Abstract
Background
Hypoxia followed by reoxygenation (H-R) observed during perinatal asphyxia is a serious complication with a high mortality and morbidity rate which may cause adverse cardiovascular effects in neonates. Our aim was to determine if oxidative stress related to H-R induces peroxynitrite-dependent modifications of the cardiac contractile protein, myosin regulatory light chain 2 (MLC2) and whether this is associated with development of cardiac systolic dysfunction.
Methods and Results
Twelve newborn piglets were acutely instrumented for hemodynamic monitoring and randomized to a control group ventilated with only atmospheric air or to the H-R study group, exposed to alveolar normocapnic hypoxia followed by reoxygenation. Afterwards, animals were euthanized and the hearts harvested for biochemical analyses. Systolic function as well as cardiac MLC2 levels decreased in H-R animals, whereas nitrates and nitrotyrosine levels increased. Negative correlations between nitrates, nitrotyrosine and MLC2 levels were observed. Moreover, H-R induced nitration of two tyrosine residues within the MLC2 protein. Similarly, in vitro exposure of MLC2 to peroxynitrite resulted in the nitration of tyrosine, which increased the susceptibility of MLC2 to subsequent degradation by matrix metalloproteinase-2 (MMP-2). Substitution of this tyrosine with phenylalanine prevented the MMP-2 dependent degradation of MLC2. In addition, a large decrease in MLC2 phosphorylation due to H-R was observed.
Conclusions
1. Oxidative stress related to asphyxia induces nitration of cardiac MLC2 protein and thus increases its degradation. This and a large decrease in MLC2 phosphorylation contribute to the development of systolic dysfunction. 2. Inhibition of MLC2 nitration and/or direct inhibition of its degradation by MMP-2 could be potential therapeutic targets aiming at reduction of myocardial damage during resuscitation of asphyxiated newborns.
Keywords: peroxynitrite, asphyxia, MLC2 nitration, newborns, heart failure
Introduction
Although neonatal asphyxia affects various organs due to poor perfusion and changes in oxygen delivery, the most serious effects are observed in the central nervous and cardiovascular systems. Asphyxia, being the third major cause of neonatal mortality, accounts for more than one million neonatal deaths worldwide annually [1]. Even though resuscitation revives three quarters of newborns [2], late complications of asphyxia still remain an important cause of morbidity and mortality in children [1,2]. These include cardiovascular dysfunction, which is among the most common problem occurring in more than 60% of asphyxiated neonates [3,4]. Therefore, it is necessary to understand the factors leading to cardiac dysfunction and to develop therapeutic strategies to prevent or reverse such detrimental outcomes.
It has been well established that changes in myocardial contractility might be due postranslational modifications of several proteins which compose the contractile machinery of the heart [4,5,6,7]. These modifications could be attributed to increased levels of reactive oxygen species (ROS), mainly peroxynitrite (ONOO−). Increased ONOO− formation has been correlated with the development of systolic dysfunction [8,9,10,11,12,13]. Recently, we showed that neonatal asphyxia results in ONOO−-dependent modification of the cardiac contractile protein, myosin essential light chain 1 (MLC1), which is followed by its subsequent degradation by matrix metalloproteinase-2 (MMP-2 leading to cardiac systolic dysfunction [14]. In fact, in several pathological models of ischemia, MMP-2, a zinc proteinase was identified as the primary enzyme responsible for degradation of extracellular matrix components as well as intracellular targets such as the contractile proteins troponin I and MLC1 [6, 15].
Myosin is the key protein of the contractile apparatus of the heart and its interaction with actin is responsible for the ability of the heart muscle to contract and, correspondingly to beat. The myosin molecule consists of two heavy chains and two types of light chains, two essential light chains (MLC1), and two regulatory light chains (MLC2) [16]. Both light chains play important structural and functional roles by supporting the structure of the myosin neck region and fine-tuning the kinetics of the actin myosin interaction (reviewed in [17,18,19]. This neck region of myosin is proposed to act as a lever arm, amplifying small conformational changes that originate at the catalytic site of myosin head into large movements allowing myosin to generate motion and force [20,21]. Two functionally important domains of MLC2 include a highly conserved N-terminal phosphorylatable serine 15 constituting a myosin light chain kinase (MLCK) phosphorylation site and a Ca2+-Mg2+ binding site comprising the N-terminal helix-loop-helix MLC2 motif [18]. Given the important role of MLC2 in muscle contraction, it is not surprising that oxidative stress due to hypoxia-reoxygenation could lead to protein modifications and its subsequent degradation. Similar to MLC1, it has been shown that the levels of MLC2 drastically decrease in cardiac myocytes during myocardial infarction [22]. We hypothesized that similar to stress-induced modifications observed in MLC1 [14], the same can be detected in MLC2 from hearts subjected to H-R.
In this study we examined the asphyxia induced modifications in MLC2, which led to a decrease in its level and resulted in the development of systolic heart failure. We aimed to elucidate: 1) whether the increase in ONOO− production caused by global hypoxia and reoxygenation results in post-translational modifications of MLC2 such as nitration and/or nitrosylation; 2) whether H-R leads to alterations in MLC2 phosphorylation, and 3) if these modifications are associated with an increase in degradation of MLC2. Finally, we assessed 4) the relevance of these pathophysiological events by determination of their effects on cardiac hemodynamic function.
This project is one of our on-going studies to identify the factors related to cardiac dysfunction after neonatal hypoxia-reoxygenation and to develop novel therapeutic strategies to treat asphyxiated neonates effectively. If our hypothesis is confirmed – that the myocardial contractile dysfunction is associated with the degradation of these cardiac contractile proteins by MMP-2, this will result in the development of new strategies including the use of a specific myocardial MMP-2 inhibitor, in addition to the conventional therapies with fluid and inotropes which have no significant effect in the outcome of these critically ill neonates [23].
Materials and Methods
Animals
Sixteen mixed breed piglets 1 to 3 days of age, weighing 1.4 to 2.3 kg, were used. This investigation conforms to the Guide to the Care and Use of Experimental Animals published by the Canadian Council on Animal Care (1996).
The instrumentation and protocol have been described previously [24]. Briefly, animals were anesthetized by inhalational halothane (2–5%) and then switched to intravenous fentanyl (0.005–0.015 mg/kg/h), midazolam (0.1–0.2 mg/kg/h) and pancuronium (0.05–0.1 mg/kg/h) after starting mechanical ventilation. Femoral arterial and venous catheters (5F, Argyl®, Sherwood Medical Co., St. Louis, MO) were placed and positioned in the abdominal aorta and right atrium, respectively. After endotracheal intubation via tracheotomy, pressure-controlled assisted ventilation was commenced (Sechrist infant ventilator model IV-100, Sechrist Industries Inc., Anaheim, CA) with pressures of 18/4 cm H2O at a rate of 15 to 20 breaths/min. A left anterior thoracotomy in the third intercostal space was performed. Following the ligation of Ductus Arteriosus, a 6-mm transonic flow probe (6SB906, Transonic Systems Inc., Ithica, NY) was placed around the main pulmonary artery for continuous measurement of blood flow, as a surrogate of cardiac output (CO).
Piglets were monitored for heart rate and mean arterial pressure (MAP) with a Hewlett Packard 78833B monitor (Hewlett Packard Co., Palo Alto, CA). Inspired oxygen concentration was measured using an Ohmeda 5100 oxygen monitor (Ohmeda Medical, Laurel, MD) and maintained at 0.21–0.24 to keep fraction of volume for oxygen saturation between 90% and 96%, which was continuously monitored with a pulse oximeter (Nellcor, Hayward, CA). Maintenance fluids during experimentation consisted of 10% dextrose in water at 10 ml/kg/h and 0.9% NaCl at 2 ml/kg/h. Piglet temperature was maintained at 38.5°C to 39.5°C using an overhead warmer and a heating pad.
Experimental protocol
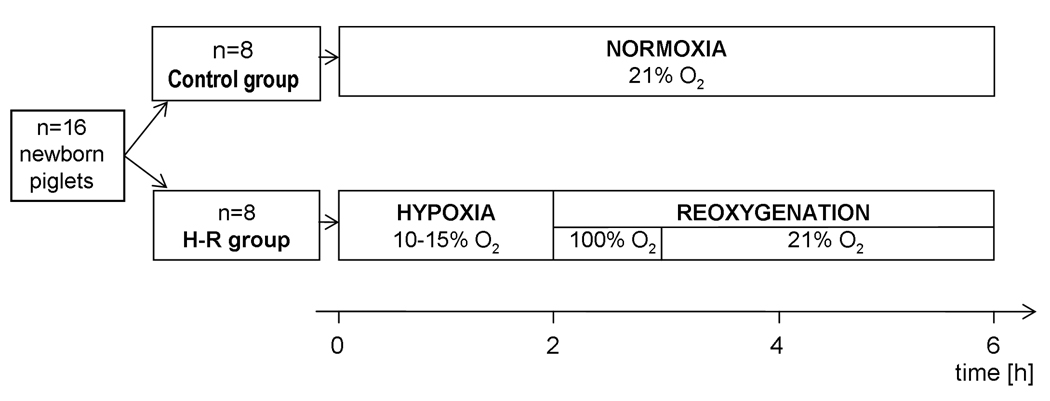
After surgical instrumentation, piglets were allowed to recover from the surgical procedure until baseline hemodynamic measures were stable (change less than 10% over 20 min). Then, the animals were separated into two groups (n=8/group): 1) a sham-operated normoxic group with no period of H-R, and 2) a H-R group where animals were exposed to alveolar normocapnic hypoxia at inspired oxygen concentrations of 10 to 15% for 120 min. At the end of hypoxia, the animals were in cardiogenic shock with decreased CO (<50% of normoxic baseline), severe hypotension and metabolic acidosis. The hypoxic period was followed by reoxygenation with 100% oxygen for 60 min and then 21% oxygen for 180 min (Figure 1). At the end of the experiment, animals were euthanized with an intravenous bolus of 100 mg/kg pentobarbital, and the left ventricular myocardium of the heart was harvested and freeze-clamped in liquid nitrogen for subsequent proteomic analysis.
Figure 1.
Experimental design. All animals were subjected to 30 min stabilization. The investigated group (H-R) was subjected to 120 min of hypoxia followed by 240 min of reoxygenation, while the control group was subjected to 360 min of normoxia.
Measurement of hemodynamic parameters
HR, MAP and CO were continuously recorded throughout the experiment. Analogue outputs of the pressure amplifiers and flow monitor were digitized by a DT 2801-A analogue to digital converter board (Data Translation, Ontario, Canada). Software was custom written using the Assist programming environment. Stroke volume (SV) was calculated by dividing the CO by HR. The hemodynamic parameters were analyzed at normoxic baseline, 60th and 120th min of hypoxia, 10th and 60th min of reoxygenation with 100% oxygen, and 60th, 120th and 180th min of reoxygenation with 21% oxygen.
Preparation of heart extracts
Protein samples for 2-dimensional electrophoresis (2-DE) were prepared by mixing frozen (−80 °C), powdered heart tissue (40 to 60 mg wet weight) with 200 µl rehydration buffer (8 mM urea, 4% CHAPS, 10 mM DTT, 0.2% Bio-Lytes 3/10 [BioRad]). Samples were sonicated on ice twice for 5 s and centrifuged for 10 min at 10000 g at 4 °C to remove any insoluble particles. Protein content of the heart extract in rehydration buffer was measured with a BioRad protein assay. The efficient solubilization of contractile proteins using this method was previously verified to be suitable [6]. For other biochemical studies, frozen heart tissue powder was homogenized on ice in 50 mM Tris-HCl (pH 7.4) containing 3.1 mM sucrose, 1 mM DTT, 10 µg/ml leupeptin, 10 µg/ml soybean trypsin inhibitor, 2 µg/ml aprotinin and 0.1% Triton X-100. Homogenates were centrifuged at 10000 g at 4 °C for 10 min, and the supernatant was collected and stored at −80 °C until use.
2-dimensional gel electrophoresis (2-DE)
After preparing the tissue extract, 100 µg of protein was applied to 11 cm immobilized linear pH gradient (3 to 10) strips (IPG, BioRad), with rehydration for 16–18 h at 20 °C. For isoelectrofocusing (IEF), the BioRad Protean IEF cell was used with the following conditions at 20 °C with fast voltage ramping: Step 1: 15 min with end voltage at 250 V; Step 2: 150 min with end voltage at 8000 V; Step 3: 35 000 V-hours (approximately 260 min). After IEF, the strips were equilibrated according to the manufacturer’s instructions. Second dimension of 2-DE was then carried out with Criterion pre-cast gels (8 to 16%) (BioRad). To minimize variations in resolving proteins during the 2-DE run, all gels were run simultaneously using a Criterion Dodeca Cell (BioRad). Because, we could only run 12 gels simultaneously using the system, 6 randomly chosen samples from each group (control and H-R) were used.
All gels were stained in the same staining bath with the Silver Staining Plus kit (BioRad). Developed gels were scanned using a GS-800 calibrated densitometer (BioRad). The intensity of protein spots from 2-DE gels was measured using PDQuest 7.1 software (BioRad). Equivalent protein loading was confirmed by determination of actin, tropomyosin and ATP synthase alpha subunit spot level.
Because the image profile of an ideal spot conforms to a Gaussian curve, PDQuest uses Gaussian modeling to create "ideal" spots that can be easily identified and quantitated. Gaussian spot is a precise three-dimensional representation of an original scanned spot.
Mass spectrometry
MLC2 protein spots were manually excised from the 2-DE gel. These spots were then processed using a MassPrep Station from Micromass using the methods supplied by the manufacturer. Briefly, the excised gel fragment containing the protein spot was first destained, reduced, alkylated, digested with trypsin and extracted. Analysis of the trypsin digest of MLC2 for ONOO− dependent modifications were performed on Q-TOF Ultima Global (Waters) and Applied Biosystems 4800 MALDI TOF-TOF analyzers. A mass deviation of 0.6 was tolerated and one missed cleavage site was allowed. Resulting values from mass spectrometry (MS/MS and MS) analysis were used to search against the NCBInr and Swiss-Prot databases with Mammalia specified. We used the Mascot (www.matrixscience.com) search engine to search the protein database.
Immunoblotting for MLC2 levels
MLC2 content in the myocardium was determined by immunoblot. We separated 20 µg of protein from each heart extract using 12% SDS-PAGE and transferred the protein to a polyvinylidene difluoride membrane (Bio-Rad). MLC2 was identified using a polyclonal anti-MLC2 antibody (from Santa Cruz Biotechnology Inc.). Band densities were measured using GS-800 calibrated densitometer and Quantity One measurement software 4.6 (BioRad).
Measurement of MMP-2 by zymography
Gelatin zymography was performed as described previously [25]. Briefly, heart extract preparations (30 µg of protein) were applied to 8% polyacrylamide gel copolymerized with 2 mg/ml gelatin. After electrophoresis gels were rinsed 3 times for 20 min each in 2.5% Triton X-100 to remove SDS. The gels were then washed twice in incubation buffer (50 mM Tris-HCl, 5 mM CaCl2, 150 mM NaCl, and 0.05% NaN3) for 20 min each at room temperature and then incubated in incubation buffer at 37°C for 24 h. The gels were stained in 0.05% Coomassie Brilliant Blue G in a mixture of methanol: acetic acid: water (2.5: 1: 6.5, v:v) and destained in aqueous 4% methanol: 8% acetic acid (v:v). Developed gels were scanned with GS-800 densitometer (BioRad) and the MMP-2 activity was measured using Quantity One measurement software 4.6.
Measurement of nitrite and nitrate (NOx)− concentration
We measured (NOx)− as a marker of induction of ONOO− biosynthesis. Myocyte homogenates were diluted 1:1 with deionized water and then deproteinized by centrifugal ultrafiltration (Ultrafree-MC micro-centrifuge tubes UFC3, Millipore Corporation, Bedford, MA). Ultrafiltrates were analyzed for total nitrate and nitrite content according to the method of Green [26]. Briefly, all nitrates were first reduced to nitrites online by passing the sample through a high-pressure liquid chromatography column packed with copper-coated cadmium. These nitrites were then reacted online with Griess reagent to produce an “azo” compound that was detected at 546 nm by means of a visible light detector. The concentrations of nitrite in each sample were quantified through generation of a standard curve using sodium nitrite.
Measurements of nitrotyrosine levels using HPLC
Nitrotyrosine and tyrosine concentrations in cardiomyocyte homogenates were determined by high-pressure liquid chromatography (HPLC) as previously described [27]. Briefly, tissue samples were sonicated in 400 µl of sodium acetate (10 mM, pH 6.5) and vortexed for 1 h. Afterwards samples were centrifuged at 12000 g for 10 min and a 50 µl aliquot was removed and used for a protein assay according to the Bradford method. Proteolysis was performed by adding 150 µl of the supernatant to 25 µl of sodium acetate buffer and 50 µl of pronase (1 mg/ml in acetate buffer) followed by heating at 50 °C for 18 h. After digestion, samples were dried in a Speed Vac system and the extract was dissolved in acetonitrile. Derivatization with 4-fluoro-7-nitrobenzeno-2-oxa-1,3-diazole was performed. A 10 µl aliquot was used for HPLC quantification as described [28].
Preparation of recombinant human cardiac MLC2
The cDNA clone for the human ventricular myosin light chain 2 (MLC2) (NCBI #AAB07462) was isolated with a two-tube RT-PCR method and Omniscript RT kit (Qiagen) using total adult human heart RNA (Stratagene), Oligo dT15 (Promega) and MLC2 specific primers. The sequence of MLC2 wild type (WT) clone was verified and confirmed. MLC2-WT DNA was used to transform BL21 (DE3) Codon Plus competent cells (Stratagene). The MLC2 protein was expressed in 8 L of enriched media consisting of 30 g of peptone/L, 20 g of select yeast extract/L, and 10 g/L of M9 minimal salts with 20 µg/mL of ampicillin and purified using column chromatography (S-Sepharose, DEAE-Sephacel). The fractions of protein purity 97–99% were pooled and stored frozen at −80°C until needed.
Preparation of human recombinant Y152F mutant MLC2
The Tyrosine 152 Phenylalanine (Y152F) mutant of MLC2 was cloned using a two-step sequential overlapping PCR method. Wild type MLC2 was used as the template DNA and specific primers were designed to introduce the Y152F mutation. Clones positive for insert were sent for DNA sequencing and confirmed clones were used for protein expression after transformation into BL21 (DE3) Codon Plus RIPL competent cells (Stratagene). Transformed colonies were screened for overexpression of the mutant MLC2-Y152F protein. Eight liters of enriched media was inoculated with a small culture of the overexpresser and grown overnight at 37 °C. Pellets were harvested by centrifugation and then resuspended in the buffer of 2 M urea, 20 mM citrate, pH=6, 0.02% NaN3, 0.1 mM PMSF, and 1 mM DTT. Pellets were stored frozen at −20 °C until needed. Frozen pellets were thawed and then sonicated for 10 min total time, in cycles of 30 s on 2 min off, while on ice using a Heat Systems XL2020 Sonicator. Sonicate was clarified by centrifugation for 45 min in a JA20 rotor at 18000 rpm at 4 °C. The resultant supernatant was loaded onto a 5 × 10 cm previously equilibrated S-Sepharose column and eluted with a linear gradient of 0–0.45M KCl. Fractions found to contain pure protein by SDS-PAGE were pooled, dialyzed into a buffer of 2 M urea, 25 mM Tris, pH=7.5, 0.02% NaN3, 0.1 mM PMSF, and 1 mM DTT and loaded onto a previously equilibrated Q-sepharose column. MLC2 mutant was eluted with a linear gradient of 0–0.45 M KCl and pure fractions determined by SDS-PAGE were pooled, aliquoted and stored for later use at −20 °C.
In vitro nitration of MLC2 followed by its degradation by MMP-2
Because a commercial preparation of pig MLC2 is not available, we used human cardiac MLC2 for in vitro degradation of MLC2 by MMP-2. Using the LALIGN peptide comparison program (www.ch.embnet.org/software/LALIGN_form.html), the primary sequence of pig cardiac ventricular MLC2 (Q8MHY0) was compared to human cardiac ventricular MLC2 (P10916). Comparison of the primary structures of pig and human MLC2 shows 93.5% identity for all amino acids.
The preparation of human cardiac MLC2 was used for assessment of ONOO− effect on in vitro degradation of MLC2 by MMP-2. Purified human cardiac MLC2 (12 µg) was pre-incubated with ONOO− at three concentrations (10 µM, 100 µM and 1 mM) for 30 min at room temperature. This was followed by incubation for 120 min with 200 ng of MMP-2 (Calbiochem) in 50 mM Tris-HCl buffer (5 mM CaCl2, 150 mM NaCl, total volume 40 µL) at 37°C for 60 min. The reaction mixtures were analyzed by 12% SDS-PAGE under reducing conditions and visualized by the Coomassie Brilliant Blue G-250 staining method. Developed gels were then scanned using a GS-800 calibrated densitometer (BioRad). The degradation of MLC2 was calculated using Quantity One 4.6 software (BioRad).
Immunoprecipitation (IP)
We incubated 500 µg of heart extract proteins/sample with 5 µg rabbit anti–nitrotyrosine IgG or 0.5 µg rabbit anti-MLC2 IgG or 12 µg rabbit anti-MMP-2 IgG in a total volume of 500 µl RIPA buffer (150 mM NaCl, 1% IGEPAL CA-630, 0.5% sodium deoxycholate (DOC), 0.1% SDS, 50 mM Tris, pH 8.0, 1 mM PMSF) overnight at 4 °C. This buffer was chosen because of its known high stringency to avoid unspecific binding. As a negative control, unrelated IgG was used instead of anti-MMP-2, anti–nitrotyrosine or anti-MLC2 IgG. We added 100 µl of slurry of protein A–Sepharose beads and incubated the mixture overnight at 4 °C. The mixture was washed three times with 0.5 ml of RIPA buffer at 4 °C and 20 µl of sample buffer was added. In order to show co-localization of MLC2 with MMP-2 the immunoprecipitates with anti-MLC2 IgG were analyzed by zymography for MMP-2 activity. The immunoprecipitates with anti-MMP-2 IgG were analyzed by immunobloting for MLC2 level. The changes in nitrated MLC2 were analyzed by immunobloting following IP with anti-nitrotyrosine IgG.
Statistical analysis
The protein spot intensities, analyzed using PDQuest measurement software, were evaluated by Mann-Whitney test and/or t-test, as appropriate. Kruskal-Wallis or Friedman ANOVA, as appropriate, were used in functional studies (repeated measurements of hemodynamic and metabolic parameters). Immunoblot results were assessed using Mann-Whitney test. Correlation was performed with Spearman test. Data are expressed as the mean±SEM.
Results
Hemodynamic function
Significant worsening of hemodynamic parameters for the cardiac function was observed in the H-R group as compared to the control group (Table 1). Heart rate measured in the 120th min of the experiment, was similar in both groups. However, during reoxygenation an increase in heart rate in the H-R group was observed. At 360 min of experiment, heart rate was significantly higher in the H-R group as compared to the control group (256±15 vs. 193±18 bpm, p<0.05, respectively) (Table 1).
Table 1. Hemodynamic characterization of both groups during the experimental period.
Comparison of systemic hemodynamic variables
| Control Group | H-R Group | |||||
|---|---|---|---|---|---|---|
| 1st minute | 120th minute | 360th minute | 1st minute | 120th minute | 360th minute | |
| 1. Stroke volume (ml/kg) | 1.05 ± 0.11 | 0.89 ± 0.12 | 0.92 ± 0.07 | 0.92 ± 0.11 | 0.59 ± 0.06* | 0.51 ± 0.08* |
| 2. Heart rate (beats/min) | 185.6 ± 17.1 | 193.0 ± 18.5 | 194.7 ± 7.8 | 201.1 ± 12.1 | 201.0 ± 13.7 | 256.5 ± 15.0*,# |
| 3. Cardiac output (ml/kg/min) | 203.1 ± 18.1 | 165.2 ± 18.2 | 181.3 ± 16.8 | 192.0 ± 12.0 | 96.0 ± 5.8* | 108.5 ± 15.6* |
| 4. Mean arterial pressure (mmHg) | 69.4 ± 7.5 | 66.2 ± 4.6 | 59.2 ± 1.9 | 73.0 ± 5.0 | 27.0 ± 0.4* | 38.6 ± 2.8*,# |
|
5. Systemic vascular resistance (mmHg*min/ml) |
0.34 ± 0.01 | 0.40 ± 0.02 | 0.32 ± 0.01 | 0.38 ± 0.01 | 0.28 ± 0.01 | 0.35 ± 0.01 |
p<0.05 vs. control group
p<0.05 vs. end of hypoxia (120th minute)
Mean arterial pressure (MAP) significantly decreased during hypoxia (27.0±0.4 vs. 66.2±4.6 mmHg in the control group, p<0.05). During reoxygenation MAP significantly increased in the H-R group, however it was still lower in comparison to the control group (38.6±2.8 vs. 59.2±1.9 mmHg, respectively, p<0.05) (Table 1).
There was a significant decrease in cardiac output (CO) during hypoxia (96±6 vs. 165±18 ml/kg/min. in the control group, p<0.05). The CO in the H-R group increased during reoxygenation, but remained significantly lower in comparison the control group (109±16 vs. 181±17 ml/kg/min, respectively, p<0.05) (Table 1).
Stroke volume (SV) was significantly lower in the H-R group in comparison to that observed in the control group, both at the 120th minute of hypoxia (0.59±0.06 vs. 0.89±0.12 ml/beat, respectively, p<0.05) and after reoxygenation at the 360th minute of experiment (0.51±0.08 vs. 0.92±0.07 ml/beat, respectively, p<0.05) (Table 1).
Markers of metabolic acidosis
During hypoxia severe lactic acidosis developed (pH=7.04±0.04 vs. 7.38±0.02, p<0.05; plasma lactates level=14.5±0.6mM vs. 3.6±0.5 mM in the control group, respectively, p<0.05), which subsequently recovered during the reoxygenation period (pH=7.34±0.02; lactates 5.7±1.1 mM at 360th minute of experimentation in the H-R group) (Table 2).
Table 2.
Markers of metabolic acidosis
| Control Group | H-R Group | |||||
|---|---|---|---|---|---|---|
| 1st minute | 120th minute | 360th minute | 1st minute | 120th minute | 360th minute | |
| 1. pH | 7.37 ± 0.02 | 7.38 ± 0.02 | 7.37 ± 0.02 | 7.41 ± 0.02 | 7.04 ± 0.04* | 7.34 ± 0.02*,# |
| 2. Lactates (mM) | 4.4 ± 0.5 | 3.6 ± 0.5 | 3.6 ± 0.5 | 4.2 ± 0.7 | 14.5 ± 0.6* | 5.7 ± 1.1*,# |
p<0.05 vs. control group
p<0.05 vs. end of hypoxia (120th minute)
Analysis of changes in MLC2 levels
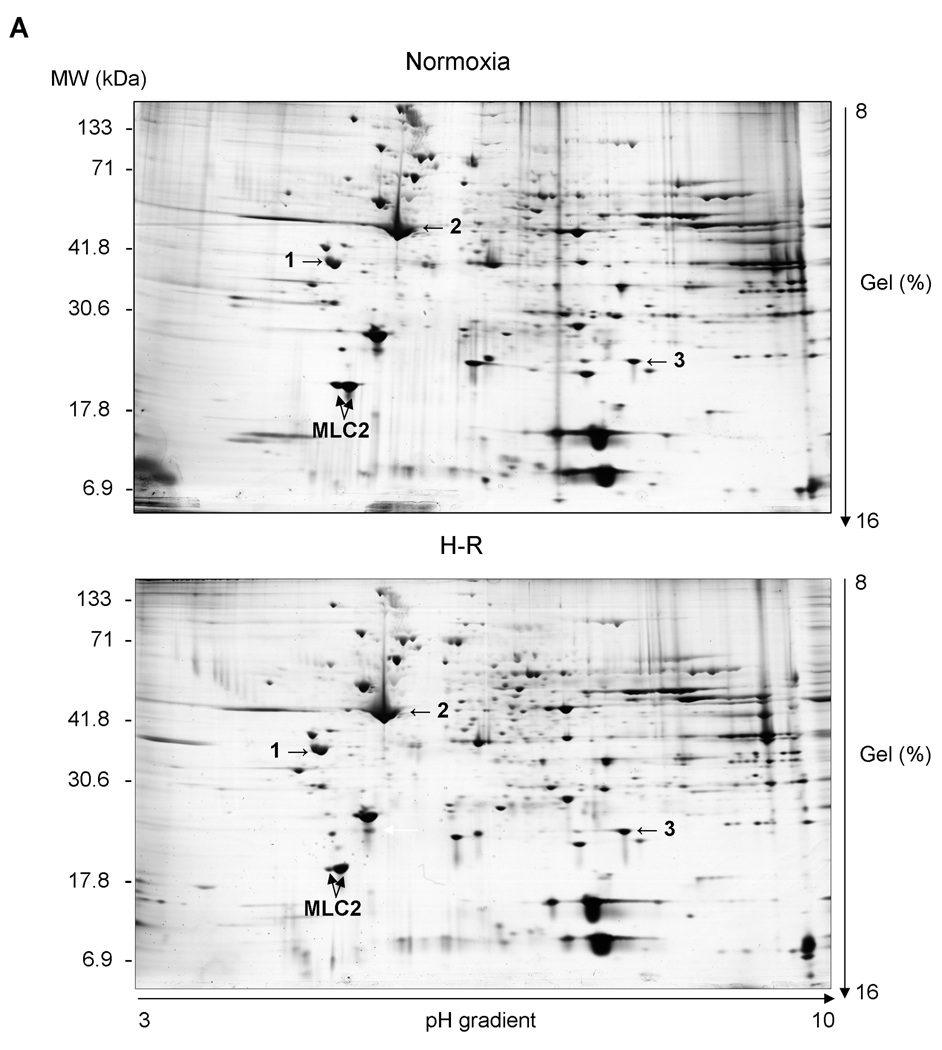
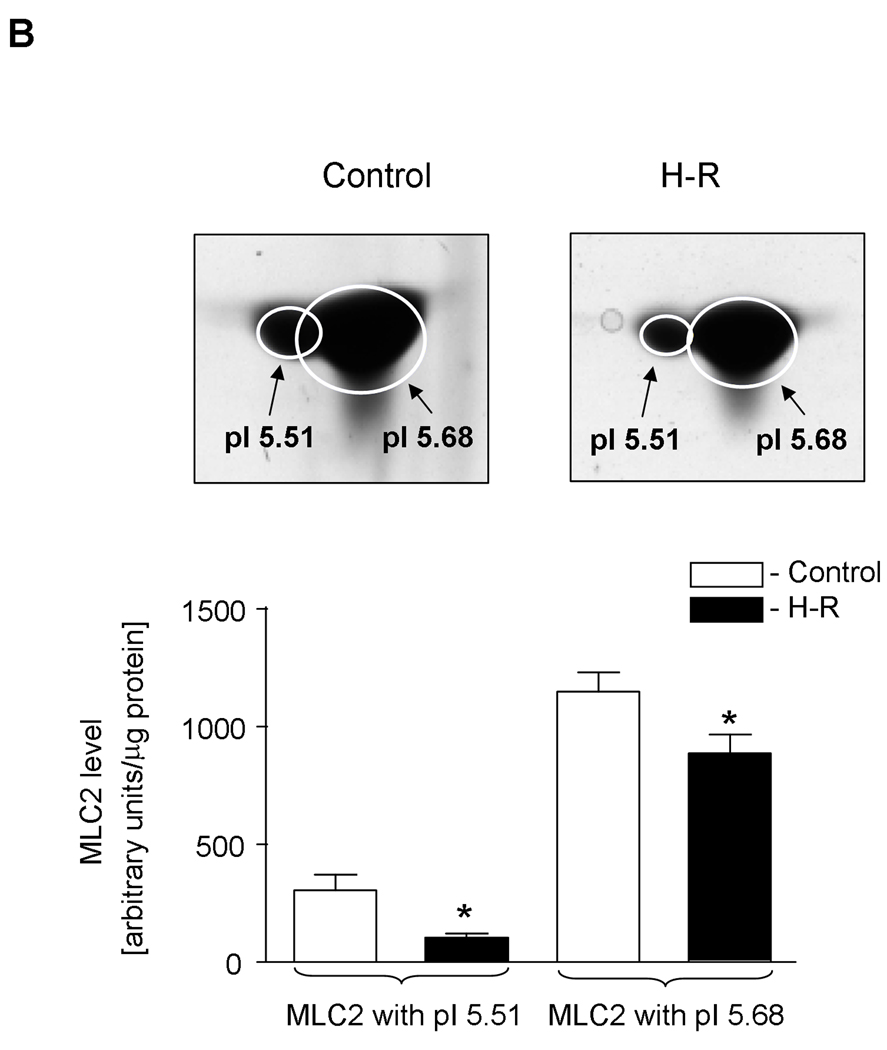
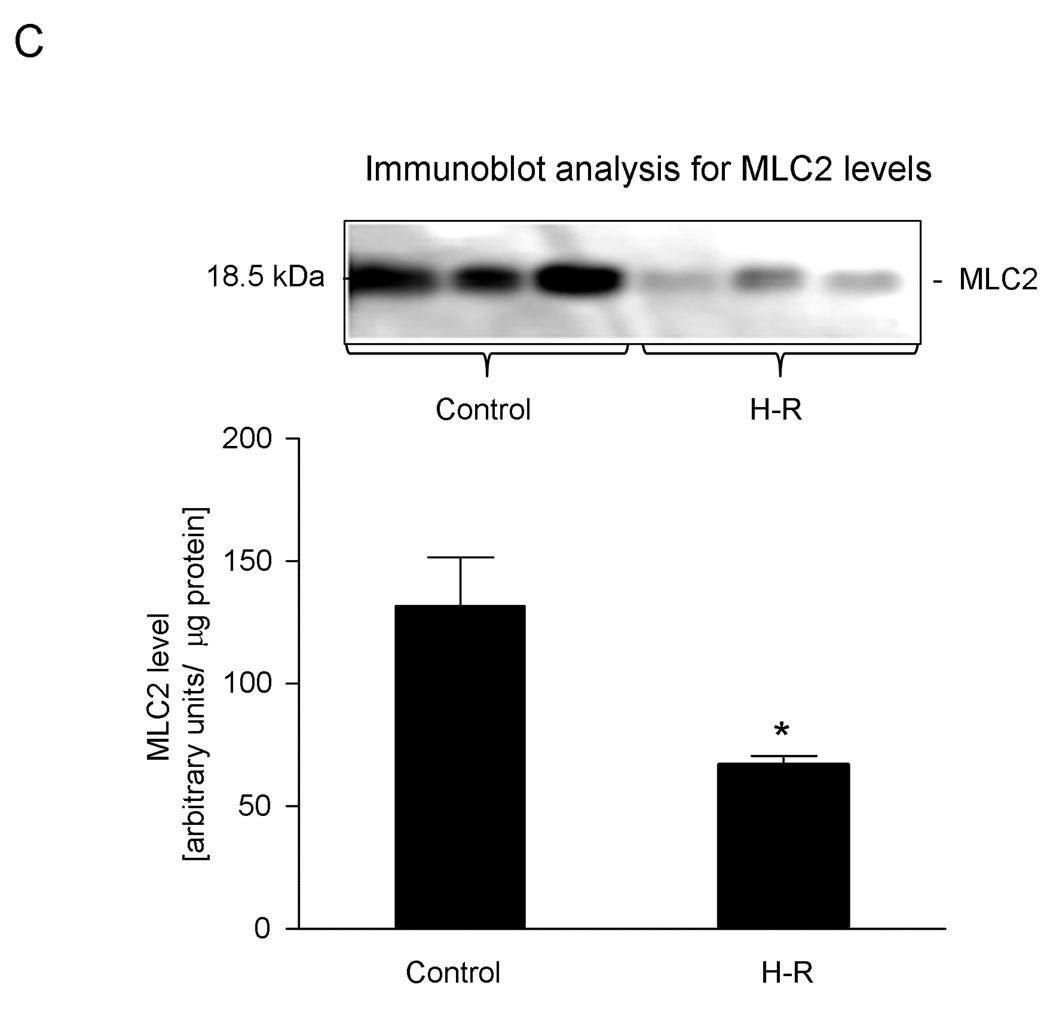
In heart homogenates we identified two forms of myosin light chain 2 (MLC2) showing identical molecular weights but differing in their isoelectric points (Figure 2A) with pI 5.51 and 5.68 (Figure 2B). The difference of 0.17 pH unit between both forms suggests that the more acidic form depicts the phosphorylated form of MLC2. Interestingly, the theoretical pI of MLC2 (calculated on the base of amino acid sequence without any modifications) is 4.86 units. The level of phosphorylated MLC2was significantly lower in the samples from H-R group compared to those from the control group, as assessed by 2-DE (103.2±18.7 AU in H-R groups vs. 305.7±66.5 AU in control form, pI 5.51) Similarly, the level of non-phosphorylated MLC2 decreased due to H-R (887±79.8 AU in H-R vs. 1149.5±80.9 AU in the control group, for spots with pI 5.68). It should be noted that while non-phosphorylated MLC2 (pI 5.68) decreased by ∼33% due to H-R, the decrease in the level of phosphorylated MLC2 (pI 5.51) was ∼66% in H-R animals (Figure 3B). Additionally the changes in total MLC2 evaluated by immunoblot were 66.8±3.7 AU (H-R) vs. 131.0±19.8 AU (control), p<0.05 (Figure 2C). The quality of identification of both forms (non-phosphorylated and phosphorylated) of MLC2 by mass spectrometry and using Mascot search engine is shown in Table 3.
Figure 2.
A. Representative 2-DE gels of proteins from piglet hearts. Arrows indicate location of the MLC2 proteins. Equivalent protein loading was confirmed by determination of tropomyosin (1), actin (2), and ATP synthase alpha subunit (3) spot level.
B. Quantitative analysis of the amount of two MLC2 protein forms (non-phosphorylated and phosphorylated) by 2-dimensional electrophoresis (2-DE) in the control and H-R hearts. Insets show representative protein spots of the MLC2 forms. Isoelectric points (pI) for each form of MLC2 are indicated.
Ellipses on the gels show the shape of each spot generated by Gaussian fitting.
C. Densitometric analysis of total MLC2 levels from immunoblotting in control and H-R groups. Inset shows a representative immunoblot.
Figure 3.
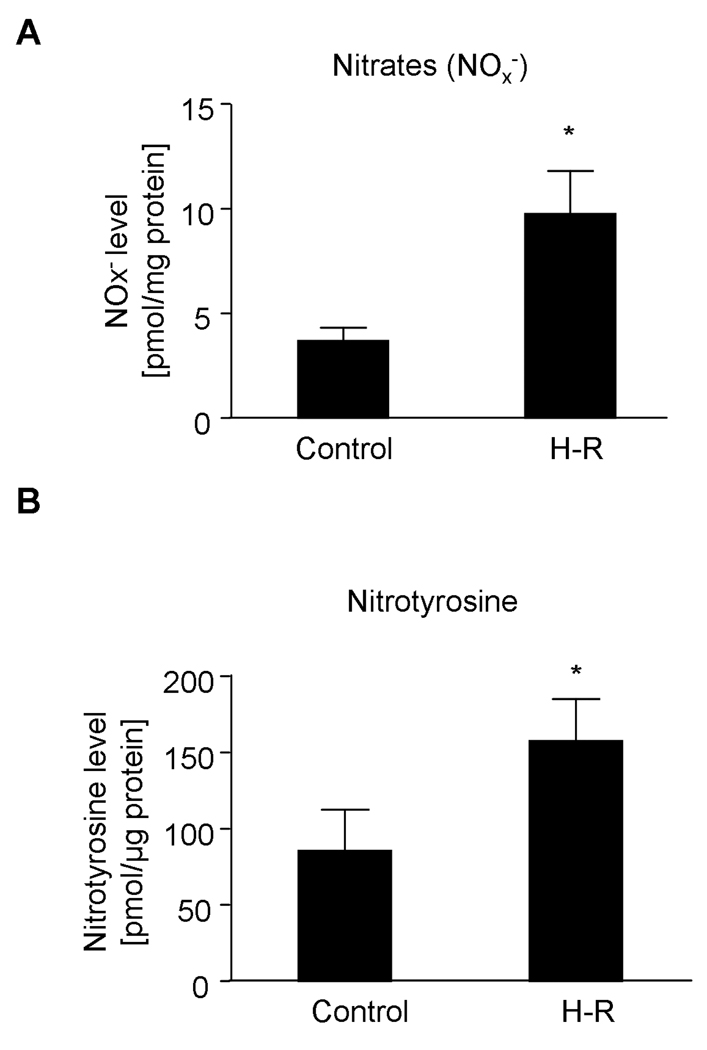
Analysis of peroxynitrite (ONOO−) generation by measurement of nitrate and nitrotyrosine levels by HPLC. A. Nitrogen oxides (NOx−) and B. Nitrotyrosine levels in the control and in the H-R group.
Table 3. Results of identification of MLC2 spot using Mascot search engine.
Identification of protein spots as MLC2
| Identification of cardiac myosin light chain-2 forms (MLC2) |
Probability Based Mowse Score* | Peptides Matched (n) |
Sequence Coverage (%) |
|
|---|---|---|---|---|
| Threshold (p<0.05) |
Observed Score | |||
| Form with Ip 5.51 | 41 | 145 | 5 | 26 |
| Form with Ip 5.68 | 41 | 249 | 7 | 33 |
−10log(P) where P is the probability that the observed match is a random event
Markers of peroxynitrite formation
In order to evaluate peroxynitrite (ONOO−) production we measured nitrogen oxides (NOx−) and nitrotyrosine levels in heart homogenates as markers of ONOO− production. Hypoxia followed by reoxygenation resulted in a significant increase in NOx− levels in comparison to the control group (9.75±2.05 pmol/mg of protein vs. 3.71±0.61 pmol/mg of protein, respectively, p<0.05). Simultaneously, a significant increase in nitrotyrosine levels in the homogenates of H-R hearts was observed (157.6±27.5 vs. 85.6±26.9 pmol/µg of protein in the control group, p<0.05) (Figure 3).
Correlations of peroxynitrite formation with heart function
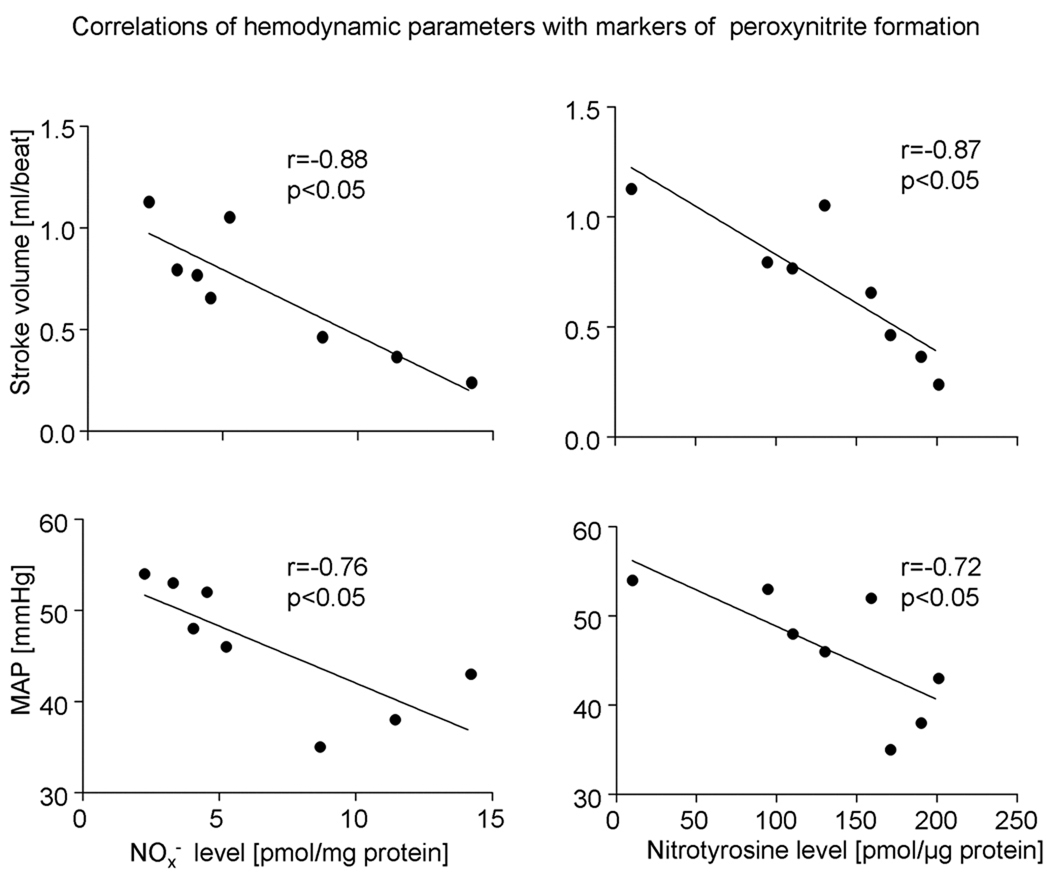
NOx− as well as nitrotyrosine levels were negatively correlated with cardiac systolic function assessed by stroke volume (r= −0.88, p<0.05 for NOx− and r= −0.87, p<0.05 for nitrotyrosine). Furthermore, a negative correlation between MAP and both NOx− and nitrotyrosine levels was observed (r=−0.76 and r=−0.72, respectively, p<0.05) (Figure 4).
Figure 4.
Correlations of stroke volume (SV) and mean arterial pressure (MAP) with markers of peroxynitrite formation.
Effect of peroxynitrite on MMP-2 dependent degradation of MLC2 in vitro
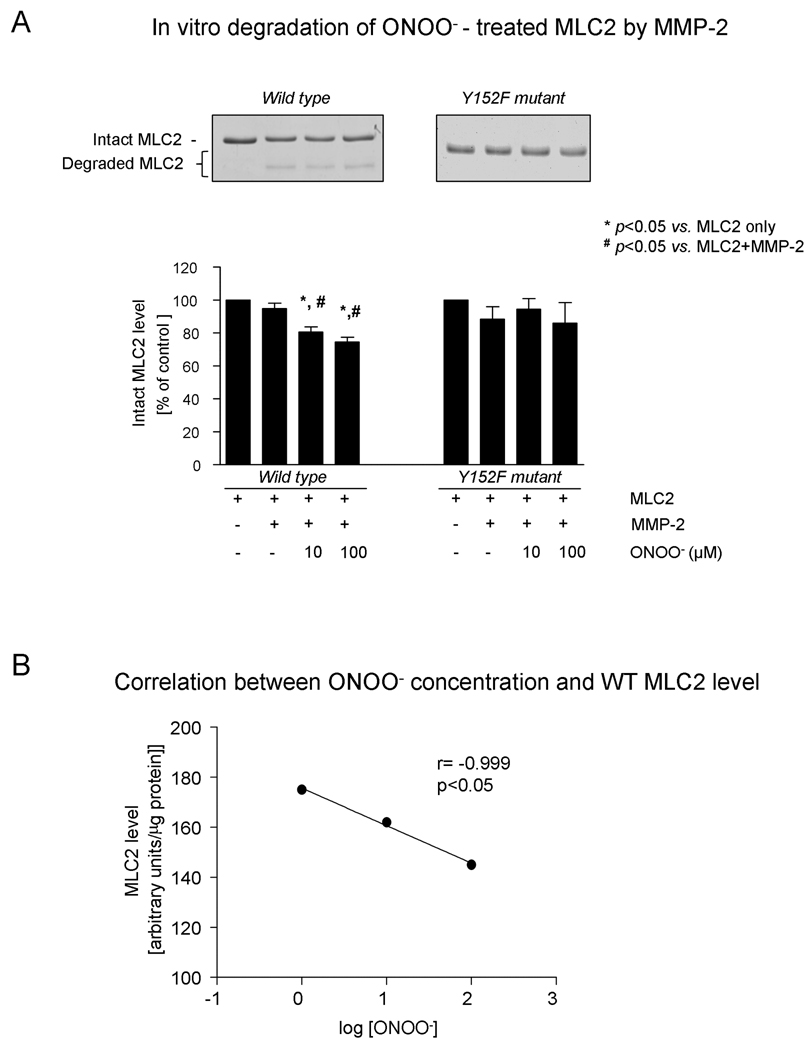
Peroxynitrite dose-dependently enhanced the degradation of human cardiac MLC2, as assessed by SDS-PAGE. Moreover, there was a strong negative correlation between MLC2 level and log [ONOO−] (r=−0.98, p<0.05) (Figures 5A and B).
Figure 5.
Effect of peroxynitrite exposure on MMP-2 - dependent degradation of MLC2 in vitro
A. Densitometric analysis of human cardiac MLC2 levels (MLC2 WT - wild type, and MLC2 Y152F - mutant) in samples subjected to ONOO− at various concentrations. Representative SDS-PAGE scans are shown in the inset.
B. Correlation between ONOO− concentration and MLC2 WT level.
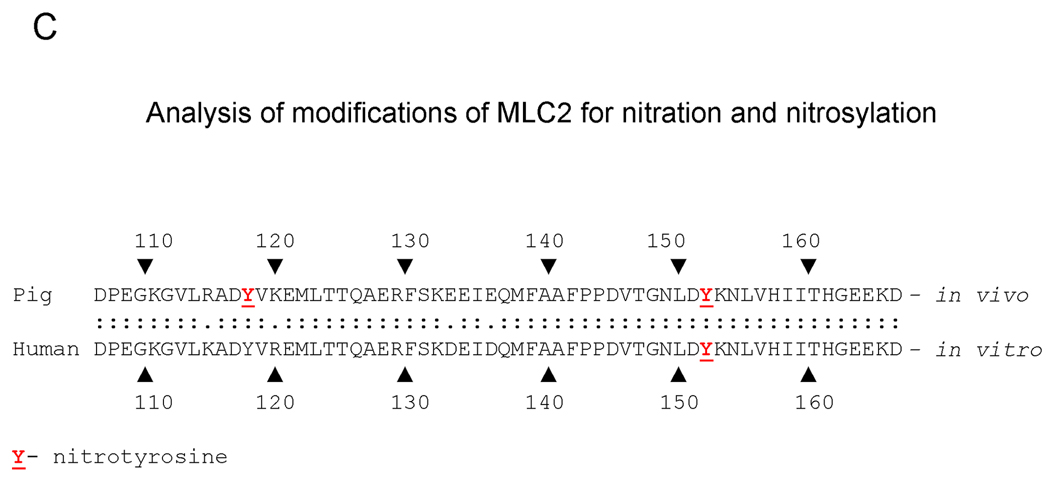
C. Detection of nitrotyrosines in MLC2 by mass spectrometry analysis from hypoxia-reoxygenated hearts and from in vitro studies with ONOO− - induced modifications of MLC2.
Substitution of tyrosine (Y152) for phenylalanine (F152) prevented ONOO− -dependent MMP-2 degradation of MLC2-Y152F mutant (Figure 5A).
Mass spectrometry analysis for nitration of MLC2
Due to the low quantity of the more acidic form of MLC2 (pI 5.51) and since the more acidic form could not be collected without contamination with the less acidic form, for the analysis of MLC2 modifications only the non-phosphorylated form with pI 5.68 was used (Figure 2B). The analysis for nitration of pig MLC2 protein has shown the nitration of tyrosine 118 (Y118) and 152 (Y152) within the protein obtained from the H-R group. No nitration/nitrosylation of MLC2 in the control group was observed.
The mass spectrometry analysis of ONOO−-induced modifications of human cardiac MLC2 protein from the in vitro experiment has shown the nitration of tyrosine 152 (Y152), (homologous to Y152 within MLC2 protein obtained from pig heart). There was no second nitration of the tyrosine within the human MLC2 (Figure 5C).
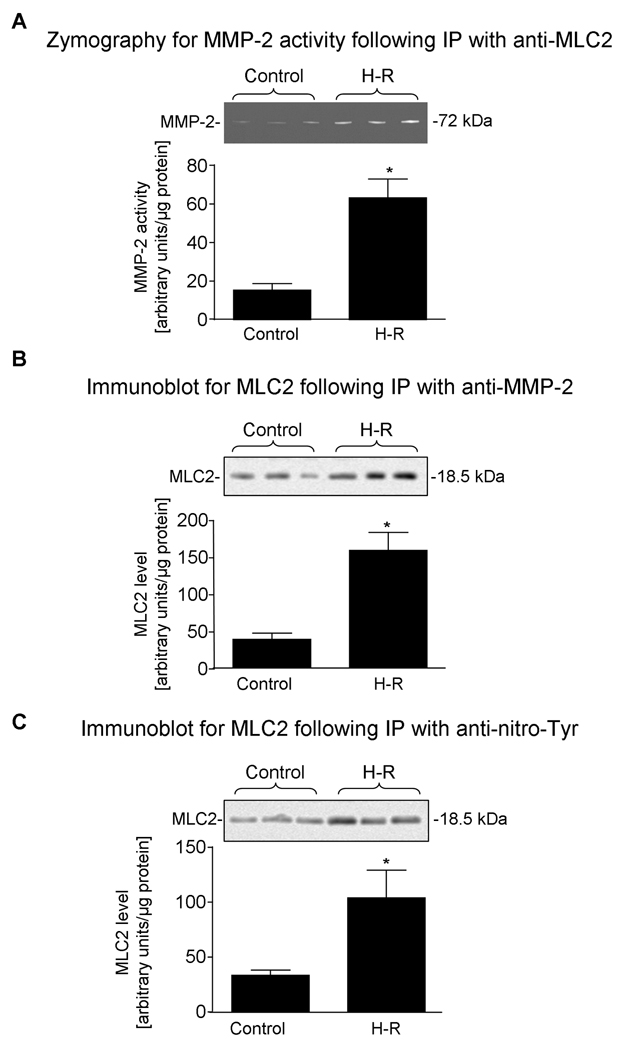
Co-localization of MMP-2 as well as of nitrotyrosine with MLC2 in vivo
Co-localization of MLC2 with MMP-2 was determined in vivo by immunoprecipitation (IP) followed by zymography and immunobloting. Zymography shows (Fiure 6A) approximately 3 fold higher activity of MMP-2 associated with MLC2 from H-R hearts then from control group. Immunoblot analysis for MLC2 level following IP with anti-MMP-2 antibody (Figure 6B) revealed a similar increased level of enzyme-substrate complex (MLC2 with MMP-2) in the H-R group, in comparison to the control group. Furthermore, the levels of nitrated MLC2 protein were similarly increased in the H-R group in comparison to the control group, as was observed when anti-nitrotyrosine antibody was used for IP followed by immunoblotting for MLC2 (Figure 6C).
Figure 6.
A. MMP-2 activity in control hearts as well as those subjected to hypoxia-reoxygenation (H-R) from immunoblot following immunoprecipitation with anti-MLC2. Inset shows a zymogram.
B. MLC2 levels in control hearts as well as those subjected to H-R from immunoblot following immunoprecipitation with anti-MMP-2 IgG. Inset shows an immunoblot.
C. MLC2 levels in control hearts as well as those subjected to H-R from immunoblot following immunoprecipitation with anti-nitrotyrosine (anti-N-Tyr). Inset shows an immunoblot.
Discussion
This is the first study to demonstrate that an increase in peroxynitrite (ONOO−) formation observed in an experimental setting of neonatal asphyxia results in nitration of the myosin light chain 2 (MLC2) protein, which subsequently leads to a decrease in its level and results in the development of systolic heart failure. We have characterized a novel functionally important, oxidative stress dependent nitration of MLC2. Importantly, as we showed earlier and in this study both contractile proteins, MLC1and MLC2, can be degraded by MMP-2. If these contractile proteins contribute to the cardiac dysfunction in hypoxia-reoxygenation, new therapeutic strategies can be developed including a specific myocardial MMP-2 inhibitor. This will open new avenues to alleviate cardiovascular morbidity and improve the outcome of asphyxiated neonates.
In this study we have observed decreased stroke volume during the entire reoxygenation period which was due to deterioration of cardiac systolic function. Decreased cardiac output, which persisted despite the reoxygenation, reflects the loss of cardiac contractility and was partially compensated by an increase in heart rate. Systemic vascular resistance, which was decreased during hypoxia, has recovered during reoxygenation as lactic acidosis was recovering. Because mean arterial pressure was still being decreased despite the normalization of vascular resistance, we can postulate that the cardiac systolic function was persistently impaired. Moreover, since the parameters of cardiac performance were negatively correlated with the markers of peroxynitrite formation, a causative role of peroxynitrite in cardiac damage might be postulated.
In order to confirm the crucial role of increased ONOO− formation (which was demonstrated by changes in NOx− and nitrotyrosine levels) in the development of cardiac systolic dysfunction, we have performed mass spectrometry analysis of the altered cardiac contractile protein, MLC2, which has revealed the nitration of two tyrosines. Since the level of MLC2 in hearts from the H-R group was decreased, we hypothesized that nitration of MLC2 increases its susceptibility to degradation by matrix metalloproteinase-2 (MMP-2), which was confirmed in the in vitro experiment with human cardiac MLC2, where ONOO− exposure of MLC2 in a dose dependent manner potentiated its subsequent degradation by MMP-2. Furthermore, substitution of nitrated tyrosine with phenylalanine within the human recombinant MLC2 protein prevented its degradation by MMP-2 after ONOO− exposure. These results were further confirmed in our in vivo model by immunoprecipitation with anti-nitrotyrosine followed by immunoblotting for MLC2 where the amount of nitrated MLC2 protein was increased in hearts subjected to H-R. Moreover, in vivo co-localization of MLC2 and MMP-2 has also been demonstrated and the amount of the enzyme-substrate complex was higher in the H-R group, as compared to the control group.
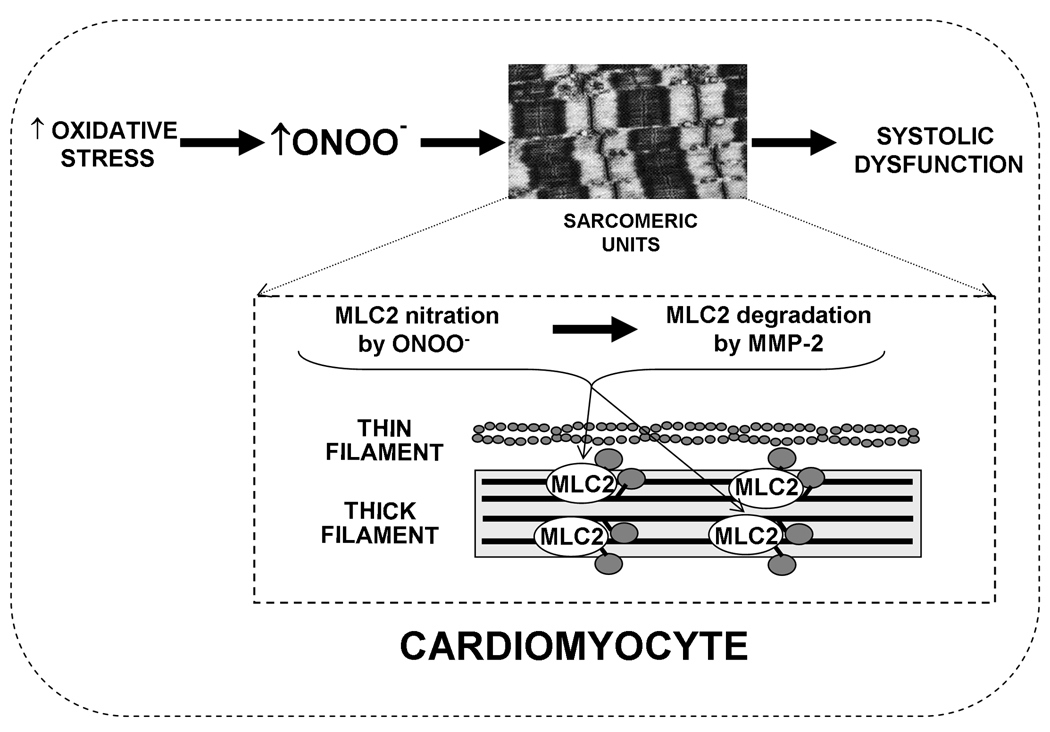
On base of the results we propose that oxidative stress triggers post-translational modifications (nitrations) of contractile proteins (MLC2). Following these changes, the proteins become a substrate for MMPs (MMP-2) which results in impairment of myocardial contractile function (Figure 7). Thus, the pharmacological inhibition of modification of contractile proteins together with inhibition of MMP-2 activity will provide adequate protection to the heart.
Figure 7.
A. Conceptual hypothesis of hypoxia-induced degradation of MLC2. During oxidative stress, such as hypoxia, there is an increase in production of peroxynitrite (ONOO−) leading to modification of MLC2 within the sarcomere of a cardiac myocyte. The modified MLC2 becomes a substrate for MMP-2, and its subsequent degradation leads to the development of cardiac systolic dysfunction.
It is well recognized that MLC2 is also phosphorylated and that this phosphorylation is crucial for the regulation of MLC2 function [18,29]. It was shown that the level of MLC2 phosphorylation does not change during systole and diastole, due to a much lower turnover rate of phosphate exchange compared to the systolic and diastolic cycles [18,30]. However, patients with compromised cardiac function as in heart failure demonstrated complete dephosphorylation of MLC2 [31]. Therefore, increasing or decreasing the level of MLC2 phosphorylation has been associated with a positive or negative inotropic state of the heart, respectively [17]. Similar to human subjects, around 30–40% of MLC2 phosphorylation was observed in pig [32], rabbit [33] and rat [34,35] cardiac fibers reflecting a quite low activity of the myosin light chain kinase (MLCK) and phosphatase (MLCP), the two enzymes catalyzing the phosphorylation-dephosphorylation processes, respectively, in the heart. Indeed, in our experimental model we were able to detect two forms of MLC2, phosphorylated with pI 5.51 and non-phosphorylated with pI 5.68. As expected, the level of phosphorylated MLC2 was largely decreased in the H-R group compared to controls (Fig. 3).
Our data suggest that nitration of tyrosine residues within the MLC2 protein by ONOO−increases its affinity for the MMP-2 enzyme and that MMP-2 can be responsible for degradation of MLC2 in hearts subjected to H-R. Furthermore, since the substitution of tyrosine within human MLC2 mutant by phenylalanine prevents its nitration by ONOO− and thus its subsequent MMP-2 dependent degradation, a crucial role of the ONOO− - MLC2 - MMP-2 axis in pathogenesis of asphyxia-induced cardiac systolic dysfunction might be postulated.
Up to date, it has been well established that multiple biochemical cascades contribute to the pathogenesis of oxidative stress induced myocardial injury. Modifications in thick filament protein content and performance are thought to underlie contraction-relaxation dysfunction in human heart failure [36]. Although it is known that some mutations in the MLC2 gene may result in cardiomyopathies [18,37], the precise role of MLC2 in the heart is still not fully understood. We are the first to demonstrate that oxidative stress induces the nitration of MLC2 which in turn leads to its subsequent degradation. Also, we report promising data pointing toward a decrease in the phosphorylation level of MLC2 due to H-R. Moreover, we establish for the first time that MMP-2 is a key player in the degradation of nitrated MLC2 in vivo. Thus, the present study confirms and extends our previous observations that degradation of cardiac contractile machinery triggered by ischemia-reperfusion in rats [6] or by H-R in newborn piglets involves MMP-2 and occurs upon oxidative stress-dependent modifications of contractile proteins [14]. Hence, the assessment of pharmacological treatment aiming at decreasing oxidative stress induced modifications of contractile proteins should be an effective intervention to minimize myocardial injury.
Acknowledgments
This project was funded by grants from Canadian Institutes of Health Research and the Saskatchewan Health Research Foundation. GS is an investigator supported by the Heart and Stroke Foundation of Canada. PYC is an investigator supported by the Canadian Institutes of Health Research and the Alberta Heritage Foundation for Medical Research. DSC is supported by NIH-HL071778.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None declared.
References
- 1.Hack M, Stork E. Resuscitation at birth and long-term follow-up. Lancet. 2009;373(9675):1581–1582. doi: 10.1016/S0140-6736(09)60228-2. [DOI] [PubMed] [Google Scholar]
- 2.Lawn JE, Cousens S, Zupan J. Lancet Neonatal Survival Steering Team: 4 million neonatal deaths: When? Where? Why? Lancet. 2005;365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 3.Shah P, Riphagen S, Beyene J, Perlman M. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2004;89(2):F152–F155. doi: 10.1136/adc.2002.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas SA, Fallavollita JA, Lee TC, Feng J, Canty JM., Jr Absence of troponin I degradation or altered sarcoplasmic reticulum uptake protein expression after reversible ischemia in swine. Circ Res. 1999;85(5):446–456. doi: 10.1161/01.res.85.5.446. [DOI] [PubMed] [Google Scholar]
- 5.Prasan AM, McCarron HC, Hambly BD, Fermanis GG, Sullivan DR, Jeremy RW. Effect of treatment on ventricular function and troponin I proteolysis in reperfused myocardium. J Mol Cell Cardiol. 2002;34(4):401–411. doi: 10.1006/jmcc.2002.1522. [DOI] [PubMed] [Google Scholar]
- 6.Sawicki G, Leon H, Sawicka J, Sariahmetoglu M, Schulze CJ, Scott PG, Szczesna-Cordary D, Schulz R. Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury. Circulation. 2005;112(4):544–552. doi: 10.1161/CIRCULATIONAHA.104.531616. [DOI] [PubMed] [Google Scholar]
- 7.White MY, Cordwell SJ, McCarron HC, Tchen AS, Hambly BD, Jeremy RW. Modifications of myosin-regulatory light chain correlate with function of stunned myocardium. J Mol Cell Cardiol. 2003;35(7):833–840. doi: 10.1016/s0022-2828(03)00141-x. [DOI] [PubMed] [Google Scholar]
- 8.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Lalu MM, Wang W, Schulz R. Peroxynitrite in myocardial ischemia-reperfusion injury. Heart Fail Rev. 2002;7:359–369. doi: 10.1023/a:1020766502316. [DOI] [PubMed] [Google Scholar]
- 10.Tecder-Unal M, Kanzyk Y. Peroxynitrite in reperfusion arrhythmias and its whole blood chemiluminescence results. Pharmacol Res. 2004;49(1):7–16. doi: 10.1016/j.phrs.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2003;138(4):532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanski J, Behring A, Pelling J, Schoneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: effects of biological aging. Am J Physiol Heart Circ Physiol. 2005;288(1):H371–H381. doi: 10.1152/ajpheart.01030.2003. [DOI] [PubMed] [Google Scholar]
- 13.Jugdutt BI. Nitric oxide and cardioprotection during ischemia-reperfusion. Heart Fail Rev. 2002;7(4):391–405. doi: 10.1023/a:1020718619155. [DOI] [PubMed] [Google Scholar]
- 14.Doroszko A, Polewicz D, Sawicka J, Richardson JS, Cheung PY, Sawicki G. Cardiac dysfunction in an animal model of neonatal asphyxia is associated with increased degradation of MLC1 by MMP-2. Basic Res Cardiol. 2009;104(6):669–679. doi: 10.1007/s00395-009-0035-1. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JR, Sawicki G, Schulz R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation. 2002;106(12):1543–1549. doi: 10.1161/01.cir.0000028818.33488.7b. [DOI] [PubMed] [Google Scholar]
- 16.Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993;261:50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- 17.Morano I. Tuning the human heart molecular motors by myosin light chains. J Mol Med. 1999;77:544–555. doi: 10.1007/s001099900031. [DOI] [PubMed] [Google Scholar]
- 18.Szczesna D. Regulatory light chains of striated muscle myosin. Structure, function and malfunction. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3(2):187–197. doi: 10.2174/1568006033481474. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez OM, Jones M, Guzman G, Szczesna-Cordary D. Myosin essential light chain in health and disease. Am J Physiol Heart Circ Physiol. 2007;292:H1643–H1654. doi: 10.1152/ajpheart.00931.2006. [DOI] [PubMed] [Google Scholar]
- 20.Howard J, Spudich JA. Is the lever arm of myosin a molecular elastic element? Proc Natl Acad Sci U S A. 1996;93(9):4462–4464. [PubMed] [Google Scholar]
- 21.Pant K, Watt J, Greenberg M, Jones M, Szczesna-Cordary D, Moore JR. Removal of the cardiac myosin regulatory light chain increases isometric force production. FASEB J. 2009 doi: 10.1096/fj.08-126672. fj.08-126672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akiyama K, Akopian G, Jinadasa P, Gluckman TL, Terhakopian A, Massey B, Bing RJ. Myocardial infarction and regulatory myosin light chain. J Mol Cell Cardiol. 1997;29(10):2641–2652. doi: 10.1006/jmcc.1997.0493. [DOI] [PubMed] [Google Scholar]
- 23.Osborn DA, Paradisis M, Evans N. The effect of inotropes on morbidity and mortality in preterm infants with low systemic or organ blood flow. Cochrane Database Syst Rev. 2007;1 doi: 10.1002/14651858.CD005090.pub2. CD005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Salam Z, Johnson S, Abozaid S, Bigam D, Cheung PY. The hemodynamic effects of dobutamine during reoxygenation after hypoxia: a dose-response study in newborn pigs. Shock. 2007;28(3):317–325. doi: 10.1097/shk.0b013e318048554a. [DOI] [PubMed] [Google Scholar]
- 25.Haase E, Bigam DL, Nakonechny QB, Rayner D, Korbutt G, Cheung PY. Cardiac function, myocardial glutathione, and matrix metalloproteinase-2 levels in hypoxic newborn pigs reoxygenated by 21%, 50%, or 100% oxygen. Shock. 2005;23(4):383–389. doi: 10.1097/01.shk.0000158962.83529.ce. [DOI] [PubMed] [Google Scholar]
- 26.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 27.Hensley K, Maidt ML, Pye QN, Stewart CA, Wack M, Tabatabaie T, Floyd RA. Quantitation of protein-bound 3-nitrotyrosine and 3,4-dihydroxyphenylalanine by high-performance liquid chromatography with electrochemical array detection. [Published erratum appears in 1998 Anal Biochem. 256: 148.] Anal Biochem. 1997;251(2):187–195. doi: 10.1006/abio.1997.2281. [DOI] [PubMed] [Google Scholar]
- 28.Zhang WZ, Lang C, Kaye DM. Determination of plasma free 3-nitrotyrosine and tyrosine by reversed-phase liquid chromatography with 4-fluoro-7-nitrobenzofuran derivatization. Biomed Chromatogr. 2007;21(3):273–278. doi: 10.1002/bmc.750. [DOI] [PubMed] [Google Scholar]
- 29.Li T, Liu L, Liu J, Ming J, Xu J, Yang G, Zhang Y. Mechanisms of Rho kinase regulation of vascular reactivity following hemorrhagic shock in rats. Shock. 2008;29(1):65–70. doi: 10.1097/shk.0b013e318063e477. [DOI] [PubMed] [Google Scholar]
- 30.Herring BP, England PJ. The turnover of phosphate bound to myosin light chain-2 in perfused rat heart. Biochem J. 1986;240:205–214. doi: 10.1042/bj2400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morano I. Effects of different expression and posttranslational modifications of myosin light chains on contractility of skinned human cardiac fibers. Basic Res Cardiol. 1992;87:129–141. doi: 10.1007/978-3-642-72474-9_11. [DOI] [PubMed] [Google Scholar]
- 32.Morano I, Osterman A, Arner A. Rate of active tension development from rigor in skinned atrial and ventricular cardiac fibres from swine following photolytic release of ATP from caged ATP. Acta Physiol Scand. 1995;154:343–353. doi: 10.1111/j.1748-1716.1995.tb09918.x. [DOI] [PubMed] [Google Scholar]
- 33.Cole HA, Frearson N, Moir AJ, Perry SV, Solaro RJ. Phosphorylation of cardiac myofibrillar proteins. Recent Adv Stud Cardiac Struct Metab. 1976;11:111–120. [PubMed] [Google Scholar]
- 34.Fitzsimons DP, Bodell PW, Baldwin KM. Phosphorylation of rodent cardiac myosin light chain 2:effects of exercise. J Appl Physiol. 1989;67:2447–2453. doi: 10.1152/jappl.1989.67.6.2447. [DOI] [PubMed] [Google Scholar]
- 35.Morano I, Lengsfeld M, Ganten U, Ganten D, Ruegg JC. Chronic hypertension changes myosin isoenzyme pattern and decreases myosin phosphorylation in the rat heart. J Mol Cell Cardiol. 1988;20:875–886. doi: 10.1016/s0022-2828(88)80142-1. [DOI] [PubMed] [Google Scholar]
- 36.Palmer BM. Thick filament proteins and performance in human heart failure. Heart Fail Rev. 2005;10(3):187–197. doi: 10.1007/s10741-005-5249-1. [DOI] [PubMed] [Google Scholar]
- 37.Greenberg MJ, Watt JD, Jones M, Kazmierczak K, Szczesna-Cordary D, Moore JR. Regulatory light chain mutations associated with cardiomyopathy affect myosin mechanics and kinetics. J Mol Cell Cardiol. 2009;46(1):108–115. doi: 10.1016/j.yjmcc.2008.09.126. [DOI] [PMC free article] [PubMed] [Google Scholar]