Abstract
Objectives
The aim of this study was to evaluate the effects of Lactobacilli reuteri (Prodentis) alone and in combination with scaling and root planing (SRP) in a double blind, randomized, placebo-controlled clinical trial of volunteers with chronic periodontitis.
Methods
Thirty, otherwise systemically healthy, chronic periodontitis patients (19 males and 11 females, aged between 34 and 50 years) were included. The study period was 42 days. ‘Split-mouth’ design was used for the SRP, which was performed on day 0; two quadrants (either right or left) were treated with SRP whereas the remaining two quadrants were left untreated. The participants received a toothbrush, toothpaste, and brushing instructions. L. reuteri Prodentis lozenges (1×108 CFU DSM17938+1×108 CFU ATCC PTA 5289) or the corresponding placebo lozenges were taken twice daily from day 21 to day 42. Statistical analysis was done for comparisons of clinical parameters (Plaque Index (PI), Gingival Index (GI), Gingival Bleeding Index (GBI), probing pocket depth (PPD), clinical attachment level (CAL)) and microbiological levels of the pathogens Aggregibacter actinomycetemcomitans (Aa), Porphyromonas gingivalis (Pg), and Prevotella intermedia (Pi). All p-values less than 0.05 were considered significant. Assessments were made on day 0 before SRP treatment, on day 21 before administration of the lozenges, and on day 42.
Results
At day 42, the PI, GI, and GBI were significantly reduced by all treatment modalities. When ranked, the amount of PI, GI and GBI reduction by the different treatments was SRP + Prodentis Prodentis SRP + placebo placebo; all differences were statistically significant. For PPD and CAL, the best result was obtained with the SRP+Prodentis treatment. PPD was reduced from 5.08±0.75 to 3.78±0.61 mm (p<0.001) and CAL from 3.93±0.93 to 2.85±0.74 mm (p<0.001). Prodentis, either alone or following SRP, reduced Aa, Pi, and Pg by 1 log10 unit (p<0.01). The SRP+placebo combination did not significantly affect the levels of the pathogens.
Conclusion
The present randomized controlled trial confirms the plaque inhibition, anti-inflammatory, and antimicrobial effects of L. reuteri Prodentis. L. reuteri Prodentis probiotic can be recommended during non-surgical therapy and the maintenance phase of periodontal treatment. Considering the beneficial effects of probiotics, this therapy could serve as a useful adjunct or alternative to periodontal treatment when SRP might be contraindicated. Further studies are required in this direction.
Keywords: scaling and root planning, DSM 17938, ATCC PTA 5289, chronic generalized periodontitis, anti-inflammatory, antimicrobial, plaque, gingivitis
There is a long tradition, particularly in parts of Europe and Asia, of ingesting microbes or food products that affect the intestinal microbiota in ways that are believed to provide beneficial health effects, i.e. intake of probiotics and prebiotics. According to the World Health Organization, probiotics are defined as viable microorganisms that confer a health benefit when administered in sufficient doses.
A few studies have revealed that probiotic Lactobacillus strains were useful in reducing gingival inflammation and the number of black-pigmented rods, including Porphyromonas gingivalis (Pg), in the saliva and subgingival plaque (1–3). Concerning periodontal conditions, Teughels and coworkers (4) have shown that application of beneficial bacteria, as an adjunct to scaling and root planing (SRP), can inhibit recolonization of pathogens in periodontal pockets and reduce bleeding on probing in dogs. Another clinical trial demonstrated a reduced prevalence of moderate to severe gingival inflammation as well as an improved Plaque Index (PI) and probing depth in adults after regular use of probiotic tablets (5). Also, probiotic chewing gums consumed over the course of 2 weeks caused a reduction in proinflammatory cytokines in gingivitis patients (6).
On the other hand, mechanical debridement by SRP is one of the basic periodontal treatment procedures known to reduce periodontal inflammatory effects (7–9). A Medline search using the key words ‘CGP’ (chronic generalized periodontitis), ‘SRP,’ and ‘Probiotics’ revealed a lack of data on Gingival Index (GI) and Gingival Bleeding Index (GBI) reduction after SRP along with probiotics in the treatment of periodontal disease. To date, the combination of SRP and probiotics has not been addressed as a treatment protocol for periodontal patients. Hence, to determine the effects of probiotics in periodontitis cases, we conducted a double blind, randomized, placebo-controlled clinical trial in patients with chronic periodontitis.
Material and methods
This was a randomized, placebo-controlled, double blind, split-mouth designed clinical study to evaluate the effect of probiotic lozenges with and without SRP on the clinical parameters and microbiological profile in subgingival plaque samples of chronic periodontitis patients.
To calculate the proper sample size, the change in probing depth at the end of 42 days was estimated to be 0.1 mm in the test group. The sample size was calculated to be approximately 15 subjects each in the test and placebo groups with a power of 0.80 and α at 0.05.
Patients of both sexes within the age limit of 35–50 years were eligible for inclusion. Patients diagnosed as suffering from chronic periodontitis as clinically evidenced by gingivitis together with mild to moderate periodontal pockets (5–7 mm) clinically and radiographic evidence of bone loss were included (10). Apart from having chronic periodontitis, the patients were in general good health and had not participated in any clinical trial during the previous 4 weeks. No patient had ongoing antibiotic treatment or any systemic disease. Patients who were pregnant, lactating, smokers, alcoholic, or who had undergone any surgical or non-surgical therapy within 6 months prior to the start of the study were not included. Written informed consent was obtained from all patients.
All subjects were assigned to one of the two groups (Prodentis group or placebo group) by using a randomization table. To maintain full blinding of the results, the randomization code was held by one of the authors remotely from all assessments and was not broken until all data had been collected and all analyses had been performed. The randomization was concealed by using sequentially numbered, identical-appearing containers of probiotic or placebo tablets.
Clinical and microbiological parameters were recorded at baseline (day 0), on day 21, and day 42. The clinical parameters recorded were PI (11), GI (12), GBI (13), probing pocket depth (PPD), and clinical attachment level (CAL) (14). PPD and CAL were measured for the indexed teeth of the Community Periodontal Index using the WHO probe (14). All parameters were assessed by a single clinical investigator experienced with the index systems. Patients were given Lactobacillus reuteri Prodentis (containing a minimum of 1×108 colony forming units (CFU) for each of the strains DSM17938 and ATCC PTA 5289 (Prodentis)). Both study products, Prodentis and placebo lozenges, were identical in shape, texture, and taste. The patients were instructed to take suck lozenge by sucking in the morning and one at night, after brushing their teeth (2). The lozenges were administered to the patients from day 21 after the clinical and microbiological parameters had been assessed and continued until day 42.
Split-mouth SRP in all patients was achieved by treating two quadrants with SRP on day 0 while two quadrants were left untreated. SRP was performed using ultrasonic (Cavitron® – BOBCAT PRO, DENTSPLY; Power-240AC 50/60Hz 80VA) and hand instruments (Universal Gracey Curettes, 2R/2L and 4R/4L Hufriedy). After SRP, the patients were instructed to perform regular oral hygiene habits, i.e. twice daily brushing by ‘roll-on technique’ for a minimum of 2 min, using a regular toothpaste (STOLIN-R® Dr. Reddy's Lab) and regular toothbrushes (Stim® toothbrush – DentAids Pvt. Ltd.), which were provided to each subject.
After recording the clinical parameters, at least 10–12 sites with 5–7 mm pocket depth were selected for subgingival pooled plaque sample collection separately for each half of the mouth. The pooled plaque samples were collected at baseline and on day 21 and day 42 from the same sites. The sample site was isolated with sterile cotton rolls and the supragingival plaque was removed using cotton rolls and air dried. A sterile curette was introduced to the base of the pocket and plaque was removed. The curette with the collected plaque was dispensed in separate vials containing transport media viz. thioglycolate broth with hemin and Vit k (transport 8 medium) and sealed tightly to avoid contamination. Samples were processed within 2 days of collection. The sample was mixed thoroughly and 5µl aliquots were was inoculated using a sterile loop onto petri plates with the following mediums: enriched trypticase soy agar for Aggregibacter actinomycetemcomitans (Aa) cultivation (15); kanamycin blood agar for Pg (16); and kanamycin and vancomycin blood agar for Prevotella intermedia (Pi) (16). The samples were then vortexed at 3000×g for 1 min to break the plaque and to obtain uniform dispersal of organisms.
The plates were kept under anaerobic conditions. The medium for Aa was incubated in a desiccator with 5% carbon dioxide at 37°C for 5 days. The plates were incubated for a minimum of 72 h. After the specified period, the colonies were characterized according to size, shape, hemolysis, and pigmentation and the number of each colony type was counted.
Aa was identified by its characteristic star-shaped colonies on the agar plate, Gram negative coccobacillary morphology, negative indole test, nitrate reduction, and glucose fermentation. Porphyromonas and Prevotella species were provisionally identified by pigment production, colony characters, cell morphology, and the variation in their susceptibility to kanamycin and vancomycin. The identity of these organisms was further confirmed by subjecting them to a series of biochemical reactions, which included indole production, nitrate reduction, and sugar fermentation tests. The sugars tested were dextrose, mannitol, lactose, sucrose, maltose, salicin, glycerol, xylose, arabinose, trehalose, rhamnose, and xylan. A uniform standardized suspension of the organisms was prepared in thioglycollate broth and inoculated into peptone yeast extract broth containing 1% of the appropriate sugar and bromocresole purple as the indicator. The tubes were incubated for 48 h in a modified gas-pak anaerobic jar and the fermentation reactions were noted by a change in the color of the indicator to yellow. Indole and nitrate reduction tests were performed by spot disc method using commercially available reagents (Hi-Media).
All clinical and microbiologic data collected were subjected to statistical analysis. Results are expressed as mean±SD and proportions as percentages. For clinical parameters, intra-group comparisons were made by paired t-test and inter-group comparison by unpaired t-test. For microbiological parameters, non-parametric methods were used for analysis since microbes were non-normally distributed; the Wilcoxon's signed rank test was used for intra-group comparison and the Mann–Whitney test for inter-group comparison. For all tests a p-value of less than 0.05 was considered statistically significant.
The study protocol was in accordance with the local ethical guidelines and in accordance with the Helsinki Declaration of Human Rights and approved by the local ethics committee.
Results
Thirty, systemically healthy, chronic periodontitis patients (19 males and 11 females, aged between 34 and 50 years) were included. Age, gender distribution, clinical and microbiological parameters were similar in both groups at day 0 (baseline) (Table 1). All subjects completed the 42-day study period. There were no significant differences in the clinical and microbiological parameters between the Prodentis and placebo groups at day 0.
Table 1.
Baseline characteristics of study participants (mean (SD))
| Prodentis group | Placebo group | Significance | |
|---|---|---|---|
| Men/women | 9/6 | 10/5 | ns |
| Age (years) | 41.4 (5.3) | 41.5 (4.9) | ns |
| PI (score) | 1.79 (0.36) | 1.77 (0.20) | ns |
| GI (score) | 1.85 (0.22) | 1.88 (0.12) | ns |
| GBI (%) | 81.6 (18.4) | 87.9 (13.5) | ns |
| PPD (mm) | 5.08 (0.75) | 5.26 (0.53) | ns |
| CAL (mm) | 3.93 (0.93) | 4.46 (1.94) | ns |
| Aa (CFU/ml×104) | 105.3 (66.8) | 103.0 (66.4) | ns |
| Pg (CFU/ml×104) | 89.7 (70.4) | 98.7 (60.4) | ns |
| Pi (CFU/ml×104) | 81.0 (67.0) | 80.3 (73.1) | ns |
The active study product of Prodentis lozenges was re-analyzed by the producer (BioGaia AB, Sweden) at the end of the study and the CFU count of both strains was above the stipulated shelf-life limit of 1×108 CFU. The presence of lactobacilli in the saliva, plaque, or gingival pockets was not assessed in this study.
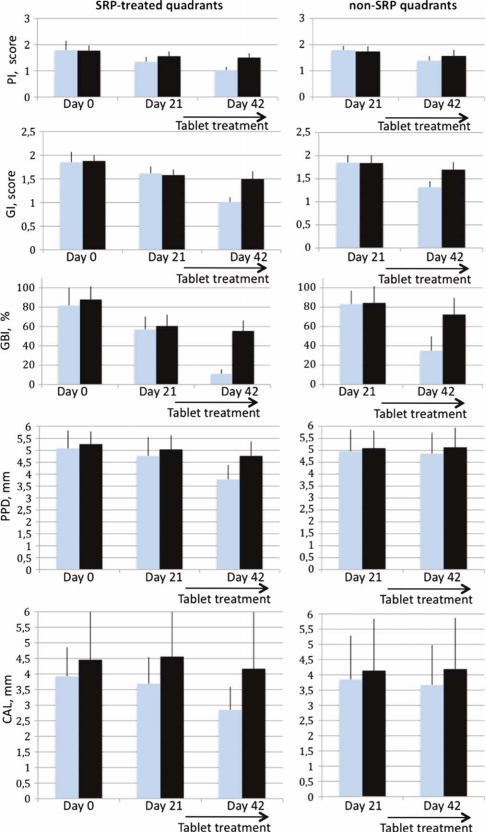
Within both the Prodentis and the placebo groups, with or without SRP, PI, GI, or GBI, the reduction was highly significant (p<0.001) over the treatment periods (Fig. 1). The maximum reduction of PI (0.76±0.29) was obtained for the combination of SRP and Prodentis, which was statistically significant when compared to all other treatment modalities (p<0.01) (Table 2). In the untreated quadrants, Prodentis was significantly better than the placebo with a reduction of 0.41±0.16 and 0.17±0.14, respectively (p<0.001). Also for the GI and GBI, the best reduction was obtained with the combination of SRP and Prodentis as compared with the other treatment modalities (p<0.001), the GI fell from 1.85±0.22 to 1.01±0.10 and the GBI from 81.6±18.4 to 11.1±4.6% over the full period. In the untreated quadrants, Prodentis was better than the placebo on both accounts, the GI was reduced by 0.53±0.12 and 0.14±0.14 units (p<0.001) and the GBI by 48.3±14.4 and 12.0±8.7 percentage units (p<0.001), respectively. An interesting observation was that Prodentis alone demonstrated a highly significant (p<0.001) GBI reduction as compared to SRP alone.
Fig. 1.
Results of the clinical assessments during the study period. Error bars indicate SD.  =Prodentis group;
=Prodentis group;  =placebo group.
=placebo group.
Table 2.
Statistical comparison (unpaired t-test) of the clinical effects between the treatment modalities after 42 days (values are given as mean (SD))
| Group | Reduction | Group | Reduction | Significance |
|---|---|---|---|---|
| PI score | ||||
| SRP+Prodentis | 0.76 (0.29) | SRP+placebo | 0.27 (0.13) | p<0.001 |
| SRP+Prodentis | 0.76 (0.29) | Prodentis | 0.41 (0.16) | p<0.001 |
| SRP+placebo | 0.27 (0.13) | Placebo | 0.17 (0.14) | p<0.05 |
| Prodentis | 0.41 (0.16) | Placebo | 0.17 (0.14) | p<0.001 |
| GI score | ||||
| SRP+Prodentis | 0.84 (0.23) | SRP+placebo | 0.38 (0.23) | p<0.001 |
| SRP+Prodentis | 0.84 (0.23) | Prodentis | 0.53 (0.12) | p<0.001 |
| SRP+placebo | 0.38 (0.23) | Placebo | 0.14 (0.14) | p<0.01 |
| Prodentis | 0.53 (0.12) | Placebo | 0.14 (0.14) | p<0.001 |
| GBI (%) | ||||
| SRP+Prodentis | 70.4 (10.7) | SRP+placebo | 32.5 (10.0) | p<0.001 |
| SRP+Prodentis | 70.4 (10.7) | Prodentis | 48.3 (14.4) | p<0.001 |
| SRP+placebo | 32.5 (10.0) | Placebo | 12.0 (8.7) | p<0.001 |
| Prodentis | 48.3 (14.4) | Placebo | 12.0 (8.7) | p<0.001 |
| PPD (mm) | ||||
| SRP+Prodentis | 1.31 (0.49) | SRP+placebo | 0.49 (0.39) | p<0.001 |
| SRP+Prodentis | 1.31 (0.49) | Prodentis | 0.10 (0.20) | p<0.001 |
| SRP+placebo | 0.49 (0.39) | Placebo | −0.04 (0.23) | p<0.001 |
| Prodentis | 0.10 (0.20) | Placebo | −0.04 (0.23) | ns |
| CAL (mm) | ||||
| SRP+Prodentis | 1.09 (0.62) | SRP+placebo | 0.29 (0.51) | p<0.001 |
| SRP+Prodentis | 1.09 (0.62) | Prodentis | 0.17 (0.26) | p<0.001 |
| SRP+placebo | 0.29 (0.51) | Placebo | −0.05 (0.26) | p<0.05 |
| Prodentis | 0.17 (0.26) | Placebo | −0.05 (0.26) | p<0.05 |
In the untreated quadrants, the PPD was only slightly changed after the placebo or Prodentis treatment, however these changes were not significant either over time or between groups. Over the 42 days, SRP alone significantly reduced PPD from 5.26±0.53 to 4.77±0.60 mm (p<0.001). The maximum PPD reduction was observed in SRP+Prodentis treatment from 5.08±0.75 to 3.78±0.61 mm, which was significantly better than SRP alone (p<0.001).
The CAL did not change significantly over time in the untreated quadrants group receiving placebo whereas there was a small decrease in the corresponding Prodentis group from 3.85±1.44 to 3.67±1.31 mm (p<0.05), which was also better than for the placebo group (p<0.05). The SRP+placebo treatment yielded a decrease from 4.46±1.94 to 4.17±1.82 mm (p<0.05). Again, the best result was obtained for the combination of SRP+Prodentis where the CAL dropped from 3.93±0.93 to 2.85±0.74 mm (p<0.001). This was also better than any other treatment modality (p<0.001).
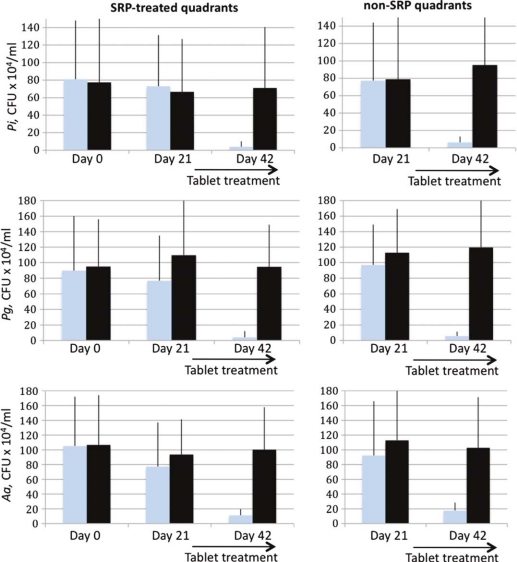
The only treatment modalities that were able to significantly reduce the CFU counts of the pathogens Aa, Pg, and Pi were those including Prodentis, either alone or in combination with SRP (Table 3 and Fig. 2). All three pathogens were equally sensitive toward Prodentis treatment and bacterial counts were reduced by approximately 1 log10 unit from 106 CFU/ml to less than 105 CFU/ml.
Table 3.
Statistical comparison (Wilcoxon's signed rank test) of the microbiological effects between the treatment modalities after 42 days (values are given as mean (SD) CFU/ml×10e4).
| Group | Reduction | Group | Reduction | Significance |
|---|---|---|---|---|
| Aa | ||||
| SRP+Prodentis | 94.0 (62.8) | SRP+placebo | 6.4 (75.7) | p<0.005 |
| SRP+Prodentis | 94.0 (62.8) | Prodentis | 74.7 (67.9) | ns |
| SRP+placebo | 6.4 (75.7) | Placebo | 10.0 (21.0) | ns |
| Prodentis | 74.7 (67.9) | Placebo | 10.0 (21.0) | p<0.01 |
| Pg | ||||
| SRP+Prodentis | 85.7 (73.5) | SRP+placebo | 0.4 (46.4) | p<0.005 |
| SRP+Prodentis | 85.7 (73.5) | Prodentis | 91.3 (51.7) | ns |
| SRP+placebo | 0.4 (46.4) | Placebo | −6.8 (32.3) | ns |
| Prodentis | 91.3 (51.7) | Placebo | −6.8 (32.3) | p<0.001 |
| Pi | ||||
| SRP+Prodentis | 77.0 (65.1) | SRP+placebo | 6.4 (67.9) | p<0.05 |
| SRP+Prodentis | 77.0 (65.1) | Prodentis | 71.0 (63.6) | ns |
| SRP+placebo | 6.4 (67.9) | Placebo | −16.4 (69.2) | ns |
| Prodentis | 71.0 (63.6) | Placebo | −16.4 (69.2) | p<0.001 |
Fig. 2.
Results of the microbiological assessments during the study period. Error bars indicate SD.  =Prodentis group;
=Prodentis group;  =placebo group.
=placebo group.
Compliance to the study treatment was appreciable and no subject rejected using the lozenges. Hypersensitivity as a symptom of root caries was not reported by any of the study subjects and no other adverse reactions were reported by the subjects or observed by the clinician during the study period.
Discussion
The effect of SRP as a non-surgical mode of periodontal therapy is well discussed in the literature in terms of a reduction of clinical and microbial parameters. According to a review in 2009 (17), the basic/initial treatment for periodontal patients in terms of SRP has not been compared with the probiotic effect in the reduction of clinical and microbiological parameters. Hence, an initial attempt has been made in this randomized, double blind clinical trial to evaluate and compare the benefits of the probiotic Prodentis alone and SRP+Prodentis in the treatment of chronic periodontitis.
In both the Prodentis and the placebo groups, PI, GI, and GBI were significantly reduced within each treatment group over the 42 days and thus even the placebo, with or without SRP, had a significant effect. However, such placebo effects are known to occur as a simple consequence of the contact between clinicians and patients (18, 19) and that they were instructed on proper brushing technique when the study started.
The effect of SRP on plaque reduction is similar to that reported by Mousquès et al. (9) and Proye et al. (20). Mousquès reported the reduction of PI and GI during the first 14 days after a single mouth SRP and Proye et al. reported the response of 128 periodontal pockets (3–7 mm) in 10 subjects after a single episode of SRP to give a significant reduction in PI and gingival findings after 1 week.
In the Prodentis group, the combination of SRP+Prodentis demonstrated a significant reduction of PI when compared to SRP and probiotic effects individually, thus the plaque reduction brought about by SRP was enhanced by the use of probiotics. Probiotics and SRP alone were similarly efficacious in plaque reduction. i.e. no difference in mean plaque reduction was observed. Krasse et al. (2) conducted a study to assess if the probiotic L. reuteri could be effective in the treatment of gingivitis and further to evaluate the influence of the probiotic on plaque and the lactobacilli population in the saliva. In their study, L. reuteri was efficacious in reducing both gingivitis and plaque in patients with moderate to severe gingivitis.
In the Prodentis group, both the GI score and GBI percentage reduction were significant both with Prodentis alone and in combination with SRP. An interesting observation was that the Prodentis alone group demonstrated a highly significant gingival bleeding reduction as compared to SRP alone. Interestingly, the gingival bleeding reduction by SRP was enhanced by the probiotic. Ma et al. (21) studied the in-vitro effects of live L. reuteri on human epithelial cells and found that L. reuteri is able to block the TNFα-induced secretion of the pro-inflammatory IL-8, upregulate NGF, and inhibit NF-kB translocation to the nucleus. Twetman et al. (6) investigated the clinical effect of a chewing gum containing probiotic bacteria on gingival inflammation and the levels of selected inflammatory mediators in gingival crevicular fluid (GCF) in patients with gingivitis. The chewing gums contained two strains of L. reuteri: ATCC 55730 and ATCC PTA 5289 (1×108 CFU/gum, respectively). They found that the pro-inflammatory cytokines IL-1β, TNFα, and IL-8 in GCF were reduced by active probiotic treatment, which gives clinical support to the findings by Ma et al. (21). This may be the proof of a principle for a probiotic approach to combating inflammation in the oral cavity and the findings by Twetman et al. (6) are confirmed in the present study as evidenced by the reduced gingivitis parameters (GI and GBI).
There was a significant improvement of PPD and CAL in the Prodentis group, which could possibly be due to the significant reduction in the GI and PI. The maximum PPD reduction was observed in those receiving SRP+Prodentis treatment and this reduction (1.31 mm) was more than twice the sum of the SRP alone reduction added to the Prodentis alone reduction (0.49+0.10 mm), suggesting a synergistic effect. An improvement was observed for CAL; SRP alone yielded 0.29 mm, Prodentis yielded 0.17 mm and the combination resulted in a CAL improvement of 1.09 mm. It should be noted that to our knowledge this is the first time that Prodentis or any other probiotic treatment has been shown to improve CAL. In a randomized, double-blinded, placebo-controlled study, Shimauchi et al. (5) reported a significant improvement of the PI and PPD in the probiotic group (L. salivarius WB21), but CAL was not assessed in their study. Moreover, in their clinical setting there was no significant difference between the probiotic and placebo group in the complete set of patients.
It has been observed that there is a substantial in vitro variation in the antimicrobial activity of Lactobacillus spp. when examined together with several oral microorganisms, including the periodontal pathogens Aa and Pg (9). Aa strains were the most susceptible species to lactobacilli under the conditions of this experiment. The in-vitro effect of probiotics on Aa, Pg, and Pi has been studied (22), and in the present study the antimicrobial effect of L. reuteri Prodentis has been tried for the first time in clinical periodontitis cases. The effect of L. reuteri Prodentis on salivary mutans streptococci was not addressed in the present study, but as reported by Caglar et al. (31), L. reuteri significantly reduces mutans streptococci when administered over a 10-day period.
In the present study, the mean CFU reduction of the three assessed pathogens was highest for the combination of SRP+Prodentis followed by Prodentis alone and the size of the reduction was approximately one log unit. The mechanisms of inhibition of periodontal pathogens have not been fully clarified. The inhibitory activity displayed by homofermentative lactobacilli against periodontal pathogens was principally related to their production of acid, not to H2O2 or bacteriocin production (23). In the present study, at baseline the 30 chronic generalized periodontitis patients demonstrated the presence of Aa, Pg, and Pi, in accordance with the findings of Socransky et al. (23). The results obtained by Mousqués et al. (9) suggest that a single session of SRP is capable of disturbing the proportions of certain bacterial forms in the subgingival periodontal flora, and that it may require approximately 42 days for the proportions to return to baseline levels. Studies designed to determine the effect of SRP on the subgingival microbial flora have consistently reported significant reductions in the percentage of motile microbes and spirochetes (9, 22, 23), Pg and other Gram negative anaerobic bacteria and a concomitant increase in the percentage of cocci and non-motile bacteria (9, 24, 25).
In contrast to the obtained effects of SRP alone on the clinical parameters, we found no significant effects of SRP alone on the microbiological parameters. However, this phenomenon has also been reported by others. Sbordone et al. (26) evaluated the recolonization patterns of the subgingival microflora of eight adult periodontitis patients after a single session of SRP. Their results indicate that a single session of SRP is clearly insufficient to maintain a healthy subgingival microflora. Mombelli et al. (27) conducted a study to determine the topographic distribution of Aa in patients with adult periodontitis before and after mechanical periodontal treatment (repeated oral hygiene instructions and systematic deep SRP) and found that Aa was present in 40% of the samples taken before and in 23% of the samples taken after treatment. Persistence of the organism after thorough debridement of root surfaces has also been reported (27).
Both the L. reuteri strains used in the present study produce the antimicrobial substance reuterin, 3-hydroxypropionaldehyde, within biofilms (28, 29) and it was recently demonstrated that reuterin induces oxidative stress in pathogenic organisms (29), which accounts for its anti-pathogenic effect. Reuterin is effective against a vast array of intestinal pathogens (30, 31) and the present study suggests a similar effect against selected periodontal pathogens. To our knowledge, the present study is the first of its kind to demonstrate the in vivo antimicrobial effect of L. reuteri Prodentis in a clinical setting.
The present randomized controlled trial confirms the plaque inhibition, anti-inflammatory, and antimicrobial effects of L. reuteri Prodentis. Hence, the L. reuteri Prodentis probiotic is suggested as an addition to mechanical debridement and during the maintenance phase of periodontal treatment. Further randomized controlled clinical trials over longer periods are required to build up stronger evidence for probiotic application of L. reuteri Prodentis supporting a periodontal treatment protocol.
Acknowledgements
We would like to thank our volunteers for their participation in this study and D.K. Sangam, Bio-statistician, J.J.M. Medical College, Davangere, India, for his valuable assistance in statistical analysis.
Conflict of interest and funding
The authors declare that there is no conflict of interest. The study was funded by the College of Dental Sciences, Davangere. The test products and a publication grant were provided by BioGaia AB, Stockholm, Sweden.
References
- 1.Ishikawa H, Aiba Y, Nakanishi M, Ohhashi Y, Koga Y. Suppression of periodontal pathogenic bacteria in the saliva of humans by the administration of Lactobacillus salivarius TI 2711. J Jpn Soc Periodontol. 2003;45:105–12. [Google Scholar]
- 2.Krasse P, Carlsson B, Dahl C, Paulsson A, Nilsson A, Sinkiewicz G. Decreased gum bleeding and reduced gingivitis by the probiotic Lactobacillus reuteri. Swed Dent J. 2006;30:55–60. [PubMed] [Google Scholar]
- 3.Matsuoka T, Sugano N, Takigawa S, Takane M, Yoshimura N, Ito K, et al. Effect of oral Lactobacillus salivarius TI 2711 (LS1) administration on periodontopathic bacteria in subgingival plaque. J Jpn Soc Periodontol. 2006;48:315–24. [Google Scholar]
- 4.Teughels W, Newman MG, Coucke W, Haffajee AD, Van Der Mei HC, Haake SK. Guiding periodontal pocket recolonization: a proof of concept. J Dent Res. 2007;86:1078–82. doi: 10.1177/154405910708601111. [DOI] [PubMed] [Google Scholar]
- 5.Shimauchi H, Mayanagi G, Nakaya S, Minamibuchi M, Ito Y, Yamaki K, et al. Improvement of periodontal condition by probiotics with Lactobacillus salivarius WB21: a randomized, double-blind, placebo-controlled study. J Clin Periodontol. 2008;35:897–905. doi: 10.1111/j.1600-051X.2008.01306.x. [DOI] [PubMed] [Google Scholar]
- 6.Twetman S, Derawi B, Keller M, Ekstrand K, Yucel-Lindberg T, Stecksén-Blicks C. Short-term effect of chewing gums containing probiotic Lactobacillus reuteri on the levels of inflammatory mediators in gingival crevicular fluid. Acta Odontol Scand. 2009;67:19–24. doi: 10.1080/00016350802516170. [DOI] [PubMed] [Google Scholar]
- 7.Morrison E, Ramfjord S, Hill R. Short-term effects of initial, non-surgical periodontal treatment (hygiene phase) J Clin Periodontol. 1980;7:199–211. doi: 10.1111/j.1600-051x.1980.tb01963.x. [DOI] [PubMed] [Google Scholar]
- 8.Badersten A, Nilveus R, Egelberg J. Effect of non-surgical periodontal therapy. II: severely advanced periodontitis. J Clin Periodontol. 1984;11:63–76. doi: 10.1111/j.1600-051x.1984.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 9.Mousquès T, Listgarten MA, Phillips RW. Effect of scaling and root planing on the composition of the human subgingival microbial flora. J Periodontal Res. 1980;15:144–51. doi: 10.1111/j.1600-0765.1980.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 10.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Silness J, Loe H. Periodontal diseases in pregnancy (II) correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;24:747–59. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 12.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 13.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229–35. [PubMed] [Google Scholar]
- 14.WHO. Oral Health Country/Area Profile Programme/CAPP: Community Periodontal Index (CPI) Available from: http://www.whocollab.od.mah.se/expl/orhcpitn97.html [cited 17 March 2009].
- 15.Slots J. Selective medium for isolation of Actinobacillus actinomycetemcomitans. J Clin Microbiol. 1982;15:606–9. doi: 10.1128/jcm.15.4.606-609.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Syed SA, Svanberg M, Svanberg G. The predominant cultivable dental plaque flora of beagle dogs with gingivitis. J Periodontal Res. 1980;15:123–36. doi: 10.1111/j.1600-0765.1980.tb00266.x. [DOI] [PubMed] [Google Scholar]
- 17.Stamatova I, Meurman JH. Probiotics and periodontal disease. Periodontology. 2008;51:141–51. doi: 10.1111/j.1600-0757.2009.00305.x. [DOI] [PubMed] [Google Scholar]
- 18.Stewart JE, Jacobs-Schoen M, Padilla MR, Maeder LA, Wolfe GR, Hartz GW. The effect of a cognitive behavioural intervention on oral hygiene. J Clin Periodontol. 1991;18:219–22. doi: 10.1111/j.1600-051x.1991.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 19.Renz A, Ide M, Newton T, Robinson P, Smith D. Psychological interventions to improve adherence to oral hygiene instructions in adults with periodontal diseases. Cochrane Database of Systematic Reviews 2007. (2) doi: 10.1002/14651858.CD005097.pub2. Art. No.: CD005097. [DOI] [PubMed] [Google Scholar]
- 20.Proye M, Caton J, Polson A. Initial healing of periodontal pockets after a single episode of root planning monitored by controlled probing forces. J Periodontol. 1982;53:296–301. doi: 10.1902/jop.1982.53.5.296. [DOI] [PubMed] [Google Scholar]
- 21.Ma D, Forsythe P, Bienenstock J. Live Lactobacillus reuteri is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression. Infect Immunol. 2004;72:5308–14. doi: 10.1128/IAI.72.9.5308-5314.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amornchat C, Rassameemasmaung S, Sirpairojthikoon W, Swasdison S. Invasion of Porphyromonas gingivalis into human gingival fibroblasts in vitro. J Int Acad Periodontol. 2003;5:98–105. [PubMed] [Google Scholar]
- 23.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 24.Magnusson I, Lindhe J, Yoneyama T, Liljenberg B. Recolonization of a subgingival microbiota following scaling in deep pockets. J Clin Periodontol. 1984;11:193–207. doi: 10.1111/j.1600-051x.1984.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 25.Hinrichs J, Wolff L, Pihlstrom B, Schaffer E, Liljemark W, Bandt C. Effects of scaling and root planing on subgingival microbial proportions standardized in terms of their naturally occurring distribution. J Periodontol. 1985;56:187–94. doi: 10.1902/jop.1985.56.4.187. [DOI] [PubMed] [Google Scholar]
- 26.Sbordone L, Ramaglia L, Gulletta E, Iacono V. Recolonization of the subgingival microflora after scaling and root planing in human periodontitis. J Periodontol. 1990;61:579–84. doi: 10.1902/jop.1990.61.9.579. [DOI] [PubMed] [Google Scholar]
- 27.Mombelli A, Gmür R, Gobbi C, Lang NP. Actinobacillus actinomycetemcomitans in adult periodontitis. I. Topographic distribution before and after treatment. J Periodontol. 1994;65:820–6. doi: 10.1902/jop.1994.65.9.820. [DOI] [PubMed] [Google Scholar]
- 28.Jones SE, Versalovic J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009;9:35. doi: 10.1186/1471-2180-9-35. Published online 11 February 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaefer L, Auchtung TA, Hermans KE, Whitehead D, Borhan B, Britton RA. The antimicrobial compound reuterin (3-hydroxypropionaldehyde) induces oxidative stress via interaction with thiol groups. Microbiology. 2010;156:1589–99. doi: 10.1099/mic.0.035642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cleusix V, Lacroix C, Vollenweider S, Duboux M, Le Blay G. Inhibitory activity spectrum of reuterin produced by Lactobacillus reuteri against intestinal bacteria. BMC Microbiol. 2007;7:101. doi: 10.1186/1471-2180-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caglar E, Kuscu OO, Cildir SK, Kuvvetli SS, Sandalli N. A probiotic lozenge administered medical device and its effect on salivary mutans streptococci and lactobacilli. Int J Paediatr Dent. 2008;18:35–9. doi: 10.1111/j.1365-263X.2007.00866.x. [DOI] [PubMed] [Google Scholar]