Abstract
Molecular targeting of cancer stem cells has therapeutic potential for efficient treatment of cancer although relatively few specific targets have so far been identified. Hypoxia-inducible factor was recently shown to regulate tumorigenic capacity of glioma stem cells under hypoxic condition. Surprisingly, we found that, under normoxia, HIF1α signaling was selectively activated in the stem cells of mouse lymphoma and human acute myeloid leukemia (AML). HIF1a ShRNA and HIF inhibitors abrogated the colony forming unit activity of mouse lymphoma and human AML CSCs. Importantly, the HIF inhibitor echinomycin efficiently eradicated mouse lymphoma and serially transplantable human AML in xenogeneic model by preferential elimination of CSCs. HIF1α maintains mouse lymphoma CSCs by repressing a negative feedback loop in the Notch pathway. Taken together, our results demonstrate an essential function of HIF1α-Notch interaction in maintaining CSCs and provide an effective approach to target CSCs for therapy of hematological malignancies.
Introduction
Many human cancers contain cancer stem cells (CSC) that are responsible for initiating and maintaining tumor growth and for resistance to therapy (Al-Hajj et al., 2003; Bao et al., 2006; Ishikawa et al., 2007; Lapidot et al., 1994; Li et al., 2007; Reya et al., 2001; Singh et al., 2004). Understanding the mechanism of self-renewal of CSC is therefore not only crucial for understanding the fundamental cancer biology, but also for providing new approaches for long-lasting cancer therapy (Wicha et al., 2006). Similar to that of normal stem cells, CSC function involves two related processes. First, the stem cells must undergo proliferation (or self-renewal) to regenerate themselves. This process involves Wnt, Hedgehog and Bmi-1 (Beachy et al., 2004; Lessard and Sauvageau, 2003; Molofsky et al., 2003; Park et al., 2003). Second, the cancer stem cells must survive throughout tumorigenesis (Naka et al., 2010). For cancer therapy, it is best to eliminate CSC, as the dormant CSC may re-enter proliferative phase once the proliferation-inhibiting drugs are cleared.
Hypoxia-inducible factors (HIF) mediate the cellular response to hypoxia. Keith and Simon proposed that a hypoxic environment is required for cancer stem cell function (Keith and Simon, 2007). In support of this notion, Li et al. showed that HIF2α, but not HIF1α, was induced during hypoxia and was critical for the tumorigenicity of glioma stem cells (Li et al., 2009). Since this mechanism operates only under hypoxia, it is unclear whether it mediates the function of CSC in hematological malignancies. Here we report that a novel HIF1α-Notch pathway is essential for maintenance of CSC in hematological malignancies under normoxia and can be targeted to selectively eliminate CSC.
Results
Essential role for HIF1α activity in the maintenance of CSC in hematological malignancies
We have recently reported that 100% of the transgenic mice (TGB) with insertional mutation of the Epm2a gene succumbed to lymphoma (Wang et al., 2006). In our search for the expression of potential stem cell markers in the TGB lymphoma cells, we found that a small subset of cells expressed both c-Kit and Sca-1. To test if these cells had CSC activity, lymphoma cells from the spleens of tumor-bearing TGB transgenic mice were sorted based on expression of both c-Kit and Sca-1. We found that this subset represented the self-renewing population among the TGB lymphoma as determined by the colony-forming units (CFU) assay (Fig. S1). To determine if the c-Kit+Sca-1+ cells are also the lymphoma-initiating cells in vivo, we injected either c-Kit+Sca-1+ cells or c-Kit−Sca-1− cells intraperitoneally (i.p.) into the syngeneic B10BR mice. As shown in Table 1, Expt 1, 3/3 mice receiving 102 c-Kit+Sca-1+ cells developed lymphomas usually within 10 weeks after injection, while none of the recipients of 104 of c-Kit−Sca-1− cells developed lymphoma even after 40 weeks of observation. Similar results were obtained when the experiments were repeated by intravenous injection (Table 1, Expt 2). The lymphomas are characterized by enlarged spleens (Fig. S1) and lymph nodes, and metastases to the liver and lung, but not thymus (data not shown), unlike the spontaneously developed lymphoma that first appeared as a thymoma and then metastasized into other organs (Wang et al., 2006). Furthermore, no constitution of other cell lineages was achieved from the c-Kit+Sca-1+ subset isolated from tumors, which indicates that the c-Kit+Sca-1+ cells are not the tumor-infiltrating HSC. In three rounds of serial transplantation (Table 1, Expt 5), the c-Kit+Sca-1+ cells, but not the c-Kit−Sca-1− cells, gave rise to lymphoma at a comparable potency. Moreover, in each rounds of serial transplantation, the c-Kit+Sca-1+ cells gave rises to all subsets of tumor cells, including c-Kit−Sca-1−, c-Kit+Sca-1− and c-Kit−Sca-1+ subsets (Fig. S2a). The tumors maintained expression of T-cell marker CD8, but gradually lost cell surface expression of the transgenic TCR (Fig. S1, 2). The significance of the phenotypic shift is unclear. More importantly, the frequency of the c-Kit+Sca-1+ cells remained around 1% throughout the serial transplantation (Supplemental Fig. S2). Thus, there are self-renewing tumor-initiating cells among the c-Kit+Sca-1+ tumor cells.
Table 1.
Identification of CSC using c-Kit and Sca-1 markers
| Expt | Donor | Recipient | Number of cells injected | |||
|---|---|---|---|---|---|---|
| 10,000 | 1,000 | 500 | 100 | |||
| 1 | c-Kit+Sca-1+ | B10.BR | - | - | 3/3 | 3/3 |
| c-Kit−Sca-1− | B10.BR | 0/3 | - | - | - | |
| 2. | c-Kit+Sca-1+ | B10.BR | - | - | 4/4 | 3/3 |
| c-Kit−Sca-1− | B10.BR | 0/3 | - | 0/3 | - | |
| 3. | c-Kit+Sca-1+ | B10.BR | - | - | 5/5 | 5/5 |
| c-Kit−Sca-1− | B10.BR | 1/5 | 0/5 | - | - | |
| 4. | c-Kit+Sca-1+ | RAG2−/− | - | - | 5/5 | 5/5 |
| c-Kit−Sca-1− | RAG2−/− | 1/5 | 0/5 | - | - | |
| 5. | Serial transplantation | |||||
| Round 1 | ||||||
| c-Kit+Sca-1+ | B10.BR | - | - | 3/3 | 3/3 | |
| c-Kit−Sca-1− | B10.BR | 1/3 | 0/3 | - | - | |
| Round 2 | ||||||
| c-Kit+Sca-1+ | B10.BR | - | - | - | 5/5 | |
| c-Kit−Sca-1− | B10.BR | 2/5 | - | - | - | |
| Round 3 | ||||||
| c-Kit+Sca-1+ | B10.BR | - | - | - | 3/3 | |
| c-Kit−Sca-1− | B10.BR | 0/3 (5000/mouse) | - | - | ||
The donor cells were isolated ex vivo from lymphoma (Expt 1 and 2) or cells that were cultured for more than 30 passages in vitro. The routes of injection were intraperitoneal (i.p.) for experiments 1, 3, and 4, and intravenous for experiment 2. There was no tumor growth (0/3) when 10 c-Kit+Sca-1+ cells were transplanted into B10.BR mice. In experiment 5, donor cells were isolated ex vivo from lymphoma and injected i.p. The lymphoma cells obtained in round 1 were sorted and injected for the second around, then harvested, resorted and injected for the third round. The tumor-free mice were observed for 22–40 weeks to confirm the lack of tumor growth. - means no cells injected.
Using the medium for assaying CFU of hematopoeitic progenitor cells, we were able to establish long term cultures of the TGB lymphoma cells. In over 30 passages, the c-Kit+Sca-1+ cells remained at about 0.5–1.5% of total lymphoma cell population and maintained the CFU in vitro (data not shown) and tumor initiation in vivo (Table 1, Expts 3 and 4), with an undiminished efficiency. As demonstrated in Fig. S2, the c-Kit+Sca-1+ cells remained at low %.
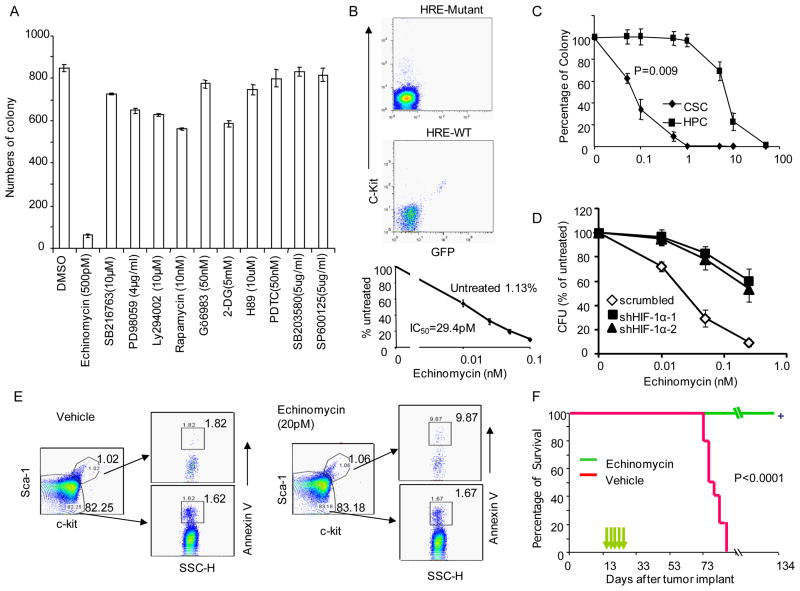
Using CFU as a surrogate assay, we set out to identify the molecular program responsible for this activity, As shown in Fig. 1a, treatment with pharmacologically effective doses of Ly294002 (inhibitor of PI-3 kinase-AKT signal pathway), Rapamycin (mTor-S6K protein synthesis pathway), SB216763 (GSK3β-beta-catenin pathway inhibitor), Gö6983 (PKC inhibitor), 2-DG (hexokinase inhibitor), H89 (PKA-CREB), PDTC (NF-κB signal pathway), PD98059, SB203580, and SP600126 (MAPK family ERK, p38, and JNK inhibitors, respectively) had no effect on CFU. In contrast, low doses of HIF1α inhibitor echinomycin (Kong et al., 2005a) abrogated the CFU.
Fig. 1.
Lymphoma CSC were abrogated by selectively by an HIF inhibitor. a. Selective ablation of lymphoma CFU by echinomycin. The cultured lymphoma cells were treated with given doses of pharmacologically effective drugs in medium for 24 hours prior to CFU assay. Data shown are means +/−SD of triplicates and have been confirmed by 3 independent experiments. b. Constitutive HIF activity among c-Kit+ cells and its sensitivity to echinomycin. The FACS profiles in the upper and middle panels show the specificity of the GFP reporter by co-expression of GFP expressing cells and c-Kit in WT HRE, but not mutant HRE lentiviral reporters. The dose response to inhibition by echinomycin is shown in the bottom panel. The lymphoma cells transfected with the HRE reporter system were cultured in the presence of different concentration of echinomycin for 12 hours, the % of c-Kit+GFP+ cells was normalized against the untreated group (1.13%, which was defined as 100%). The dose that resulted in 50% reduction of the c-Kit+GFP+ cells is defined as IC50. Detailed description of the reporter and its specificity is presented in supplemental data Fig. S3a. c. Selectivity of HIF inhibitor for CFU of lymphoma CSC over the CFU from hematopoietic progenitor cells (HPC). c-Kit+Sca-1+ cells from either TGB or normal bone marrow were treated with given concentration of echinomycin overnight prior to CFU assay. The data shown were % of untreated controls, and were means +/− S.D. of triplicates. d. ShRNA silencing reduces susceptibility of CFU activity to echinomycin. Lymphoma cells were transduced three times with either scrambled vector or Hif1a ShRNA vector and tested their response to inhibition by echinomycin. Note that the ShRNA groups yielded much reduced number CFU activity. For better comparison, CFU activity in the groups that received no echinomycin treatment is defined as 100%. In shRNA group, 100% is defined as 88 (sh1) and 132 (Sh2) CFU per 200,000 cells. In the control group, 100%= 448 CFU per 200,000 cells. e. Echinomycin selectively induces apoptosis of CSC. The cultured lymphoma cells were treated with 20 pM echinomycin or vehicle in medium for 16 hours. The treated cells were stained with c-Kit and Sca-1, followed by staining with Annexin V. f. Therapeutic effect of a low dose of echinomycin. Cultured lymphoma cells (1X106/mouse) were injected i.p. into immune competent B10.BR mice. Fourteen days later, 10μg/Kg/injection of echimomycin was injected at a two-day interval for a total of 5 times. Control mice received vehicle only. The mice were observed daily for survival. Data shown are representative of 2–3 independent experiments. See supplemental Fig. S1 and S2 for characterization of CSC, Fig. S3 for reporter of HIF activity and HIF activity of in vitro propagated and spontaneous lymphoma.
In order to monitor the HIF1α activity of the CSC, we established a lentiviral reporter consisting of triple HIF1 responsive elements (HRE) in the upstream of a minimum TATA box sequence and an enhanced green fluorescence protein (EGFP) cDNA, as shown in supplemental Fig. S3a. A pause sequence was introduced to eliminate LTR promoter activity. To validate the reporter, we first transiently transfected the HEK293 cells with either control vector or a mutant HIF1α (P402A/P564A) cDNA in conjunction with either WT or mutant HRE-driven EGFP reporters. The mutations made HIF1α functional under normoxia condition by resisting prolyl hydroxylation-mediated degradation (Pereira et al., 2003). As expected, the HER-EGFP reporter was specifically induced by HIF1α but not by the control vector. In contrast, the mutant HRE-EGFP reporter did not respond to HIF1α (Fig. S3a). Using this lentiviral vector, we determined the effect of echinomycin on the % of cells with HIF activity. As shown in Fig. 1b lower panel, a distinct GFP+ population of cells that expressed both c-Kit and Sca-1 markers was found among in vitro propagated lymphoma cells (Fig. 1b and supplemental Fig. S3). The expression of GFP reflected HIF activity as it can be abrogated by mutation of the HRE (Fig. 1b, upper panel). We further tested the sensitivity of this subset to echinomycin. As shown in Fig. 1b lower panel, echinomycin abrogated the CSC with an IC50 of 29.4 pM, which is considerably more sensitive than other cell types, where IC50 is in the nM range (Kong et al., 2005b).
It should be noted that the c-Kit+Sca-1− and c-Kit−Sca-1+ cells were also present in ex vivo tumor cells (Fig. S1). Since the c-Kit+Sca-1− subset also have significant HIF activity (Fig. S3b), it remains possible that the subset may also have CSC activity. However, c-Kit+Sca-1− and c-Kit−Sca-1+ cells disappeared over time during in vitro culture (Fig. 1e and Fig. S3c, for examples). Since their disappearance did not cause loss of CSC activity and since c-Kit+Sca-1− and c-Kit−Sca-1+ cells are progenies of the c-Kit+Sca-1+ cells, we have decided to focus on the c-Kit+Sca-1+ population for our subsequent analyses of molecular program and therapeutic elimination of CSC.
To substantiate the role of HIF1 activity in CSC function, we tested the effect of HIF inhibitors for both CFU in vitro and tumor-initiating activity in vivo. Since the CFU from the lymphoma CSC and normal hematopoeitic progenitor cells (HPC) can be assayed under similar conditions, we tested the selectivity of echinomycin for HPC vs lymphoma CSC. As shown in Fig. 1c, lymphoma CSC was approximately 100-fold more sensitive to echinomycin than HPC.
Although the echinomycin is also known to inhibit c-Myc activity, its IC50 is in the nM range (Vlaminck et al., 2007). As shown in Fig. S4a, in the ranges used in this study, echinomycin strongly inhibited HIF1α but had no effect on c-Myc function. It is also predicted that if echinomycin targets HIF1a, knockdown of the Hif1a should convey resistance to the drug. To test this hypothesis, we tested the impact of shRNA silencing of the Hif1a gene on susceptibility of CFU activity to echinomycin. Since the shRNA also reduced the CFU (see below), we normalized the CFU in the untreated groups to 100%. As shown in Fig. 1d, silencing Hif1a reduced the sensitivity of CFU to echinomycin. The apparent “synthetic lethality” provides strong genetic evidence that Hif1a is the major target of echinomycin.
To test if HIF activity was selectively required for survival of the c-Kit+Sca-1+ CSC, we treated the tumor cell culture with low doses of echinomycin (20 pM) for 16 hours and analyzed the % of apoptotic c-Kit+Sca-1+ and c-Kit−Sca-1− tumor cells. In the vehicle treated group, approximately 1.8% of c-Kit+Sca-1+ and c-Kit−Sca-1− tumor cells bound to Annexin V. Echinomycin increased Annexin V+ cells in the c-Kit+Sca-1+ tumor cells by 6-fold, to about 10%. No effect was observed in the c-Kit−Sca-1− tumor cells (Fig. 1e).
We injected 1×106 of cultured lymphoma cells i.p. into immune competent B10BR mice. Four or 14 days later, the mice that received lymphoma cells were either treated with vehicle only or 3 (Fig. S4b) or 5 (Fig. 1f) injections of 10 μg/Kg/injection/mouse of echinomycin at 2 day intervals. As shown in Fig. 1f and supplemental Fig. S4b, the untreated mice survived only 6–10 weeks, while all treated mice lived until euthanasia at 134 or 252 days after tumor cell injection, with no sign of tumor development upon necropsy. Two other known HIF inhibitors, 2-methoxyestradiol (Mabjeesh et al., 2003) and Geldanamycin (Minet et al., 1999), also reduced both CFU and tumor initiation of CSC, albeit at less efficacy (supplemental Fig. S4c, d). The difference in efficacy may be due to different mechanisms of action and bio-availability. The therapeutic efficacy of these HIF inhibitors demonstrated that HIF may serve as an effective therapeutic target.
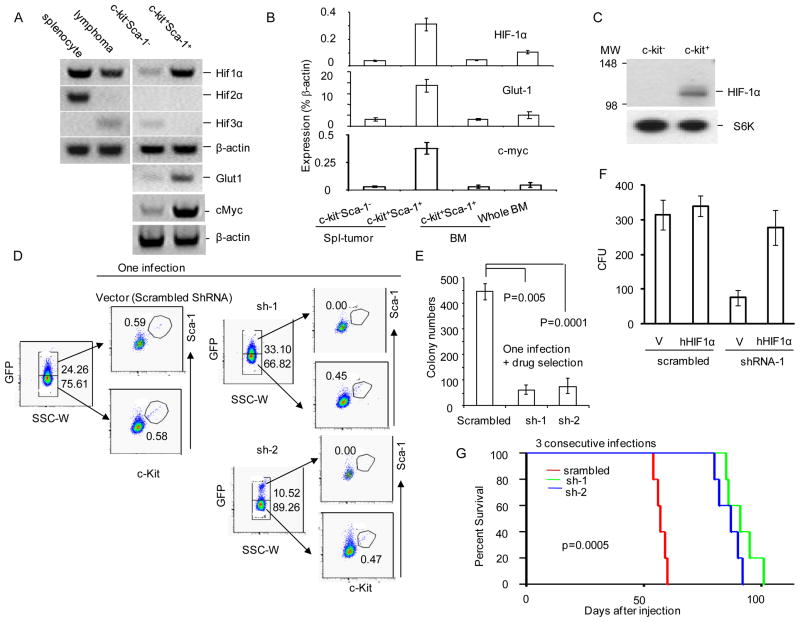
To determine the molecular mechanism for the high HIF1α activity in the CSC, we sorted the lymphoma cells into c-Kit+Sca-1+ or c-Kit−Sca-1− subsets and analyzed HIF1a, HIF2a and HIF3a expression by RT-PCR. As illustrated in Fig. 2a and quantitated in Fig. 2b, c-Kit+Sca-1+ cells expressed HIF1α at a level that was 4-fold higher than the c-Kit−Sca-1− cells. No expression of HIF2α or HIF3α was detected in the c-Kit+Sca-1+ cells. Consistent with higher levels of HIF activity, expression of glucose transporter Glut1and cMyc, two known target genes of HIF1α, was also highly elevated in the c-Kit+Sca-1+ cells (Fig. 2a, b). In contrast, no up-regulation of HIF1a and Glut1 was observed in the c-Kit+Sca-1+ bone marrow cells. An increased accumulation of the HIF1α protein was also observed in the c-Kit+ cells in comparison to the c-Kit− cells (Fig. 2c).
Fig 2.
Constitutively active HIF1α is essential for maintenance of CSC. a &b. Gene expression. The cultured lymphoma cells were sorted by BD FACSAria sorting system into c-Kit+Sca-1+ or c-Kit-Sca-1− fractions. The transcripts of HIF1a, HIF2a, HIF3a, Vhl, Glut1 and control gene βactin were determined by RT-PCR. a. Photograph of RT-PCR products. b. Relative expression as measured by real-time PCR. Relative expression of Hif1a, Glut1 and cMyc transcripts in FACS sorted c-Kit+Sca-1+ cells from either TGB tumor (Spl-tumor) or bone marrow (BM). The expression levels were expressed as fractions of house-keeping gene, βactin. c. Selective accumulation of Hif1α protein in the c-Kit+ but not c-Kit− TGB tumor cells, as determined by Western blot. The c-Kit+ (purity 83%) and c-Kit− (purity 99%) subsets were purified by MAC beads and lysed for Western blot. d. Silencing Hif1a abrogates the c-Kit+Sca-1+ CSC. TGB tumor cells were infected with either lentiviral vector with scrambled shRNA or lentiviral vector expressing two independent shRNA (sh-1 or sh-2). Three days after infection, the bulk tumor cells were analyzed by flow cytometry. The GFPhi and GFPlo cells were gated and analyzed for expression of c-Kit and Sca-1. e. Hif1a shRNA reduces CFU. The cultured lymphoma cells were infected with either lentiviral Hif1a shRNA or scrambled ShRNA by spinoculation, and the infected cells were selected with 5μg/ml of blasticidin for one week. The infected cells were seeded into 1% of methylcellulose culture medium at the density of 2X105/well. The colony numbers were counted under a microscope. Data shown are means +/− SD of colony numbers in triplicates and are representative of three independent experiments. f. Complementation of shRNA-induced defects by human HIF1A cDNA. TGB tumor cells were transduced with either lentiviral vector control or vector encoding human HIF1α protein. After blasticidin selection, the cells were transduced with lentiviral vector deliverying either scrambled shRNA or Hif1a shRNA-1 that has mismatch with human HIF1A. The CFU derived from transduced cells were counted based on expression of EGFP. g. HIF1a shRNAs abrogate tumor-initiating activity. TGB tumor cells were infected 3 times with lentiviral expressing scrambled ShRNA or HIF1α shRNA and then injected into B10.BR mice (9X105/mouse, i.p.). The survival of the recipient mice (n=5) was compared by Kaplan-Meier analysis. All mice that succumbed had developed lymphoma as confirmed by necropsy. All data presented in this figure have been repeated at least twice. See Fig. S5 for lentiviral transduction efficacy.
To establish the significance of HIF1a up-regulation in the c-Kit+Sca-1+ cells, we first used lentiviruses expressing HIF1a shRNA to transduce the lymphoma cells. We used GFP to track cells expressing the lentiviral vector. As shown in Fig. 2d, in the vector control group with scrambled shRNA, equal numbers of c-Kit+Sca-1+ cells were found in GFPhi and GFPlow subsets. In contrast, in two shRNA-transduced tumors, the GFPhi population was essentially devoid of the c-Kit+Sca-1+ cells, which indicated that the silencing of HIF1α eliminated the c-Kit+Sca-1+ subset. Since more than 50-fold reduction of CSC was observed on day 3 after transduction, we concluded that HIF activity is required for the maintenance of the c-Kit+Sca-1+ CSC.
Consistent with this notion, after drug selection to enrich the transduced cells, the colony formation assay revealed 70–80% reduction in the HIF1α shRNA-transduced cells (Fig. 2e). The defects caused by the Hif1a shRNA were complemented by transduction with human HIF1a cDNA which is not targeted by the Hif1a shRNA-1 (Fig. 2f). Therefore, the observed defects were not due to off-target effect of ShRNA. To test the role for HIF1α in tumor-initiating activity, we transplanted control vector (with scrambled shRNA) or HIF1α shRNA (either sh1 or sh2)-transduced tumor cells into B10.BR mice after three rounds of transduction. As shown in Fig. 2g, transduction with either HIF1a shRNA significantly reduced tumor-initiating activity as judged by the significant delay of tumor-related death. However, since the transduction efficiency is approximately 70% (Fig. S5), shRNA is less efficient than echinomycin in treatment of lymphoma.
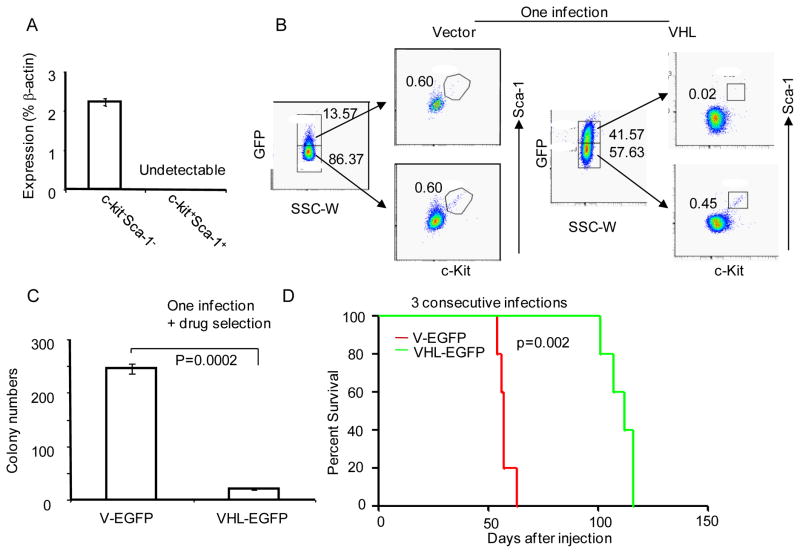
Since HIF1α is normally degraded under normoxia by a VHL-dependent mechanism (Kondo et al., 2002; Maranchie et al., 2002), we also tested the expression of Vhl in the CSC. Our data demonstrated that the Vhl transcript was undetectable in c-Kit+Sca-1+ cells, although significant levels were found in the c-Kit−Sca-1− subset (Fig. 3a). To determine the significance of Vhl down-regulation, the tumor cells were infected with Vhl-expressing lentivirus that also expresses GFP. To test the impact of Vhl expression on the number of CSC, the GFPhi and GFPlo subsets were compared for the abundance of the c-Kit+Sca-1+ subset. GFPhi subset contained no c-Kit+Sca-1+ cells (Fig. 3b). Thus, high Vhl expression ablated the c-Kit+Sca-1+ cells. Consistent with this, the ectopic expression of Vhl significantly reduced the colony forming activity of the tumor cells (Fig. 3c). To test the role for reduced Vhl in tumor-initiating activity, we transplanted vector and VHL cDNA transduced tumor cells into B10.BR mice. As shown in Fig. 3d, transduction with lentivirus expressing Vhl cDNA significantly reduced tumor-initiating activity as judged by the onset of tumor-related death of the recipients. Taken together, our data presented in Figs. 2&3 demonstrate that both over-expression of HIF1α and reduction in VHL are essential for CSC activity.
Fig. 3.
Down-regulation of the Vhl gene is essential for maintenance of CSC. a. Down-regulation of Vhl transcript in c-Kit+Sca-1+ cells. TGB thymoma cells were sorted into c-Kit+Sca-1+ and c-Kit−Sca-1− subsets, as described in Fig. S1, the levels of Vhl transcripts were determined by real-time PCR. b. Ectopic expression of Vhl ablated CSC. TGB tumor cells were infected with either lentiviral vector control, or lentiviral vector expressing Vhl cDNA. Three days after infection, the bulk tumor cells were analyzed by flow cytometry. The GFPhi and GFPlo cells were gated and analyzed for expression of c-Kit and Sca-1. c. Vhl expression reduces tumor CFU. The cultured lymphoma cells were infected with either lentiviral Vhl cDNA or vector by spinoculation, and the infected cells were selected with 5μg/ml of blasticidin for one week. The transduced cells were seeded into 1% of methylcellulose culture medium at a density of 2X105/well. The colony numbers were counted under a microscope. Data shown are means +/− SD of colony numbers in triplicate and are representative of three independent experiments. d. Ectopic expression of Vhl cDNA inhibits tumor-initiating activity. TGB tumor cells were infected 3 times with lentiviral expressing vector alone or Vhl cDNA and then injected into B10.BR mice (9X105/mouse, i.p.). The survival of the recipient mice (n=5) was compared by Kaplan-Meier analysis. The development of lymphoma was confirmed by necropsy. This experiment has been repeated twice.
HIF acts in concert with the Notch pathway in self-renewal of CSC
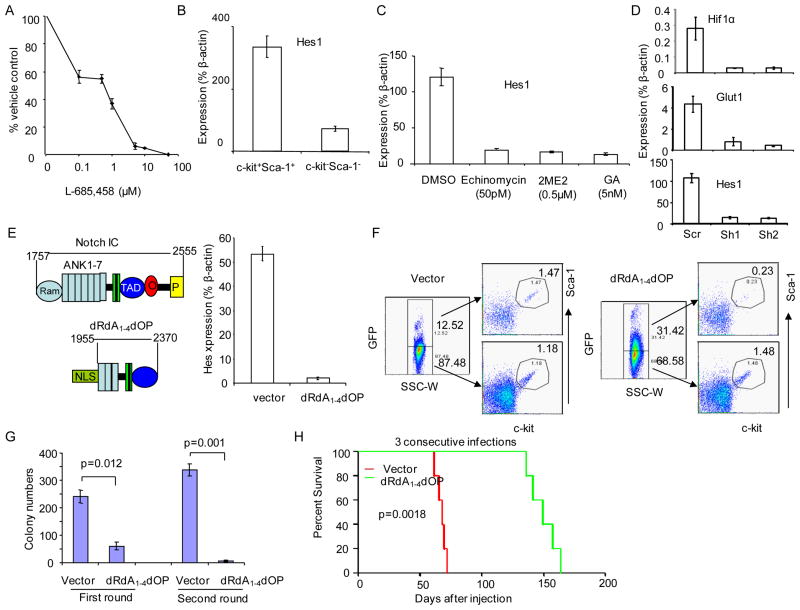
In order to determine the underlying molecular mechanisms by which HIF1α activation promotes self-renewal of CSC, we examined the potential involvement of Wnt and Notch pathways. Despite activation of the Wnt signaling in the TGB tumor (Wang et al., 2006), our data demonstrated that the dominant negative TCF-1, which we showed to inhibit tumor growth associated with Epm2a down regulation (Wang et al., 2006), does not affect the CFU of the TGB CSC (data not shown). In contrast, γ-secretase inhibitor, L-685,458, an inhibitor of the Notch pathway, potently blocked the CFU (Fig. 4a). To determine whether the Notch signaling is over-activated in the CSC, we sorted the c-Kit+Sca-1+ cells and compared them with the bulk c-Kit−Sca-1− cells for expression of the Notch target gene Hes1. As shown in Fig. 4b, sorted CSC had an approximately 3.5 fold increase in expression of Hes1. To determine whether the increased Notch activated relates to Notch mutations, we randomly selected 5 TGB tumors and sequenced the exons 26, 27 and 34, which have been shown the mostly frequent mutations in T cell lymohoma. Since no mutations were detected, Notch mutation is not responsible for elevated Notch activity in the TGB CSC.
Fig. 4.
HIF works in concert with Notch pathway to maintain CSC. a. Inhibition of tumor CFU by Notch inhibitor L685458. The cultured lymphoma cells were treated with given doses of L685, 458 for 24 hours and subjected to CFU assay. Data shown are means +/− SD of triplicate samples and are representative of those of at least 3 independent experiments. b. Enhanced Notch activity in CSC, as indicated by the levels of Hes1 transcripts. The lymphoma cells from spleen with TGB tumor were sorted into c-Kit+Sca-1+ or c-Kit-Sca-1− fractions. The expressions of Hes1and mRNA in these two fractions were measured by real time PCR. c. Inhibition of Notch activity by 3 distinct HIF inhibitors. Cultured TGB lymphoma cells were stained with APC conjugated anti-c-Kit- antibody and enriched twice using anti-APC coated MACS beads according to manufacture’s protocol (Militenyi Biotec). The c-kit positive cells-enriched samples (60.4% c-Kit+ cells) were treated with inhibitors of HIF for 16 hours. The mRNA from the treated cells were extracted for PCR. Data shown are means +/− SD of triplicates and represent those from at least 3 independent experiments. 2ME2, 2-methoxyestradiol; GA, geldamycin. d. ShRNA silencing of Hif1a reduced expression of Hes1. TGB tumor cells were transduced with Hif1a shRNA. The transduced cells were selected with blasticidin and were analyzed for Hif1a, Glut1 and Hes1 transcripts by real-time PCR. e–h. A critical role for Notch in maintenance of CSC, as revealed by ectopic expression of Notch I-C dRdA1-42dOP. e. A truncated Notch gene with potent dominant negative activity in inhibiting the expression of Notch target gene Hes1. The upper left panel shows the diagram of the intracellular portion of Notch protein, with the position of RAM, 7 ankyrin repeats (ANK1-7), transcriptional activation domain (TAD), C-terminal OPA (O) and PEST (P) sequence are marked. The lower left panel showed the composition of the dRdA1-4dOP mutant lacking RAM, ANK1-4 and C-terminal O and P sequence, but with insertion of nuclear localization sequence (NLS). The right panel show dominant inhibition of Hes expression. After three consecutive transductions by either vector control or the dRdA1-4dOP mutant, the RNA were isolated and the transcripts of Hes measured by quantitative PCR. Data shown are means of triplicates and have been reproduced by two independent experiments. f. dRdA1-4dOP abrogates the c-Kit+Sca-1+ subset. TGB tumor cells were infected with either lentiviral vector control, or lentiviral vector expressing dRdA1-4dOP. Three days after infection, the bulk tumor cells were analyzed by flow cytometry. The GFPhi and GFPlo cells were gated and analyzed for expression of c-Kit and Sca-1. g. Notch IC- dRdA1-42dOP reduces in vitro self-renewal activity of CSC. The cultured lymphoma cells were infected with either lentiviral vector encoding dRdA1-4dOP or vector control by three consecutive spinoculation. Data shown are the means +/− SD of the colony numbers in triplicate plates, and are representative of those from at least three independent experiments. h. dRdA1-4dOP abrogates tumor-initiating activity. TGB tumor cells were infected 3 times with lentiviral expressing vector alone dRdA1-4dOP and then injected into B10.BR mice (1X106/mouse, i.p.). The survival of the recipient mice was compared by Kaplan-Meier analysis with statistical significance determined by log-rank tests. All mice that died had developed lymphomas. This experiment has been repeated twice. See Fig. S6 for expression of Notch1 and 2 in CSC.
In order to test whether the increased Notch activity depended on HIF activity, we used three different HIF inhibitors to block the up-regulation of Notch targets in total TGB lymphoma cells and CSC enriched for c-Kit+ cells. The data in Fig. 4c demonstrated that all HIF inhibitors blocked expression of Hes1 among the c-Kit+ CSC. To validate the role of HIF1α, we tested if knockdown of the Hif1a reduced Hes1 expression. As shown in Fig. 4d, compared with cells transduced with lentivirus encoding the scrambled RNA, those transduced with Hif1a shRNA had approximately 5-fold lower levels of Hes1 transcripts.
We analyzed expression of Notch1-4 in c-Kit+Sca-1+ and the c-Kit−Sca-1− tumor cells. As shown in supplemental Fig. S6a, Notch1 and 2, but not Notch 3 and 4 are expressed in the TGB tumor cells. Since two Notch genes are expressed, we searched for an effective dominant negative mutant to suppress Notch signaling. By trial and error, we identified a potent dominant negative mutant of Notch (AA1955-2370), comprising intracellular domains of Notch1 with truncation in both N and C-termini. A nuclear localization sequence (NLS) from SV40 virus was inserted in the N-terminus to facilitate its translocation into the nuclei. Based on the structure of Notch I-C/CSL/Mastermind/DNA complex (Wilson and Kovall, 2006), the deletion included both the DNA binding RAM domain and the 4 ankyrin repeats responsible for binding N-terminus of mastermind. As such, it is predicted to act as a dominant negative regulator of Notch signaling by preventing the formation of mastermind-CSL-Notch IC-DNA complex. We called the mutant dRdA1-4dOP (Fig. 4e, left panel). As shown in Fig. 4e right panel, transduction of the dominant mutant resulted in about 30-fold reduction of the Hes1 transcripts. To substantiate the role for Notch signaling, we transduced the TGB lymphoma with either control lentiviral vector or that expressing dRdA1-4dOP. The transduced cells were marked with GFP. As shown in Fig. 4f, in the vector control group, the % of c-Kit+Sca-1+ cells were comparable in both GFPhi and GFPlo cells. In contrast, in the dRdA1-4dOP-transduced group, more than 5-fold reduction in the % c-Kit+Sca-1+ cells was observed in the GFPhi subset. These data demonstrate that inactivation of the Notch pathway prevents the survival of the c-Kit+Sca-1+ cells. In serial plating experiments, transduction of dRdA1-4dOP reduced self-renewal activity as revealed by CFU assay (Fig. 4g). Moreover, when the dRdA1-4dOP- or control vector-transduced cells were transplanted into syngeneic mice, it was clear that dRdA1-4dOP -transduction significantly delayed the development of lymphoma, as demonstrated by the survival analysis (Fig. 4h).
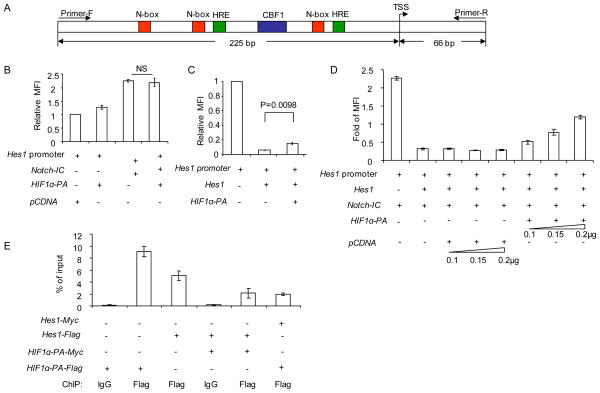
Previous studies demonstrated that HIF1α may interact with Notch directly to activate its target gene, Hey2, under hypoxia conditions (Gustafsson et al., 2005). Using the reporter for Hes1 promoter activity, however, we did not observe significant enhancement of Notch signaling by the mutant HIF1α (P402A/P564A) (Fig. 5a, b). We therefore explored alternative explanations for the function of HIF1α in Hes1 expression. It is well established that, in response to Notch signaling, Hes1 expression is self-limiting, and that the negative feedback is mediated by Hes1 binding to the N-boxes in the Hes1 promoter region (Hirata et al., 2002; Takebayashi et al., 1994). Interestingly, based on consensus sequence (RCGTG) (Camenisch et al., 2001), we identified a bona fide HRE immediately after each of the two critical N-boxes, (Fig. 5a and supplemental Fig. S6b). Given their proximity, we hypothesized that HIF1α may directly inhibit autoregulation of Hes1. Indeed, transfection of Hes1 cDNA reduced the Hes1 promoter activity by about 10-fold. This inhibition was partially reversed by the oxygen-resistant HIF1α (Fig. 5c). Likewise, in the presence of Notch IC cDNA, Hes1 also repressed its own promoter (Fig. 5d). HIF1α reversed the repression in a dose dependent manner (Fig. 5d). Using chromatin immunoprecipitation (Fig. 5e), we observed significant binding of HIF1α to the region bound by Hes1. Interestingly, the HIF1α and Hes1 competed with each other in binding to the region. Our data suggest that HIF1α enhanced Notch-induced Hes1 expression by antagonizing the autoregulation of the Hes1 gene.
Fig. 5.
HIF1α inhibits negative feedback regulation of Hes1 by preventing Hes1 binding to the N-boxes in the Hes1 promoter. a. Diagram of Hes1 promoter. Detail sequence is provided in supplemental Fig. S6b. b. HIF1α did not cooperate with Notch directly in activating Hes1 promoter. The Hes1 promoter sequence (−225 to +65, TSS as +1) was linked to GFP and transfected into 293 cells in conjunction with vector controls, or vector containing cDNA encoding HIF1α (P402, 564>A, called HIF1α-PA), Notch-IC cDNA or Notch-IC+ HIF1αPA. The promoter activity is measured by the green fluorescence intensity of transfected cells. Data shown were relative intensities. The intensity of Hes1-GFP reporter is defined as 1.0. Transfection efficiency is normalized by co-transfected Renilla lucifease. c. HIF1α partially inhibited Hes1-mediated repression of the Hes1 promoter. As in b, except that the Hes1 or mutant HIF1a cDNA are used. d. HIF1α diminishes the negative auto-regulation of Hes1 expression in Notch signaling. As in b, except different combination of cDNAs were used. Activity of Hes1 reporter in the absence of transfected Hes, HIF1α-PA and Notch is defined as 1.0. e. Competitive inhibition between HIF1αPA and Hes1 to Hes promoter, as revealed by ChIP. cDNAs encoding Flag or Myc-tagged Hes1 and HIF1αPA were transfected into 293 cells. Thirty-six hours after transfection, the transfectants were subject to ChIP analysis. Equal fractions of cells in each group were used for Western blot to confirm essentially identical levels of protein expression when cDNA encoding Hes1 and HIF1αPA were transfected alone or in combination (data not shown). The data present are means+/− S.D. (n=3) of % of input DNA, as measured by real-time PCR using primers marked in Fig. S6b. Data shown are means+/−S.D. of triplicates. The experiments have been repeated at least 3 times. Transfection was performed in 24-well plate for promoter assay or in 6-well plate for CHIP assay. Total DNA amounts used for the transfection are 0.5 μg/per well of 24-well plate and 1.5 μg/per well of 6-well plate. See Fig. S6b for promoter sequence of Hes1, with HRE and N-box and primer sequences marked.
Therapeutic elimination of leukemia stem cells (LSC) for AML
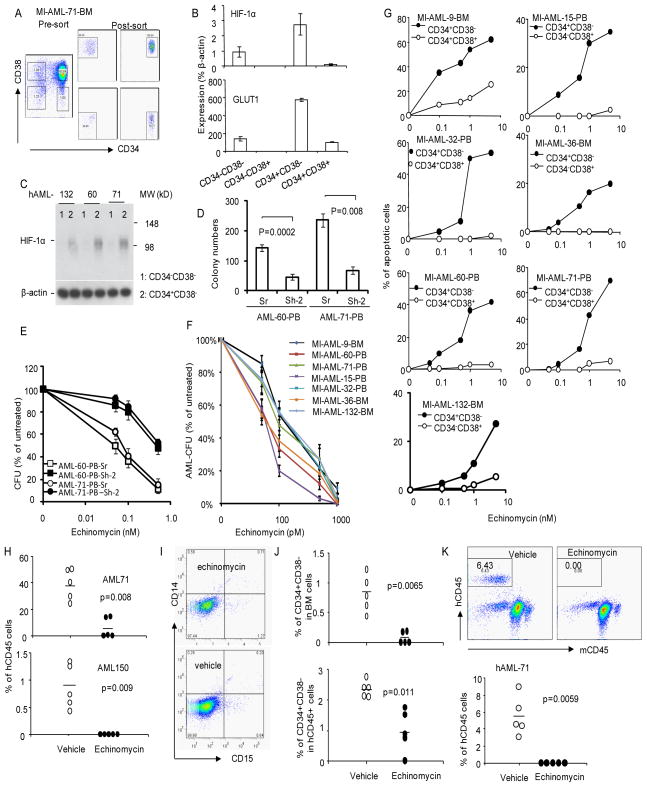
Based on these observations, we explored the therapeutic potential of HIF inhibitors for human hematological malignancies. Since the HIF function in lymphoma CSC was independent of hypoxic environment, it is plausible that the same pathway may also function in LSC. We choose AML because the phenotype of AML-LSC was well characterized and as the xenogeneic model had been established to assay the AML-LSC function in vivo (Lapidot et al., 1994). The classic studies by Dick and colleagues, using the NOD-Scid model, demonstrated that the AML-LSC has a phenotype of CD34+CD38− (Lapidot et al., 1994). To determine whether the HIF1α gene is over-expressed in this subset, we sorted the CD34+CD38−, CD34+CD38+, CD34−CD38− and the CD34−CD38+ subsets by FACS (Fig. 6a) and analyzed the expression of HIF1a and its target GLUT1. As shown in Fig. 6b, the CD34+CD38− subset had the highest levels of HIF1α transcript. Correspondingly, GLUT1 transcript is also elevated in the CD38−CD34+ cells. All 6 cases of AML tested showed increased expressions of HIF1α and GLUT1 in the CD38−CD34+ subset (data not shown), which indicate that increased HIF1α expression is a general feature of those cells bearing markers of AML-LSC cells. The HIF1α protein is also selectively enriched in the CD38−CD34+ subsets in comparison to that in the CD38−CD34− subset (Fig. 6c).
Fig. 6.
HIF1α is a target for therapeutic elimination of human AML in xenogenic mouse model. a. Isolation of 4 subsets of tumor cells in AML samples. Bone marrow cells from AML patient MI-AML-71 were stained for CD34 and CD38 and sorted into 4 subsets for RNA isolation. The presort samples and the gates used for sorting are shown in the left panel and the post-sorted populations are shown in the middle and right panels. The percentages of cells in each gate are provided in the panels. b. Expression of HIF1a (top) and GLUT1 in the subsets. Data shown are means+/−S.D. of transcript levels of the genes, presented as % of βactin from the same samples. Enhanced expression in the CD34+CD38− samples have been observed in all 6 AML samples tested. c. Increased accumulation of HIF1α protein in CD34+CD38− AML cells. CD38+ cells were depleted by negative selection with anti-CD38-conjugated magnetic beads. The remaining cells were further separated into CD38−CD34+ (purity 72–78%) and CD38−CD34− (purity 96–100%) cells by positive selection, Lysates from the two populations were used for Western blot. d. HIF1α activity is essential for AML-CFU. AML-60 and AML-71 were transduced with either scrambled (Sr) or HIF1a shRNA (Sh-2). The CFU of transduced AML cells were counted based on EGFP signal. The transduction efficacy were measured by FACS prior to plating. The % of GFP+ cells for the experiment were: AML60: Sr, 32.6; Sh-2:32.48. AML-71: Sr: 45.70; Sh-2: 40.19. The shRNA used, Sh-2, targets shared sequenced between mouse and human HIF1a genes. Data showing are CFU per 2x105 cells. e. HIF1a silencing increased the resistance to CFU to echinomycin. As in e, except the transduced cells were treated with given concentration of echinomycin. Data shown were % of CFU after normalized to untreated group (defined as 100%). The number of colonies in the control group are shown in Fig. 6d. Data in d and e have been repeated once with the same conclusions. f. AML-CFU in all 7 AML samples are highly sensitive to echinomycin. AML samples (2.5x105/ml) from either peripheral blood (PB) or bone marrow (BM) were pretreated with given concentrations of echinomycin in 2 ml medium for 24 hours. Treated viable cells were then plated at 105/well for CFU assay in triplicates. The colony numbers were counted 7–10 days later. The data shown are % means+/− S.D. of untreated controls. g. Echinomycin selectively eliminates the CD34+CD38− subset of AML cells. Primary AML samples were thawed from liquid nitrogen. After overnight recovery, they were cultured with given doses of echinomycin or vehicle control for 30 hours in RPMI 1640 containing 10% fetal calf serum and human cytokine cocktail consisting of CSF, GM-CSF and IL-3 at a density of 5X105/ml. The cells were stained with antibodies against CD34, CD38 in conjunction with Annexin V and DAPI. Data shown are the % of Annexin V+DAPI+/− cells with the specified markers. The Annexin V+ cells % in vehicle treated group has been subtracted. The filled symbols show the data for the CD34+CD38− subsets, while the open symbols show data for the bulk leukemia cells (CD34+CD38+ for AML9, AML32, AML60 and AML71 and CD34−CD38+ for AML15, AML36 and AML132). These data have been repeated twice. h. Therapeutic effect of human AML in NOD-SCID mice, data shown are % of human CD45 (hCD45)+ cells in the bone marrow of the recipient mice at 40 days after last treatment. The therapeutic effect has been repeated twice. i. Echinomycin does not induce differentiation of AML cells in vivo, as revealed by lack of mature myeloid markers on the bulk of human cells in treated and untreated group. Data shown are profiles of CD14 and CD15 among human CD45+ cells. j. Selective depletion of the CD34+CD38− subset by echinomycin. Data shown in the top panels are the abundance of CD34+CD38− subsets in mouse bone marrow, while the lower panel shows that within human leukemia cells. k. Despite presence of leukemia cells, bone marrow from echinomycin-treated mice failed to reinitiate leukemia in the new NOD-SCID mice. Representative profiles are presented in the top panel, while the summary data from 5 mice per group are presented in the lower panel. See Fig. S7 for the phenotype of AML cells in the xenogeneic model.
The CD34+CD38− are also known to form AML-colonies in vitro (Lapidot et al., 1994), which provides us with a simple assay to test the significance of HIF1α. To determine the role for HIF1a in CFU, we transduced AML with either scrambled or Hif1a shRNA-2 that targets conserved sequence between mouse and human HIF1a. Since the transduction efficiencies of both scrambled and ShRNA vector, as measured by GFP+% cells, were comparable between scrambled and shRNA silenced samples (Fig. 6d legends), the function of the HIF1a gene can be evaluated by the number of GFP+ colonies. As shown in Fig. 6d, in two independent AML samples tested, HIF1a shRNA reduced by 60–70% when compared the scrambled shRNA control. Moreover, HIF1a shRNA greatly reduced the sensitivity of the CFU to echinomycin (Fig. 6e). These data demonstrated that echinomycin also target HIF1a in human AML samples. To test the role for HIF1α in more clinical samples, we tested 7 independent cases (Table S1) for their CFU sensitivity to echinomycin. As shown in Fig. 6f, for all cases tested, echinomycin inhibited colony formation with IC50 between 50–120 pM. These data, together with the specific reduction of echinomycin sensitivity by HIF1a gene silencing (Fig. 6e), demonstrated a critical role for HIF1a in CFU activity of the 7 clinical AML samples. Given the fact that the AML cases used have diverse genetic alterations (Table S1), the broad inhibition by echinomycin is consistent with an important function of HIF1α in AML-CFU, which include the CD34+CD38− AML-LSC.
Conventional cancer therapy appears to enrich cancer stem cells (Baumann et al., 2008; Dean et al., 2005; Phillips et al., 2006; Yu et al., 2007). An NFkB inhibitor, dimethylamino analog of parthenolide, showed some selectivity for AML-LSC (Guzman et al., 2007). Since the HIF1α activity appears to be selectively activated in AML, we were intrigued by the possibility that AML-LSC can be targeted selectively by echinomycin. As shown in Fig. 6g, echinomycin was about 100-fold more efficient in inducing apoptosis of the CD34+CD38− population, in comparison to the dominant AML cells (CD34+CD38+ for most but CD34−CD38+ for two AML cases).
To test whether echinomycin can be used as a potential therapeutic agent for AML, we established a xenogeneic AML model using human AML samples. As shown in Fig. S7, primary clinical samples from AML patients reconstituted irradiated NOD.SCID mice with immature human myeloid cells, characterized by the expression of human CD45, CD11b and CD33 but not mature myeloid markers CD14 and CD15 and B cell marker CD19. Remarkably, a short term treatment with echinomycin starting at 15 days after transplantation completely eliminated human cells from sample AML 150 and dramatically reduced the burden of human leukemia of AML 71 (Fig. 6h). The residual cells in echinomycin treated mice were not differentiated as judged by the lack of mature myeloid markers CD14 and CD15 (Fig. 6i).
The incomplete remission of AML71 also allowed us to evaluate if echinomycin selectively reduce the AML-LSC, using the CD34+CD38− markers (Lapidot et al., 1994). Echinomycin reduced % of CD34+CD38− cells in bone marrow by more than 10-fold (Fig. 6j, top panel). Even among the human CD45+ compartment, the relative abundance of AML-LSC was also reduced (Fig. 6j, lower panel). To substantiate the impact of echinomycin on the leukemia initiating cells, we carried out serial transplantation studies to determine whether echinomycin reduced self-renewal of AML-LSC. As shown in Fig. 6k, while bone marrow from untreated mice induced leukemia in all new recipients, bone marrow from echinomycin treated mice failed to do so in any recipients. Therefore, the residual cells in the echinomycin-treated bone marrow are devoid of AML-LSC.
Discussion
We have used both in vitro CFU and in vivo tumor initiating properties to characterize the molecular mechanism for self-renewal of CSC. Our screening of inhibitors for major signal transduction pathways revealed a potent inhibition of CFU and tumorigenesis by echinomycin, a known inhibitor of HIF (Kong et al., 2005a). The specificity of HIF targeting by echinomycin is supported by qualitatively similar effect of multiple HIF inhibitors and by a requirement of HIF1a expression for sensitivity to the drug. Since the short-term treatment with echinomycin resulted in a specific increase of apoptosis in the c-Kit+Sca-1+ cells, the HIF1α is selectively required for survival of CSC. The specificity of HIF1a shRNA is confirmed by genetic complementation. Interestingly, the increased HIF activity is observed under normoxia in CSC of both leukemia and lymphoma. In the murine lymphoma, CSC maintenance also depends on down-regulation of Vhl, which explains why the increased HIFα activity no longer requires hypoxic environment. In contrast, reported HIF function in glioma stem cells is strictly hypoxia-dependent (Li et al., 2009). The hypoxia-independent activation HIF expands the spectrum of cancer in which HIF can play a critical role in pathogenesis.
In our effort to understand the molecular pathway responsible for the maintenance of CSC, we uncovered that HIF1α potentiates the induction of Hes1 by Notch. Hes1 is an important Notch target known to be critical for stem/progenitor cell functions (Janzen et al., 2006; Tomita et al., 1999; Yu et al., 2006). In contrast with the previous studies using Hey2 promoter as readout (Gustafsson et al., 2005), we did not observe a direct co-operation between HIF1α and Notch IC in the induction of the Hes1 gene. Rather, HIF1α blocks a negative feedback regulation of Hes1 by preventing Hes1 binding to the N-box of Hes1 promoter. Given the general, although not necessarily universal role of Notch in maintenance of a variety of tissue stem cells (Duncan et al., 2005; Fre et al., 2005; van Es et al., 2005; Wilson and Radtke, 2006), our data suggest an important functional conservation between CSC and tissue stem cells.
In contrast to conventional chemotherapeutic drugs and radiation therapy that appears to spare CSC (Baumann et al., 2008; Costello et al., 2000; Dean et al., 2005; Ishikawa et al., 2007; Phillips et al., 2006; Yu et al., 2007), echinomycin selectively eliminated CSC and conveyed remarkable therapeutic efficacy for leukemia and lymphoma. On a per molar basis, echinomycin is 10,000-fold more effective than what has been reported for dimethylamino-parthenolide, which also showed some selectivity for AML stem and progenitor cells (Guzman et al., 2007). The long-lasting elimination of lymphoma CSC is demonstrated by complete lack of recurrence in echinomycin treated mice. The high efficacy is shown by the fact that less effective therapy was achieved by as little as 200 ng of echinomycin in vivo and pico molar range IC50 as measured by CFU assay in vitro.
In the xenogeneic model of human AML, we observed that short-term treatment by echinomycin prevented serial transplantation of AML. These data, together with preferential induction of apoptosis of CD34+CD38− cells in vitro as well as selective reduction of CD34+CD38− cells within the hCD45+ cells, support the notion that the drug preferentially targets AML stem cells. It is worth noting that while the frequency of CD34+CD38− cells in echinomycin-treated recipient is reduced by 5-fold, there were still approximately 2,000 CD34+CD38− cells per inoculate for the secondary recipients. As previous studies indicate that 1000–5000 cells is sufficient to initiate AML in the NOD-SCID model (Bonnet and Dick, 1997). The fact that none of the 5 recipients had detectable AML suggests that in addition to physical reduction of CD34+CD38− cells, echinomycin may also abrogates the LSC activity of the remaining CD34+CD38− AML cells. Our approach complements other recent studies targeting CSC (Dierks et al., 2008; Ginestier et al., 2009; Jin et al., 2006; Zhao et al., 2009), although these drugs have not been shown to target intrinsic survival program of CSC. Since echinomycin is a competitive inhibitor of HRE in multiple HIF target genes (Kong et al., 2005a), it is theoretically more difficult to mutate multiple target genes to develop resistance to echinomycin. In contrast, resistance to an inhibitor of the Hedgehog pathway appears to develop readily due to mutation of Smoothened (Yauch et al., 2009).
Selective targeting of cancer cells is a key to reduce side effect of cancer therapy. Despite similarity between CSC and normal tissue stem cells, we showed that echinomycin selectively inhibited CFU of lymphoma CSC without affecting the CFU of HPC of normal bone marrow in mice. Remarkably, based on per square meter of body surface, echinomycin at doses that are nearly 50-fold higher than what was used in this study, were well tolerated in human (Muss et al., 1990), although the impact of the drug on CSC was not evaluated because these trials predated the reemergence of CSC concept (Lapidot et al., 1994). The remarkable efficacy of a previously abandoned cancer drug demonstrates the need to test drugs based on the concept of cancer stem cells (Diehn et al., 2009). The selective targeting of CSC validated a key prediction of cancer stem cell hypothesis, namely, identification of the cell-intrinsic molecular program for CSC maintenance enables cancer eradication by elimination of CSC (Park et al., 2009).
Experimental Procedures
AML samples
AML patients diagnosed at the University of Michigan Comprehensive Cancer Center between 2005 and 2009 were enrolled into this study. The study was approved by the University of Michigan Institutional Review Board. Written informed consent was obtained from all patients prior to enrollment. We used the same AML diagnostic criteria (>= 20% myeloblasts in the bone marrow or peripheral blood) and determined FAB subclassification through review of both laboratory and pathology reports dated at the time of diagnosis and interpreted by hematopathologists. Cytogenetic risk stratification was determined according to SWOG/ECOG criteria (Slovak et al., 2000).
ShRNA-mediated knockdown of HIF1a and ectopic expression of Vhl and dRdA1, 2dOP
The lentiviral vector plenti6/V5-TOPO, obtained from Invitrogen, was modified by replacing CMV promoter with U6 promoter to drive shRNA expressing cassette. This is followed by a pGK-driven EGFP cassette and a blasticidin-ressitant cassette. The core sequence of HIF1α– ShRNA-1 (Sh-1) is 5′-ctagagatgcagcaagatc-3′, while that of HIF1α– ShRNA-2 (Sh-2) is 5′-gagagaaatgcttacacac-3′. The T7-blasticidin cassette in plenti6/V5-TOPO vector was replaced with a PGK-GFP cassette to express full length Vhl cDNA, and dominant negative Notch mutant dRdA1-42dOP (AA1995-2370) in experiments that involve no drug selection. For those studies that involve drug selection, we replaced the CMV promoter with a CMV-IRES-EGFP cassette and cloned Vhl cDNA after the CMV promoter. The tumor cell cultures were infected with either control lentivirus or lentivirus encoding HIF1a shRNA, dRdA1-42dOP or Vhl cDNA by spinoculation. The cultures were selected with 5μg/ml of blasticidin for 5 days to remove uninfected cells.
In vitro colony formation assay for AML, TGB tumor cells and bone marrow cells was carried out as previously described (Wang et al., 2006).
In vivo tumorigenicity assay-TGB
Given numbers of total tumor cells or sorted subsets were injected into either immune competent B10.BR mice or RAG-2−/−BALB/c mice. Moribund mice were considered to have reached experimental endpoint and were euthanized. The therapeutic effects were analyzed by Kaplan Meier survival analysis.
Xenogeneic tumor model
NOD-SCID mice received 1.8Gy of irradiation and i.v. injection with 8X106 of peripheral blood cells from patients AML-71 and AML-150. Fifteen days later, 10 μg/kg/injection of echinomycin was injected daily for 5 consecutive days followed by 2 days rest, and then the cycle was repeated once. Control mice received vehicle only. Forty days later, the treated mice were sacrificed and the BM cells were collected and stained with antibodies specific for human (hCD45) and mouse leukocyte markers (mCD45) in conjunction with CD34 and CD38 or with CD19, CD33, CD14 and CD15. In some experiments, the vehicle or echinomycin-treated bone marrow cells from AML71-reconstituted mice were pooled and serially transplanted into new recipients (1.5×106 per mouse at one day after irradiation). The % of AML cells and stem cells are presented in Fig. 6h and j, respectively. The recipients received no further treatment, and sacrificed at 8 weeks after transplantation to analyze the reconstitution of human cells.
Statistics
Student t-tests were used to determine significance of the difference between two groups, while log-rank tests were used for survival analysis.
Supplementary Material
Acknowledgments
We thank Dr. Zhaohui Xu for advice on the structure of Notch signaling complex, Dr. Xing Chang for helping with flow cytometry, Drs. Eric Fearon and Yuan Zhu for helpful discussions, and Dr. Judith Connett and Ms Darla Kroft for editorial assistance. This study is supported by grants from National Institutes of Health, USA. All authors are co-inventor of a pending patent application that was licensed to OncoImmune, Inc. Yang Liu and Pan Zheng are among the co-founders with equity interest in OncoImmune, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer. 2008;8:545–554. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Camenisch G, Stroka DM, Gassmann M, Wenger RH. Attenuation of HIF-1 DNA-binding activity limits hypoxia-inducible endothelin-1 expression. Pflugers Arch. 2001;443:240–249. doi: 10.1007/s004240100679. [DOI] [PubMed] [Google Scholar]
- Costello RT, Mallet F, Gaugler B, Sainty D, Arnoulet C, Gastaut JA, Olive D. Human acute myeloid leukemia CD34+/CD38− progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Res. 2000;60:4403–4411. [PubMed] [Google Scholar]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- Diehn M, Cho RW, Clarke MF. Therapeutic implications of the cancer stem cell hypothesis. Semin Radiat Oncol. 2009;19:78–86. doi: 10.1016/j.semradonc.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks C, Beigi R, Guo GR, Zirlik K, Stegert MR, Manley P, Trussell C, Schmitt-Graeff A, Landwerlin K, Veelken H, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14:238–249. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2009;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Developmental cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Guzman ML, Rossi RM, Neelakantan S, Li X, Corbett CA, Hassane DC, Becker MW, Bennett JM, Sullivan E, Lachowicz JL, et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110:4427–4435. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Yoshiura S, Ohtsuka T, Bessho Y, Harada T, Yoshikawa K, Kageyama R. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science. 2002;298:840–843. doi: 10.1126/science.1074560. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, Nakamura R, Tanaka T, Tomiyama H, Saito N, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG., Jr Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–246. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, Fisher RJ, Shoemaker RH, Melillo G. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005a;65:9047–9055. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, Fisher RJ, Shoemaker RH, Melillo G. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005b;65:9047–9055. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, Stackhouse T, Kuzmin I, Modi W, Geil L, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- Maranchie JK, Vasselli JR, Riss J, Bonifacino JS, Linehan WM, Klausner RD. The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell. 2002;1:247–255. doi: 10.1016/s1535-6108(02)00044-2. [DOI] [PubMed] [Google Scholar]
- Minet E, Mottet D, Michel G, Roland I, Raes M, Remacle J, Michiels C. Hypoxia-induced activation of HIF-1: role of HIF-1alpha-Hsp90 interaction. FEBS Lett. 1999;460:251–256. doi: 10.1016/s0014-5793(99)01359-9. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muss HB, Blessing JA, Malfetano J. Echinomycin (NSC 526417) in squamous-cell carcinoma of the cervix. A phase II trial of the Gynecologic Oncology Group. Am J Clin Oncol. 1990;13:191–193. doi: 10.1097/00000421-199006000-00002. [DOI] [PubMed] [Google Scholar]
- Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, Kondo Y, Nakao S, Motoyama N, Hirao A. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463:676–680. doi: 10.1038/nature08734. [DOI] [PubMed] [Google Scholar]
- Park CY, Tseng D, Weissman IL. Cancer stem cell-directed therapies: recent data from the laboratory and clinic. Mol Ther. 2009;17:219–230. doi: 10.1038/mt.2008.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Pereira T, Zheng X, Ruas JL, Tanimoto K, Poellinger L. Identification of residues critical for regulation of protein stability and the transactivation function of the hypoxia-inducible factor-1alpha by the von Hippel-Lindau tumor suppressor gene product. J Biol Chem. 2003;278:6816–6823. doi: 10.1074/jbc.M209297200. [DOI] [PubMed] [Google Scholar]
- Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- Takebayashi K, Sasai Y, Sakai Y, Watanabe T, Nakanishi S, Kageyama R. Structure, chromosomal locus, and promoter analysis of the gene encoding the mouse helix-loop-helix factor HES-1. Negative autoregulation through the multiple N box elements. J Biol Chem. 1994;269:5150–5156. [PubMed] [Google Scholar]
- Tomita K, Hattori M, Nakamura E, Nakanishi S, Minato N, Kageyama R. The bHLH gene Hes1 is essential for expansion of early T cell precursors. Genes Dev. 1999;13:1203–1210. doi: 10.1101/gad.13.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Vlaminck B, Toffoli S, Ghislain B, Demazy C, Raes M, Michiels C. Dual effect of echinomycin on hypoxia-inducible factor-1 activity under normoxic and hypoxic conditions. The FEBS journal. 2007;274:5533–5542. doi: 10.1111/j.1742-4658.2007.06072.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu Y, Wu C, Zhang H, Zheng X, Zheng Z, Geiger TL, Nuovo GJ, Liu Y, Zheng P. Epm2a suppresses tumor growth in an immunocompromised host by inhibiting Wnt signaling. Cancer Cell. 2006;10:179–190. doi: 10.1016/j.ccr.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. discussion 1895–1886. [DOI] [PubMed] [Google Scholar]
- Wilson A, Radtke F. Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Lett. 2006;580:2860–2868. doi: 10.1016/j.febslet.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Wilson JJ, Kovall RA. Crystal structure of the CSL-Notch-Mastermind ternary complex bound to DNA. Cell. 2006;124:985–996. doi: 10.1016/j.cell.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, Pujara K, Stinson J, Callahan CA, Tang T, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science (New York, NY) 2009;326:572–574. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- Yu X, Alder JK, Chun JH, Friedman AD, Heimfeld S, Cheng L, Civin CI. HES1 inhibits cycling of hematopoietic progenitor cells via DNA binding. Stem Cells. 2006;24:876–888. doi: 10.1634/stemcells.2005-0598. [DOI] [PubMed] [Google Scholar]
- Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.