Abstract
Background
Direct microscopy, anaerobic culture and DNA–DNA hybridization have previously demonstrated an association between microorganisms and osteoradionecrosis (ORN). The purpose of our study was to use culture independent molecular techniques to detect bacteria in necrotic bone lesions of the mandible after radiation therapy.
Design
Bacterial DNA was extracted from eight deep medullar specimens from resected mandibles (six cases), including one patient with relapse. 16S rRNA genes were PCR amplified, cloned, transformed into Escherichia coli and sequenced to determine species identity and closest relatives.
Results
From the analysis of 438 clones, 59 predominant species were detected, 27% of which have not been cultivated. The predominant species detected from radionecrotic mandibles were Campylobacter gracilis, Streptococcus intermedius, Peptostreptococcus sp. oral clone FG014, uncultured bacterium clone RL178, Fusobacterium nucleatum, and Prevotella spp. The study demonstrated intersubject variability of the bacteria present in ORN. In contrast to the diverse bacterial profile detected in primary infection, only a few members of the oral indigenous flora were identified from the relapse case.
Conclusions
Diverse bacterial profiles in specimens of ORN in marrow spaces of the mandible were detected by culture independent molecular techniques. To better understand the pathogenesis and to improve the therapy of the infection, detection of all members of the complex bacterial flora associated with ORN is necessary.
Keywords: osteoradionecrosis, bacteria, 16S rRNA genes
Osteoradionecrosis (ORN) is a late complication and calculated risk to conventional radiation therapy used in treatment of cancer in the craniofacial skeleton. As a consequence, adverse effects of radiation therapy affect normal tissue cells and the vascular system of bone, cartilage, and soft tissue. The complexity of the disease still leaves the definition, etiology, and pathogenesis of ORN unclear (1, 2). ORN was earlier attributed to secondary infection in the traumatized irradiated tissue following the non-healing wounds and exposed bone (3, 4). Marx stated in 1983 that infection associated with ORN was only superficial and secondary, and that the microorganisms found in resections were surface contaminants. The statement was based on a study that failed to describe microorganisms in the medullar parts of the resections (5). Støre and Olsen (6) demonstrated the existence of a diverse microbiota of the medullar parts of the mandible visualized by scanning and transmission electron microscopy and by DNA–DNA hybridization in a checkerboard assay (7). The detection of anaerobes indicates that infection might play an important role in the pathogenesis of ORN (6, 7). Based on analysis of irradiated bone biopsies from a high number of patients with post-operative complications, Hansen et al. (1, 8) also demonstrated an association between Actinomyces and infected osteoradionecrosis (IORN). Nason and Chole (2) described formation of biofilm in association with ORN of the temporal bone after external beam radiation. Phylogenetic analysis of complex bacterial communities in biofilms relies on the sequences of housekeeping genes in bacteria, like the 16S rRNA gene, and includes both the cultivable and not-yet cultivable segment of the bacterial flora. By using modern molecular techniques, studies have shown the breadth of microbial diversity of the whole gastrointestinal tract in health and disease, and it has been discovered that as much as 50% of the oral (9) and 80% of the intestinal (10) indigenous bacterial flora consist of uncultured phylotypes. Detection of all members of the complex bacterial communities is necessary to better understand the role of infection in the pathogenesis of ORN. The aim of this study was to use culture independent molecular techniques to unveil the bacteria in necrotic bone lesions of the mandible after radiation therapy.
Materials and methods
Specimens and subjects
Eight specimens (six consecutive cases) from mandibular ORN and one relapse subject were used in this study. All subjects recruited for the study required a full segmental resection larger than 5 cm during the course of treatment. The participants received a conservative treatment regimen of local wound care and continuous treatment with tetracycline (doxycycline 100 mg daily). In cases of clinical exacerbation this dose was doubled, or a limited supplementary regimen of clindamycin, 150 mg three times daily, was prescribed.
Bone samples
All resections were made from the mandibular body region. Deep medullar bone specimens were obtained using sterile trepan burrs (3 mm wide), collecting bicortical cylinders from areas previously covered by mucoperiost and at a minimum distance of 3 cm from any surface exposed by oro-cutaneous fistulas. The cortical segments were removed from the specimens and the marrow part was suspended in 50 mM tris buffer (pH 7.6), and 1 mM EDTA, pH 8, for further analysis. The size of the lesion in the specimen from subject 1 allowed collection of two bicortical cylinders. The bone marrow samples were analyzed separately to assess the reproducibility of the molecular techniques. After primary infection and the subsequent treatment, subject 2 experienced a relapse resulting in a second resection. Specimens of the primary infection (p) and the relapse infection (r) were analyzed independently (Fig. 1).
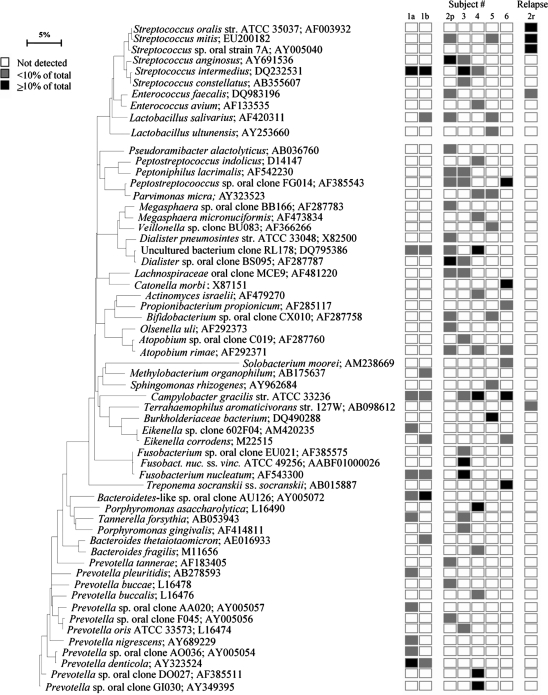
Fig. 1.
Bacterial profiles of mandibular osteoradionecrosis. Subject 1 is represented with two separate specimens (1a and 1b) from one lesion and subject 2 with a primary- (2p) and a relapse infection (2r). Distribution and levels of bacterial species/phylotypes among the eight samples from six subjects are shown by the columns of boxes to the right of the tree as either not detected (clear box), <10% of the total number of clones assayed (shaded/gray box), or ≥10% of the total number of clones assayed (dark box). 10% level was chosen arbitrarily. GenBank accession numbers are provided. Marker bar represents a 5% difference in nucleotide sequences.
DNA extraction
The marrow part of small bone cylinders was ground in liquid nitrogen to fine powder in a sterile mortar in a laminar airflow cabinet. The powder was suspended in 50 mM tris-HCl and DNA was extracted from the bone tissue samples by using the ChargeSwitch Forensic DNA Purification Kit (Invitrogen, San Diego, CA) according to the protocol of the manufacturer.
Amplification of 16S rRNA gene
The 16S rRNA gene was amplified under standardized conditions using a universal forward primer (5′-GAG AGT TTG ATY MTG GCT CAG-3′) and a universal reverse primer (5′-GAA GGA GGT GWT CCA RCC GCA-3′) (11). PCR was performed with the GeneAmp PCR systems 9700 (ABI, Foster City, CA). One microliter of DNA template was added to a reaction mixture (final volume, 50 µl) containing 20 pmol of each primer, 40 nmol of deoxytriphosphates, and 1 U of Platinum Taq polymerase (Invitrogen). In a hot-start protocol, the samples were preheated at 95°C for 4 min, followed by amplification under the following conditions: 95°C for 45 s, 60°C for 45 s, and 72°C for 1.5 min, with an additional 5 s for each cycle. A total of 30 cycles were performed, which was followed by a final elongation step at 72°C for 15 min. The results of the PCR amplification were examined by electrophoresis in a 1% agarose gel. DNA was stained with Ethidium bromide and visualized under short-wavelength UV light.
Cloning procedures
Cloning of PCR-amplified DNA was performed with the TOPO TA cloning kit (Invitrogen) according to the manufacturer's instruction. Briefly, transformation was done with competent Escherichia coli TOP10 cells. The transformed cells were plated onto Luria-Bertani agar plates supplemented with kanamycin (50 µg/ml), and the plates were incubated overnight at 37°C. Colonies were transferred to 70 µl of 10 mM tris-HCl. Correct sizes of the inserts were determined in a PCR with a forward primer M13 and a reverse primer M13 (Invitrogen). Prior to sequencing, the amplified PCR 16S rRNA fragments were purified and concentrated by using the QIAquick PCR purification kit (Qiagen, Valencia, CA).
16S rRNA gene sequencing and data analysis
Purified DNA from the PCR was sequenced with an ABI Prism cycle sequencing kit (BigDye Terminator Cycle Sequencing Kit with AmpliTaq DNA Polymerase FS, GeneAmp PCR systems 2700 and 9700; ABI). The protocol and primers used for sequencing have been described previously (11). The sequencing reactions were run on an ABI 3730 DNA sequencer (ABI). The number of sequenced clones per sample ranged from 45 to 84, with an average of 62.6 clones. A sequence of approximately 500 bases was obtained first to determine identity or approximate phylogenetic position. For identification of closest relatives, the sequences of the inserts were compared to the 16S rRNA gene sequences of over 100,000 sequences in the Ribosomal Database Project (12), EMBL (http://www.ebi.ac.uk/embl/), NCBI GenBank databases (http://www.ncbi.nlm.nih.gov/), and the Human Oral Microbiome Database (http://www.homd.org). The similarity matrices were corrected for multiple base changes at single positions by the method of Jukes and Cantor (13). Similarity matrices were constructed from the aligned sequences by using only those sequence positions for which data were available for 90% of the strains tested. The cutoff for species identification was 98% similarity. Phylogenetic trees were constructed by the neighbor-joining method of Saitou and Nei (14). TREECON, a software package for the Microsoft Windows environment, was used for the construction and drawing of evolutionary trees (15). We are aware of the potential creation of 16S rRNA chimera molecules assembled during the PCR (16). Chimeric sequences were identified by using the Chimera Check program in the Ribosomal Database Project, by treeing analysis, or by base signature analysis. Sequences suspected or identified as chimeric were excluded from phylogenetic construction and analysis. Nucleotide sequences are available for electronic retrieval in the EMBL, GenBank, and DDBJ (http://www.ddbj.nig.ac.jp/index-j.html) nucleotide sequence databases under accession numbers included in Fig. 1.
Results and discussion
Scanning and transmission electron microscopy (6) demonstrated the presence of bacteria in marrow spaces of the mandible. Another study based on DNA–DNA hybridization (7) from our group documented the presence of cultivable bacterial flora in ORN specimens. In the present study, eight specimens from six additional subjects with ORN were selected for further analysis with culture independent molecular techniques for identification of both cultivable and not yet cultivable bacteria. From a total of 438 clones, a diverse bacterial flora of 59 taxa was detected from eight specimens of ORN. Six phyla were identified, including Firmicutes, Actinobacteria, Proteobacteria, Fusobacteria, Spirochaetes, and Bacteroidetes. Of the identified bacteria, 27% cannot be cultivated. The two bone marrow samples collected from the lesion in subject 1 (1a, 1b) were analyzed separately to test whether the microbial profiles of the same lesion were uniform or differed in composition. The analyzed results show how the profiles clustered (Fig. 1). They indicated that bicortical cylinders when analyzed with sensitive molecular techniques reveal representative microbial flora associated with the actual lesion. The specimens analyzed from subject 2, represented primary (2p) and relapse infection (2r) after treatment (Fig. 1). The bacterial profile after primary infection was dominated by Streptococcus anginosus, Dialister spp. and Prevotella spp. S. anginosus is a member of the indigenous flora of the gastrointestinal tract, but has previously been detected in osteoarthritis, endocarditis, spondylodiscitis, osteomyelitis, and subdural empyema (17–20). From the relapse specimen only five bacterial species were identified compared to the 18 species detected in the primary infection. Members of the normal oral flora, such as Streptococcus oralis, Streptococcus mitis, and Streptococcus sp. oral strain 7A, dominated among the species detected, indicating an opportunistic situation due to treatment failure.
In the last decade, culture independent techniques have underlined the need to include all members of the bacterial flora to understand the role of bacteria in health and disease. Despite the challenges related to DNA extraction from bone tissue, Fenollar et al. (21) reported bacterial diversity, which included not cultivated phylotypes, from 525 samples of infected bones and joints. The bone and joint samples were collected from the whole body. Their results confirmed previous findings (7, 22) suggesting that anaerobes are underestimated and can play a central role in polymicrobial infections in bone. From the ORN data in the present study, we also recognized a predominance of anaerobes (Fig. 1). In accordance to Fenollar et al. (21), who detected several human pathogens not previously reported or rare in bone and joint infections, species like Parvimonas micra, Peptoniphilus lacrimalis, Porphyromonas asaccharolytica, and Prevotella buccalis (Fig. 1) were found in the present study.
From the eight specimens analyzed, Actinomyces israelii was only detected at a low level in one subject (Fig. 1). This result differed from a previous study from our group, based on DNA–DNA hybridization, where Actinomyces spp. were detected in all 12 specimens of ORN (7). In a retrospective study of 50 subjects with ORN of the jaws, Curi et al. (23) diagnosed Actinomyces in six cases (12%) with ORN. From culture dependent and histochemical techniques, Hansen et al. (1) found Actinomyces colonies in 20 of 31 patients (64.5%) that underwent high-dose irradiation with the typical clinical symptoms of IORN. A semi-nested PCR testing based on 16S rRNA genes for the presence of Actinomyces species was performed in three cases, which confirmed the presence of A. israelii. In a material of head and neck neoplasm where bone biopsies of radiographically affected mandibulas were analyzed, Hansen et al. (8) demonstrated with histology Actinomyces spp. in 16 out of 25 patients. The variation in Actinomyces recovery may be ascribed to differences in techniques and microfloras.
Streptococcus intermedius was among the predominant bacterial species detected in the present study (Fig. 1). S. intermedius is a member of the indigenous bacterial flora of the mouth and the upper respiratory tract. In the healthy oral cavity, S. intermedius is a commensal of supra-/sub-gingival habitats of the teeth and in the crypts of the tonsils (9). In addition to being a member of the normal flora, S. intermedius is associated with pyogenic infections like endocarditis, pneumonia, abdominal, or cerebral abscess (24–27) Calza et al. (28) concluded that S. intermedius and the ‘milleri group’ should be considered as elusive pathogens in osteomyelitis and muscular abscess as well.
The present study defined diverse bacterial profiles of specimens of ORN in marrow spaces of the mandible through culture independent molecular techniques. Complex bacterial communities, represented by cultivable and not yet cultivable bacteria, in a wide range of bacterial taxa, emphasize the need to include all the members of putative pathogens for the future clinical management and choice of antibacterial therapy in patients suffering from ORN.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors. The study was supported by the Faculty of Dentistry, University of Oslo and Rikshospitalet University Hospital, Oslo, Norway.
References
- 1.Hansen T, Kunkel M, Kirkpatrick CJ, Weber A. Actinomyces in infected osteoradionecrosis—underestimated? Hum Pathol. 2006;37:61–7. doi: 10.1016/j.humpath.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Nason R, Chole RA. Bacterial biofilms may explain chronicity in osteoradionecrosis of the temporal bone. Otol Neurotol. 2007;28:1026–8. doi: 10.1097/MAO.0b013e318157f102. [DOI] [PubMed] [Google Scholar]
- 3.Meyer I. Infectious diseases of the jaws. J Oral Surg. 1970;28:17–33. [PubMed] [Google Scholar]
- 4.Rankow RM, Weissman B. Osteoradionecrosis of the mandible. Ann Otol Rhinol Laryngol. 1971;80:603–11. doi: 10.1177/000348947108000426. [DOI] [PubMed] [Google Scholar]
- 5.Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;48:283–9. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- 6.Støre G, Olsen I. Scanning and transmission electron microscopy demonstrates bacteria in osteoradionecrosis. Int J Oral Maxillofac Surg. 2005;34:777–81. doi: 10.1016/j.ijom.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Støre G, Eribe ERK, Olsen I. DNA–DNA hybridization demonstrates multiple bacteria in osteoradionecrosis. Int J Oral Maxillofac Surg. 2005;34:193–6. doi: 10.1016/j.ijom.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Hansen T, Wagner W, Kirkpatrick CJ, Kunkel M. Infected osteoradionecrosis of the mandible: follow-up study suggests deterioration in outcome for patients with Actinomyces-positive bone biopsies. Int J Oral Maxillofac Surg. 2006;35:1001–4. doi: 10.1016/j.ijom.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–32. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–83. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–5. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jukes TH, Cantor CR. Evolution of protein molecules. Mammalian protein metabolism. In: Munro HN, editor. Vol. 3. New York, NY: Academic Press; 1969. pp. 21–132. [Google Scholar]
- 14.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 15.Van de Peer Y, De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci. 1994;10:569–70. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- 16.Liesack W, Weyland H, Stackebrandt E. Potential risk of gene amplification by PCR as determined by 16S rDNA analysis of a mixed-culture of strict barophilic bacteria. Microb Ecol. 1991;21:191–8. doi: 10.1007/BF02539153. [DOI] [PubMed] [Google Scholar]
- 17.Mateo Soria L, Miquel Nolla Solé J, Rozadilla Sacanell A, Valverde García J, Roig Escofet D. Infectious arthritis in patients with rheumatoid arthritis. Ann Rheum Dis. 1992;51:402–3. doi: 10.1136/ard.51.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woo PC, Tse H, Chan KM, Lau SK, Fung AM, Yip KT, et al. “Streptococcus milleri” endocarditis caused by Streptococcus anginosus . Diagn Microbiol Infect Dis. 2004;48:81–8. doi: 10.1016/j.diagmicrobio.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Weber M, Gubler J, Fahrer H, Crippa M, Kissling R, Boos N, et al. Spondylodiscitis caused by Viridans Streptococci: three cases and a review of the literature. Clin Rheumatol. 1999;18:417–21. doi: 10.1007/s100670050130. [DOI] [PubMed] [Google Scholar]
- 20.Greenlee JE. Subdural empyema. Curr Treat Options Neurol. 2003;5:13–22. doi: 10.1007/s11940-003-0019-7. [DOI] [PubMed] [Google Scholar]
- 21.Fenollar F, Roux V, Stein A, Drancourt M, Raoult D. Analysis of 525 samples to determine the usefulness of PCR amplification and sequencing of the 16S rRNA gene for diagnosis of bone and joint infections. J Clin Microbiol. 2006;44:1018–28. doi: 10.1128/JCM.44.3.1018-1028.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis RP, Sutter VL, Finegold SM. Bone infections involving anaerobic bacteria. Medicine (Baltimore) 1978;57:279–305. doi: 10.1097/00005792-197807000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Curi MM, Dib LL, Kowalski LP, Landman G, Mangini C. Opportunistic actinomycosis in osteoradionecrosis of the jaws in patients affected by head and neck cancer: incidence and clinical significance. Oral Oncol. 2000;36:294–9. doi: 10.1016/s1368-8375(99)00080-9. [DOI] [PubMed] [Google Scholar]
- 24.Piscitelli SC, Shwed J, Schreckenberger P, Danziger LH. Streptococcus milleri group: renewed interest in an elusive pathogen. Eur J Clin Microbiol Infect Dis. 1992;11:491–8. doi: 10.1007/BF01960802. [DOI] [PubMed] [Google Scholar]
- 25.Shinzato T, Saito A. The Streptococcus milleri group as a cause of pulmonary infections. Clin Infect Dis. 1995;21:S238–43. doi: 10.1093/clind/21.supplement_3.s238. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto M, Fukushima T, Ohshiro S, Go Y, Tsugu H, Kono K, et al. Brain abscess caused by Streptococcus intermedius: two case reports. Surg Neurol. 1999;51:219–22. doi: 10.1016/s0090-3019(97)00505-3. [DOI] [PubMed] [Google Scholar]
- 27.Herskovitz MY, Goldsher D, Finkelstein R, Bar-Lavi Y, Constantinescu M, Telman G. Multiple brain abscesses associated with tongue piercing. Arch Neurol. 2009;66:1292. doi: 10.1001/archneurol.2009.204. [DOI] [PubMed] [Google Scholar]
- 28.Calza L, Manfredi R, Briganti E, Attard L, Chiodo F. Iliac osteomyelitis and gluteal muscle abscess caused by Streptococcus intermedius . J Med Microbiol. 2001;50:480–2. doi: 10.1099/0022-1317-50-5-480. [DOI] [PubMed] [Google Scholar]