Abstract
How type I skeletal muscle inherently maintains high oxidative and vascular capacity in absence of exercise in unclear. We show that nuclear receptor ERRγ is highly expressed in type I muscle and when transgenically expressed in anaerobic type II muscles (ERRGO mice), dually induces metabolic and vascular transformation in absence of exercise. ERRGO mice show increased expression of genes promoting fat metabolism, mitochondrial respiration and type I fiber specification. Muscles in ERRGO mice also display an activated angiogenic program marked by myofibrillar induction and secretion of pro-angiogenic factors, neo-vascularization and a 100% increase in running endurance. Surprisingly, the induction of type I muscle properties by ERRγ does not involve PGC1α. Instead, ERRγ genetically activates the energy sensor AMPK, in mediating the metabo-vascular changes in the ERRGO mice. Therefore, ERRγ represents a previously unrecognized determinant that specifies intrinsic vascular and oxidative metabolic features that distinguish type I from type II muscle.
Keywords: ERRγ, slow-twitch muscles, AMPK, neo-vascularization, Therapeutic transcription
INTRODUCTION
Tissue vascular supply is tightly coupled to its oxidative capacity. This is especially evident in skeletal muscle beds, each enriched in either oxidative slow-twitch or glycolytic fast-twitch myofibers (Fluck and Hoppeler, 2003; Pette and Staron, 2000). Slow-twitch muscles are characterized by high mitochondrial content, fatigue resistant (type I) fibers and dense vascularity to ensure a steady and prolonged supply of oxygen and nutrients (Annex et al., 1998; Cherwek et al., 2000; Ripoll et al., 1979). Fast-twitch (type II) muscles generally have lower oxidative capacity, a reduced blood supply and are fatigue sensitive. How the type I vs. the type II muscle vasculature is specified to match oxidative capacity is unclear.
Previous studies have established that nuclear receptors such as PPARα, PPARδ and ERRα along with co-regulators PGC1α, PGC1β and Rip140 control diverse aspects of aerobic respiration including fatty acid oxidation, oxidative phosphorylation and mitochondrial biogenesis in skeletal muscle (Arany et al., 2007; Huss et al., 2004; Lin et al., 2002; Minnich et al., 2001; Muoio et al., 2002; Seth et al., 2007; Wang et al., 2004). While signaling factors such as TGFβ1, platelet-derived growth factor, fibroblast growth factor (FGF) 1 and 2, and vascular endothelial growth factor (VEGF) are known to stimulate angiogenesis (Carmeliet, 2000; Ferrara and Kerbel, 2005; Gustafsson and Kraus, 2001), whether and how these factors orchestrate dense vascularization of aerobic muscles is unclear. One possibility is vascular arborization by co-activator PGC1α that is induced by hypoxia and exercise (Arany et al., 2008). However, PGC1α knockout mice are viable, still retain oxidative muscle, and have normal vasculature (Arany et al., 2008; (Lin et al., 2004). Since the intrinsic enrichment of blood flow to aerobic muscles in the absence of exercise is unlikely to depend on PGC1α induction, we speculate the existence of a novel regulatory angiogenic pathway.
Estrogen receptor-related receptor γ (ERRγ), like other members of the ERR subfamily, is a constitutively active orphan nuclear receptor, though unlike ERRα and β, it is more selectively expressed in metabolically active and highly vascularized tissues such as heart, kidney, brain and skeletal muscles (Giguere, 2008; Heard et al., 2000; Hong et al., 1999). In vitro studies suggest that ERRγ activates genes such as PDK4 and MCAD that play a regulatory role in oxidative fat metabolism (Huss et al., 2002; Zhang et al., 2006). Furthermore, a comprehensive gene expression analysis identified ERRγ as a key regulator of multiple genes linked to both fatty acid oxidation and mitochondrial biogenesis in cardiac muscles (Alaynick et al., 2007; Dufour et al., 2007). Expression of ERRγ is also induced in variety of tumors with hyper-metabolic demands and abundant vasculature (Ariazi et al., 2002; Cheung et al., 2005; Gao et al., 2006). Therefore, we explored the potential of ERRγ in controlling the intrinsic angiogenic pathway in oxidative slow-twitch muscles. We found ERRγ to be exclusively and abundantly expressed in oxidative (type I) slow-twitch muscles. Transgenic expression of ERRγ in fast-twitch type II muscle triggers aerobic transformation, mitochondrial biogenesis, VEGF induction and robust myofibrillar vascularization, all in the absence of exercise. These intrinsic effects of ERRγ do not depend on PGC1α induction, but rather are linked to activation of the metabolic sensor AMPK. These findings reveal an exercise-independent ERRγ pathway that promotes and coordinates vascular supply and metabolic demand in oxidative slow-twitch muscles.
RESULTS
Skeletal muscle ERRγ expression
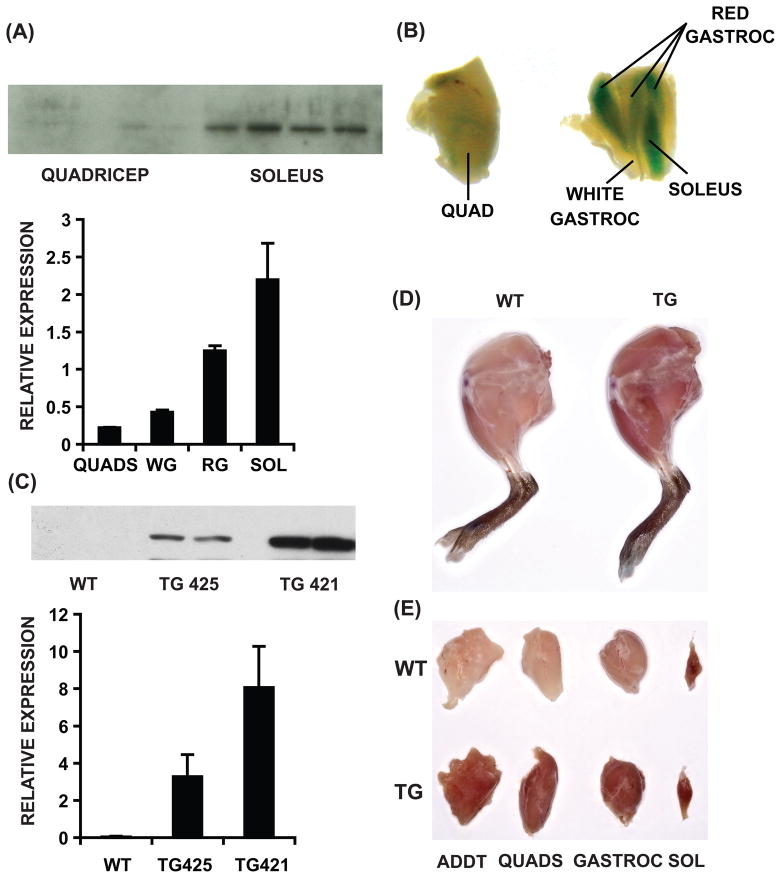
Because skeletal muscle is a functionally heterogeneous tissue consisting of both aerobic slow-twitch and glycolytic fast-twitch muscles, we re-evaluated ERRγ expression in the context of different myofibrillar beds. We found that ERRγ transcript is highly expressed in oxidative muscles such as soleus and red gastrocnemius, with minimal expression in glycolytic quadriceps and white gastrocnemius (Figure 1A, lower panel). ERRγ protein is undetectable in quadriceps, but highly expressed in soleus (Figure 1A, upper panel).
Figure 1. Skeletal muscle ERRγ expression.
(A) ERRγ gene (lower panel) and/or protein (upper panel) expression in quadriceps (QUADS), white gastrocnemius (WG), red gastrocnemius (RG) and soleus (SOL) isolated from C57Bl/6J mice (N=4). (B) Representative images of β-galactosidase stained muscles. (C) Expression of transgene transcript (lower panel) and protein (upper panel) in quadriceps of wild type (WT), founder TG 425 and 421. (D) Representative hindlimbs from WT and transgenic mice. (E) Dissected hindlimb muscle beds [adductor (ADDT), quadriceps, gastrocnemius (GASTROC) and soleus]. In (A) and (C) data are presented as mean ± SD (N=4). See Supplemental Figure S1.
Previously, we described viable ERRγ +/− mice in which a β-galactosidase protein-coding region without the promoter was introduced in-frame with the initiation site of the Esrrg gene (Alaynick et al., 2007) such that the enzyme mimics the expression of endogenous ERRγ. β-Galactosidase staining of different muscle beds from ERRγ +/− adult mice further confirmed that the receptor is highly expressed in oxidative (e.g. soleus and red gastrocnemius) compared to the minimal levels in glycolytic muscles (e.g. quadriceps, white gastrocnemius) (Figure 1B).
Transgenic muscle-specific ERRγ over-expression
The above expression pattern of ERRγ supports its presumptive role in oxidative and slow-twitch muscle biology. To test this idea, we generated transgenic mice selectively expressing ERRγ in skeletal muscles under the control of the human alpha-skeletal actin promoter (Muscat and Kedes, 1987; Wang et al., 2004). Two ERRγ over-expressing (ERRGO) transgenic lines were obtained (TG 421 and 425) showing both transcript (lower panel) and protein (upper panel) in fast-twitch quadriceps (Figure 1C). Gross anatomical analysis of hindlimb muscles (Figure1D) and dissection of individual muscle beds (Figure 1E) revealed enhanced red coloration (characteristic of oxidative fibers) in transgenic compared to wild type muscle. Importantly, slow-twitch (soleus) muscle, already high in ERRγ expression, was not affected (Figure 1E), presumably because it is already fully oxidative. In addition, oxidative biomarkers myoglobin and cytochrome c were induced in the quadriceps of both the transgenic lines compared to wild type mice (Supplemental Figure S1). For subsequent studies we focused on TG 421 due to slightly higher biomarker expression in this progeny.
Fast to slow-twitch transformation of skeletal muscle by ERRγ
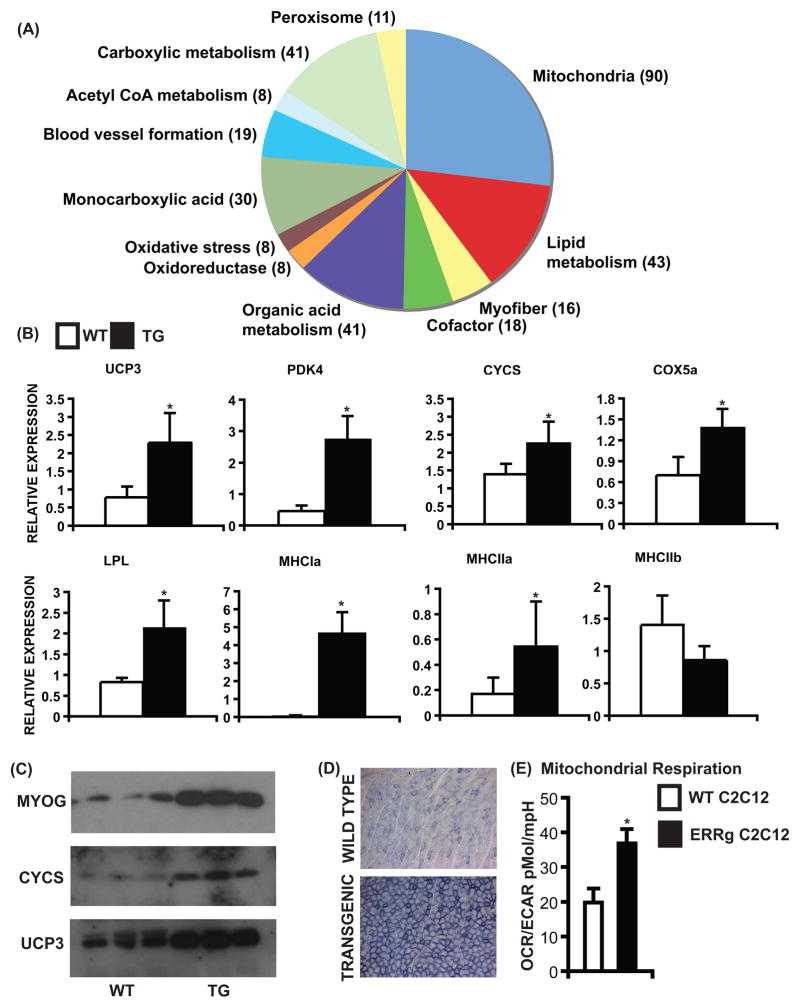
To study the transcriptional effect of ERRγ, muscle gene expression was measured in quadriceps from wild type and ERRGO mice. In gene array analysis, we found that ERRγ regulated a total of 1123 genes in skeletal muscles, of which 623 genes were induced. Gene ontology-based classification of these genes is presented in Figure 2A. The majority of the up-regulated genes belong to either mitochondrial biology (90) or oxidative metabolism (43) encoding various components of fatty acid oxidation pathway as well as the oxidative respiratory chain reflective of aerobic adaptation (described in Supplementary Table 1). Furthermore, contractile genes, especially ones associated with slow myofibers, were also activated raising the possibility of fast-to-slow transformation linked to the metabolic switch (Supplementary Table 2).
Figure 2. ERRγ promotes oxidative muscle transformation.
(A) Gene ontology classification of positively regulated genes. Gene selection was based on p<0.05 on Bonferroni’s multiple comparison test for fold change (N=3). (B) ERRγ increases expression of oxidative metabolism (Ucp3, Pdk4, Cycs, Cox5a, Lpl), oxidative muscle (Mhc1a, Mhc2a) but not glycolytic muscle (Mhc2b) biomarker genes. Data are presented as mean ± SD from N=6 samples. (C) ERRγ increases protein expression of myoglobin, cytochrome c and uncoupling protein 3 (N=3). (D) Representative images of SDH stained WT and transgenic gastrocnemius cryo-sections. Similar results were obtained from N=4 mice. (E) OCAR/ECAR ratio representing a shift in cellular energy production to oxidative phosphorylation. Data is presented as mean ± SD. * represents statistically significant difference between WT and transgenic mice or between WT and ERRγ over-expressing C2C12 cells (p<0.05, unpaired Student’s t-test). See Supplemental Figure S2, Table S1, S2 and S4.
We confirmed that key biomarker genes associated with oxidative metabolism [Ucp3, Pdk4, Cycs, Cox5a, Lpl] and oxidative myofibers [Mhc Ia, Mhc IIa], but not glycolytic myofibers [Mhc IIb] were induced by ERRγ in quadriceps of transgenic mice (Figure 2B). Conversely, many of the biomarker genes tested [Ucp3, Cycs, Acscl1, Cox6a2, Ppara] were found to be down-regulated by siRNA-mediated ERRγ knockdown in primary cultured myotubes (Supplementary Figure S2 A) isolated from oxidative muscles (soleus and red gastrocnemius). Moreover, the oxidative changes were confirmed at the protein level as exemplified by increased expression of myoglobin, cytochrome c and UCP3 in transgenic relative to wild type muscle (Figure 2C). Furthermore, staining of gastrocnemius cryo-sections for defining oxidative mitochondrial enzyme SDH activity revealed an increase in oxidative myofibers in ERRGO compared to wild type mice (Figure 2D), which was confirmed by electron microscopy (data not shown).
To access the metabolic effects of ERRγ at the cellular level, we measured the mitochondrial bioenergetics in wild type and ERRγ over-expressing C2C12 cells using an extracellular flux analyzer. Specifically, we determined the oxygen consumption rate (OCR) (an indicator of mitochondrial respiration) along with the extracellular acidification rate (ECAR) (a measure of glycolysis) in these cells (Supplemental Figure S2 B–C). ERRγ expression significantly induced mitochondrial respiration (OCR), reduced cellular glycolysis (ECAR) resulting in an 85% shift in the cellular energy production ratio towards oxidative phosphorylation (Figure 2E).
The above observations show that ERRγ promotes an overt conversion of glycolytic fast-twitch muscles such as quadriceps to an oxidative slow-twitch phenotype.
ERRγ promotes skeletal muscle vascularization
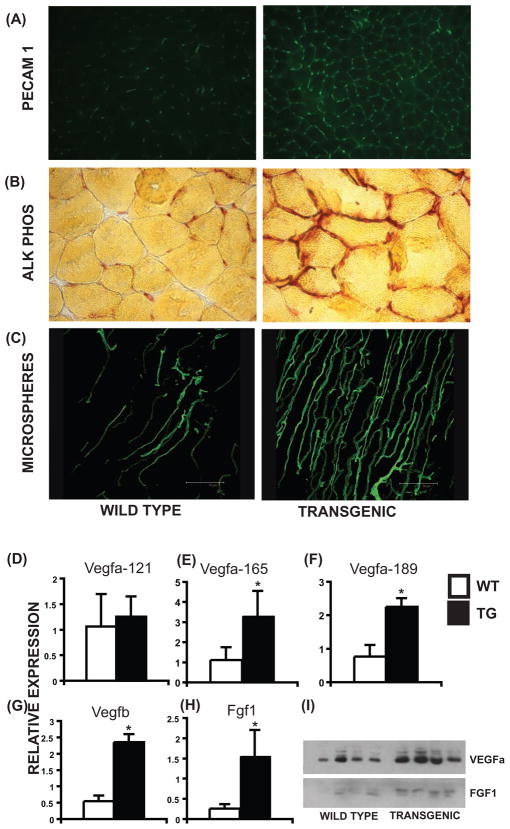
Intrinsic vascularization of slow-twitch myofibers enables a baseline of exercise independent fatigue resistance. We speculated that ERRγ, by virtue of its restricted expression to type I fibers could, in addition to promoting oxidative metabolism, simultaneously induce vessel formation to match the increased oxidative demand. To test this we first stained muscle cryo-sections for PECAM 1 (CD31), an endothelial cell marker that is routinely used to detect angiogenesis and changes in tissue vasculature. We found that transgenic muscles showed increased PECAM 1 (Figure 3A) staining compared to wild type. Similarly, transgenic muscle cryo-sections showed an increase in alkaline phosphatase staining, an alternative marker for tissue endothelium (Figure 3B). These findings point toward a possible induction of angiogenesis and muscle vascularization by ERRγ. To test whether ERRγ supports formation of functional non-leaky blood vessels we used micro-angiography following intra-ventricular perfusion of a fluorescent microspheres (0.1uM). The impermeability of the microspheres allows their vascular retention, enabling confocal angiographic “vascular mapping” of intact and mature blood vessels. Examination of perfused microspheres in wild type and transgenic gastrocnemius revealed an increase in muscle vascularity by ERRγ (Figure 3C) showing that ERRγ dually promotes oxidative fiber specification and neo-vascularization.
Figure 3. ERRγ increases muscle vascularization.
(A) Increased PECAM 1 staining in transgenic compared to WT gastrocnemius. (B) Increased alkaline phosphatase staining in transgenic compared to WT tibialis muscles. (C) Confocal images of microsphere perfused WT and transgenic quadriceps. Similar results were obtained from N=4 experiments in (A–C). (D–H) Expression of Vegfa-121, Vegfa-165, Vegfa-189, Vegfb and Fgf1 transcript levels in WT and transgenic quadriceps. Data are presented as mean ± SD from N=6 samples. (I) ERRγ increases VEGFa and FGF1 protein expression (N=4). * represents significant difference between WT and transgenic mice (p<0.05, unpaired Student’s t-test).
Paracrine regulation of muscle vascularization of ERRγ
How might ERRγ expressed in myofibers regulate proximal vascular development? Gene expression studies (Figure 2A and Supplementary Table 3) revealed increased expression of 25 angiogenic genes, including vascular endothelial growth factor A (Vegfa) in ERRGO quadriceps. Real time PCR confirmed induction of two Vegfa isoforms (165 & 189) along with Vegfb and Fgf1 in transgenic muscles (Figure 3D–H). Moreover, ERRγ as well as ERRα & ERRβ increased the transcription of a Vegfa promoter-driven luciferase reporter in AD 293 cells (Supplementary Figure S3). In addition, we confirmed that the protein levels of Vegfa and Fgf1 were increased in the quadriceps of the transgenic mice (Figure 3H), raising the specter that muscle ERRγ activates paracrine networks that are released into the microenvironment to promote neo-vascularization.
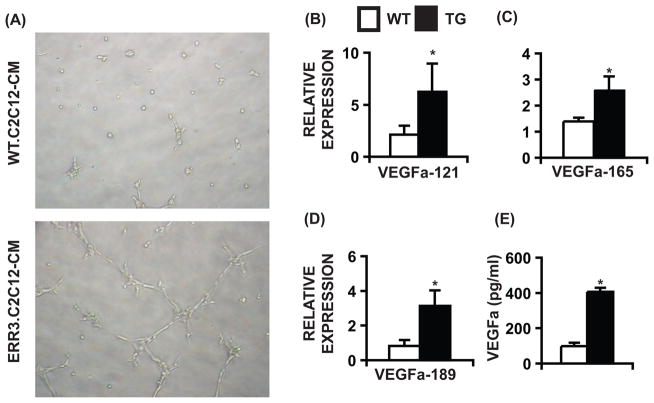
To directly test whether ERRγ triggers paracrine angiogenesis we employed an SVEC4–10 (murine endothelial cells) tube formation assay. We reasoned that conditioned media from ERRγ over-expressing muscle cells would contain the appropriate signals to induce tube formation in endothelial cells. Indeed, treatment of SVEC4–10 cells with conditioned media from ERRγ over-expressing C2C12 myotubes stimulated tube formation in 7–8 hr (Figure 4A). To confirm that the conditioned media contains angiogenic signals, we examined the gene expression in cells and protein levels in the media (by ELISA) of a representative angiokine, Vegfa. We found that over-expression of ERRγ in C2C12 myotubes increases expression of Vegfa-121, 165 and 189 genes (Figure 4B–D) and increases total Vegfa secretion (by 4-fold) in the media (Figure 4E). These results demonstrate that ERRγ can induce angiogenic factors such as myocellular Vegfa to increase angiogenesis in a paracrine fashion.
Figure 4. Paracrine stimulation of angiogenesis by ERRγ.
(A) Tube formation in SVEC4–10 cells treated for 7–8 hr with conditioned media from WT and ERRγ over-expressing C2C12 myotubes. Similar results were obtained from 4–6 experiments. (B–D) Expression of Vegfa isoforms in WT and ERRγ over-expressing C2C12 myotubes (N=6). (E) Vegfa concentrations (pg/ml) in conditioned media from 2 day differentiated WT and ERRγ over-expressing C2C12 myotubes (N=3). Data in (B–E) are presented as mean ± SD. * represents significant difference between WT and transgenic mice (p<0.05, unpaired Student’s t-test). See Supplemental Figure S3 and Table S3.
Physiological effects of ERRγ remodeled muscle
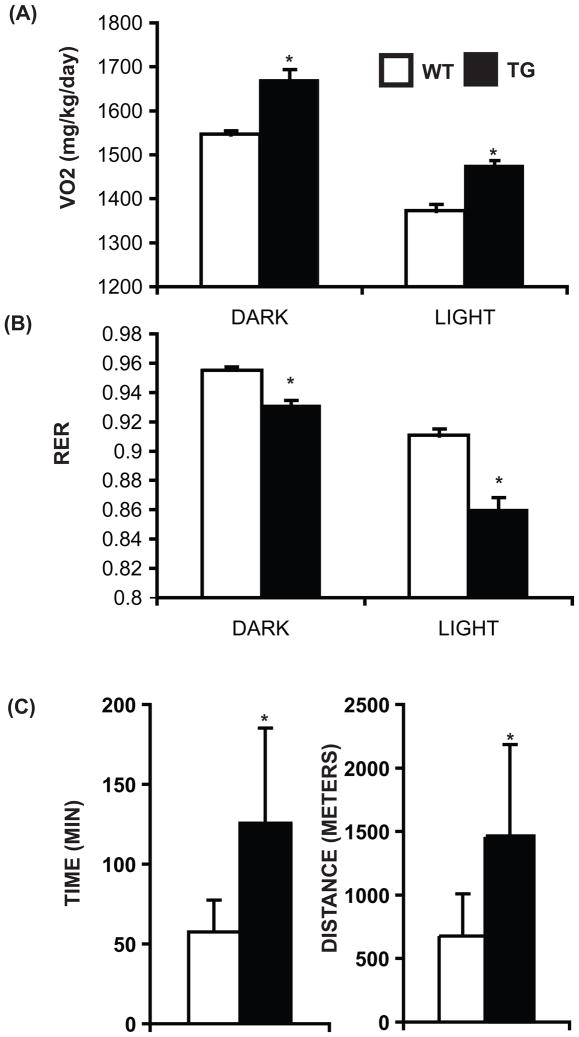
Aerobic exercise-induced remodeling of skeletal muscles depends on both an increase in oxidative capacity and new blood vessel formation; changes that are a critical part of the physiologic adaptation to training (Bloor, 2005; Egginton, 2008; Gavin et al., 2007; Gustafsson and Kraus, 2001; Jensen et al., 2004; Waters et al., 2004). Therefore, we investigated the potential of ERRγ to promote physiological re-modeling. First, in metabolic cage oxymetric studies, we found that the transgenic mice exhibited an increase in oxygen consumption (during both the light and dark cycles) in concert with the observed increased oxidative metabolism and blood supply to skeletal muscles (Figure 5A). Second, the ERGGO mice have a lower Respiratory Exchange Ratio (RER) compared to the wild type mice indicative of a tendency to preferentially oxidize fat over carbohydrate in the transgenic skeletal muscles (Figure 5B). The ambulatory activities of wild type and transgenic mice were comparable, and therefore unlikely to contribute to changes in oxymetric parameters (Supplemental Figure S4 A). These combined changes led us to explore whether ERRGO mice acquired enhanced running endurance. ERRγ transgenic mice were able to run longer and further compared to the wild type littermates (Figure 5C). Finally, the ERRGO mice were subjected to a high fat-high carbohydrate diet to establish whether the induction of endurance muscle and oxidative RER affected global metabolic balance. As expected ERRGO mice gained 35% less weight than wild type controls on a high fat diet (Supplemental Figure S4 B). These findings demonstrate that targeting of ERRγ increases oxidative metabolism and blood supply to skeletal muscle leading to increased oxygen consumption, better endurance and resistance to weight gain.
Figure 5. Physiological effect of ERRγ over-expression.
(A) Average oxygen consumption (N=6–7) and (B) average RER (N=6–7) during the light and the dark cycle over a period of 24 hr in WT and transgenic mice. (C) Running endurance as a function of time and distance (N=6). Data are presented as mean ± SEM in (A) and (B) and as mean ± SD in (C). * indicates statistically significant difference between the two groups. (p<0.05, unpaired Student’s t-test). See Supplemental Figure S4.
PGC1α-independent regulation of aerobic muscle by ERRγ
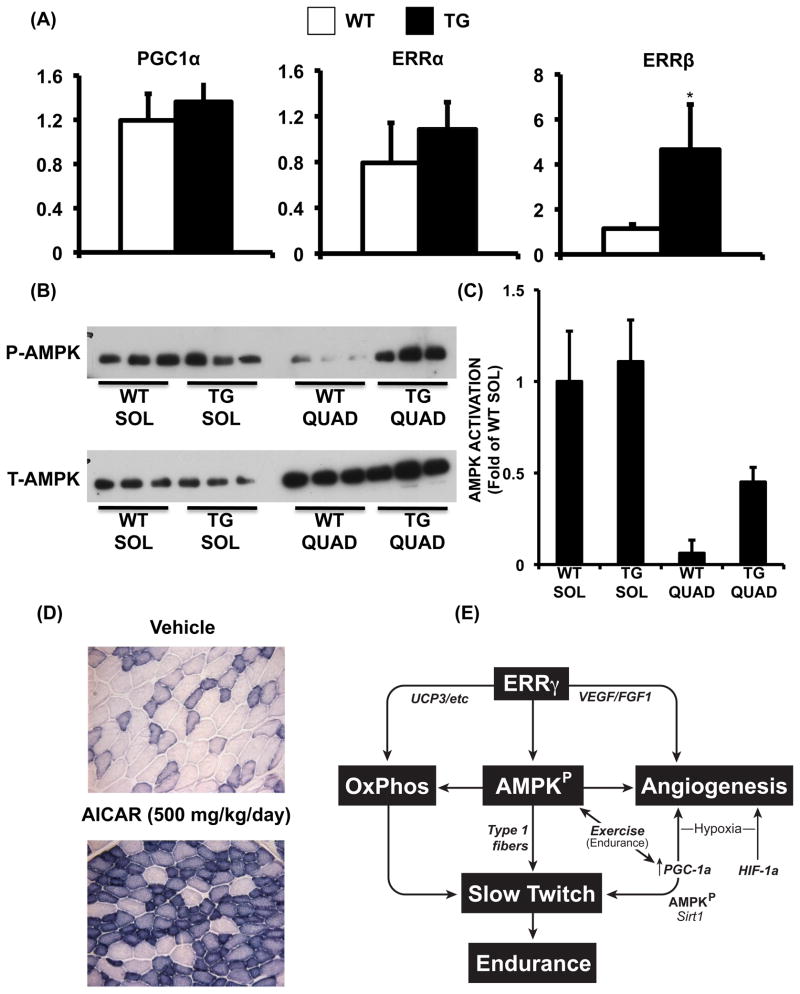
PGC1α is induced by hypoxia and exercise to promote HIF1α-independent vascularization of type II muscle (Arany et al., 2008) and further activated by post-translational modifications such as deacetylation (Jager et al., 2007; Puigserver et al., 2001; Rodgers et al., 2005). Therefore, we asked whether the ERRγ-induced changes in the muscle were due to the induction and/or activation of PGC1α. The levels of PGC1α mRNA, protein and acetylation remained unchanged in the ERRγ-transformed skeletal muscle (Figure 6A and Supplemental Figure S5 A). Interestingly, of the two additional ERR isoforms that can mediate PGC1α signaling, ERRβ but not ERRα was also significantly induced in transgenic muscle (Mootha et al., 2004; Schreiber et al., 2003; Huss et al., 2002)
Figure 6. PGC1α-independent regulation by ERRγ.
(A) Relative expression of Pgc1a, Erra and Errb genes in WT and transgenic muscle (N=6). Data are presented as mean ± SD. * represents significant difference between WT and transgenic mice (p<0.05, unpaired Student’s t-test). (B) Phospho (upper panel) and total (lower panel) AMPK in soleus (SOL) and quadriceps (QUAD) of WT and transgenic mice (N=3). (C) Quantification of AMPK activation (phospho to total AMPK ratio) by densitometric analysis, presented as fold of WT soleus (N=3). Data is presented as mean ± SD. (D) Representative images of SDH staining of muscle cryo-sections from vehicle and AICAR (500mg/kg/day for 4 weeks) treated mice. Similar results were obtained from N=3 mice. (E) Synchronization of metabolism and vasculature by ERRγ in aerobic muscle. See Supplemental Figure S5.
How might ERRγ control metabolism, VEGF induction and vasculature remodeling in ERRGO mice in absence of enhanced PGC1α signaling? We focused on the alternative aerobic master-regulator–AMPK–because of its known role in metabolic (Fujii et al., 2008; Fujii et al., 2007) and vascular adaptation (Zwetsloot et al., 2008). While AMPK is normally induced by exercise or hypoxia, surprisingly we found it to be constitutively activated in ERRGO muscle (Figure 6B and C). The AMPK activation was further validated by measuring phospho-ACC levels (an AMPK target and a bio-marker of AMPK activity), which we found to be higher in the transgenic compared to the wild type muscles (Supplemental Figure S5 B). ATP consumption is critical to AMPK activation as AMP stimulates and ATP inhibits the enzyme (Xiao et al., 2007). Indeed, we found that ATP levels were lower in ERRγ over-expressing compared to control C2C12 muscle cells, providing a biochemical basis for the observed AMPK activation (Supplemental Figure S5 C). (Note that we use cultured muscle cells for measuring ATP levels because ERRγ over-expression promotes both angiogenic gene expression as well as oxidative respiration in a fashion similar to transgenic muscle). Interestingly, in wild type mice, we found that AMPK is more active in predominantly oxidative slow-twitch compared to predominantly glycolytic fast-twitch muscle, in resting state (Figure 6B and C). Indeed, a synthetic activator AICAR, at a dose (500mg/kg/day) previously shown to stimulate AMPK in anaerobic muscle and improve aerobic performance (Narkar et al., 2008), was able to direct aspects of skeletal muscle transformation in a fashion similar to ERRγ (Figure 6D). These observations suggest a convergence between ERRγ and AMPK pathways that comprise an exercise-independent mechanism to direct intrinsic vascularization and oxidative metabolism in type I muscle, as depicted in Figure 6E.
DISCUSSION
Oxidative slow-twitch muscle beds are highly vascularized, pointing to an underlying regulatory network that integrates blood flow to myocellular metabolism. A transcriptional pathway specifying intrinsic differences between type I and II muscles has not previously been identified. Discovery of the components of this network has implications in treating cardiovascular diseases commonly linked to peripheral vascular degeneration due to ischemia. Here we show that in the skeletal muscle, ERRγ is exclusively expressed in highly vascularized aerobic muscles. Transgenic over-expression of ERRγ is sufficient to enable anaerobic muscles to acquire enhanced oxidative capacity and dense vasculature. The observed morphological remodeling is linked to induction by ERRγ of genes controlling oxidative phosphorylation, fatty acid oxidation and oxidative slow-twitch myofibers as well as a parallel induction of pro-angiogenic genes involved in paracrine regulation of vasculature. At a functional level, these genetic changes impart high oxygen consuming and exercising capacity as well as resistance to diet-induced obesity to the ERRGO mice. Surprisingly, these effects are independent of PGC1α, but instead are associated with ERRγ-directed AMPK activation in the muscle. Therefore, ERRγ regulates blood supply to aerobic muscles, and perhaps is a transcriptional gauge of myo-cellular supply and demand.
Although skeletal muscle adapts to exercise by increasing oxidative metabolism and vascular supply via induction of transcriptional regulators such as PGC1α (Arany et al., 2008; Baar et al., 2002; Huss et al., 2002; Pilegaard et al., 2003; Russell et al., 2003; Russell et al., 2005), how type I fibers achieve intrinsic vascularization even in absence of exercise is poorly understood. We show here that one such molecular pathway involves nuclear receptor ERRγ–highly expressed in oxidative slow-twitch muscles. Targeted expression of ERRγ to quadriceps and white gastrocnemius, where the receptor is typically not expressed, morphologically endows these muscles with dense vascular supply and numerous slow-twitch characteristics. Recently, it was reported that muscle-specific over-expression of a constitutively active ERRγ (VP16-ERRγ) imparts an oxidative metabolic phenotype to the skeletal muscle (Rangwala et al., 2010). However, the effect of VP16-ERRγ on muscle vascularization was not evaluated in these mice.
Genome-wide expression analysis revealed that ERRγ acts by coordinately inducing gene networks promoting mitochondrial biogenesis, oxidative transformation and angiogeneis. The ERRγ program includes mobilization and oxidation of fat [e.g. Acadl, Acadm, Cpt1b, Cpt2, Lpl], electron transport [e.g. Atp5h, Cox6a2, Ndufab1, Ndufb2m Ndufv1, Sdhb], mitochondrial biogenesis [e.g. Mfn1], and formation of energy efficient slow-contractile muscle [e.g. Tnnc1, Tnni1, Tnnt1]. The observed changes constituting transformation of the contractile apparatus to a slow phenotype and increase in oxidative metabolic genes reflected in profound increase in mitochondrial (SDH) staining represents a fiber type switch. Notably, ERRγ also induces key transcriptional inducers of oxidative metabolism including Esrrb, Ppara, Ppard and Ppargc1b (Supplemental Table 4) (Lin et al., 2002; Minnich et al., 2001; Muoio et al., 2002; Wang et al., 2004). Therefore, it is likely that ERRγ is a critical upstream genetic switch that may determine metabolic fate by presiding over the expression of multiple aerobic regulators.
We hypothesize that the vascular program triggered by myocellular ERRγ activates a transcriptional program that directs secretion of paracrine signals into skeletal muscle microenvironment to induce angiogenesis. This model is strongly supported by our observation that conditioned media from ERRγ over-expressing C2C12 myotubules is able to induce endothelial cell tube formation in culture. Indeed, ERRγ transcriptionally induced all isoforms of angiokine Vegfa in C2C12 myotubes, resulting in increased Vegfa secretion into the media. Vegfa is a key regulator of angiogenesis critical for guiding endothelial cells to their targets (Grunewald et al., 2006; Springer et al., 1998). Furthermore, ERRγ stimulates the Vegfa promoter containing putative ERR binding sites that is known to transcribe all Vegfa isoforms (Arany et al., 2008). Vegfa mRNA and protein expression is also induced in ERRGO muscle. These findings collectively raise the possibility of direct transcriptional activation of angiogenic genes by ERRγ. However, it is important to note that the angiogenic effects of ERRγ cannot be solely attributed to Vegfa induction and secretion. For example ERRγ additionally activates the expression of Fgf1 and Cxcl12, known to regulate endothelial cell proliferation and migration (Forough et al., 2006; Gupta et al., 1998; Partridge et al., 2000; Shao et al., 2008; Zheng et al., 2007), along with ephrin B2 proposed to recruit mural cells that are required for vessel maturation (Foo et al., 2006). Additionally, up-regulated factors such as Notch4 as well as SOX17 are transcriptional regulators of vasculogenesis (Hainaud et al., 2006; Leong et al., 2002; Matsui et al., 2006). In this aspect, ERRγ seems to serve a function similar to HIF1α, a known master regulator of angiogenesis during hypoxia (Pajusola et al., 2005). Interestingly, it was recently demonstrated that ERRs might physically interact with HIF1α in regulating its transcriptional activity (Ao et al., 2008). Whether such a mechanism is relevant to our model remains to be determined. Along these lines, HIF1α mRNA levels–a marker for chronic hypoxia–did not change in ERRGO compared to wild type muscles (data not shown) indicating an absence of hypoxia or its involvement in the vascular effects of ERRγ (Hoppeler and Vogt, 2001a, b). Furthermore, HIF1α is known to negatively regulate oxidative metabolism (Mason et al., 2004; Mason et al., 2007) and is therefore unlikely to contribute to ERRγ-mediated remodeling of skeletal muscles.
ERRGO mice exhibited increased oxygen consumption, decreased respiratory exchange ratio, high running endurance and resistance to diet-induced weight gain. These changes are physiological hallmarks of increased aerobic capacity in mice, and are a direct consequence of engineering highly oxidative and vascularized muscle by ERRγ. While similar remodeling of skeletal muscle and aerobic physiology are triggered by exercise, our data prove that generation of a fully functional “endurance vasculature” is not exercise dependent (Bloor, 2005; Egginton, 2008; Gavin et al., 2007; Gustafsson and Kraus, 2001; Jensen et al., 2004; Waters et al., 2004). Reciprocally, the extent to which ERRγ signaling in skeletal muscle contributes to exercise adaptation remains to be determined.
A surprising finding of our study was lack of change in the expression of PGC1α, a known and inducible regulator of aerobic muscles, in the ERRγ-transformed muscle. One alternative possibility is post-translational activation of PGC1α without change in its expression (Jager et al., 2007; Puigserver et al., 2001; Rodgers et al., 2005). De-acetylation of PGC1α is critical for its activation in the skeletal muscle (Canto et al., 2010; Gerhart-Hines et al., 2007; Lagouge et al., 2006). However, ERRγ over-expression did not lead to de-acetylation of PGC1α, which remained comparably acetylated in both the wild type and ERRGO muscles. The lack of post-translational activation of the co-factor in ERRGO mice is further underscored by a previous report that non-genomic activation of PGC1α typically leads to its transcriptional induction, which we did not observe in these studies (Jäger et al., 2007). Along the same lines, it was recently shown that both PGC1α and β are dispensable for fiber type specification in the skeletal muscle (Zechner et al., 2010). In contrast, we find that an alternative aerobic master regulator, AMPK, is activated by ERRγ in the skeletal muscles. AMPK is typically activated by exercise (Fujii et al., 2000; Winder and Hardie, 1996; Wojtaszewski et al., 2000) and is essential for exercise-mediated switch to aerobic myofibers in the skeletal muscle (Rockl et al., 2007). Indeed, transgenic activation of AMPK in the skeletal muscle increases the proportions of oxidative myofibers in absence of any exercise (Rockl et al., 2007). Similarly we found that chemical activation of AMPK by AICAR triggers aerobic transformation of type II muscle. However, AMPK alone is unlikely to mediate all the ERRγ effects, and contribution by additional metabolic regulators (e.g. calcineurin, SIRT1, etc) in ERRGO mice cannot be ruled out. This is possible because, unlike ERRγ, AMPK activation apparently does not lead to a complete transformation to a type I phenotype, but to a more intermediate type IIa and IIx oxidative myofibers (Rockl et al., 2007). In this context, it is peculiar that we found AMPK to be naturally and selectively active in soleus (pre-dominantly type I myofibers) compared to quadriceps (predominantly type II myofibers). Previous studies have suggested AMPK activity to be similar between soleus and EDL (also pre-dominantly made up of type II myofibers) (Dzamko et al., 2008; Jensen et al., 2007; Jorgensen et al., 2004). Speculatively, this discrepancy may have technical attributes or may even be linked to possible differences in recruitment of EDL and quadriceps for postural activity that might affect basal AMPK activation. Nevertheless, our results demonstrate that in the context of over-expression, ERRγ is sufficient to initiate both metabolic and vascular pathways to drive aerobic remodeling of sedentary muscle independent of PGC1α by recruiting alternative regulators such as AMPK (see Figure 6E).
Multiple diseases including obesity and diabetes are commonly linked to deregulation of both oxidative metabolism and vascularity. A shared therapeutic approach to these conditions includes exercise that activates a plethora of transcriptional pathways to increase aerobic metabolism and vascularization to ultimately enhance performance (Bloor, 2005; Egginton, 2008; Gavin et al., 2007; Gustafsson and Kraus, 2001; Jensen et al., 2004; Waters et al., 2004). Our findings present a possibility of therapeutically exploiting ERRγ to simultaneously regulate oxidative capacity and vascularity. High expression levels of this receptor in tissues most prone to metabolic and vascular diseases (e.g. heart, skeletal muscle, brain and kidney) further potentiates its value as a potential pharmacologic target (Ariazi et al., 2002; Cheung et al., 2005; Gao et al., 2006; Giguere, 2008; Heard et al., 2000; Hong et al., 1999). In summary, our studies show that ERRγ controls mitochondrial function and metabolism, together with angiogenesis that anatomically synchronizes vascular arborization to oxidative metabolism.
Experimental Procedures
Animals
Mouse ERRγ cDNA was placed downstream to the human α-skeletal actin promoter and upstream of the SV40 intron/poly (A) sequence. The purified transgene was injected into C57BL/6J x CBA F1 zygotes. Two transgenic founders (TG 425 and 421) were obtained that were backcrossed for 5 generations with C57BL/6J. All experiments used age (2–3 months) and sex (male) matched transgenic and wild type (WT) littermates. Mice were maintained on a normal chow diet. ERRγ +/− mice and tissue β-galactosidase staining has been described previously (Alaynick et al., 2007).
Drug treatment
Male C57Bl/6J mice (8 weeks old) were intra-peritoneally injected with vehicle or AICAR (500mg/kg/day), as previously described (Narkar et al., 2008).
Gene and protein expression analysis
RNA was extracted using Trizol extraction method from quadriceps or soleus isolated from WT and transgenic mice. Additionally, protein lysates were prepared from quadriceps and analyzed by western blotting with myoglobin (Dako), CYCS (Santacruz), UCP3 (Affinity Bioreagents), phospho-AMPK alpha (Cell Signaling, Cat no # 2535) and total-AMPK alpha (Cell Signaling, Cat no # 2532) antibodies. Note that the AMPK antibodies detect both the alpha 1 and 2 catalytic subunits of AMPK (Narkar et al., 2008).
Microarray Analysis
Global gene expression analysis was performed in quadriceps from WT and transgenic mice, as previously described (Narkar et al., 2008).
Muscle Staining and Immunohistochemistry
SDH, PECAM/CD31 and alkaline phosphates staining are described in the Supplemental Methods.
Fluorescence Micro-angiography
Blood vessel mapping was performed as previously described (Johnson et al., 2004; Springer et al., 2000). Briefly, a red fluorescent microsphere (0.1μM) suspension was intra-ventricularly perfused (10 ml, 1ml/min) followed by euthanasia and tissue collection. Longitudinal cryo-sections (10μM) of frozen gastrocnemius were processed and subjected to confocal microscopy to image skeletal muscle vasculature.
Cell culture, in vitro angiogenesis and Vegfa ELISA
See Supplemental Methods.
Oxymetery and treadmill assays
Oxygen consumption, respiratory exchange ratio and ambulatory activity were measured in 3 month old, WT and transgenic male mice (N=6–7/group) of comparable weight using Comprehensive Lab Animal Monitoring System to obtain oxymetric measurements (Columbus Instruments). These mice were first acclimated in the monitoring system for 1 day, followed by data collection for 24 hr to include a 12 hr light and dark cycle. For each animal, the average of all the data points within the light or dark phase was used as a representative value of the respective cycle. Diurnal differences between the light and dark cycles were detectable in all animals, validating the method of data collection.
Endurance was determined in WT and transgenic (N=6 mice/group), as previously described (Narkar et al., 2008). Treadmill protocol is described in Supplemental Methods.
Data Analysis
Data was analyzed using either one way ANOVA with an appropriate post hoc test, or unpaired student’s t-test, as indicated.
The global gene expression data has been deposited in the NCBI Gene Expression Omnibus under the GEO series accession number (pending).
Supplementary Material
Acknowledgments
We thank Dr. Li-Jung Tai for suggestions on in vitro angiogenesis assay, Dr. Ellen Potter for comments on the manuscript and S. Ganley and E. Ong for administrative assistance. R.M.E is an investigator of the Howard Hughes Medical Institute at the Salk Institute for Biological Studies and March of Dimes Chair in Molecular and Developmental Biology. V.A.N was supported by a postdoctoral fellowship from NIAMS (AR053803-03). J.W.J was supported by the Human Frontier Science Program and the Netherlands Organization for Scientific Research. The Howard Hughes Medical Institute, Hilblom foundation, the Nuclear Receptor Signaling Atlas U19DK62434-01, the Helmsley foundation and NIH grants HD027183 and DK057978 primarily supported this work. A part of this work was also supported by a grant from Muscular Dystrophy Association (174408) to V.A.N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguere V, et al. ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab. 2007;6:13–24. doi: 10.1016/j.cmet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Annex BH, Torgan CE, Lin P, Taylor DA, Thompson MA, Peters KG, Kraus WE. Induction and maintenance of increased VEGF protein by chronic motor nerve stimulation in skeletal muscle. Am J Physiol. 1998;274:H860–867. doi: 10.1152/ajpheart.1998.274.3.H860. [DOI] [PubMed] [Google Scholar]
- Ao A, Wang H, Kamarajugadda S, Lu J. Involvement of estrogen-related receptors in transcriptional response to hypoxia and growth of solid tumors. Proc Natl Acad Sci U S A. 2008;105:7821–7826. doi: 10.1073/pnas.0711677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 2007;5:35–46. doi: 10.1016/j.cmet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Ariazi EA, Clark GM, Mertz JE. Estrogen-related receptor alpha and estrogen-related receptor gamma associate with unfavorable and favorable biomarkers, respectively, in human breast cancer. Cancer Res. 2002;62:6510–6518. [PubMed] [Google Scholar]
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- Bloor CM. Angiogenesis during exercise and training. Angiogenesis. 2005;8:263–271. doi: 10.1007/s10456-005-9013-x. [DOI] [PubMed] [Google Scholar]
- Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Cherwek DH, Hopkins MB, Thompson MJ, Annex BH, Taylor DA. Fiber type-specific differential expression of angiogenic factors in response to chronic hindlimb ischemia. Am J Physiol Heart Circ Physiol. 2000;279:H932–938. doi: 10.1152/ajpheart.2000.279.3.H932. [DOI] [PubMed] [Google Scholar]
- Cheung CP, Yu S, Wong KB, Chan LW, Lai FM, Wang X, Suetsugi M, Chen S, Chan FL. Expression and functional study of estrogen receptor-related receptors in human prostatic cells and tissues. J Clin Endocrinol Metab. 2005;90:1830–1844. doi: 10.1210/jc.2004-1421. [DOI] [PubMed] [Google Scholar]
- Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguere V. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab. 2007;5:345–356. doi: 10.1016/j.cmet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Dzamko N, Schertzer JD, Ryall JG, Steel R, Macaulay SL, Wee S, Chen ZP, Michell BJ, Oakhill JS, Watt MJ, et al. AMPK-independent pathways regulate skeletal muscle fatty acid oxidation. J Physiol. 2008;586:5819–5831. doi: 10.1113/jphysiol.2008.159814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egginton S. Invited review: activity-induced angiogenesis. Pflugers Arch. 2008 doi: 10.1007/s00424-008-0563-9. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- Fluck M, Hoppeler H. Molecular basis of skeletal muscle plasticity--from gene to form and function. Rev Physiol Biochem Pharmacol. 2003;146:159–216. doi: 10.1007/s10254-002-0004-7. [DOI] [PubMed] [Google Scholar]
- Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Forough R, Weylie B, Collins C, Parker JL, Zhu J, Barhoumi R, Watson DK. Transcription factor Ets-1 regulates fibroblast growth factor-1-mediated angiogenesis in vivo: role of Ets-1 in the regulation of the PI3K/AKT/MMP-1 pathway. J Vasc Res. 2006;43:327–337. doi: 10.1159/000093198. [DOI] [PubMed] [Google Scholar]
- Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, et al. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- Fujii N, Ho RC, Manabe Y, Jessen N, Toyoda T, Holland WL, Summers SA, Hirshman MF, Goodyear LJ. Ablation of AMP-activated protein kinase alpha2 activity exacerbates insulin resistance induced by high-fat feeding of mice. Diabetes. 2008;57:2958–2966. doi: 10.2337/db07-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N, Seifert MM, Kane EM, Peter LE, Ho RC, Winstead S, Hirshman MF, Goodyear LJ. Role of AMP-activated protein kinase in exercise capacity, whole body glucose homeostasis, and glucose transport in skeletal muscle -insight from analysis of a transgenic mouse model. Diabetes Res Clin Pract. 2007;77(Suppl 1):S92–98. doi: 10.1016/j.diabres.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Gao M, Sun P, Wang J, Zhao D, Wei L. Expression of estrogen receptor-related receptor isoforms and clinical significance in endometrial adenocarcinoma. Int J Gynecol Cancer. 2006;16:827–833. doi: 10.1111/j.1525-1438.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- Gavin TP, Ruster RS, Carrithers JA, Zwetsloot KA, Kraus RM, Evans CA, Knapp DJ, Drew JL, McCartney JS, Garry JP, et al. No difference in the skeletal muscle angiogenic response to aerobic exercise training between young and aged men. J Physiol. 2007;585:231–239. doi: 10.1113/jphysiol.2007.143198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev. 2008;29:677–696. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Lysko PG, Pillarisetti K, Ohlstein E, Stadel JM. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem. 1998;273:4282–4287. doi: 10.1074/jbc.273.7.4282. [DOI] [PubMed] [Google Scholar]
- Gustafsson T, Kraus WE. Exercise-induced angiogenesis-related growth and transcription factors in skeletal muscle, and their modification in muscle pathology. Front Biosci. 2001;6:D75–89. doi: 10.2741/gustafss. [DOI] [PubMed] [Google Scholar]
- Hainaud P, Contreres JO, Villemain A, Liu LX, Plouet J, Tobelem G, Dupuy E. The role of the vascular endothelial growth factor-Delta-like 4 ligand/Notch4-ephrin B2 cascade in tumor vessel remodeling and endothelial cell functions. Cancer Res. 2006;66:8501–8510. doi: 10.1158/0008-5472.CAN-05-4226. [DOI] [PubMed] [Google Scholar]
- Heard DJ, Norby PL, Holloway J, Vissing H. Human ERRgamma, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: tissue-specific isoforms are expressed during development and in the adult. Mol Endocrinol. 2000;14:382–392. doi: 10.1210/mend.14.3.0431. [DOI] [PubMed] [Google Scholar]
- Hong H, Yang L, Stallcup MR. Hormone-independent transcriptional activation and coactivator binding by novel orphan nuclear receptor ERR3. J Biol Chem. 1999;274:22618–22626. doi: 10.1074/jbc.274.32.22618. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Vogt M. Hypoxia training for sea-level performance. Training high-living low. Adv Exp Med Biol. 2001a;502:61–73. doi: 10.1007/978-1-4757-3401-0_6. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Vogt M. Muscle tissue adaptations to hypoxia. J Exp Biol. 2001b;204:3133–3139. doi: 10.1242/jeb.204.18.3133. [DOI] [PubMed] [Google Scholar]
- Huss JM, Kopp RP, Kelly DP. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J Biol Chem. 2002;277:40265–40274. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- Huss JM, Torra IP, Staels B, Giguere V, Kelly DP. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol. 2004;24:9079–9091. doi: 10.1128/MCB.24.20.9079-9091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen L, Bangsbo J, Hellsten Y. Effect of high intensity training on capillarization and presence of angiogenic factors in human skeletal muscle. J Physiol. 2004;557:571–582. doi: 10.1113/jphysiol.2003.057711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TE, Rose AJ, Jorgensen SB, Brandt N, Schjerling P, Wojtaszewski JF, Richter EA. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am J Physiol Endocrinol Metab. 2007;292:E1308–1317. doi: 10.1152/ajpendo.00456.2006. [DOI] [PubMed] [Google Scholar]
- Johnson C, Sung HJ, Lessner SM, Fini ME, Galis ZS. Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues: potential role in capillary branching. Circ Res. 2004;94:262–268. doi: 10.1161/01.RES.0000111527.42357.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Leong KG, Hu X, Li L, Noseda M, Larrivee B, Hull C, Hood L, Wong F, Karsan A. Activated Notch4 inhibits angiogenesis: role of beta 1-integrin activation. Mol Cell Biol. 2002;22:2830–2841. doi: 10.1128/MCB.22.8.2830-2841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Mason SD, Howlett RA, Kim MJ, Olfert IM, Hogan MC, McNulty W, Hickey RP, Wagner PD, Kahn CR, Giordano FJ, et al. Loss of skeletal muscle HIF-1alpha results in altered exercise endurance. PLoS Biol. 2004;2:e288. doi: 10.1371/journal.pbio.0020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason SD, Rundqvist H, Papandreou I, Duh R, McNulty WJ, Howlett RA, Olfert IM, Sundberg CJ, Denko NC, Poellinger L, et al. HIF-1alpha in endurance training: suppression of oxidative metabolism. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2059–2069. doi: 10.1152/ajpregu.00335.2007. [DOI] [PubMed] [Google Scholar]
- Matsui T, Kanai-Azuma M, Hara K, Matoba S, Hiramatsu R, Kawakami H, Kurohmaru M, Koopman P, Kanai Y. Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J Cell Sci. 2006;119:3513–3526. doi: 10.1242/jcs.03081. [DOI] [PubMed] [Google Scholar]
- Minnich A, Tian N, Byan L, Bilder G. A potent PPARalpha agonist stimulates mitochondrial fatty acid beta-oxidation in liver and skeletal muscle. Am J Physiol Endocrinol Metab. 2001;280:E270–279. doi: 10.1152/ajpendo.2001.280.2.E270. [DOI] [PubMed] [Google Scholar]
- Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, et al. Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci U S A. 2004;101:6570–6575. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio DM, Way JM, Tanner CJ, Winegar DA, Kliewer SA, Houmard JA, Kraus WE, Dohm GL. Peroxisome proliferator-activated receptor-alpha regulates fatty acid utilization in primary human skeletal muscle cells. Diabetes. 2002;51:901–909. doi: 10.2337/diabetes.51.4.901. [DOI] [PubMed] [Google Scholar]
- Muscat GE, Kedes L. Multiple 5′-flanking regions of the human alpha-skeletal actin gene synergistically modulate muscle-specific expression. Mol Cell Biol. 1987;7:4089–4099. doi: 10.1128/mcb.7.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajusola K, Kunnapuu J, Vuorikoski S, Soronen J, Andre H, Pereira T, Korpisalo P, Yla-Herttuala S, Poellinger L, Alitalo K. Stabilized HIF-1alpha is superior to VEGF for angiogenesis in skeletal muscle via adeno-associated virus gene transfer. Faseb J. 2005;19:1365–1367. doi: 10.1096/fj.05-3720fje. [DOI] [PubMed] [Google Scholar]
- Partridge CR, Hawker JR, Jr, Forough R. Overexpression of a secretory form of FGF-1 promotes MMP-1-mediated endothelial cell migration. J Cell Biochem. 2000;78:487–499. [PubMed] [Google Scholar]
- Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech. 2000;50:500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001;8:971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- Rangwala SM, Wang X, Calvo JA, Lindsley L, Zhang Y, Deyneko G, Beaulieu V, Gao J, Turner G, Markovits J. Estrogen-related receptor gamma is a key regulator of muscle mitochondrial activity and oxidative capacity. J Biol Chem. 2010;285:22619–22629. doi: 10.1074/jbc.M110.125401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoll E, Sillau AH, Banchero N. Changes in the capillarity of skeletal muscle in the growing rat. Pflugers Arch. 1979;380:153–158. doi: 10.1007/BF00582151. [DOI] [PubMed] [Google Scholar]
- Rockl KS, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes. 2007;56:2062–2069. doi: 10.2337/db07-0255. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, et al. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes. 2003;52:2874–2881. doi: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- Russell AP, Hesselink MK, Lo SK, Schrauwen P. Regulation of metabolic transcriptional co-activators and transcription factors with acute exercise. FASEB J. 2005;19:986–988. doi: 10.1096/fj.04-3168fje. [DOI] [PubMed] [Google Scholar]
- Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRalpha) J Biol Chem. 2003;278:9013–9018. doi: 10.1074/jbc.M212923200. [DOI] [PubMed] [Google Scholar]
- Seth A, Steel JH, Nichol D, Pocock V, Kumaran MK, Fritah A, Mobberley M, Ryder TA, Rowlerson A, Scott J, et al. The transcriptional corepressor RIP140 regulates oxidative metabolism in skeletal muscle. Cell Metab. 2007;6:236–245. doi: 10.1016/j.cmet.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H, Tan Y, Eton D, Yang Z, Uberti MG, Li S, Schulick A, Yu H. Statin and stromal cell-derived factor-1 additively promote angiogenesis by enhancement of progenitor cells incorporation into new vessels. Stem Cells. 2008;26:1376–1384. doi: 10.1634/stemcells.2007-0785. [DOI] [PubMed] [Google Scholar]
- Springer ML, Chen AS, Kraft PE, Bednarski M, Blau HM. VEGF gene delivery to muscle: potential role for vasculogenesis in adults. Mol Cell. 1998;2:549–558. doi: 10.1016/s1097-2765(00)80154-9. [DOI] [PubMed] [Google Scholar]
- Springer ML, Ip TK, Blau HM. Angiogenesis monitored by perfusion with a space-filling microbead suspension. Mol Ther. 2000;1:82–87. doi: 10.1006/mthe.1999.0006. [DOI] [PubMed] [Google Scholar]
- Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters RE, Rotevatn S, Li P, Annex BH, Yan Z. Voluntary running induces fiber type-specific angiogenesis in mouse skeletal muscle. Am J Physiol Cell Physiol. 2004;287:C1342–1348. doi: 10.1152/ajpcell.00247.2004. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270:E299–304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528(Pt 1):221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, Walker PA, Haire L, Eccleston JF, Davis CT, et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- Zechner C, Lai L, Zechner JF, Geng T, Yan Z, Rumsey JW, Collia D, Chen Z, Wozniak DF, Leone TC, et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab. 2010;12:633–642. doi: 10.1016/j.cmet.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ma K, Sadana P, Chowdhury F, Gaillard S, Wang F, McDonnell DP, Unterman TG, Elam MB, Park EA. Estrogen-related receptors stimulate pyruvate dehydrogenase kinase isoform 4 gene expression. J Biol Chem. 2006;281:39897–39906. doi: 10.1074/jbc.M608657200. [DOI] [PubMed] [Google Scholar]
- Zheng H, Fu G, Dai T, Huang H. Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathway. J Cardiovasc Pharmacol. 2007;50:274–280. doi: 10.1097/FJC.0b013e318093ec8f. [DOI] [PubMed] [Google Scholar]
- Zwetsloot KA, Westerkamp LM, Holmes BF, Gavin TP. AMPK regulates basal skeletal muscle capillarization and VEGF expression, but is not necessary for the angiogenic response to exercise. J Physiol. 2008;586:6021–6035. doi: 10.1113/jphysiol.2008.159871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.