Abstract
Ras genes are commonly mutated in human cancers of the skin and other tissues. Oncogenic Ras signals through multiple effector pathways, including the Erk1/2 MAPK, phosphatidylinositol-3 kinase (PI3K), and the Ral guanine nucleotide exchange factor (RalGEF) cascades. In epidermis, activation of oncogenic Ras induces hyperplasia and inhibits differentiation, features characteristic of squamous cell carcinoma (SCC). The downstream effector pathways required for oncogenic Ras effects in epidermis, however, are undefined. In this study we investigated the direct contribution of Mek1 and Mek2 MAPKKs to oncogenic Ras signaling. The response of murine epidermis to conditionally active oncogenic Ras was unimpaired by deletion of either Mek1 or Mek2 MAPKKs individually. In contrast, Ras effects were entirely abolished by combined deletion of all Mek1/2 alleles while epidermis retaining only one allele of either Mek1 or Mek2 showed intermediate responsiveness. Thus, the effects of oncogenic Ras on proliferation and differentiation in skin display a gene dosage-dependent requirement for the Erk1/2 MAPK cascade at the level of Mek1/2 MAPKKs.
Keywords: MAP kinase, Mek, Ras, cancer, apoptosis
Introduction
The epidermis is a stratified squamous epithelium that provides an essential barrier between the interior and exterior of an organism by blocking the entry of foreign microorganisms and the exit of body fluids. It is maintained by stem cells that possess the ability to self-renew and generate daughter cells that differentiate along the lineages of the interfollicular epidermis, hair follicles, and sebaceous glands (Fuchs and Horsley, 2008). The epidermis relies on a finely calibrated homeostatic balance between cell division in the undifferentiated basal cell layers and terminal differentiation in the outer cell layers, such that the epidermis is continually regenerated. Epidermal homeostasis, however, is perturbed in many skin diseases, including cancer, in which cell proliferation is inappropriately increased and differentiation is inhibited. Ras, a small GTPase protein mutated in approximately 30% of all cancers (Castro et al., 2005; Hoshino et al., 1999; Pierceall et al., 1991), is activated by a variety of receptors, including growth factor receptor tyrosine kinases and integrins. Activating Ras mutations have been found in epidermal SCCs at frequencies as high as 46% (Pierceall et al., 1991), and tumors lacking Ras mutations display increased levels of active, GTP-bound Ras in a majority of cases (Dajee et al., 2003). The finding that Ras is a potent oncogene in skin and other tissues has stimulated efforts to treat Ras-associated tumors by targeting Ras effectors.
Ras signals via a host of effector pathways, among which are the Raf/Mek/Erk pathway, the type I phosphatidylinositol-3 kinases pathway, and the Ral guanine nucleotide exchange factors pathway (Dhillon et al., 2007; Shaul and Seger, 2007). Recent work has identified the Erk1/2 mitogen-activated protein kinase (MAPK) cascade as an essential regulator of epidermal homeostasis. This three-tiered kinase cascade—comprising Raf MAPKKKs, Mek MAPKKs, and Erk1/2 MAPK—translates extracellular signals into changes in gene expression and protein activity to regulate a wide range of cellular functions, including proliferation, differentiation, and survival (Chambard et al., 2007; Sturgill, 2008). Upon activation, Ras recruits Raf (Raf-1, B-Raf, and A-Raf) to the cell membrane, where it is phosphorylated. Raf then phosphorylates Mek (Mek1 and Mek2), which in turn phosphorylates Erk1 and Erk2, the only known Mek1/2 substrates. To date, only Mek1 and Mek2 are known to activate Erk1/2, which in turn phosphorylate over 160 proteins, including numerous cytoplasmic and nuclear targets, such as kinases, phosphatases, transcription factors, and cytoskeletal proteins (Yoon and Seger, 2006).
Mek1 and Mek2 are highly homologous proteins with 85% sequence identity. They are activated not only by Raf kinases, but also by c-mos, tpl-2, and Mek kinase 1. Selective knockouts in mice have been valuable in delineating the functional roles of Mek1 and Mek2 in development. Mek1 knockout mice die during embryogenesis, but further analysis demonstrated that Mek1 is required only for extraembryonic ectoderm formation, since depletion of Mek1 specifically in the embryo proper had no effect on development (Bissonauth et al., 2006; Giroux et al., 1999). In contrast, Mek2 knockout animals are viable, fertile, and phenotypically normal (Belanger et al., 2003). A recent study established the necessity of Mek1/2 function in mammals by showing that combined Mek1/2 deletion in mouse skin results in tissue hypoplasia, hypo-proliferation, and perinatal lethality (Scholl et al., 2007). Depletion of Mek1 in the skin of adult Mek2 knockout mice led likewise to hypoplasia, hypo-proliferation, and apoptosis. Similarly, combined depletion of Mek1/2 in human skin grafts through siRNA resulted in hypoplasia and hypo-proliferation (Scholl et al., 2007). Thus, function of the Erk1/2 MAPK pathway in epidermis is required for viability in mammals and displays functional redundancy at the level of Mek1/2 MAPKKs.
The Erk1/2 MAPK pathway has gained recent attention due to the unexpected finding that gain of function mutations in Ras, Raf, Mek1, and Mek2 underlie three clinically overlapping human genetic disorders: Noonan, cardio-facio-cutaneous, and Costello syndromes (Aoki et al., 2008; Kratz et al., 2007). The Erk1/2 MAPK pathway has largely been studied in vitro, however. Conflicting data have been presented for keratinocytes in these studies, and limited in vivo data are available. Mouse studies have shown that dominant negative Ras induces epidermal hypoplasia and increases differentiation when expressed in the basal epidermal layer, but has no effect when expressed in the suprabasal layer, indicating that Ras activity is required in basal layer keratinocytes, where it acts to promote proliferation and oppose differentiation (Dajee et al., 2002). Mice expressing oncogenic Ras in suprabasal layers of the epidermis develop papillomas (Bailleul et al., 1990; Brown et al., 1998; Greenhalgh et al., 1993). Furthermore, expression of constitutively active Ras in the basal layer of mouse skin effects epidermal hyperplasia marked by increased cell proliferation, increased integrin expression, and reduced expression of differentiation markers (Oh et al., 2007; Tarutani et al., 2003). Transgenic mice expressing active forms of Raf-1 and Mek1 similarly display hyperplasia and decreased differentiation marker expression in skin (Scholl et al., 2004; Tarutani et al., 2003), indicating that the Erk1/2 MAPK pathway may be an important mediator of the effects of oncogenic Ras in epidermis. The requirement for Erk1/2 MAPK pathway function in mediating oncogenic Ras effects in epidermis, however, is unknown.
Other major pathways downstream of Ras have also been implicated in Ras-driven hyperplasia. Activation of the PI3K pathway in skin has been shown to induce hyperplasia and promote hair growth (Murayama et al., 2007). PI3K consists of four isoforms: p110α, p110β, p110δ, and p110γ. p110α has been associated with cancer-specific mutations and is required for Ras-driven tumorigenesis in mice (Denley et al., 2008a; Gupta et al., 2007). The functions of the PI3K isoforms may not be completely independent from the Erk1/2 MAPK pathway, for the Erk pathway is required for p110γ and p100β transformation capacity (Denley et al., 2008b). In addition, the RalGEF pathway has been implicated in cancer, as loss of RalGDS reduces tumor incidence in the DMBA/TPA skin carcinogenesis model due to reduced survival of RalGDS knockout cells (Gonzalez-Garcia et al., 2005). To understand the signaling targets of oncogenic Ras and to design therapies against Ras-driven tumors, it will be important to delineate the necessity of each effector cascade in mediating the effects of Ras. Indeed, the Erk1/2 MAPK pathway has been a major focus for drug development, with Ras, Raf and Mek1/2 as the main targets, although no isoform-specific inhibitors for Mek1/2 are yet available (McCubrey et al., 2008; Wallace et al., 2005). Genetic studies designed to define the effector pathways required for oncogenic Ras effects in cancer-relevant tissues may help focus drug development efforts targeting Ras-driven tumors.
Here we investigated the importance of Mek1 and Mek2 MAPKKs in mediating Ras-induced hyperplasia in epidermis, a common tissue site of Ras-driven tumors. To do so, oncogenic Ras was conditionally activated in the skin of mice deficient in Mek1, Mek2, or both genes. Oncogenic Ras activation in skin in which two of the four Mek alleles were replaced by null alleles was reactive to oncogenic Ras signals in a manner indistinguishable from that of wild-type skin. In contrast, skin retaining only one wild-type Mek allele displayed dramatically reduced hyperplasia in response to oncogenic Ras activation, and skin lacking all Mek alleles was refractory to the oncogenic Ras stimulus. These data indicate that Mek1/2 MAPKKs are required in a gene dosage-dependent manner to mediate oncogenic Ras effects in epidermis.
Results
Mek1 is dispensable for oncogenic Ras-induced epidermal hyperplasia
To study the extent to which Mek1 disruption affects Ras signaling, we analyzed the effect of Ras activation in adult mouse skin lacking the Mek1 MAPK. Transgenic mice expressing a 4-hydroxytamoxifen (4OHT) inducible oncogenic Ras construct driven by the keratin 14 promoter (K14-ER:Ras mice, denoted in figures as ER:Ras) were crossed to skin-restricted Mek1 knockout mice. As previously described, activation of Ras in wild-type skin (Mek1fl/flER:Ras) induced massive hyperplasia and hyperproliferation after as few as 5 days, with the first clinical signs of hyperplasia appearing after 3 days of 4OHT treatment (Tarutani et al., 2003). We observed the same level of hyperplasia in Mek1 heterozygous (Mek1fl/+CreER:Ras) and Mek1 knockout animals (Mek1fl/flCreER:Ras) following Ras activation (Figure 1). In all cases, Ras-induced hyperplasia was marked by increased proliferation as well as increased expression of β4 integrin and the proliferation-associated keratin 6. Further, as expected, differentiation marker expression, including that of loricrin and keratin 10, was reduced. These results reflect a consequence of Ras activation in the skin, since ethanol vehicle treatment alone had no effect on either skin thickness or the expression of any marker analyzed. Thus, Mek1 expression is dispensable for Ras-induced hyperplasia.
Figure 1.
Mek1 deletion fails to alter oncogenic Ras-induced epidermal hyperplasia. Histology and immunofluorescence staining of adult mouse skin after 5 days of treatment with ethanol (vehicle) or 4-hydroxytamoxifen (4OHT). Tissue genotypes and treatment groups are noted at the top of each column. Skin sections were stained with antibodies against keratin 6, loricrin, keratin 10, keratin 5, Ki67, and β4 integrin (orange), and counterstained with Hoechst 33342 nuclear dye (blue) as noted at the right of each corresponding row; scale bars = 50 μm.
Recently, it was reported that Cre recombinase has toxic effects that may have complicated interpretation of several mouse knockout models (Editorials, 2007; Naiche and Papaioannou, 2007; Schmidt-Supprian and Rajewsky, 2007). To rule out a toxic effect of Cre in our experiments, we analyzed our transgenic mouse lines, which express Cre or inducible Cre (Cre:ER) from the keratin 14 promoter, which restricts Cre expression to stratified epithelial tissues. Five days of treatment with ethanol or 4OHT had no effect on adult mouse skin from either the Cre line or the Cre:ER line, similar to observations made in wild-type animals (Supplemental Figure 1). Further, no differences in expression of keratin 6, loricrin, keratin 10, keratin 5, Ki67, or β4 integrin were seen. We thus observe no Cre toxicity in our transgenic mouse lines with the treatment used in our experiments. Similarly, the epidermis of skin-restricted Mek1 knockout mice, inducible skin-restricted Mek1 knockout mice, and Mek1/2 double knockout mice was investigated in regard to potential nonspecific or toxic Cre side effects. Both types of conditional Mek1 knockout mice showed no response to ethanol or 4OHT treatments, similar to wild-type mice (Supplemental Figure 2). In contrast, as previously reported (Scholl et al., 2007), knockout of both Mek1/2 proteins led to apoptosis, since activation of Cre results in a total loss of Mek1/2 and abolishes function of the Erk1/2 MAPK signaling cascade, which is crucial for cell survival (Supplemental Figure 2). Taken together, these data demonstrate the absence of Cre recombinase toxicity in this setting.
Mek2 is dispensable for oncogenic Ras-induced epidermal hyperplasia
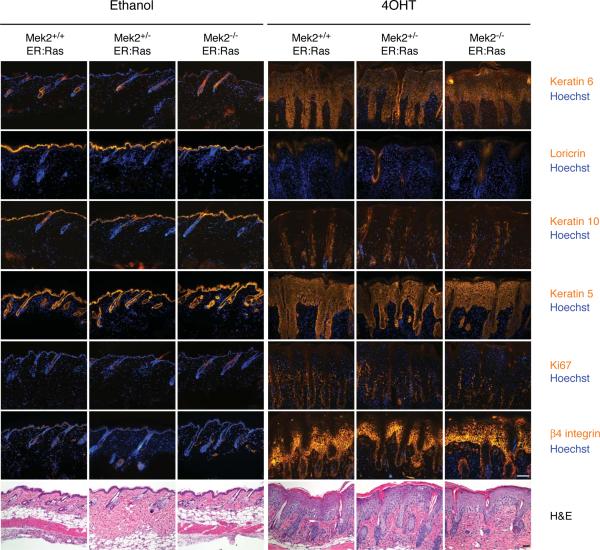
We next assessed the requirement for Mek2 in Ras-induced epidermal hyperplasia. Mek2 knockout animals were crossed with K14-ER:Ras transgenic mice. Wild-type (Mek2+/+), Mek2 heterozygous (Mek2+/−), and Mek2 knockout (Mek2−/−) ER:Ras transgenic mice were treated topically with either ethanol or 4OHT for 5 days. Activation of Ras, achieved by 4OHT treatment, led to hyperproliferation and hyperplasia that did not depend on the presence of Mek2 (Figure 2). Furthermore, loss of Mek2 did not alter the expression of keratin 5, 6, and 10, loricrin, Ki67, or β4 integrin. As seen in wild-type and Mek1 knockout skin (Figure 1), activation of Ras in Mek2 knockout skin caused acanthosis, hypogranulosis, and hyperkeratosis. As a control, ethanol treatment alone had no effect on the skin of any genotype. Together, these data demonstrate that Mek2 deletion does not alter epidermal response to Ras-mediated signals in skin.
Figure 2.
Ras-induced hyperplasia is not dependent on Mek2. Histology and immunofluorescence staining of adult mouse skin after 5 days of treatment with ethanol (vehicle) or 4-hydroxytamoxifen (4OHT). Tissue genotypes studied and treatment groups are noted at the top of each column, and markers stained are noted to the right. Scale bars = 50 μm.
Mek1/2 gene dosage influences Ras-induced epidermal hyperplasia
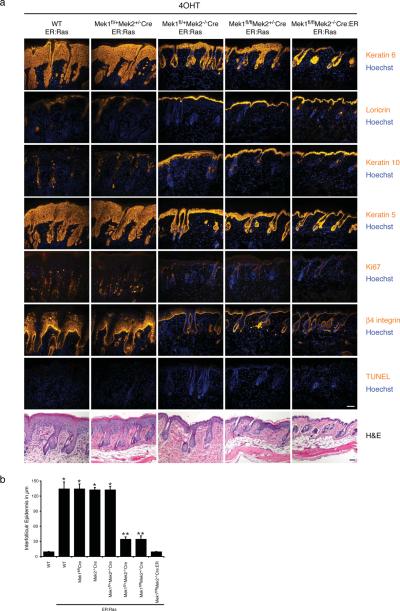
Because Mek1 and Mek2 show high structural and functional similarities, it is possible that our inability to see an effect on Ras-induced hyperplasia after deleting one isoform of Mek was due to functional compensation by the remaining Mek isoform. To further investigate the necessity of the Erk1/2 MAPK pathway in mediating Ras-induced hyperplasia, we generated mice having combinations of Mek1 and Mek2 wild-type and null alleles in the background of the K14-ER:Ras transgene. Upon Ras activation, mice having at least two functional alleles of either Mek1 or Mek2 showed hyperplasia indistinguishable from that induced by Ras activation in wild-type skin (Figure 3a). This massive hyperplasia, which caused a 13.5-fold increase in skin thickness as compared to normal skin, was always accompanied by hypogranulosis, hyperkeratosis, and some inflammation. In contrast, mice having solely one Mek1 or Mek2 allele (Mek1fl/+Mek2−/−CreER:Ras, Mek1fl/flMek2+/−CreER:Ras) displayed only mild hyperplasia, with a 3.5-fold increase in skin thickness as compared to normal skin (Figure 3b). Furthermore, skin lacking all alleles of Mek1/2 was entirely unresponsive to Ras-induced hyperplasia and resembled normal mouse skin. No epidermal apoptosis, as assessed by TUNEL staining, was visible after 5 days of 4OHT treatment in any of the genotypes studied; a magnified view of the histological appearance of each genotype is shown in Supplemental Figure 3. As expected, treatment of skin of any genotype with ethanol alone had no effect on thickness or differentiation marker expression (Supplemental Figure 4). The lack of Mek1/2 expression in the mutant mouse skin was previously demonstrated (Scholl et al., 2007). Immunohistochemical staining of mutant mouse skin with an antibody that recognizes both Mek1 and Mek2 further confirmed the lack of epidermal Mek1/2 expression (Figure 4c and Supplemental Figure 5).
Figure 3.
Ras-induced hyperplasia is Mek gene dosage-dependent. Whereas the presence of two Mek alleles is sufficient for full hyperplasia, the presence of one Mek allele is associated with reduced hyperplasia, and no hyperplasia is observed in skin with no Mek alleles. (a) Histology and immunofluorescence staining of adult mouse skin after 5 days of treatment with 4-hydroxytamoxifen (4OHT). Tissue genotypes are noted at the top of each column and markers stained are noted to the right. Scale bars = 50 μm. (b) Quantification of hyperplasia. The interfollicular epidermis was measured using a micrometer. Error bars represent the mean ± SEM (n = 5 per genotype). The difference in skin thickness between wild-type (WT) skin versus ER:Ras induction in 2–4 allele Mek skin (*, p < 2×10−5) and WT skin versus ER:Ras induction in 1 allele Mek skin (**, p < 0.0008) are statistically significant by Student's t test analysis.
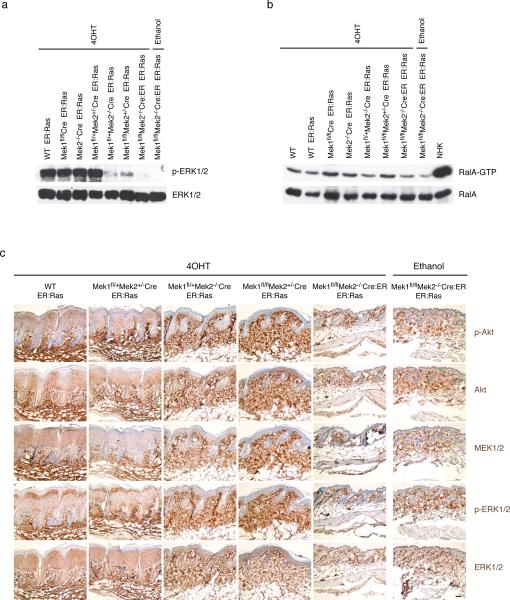
Figure 4.
Changes in Ras effector pathways following ablation of Mek1/2 alleles. (a) Epidermal extracts of the noted genotype, after treatment with either 4OHT or ethanol diluent control, were probed with the antibodies indicated at right. (b) GTP-bound Ral recovered with GST-RalBD (upper panel) and Ral present in the epidermal mouse extract or EGF-stimulated normal human keratinocytes (NHK) (lower panel) were identified by Western analysis using a RalA specific antibody. (c) Immunohistochemistry of adult mouse skin after 5 days of treatment with 4OHT or ethanol. The genotypes are noted on top, the antibodies used at right. Scale bar = 50 μm.
Thus, Mek1/2 gene dosage strongly influences Ras-induced hyperplasia in mouse skin. Whereas loss of any two Mek alleles does not affect Ras-induced hyperplasia, skin lacking three Mek alleles is minimally responsive to Ras-induced hyperplasia, and Mek1/2 double knockout skin is entirely refractory to the actions of oncogenic Ras.
Activation of Ras effector pathways is altered depending on degree of Mek1/2 loss
Ras signals through a multitude of effector pathways. Therefore, the effect of Mek1/2 loss in inducible oncogenic Ras activated skin on the three main downstream signaling pathways was assessed, namely the Erk1/2 MAPK pathway, the PI3K pathway, and the RalGEF pathway. Epidermal skin extracts were obtained after 5 days of daily 4OHT or ethanol treatment and phospho-Erk1/2 levels were assessed by Western blot. Activation of oncogenic Ras led to a massive increase in Erk1/2 phosphorylation over the basal level of Erk1/2 phosphorylation in mouse skin, which can only be detected with longer exposures (data not shown). No change in the amount of Ras-induced Erk1/2 phosphorylation was seen as long as at least two Mek alleles were present, regardless of whether these alleles are Mek1 or Mek2. In contrast, a single allele of Mek1 or Mek2 was capable of inducing only a minimal amount of Erk1/2 phosphorylation (Figure 4 a). Thus, the level of Erk1/2 phosphorylation induced by oncogenic Ras correlates with the increase in epidermal thickness stimulated by this oncogene. Immunohistochemical staining of phospho-Erk1/2 in treated mouse skin sections confirmed the Western blot findings (Figure 4a,c and Supplemental Figure 5).
To assess the activation of the Ral pathway, RalA-GTP level in epidermal extracts was measured by a pulldown assay. As expected, Ras activation in mouse skin led to an increase of RalA-GTP, but this increase was very modest as compared to that of human keratinocytes activated by EGF (Figure 4b). The number of Mek alleles did not broadly affect the extent of Ras-induced RalA-GTP increase, although loss of all four Mek1/2 alleles appeared to reduce the RalA-GTP increase slightly.
Activation of the PI3K pathway was assessed by immunohistochemical staining of phospho-Akt in treated mouse skin sections. The level of phospho-Akt activation correlated with epidermal hyperplasia, with tissue containing 2–4 Mek alleles showing pronounced staining (Figure 4c and Supplemental Figure 5). There was no increase of phospho-Akt staining in Mek1/2 double knockout skin in which Ras is activated, as compared to that of skin in which Ras was not activated.
Long-term activation of oncogenic Ras in skin results in papilloma-like hyperplasia, which is Mek1/2 gene dosage-dependent
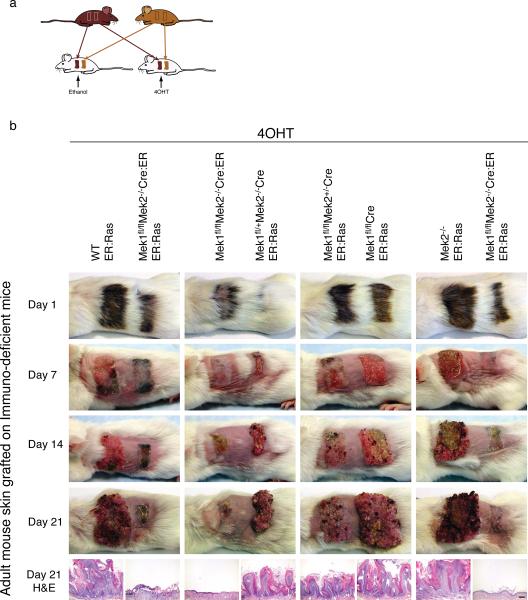
We next wanted to assess the Mek1/2 dependence of oncogenic Ras over a longer period of Ras action. Long-term topical 4OHT treatment influences not only lower back skin but also the mouth epithelium—perhaps due to mouse grooming—and impairs long-term viability (Scholl, FA unpublished observations). To circumvent these effects, we grafted skin from genetically altered mice onto immune-deficient mice. Dorsal skin was harvested from ER:Ras, Mek1fl/flCreER:Ras, Mek2−/−ERRas, Mek1fl/flMek2−/−Cre:ER, Mek1fl/+Mek2−/−CreER:Ras, Mek1fl/flMek2+/−CreER:Ras, and Mek1fl/flMek2−/−Cre:ERER:Ras mice. Two skin pieces, each from a different mouse, were grafted onto individual CB-17 scid mice. This procedure was performed in duplicate to generate identical mouse pairs. After the grafts were allowed to heal for 3 months, the animals were shaved, and for each pair of identical mice, one was treated with 4OHT and the other with ethanol (Figure 5a).
Figure 5.
Long-term activation of Ras in mouse skin. (a) Skin strips from 5-6 week old transgenic mice were grafted adjacently onto immune-deficient mice. This procedure was performed in duplicate to generate identically grafted mice, which were subsequently treated topically with either ethanol or 4OHT. (b) Mice were treated with 4OHT over the course of 21 days; time points are noted to the left. Genotypes are noted at the top of each column over each corresponding graft. The lower panel displays the histologic appearance of each graft at day 21. Scale bar = 200 μm.
Consistent with our previous results (Scholl et al., 2007) and depicted in Supplemental Figure 2, combined Mek1/2 deletion led to tissue death, as treatment of Mek1fl/flMek2−/−Cre:ER grafts with 4OHT led to epidermal erosions and eschar formation after 14 days, and progressed over the next 7 days to skin graft loss. In contrast, treating the skin of any genotype with ethanol had no effect (Supplemental Figure 6). As observed previously, activation of the ER:Ras transgene with 4OHT in wild-type, Mek1fl/flCre, or Mek2−/− skin resulted in massive hyperplasia that was equivalent in each genotype (Figure 5b). The whole skin graft became hyperplastic, resembling a large papilloma (Figure 5b, left graft in first column). Skin grafts containing only one Mek1 or Mek2 allele (Mek1fl/+Mek2−/−CreER:Ras, Mek1fl/flMek2+/−CreER:Ras) also responded to Ras activation, but with formation of smaller papillomas, consistent with our short-term studies. In contrast, activation of oncogenic Ras in the setting of combined Mek1/2 deletion failed to produce either papillomatosis or tissue death: treatment of Mek1fl/flMek2−/−Cre:ERER:Ras mouse skin with 4OHT initially caused some eschar formation, but no epidermal erosions were seen, and after 21 days of treatment, the skin was normal or minimally hyperkeratotic. These data thus show that combined deletion of Mek1 and Mek2 disrupts the epidermal response to long-term activation of oncogenic Ras.
Discussion
Here we have observed that the Mek1/2 MAPKK components of the Erk1/2 MAPK pathway are required for the epidermal hyperplasia and impaired differentiation induced by oncogenic Ras and that this relationship is gene dosage-dependent. To demonstrate this, mice expressing conditionally active, 4OHT-responsive oncogenic Ras in the epidermis, skin-restricted Mek1 knockout mice, and Mek2 knockout mice were used. The presence of any combination of two or more of the four total alleles of Mek1 and Mek2 were sufficient, in the context of oncogenic Ras activation, to mediate hyperplasia, hyperproliferation, and reduced expression of differentiation markers in skin. In contrast, the presence of a single allele of Mek1 or Mek2 was not sufficient to support maximal oncogenic Ras signaling, as hyperplasia was reduced significantly in these genotypes. Finally, Mek null skin was completely refractory to the effects of oncogenic Ras, confirming the primary importance of the Erk1/2 MAPK cascade in mediating the effects of this oncogene (Supplemental Figure 7).
Functional redundancy of Mek1 and Mek2
Our results indicate overlapping functions for Mek1 and Mek2 in oncogenic Ras-induced hyperplasia. This is in concordance with normal homeostasis, where loss of Mek1 or Mek2 in skin has no effect on epidermal development or maintenance (Belanger et al., 2003; Bissonauth et al., 2006). Further, mice expressing only one of the four total Mek1/2 alleles in skin, whether Mek1 or Mek2, are viable and fertile, show no skin abnormalities, and are indistinguishable from normal mice (Scholl et al., 2007). Therefore, a single Mek allele is sufficient for normal skin homeostasis, but is not adequate to transmit the total extent of oncogenic Ras action. This activity appears to require at least two Mek1/2 alleles, whether two Mek1, two Mek2, or one Mek1 and one Mek2 allele. These results thus suggest that Mek1 and Mek2 contribute similarly to Ras-induced hyperplasia and that both may have to be targeted by therapeutics aimed at Ras-associated tumors in this tissue.
In contrast, a previous report (Scholl et al., 2004) investigating the effect of expression of active forms of Mek1 and Mek2 in mouse as well as human skin showed that constitutively active Mek1 promotes proliferation and hyperplasia, whereas constitutively active Mek2 has no effect. The apparent functional difference between these two isoforms may have been due to the use of oncogenic forms the proteins, as overexpression of mutant proteins could have allowed for nonphysiologic activity. Interestingly, MEK1 mutations have been identified in cancer, but MEK2 mutants have not (Estep et al., 2007; Marks et al., 2008).
Gene dosage and oncogenic Ras-responsiveness
Limiting the amount of Mek1/2 directly limits the extent of hyperplasia, hyperproliferation, and hyperkeratosis induced by oncogenic Ras. Similarly, the hyperplasia of three different lines of Ras transgenic mice was directly proportional to the level of Ras expression in transgenic lines (Tarutani et al., 2003). Another study demonstrates as well that the level of oncogenic H-Ras correlates with tumorigenicity and malignancy (Sun et al., 2008). Thus, the effects of oncogenic Ras signaling are limited not only by the amount of activated Ras expressed in cells, but also by the levels of the downstream kinases Mek1/2.
Interestingly, although Mek1/2 double knockout skin is phenotypically normal after 5 days of Ras activation and shows no hyperplasia or cell death, Mek1/2 double knockout skin displays apoptosis after 5 days of Raf-1 activation (Scholl et al., 2007). Whereas Raf activates only the Erk1/2 MAPK cascade, Ras activates many additional signaling pathways, and it is likely that one of these pathways is able to rescue the cell death induced by Mek1/2 loss. Long-term, 21-day treatment of skin grafts demonstrates that skin possessing a single Mek allele is capable of substantial hyperplasia, but this hyperplasia remains reduced as compared to that of epidermis having 2–4 Mek1/2 alleles. Mek1/2 double knockout skin, however, is barely hyperproliferative, even at this late time point. Thus, gene dosage appears to be a central determinant of tissue responsiveness to Ras and Erk1/2 MAPK signaling during both short and long timeframes.
Oncogenic Ras activates multiple effector pathways, including the Erk1/2 MAPK, RalGEF, and PI3K cascades. Loss of all Mek1/2 alleles blocks the hyperplasia induced by oncogenic Ras in epidermis, suggesting that the skin phenotype observed in response to Ras activation is transmitted predominantly through the Erk1/2 MAPK pathway. Interestingly, it was shown that activation of the PI3K pathway through expression of active Akt in skin results in hyperplasia and hair growth (Murayama et al., 2007), which appears to contradict our observations. However, our studies focused on the requirement for Erk1/2 MAPK function in oncogenic Ras-driven effects and it is possible that PI3K/Akt signaling contributes in a synergistic fashion with Erk1/2 MAPK action. Consistent with this, a recent report, based on in vitro data, demonstrates that two out of the four subunits of PI3K depend on a functional Erk1/2 MAPK pathway for their ability to transform cells (Denley et al., 2008b). Our study shows that Akt activation is higher in hyperplastic skin than in nonhyperplastic skin, suggesting that Erk activity aids activation of Akt, which provides support for a functional interaction model. Further, no increased phoshpo-Akt staining was seen in Mek1/2 double knockout skin in which Ras is activated, as compared to the skin in which Ras is not activated, suggesting that Akt activation does not explain the reduced apoptosis observed in this genotype. It cannot be excluded that the crucial time point was missed, however, and that transient Akt activation explains the lack of apoptosis in the presence of oncogenic Ras. It will be interesting to further investigate the interactions between these pathways in vivo. Further, the extent of oncogenic Ras-induced RalA-GTP increase was not significantly affected by the number of Mek alleles present, although loss of all Mek1 and Mek2 alleles appeared to slightly reduce Ral-GTP levels.
Materials and methods
Mice
Mek2−/− mice and Mek1fl/fl mice were maintained in a 129/SvEv background (Belanger et al., 2003; Bissonauth et al., 2006). K14-ER:Ras transgenic mice, Jax stock number 006403, were kept in a 129/SvEv background (Tarutani et al., 2003). K14-Cre transgenic mice were obtained from Elaine Fuchs (Vasioukhin et al., 1999) and K14-Cre:ER mice were provided by Pierre Chambon (Li et al., 2000); each line was backcrossed to 129/SvEv mice. All mouse husbandry and experimental procedures were conducted in compliance with the protocols established by the Stanford University Animal Care and Use Committee. Mice were genotyped as previously described (Scholl et al., 2007). The ER:Ras transgene was detected with either the following primers: 5'-CACCACCAGCTCCACTTCAGCACATT-3' and 5'-CGCACCAACGTGTAGAAG GCATCCTC-3' or by genotyping performed by Transnetyx Inc. (Cordova, TN). The lower backs of mice were shaved and a 5-day, once-daily topical application of 4-hydroxytamoxifen (4OHT, Sigma) (1 mg/0.1 ml ethanol) was used to activate the ER:Ras and Cre:ER transgenes.
Grafting of mouse skin onto immune-deficient mice
Skin samples from 5–6 week old mice were harvested, stored overnight at 4°C in PBS with 100 U/ml penicillin and 100 μg/ml streptomycin, and then grafted onto 6 – 8 week old female CB-17 scid mice (Charles River). Grafts of adult mouse skin were maintained for three months after surgery, at which point they began a three-week regimen of once-daily 4OHT (1mg/0.1ml ethanol) or ethanol (vehicle) treatment. Samples were harvested 24 hours after the final treatment.
Active Ral pull-down assay
Pull-down assay to analyze levels of GTP-bound RalA was performed under nonsaturated conditions as described (Wolthuis et al., 1998). Briefly, bacterially produced GST-RalBD lysate was pre-coupled to glutathione-Sepharose beads (12μl/sample, Amersham) at room temperature for 30 min with shaking. After washing, 100 μg of epidermal tissue extract was added at 4°C for 1 hour with shaking. After 3 washes, the samples were separated on 12% SDS PAGE. Levels of active Ral protein were detected by a monoclonal anti-RalA antibody (1:1000, Transduction Laboratories).
Western Blot
Mice were treated with 4OHT or ethanol daily for 5 days. For immunoblotting, epidermal skin extract was obtained by incubating the dorsal skin in 1:1 dispase/PBS (Invitrogen, Carlsbad, CA) at 37°C for 3 hours to separate the epidermis from the dermis. The epidermis was lysed in lysis buffer and run on Western blots as previously described (Scholl et al., 2007). Antibodies used for immunoblotting were goat anti-RalA (1:1,000, R&D), rabbit anti-phospho-p44/42 MAPK (1:1,000, Cell Signaling Technologies, Danvers, MA), rabbit anti-p44/42 MAPK (1:1,000, Cell Signaling Technologies, Danvers, MA), donkey anti-rabbit IgG conjugated to horseradish peroxidase (HRP) (1:40,000, Amersham Biosciences, Piscataway, NJ) and bovine anti-goat HRP (1:10,000, Santa Cruz Biotechnology, Santa Cruz, CA).
Histological and immunohistochemical analyses of tissues
Skin samples of the lower backs of treated mice were harvested, fixed overnight in 10% neutral buffered formalin (Accustain, Sigma-Aldrich, USA), embedded in paraffin, cut into 5 μm sections, and stained with hematoxylin and eosin (H&E) or by immunohistochemistry according to standard methods. Permeabilization for antigen retrieval was achieved by microwaving samples in Antigen Unmasking Solution (Vector Laboratories, Burlingame, CA), after which the sections were stained with rabbit anti-phosho-Akt (1:150), rabbit anti-Akt (1:900), rabbit anti-MEK (1:150), rabbit anti-phospho-p44/p42 MAPK (1:600), and rabbit anti-p44/42 MAPK (1:750) Cell Signalling Technologies) as primary antibodies and biotinylated horse anti-rabbit IgG as secondary antibody (RTU Vectastain Universal Elite ABC Kit, Vector Laboratories). Staining and development were performed using the Elite ABC Reagent (Vector Laboratories) and DakoCytomation liquid DAB+ substrate chromogen system (Dako). The slides were counterstained with hematoxylin and PBS blueing. A minimum of three specimens was analyzed per genotype. Tissue stains were examined using a Leica DM LB microscope. The thickness of the interfollicular epidermis was measured from the basal layer to the top of the stratified layer using a micrometer (the cornified layer was not included).
Immunostaining
For each mouse genotype, specimens from at least 3 individual mice were harvested and analyzed. Samples from all genotypes were stained simultaneously and representative pictures were taken using a Zeiss 100M Axiovert microscope. Lower back skin samples were frozen in OCT (Sakura Finetek, Torrance, CA), cut into 7 μm cryosections, and fixed with either acetone or 4% paraformaldehyde. Detailed procedures and antibody information were previously described (Scholl et al., 2007). TUNEL assays were performed on skin sections as recommended by the manufacturer (Roche Diagnostics).
Statistics
ANOVA and subsequent post hoc comparisons using Student's t test were performed.
Supplementary Material
Acknowledgements
We thank A. Truong and K. Jameson for critical reading of the manuscript, E. Fuchs for K14-Cre mice, P. Chambon for K14-CreER mice, C. Enrile for histological work, H. Bernstein, P. Bernstein and N. Griffiths for support. This work was supported by the U.S. VA Office of Research and Development and by AR49737 from NIAMS/NIH to P.A.K., the Swiss cancer foundation grant BIL SKL-01236-02-2002 to F.A.S, and MOP-67208 from CIHR to J.C.
References
- Aoki Y, Niihori T, Narumi Y, Kure S, Matsubara Y. The RAS/MAPK syndromes: novel roles of the RAS pathway in human genetic disorders. Hum Mutat. 2008;29:992–1006. doi: 10.1002/humu.20748. [DOI] [PubMed] [Google Scholar]
- Bailleul B, Surani MA, White S, Barton SC, Brown K, Blessing M, et al. Skin hyperkeratosis and papilloma formation in transgenic mice expressing a ras oncogene from a suprabasal keratin promoter. Cell. 1990;62:697–708. doi: 10.1016/0092-8674(90)90115-u. [DOI] [PubMed] [Google Scholar]
- Belanger LF, Roy S, Tremblay M, Brott B, Steff AM, Mourad W, et al. Mek2 is dispensable for mouse growth and development. Molecular & Cellular Biology. 2003;23:4778–87. doi: 10.1128/MCB.23.14.4778-4787.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonauth V, Roy S, Gravel M, Guillemette S, Charron J. Requirement for Mapk2k1 (Mek1) in extraembryonic ectoderm during placentogenesis. Development. 2006;133:3429–40. doi: 10.1242/dev.02526. [DOI] [PubMed] [Google Scholar]
- Brown K, Strathdee D, Bryson S, Lambie W, Balmain A. The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Curr Biol. 1998;8:516–24. doi: 10.1016/s0960-9822(98)70203-9. [DOI] [PubMed] [Google Scholar]
- Castro AF, Rebhun JF, Quilliam LA. Measuring Ras-family GTP levels in vivo--running hot and cold. Methods. 2005;37:190–6. doi: 10.1016/j.ymeth.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Chambard JC, Lefloch R, Pouyssegur J, Lenormand P. ERK implication in cell cycle regulation. Biochim Biophys Acta. 2007;1773:1299–310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, et al. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639–43. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- Dajee M, Tarutani M, Deng H, Cai T, Khavari PA. Epidermal Ras blockade demonstrates spatially localized Ras promotion of proliferation and inhibition of differentiation. Oncogene. 2002;21:1527–38. doi: 10.1038/sj.onc.1205287. [DOI] [PubMed] [Google Scholar]
- Denley A, Gymnopoulos M, Hart JR, Jiang H, Zhao L, Vogt PK. Biochemical and Biological Characterization of Tumor-Associated Mutations of p110alpha. Methods Enzymol. 2008a;438:291–305. doi: 10.1016/S0076-6879(07)38020-8. [DOI] [PubMed] [Google Scholar]
- Denley A, Kang S, Karst U, Vogt PK. Oncogenic signaling of class I PI3K isoforms. Oncogene. 2008b;27:2561–74. doi: 10.1038/sj.onc.1210918. [DOI] [PubMed] [Google Scholar]
- Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Editorials Toxic alert. Nature. 2007;449:378. doi: 10.1038/449378a. [DOI] [PubMed] [Google Scholar]
- Estep AL, Palmer C, McCormick F, Rauen KA. Mutation analysis of BRAF, MEK1 and MEK2 in 15 ovarian cancer cell lines: implications for therapy. PLoS ONE. 2007;2:e1279. doi: 10.1371/journal.pone.0001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Horsley V. More than one way to skin. Genes Dev. 2008;22:976–85. doi: 10.1101/gad.1645908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux S, Tremblay M, Bernard D, Cardin-Girard JF, Aubry S, Larouche L, et al. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Current Biology. 1999;9:369–72. doi: 10.1016/s0960-9822(99)80164-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia A, Pritchard CA, Paterson HF, Mavria G, Stamp G, Marshall CJ. RalGDS is required for tumor formation in a model of skin carcinogenesis. Cancer Cell. 2005;7:219–26. doi: 10.1016/j.ccr.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Greenhalgh DA, Rothnagel JA, Quintanilla MI, Orengo CC, Gagne TA, Bundman DS, et al. Induction of epidermal hyperplasia, hyperkeratosis, and papillomas in transgenic mice by a targeted v-Ha-ras oncogene. Mol Carcinog. 1993;7:99–110. doi: 10.1002/mc.2940070208. [DOI] [PubMed] [Google Scholar]
- Gupta S, Ramjaun AR, Haiko P, Wang Y, Warne PH, Nicke B, et al. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell. 2007;129:957–68. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- Hoshino R, Chatani Y, Yamori T, Tsuruo T, Oka H, Yoshida O, et al. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999;18:813–22. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–31. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz CP, Niemeyer CM, Zenker M. An unexpected new role of mutant Ras: perturbation of human embryonic development. J Mol Med. 2007;85:227–35. doi: 10.1007/s00109-006-0135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Indra AK, Warot X, Brocard J, Messaddeq N, Kato S, et al. Skin abnormalities generated by temporally controlled RXRalpha mutations in mouse epidermis. Nature. 2000;407:633–6. doi: 10.1038/35036595. [DOI] [PubMed] [Google Scholar]
- Marks JL, Gong Y, Chitale D, Golas B, McLellan MD, Kasai Y, et al. Novel MEK1 mutation identified by mutational analysis of epidermal growth factor receptor signaling pathway genes in lung adenocarcinoma. Cancer Res. 2008;68:5524–8. doi: 10.1158/0008-5472.CAN-08-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, Milella M, Tafuri A, Martelli AM, Lunghi P, Bonati A, et al. Targeting the Raf/MEK/ERK pathway with small-molecule inhibitors. Curr Opin Investig Drugs. 2008;9:614–30. [PubMed] [Google Scholar]
- Murayama K, Kimura T, Tarutani M, Tomooka M, Hayashi R, Okabe M, et al. Akt activation induces epidermal hyperplasia and proliferation of epidermal progenitors. Oncogene. 2007;26:4882–8. doi: 10.1038/sj.onc.1210274. [DOI] [PubMed] [Google Scholar]
- Naiche LA, Papaioannou VE. Cre activity causes widespread apoptosis and lethal anemia during embryonic development. Genesis. 2007;45:768–75. doi: 10.1002/dvg.20353. [DOI] [PubMed] [Google Scholar]
- Oh WJ, Rishi V, Pelech S, Vinson C. Histological and proteomic analysis of reversible H-RasV12G expression in transgenic mouse skin. Carcinogenesis. 2007;28:2244–52. doi: 10.1093/carcin/bgm127. [DOI] [PubMed] [Google Scholar]
- Pierceall WE, Goldberg LH, Tainsky MA, Mukhopadhyay T, Ananthaswamy HN. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196–202. doi: 10.1002/mc.2940040306. [DOI] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8:665–8. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- Scholl FA, Dumesic PA, Barragan DI, Harada K, Bissonauth V, Charron J, et al. Mek1/2 MAPK kinases are essential for Mammalian development, homeostasis, and Raf-induced hyperplasia. Dev Cell. 2007;12:615–29. doi: 10.1016/j.devcel.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Scholl FA, Dumesic PA, Khavari PA. Mek1 alters epidermal growth and differentiation. Cancer Research. 2004;64:6035–40. doi: 10.1158/0008-5472.CAN-04-0017. [DOI] [PubMed] [Google Scholar]
- Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–26. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Sturgill TW. MAP kinase: It's been longer than fifteen minutes. Biochem Biophys Res Commun. 2008;371:1–4. doi: 10.1016/j.bbrc.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Sun B, Gao Y, Deng L, Li G, Cheng F, Wang X. The level of oncogene H-Ras correlates with tumorigenicity and malignancy. Cell Cycle. 2008;7:934–9. doi: 10.4161/cc.7.7.5622. [DOI] [PubMed] [Google Scholar]
- Tarutani M, Cai T, Dajee M, Khavari PA. Inducible activation of Ras and Raf in adult epidermis. Cancer Research. 2003;63:319–23. [PubMed] [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8551–6. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace EM, Lyssikatos JP, Yeh T, Winkler JD, Koch K. Progress towards therapeutic small molecule MEK inhibitors for use in cancer therapy. Curr Top Med Chem. 2005;5:215–29. doi: 10.2174/1568026053507723. [DOI] [PubMed] [Google Scholar]
- Wolthuis RM, Zwartkruis F, Moen TC, Bos JL. Ras-dependent activation of the small GTPase Ral. Curr Biol. 1998;8:471–4. doi: 10.1016/s0960-9822(98)70183-6. [DOI] [PubMed] [Google Scholar]
- Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.