Abstract
Innate immune cells must be able to distinguish between direct binding to microbes and detection of components shed from the surface of microbes located at a distance. Dectin-1 is a pattern recognition receptor expressed by myeloid phagocytes (macrophages, dendritic cells and neutrophils) that detects β-glucans in fungal cell walls and triggers direct cellular anti-microbial activity, including phagocytosis and production of reactive oxygen species1, 2. In contrast to inflammatory responses stimulated upon detection of soluble ligands by other pattern recognition receptors, such as Toll-like receptors (TLRs), these responses are only useful when a cell comes into direct contact with a microbe and must not be spuriously activated by soluble stimuli. In this study we show that despite its ability to bind both soluble and particulate β-glucan polymers, Dectin-1 signalling is only activated by particulate β-glucans, which cluster the receptor in synapse-like structures from which regulatory tyrosine phosphatases CD45 and CD148 are excluded (Supplementary Figure 1). The “phagocytic synapse” now provides a model mechanism by which innate immune receptors can distinguish direct microbial contact from detection of microbes at a distance, thereby initiating direct cellular anti-microbial responses only when they are required.
Studies in mice and humans have demonstrated an important role for Dectin-1 in anti-fungal defense3-6. Dectin-1 signals activate anti-microbial (phagocytosis, production of reactive oxygen species) and inflammatory (cytokine and chemokine production) innate immune responses, and influence the development of adaptive immunity (reviewed in 1, 2). Although Dectin-1 has been demonstrated to collaborate with TLR signals to orchestrate immune responses to fungi7, 8, it activates a distinctly different signalling cascade. Dectin-1 signals via a motif in its cytoplasmic tail that resembles an immunoreceptor tyrosine-based activation motif (ITAM; reviewed in 1, 2). Like other ITAM-based receptors, including Fc Receptors (FcR), T cell receptors (TCR), and B cell receptors (BCR), Dectin-1 signalling relies on activation of Src and Syk family kinases. However, in contrast to conventional ITAMs which comprise dual YXXL sequences, Dectin-1’s “hemITAM” has only a single YXXL2, 9. Despite its unusual ITAM, Dectin-1 ligation by β-glucan-containing particles, such as zymosan and curdlan, triggers Src/Syk-dependent downstream signals in myeloid cells (macrophages, dendritic cells (DC) and neutrophils) to activate MAP kinases, as well as NF-κB and NFAT transcription factors (reviewed in1, 2). In addition to inflammatory responses which are also triggered by TLRs, Dectin-1 induces distinct anti-microbial responses. Dectin-1 is a key phagocytic receptor for fungi and triggers a massive oxidative burst in response to fungal exposure5, 6, 10, 11.
TLRs sense soluble microbial stimuli and are activated by dimerization of intracellular signalling domains. The decades-old use of the small (6-8 kDa), soluble β-glucan laminarin (from Laminara digitata) to “block” β-glucan receptors on macrophages rather than activate them suggests that Dectin-1 may behave very differently12. Indeed, in previous studies we have failed to detect Src, Syk and NFAT activation following treatment of macrophages with laminarin even though the material is a polymer of pure ligand11, 13. We hypothesized that a larger molecule may be required to provide a greater degree of receptor crosslinking to permit activation. We therefore compared the ability of whole glucan particles (WGP), a particulate Saccharomyces cerevisiae β-glucan preparation that lacks TLR-stimulating activity (Supplementary Figure 2), with various molecular weight fractions of soluble S. cerevisiae β-1,3/1,6-glucans (see Figure 1a).
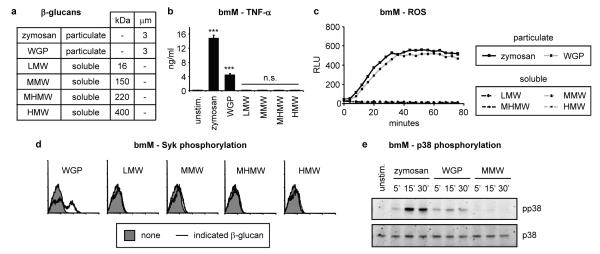
Figure 1. Particulate, but not soluble, β-glucans induce Dectin-1 signalling.
a Size (molecular weight or diameter) of β-glucan preparations used in this study. b-e Bone marrow-derived macrophages (bmM; b-d IFN-γ-primed overnight) were stimulated with 50 μg/ml β-glucans. b TNF-α production (24 h) was assessed by ELISA; data are means plus standard deviations of triplicate culture (*** p<0.001, n.s. not significant). c ROS production was assessed by luminol-ECL; datapoints are means of triplicate culture. d Syk activation (10 min) was assessed by intracellular flow cytometry. e p38 MAP kinase activation at the indicated times was assessed by immunoblotting. All data are representative of at least 3 independent experiments.
WGP, like zymosan and curdlan, induced robust Dectin-1-dependent responses, including phagocytosis, induction of TNF-α and IL-6 and production of reactive oxygen species (ROS) by bone marrow-derived macrophages and DC (bmM and bmDC; Figure 1b and c, Supplementary Figures 3-5, and data not shown). In contrast, none of the soluble β-glucans, not even the high molecular weight fraction, induced ROS, TNF-α or IL-6 production by either bmM or bmDC. Similar data were obtained using murine neutrophils, human monocytes and monocyte-derived macrophages (Supplementary Figures 4 and 6, and data not shown). Like zymosan, WGP induced Dectin-1 signalling (activation of Syk, p38 MAP kinase, NF-κB and NFAT; Figure 1d and e, and Supplementary Figures 5d, 7-9). In contrast, none of the soluble β-glucans induced Dectin-1 signals. Thus simply increasing the size of the β-glucan polymer is not sufficient to activate Dectin-1 signalling.
Adams et al. previously demonstrated that a 150 kDa soluble S. cerevisiae β-glucan interacts with purified Dectin-1 with pM affinity14. We used a variety of approaches to verify that our S. cerevisiae β-glucans bind directly to cell surface Dectin-1. Fluorescently labelled soluble β-glucans bound to bmM and bmDC surfaces in a Dectin-1-dependent manner and all molecular weight fractions efficiently blocked (by at least 50%) binding of anti-Dectin-1 antibodies at the dose (50 μg/ml) used in this study (Figure 2a, Supplementary Figures 10 and 11, and data not shown). Furthermore, we observed significant soluble β-glucan binding to Dectin-1-expressing RAW264.7 macrophages, which, like primary macrophages/DC, respond robustly to particulate but not soluble β-glucans (Supplementary Figures 12 and 13). In contrast, parental RAW264.7 macrophages, which express very little Dectin-1 and mount only low responses to β-glucan particles, failed to bind soluble β-glucans (Supplementary Figure 12a and data not shown). Furthermore, like laminarin, all the soluble β-glucans blocked Dectin-1-mediated particulate β-glucan responses in primary bmM and bmDC (Supplementary Figures 14 and 15). Thus, despite efficient binding to Dectin-1, soluble β-glucans are incapable of activating the receptor.
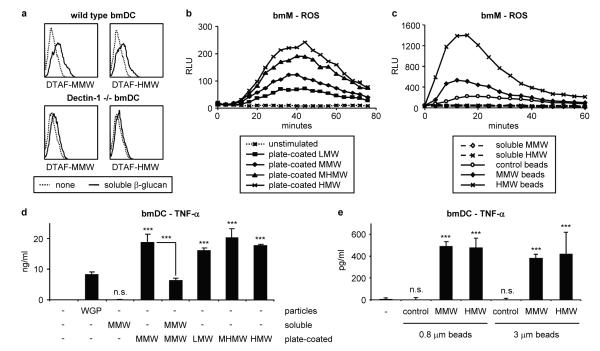
Figure 2. Immobilised β-glucans induce Dectin-1 signalling.
a Soluble β-glucan binding (50 μg/ml, 10 min) to wild type and Dectin-1 -/- bone marrow-derived DC (bmDC) was assessed by flow cytometry. b, c IFN-γ-primed bmM were stimulated with soluble β-glucans (50 μg/ml) or β-glucans immobilised on either tissue culture plates (b – plate-coated) or 0.8 μm polystyrene latex beads (c – beads). ROS production was measured by luminol-ECL; datapoints are means of triplicate culture. d, e TNF-α production (24 h) by bmDC exposed to soluble/particulate (50 μg/ml), plate-immobilised or bead-coated soluble β-glucans was assessed by ELISA; data are expressed as means plus standard deviations of triplicate culture (*** p<0.001, n.s. not significant). All data are representative of at least 3 independent experiments.
These data indicate that the mode of presentation of the β-glucan may be critical for Dectin-1 signalling. We therefore examined whether immobilisation of soluble β-glucans is sufficient to trigger Dectin-1 signalling. Like the β-glucan particles, soluble β-glucans immobilised on tissue culture plates or polystyrene latex beads (0.5 μm diameter or larger) stimulated robust Dectin-1-dependent responses (Figure 2b-e and Supplementary Figures 16-18).
The above data collectively demonstrate that in order to activate Dectin-1, β-glucans must be presented in an immobilised form e.g. on the surface of a phagocytosable particle such as a yeast cell. This scenario is reminiscent of the requirement for antigen presentation to the TCR by an antigen presenting cell (APC). TCR signalling is regulated by CD45, a membrane protein with a large extracellular domain and intrinsic tyrosine phosphatase activity15, 16. CD45 is initially required for removal of an inhibitory phosphate to permit activation of Src family kinases, but subsequently must be isolated from the TCR complex due to its negative regulation of ITAM signalling. We therefore investigated whether Dectin-1 signalling is similarly regulated by CD45 and/or CD148, a CD45-related membrane tyrosine phosphatase with overlapping function that regulates ITAM signalling by the TCR, BCR and FcγR17, 18.
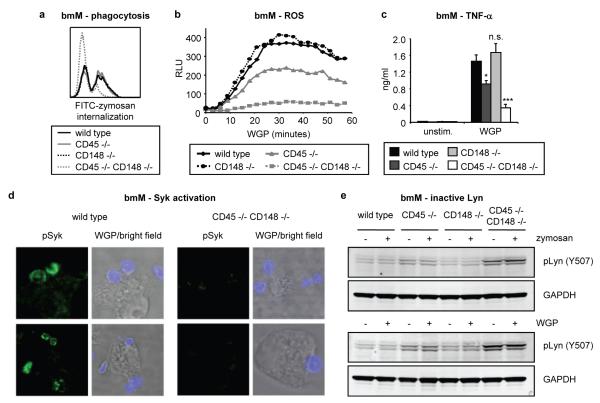
CD45-deficient bmM exhibited normal zymosan phagocytosis and only partially compromised WGP-induced ROS and TNF-α, while CD148-deficient bmM showed no defect (Figure 3a-c). In contrast, these Dectin-1 responses were severely compromised in bmM deficient in both CD45 and CD148 (Figure 3a-c). TNF-α induction by various other stimuli was unaffected (Supplementary Figure 19a) and Dectin-1 surface expression was normal (Supplementary Figure 19b). WGP failed to induce Syk activation in bmM deficient in both phosphatases (Figure 3d), which as previously reported18 had elevated basal levels of phosphorylation of the Src family kinase Lyn at its inhibitory tyrosine (Y507; Figure 3e). Thus CD45 and CD148 have overlapping function in the regulation of Dectin-1 signalling in macrophages.
Figure 3. CD45 and CD148 regulate Dectin-1 signalling.
Responses of wild type and CD45/CD148-deficient bmM (b IFN-γ-primed) were examined. a Internalization of FITC-labelled zymosan particles (20 μg/ml, 10 min) was assessed by flow cytometry. b ROS production was measured by luminol-ECL; datapoints are means of triplicate culture. c TNF-α production (50 μg/ml WGP, 24 h) was assessed by ELISA; data are expressed as means plus standard deviations of triplicate culture (* p<0.01, *** p<0.001, n.s. not significant). d Syk activation (pSyk; green) by AlexaFluor647-labelled WGP (20 μg/ml, 1 min; blue) was assessed by confocal microscopy. e Inactive Lyn (pY507) levels following zymosan/WGP stimulation (50 μg/ml, 10 min) were assessed by immunoblotting. All data are representative of 3 independent experiments.
TCR activation is characterized by formation of an “immunological synapse” between a T cell and an APC. Surface molecules on the two interacting cells are reorganized at the cell-cell interface to permit TCR signalling15. Following the initial CD45-promoted activation of Src family kinases, CD45 must be isolated from the TCR complex in order to remove its inhibitory phosphatase activity and permit propagation of the ITAM signal. We hypothesized that following binding of β-glucan-containing particles, CD45 and CD148 would similarly be sequestered from Dectin-1 to permit activation of Dectin-1 signalling.
We therefore examined macrophage surface molecule rearrangement following β-glucan particle binding. As we and others have previously observed8, 19, Dectin-1 clustered at β-glucan particle contact sites on the surface of Dectin-1-expressing RAW264.7 macrophages and phagocytic cups formed within 1 minute of binding (Figure 4a, Supplementary Figure 20a). Recruitment of active Src and Syk kinases, as well as other tyrosine-phosphorylated proteins, to the contact sites of β-glucan particles with macrophages (Dectin-1-expressing RAW264.7 and primary murine macrophages) was also seen at this early timepoint (Figure 4b-e and Supplementary Figures 20b-c and 21-23).
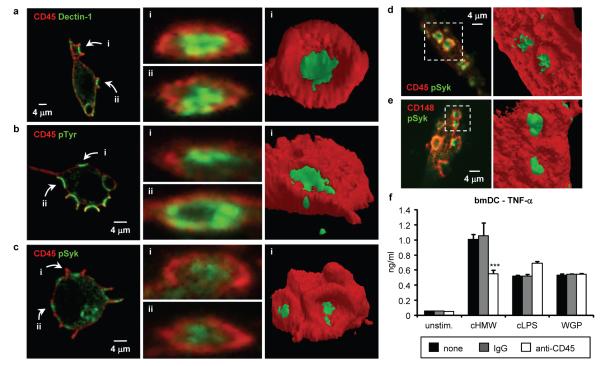
Figure 4. CD45 and CD148 phosphatases are excluded from the β-glucan particle contact site.
a-c Confocal microscopy of SBPc-tagged Dectin-1-expressing RAW264.7 macrophages stimulated with zymosan (20 μg/ml, 1 min) and stained for CD45 (red) and SBPc tag (Dectin-1; green), phospho-tyrosine (pTyr; green) or active Syk (pSyk; green). z-stacks were analyzed to visualise the indicated particle contact sites (left panels) in cross-section (center panels). 3-dimensional isosurface models of the indicated contact sites were generated using ImageJ and ImageSurfer (right panels). d Resident peritoneal macrophages stimulated with WGP (20 μg/ml, 1 min) were stained (left panel) for CD45 (red) and active Syk (pSyk; green). e Dectin-1-expressing RAW264.7 macrophages stimulated with WGP (20 μg/ml, 1 min) were stained (left panel) for CD148 (red) and active Syk (pSyk; green). Isosurface models (d, e right panels) are of the indicated particle contact sites. f bmDC were added to tissue culture plates pre-coated with HMW soluble β-glucan (cHMW) or LPS (cLPS) and/or anti-CD45 or control IgG; some bmDC were stimulated with WGP (50 μg/ml). TNF-α production (24 h) was assessed by ELISA; data are means plus standard deviations of triplicate culture (*** p<0.001). All data are representative of at least 3 independent experiments.
In contrast, CD45 was uniformly distributed on the surface of unstimulated macrophages, but upon contact with β-glucan particles was specifically absent from regions of membrane enriched for Dectin-1, active Src family and Syk kinases, and phospho-tyrosine staining (Figure 4a-d and Supplementary Figures 20-24). Timecourse experiments showed that CD45 exclusion from the region of Dectin-1 clustering occurs prior to initiation of phagocytic cup formation (Supplementary Figure 25 and Supplementary Movie 1), and is not dependent on actin dynamics (Supplementary Figure 26). CD148 was similarly excluded from regions of Dectin-1 clustering and signalling induced by β-glucan particle binding (Figure 4e). 3-dimensional images clearly show a “bulls-eye” pattern of staining with a central Dectin-1 signalling region, from which CD45 and CD148 are excluded (Figure 4a-e, Supplementary Figure 27 and Supplementary Movie 2). We also observed clustering of Dectin-1 and exclusion of CD45 upon contact of Dectin-1-expressing RAW264.7 macrophages with live fungi (S. cerevisiae, Candida albicans and Aspergillus fumigatus) and β-glucan-coated plates (Supplementary Figures 28-32). In contrast, upon binding of soluble β-glucans to Dectin-1, CD45 remained colocalized with Dectin-1 at the cell surface (Supplementary Figure 33).
CD45 and CD148 exclusion from the contact site of Dectin-1 with the β-glucan particle in the forming phagosome (or the contact surface during frustrated phagocytosis) is consistent with the hypothesis that following the initial activation of Src family kinases, the phosphatases must be isolated from the receptor to permit productive signalling. This model predicts that co-coating anti-CD45 antibodies on plates with β-glucans to prolong CD45 colocalization with β-glucan-bound Dectin-1 would suppress Dectin-1 signalling. Indeed, co-coated anti-CD45 reduced immobilised β-glucan-induced TNF-α production by about 45%, but did not affect TNF-α induction by WGP (which contact cells at points not bound to anti-CD45) or immobilised LPS (Figure 4f).
Collectively, our data suggest a mechanism by which Dectin-1, unlike other innate pattern recognition receptors such as TLRs, discriminates between soluble and particulate ligands (Supplementary Figure 1). Binding of particulate β-glucans (such as yeast) to Dectin-1 triggers phagocytosis, a process that involves massive reorganization of membrane proteins and membrane movement coordinated by the actin cytoskeleton. We have shown that during this process membrane tyrosine phosphatases, which are well characterized regulators of ITAM signalling16, are excluded from the particle contact site. In a manner analogous to the formation of immunological synapses between APCs and T cells, “phagocytic synapses” are required for activation of Dectin-1 when myeloid cells encounter β-glucan-containing microbes. In contrast, it appears that upon detection of soluble β-glucans, the inhibitory activity of membrane tyrosine phosphatases cannot be sufficiently isolated from the crosslinked receptors, and thus Dectin-1 signalling is aborted.
Future studies are required to determine whether the unique nature of the Dectin-1 hemITAM underlies its requirement for ligand immobilisation. We suspect that the C-type lectin family member CLEC-2 may be similarly regulated. CLEC-2 also contains a hemITAM and signals in an apparently identical manner to Dectin-120. In addition to playing a role in platelet aggregation, CLEC-2 is expressed on the surface of neutrophils and is capable of inducing phagocytosis of anti-CLEC-2-coated beads21. Interestingly, Dectin-1/CLEC-2 chimeras can be activated to induce TNF-α production by zymosan but this activation is blocked by soluble β-glucans21 (which presumably bind but fail to activate the receptor), indicating that activation of CLEC-2 signalling may be dependent on the formation of a phagocytic synapse.
It is widely appreciated that the nature of an innate immune response to a microbe is defined by the types of pattern recognition receptors that detect it. Thus receptors that detect viral nucleotides induce responses appropriate for killing viruses. Activation of phagocytic receptors is only appropriate when they bind intact microbes. While models exist for the detection of soluble stimuli (e.g. receptor dimerization, induction of conformational changes), we are currently lacking good models for the discrimination of soluble versus particulate ligands. Our data present the phagocytic synapse as a mechanistic model for the specific detection of ligands associated with a microbial surface, as opposed to those released from distantly located organisms.
Methods Summary
β-glucan preparations
Particulate S. cerevisiae β-glucans - zymosan (Sigma) and whole glucan particles (WGP - Wellmune WGP® from Biothera) - were used as described previously8, 22. Soluble S. cerevisiae β-glucans were prepared by acid hydrolysis of WGP and fractionated by preparative gel permeation chromatography. The molecular weight distribution of each soluble β-glucan was determined by gel permeation chromatography (GPC) with multi-angle laser light scattering photometry (MALLS); the polydispersities of the LMW, MMW, MHMW and HMW soluble β-glucans were 1.5, 1.6, 1.2 and 1.4 respectively. All β-glucan preparations were endotoxin-free and used at 50 μg/ml unless otherwise stated.
Confocal microscopy
Cells were plated on glass coverslips overnight prior to addition of β-glucan particles, brief centrifugation to ensure particle contact with the cells, and incubation at 37°C for the times indicated. Cells were washed to remove unbound particles, fixed with 10% formalin, permeabilised with ice-cold acetone, blocked and stained with primary and secondary antibodies. Coverslips were mounted and examined using a Leica TCS SP5 confocal microscope. Image analysis was performed using Leica LAS AF software, as well as ImageJ and ImageSurfer23.
β-glucan immobilisation and CD45 co-immobilisation
Soluble β-glucans were immobilised on tissue culture plates or polystyrene latex beads by incubation with PBS/EDTA containing 100 μg/ml soluble β-glucan for 1 h at 37°C. Plates or beads were then washed to remove unbound β-glucans, and blocked with media containing 10% FCS prior to use. For CD45 co-immobilisation assays, HMW soluble β-glucan (20 μg/ml) and LPS (100 ng/ml) were immobilised on tissue culture plates in PBS/EDTA in the presence/absence of 10 ng/ml anti-CD45 or rat IgG for 1 h at 37°C, and plates were washed and blocked as above prior to addition of bmDC.
Methods
β-glucan preparations
Particulate S. cerevisiae β-glucans - zymosan (Sigma) and whole glucan particles (WGP - Wellmune WGP® from Biothera) - were used as described previously8, 22. Soluble S. cerevisiae β-glucans were prepared by acid hydrolysis of WGP and fractionated by preparative gel permeation chromatography. The molecular weight distribution of each soluble β-glucan was determined by gel permeation chromatography (GPC) with multi-angle laser light scattering photometry (MALLS); the polydispersities of the LMW, MMW, MHMW and HMW soluble β-glucans were 1.5, 1.6, 1.2 and 1.4 respectively. All β-glucan preparations were endotoxin-free and used at 50 μg/ml unless otherwise stated.
Live yeast
Live Saccharomyces cerevisiae and Candida albicans yeast were cultured in Saboraud Dextrose Broth. Aspergillus fumigatus conidia were prepared from mature colonies grown on potato dextrose agar by flushing with PBS containing 0.05% Tween-80, and incubated in RPMI for 4 h to generate swollen conidia or 12 h to induce germ tube formation.
Cell culture, and functional and biochemical assays
RAW264.7 macrophages stably expressing SBPc-tagged Dectin-1 or the ELAM-luciferase reporter have been described previously8. Dectin-1-deficient mice were provided by Gordon D. Brown, University of Aberdeen. Culture of primary mouse macrophages and DC was performed as in previous studies22. Human monocytes were obtained from peripheral blood, and macrophages were derived by 7-day culture with 50 ng/ml rhM-CSF. Cytokine and reactive oxygen species production, Syk phosphorylation, MAP kinase and NF-κB activation, and Egr2/3 induction were assessed as previously described11, 13, 22. Active phospho-Syk (Y525/Y526), active phospho-Src family kinases (Y416) and inactive phospho-Lyn (Y507) antibodies were from Cell Signalling Technology.
β-glucan immobilisation and CD45 co-immobilisation
Soluble β-glucans were immobilised on tissue culture plates or large polystyrene latex beads (0.8 and 3 μm – Sigma) by incubation with PBS/EDTA containing 100 μg/ml soluble β-glucan for 1 h at 37°C. Plates or beads were then washed 3 times with PBS/EDTA to remove unbound β-glucans, and blocked with media containing 10% FCS for 30 min prior to use. For CD45 co-immobilisation assays, soluble HMW β-glucan (20 μg/ml) and LPS (100 ng/ml) were immobilised on tissue culture plates in PBS/EDTA in the presence/absence of 10 ng/ml anti-CD45 or rat IgG for 1 h at 37°C, and washed and blocked as above prior to the addition of bmDC. For assays using small polystyrene latex beads (0.05, 0.2 and 0.5 mm – Polysciences), coating was achieved by incubating beads in PBS/EDTA containing 50 μg/ml DTAF-labelled soluble HMW β-glucan, and beads were diluted to a final concentration of 0.05 μg/ml β-glucan for stimulation, a dose at which the soluble β-glucans are too dilute to block Dectin-1 signalling by β-glucan particles (data not shown). Beads were fed to cells at a dose that achieves presentation of an equivalent total β-glucan-coated surface area per cell (approximately 40:1 0.5 μm beads:cell, 250:1 0.2 μm beads:cell, and 4000:1 0.05 μm beads:cell).
Soluble β-glucan binding to Dectin-1
Parental RAW264.7 macrophages or RAW264.7 macrophages stably expressing SBPc-tagged-Dectin-1 were incubated for 2 h at 37°C with 100 μg/ml unlabelled MMW soluble β-glucan, and binding was detected by flow cytometry using a mouse IgM monoclonal antibody specific for β–(1,3)-linked glucan specific antibody (BfD IV; clone 10C624) and a FITC-conjugated goat anti-mouse secondary antibody. Dectin-1 expression by the macrophages was assessed by flow cytometry using a FITC-conjugated anti-Dectin-1 antibody (2A11) from Serotec. Macrophages and DC incubated with DTAF-labelled soluble β-glucans were washed and fixed prior to analysis by flow cytometry.
Anti-Dectin-1 competition assay
Cells were incubated on ice in media containing 0.4 μg/ml FITC-anti-Dectin-1 and the indicated concentrations of unlabelled soluble β-glucans for 30 min, and then washed and fixed prior to assessment of anti-Dectin-1 binding by flow cytometry.
Confocal microscopy – fixed cells
Cells were plated on glass coverslips overnight prior to the addition of β-glucan particles (unlabelled zymosan or AlexaFluor647-labelled WGP), brief centrifugation to ensure particle contact with the cells, and incubation for 1 min at 37°C. Cells were then washed with ice-cold PBS to remove unbound particles, fixed with 10% formalin for 20 min, and permeabilised with ice-cold acetone for 30 sec. Non-specific binding was blocked by incubation with TBS + 5% FCS for 10 min. Cells were stained with unconjugated primary antibodies for 1 h as follows: SBPc-tagged Dectin-1 – mouse anti-protein C tag (HPC4; Amersham Biosciences), pSyk – rabbit anti-pSyk (Y525/Y526) and pSrc – rabbit anti-pSrc (Y416) (Cell Signalling Technology), pTyr – mouse anti-pTyr (Cell Signalling Technology), CD45 – rat anti-CD45 (AbD Serotec), and CD148 – hamster anti-CD14817. Cells were then washed and incubated with secondary antibodies for 30 min as follows: Dectin-1 and pTyr – AlexaFluor488-conjugated anti-mouse, pSyk and pSrc - FITC-conjugated anti-rabbit, CD45 – AlexaFluor568-conjugated anti-rat, and CD148 – AlexaFluor568-conjugated anti-hamster (Invitrogen). AlexaFluor647-conjugated cholera toxin (Invitrogen) was used to stain the plasma membrane of unpermeabilised cells. Coverslips were mounted and examined using a Leica TCS SP5 confocal microscope. Image analysis was performed using Leica LAS AF software, as well as ImageJ and ImageSurfer23, as well as Volocity (Perkin Elmer).
Confocal microscopy – live cell imaging
RAW264.7 macrophages stably expressing Dectin-1 tagged with GFP at the carboxy-terminus and CD45 tagged with DsRed at the carboxy-terminus were plated on chamber slides overnight prior to stimulation and maintained at 37°C during confocal imaging. For assessment of cell contact with β-glucan-coated surfaces, chamber slides were incubated with PBS/EDTA control or 100 μg/ml β-glucan in PBS/EDTA for 1 h at 37°C and washed 3 times with PBS/EDTA prior to macrophage addition.
Statistics
Statistical significance was assessed using a Student’s t-test.
Supplementary Material
Acknowledgements
The authors would like to thank Kolja Wawrowsky for help with confocal microscopy, and Gordon D. Brown for Dectin-1-deficient mice. This study was funded by grants from the NIH (D.M.U. – AI071116, and A.W. – AI066120) and the American Heart Association (D.M.U.). H.S.G. held a Research Fellowship Award from the Crohn’s and Colitis Foundation of America. D.M.U. holds the Janis and William Wetsman Family Chair in Inflammatory Bowel Disease at Cedars-Sinai Medical Center.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature
Reprints and permissions information is available at www.nature.com/reprints
The authors declare no competing financial interests.
References
- 1.Goodridge HS, Wolf AJ, Underhill DM. Beta-glucan recognition by the innate immune system. Immunol Rev. 2009;230:38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerrigan AM, Brown GD. Syk-coupled C-type lectin receptors that mediate cellular activation via single tyrosine based activation motifs. Immunol Rev. 2010;234:335–52. doi: 10.1111/j.0105-2896.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferwerda B, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–7. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plantinga TS, et al. Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49:724–32. doi: 10.1086/604714. [DOI] [PubMed] [Google Scholar]
- 5.Saijo S, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 6.Taylor PR, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–8. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennehy KM, et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–6. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–17. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nat Immunol. 2006;7:1258–65. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- 10.Brown GD, et al. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med. 2002;196:407–12. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106:2543–50. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czop JK, Austen KF. A beta-glucan inhibitable receptor on human monocytes: its identity with the phagocytic receptor for particulate activators of the alternative complement pathway. J Immunol. 1985;134:2588–93. [PubMed] [Google Scholar]
- 13.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178:3107–15. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 14.Adams EL, et al. Differential high-affinity interaction of dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branching. J Pharmacol Exp Ther. 2008;325:115–23. doi: 10.1124/jpet.107.133124. [DOI] [PubMed] [Google Scholar]
- 15.Fooksman DR, et al. Functional anatomy of T cell activation and synapse formation. Annu Rev Immunol. 2010;28:79–105. doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermiston ML, Zikherman J, Zhu JW. CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signalling networks in immune cells. Immunol Rev. 2009;228:288–311. doi: 10.1111/j.1600-065X.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin J, Weiss A. The tyrosine phosphatase CD148 is excluded from the immunologic synapse and down-regulates prolonged T cell signalling. J Cell Biol. 2003;162:673–82. doi: 10.1083/jcb.200303040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu JW, Brdicka T, Katsumoto TR, Lin J, Weiss A. Structurally distinct phosphatases CD45 and CD148 both regulate B cell and macrophage immunoreceptor signalling. Immunity. 2008;28:183–96. doi: 10.1016/j.immuni.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown GD, et al. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–24. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuller GL, et al. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signalling cascade. J Biol Chem. 2007;282:12397–409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerrigan AM, et al. CLEC-2 is a phagocytic activation receptor expressed on murine peripheral blood neutrophils. J Immunol. 2009;182:4150–7. doi: 10.4049/jimmunol.0802808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodridge HS, et al. Differential use of CARD9 by dectin-1 in macrophages and dendritic cells. J Immunol. 2009;182:1146–54. doi: 10.4049/jimmunol.182.2.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng D, et al. Stepping into the third dimension. J Neurosci. 2007;27:12757–60. doi: 10.1523/JNEUROSCI.2846-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavigne LM, Albina JE, Reichner JS. Beta-glucan is a fungal determinant for adhesion-dependent human neutrophil functions. J Immunol. 2006;177:8667–75. doi: 10.4049/jimmunol.177.12.8667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.