Abstract
Epidermal growth factor receptor (EGFR)-mediated signaling helps regulate bone development and healing through its effects on osteogenic cells. Here, we show how EGFR activity and osteogenic differentiation responses in primary human bone marrow-derived multipotent stromal cells (MSCs) are influenced by presenting covalently tethered epidermal growth factor (tEGF) on the culture substratum, a presentation mode that reduces EGFR internalization and restricts signaling to the cell surface. In both absence and presence of tEGF, MSCs increase expression levels of EGFR and its heterodimerization partner HER2 during the course of osteogenic differentiation. tEGF substrata increased levels of phosphorylated EGFR and phosphorylated extracellular regulated kinase (ERK) compared to control substrata, and these elevations were associated with a 2-fold enhancement of MSC alkaline phosphatase activity at day 7 and matrix mineralization at day 21. Surprisingly, addition of soluble EGF (sEGF) to cells cultured on tEGF substrata reduces osteogenic differentiation, even though EGFR signaling is more strongly activated in acute, short-term manner by sEGF treatment than by tEGF treatment. A striking concomitant result of the sEGF effects is near-complete downregulation of EGFR and HER2, demonstrating that the tEGF/EGFR interaction is dynamically reversible even though temporally sustained. Taken together, our results show that enhanced MSC osteogenic differentiation corresponds to a sustained combination of receptor expression and ligand presentation, both of which are maintained by tEGF.
Keywords: Multipotent stromal cells, Epidermal growth factor receptor, Differentiation, Stem Cells
Introduction
A wide variety of epithelial and mesenchymal cells express epidermal growth factor receptor (EGFR), a receptor tyrosine kinase that binds a diverse family of extracellular ligands and activates intracellular signaling cascades that influence cell migration, proliferation, and differentiation (Fan et al., 2007; French et al., 1995; Harrington et al., 2007; Harris et al., 2003; Muthuswamy et al., 1999; Reddy et al., 1994; Schmidt et al., 2003; Wiley, 2003). EGF, the prototypical ligand for EGFR, induces EGFR dimerization, autophosphorylation, and rapid internalization of the EGF-EGFR complex, which can continue to signal as it is being trafficked through endosomal compartments toward degradation (French et al., 1995; Harris et al., 2003; Haugh, 2002; Wiley, 2003). Other ligands known to stimulate EGFR, including TGFα, amphiregulin, and epiregulin, can elicit phenotypically different responses than EGF. In some cases these differences have been linked to alterations in intracellular trafficking, with resulting alterations in the duration and relative strength of the various cell surface and cytoplasmic signaling pathways elicited by EGFR activation (Haugh, 2002; Iyer et al., 2008; Wang et al., 2002; Wells et al., 1990; Wiley, 2003; Wu et al., 2000).
EGFR-mediated signaling has been implicated in many different steps of bone development and regeneration. Hence, stimulation of EGFR signaling may be useful in regenerative medicine strategies for bone. Unfortunately for rationally designed technologies aimed toward this goal, the role of EGFR signaling in bone is poorly understood. For instance, loss of EGFR signaling has been reported to enhance the chronology of bone formation developmentally (Sibilia et al., 2003), and reduced levels of EGFR activation upon stimulation by soluble EGF are observed in human primary marrow stromal cells that form bone in a transplant assay compared to those that do not form bone (Satomura et al., 1998). In contrast, however, activation of EGFR is also associated with enhanced proliferation of the stem or progenitor cell compartment with no impairment of differentiation (Krampera et al., 2005; Tamama et al., 2006), or even enhancement of differentiation (Kratchmarova et al., 2005). These disparate effects in reported outcomes may arise from differences in the spatial and temporal nature of EGFR activation by different EGFR ligands, as most of the EGFR ligands are known to be expressed in the osteogenic compartment in bone (Chen et al., 2008; Krampera et al., 2005; Qin et al., 2005).
Several therapeutic tissue regeneration strategies involve transplantation of fresh or culture-expanded bone marrow-derived multi-potent stromal cells (MSCs). MSCs are versatile progenitor cells capable of differentiating into bone, cartilage, and fat cells (Friedenstein et al., 1966; Pittenger et al., 1999; Sekiya et al., 2002), and have potential for regeneration of bone and other connective tissues. Freshly isolated MSCs express very low levels of EGFR and HER2, but expression increases during culture (Sekiya et al., 2002). Relevant to scaffold-guided transplantation strategies, we have previously shown that when EGF is covalently tethered to the culture substrate, surface-restricted signaling of EGFR by tethered EGF (tEGF) induces sustained ERK activation and improved survival of MSCs challenged with the pro-death factor Fas ligand (Fan et al., 2007). This suggests that tEGF may be therapeutically useful in protecting MSC in the immediate post-transplant period. Several investigators have analogously shown phenotypic differences in cell responses to sustained versus transient signaling through EGFR in fibroblasts and other cell types using EGFR mutants or extracellular matrix immobilized EGF-like domains (Iyer et al., 2008; Schmidt et al., 2003; Sigismund et al., 2008; Taub et al., 2007; Vieira et al., 1996). However, whether restricting EGFR signaling to the cell surface via mechanical restraint of the EGFR ligand (i.e., the tEGF approach we employ) is able to influence MSC differentiation behaviors is unknown.
Here, we investigate EGFR and HER2 expression levels and signaling during proliferation and osteogenic differentiation of primary human MSCs. We further illuminate the role of EGFR during this process based on sustained or transient downstream signaling using different modes of ligand presentation in combination with kinase inhibitors.
Materials and Methods
Cell culture
Human telomerase reverse transcriptase (hTERT)-immortalized human MSC (hTMSC) were a gift from Dr. Junya Toguchida (Kyoto University, Kyoto, Japan) (Okamoto et al., 2002) and were maintained in a standard medium formulation containing Dulbecco’s modified Eagle’s medium, 10% fetal bovine serum (FBS), 1 mM pyruvate, 1 mM L-glutamine, 1 μM nonessential amino acids, and 100 units/ml penicillin-streptomycin (Invitrog en), at 37°C in a humidified atmosphere of 5% CO2/95% air.
Primary human multi-potent stromal cells were obtained from Tulane Center for Gene Therapy, and maintained according to prescribed protocols. Briefly, MSCs were expanded in expansion medium (“Exp”) comprising α-MEM with L-glutamine and without ribonucleosides or deoxyribonucleosides (Invitrogen/GIBCO) supplemented with 16.5% fetal bovine serum (Atlanta Biologicals), L-glutamine (200 mM), and penicillin-streptomycin (100 units/ml -100 μg/ml). For osteogenic differentiation, medium was supplemented with 50 μM L-Ascorbic acid 2-phosphate, 20 mM β-glycerophosphate, and 10 nM dexamethasone (“OS” medium). Soluble EGF was added to either Exp or OS medium where indicated. In experiments comparing cell behaviors in Exp or OS medium, cells were plated in Exp medium, and media were changed to the respective conditions 24 hr after plating. For all conditions, media were changed every third day. MSCs were received from Tulane Gene Therapy Center at P1, thawed and culture expanded to 80% confluence, then passaged and frozen down (P2) for liquid nitrogen storage. Prior to experiments, MSCs were thawed overnight, trypsinized (P3) and seeded onto surfaces. Cells from two donors were used for all experiments.
Polymeric substrate preparation
Polymeric substrates were prepared as previously described (Fan et al., 2007) using a mixture of two different poly(methyl methacrylate)-graft-poly(ethylene oxide) (PMMA-g-PEO) comb polymers (Irvine et al., 2001). PMMA-g-PEO comprising 33 wt% PEO is resistant to cell adhesion, but allows presentation of tethered EGF in a locally dense concentration suitable for allowing receptor homodimerization (tethered EGF [tEGF]-polymer) (Fan et al., 2007; Kuhl and Griffith-Cima, 1996). Substrates were formed by activating this polymer on the hydroxyl chain ends with 4-nitrophenyl chloroformate, and mixing it with a 20 wt % PMMA-g-PEO diluent polymer at a 40:60 tEGF-polymer:diluent ratio. PMMA-g-PEO comb copolymer comprising 20 wt% PEO allows protein adsorption and is cell adhesive..
Specifically, glass coverslips (18 mm in diameter) were silanized with Siliclad (Gelest Inc) prior to spin-coating of the polymer mixture to form an ~100-nm thin transparent film on the substrate. Murine EGF was coupled to the activated side chains of the spin-coated polymer by incubation with a solution of 25 μg/ml EGF in 0.1 M phosphate buffer, pH 8.7, at room temperature for 22–24 hours. Control surfaces were prepared using phosphate buffer without EGF. Surfaces were then blocked in 100 mM Tris buffer (pH 9) at room temperature for 2 hours, followed by PBS rinses to achieve a surface density of approximately 5,000 –7,000 tethered EGF molecules per μm2. Substrates were stored in PBS at 4°C until use. For in vitro experiments, each substrate was placed in individual wells of a 12-well plate and seeded with ~25,000 cells/cm2 (50,000 cells per well). After the culture period and treatment of the cells, prior to biochemical assay, surfaces were transferred to new 12 well plate.
Total protein determination
Total protein levels were determined using the BCA kit (Pierce).
Immunoassays for quantifying amounts and activation levels of signaling proteins
Bioplex® bead kits were used for phosphorylated EGFR determination (BioRad), and Novagen® bead kits were used for total EGFR determination (EMD Sciences). Both assays use antibodies immobilized to fluorescent beads to bind target proteins in cell lysate or supernatant preparations, and are designed to work with a Bioplex 200 System using Luminex technology (Bio-Rad). Lysates were prepared according to manufacturer’s instructions and 10 μg protein from each sample were incubated overnight in filter plates (Millipore) with the appropriate antibody-bead conjugates. Unbound proteins were washed away by vacuum filtration of the plate, trapping the beads in the well. Beads were rinsed with vendor-supplied buffers and incubated with a biotinylated antibody specific for a second epitope on the target. Beads were rinsed again and incubated with streptavidin phycoerythin (Strep-PE), fluorescently tagging the antibody bound to the second epitope. The beads are intrinsically fluorescent at a wavelength matched to the target protein, hence, intensity of PE fluorescence relative to the fiduciary fluorescence of the bead allows quantification of the target protein. Total EGFR fluorescence was normalized to a standard curve generated with increasing concentrations of the extracellular domain of EGFR and HER2 provided by the manufacturer (Novagen). Phosphorylated EGFR signal was normalized to the signal of a master lysate prepared in bulk, separated into single thaw aliquots, and used with each experiment as a standard.
Western blotting
Cells were lysed with proprietary buffer from the Bioplex® kits after surfaces were transferred to new 12-well plates. Total protein concentration was determined and equal amounts of protein were loaded into a 4–20% gradient SDS-PAGE NuPage® precast gel (Invitrogen). Protein was transferred to a nitrocellulose membrane (BioRad) and probed with a rabbit polyclonal anti-EGFR antibody (Cell Signaling), rabbit polyclonal anti-phospho-ERK antibody (Cell Signaling) and a mouse anti-GAPDH antibody (Calbiochem). Secondary antibodies conjugated to infrared fluorescent dyes bound the primary antibodies and the signal was detected with the Li-Cor Odyssey System. Densitometry of each band for the target protein was calculated for each surface condition and treatment, and those three biological replicates were used for statistical analysis.
Fluorescent EGF staining
MSCs were placed on ice before a final concentration of 100 ng/ml of rhodamine labeled EGF (Invitrogen) was added. Cells were then washed with medium and transferred to 37ºC for 30 minutes to allow internalization, then fixed with 4% paraformaldehyde for 20 minutes. Following rinsing with PBS, cells were imaged an Axiovert 135 Microscope (Zeiss) using the same exposure settings in OpenLab for comparison of fluorescence intensity. Granularity was removed with Adobe Photoshop, and phase images and fluorescent images were combined in ImageJ.
Quantitative reverse transcription polymerase chain reaction
Control and tEGF surfaces with attached cells were moved to new 12 well dishes and rinsed with PBS prior to cell lysis. RNA was isolated using the Qiagen RNEasy kit according to manufacturer’s instructions. 200 ng of total RNA was reverse transcribed with Superscript III (Invitrogen) to synthesize first-strand cDNA according to manufacturer’s instructions. The cDNA was amplified with SYBR green PCR master mix (Qiagen) on an ABI7500 instrument (Applied Biosystems). Program set for 30 seconds denaturation at 95°C followed by 60 seconds of annealing and extension at 60°C (except for TGFα which was 64°C) Primer sequences are as follows EGF: forward 5′-CAGGGAAGATGACCACCACT-3′ reverse 5′-CAGTTCCCACCACTTCAGGT-3′ (Morita et al., 2007); TGFα forward 5′-TGATACACTGCTGCCAGGTC -3′; reverse: 5′-ATCTCTGGCAGTGCTGTCCT-3′ (Morita et al., 2007) Alkaline phosphatase Forward 5′-CTTCAAACCGAGATACAAGCAC-3′ reverse 5′-CTGGTAGTTGTTGTGAG CATAG-3′ (Xia et al., 2008). Glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) forward 5’-ACCACAGTCCATGCCATCAC-3’ reverse 5’-TCCACCACCCTGTT GCTGTA- 3’. EGFR mRNA was detected using proprietary Taqman® probes (Applied Biosystems) with the same PCR program. Normalization and fold changes were calculated using the ΔΔCt methodt.
Alkaline phosphatase activity assay
Surfaces with attached cells were moved to new 12-well dishes and rinsed with PBS. Cells were lysed in 200 μL of 0.2% NP-40 in 1 mmol/L MgCl2, scraped with a rubber policeman, and collected. After one minute of sonication in a water bath, equal volumes of lysates were diluted by 10 and 100 fold with lysis buffer. Diluted sample lysate and lysis buffer were placed in a 96 well plate with an all lysis buffer sample used as a background control. A 1:1 solution of 2-Amino-2-methyl-1-propanol, 1.5 mol/L, pH 10.3 at 25°C (Sigma) and stock substrate solution of p-nitrophenyl phosphate, disodium (Sigma) was added to the samples and incubated for 30 minutes at 37°C; sodium hydroxide was added to stop the reaction. Absorbance at 405 nm was read using the SpectraMax platereader and background signal from the blank control was subtracted from the readings. Increasing concentrations of p-nitrophenol in sodium hydroxide was used to generate a standard curve to fit the data into Sigma units: that amount of enzyme which catalyses the liberation of 1 micromole p-nitrophenol per minute at 37°C. Total protein was then determined from lysates.
Alizarin Red S staining
At the end of 21 days of culture, polymeric surfaces were transferred to a new 12-well plate, and rinsed with PBS. 10% buffered formalin (Sigma) was used to fix cells at room temperature for 1 hour. Surfaces were rinsed with DI water, then incubated with 1% w/vol Alizarin Red S (Sigma) for 20 mins at room temperature. Surfaces were rinsed with DI water and images were captured to visualize staining. Plates were then washed four times with PBS before the addition of 0.1 ml of 10% (wt%) cetylpyridinium chloride for 30 min to release the calcium-bound Alizarin Red S. The solution was collected, on a SpectraMAX microplate reader diluted at a ratio of 1:10 and read at OD570 (Molecular Devices).
Statistical tests
Significance was determined using unpaired student’s t-test or ANOVA, with P values < 0.05 considered to represent results not likely arising from random chance.
Results
Tethered EGF induces sustained EGFR-mediated signaling in immortalized and primary human MSCs
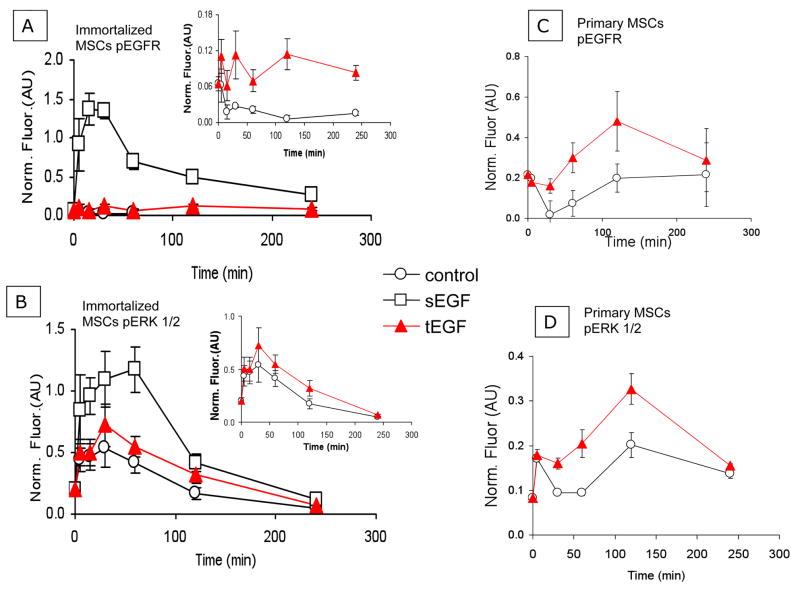
We first confirmed our previously-published observations that tethered EGF (tEGF) stimulates EGFR-mediated signaling in human MSCs immortalized with telomerase reverse-transcriptase (htMSC). Immortalized htMSCs were seeded on control and tEGF surfaces in serum free medium at a density of ~25,000 cells/cm2 and incubated for defined time periods. Separate samples were also stimulated on control surfaces with soluble EGF (1.7 nM). Cells were lysed, and equal amounts of protein were used to quantify phosphorylation of EGFR and ERK 1/2 using a quantitative bead based immunoprecipitation fluorescent assay. Soluble EGF induced rapid activation of both EGFR and the downstream target ERK 1/2 with peaks in phosphorylation after 30 and 60 minutes, respectively (Fig 1A,B) and a return to near-baseline levels by 4 hr. In contrast, tEGF induces modest and sustained activation of EGFR compared to soluble EGF (Fig 1A) resulting in a transient peak of ERK phosphorylation that follows similar kinetics to that of soluble EGF (Fig 1B).
Figure 1. Tethered EGF induces sustained activation in primary MSCs.
Immortalized human MSCs were seeded onto control or tEGF surfaces. 10 ng/ml soluble EGF was added to the media of a set of MSCs on control surfaces (sEGF). After defined time periods up to 4 hours, both suspended and adhered cells were collected and lysed with Bioplex lysis buffer, total protein determined with BCA kit, and phosphorylation of EGFR (A, C) and ERK 1/2 (B, D) were quantified using a Bioplex quantitative bead based immunoprecipitation fluorescent assay. Arbitrary units of fluorescence (AU) were normalized to a master lysates used to compare between experiments (Norm. Fluor.). Insets in A and B are close up views of control and tEGF.. Data shown are from one representative experiment with three biological replicates.
We then examined the responses of primary human MSCs in the presence of serum, a more relevant model for therapeutic application. Using the same protocol described above but with primary human MSC (passage 3), we found that tEGF induces greater phosphorylation of EGFR and ERK 1/2 compared to MSCs on control surfaces without exogenous EGF (Fig 1C,D), even in the presence of serum. This showed that tEGF stimulates EGFR and ERK phosphorylation in primary MSCs above the background of exogenous growth factors present in the serum-containing culture medium.
Stimulation of primary human MSCs by tEGF alters EGFR protein and activation levels during expansion and osteogenic differentiation
Soluble EGF (sEGF) causes internalization and degradation of EGFR, a phenomenon that can result in acute or chronic EGFR downregulation when high (≫KD) concentrations are used for stimulation. Following acute downregulation, the number of surface EGFR may rebound via recycling of internalized receptors and, over a longer time scale, de novo synthesis of EGFR (Vieira et al., 1996). Although EGFR bound to tEGF cannot be endocytosed and degraded, temporal evolution of EGFR protein levels may result from changes in transcription, translation, or internalization and degradation via alternate mechanisms (Huang et al., 2007; Huang et al., 2006; Kuhl and Griffith-Cima, 1996).
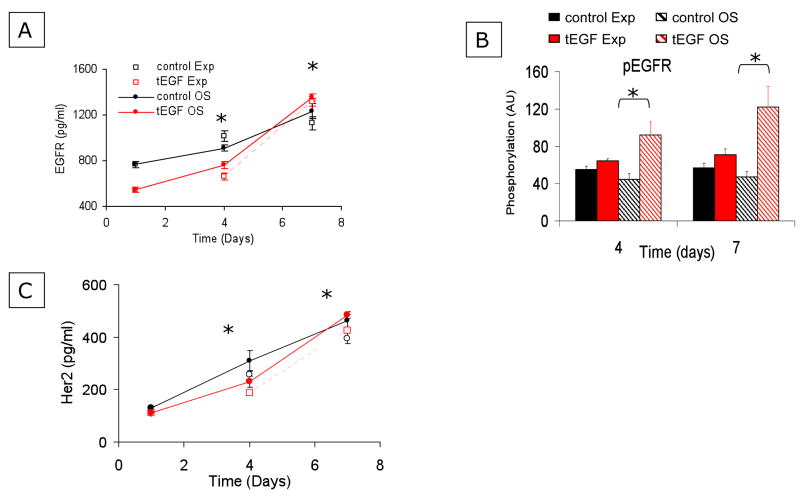
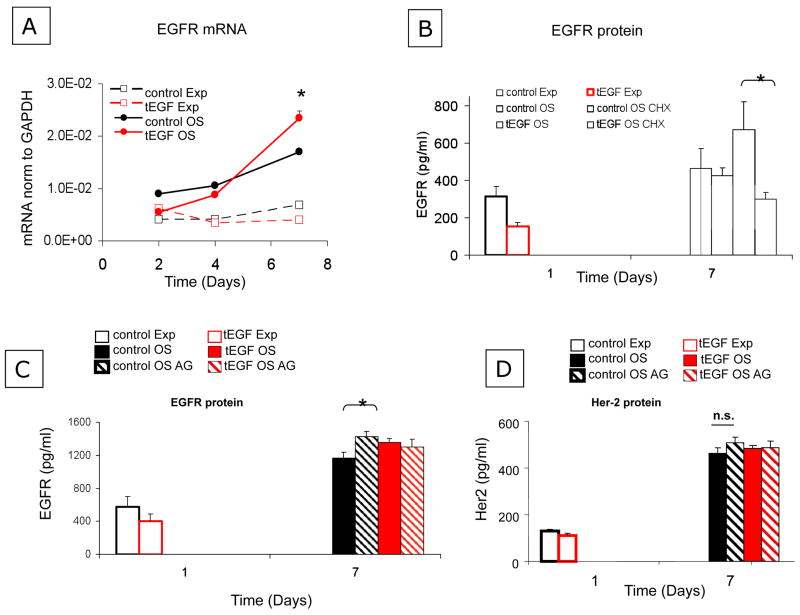
It has been reported that freshly isolated MSCs have low, barely detectable levels of EGFR, but increase expression of EGFR as they become more differentiated (Sekiya et al., 2002). To examine the dynamic changes in MSC EGFR number during culture and osteogenic differentiation, human primary MSCs were cultured on control or tEGF surfaces in expansion (Exp) medium that maintains the undifferentiated MSC phenotype or osteogenic (OS) medium that contains the osteoinductive factors betaglycerolphosphate, ascorbic acid, and dexamethasone. MSCs were seeded at a density of ~25,000 cells/cm2 in Exp medium. After 24 hours, medium was changed to either fresh Exp or OS media. Cells were lysed after 1, 4, and 7 days of culture and lysates were examined by bead-based immunoassay (Fig 2A) for total EGFR protein levels. At the early time-point (day 1), MSCs cultured on control surfaces had a significantly greater number of EGFR compared to cells on tEGF. EGFR expression increased in a comparable fashion between days 1–4 for all conditions, such that on day 4, EGFR expression for MSCs on control surfaces was still significantly greater than on tEGF surfaces. EGFR expression by MSC on tEGF slightly exceeded control levels by day 7 (Fig 2A). Additionally, cell lysates were collected for analysis of phosphorylation of EGFR. Surprisingly, although there are fewer total EGFR expressed in MSCs cultured on tEGF on day 4, the absolute amount of phosphorylated EGFR was greater for cells on tEGF compared to controls at day 4 (Fig 2B), and the ratio of pEGFR/total EGFR for cells on tEGF exceeded that of controls by about 2-fold for both Exp and OS medium. This same trend was observed on day 7 (Fig 2B): cells on tEGF exhibited greater absolute levels of pEGFR compared to controls, and the ratio of pEGFR/total EGFR for cells on tEGF exceded that for controls by about 2-fold for cells in OS medium.
Figure 2. Tethered EGF delays temporal increase in EGFR protein levels.
Human primary MSCs were seeded onto control or tEGF surfaces in Exp medium. After 24 hours, the medium was changed to OS differentiating medium or fresh Exp medium and cells were lysed for Novagen (A, C), or Bioplex (B) bead-based immunoprecipitation assays to detect total EGFR (A), phosphorylated EGFR (B), or total HER2 (C). *p<.05, n=3 comparing day 4 and day 7 EGFR and HER2 levels to day 1 levels.
The EGFR family member HER2 has no known ligands but is active in heterodimers with other members of the EGFR family including EGFR (Citri et al., 2003; Citri and Yarden, 2006; Hendriks et al., 2003a; Hendriks et al., 2003b). Heterodimers of EGFR and HER2 signal very efficiently through EGFR-activated pathways. Hence, HER2 expression levels may provide useful information concerning the potential for EGFR-mediated signaling. When we analyzed HER2 expression levels in lysates generated for Fig 2B using the immunobead assay, we found that the trends in expression of HER2 by MSC mirrored those of EGFR, though the absolute magnitudes of expression are lower for HER2 than for EGFR (Fig 2C).
Soluble EGF binds EGFR of MSCs cultured on tethered EGF
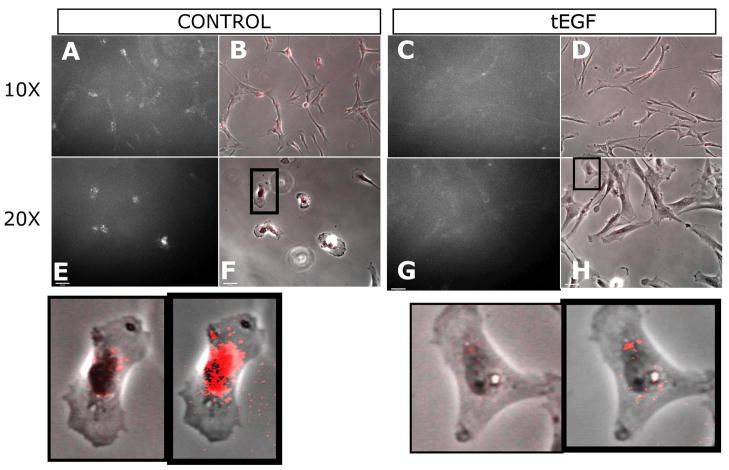
We visualized uptake of sEGF by MSCs on control and tEGF substrata as a complementary means of comparing relative EGFR expression levels at early days of culture. MSCs were cultured for two days on control or tEGF surfaces then placed on ice and incubated with 100 ng/ml of rhodamine labeled EGF for one hour, a protocol that would allow near-complete saturation of surface EGFR with labeled soluble EGF for adherent cells, based on ligand exchange kinetics for the EGF-EGFR complex on intact cells at 0°C (Sato et al., 2005). Cells were washed to remove unbound rhodamine-EGF, transferred to 37ºC for 30 minutes to allow internalization of the complexed receptors, and then fixed and imaged. MSCs cultured on control surfaces internalized more rhodamine-labeled EGF than those on tEGF surfaces (Fig 3), supporting the results obtained by immunobead assay that EGFR levels are attenuated on tEGF compared to control conditions up through day 4. We cannot rule out, however, that the kinetics for replacing bound ligand on cells contacting tEGF are delayed compared to those for replacing bound ligand on cells in contact with soluble EGF.
Figure 3. Soluble, exogenous EGF family ligands still bind EGFR of MSCs cultured on control or tethered EGF surfaces.
MSCs were cultured for two days on control (A, B, E, F) or tEGF (C, D, G, H) surfaces then placed on ice 1 hour and incubated with 100 ng/ml of rhodamine labeled EGF. Cells were then washed and transferred to 37ºC for 30 minutes to allow internalization, then fixed and imaged. MSCs cultured on tEGF do not internalize as much rhodamine labeled EGF as those cultured on control surfaces , suggesting that they have fewer surface receptors. A, C, E, G are fluorescent images captured by camera with same settings, and B, D, F, H are computerized color merged with phase images of cells to visualize location of rhodamine EGF. Inset is close-up view of selected cell, unretouched (left image in inset) or oversaturated to view location (right image in inset).
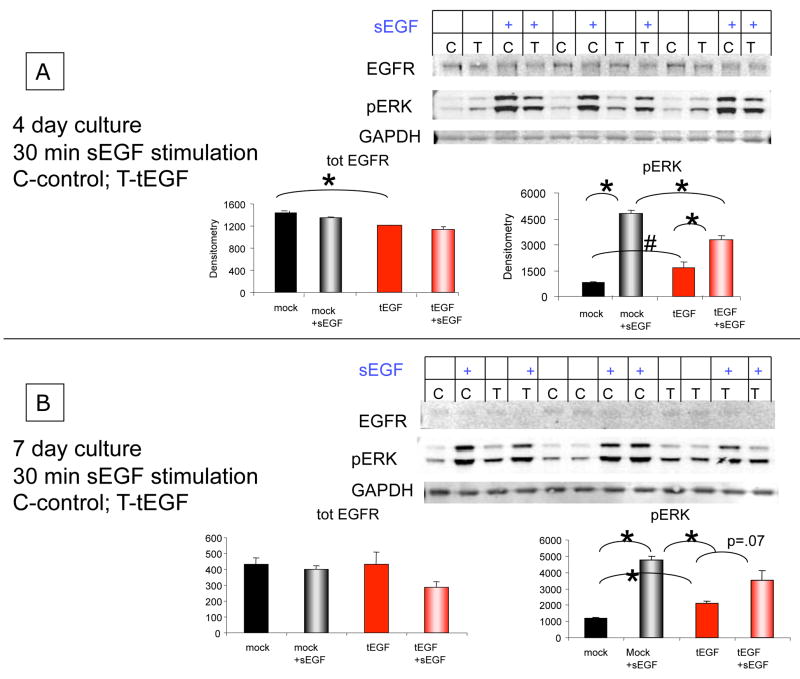
To further test the hypothesis that a soluble EGFR ligand could drive receptor internalization in the presence of tEGF, a protocol omitting the pre-equilibration at low temperature and using EGF concentrations in the physiological range was used. Human primary MSCs were cultured on control or tEGF surfaces for 4 or 7 days and then stimulated for 30 minutes with soluble EGF at a concentration of 1.7 nM (10 ng/ml), a value close to the measured KD of 2.9 nM for EGF binding to EGFR on htMSC (Tamama 2006). Prior to EGF stimulation, MSCs on tEGF had fewer EGFR at day 4 (Fig 4A) but comparable levels at day 7 (Fig 4B) as seen in previous experiments (Fig 2A). After stimulation with 1.7 nM of soluble EGF for 30 minutes, there is some reduction of total EGFR levels although not to the extent of statistical significance after densitometric analysis of Western blots of biological triplicates (Fig 4).
Figure 4. Exogenous soluble EGF elicits signaling in MSCs on control and tEGF surfaces.
MSCs were cultured for 4 (A) or 7 (B) days on control or tEGF surfaces, then stimulated with 10 ng/ml EGF for 30 minutes followed by cell lysis. Equal amounts of protein were loaded for Western blots of total EGFR, phosphorylated ERK 1/2, and GAPDH. Graphs display the average densitometry of the target bands from the biological triplicates for each treatment (*n=3, p<.01; # n=3, p<.05). GAPDH is provided for illustration of loading control for replicate experiments.
For cells cultured on tEGF, at least some fraction of EGFR appear to be engaged by tEGF, as inferred by activation of EGFR-mediated signaling (Figs 1, 2), but the fraction of free EGFR is difficult to ascertain. In these experiments, therefore, exogenous soluble EGF would have to reduce EGFR number by binding to free EGFR or by successfully competing with tEGF for binding, replacing it, and driving internalization and degradation of the receptor. Interestingly, phosphorylation of ERK is chronically and significantly increased in the presence of tEGF (Fig 4), but cells cultured in the presence of tEGF had a less robust ERK phosphorylation response upon further stimulation with soluble EGF ligand compared to the previously unstimulated MSCs on control surfaces (Fig 4). The muted ERK phosphorylation response of cells cultured in the presence of tEGF cannot be attributed to their having less total ERK, as cells on tEGF have similar amounts of total ERK at day 4 and day 7 (Supplementary Figure 1). This muted response may arise from having relatively few free EGFR available for stimulation supporting the earlier results (Figs. 2 and 3), although we do not negate the possibility of upregulated phosphatase activity. The response was measured at 30 min, and although this time scale should be sufficient for most EGFR to go through at least one cycle of ligand binding/unbinding for, the localization of the tEGF-EGFR bond to the cell substrata may delay these kinetics, particularly since the concentration of local tEGF ligand is relatively high in the small volume between the cell membrane and the substrate.
During osteogenic differentiation, temporal changes in MSC EGFR number are regulated at the mRNA level, and not specifically via EGFR phosphorylation
The observation that total EGFR levels are lower for cells cultured on tEGF compared to controls at days 2 and 4 was at first take an unexpected finding from the perspective of ligand-mediated downregulation, as cells on tEGF are presumed to be resistant to ligand-mediated internalization and degradation of EGFR. However, expression levels of EGFR may be controlled at the transcriptional, post-transcriptional, or translational level (Seth et al., 1999).
To further illuminate the mechanisms regulating the observed EGFR protein levels under these different conditions, RNA was collected for 2, 4, and 7 days, and quantitative real time PCR analysis of EGFR was performed using GAPDH mRNA as a loading control. For cells maintained in osteogenic medium, the mRNA levels of EGFR closely approximated the trend seen in the total EGFR protein levels (Fig 2A): the amount of EGFR mRNA in cells on control substrates was greater than that in cells on tEGF through day 4, but EGFR mRNA in cells on tEGF exceeded that in cells on control substrates by day 7 (Fig 5A). In contrast, cells maintained in Exp medium showed no significant changes in EGFR mRNA levels as a function of culture time (Fig 5A), despite increases in EGFR protein levels over the 7 day culture period (Fig 2A). The correlation between the EGFR mRNA and protein level changes in OS medium suggests transcriptional regulation is the primary means of controlling EGFR protein levels in MSCs during osteoblastic differentiation. To further examine mechanisms regulating changes in EGFR protein levels when cells are cultured in OS medium, we examined EGFR expression under conditions where protein synthesis was inhibited by cycloheximide (CHX) during the day 5–7 period when EGFR mRNA and protein levels both increase. Inhibiting translation resulted in little change in EGFR protein for cells cultured on control substrates in OS medium (Fig 5B), suggesting that EGFR turnover is slow and that very modest levels of synthesis and degradation occur in this time period. The presence of cycloheximide did not alter cell morphology (Supplemental figure 2). For cells on tEGF in OS medium, however, where there is a much more marked increase in EGFR over the 1-4-7 day period (Fig 2A), CHX causes a significant reduction in EGFR levels at Day 7 compared to untreated cells cultured on tEGF (Fig 5B). The data suggest that synthesis of new EGFR is inhibited but that degradation is not significant.
Figure 5. During osteogenic differentiation, temporal changes in MSC EGFR number are regulated at the mRNA level and not specifically by EGFR phosphorylation.
MSCs were cultured on control or tEGF surfaces for 2, 4, and 7 days and total RNA was collected. 200 ng total RNA was reverse transcribed for quantitative real time PCR analysis of EGFR (A, *p<.05, n=3 between tEGF OS and control OS); GAPDH mRNA was used as a loading control. In parallel cultures of MSCs cultured in OS conditions after 5 days, 0.5 μM cycloheximide or DMSO vehicle was added to cultures and cells were lysed on Day 7 for Novagen bead-based immunoprecipitation assays to determine total EGFR (B, *p<.05, n=3 between day 7 tEGF veh and tEGF CHX). MSCs were also cultured in OS conditions in the presence or absence of 1 μM AG1478 and lysed on Day 7 for Novagen bead-based immunoprecipitation assays to determine total EGFR and total HER2. *p<.05, n=3–6 (C, D).
To assess whether activation of EGFR stimulates its own delayed increased synthesis, MSCs were cultured for 7 days under osteogenic conditions in the presence of AG1478, an EGFR kinase inhibitor, on control or tEGF substrates, and lysed for total EGFR protein. Inhibition of EGFR kinase activity with AG1478 did not block the increase in EGFR or HER2 between days 4 and 7, suggesting that the increase in total EGFR protein is not EGFR phosphorylation-dependent (Fig 5C, D). There was a modest but significant increase in EGFR protein on control substrates.
Osteogenic differentiating MSCs inversely regulate EGF and TGFα at the mRNA level, with preference for TGFα
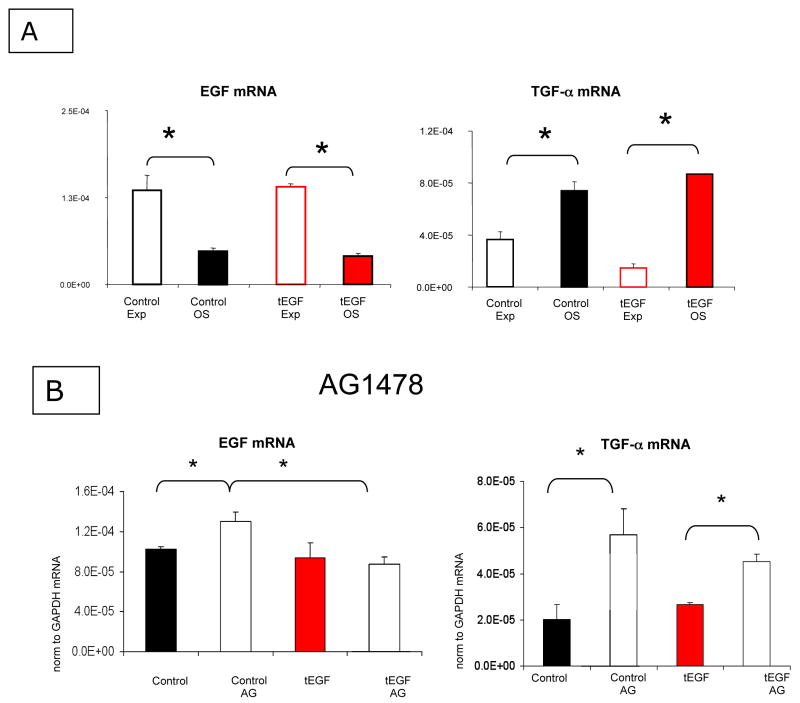
Having established that soluble ligand can still bind EGFR and elicit signaling in the presence of the tEGF-EGFR complex (Fig 4), we hypothesized that production of autocrine ligands by MSCs may influence EGFR number in our system. EGF-like family members EGF, TGFα, HB-EGF, amphiregulin, betacellulin, and epiregulin, were all detected by mRNA in primary rat calvarial osteoblasts and the rat cell line UMR 106-01 (Qin et al., 2005) and human, but MSCs have only been reported to express EGF mRNA and secreted protein (Chen et al., 2008) and specifically not HB-EGF (Krampera et al., 2005). We thus examined expression levels of EGF and TGFα, two EGF-family ligands that are processed similarly by the cell but direct traffic of bound EGFR differently due to their dissociation rates and binding affinities. EGF targets receptors for degradation but TGF-α targets receptors for recycling to the cell surface sustaining EGFR signaling via maintenance of EGFR surface protein (French et al., 1995). MSCs were cultured on control or tEGF surfaces for 7 days, and total RNA was collected and prepared for quantitative reverse transcription PCR. The pattern of EGF and TGFα expression was inversely related for cells in Exp vs. OS conditions on both the control and tEGF substrates (Fig 6A). On both substrates, EGF mRNA is significantly reduced in OS conditions, but TGFα mRNA is significantly increased in OS compared to Exp medium (Fig 6A).
Figure 6. Osteogenic differentiating MSCs inversely regulate EGF and TGFα at the mRNA level.
MSCs were cultured on control or tEGF surfaces for 7 days in expansion (Exp) or osteogenic (OS) media (A) in the presence or absence of 1 μM AG1478 (B), and total RNA was collected. 200 ng total RNA was reverse transcribed for quantitative real time PCR analysis of EGFR, EGF ligand, and TGF-α; GAPDH was used as a loading control (*p<.05, n=3–6 comparing conditions noted by the bars).
To examine effects of EGFR activation on mRNA levels, AG1478, an EGFR kinase inhibitor, was used. MSCs were cultured for 7 days on control or tEGF surfaces in the presence or absence of 1 μM AG1478, and total RNA was collected. When EGFR kinase activity is blocked with AG1478, TGF α mRNA undergoes a greater fold change increase compared to EGF mRNA (Fig 6B); this was even greater for control surfaces.
Sustained EGFR signaling by tEGF increases osteogenic differentiation and matrix mineralization of primary MSCs
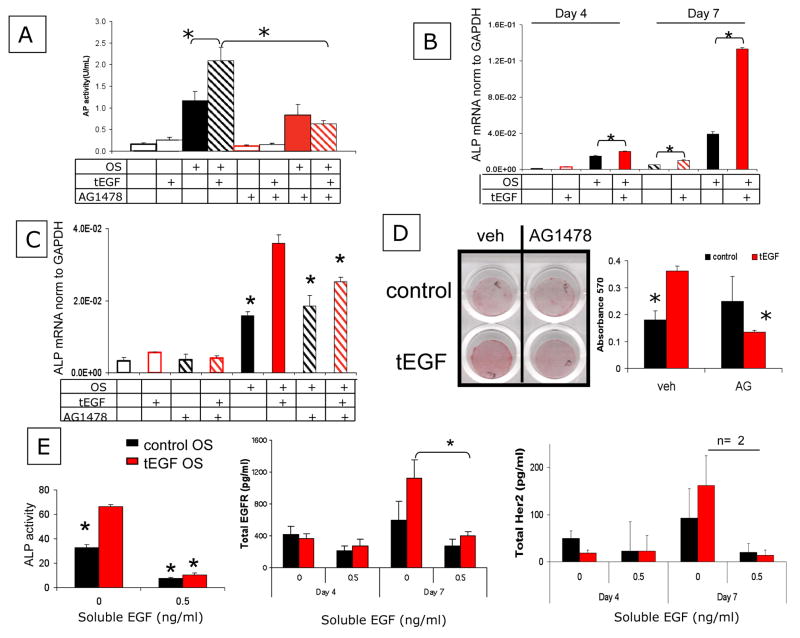
In several cell types, sustained signaling from EGFR results in different phenotypes compared to transient stimulation (Schmidt et al., 2003; Sigismund et al., 2008; Sigismund et al., 2005). After observing that tethered EGF modulates EGFR protein levels and results in sustained activation under conditions that induce osteogenic differentiation, we sought to determine whether tEGF influenced MSC differentiation. MSCs were cultured for 7 days on control and tEGF surfaces in either Exp or OS medium and lysed for alkaline phosphatase activity, an early marker of bone differentiation. MSCs cultured on tEGF in OS medium had two-fold greater alkaline phosphatase activity than MSCs maintained on control substrates (Fig. 7A). This pronounced effect of tEGF on alkaline phophatase activity at day 7 was abrogated when cells were cultured in the presence of the EGFR kinase inhibitor AG1478 (Fig 7A). Gene expression of alkaline phosphatase by quantitative reverse transcription PCR measure of mRNA levels corroborated the increase in osteoblastic differentiation on tEGF after 7 days (Fig 7B). Interestingly, MSCs on tEGF had greater alkaline phosphatase mRNA levels even in expansion media which, by itself, is not pro-osteogenic. Inhibiting EGFR kinase activity also reduced alkaline phosphatase mRNA expression when MSCs were cultured with OS medium (Fig 7C).
Figure 7. Sustained EGFR signaling by tethering EGF increases osteogenic differentiation and matrix mineralization of primary MSCs.
Human primary MSCs were cultured on control surfaces or on tethered EGF surfaces in expansion (Exp) medium or osteogenic (OS) differentiating media in the presence or absence of 1 uM AG1478, the EGFR kinase inhibitor for 7 days and lysed for alkaline phosphatase (ALP) activity, an early marker of OS differentiation *p<.05, n=3 (A). 200 ng total RNA was reverse transcribed for quantitative real time PCR analysis of alkaline phosphatase (B); GAPDH mRNA was used as a loading control. MSCs were also incubated for 6 days in the presence or absence of 1 μM AG1478, the EGFR kinase inhibitor before RNA collection *p<.05, n=3 (C). MSCs were cultured on control or tEGF surfaces for 21 days in osteogenic media in the presence or absence of 1 uM AG1478, then stained with Alizarin Red (D). Images were captured. Subsequently, cetylpyridinium chloride was used to extract bound Alizarin Red and absorbance of that solution was read at 570 nm to quantify Alizarin Red staining (*p<.01, n=3 compared to tEGF vehicle). Human primary MSCs were cultured for 7 days in the absence or presence of 0.5 ng/ml EGF added to expansion or osteogenic differentiating media and then lysed for alkaline phosphatase activity assay, * p<0.05, n=3 compared to tEGF 0 ng/ml EGF sample (E). Parallel cultures were also lysed after 4 and 7 days culture for total EGFR and HER2.
With evidence that tEGF influences the early stage of commitment to bone formation, we then examined its effects on matrix deposition and mineralization as an indicator of later development and bone formation. MSCs were cultured on control or tEGF surfaces for 21 days in the presence or absence of AG1478 and stained with Alizarin Red to visualize mineral deposition. Images were captured (Fig 7D), and then bound Alizarin Red was extracted and quantified by colorimetric assay. MSCs cultured in OS medium on tEGF showed two fold higher mineralization compared to MSC on control substrates, and mineralization was significantly reduced in the presence of the EGFR kinase inhibitor AG1478 (Fig 7D).
These data suggest that sustained EGFR-mediated signaling elicited by tethered EGF ligand increased MSC differentiation into bone forming cells and this effect could be blocked by inhibiting EGFR kinase. MSC cultured on tEGF exhibited altered EGFR protein levels compared to controls (Fig 2), and sEGF could compete with tEGF (Fig 4). We next sought to test if altering EGFR number with soluble ligand competition would affect MSC osteogenic differentiation to establish the receptor number as an important player in tEGF mediated increases. To look at the effects of chronic culture with soluble EGF ligand, MSCs were cultured on control or tEGF surfaces in the presence or absence of 0.5 ng/ml of soluble EGF in OS medium with fresh EGF added every third day with media change. This concentration was used because it was great enough to activate EGFR, yet low enough that we expected most soluble ligand to be depleted in between medium changes (see estimated depletion rates in Supplemental Figure 3). After 4 and 7 days, the cells were lysed for quantification of EGFR protein. Soluble ligand caused a reduction in EGFR on both control and tEGF surfaces at day 4. By day 7, when the receptor numbers are higher for both surface conditions (Fig 2A), there are still significantly fewer EGFR in the presence of the soluble ligand (Fig 7E). The trends observed for EGFR expression were mirrored by HER2, which showed similar downregulation in the presence of soluble ligand.
Discussion
Previous studies of EGFR-mediated effects on MSC osteogenic differentiation have produced diverse findings (Krampera et al., 2005; Sibilia et al., 2003; Tamama et al., 2006), reflecting the intricate dynamics of EGF ligand/receptor system trafficking and signaling. Here, we studied MSC responses to EGFR stimulation using a mode of ligand presentation that offers a relatively constant exogenous EGF stimulus to the cells. Our experiments show that stimulation of primary human MSC with tEGF increases osteogenic differentiation markers ALP (at day 7) and mineralization (day 21). This phenotypic behavior is associated with sustained activation of the EGFR (Figure 2B, Figure 4) and Erk (Figure 4) and increased ratios of pEGFR/total EGFR for cells on tEGF compared to control. The physical tethering presumably constrains ligand/receptor complexes from undergoing endocytic internalization, protecting activated EGFR from a degradation fate associated with EGF-mediated internalization. Consistent with the concept that sustained EGFR activation in the day 4–7 period enhances osteogenic differentation, both EGFR downregulation by soluble EGF (sEGF) and inhibition of EGFR kinase activity by a pharmacological agent each independently served to attenuate tEGF-induced increase in alkaline phosphatase expression (Fig 7). The delay in EGFR (and HER2) expression level increase on tEGF substrata following initial cell seeding (Figs 2A,C) coupled with the concomitant greater EGFR phosphorylation level (Fig 2B) indicates that it is the integrated combination of receptor expression and ligand availability during the first few days of osteoinduction that facilitates enhanced downstream signaling activities governing MSC differentiation fate decisions by day 7.
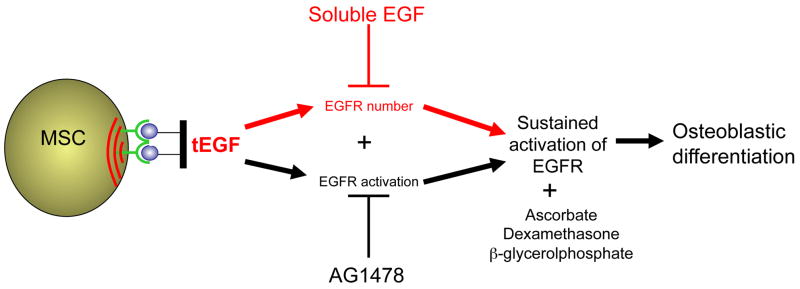
Our results taken together suggest the model shown schematically in Fig 8: the maintenance of higher levels of pEGFR, due in part to inhibition of EGFR internalization and in part to sustained stimulation by externally-restrained EGF ligand, both contribute to enhancement of differentiation.
Figure 8. Conceptual illustration of tEGF effects in MSC osteogenic differentiation.
Sustained maintenance of EGFR levels combined with constant EGF presentation availability facilitates EGFR-mediated increase in osteogenic fate.
The literature has conflicting reports regarding the effects of EGF on osteogenic differentiation of MSCs. 0.4 ng/ml EGF (66.4 pM) (Kumegawa et al., 1983), 5–50 ng/ml (0.5–5 nM) of amphiregulin (Qin et al., 2005), and 50 ng/ml (2.3 nM) HB-EGF, (Krampera et al., 2005) all have been found to inhibit osteogenic differentiation. In contrast, addition of 50 ng/ml (83 nM) EGF with media changes every 4 days (Kratchmarova et al., 2005) and 5 ng/ml EGF (8.3 nM) (Kim et al., 2008) were observed to increase osteogenic differentiation. Different EGF concentrations, treatment durations, cross-binding to other EGFR family members (by amphiregulin and HB-EGF), and cell culture histories render these reports difficult to interpret as a whole, particularly since the different bone progenitor cell types used for these experiments have different levels of EGFR (Tamama et al., 2006).
We have moreover found differential regulation of EGF and TGFα synthesis (Fig 6), offering an additional complicating feature in understanding EGFR-mediated regulation of MSC differentiation but one that is consistent with our notion of the central importance of time-integrated combination of receptor level and ligand availability, as these two ligands can have very different consequences for receptor and ligand endocytic degradation. In fibroblasts, EGF binding predominantly traffics ligand/receptor complexes to lysosomes for degradation when receptor expression is relatively low, whereas TGFα bindings instead favors trafficking to recycling (French et al., 1995; Wiley, 2003). We observe here that inhibition of EGFR kinase activity yields an increase of TGFα mRNA (Fig 6B) with greater fold increase than of EGF mRNA (Fig 6B). We speculate that this differential regulation in cells with depressed EGFR signaling provides a mechanism for the cells to sustain EGFR signaling via TGFα by sparing EGFR number in the longer-term (Joslin et al., 2007).
Future work will be required to elucidate the complex relationships between particular signals – including magnitude and duration – and MSC differentiation fate downstream of EGFR activity. ERK signaling has been implicated in osteogenesis (Ge et al., 2007; Jaiswal et al., 2000), and tuning of the ERK signaling pathway with inhibitors promoted differentiation of MSCs and preosteoblasts (Higuchi et al., 2002). Thus, modulating this key pathway with a sustained stimulus may help promote osteogenic differentiation analogously. In fact, our highly controllable tethered ligand system may offer prospects for generating novel, beneficial signaling network dynamics. It is possible that tEGF-generated sustained activation and more continuously engaged downstream pathway components (such as ERK) elicits enhancement of phosphatase-related feedback loops, with subsequent muting of cell response to other exogenous signals. Such desensitization has been shown in NIH-3T3 cells: adhesion to extracellular matrices activated ERK intracellular signaling but desensitized the cell to later growth factor stimulation and responsiveness (Galownia et al., 2007). Full activation of ERK and other mitogen-activated kinases (MAPKs) typically develops only following normal endocytic trafficking with hyperphosphorylation of EGFR in the endosomal compartment (Vieira et al., 1996). Receptors constrained at the cell plasma membrane by tethered ligand would not be expected to activate pathways preferentially localized to endosomal compartments.
Restricting EGFR to the plasma membrane via tEGF may also preferentially activate PI3K/Akt paths (Watton and Downward, 1999; Wu et al., 2000). Although inhibition of the PI3K pathway in immortalized MSCs has been determined to increase osteogenic differentiation (Kratchmarova et al., 2005), their protocol of stimulation with a large bolus of sEGF may have activated a different network and effectors, and other groups have shown that inhibition of PI3K decreases osteogenic differentiation (Kundu et al., 2008).
The phenotypic outcomes we measured here, ALP and matrix mineralization, are influenced by activation of osteoblastic transcription factors Runx2 and Osterix. Future studies directed at illuminating how the EGFR pathways influence MSC fate choices may productively include these features of the network, as motivated by our results here. This work shows that tethering strategies that alter receptor dynamics and signaling, can, ultimately, be used to modify MSC behavior in ways that may be useful for regenerative medicine strategies.
Supplementary Material
Acknowledgments
The authors are deeply grateful to Linda Stockdale for assistance with preparing the surfaces used in these experiments. Funding for this work was provided by NIH R01-GM059870-07 (L.G.G.); R01 DE 019523-10, UNCF/Merck Postdoctoral Fellowship (M.O.P.), and Georgia Tech FACES Fellowship (M.O.P.). Some of the materials employed in this work were provided by the Tulane Center for Gene Therapy through the NCRR grant P40RR017447.
Contract grant sponsor: NIH; Contract grant number: R01-GM059870-07
References
- Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3(4):e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res. 2003;284(1):54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- Fan VH, Tamama K, Au A, Littrell R, Richardson LB, Wright JW, Wells A, Griffith LG. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25(5):1241–1251. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- French AR, Tadaki DK, Niyogi SK, Lauffenburger DA. Intracellular trafficking of epidermal growth factor family ligands is directly influenced by the pH sensitivity of the receptor/ligand interaction. J Biol Chem. 1995;270(9):4334–4340. doi: 10.1074/jbc.270.9.4334. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381–390. [PubMed] [Google Scholar]
- Galownia NC, Kushiro K, Gong Y, Asthagiri AR. Selective desensitization of growth factor signaling by cell adhesion to fibronectin. J Biol Chem. 2007;282(30):21758–21766. doi: 10.1074/jbc.M703577200. [DOI] [PubMed] [Google Scholar]
- Ge C, Xiao G, Jiang D, Franceschi RT. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J Cell Biol. 2007;176(5):709–718. doi: 10.1083/jcb.200610046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington M, Pond-Tor S, Boney CM. Role of epidermal growth factor and ErbB2 receptors in 3T3-L1 adipogenesis. Obesity (Silver Spring) 2007;15(3):563–571. doi: 10.1038/oby.2007.562. [DOI] [PubMed] [Google Scholar]
- Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284(1):2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- Haugh JM. Localization of receptor-mediated signal transduction pathways: the inside story. Mol Interv. 2002;2(5):292–307. doi: 10.1124/mi.2.5.292. [DOI] [PubMed] [Google Scholar]
- Hendriks BS, Opresko LK, Wiley HS, Lauffenburger D. Coregulation of epidermal growth factor receptor/human epidermal growth factor receptor 2 (HER2) levels and locations: quantitative analysis of HER2 overexpression effects. Cancer Res. 2003a;63(5):1130–1137. [PubMed] [Google Scholar]
- Hendriks BS, Opresko LK, Wiley HS, Lauffenburger D. Quantitative analysis of HER2-mediated effects on HER2 and epidermal growth factor receptor endocytosis: distribution of homo- and heterodimers depends on relative HER2 levels. J Biol Chem. 2003b;278(26):23343–23351. doi: 10.1074/jbc.M300477200. [DOI] [PubMed] [Google Scholar]
- Higuchi C, Myoui A, Hashimoto N, Kuriyama K, Yoshioka K, Yoshikawa H, Itoh K. Continuous inhibition of MAPK signaling promotes the early osteoblastic differentiation and mineralization of the extracellular matrix. J Bone Miner Res. 2002;17(10):1785–1794. doi: 10.1359/jbmr.2002.17.10.1785. [DOI] [PubMed] [Google Scholar]
- Huang F, Goh LK, Sorkin A. EGF receptor ubiquitination is not necessary for its internalization. Proc Natl Acad Sci U S A. 2007;104(43):16904–16909. doi: 10.1073/pnas.0707416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol Cell. 2006;21(6):737–748. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Irvine DJ, Mayes AM, Griffith LG. Nanoscale clustering of RGD peptides at surfaces using Comb polymers. 1. Synthesis and characterization of Comb thin films. Biomacromolecules. 2001;2(1):85–94. doi: 10.1021/bm005584b. [DOI] [PubMed] [Google Scholar]
- Iyer AK, Tran KT, Griffith L, Wells A. Cell surface restriction of EGFR by a tenascin cytotactin-encoded EGF-like repeat is preferential for motility-related signaling. J Cell Physiol. 2008;214(2):504–512. doi: 10.1002/jcp.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275(13):9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- Joslin EJ, Opresko LK, Wells A, Wiley HS, Lauffenburger DA. EGF-receptor-mediated mammary epithelial cell migration is driven by sustained ERK signaling from autocrine stimulation. J Cell Sci. 2007;120(Pt 20):3688–3699. doi: 10.1242/jcs.010488. [DOI] [PubMed] [Google Scholar]
- Kim SM, Jung JU, Ryu JS, Jin JW, Yang HJ, Ko K, You HK, Jung KY, Choo YK. Effects of gangliosides on the differentiation of human mesenchymal stem cells into osteoblasts by modulating epidermal growth factor receptors. Biochem Biophys Res Commun. 2008;371(4):866–871. doi: 10.1016/j.bbrc.2008.04.162. [DOI] [PubMed] [Google Scholar]
- Krampera M, Pasini A, Rigo A, Scupoli MT, Tecchio C, Malpeli G, Scarpa A, Dazzi F, Pizzolo G, Vinante F. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood. 2005;106(1):59–66. doi: 10.1182/blood-2004-09-3645. [DOI] [PubMed] [Google Scholar]
- Kratchmarova I, Blagoev B, Haack-Sorensen M, Kassem M, Mann M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308(5727):1472–1477. doi: 10.1126/science.1107627. [DOI] [PubMed] [Google Scholar]
- Kuhl PR, Griffith-Cima LG. Tethered epidermal growth factor as a paradigm for growth factor-induced stimulation from the solid phase. Nat Med. 1996;2(9):1022–1027. doi: 10.1038/nm0996-1022. [DOI] [PubMed] [Google Scholar]
- Kumegawa M, Hiramatsu M, Hatakeyama K, Yajima T, Kodama H, Osaki T, Kurisu K. Effects of epidermal growth factor on osteoblastic cells in vitro. Calcif Tissue Int. 1983;35(4–5):542–548. doi: 10.1007/BF02405091. [DOI] [PubMed] [Google Scholar]
- Kundu AK, Khatiwala CB, Putnam AJ. Extracellular Matrix Remodeling, Integrin Expression, and Downstream Signaling Pathways Influence the Osteogenic Differentiation of Mesenchymal Stem Cells on Poly(Lactide-Co-Glycolide) Substrates. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2008.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S, Shirakata Y, Shiraishi A, Kadota Y, Hashimoto K, Higashiyama S, Ohashi Y. Human corneal epithelial cell proliferation by epiregulin and its cross-induction by other EGF family members. Mol Vis. 2007;13:2119–2128. [PubMed] [Google Scholar]
- Muthuswamy SK, Gilman M, Brugge JS. Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers. Mol Cell Biol. 1999;19(10):6845–6857. doi: 10.1128/mcb.19.10.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Aoyama T, Nakayama T, Nakamata T, Hosaka T, Nishijo K, Nakamura T, Kiyono T, Toguchida J. Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochem Biophys Res Commun. 2002;295(2):354–361. doi: 10.1016/s0006-291x(02)00661-7. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Qin L, Tamasi J, Raggatt L, Li X, Feyen JH, Lee DC, Dicicco-Bloom E, Partridge NC. Amphiregulin is a novel growth factor involved in normal bone development and in the cellular response to parathyroid hormone stimulation. J Biol Chem. 2005;280(5):3974–3981. doi: 10.1074/jbc.M409807200. [DOI] [PubMed] [Google Scholar]
- Reddy CC, Wells A, Lauffenburger DA. Proliferative response of fibroblasts expressing internalization-deficient epidermal growth factor (EGF) receptors is altered via differential EGF depletion effect. Biotechnol Prog. 1994;10(4):377–384. doi: 10.1021/bp00028a006. [DOI] [PubMed] [Google Scholar]
- Sato H, Kuwashima N, Sakaida T, Hatano M, Dusak JE, Fellows-Mayle WK, Papworth GD, Watkins SC, Gambotto A, Pollack IF, Okada H. Epidermal growth factor receptor-transfected bone marrow stromal cells exhibit enhanced migratory response and therapeutic potential against murine brain tumors. Cancer Gene Ther. 2005;12(9):757–768. doi: 10.1038/sj.cgt.7700827. [DOI] [PubMed] [Google Scholar]
- Satomura K, Derubeis AR, Fedarko NS, Ibaraki-O'Connor K, Kuznetsov SA, Rowe DW, Young MF, Gehron Robey P. Receptor tyrosine kinase expression in human bone marrow stromal cells. J Cell Physiol. 1998;177(3):426–438. doi: 10.1002/(SICI)1097-4652(199812)177:3<426::AID-JCP6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Schmidt MH, Furnari FB, Cavenee WK, Bogler O. Epidermal growth factor receptor signaling intensity determines intracellular protein interactions, ubiquitination, and internalization. Proc Natl Acad Sci U S A. 2003;100(11):6505–6510. doi: 10.1073/pnas.1031790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20(6):530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- Seth D, Shaw K, Jazayeri J, Leedman PJ. Complex post-transcriptional regulation of EGF-receptor expression by EGF and TGF-alpha in human prostate cancer cells. Br J Cancer. 1999;80(5–6):657–669. doi: 10.1038/sj.bjc.6690407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibilia M, Wagner B, Hoebertz A, Elliott C, Marino S, Jochum W, Wagner EF. Mice humanised for the EGF receptor display hypomorphic phenotypes in skin, bone and heart. Development. 2003;130(19):4515–4525. doi: 10.1242/dev.00664. [DOI] [PubMed] [Google Scholar]
- Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15(2):209–219. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci U S A. 2005;102(8):2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamama K, Fan VH, Griffith LG, Blair HC, Wells A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24(3):686–695. doi: 10.1634/stemcells.2005-0176. [DOI] [PubMed] [Google Scholar]
- Taub N, Teis D, Ebner HL, Hess MW, Huber LA. Late endosomal traffic of the epidermal growth factor receptor ensures spatial and temporal fidelity of mitogen-activated protein kinase signaling. Mol Biol Cell. 2007;18(12):4698–4710. doi: 10.1091/mbc.E07-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274(5295):2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- Wang Y, Pennock S, Chen X, Wang Z. Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival. Mol Cell Biol. 2002;22(20):7279–7290. doi: 10.1128/MCB.22.20.7279-7290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watton SJ, Downward J. Akt/PKB localisation and 3' phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr Biol. 1999;9(8):433–436. doi: 10.1016/s0960-9822(99)80192-4. [DOI] [PubMed] [Google Scholar]
- Wells A, Welsh JB, Lazar CS, Wiley HS, Gill GN, Rosenfeld MG. Ligand-induced transformation by a noninternalizing epidermal growth factor receptor. Science. 1990;247(4945):962–964. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]
- Whitty A. Cooperativity and biological complexity. Nat Chem Biol. 2008;4(8):435–439. doi: 10.1038/nchembio0808-435. [DOI] [PubMed] [Google Scholar]
- Wiley HS. Trafficking of the ErbB receptors and its influence on signaling. Exp Cell Res. 2003;284(1):78–88. doi: 10.1016/s0014-4827(03)00002-8. [DOI] [PubMed] [Google Scholar]
- Wu CJ, Chen Z, Ullrich A, Greene MI, O'Rourke DM. Inhibition of EGFR-mediated phosphoinositide-3-OH kinase (PI3-K) signaling and glioblastoma phenotype by signal-regulatory proteins (SIRPs) Oncogene. 2000;19(35):3999–4010. doi: 10.1038/sj.onc.1203748. [DOI] [PubMed] [Google Scholar]
- Xia Z, Locklin RM, Triffitt JT. Fates and osteogenic differentiation potential of human mesenchymal stem cells in immunocompromised mice. Eur J Cell Biol. 2008;87(6):353–364. doi: 10.1016/j.ejcb.2008.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.