: 10.1111/j.1460-9568.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Melchitzky DS, Sesack SR, Fish KN, Lewis DA. Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience. 2010;169:1651–1661. doi: 10.1016/j.neuroscience.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat. Neurosci. 2005;8(12):1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Erickson SL, Lewis DA. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J. Comp. Neurol. 2002;448:186–202. doi: 10.1002/cne.10249. [DOI] [PubMed] [Google Scholar]
- Estroff TW, Schwartz RH, Hoffmann NG. Adolescent cocaine abuse: addictive potential, behavioral and psychiatric effects. Clinical Pediatrics. 1989;28:550–555. doi: 10.1177/000992288902801201. [DOI] [PubMed] [Google Scholar]
- Fahy FL, Riches IP, Brown MW. Neuronal activity related to visual recognition memory: Long-term memory and the encoding of recency and familiarity information in the primate anterior and medial inferior temporal and rhinal cortex. Exp. Brain Res. 1993;96:457–472. doi: 10.1007/BF00234113. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J. Neurophysiol. 1989;61(2):331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173(997):652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Harrison S. Reversal learning by fornix-transected monkeys. Q. J. Exp. Psychol. B. 1984;36(3):223–234. doi: 10.1080/14640748408402204. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman PS, Alexander GE. Maturation of prefrontal cortex in the monkey revealed by local reversible cryogenic depression. Nature. 1977;267(5612):613–615. doi: 10.1038/267613a0. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE. Localization of function within the dorsolateral prefrontal cortex of the rhesus monkey. Exp. Neurol. 1970;27(2):291–304. doi: 10.1016/0014-4886(70)90222-0. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE. The effects of selective caudate lesions in infant and juvenile Rhesus monkeys. Brain Res. 1972;43(1):53–66. doi: 10.1016/0006-8993(72)90274-0. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE, Vest B, Galkin TW. Analysis of the delayed-alternation deficit produced by dorsolateral prefrontal lesions in the rhesus monkey. J. Comp. Physiol. Psychol. 1971;77(2):212–220. doi: 10.1037/h0031649. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Devel. 1987;58(3):601–622. [PubMed] [Google Scholar]
- Guerin L, Stroup WW. A simulation study to evaluate PROC. MIXED analysis of repeated measuRes. data; Proceedings of the 12th Kansas State University Conference on Applied Statistics in Agriculture; 2000. pp. 170–203. [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, De Courten C, Garey LJ, Van der Loos H. Synaptic development in human cerebral cortex. Int. J. Neurol. 1982–1983;16–17:144–154. [PubMed] [Google Scholar]
- Jarvik ME, Goldfarb TL, Carley JL. Influence of interference on delayed matching in monkeys. J.Exp. Psychol. 1969;81:1–6. [Google Scholar]
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus--reinforcement associations. Exp. Neurol. 1972;36(2):362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139(1):181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Keppel G, Underwood BJ. Retroactive inhibition of R-S associations. J. Exp. Psychol. 1962;64:400–404. doi: 10.1037/h0040424. [DOI] [PubMed] [Google Scholar]
- Kowalska DM, Bachevalier J, Mishkin M. The role of the inferior prefrontal convexity in performance of delayed nonmatching-to-sample. Neuropsychologia. 1991;29(6):583–600. doi: 10.1016/0028-3932(91)90012-w. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Gazzaley A, D’Esposito M. Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Res. 2007;1141:168–177. doi: 10.1016/j.brainres.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Kutcher S, Sokolov S. Adolescent depression: neuroendocrine aspects. In: Goodyer IM, editor. The depressed child and adolescent: developmental and clinical perspectives. Cambridge, UK: Cambridge University Press; 1995. pp. 195–224. [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc. Natl. Acad. Sci. U. S. A. 2002;99(20):13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16(6):385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS® for Mixed Models. second ed. Cary, NC: SAS Institute Inc.; 2006. [Google Scholar]
- Liu S, Heitz RP, Bradberry CW. A touch screen based Stop Signal Response Task in rhesus monkeys for studying impulsivity associated with chronic cocaine self-administration. J. Neurosci. Methods. 2009;177(1):67–72. doi: 10.1016/j.jneumeth.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35(7):999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science. 1991;254:1377–1379. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Delacour J. An analysis of short-term visual memory in the monkey. J. Exp. Psychol. Anim. Behav. Process. 1975;1(4):326–334. doi: 10.1037//0097-7403.1.4.326. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Manning FJ. Non-spatial memory after selective prefrontal lesions in monkeys. Brain Res. 1978;143(2):313–323. doi: 10.1016/0006-8993(78)90571-1. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Prockop ES, Rosvold HE. One-trial objectdiscrimination learning in monkeys with frontal lesions. J. Comp. Physiol. Psychol. 1962;55:178–181. doi: 10.1037/h0046525. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. A non-human primate test of abstraction and set shifting, an automated adaptation of the Wisconsin Card Sorting Test. J. Neurosci. Methods. 2005;146(2):165–173. doi: 10.1016/j.jneumeth.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Owen AM, Milner B, Petrides M, Evans AC. Memory for object featuRes. versus memory for object location: a positron-emission tomography study of encoding and retrieval processes. Proc. Natl. Acad. Sci. U. S. A. 1996;93(17):9212–9217. doi: 10.1073/pnas.93.17.9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Stern CE, Look RB, Tracey I, Rosen BR, Petrides M. Functional organization of spatial and nonspatial working memory processing within the human lateral frontal cortex. Proc. Natl. Acad. Sci. U. S. A. 1998;95(13):7721–7726. doi: 10.1073/pnas.95.13.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE. The brain and intelligence. Brain Behav. Evol. 1975;11(1):1–15. doi: 10.1159/000123620. [DOI] [PubMed] [Google Scholar]
- Petrides M. Functional organization of the human frontal cortex for mnemonic processing. Evidence from neuroimaging studies. Ann. N.Y. Acad. Sci. 1995;769:85–96. doi: 10.1111/j.1749-6632.1995.tb38133.x. [DOI] [PubMed] [Google Scholar]
- Postle BR, D'Esposito M. "What"-Then-Where" in visual working memory: an event-related fMRI study. J. Cogn. Neurosci. 1999;11(6):585–597. doi: 10.1162/089892999563652. [DOI] [PubMed] [Google Scholar]
- Postle BR, Stern CE, Rosen BR, Corkin S. An fMRI investigation of cortical contributions to spatial and nonspatial visual working memory. Neuroimage. 2000;11(5 Pt 1):409–423. doi: 10.1006/nimg.2000.0570. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Jackson WJ, Terry AV, Jr, Kille NJ, Arneric SP, Decker MW, Buccafusco JJ. Age-related differences in distractibility and response to methylphenidate in monkeys. Cereb. Cortex. 1998;8(2):164–172. doi: 10.1093/cercor/8.2.164. [DOI] [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 1998;393(6685):577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- Rao SC, Rainer G, Miller EK. Integration of what and where in the primate prefrontal cortex. Science. 1997;276:821–824. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119(5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Riches IP, Wilson FA, Brown MW. The effects of visual stimulation and memory on neurons of the hippocampal formation and the neighboring parahippocampal gyrus and inferior temporal cortex of the primate. J. Neurosci. 1991;11:1763–1779. doi: 10.1523/JNEUROSCI.11-06-01763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, McInnes L, Rabbitt PM. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. J. Int, NeuroPsychol. Soc. 1998;4:474–490. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt PM. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Rodman HR. Development of inferior temporal cortex in the monkey. Cereb. Cortex. 1994;5:484–498. doi: 10.1093/cercor/4.5.484. [DOI] [PubMed] [Google Scholar]
- Rodriguez JS, Morris SM, Hotchkiss CE, Doerge DR, Allen RR, Mattison DR, Paule MG. The effects of chronic methylphenidate administration on operant test battery performance in juvenile rhesus monkeys. Neurotoxicol. Teratol. 2010;32(2):142–151. doi: 10.1016/j.ntt.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304(5668):307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- Rosenberg DP. "Critical Care". In: Bennett BT, Abee CR, Henrickson R, editors. Nonhuman Primates in Biomedical Research: Biology and Management. New York: Academic press; 1995. p. 328. [Google Scholar]
- Rosso IM, Young AD, Femia LA, Yurgelun-Todd DA. Cognitive and emotional components of frontal lobe functioning in childhood and adolescence. Ann. N. Y. Acad. Sci. 2004;1021:355–362. doi: 10.1196/annals.1308.045. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum. Brain Mapp. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels JF. Recent advances in the genetics of obsessive-compulsive disorder. Curr. Psychiatry Rep. 2009;11(4):277–282. doi: 10.1007/s11920-009-0040-y. [DOI] [PubMed] [Google Scholar]
- Schon K, Tinaz S, Somers DC, Stern CE. Delayed match to object or place, an event-related fMRI study of short-term stimulus maintenance and the role of stimulus pre-exposure. Neuroimage. 2008;39(2):857–872. doi: 10.1016/j.neuroimage.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. BioBehav. Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stellar JR, Stellar E. The Neurobiology of Motivation and Reward. New York: Springer-Verlag; 1985. p. 73. [Google Scholar]
- Stern CE, Sherman SJ, Kirchhoff BA, Hasselmo ME. Medial temporal and prefrontal contributions to working memory tasks with novel and familiar stimuli. Hippocampus. 2001;11(4):337–346. doi: 10.1002/hipo.1048. [DOI] [PubMed] [Google Scholar]
- Szatkowska I, Bogorodzki P, Wolak T, Marchewka A, Szeszkowski W. The effect of motivation on working memory, an fMRI and SEM study. Neurobiol. Learn Mem. 2008;90(2):475–478. doi: 10.1016/j.nlm.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Davis SD, Gutierrez T, Gold LH. Ketamine impairs multiple cognitive domains in rhesus monkeys. Drug Alcohol. Depend. 2002;68:175–187. doi: 10.1016/s0376-8716(02)00194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubes G, Mann CC. Epidemiology faces its limits. Science. 1995;269(5221):164–169. doi: 10.1126/science.7618077. [DOI] [PubMed] [Google Scholar]
- Van Hoesen G, Pandya DN, Butters N. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. II. Frontal lobe afferents. Brain Res. 1975;95(1):25–38. doi: 10.1016/0006-8993(75)90205-x. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Lynch L, Fahey MA, Fryer AK, Miller GM, Madras BK. MDMA-induced impairment in primates: antagonism by a selective norepinephrine or serotonin, but not by a dopamine/norepinephrine transport inhibitor. J. Psychopharmacol. 2008;22(2):187–202. doi: 10.1177/0269881107083639. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur. J. Neurosci. 2003;18(7):2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Weed MR, Bryant R, Perry S. Cognitive development in macaques: attentional set-shifting in juvenile and adult rhesus monkeys. Neuroscience. 2008;157(1):22–28. doi: 10.1016/j.neuroscience.2008.08.047. [DOI] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, Bloom FE, Gold LH. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Brain Res. Cogn. Brain Res. 1999;8(3):185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260(5116):1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- Xiang J-Z, Brown MW. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacol. 1998;37:657–676. doi: 10.1016/s0028-3908(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Zürcher NR, Rodriguez JS, Jenkins SL, Keenan K, Bartlett TQ, McDonald TJ, Nathanielsz PW, Nijland MJ. Performance of juvenile baboons on neuropsychological tests assessing associative learning, motivation and attention. J. Neurosci. Methods. 2010;188(2):219–225. doi: 10.1016/j.jneumeth.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]