Abstract
The progression of ovarian cancer, from cell transformation through invasion of normal tissue, relies on communication between tumor cells and their adjacent stromal microenvironment. Through a natural selection process, an autocrine-paracrine communication loop establishes reciprocal reinforcement of growth and migration signals. Thus, the cancer-activated stromal response is similar to an off-switch-defective form of the normal, universal response needed to survive insult or injury. It is becoming clearer within the cancer literature base that tumor stroma plays a bimodal role in cancer development: it impedes neoplastic growth in normal tissue while encouraging migration and tumor growth in a co-opted desmoplastic response during tumor progression. In this review, we discuss this reciprocal influence that ovarian cancer epithelial cells may have on ovarian stromal cell-reactive phenotype, stromal cell behavior, disrupted signaling networks, and tumor suppressor status in the stroma, within the context of cancer fibroblast studies from alternate cancer tissue settings. We focus on the exchange of secreted factors, in particular interleukin 1β and SDF-1α, between activated fibroblasts and cancer cells as a key area for future investigation and therapeutic development. A better understanding of the bidirectional reliance of early epithelial cancer cells on activated stromal cells could lead to the identification of novel diagnostic stromal markers and targets for therapy.
Introduction
Epithelial ovarian cancer is the most lethal gynecologic malignancy among women in the United States and other industrialized nations, resulting in approximately 15,000 deaths and nearly 22,000 new cases in 2009 [1]. Survival rates approaching 90% are achievable among ovarian cancer patients diagnosed at an early stage. Nonetheless, early detection is challenging because nonspecific symptoms of early ovarian lesions go unnoticed until the patient presents with an abdominal distension due to late-stage tumor growth and accumulation of ascites fluid. Despite extensive surgical debulking followed by an aggressive platinum/taxane-based chemotherapy and radiotherapy regimen, recurrence and dissemination occurs frequently. Late-stage high-grade ovarian cancer metastasizes rapidly to the omentum and surrounding abdominal organ surfaces [2]. Several studies have noted that the defined histological categories of ovarian carcinoma tend to associate with particular underlying molecular mechanisms, including genetic mutations (e.g. KRAS, p53, BRCA1/2), allelic amplification, and carcinogens [3–6]. Thus, specific genetic mutations among diverse histomorphologic ovarian cancer subtypes allow pathologists to identify and diagnose tumor specimens by microscopy [7,8]. However, the origin and causes of ovarian carcinoma, particularly the cooperative interaction with an activated stromal tumor microenvironment, remain to be elucidated.
Ovarian tumorigenesis is initiated by the malignant transformation of epithelial cells derived from the pelvic müllerian duct, likely originating either from the continuous outer ovarian surface epithelial cell layer or from fallopian tube epithelial cells [9]. Of note, there is accumulating evidence implicating fallopian tube epithelial cells, especially those derived from the fimbriated ends, as the likely origin for high-grade serous carcinoma [10]. In contrast to the dedifferentiation observed after transformation in most epithelial cancers, ovarian cancer progression results in distinct histological subtypes (or histotypes) that are reminiscent of the differentiated morphology of the surrounding gynecological anatomy: high-grade and low-grade serous, endometrioid, clear cell, mucinous carcinoma, and tumors of low malignant potential [11]. These distinct histotypes allow clinicians to monitor for increased levels of serum markers for early detection of ovarian cancer, such as CA125 [11]. Monitoring secreted communication signals sent by ovarian epithelial cancer cells is a mainstay of ovarian cancer patient follow-up; however, these signals are only a part of the epithelial-stroma communications network.
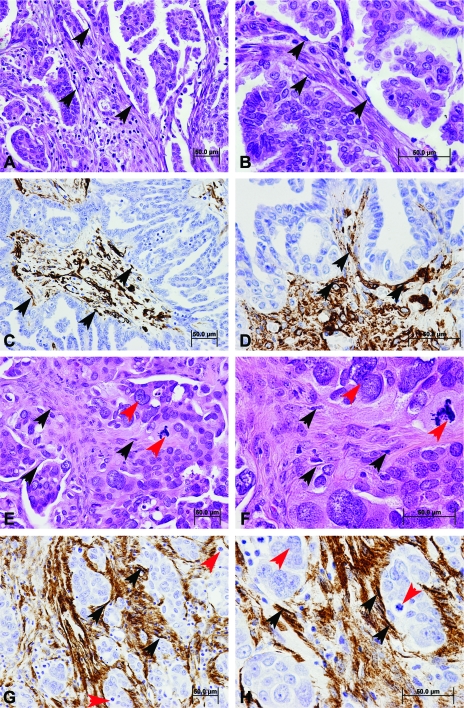
The histomorphologic complexion of ovarian cancer varies according to the histotype and grade of the developing carcinoma. Low-grade serous carcinoma displays a consistent, differentiated papillary growth architecture, with a key feature being uniform nuclei and numerous psammoma bodies [12] and a comparatively high proportional contribution of cancer stromal cells and expansive tumor microenvironment (Figure 1, A and B, fibroblasts indicated by black arrows), highlighted by the reactive stroma marker α-smooth muscle actin (α-SMA) (Figure 1, C and D). Low-grade and high-grade serous carcinomas likely initiate by means of independent genetic pathways, as evidenced by molecular analysis, clinical appearance, and morphologic features [13]. High-grade serous carcinoma is characterized by a distinctive growth pattern of highly stratified and fenestrated epithelium, a very high mitotic rate, a threefold variation in nuclear size and pleomorphism [12], and a relatively low proportion of cancer-associated stromal cells (Figure 1, E–H, fibroblasts highlighted by black arrows and positive staining for α-SMA immunohistochemical staining; nuclear atypia highlighted by red arrows). Endometrioid carcinoma, so named for a resemblance to the cribriform morphology of the endometrium, exhibits clusters of tube-shaped glandular lumens that are lined by stratified, potentially squamous (in the case of morules), epithelium lacking mucinous deposits [12], and displays a moderate to a low proportion of cancer-associated stroma. Mucinous carcinoma is a heterogeneous designation, ranging in morphology from expansile glandular to multilobular, pilus-like, papillary, to a solid infiltrative epithelial phenotype, with a key unifying feature being extensive mucus-like deposition from goblet cells [14], and a relatively large proportional contribution of stromal cells. Clear cell carcinoma displays characteristic microscopic features that include multiple complex papillae with densely hyaline basal lamina cores and occasional hyaline bodies, with epithelial cells displaying enlarged, clear cytosolic bodies [12], and a relatively dense extracellular matrix (ECM) that lacks the high fibroblastic concentration of other histotypes. Overall, the relative proportion of stroma in advanced ovarian cancer ranges from 7% to 83% of tissue composition, with a median of 50% contribution, and this estimation does not vary significantly according to histotype, International Federation of Gynecology and Obstetrics stage, or grade [15].
Figure 1.
Histomorphology and interaction of ovarian cancer epithelial cells and CAFs. The morphologic characteristics of low-grade serous ovarian carcinoma include a papillary growth architecture and uniform nuclei (A and B; hematoxylin and eosin stain), with a comparatively high proportional contribution of cancer stromal cells identified by immunohistochemistry for α-SMA (C and D), a marker that highlights activated reactive CAFs (indicated by black arrows). The altered histomorphology of high-grade serous carcinoma is characterized by a distinctive growth pattern of stratified epithelium with high mitotic rate and a threefold variation in nuclear size and pleomorphism (E and F; hematoxylin and eosin stain). Nuclear atypia are highlighted by red arrows. High-grade serous carcinoma displays a high proportion of CAFs (G and H) highlighted here by immunohistochemistry for α-SMA (black arrows). Scale bars, 50 µm. Magnification, x200 (A, C, E, and G); x400 (B, D, F, and H).
The progression of ovarian cancer tumor cell populations, from cell transformation through invasion of normal tissue and eventual metastasis, likely relies on a critical secretory reciprocal communication with their adjacent stromal microenvironment. A component that is vital to our understanding is how synergistic communication signals sent by cancer epithelial cells are interpreted and translated into a non-cell-autonomous secretion- and growth-activating response in cancer-associated fibroblasts (CAFs). Deciphering the role of this paracrine and reciprocal cancer-stromal communication network in the early initiatory stages of ovarian cancer is fundamental to understanding abnormal acute and chronic fibroblast activation. Although multiple cell types are present in the stromal ECM compartment of the various ovarian cancer histotypes, CAFs have been shown to play a critical role in determining overall clinical outcome of cancers throughout the body. Gene expression profiling of CAFs in multiple cancers has identified genes that are differentially expressed in comparison to normal fibroblasts, and these genes may shed light on malignant epithelium-activated fibroblast secretory interaction and cooperative cellular behavior. Further, molecular indicators of an activated ovarian CAF state may enable the development of non-cancer cell markers for early-stage detection of the extent of aggressive growth promotion and may thus yield additional candidates for therapeutic intervention. Therefore, in this review, we focus on putative intracellular and intercellular signaling activators and pathways in CAFs, including ovarian, that affect communication with cancer cells and their normal neighbors. First, we give a brief perspective on the role of the stromal microenvironment in cancer. Then, we discuss the influence that ovarian cancer epithelial cells have on stromal cell behavior, disrupted pathway signaling in CAFs of various cancer types that may be involved in ovarian CAFs, and tumor suppressor status in CAFs of ovarian, breast, and prostate carcinomas. Finally, we summarize the status of identifying ovarian CAF contribution to ovarian carcinoma and discuss future hypotheses. Overall, our focus is on the exchange of secreted factors between activated fibroblasts and cancer cells as a key area for future investigation and therapeutic development in ovarian cancer treatment.
A Historical Perspective: Selective Activation in the Tumor Stromal Microenvironment
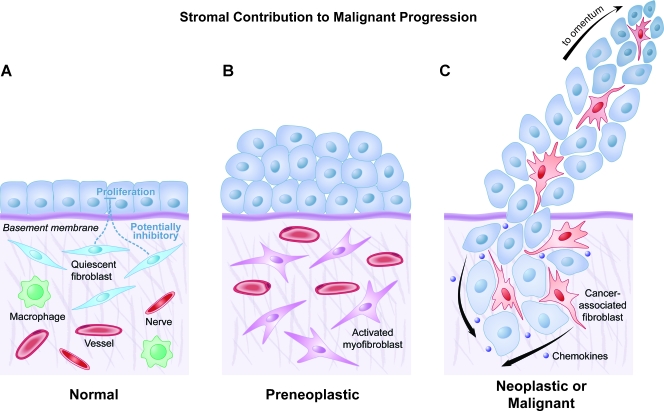
Although some cancers develop stromal independence through epithelial-mesenchymal transition (EMT) before metastasis, successful tumorigenic initiation likely requires a coevolutionary stimulus of the stromal microenvironment whereby cancer-associated stromal cells receive acute, prolonged activation signals to form a reactive, secretory phenotype [16–18]. Activated stromal response in cancer was first described in 1979, when Seemayer et al. [19] observed that myofibroblasts, or activated fibroblasts, played a critical role in the desmoplastic response to neoplastic mammary carcinoma. In 1983, an initial distinction was made between the migratory behavior of CAFs and that of normal fibroblasts, when Mensing et al. [20] noted that dermal tumor-associated fibroblasts displayed a more differential chemotactic response to fibronectin than did normal fibroblasts. Shortly thereafter, Strauli et al. [21] and Haemmerli et al. [22] used the rabbit V2 carcinoma model and observed that V2 cancer cell invasion into rabbit mesentery was mediated, in part, by a multiplication of co-opted connective tissue cells and enhanced ECM deposition, characterized by a transdifferentiation of recruited fibrocytes (circulating fibroblast progenitor cells) into myofibroblasts. In the late 1980s, studies showed that interactions between lung tumor cells and lung tumor-associated fibroblasts are likely to play a critical role in ECM degradation, as well as in the selection of tumor cells that eventually metastasize [23], and that activated immune cells and tumor cells increased the ECM-degrading capacity of normal lung fibroblasts [24]. In 1989, Carnemolla et al. [25] observed an oncogenesis-specific secretion from activated stroma: the alternatively spliced fibronectin isoform B was expressed only by transformed lung fibroblasts. In a seminal publication, Nagy et al. [26] noted that generation of generic fibrinolytic tumor stroma is perpetual and critical to successful tumorigenesis and, therefore, cancer resembles a wound that is unable to heal. Thus, stromal fibroblasts possess a bimodal functional role in tissue biology. Normal stromal fibroblasts can impede the abnormal growth of preneoplastic epithelial cells in a variety of normal tissues (Figure 2). However, stromal fibroblasts can also be protumorigenic, responding in a co-opted desmoplastic response where they encourage both migration and invasion of epithelial cancer cells [27–31] (Figure 2, last panel).
Figure 2.
The contribution of CAFs to malignant progression of ovarian cancer. (A) Normal ovarian tissue is composed of ovarian or fallopian tubal epithelial cells and an extracellular microenvironment consisting of quiescent fibroblasts and other supportive mesenchymal cell types that collectively inhibit inappropriate or preneoplastic epithelial proliferation. (B) Transition from normal to preneoplastic, or neoplastic, ovarian or fallopian tubal epithelial cells involves reciprocal secretory communication with activated myofibroblasts, whether tissue resident or recruited from circulation. (C) Progression to malignant ovarian cancer relies, at least initially, on a secretory co-opting of ovarian CAFs through exchange of intercellular secreted factors (e.g., chemokines like IL-1β and GRO-α) with CAFs to facilitate dissemination to theomentum. CAFs may directly facilitate this metastatic movement, although it is likely that EMT of ovarian or fallopian tumor cells provides a reservoir of secretory, ECM-digesting, migratory CAF-like cells.
These seminal publications have led to the viewpoint that aggressive malignancies selectively perpetuate within stromal microenvironments that are richly populated with activated and reactive cells (CAFs, myofibroblasts, angiogenic precursors, immune cells, and others), which can be collectively reprogrammed to support overall tumor growth [32]. A functional, reciprocal interaction is now currently acknowledged between tumor cells and the tumor microenvironment, wherein tumor stromal cells very likely facilitate a critical role during both tumor cell initiation and growth progression [33]. Thus, epithelial cancers are no longer considered as isolated clusters of transformed epithelial cells that invade passive, uninvolved neighboring regions, namely, stroma. An alternate perspective has acquired increased stature within the past few decades: a tumor-promoting stromal microenvironment selectively facilitates, through reciprocal juxtacrine communication, the proliferation and invasion of epithelial cancer cells. Therefore, cancer cells that activate and maintain a protumorigenic, anti-immunogenic niche would retain a selective growth advantage [32]. This natural selection process is strikingly similar both to Dvorak's wound healing analogy and to some processes in embryogenesis [34], where an autocrine-paracrine communication loop establishes reciprocal reinforcement of growth and migration signals, thereby limiting healing or development (Figure 2). This perspective, which has been pioneered by Dvorak [36], Rowley [18], and others, implies that the cancer-activated reactive stromal response is an off-switch-defective form of the normal, universal biologic response needed to survive tissue insult or injury [35].
Cancer Epithelial Cell Activation of the Reactive Stromal Phenotype in Ovarian and Other Cancers
Activation of fibroblasts to a myofibroblast phenotype through reciprocal paracrine interaction with neoplastic and transformed epithelial cells has been observed in several in vitro studies using ovarian cells. For example, medium conditioned by SKOV3 cells, an established, malignant ovarian cancer cell line, induced transdifferentiation of normal ovarian fibroblast to a myofibroblast phenotype characterized by elevated reactive stroma marker α-smooth muscle actin (α-SMA) [37]. Further, the authors identified chloride intracellular channel-like 4 (CLIC4) and various reactive oxygen species as potential SKOV3-secreted factors that could yield the same ovarian myofibroblast stromal phenotype in vitro [37]. Analogously, breast carcinoma progression was associated with increased expression of CLIC4 in breast cancer-associated stromal cells, relative to a reduced expression in normal breast epithelial cells [38]. Moreover, CLIC4 induced up-regulation of α-SMA in breast cancer CAFs in vitro, and CLIC4-overexpressing breast myofibroblasts stimulated xenograft tumorigenesis [38]. A recent study showed that normal human fibroblasts selectively inhibited proliferation of prostate and lung cancer-derived tumor cells in a direct cell contact-dependent manner [39]. Similarly, it was shown that normal human breast-associated fibroblast inhibition of tumorigenic breast cancer cells was significantly enhanced in direct coculture compared with indirect coculture, relative to breast CAF stimulation of breast tumor cell proliferation [40]. Ovarian stromal cell type is critical in determining whether metastasis occurs because it was observed that fibroblasts derived from the omentum, the richly vascularized fatty subperitoneal layer draping the ovaries, augmented ovarian cancer cell adhesion and invasive behavior, whereas omentum-derived mesothelial cells functionally inhibited ovarian cancer cell aggressiveness [41]. Very recently, a study used the reactive stroma markers α-SMA and fibroblast activation protein (FAP) to correlate the presence of ovarian CAFs to ovarian cancer patient clinical outcome, finding a significant association with the occurrence of lymph node and omentum metastases, as well as elevated lymphatic vessel density and microvessel density [42]. Moreover, the authors showed that CAFs isolated from ovarian cancer tissue induced ovarian cancer cell invasion and migration in vitro [42]. These data demonstrate that ovarian CAFs are directly related to ovarian cancer progression and metastasis. However, more work must be done in identifying specific ovarian cancer epithelial cell-secreted factors that directly facilitate ovarian fibroblast activation and protumorigenic and prodissemination secretory activation.
Activated CAFs, across most cancers, secrete a wide variety of growth factors, chemokines, collagens, and matrix-modifying enzymes, collectively supplying a communication network and altered three-dimensional ECM scaffold that governs the proliferation of cancer cells, tumor invasion, and metastasis across tissue types [43]. Therefore, it is of interest whether the proportion of tumor stroma cells in the tumor microenvironment within cancer reflects a mutual growth pattern. Interestingly, the impact of the proportional representation of the stromal compartment on tumor invasiveness and histological dedifferentiation has been studied in prostate disease [44], colorectal cancer [45], breast carcinoma [46], and pulmonary carcinoma [47]. Specifically, in ovarian cancer, component aspects of the tumor stromal compartment have been described as prognostic patient severity indicators, including blood vessel architecture [48], extent and type of inflammatory cells [49], and ECM-interacting, ovarian fibroblast-secreted factors, including the glycosaminoglycan hyaluronic acid [50] and a hyaluronan-partner glycoprotein versican [51]. In another study, conditioned medium from ovarian clear cell carcinoma (ES-2) cells included paracrine-acting cytokine signals, which upregulated ovarian fibroblast transcription of urokinase-type plasminogen activator, a key enzyme in cancer cell invasion and metastasis [52]. Moreover, parallel epithelial and stromal expression patterns were observed in tissue samples from patients with aggressive ovarian cancer for the paracrine-secreted markers cyclooxygenase 1 (COX-1), COX-2, microsomal prostaglandin E synthase-1 (mPGES-1), and EP1-2, factors that collectively promote angiogenesis and proliferation while simultaneously discouraging apoptosis [53]. Moreover, the relative stromal abundance and composition of the ovarian tumor microenvironment itself was found to have an independent, statistically significant prognostic value, particularly in late-stage epithelial ovarian cancer patients [15]. These data demonstrate that women with a high proportion of ovarian tumor stroma display decreased overall survival. Interestingly, this study did not identify a significant relationship between the proportion of ovarian tumor stroma and ovarian tumor histotype [15]. This suggests that the proportion or percentage of ovarian tumor stroma may functionally activate a universal ovarian stromal communication mechanism that is independent of the gynecological tissue or cell type of origin. The authors theorized that decreased survival of ovarian cancer patients with a high proportion of cancer stroma may potentially reflect an insufficient penetration, or altered resistance mechanism, to drug treatment based on cell adhesion [15]. Further, another study focusing on ovarian carcinosarcomas identified that patients with late-stage disease and a high percentage of ovarian cancer stroma displayed reduced survival and poor clinical outcome [54]. Therefore, it is becoming clear that ovarian cancer cell-secreted factors, within the context of altered interaction from cell-cell communication, directly promote the activation of stromal cell secretion, migration, and function (Figure 3A).
Figure 3.
Model of critical pathways and mechanisms in the activation of ovarian fibroblasts by secretory interaction with neoplastic ovarian cancer cells. (A) Secreted activation signals, such as IL-1β, IL-6, and IL-8 or GRO-α, from neoplastic ovarian or fallopian tubal cancer epithelial cells stimulate a stromal phenotypic shift from quiescent to activated ovarian or omental fibroblasts. Activated CAFs are proliferative, are migratory, and secrete a variety of ECM-restructuring factors (e.g., FAP-1α, urokinase-type plasminogen activator), soluble cancer-activating chemokines (e.g., SDF-1α), and cell surface proteins (e.g., CLIC4). Cancer cell-mediated fibroblast activation selectively promotes tumor angiogenesis, adhesion, migration, and invasion while reducing apoptotic inhibition. (B) Activation of chemokine receptors on ovarian CAFs, including IL-1 receptor 1 (IL-1R1), CD126/CD130, and CXCR2, by ovarian cancer cells likely activates intracellular signaling cascade mediators in ovarian CAFs including IL-1R-associated kinases (IRAK) 1, 2, and 4; tumor necrosis factor α receptor-associated factor 6 (TRAF-6); IL-1-induced activation of c-Jun N-terminal kinase and NF-γB; as well as the c-Jun N-terminal kinase/Janus kinase (JAK)/STAT family members. In ovarian CAFs, these signaling mediators may activate AKT/extracellular signal-regulated kinase 1/2 (ERK1/2)/RBPJγ-mediated transcriptional up-regulation of ovarian CAF-secreted factors that impact epithelial ovarian cancer tumor cell aggressiveness, including the glycoprotein tenascin-C, protease FAP-1α, and myriad interleukins, especially IL-8. In parallel, downstream signals in ovarian CAFs from chemokine-activated receptors facilitate transcriptional or translational inactivation of tumor suppressors, like p53, by yet uncharacterized mechanisms. Once tumor suppressors like p53, PTEN, or Rb are inhibited/inactivated, two intracellular pathways are initiated that are not understood at all: 1) increased cellular production of chemokines, including IL-1β, IL-8, IL-6, and SDF-1α, and 2) chemokine receptors, like IL-1R1, are upregulated. Collectively, ovarian fibroblast activation leads to paracrine ovarian cancer cell stimulation and autocrine stimulation, co-opting a desmoplastic-like stromal response for tumor cell initiation, survival, and inappropriate growth.
Critical Factors Mediating the Activated Response of CAFs from Diverse Cancers, Including Ovarian Carcinoma
Several recent reviews have focused on the therapeutic potential of targeting the activated cancer-associated stromal compartment [55,56]. Some putative CAF markers that have been correlated with cancer incidence or progression in other cancer types are also likely to play a critical role in ovarian tumorigenesis (Figure 3B). It has been shown that an oncogenic cancer epithelium results in a tumor microenvironment replete with inflammatory mediators, growth factors, matrix remodeling enzymes, and angiogenic factors [31,57,58]. The net result of this milieu leads to a recruitment of associated, activated fibroblasts, ostensibly due to reciprocal interaction through inflammatory factors.
Chemokines and Cytokines
Generalized inflammation of the female peritoneum, typically associated with ovarian micrometastases and stromal invasion, has been tied to elevated expression of IL-8 [59,60]. Moreover, up-regulation of tumor-regulated IL-6, IL-8, and IL-1β is associated with the inflammatory network, promoting tumorigenesis, angiogenesis, and metastasis in various cancers, including those of the breast, prostate, and pancreas [61]. Our laboratory has identified several stromal-activating chemokines, including IL-6, IL-8, and growth-regulated oncogene a (GRO-α), which are significantly elevated in transformed, neoplastic ovarian surface epithelial cells [62]. Moreover, we have demonstrated that GRO-α-overexpressing ovarian fibroblasts with inhibited p53 significantly increased ovarian cancer epithelial proliferation and tumorigenic growth in a mouse xenograft model [63]. Furthermore, our laboratory demonstrated that IL-1β is significantly elevated in Ras-transformed ovarian carcinoma cells [62], and we have evidence that ovarian cancer cell-secreted IL-1β attenuates p53 in neighboring ovarian fibroblasts (unpublished observations). As a comprehensive signaling axis, the IL-1β/IL-1 receptor 1 (IL-1R1) activates a defined set of downstream signaling effectors and binding partners [64]. IL-1R1 axis effectors include several isoforms of the IL-1 receptor-associated kinases (IRAK proteins), as well as the protein-protein interaction effector tumor necrosis factor-α receptor-associated factor 6 [65–67]. Nuclear localization of IRAK-1 has been correlated with enhanced malignancy in lung cancer [68] and prostate cancer [69] and has been shown to bind directly to the IγBα promoter and enhance binding of nuclear factor-γB (NF-γB) p65 subunit in skin fibroblasts [70]. In breast cancer, CAFs were found to initiate and mediate tumorigenesis through a macrophage-recruitment inflammatory signature that was highly dependent on breast CAF NF-γB signaling [71]. Thus, as pathway effectors activated by the IL-1β/IL-1R1 signaling axis function both in the nucleus and cytoplasm of fibroblasts, these IL-1R1 pathway mediators may perhaps be responsible for eliciting IL-1β-mediated transcriptional or translational attenuation of tumor suppressors in ovarian CAFs (Figure 2B).
Stromal-derived factor-1α (SDF-1α), also called chemokine (CXC motif ) ligand 12 (CXCL12), is a glutamic acid-leucine-arginine motif-negative chemokine lacking chemotactic influence on immune cells [71]. SDF-1α signals primarily through the CXC chemokine receptor 4 (CXCR4) [58], and the SDF-1α/CXCR4 signaling axis is deregulated in multiple malignancies, playing a critical role in promoting cancer cell migration and metastasis of many tumor types, including leukemia [72] where there is a preponderance of SDF-1α publications, ovarian and cervical cancer [73–77], prostate [78], breast [79], liver [80,81], colorectal [82], pancreatic [83], lung [84], and multiple myeloma [85]. Moreover, a few recent articles address the potential for CAF expression of SDF-1α contributing to cancer progression [86–89]. Several reports have discussed the involvement of the SDF-1α/CXCR4 signaling axis in epithelial ovarian cancer cell lines in vitro, or using tissue immunohistochemistry quantitation to determine predictive ability [77,90–94]. One recent publication showed elevated peritoneal dissemination in vivo after intraperitoneal nude mouse injection of 5 million ES-2 cells, a clear cell ovarian cancer line, with daily injection of systemic, exogenous high-concentration SDF-1α [95]. Moreover, a recent review of the role of the SDF-1α/CXCR4 signaling axis in ovarian cancer cell stated that SDF-1α overexpression coincides with ovarian cancer cell proliferation and metastasis, and identified several recently developed therapies that target either SDF-1α or CXCR4 [96]. CXCR4 is expressed not only in cancer cells; it can also be upregulated in fibroblasts through PDGF, IL-1β, and HIF-1α and can be attenuated in fibroblasts by activation of phosphatidylinositol 3-kinase and mammalian target of rapamycin [97,98]. Both exogenous IL-1α and oral squamous cell carcinoma (OSCC)-conditioned medium stimulated SDF-1α expression in CAFs, whereas CAF-conditioned medium stimulated OSCC cell invasion in vitro [99]. However, when exogenous SDF-1α was applied to OSCC cells in the absence of CAFs, the pattern of invasion in culture was different from that with CAF-conditioned medium, implying a complex multifactor intracellular communication [99]. Relating this chemokine pathway to tumor suppressor function, p53 can repress the expression of SDF-1α in embryonic lung fibroblasts [100], likely resulting in a microenvironment less conducive to tumor cell migration and survival. p53 activation directly attenuated invasion and migration of breast cancer cells through repressed SDF-1α gene transcription [100]. Thus, loss of p53 expression in CAFs may contribute to inappropriate activation of the CXCR2/4 signaling axis in tumorigenesis (Figure 2B). However, the functional role of the SDF-1α/CXCR4 signaling axis in ovarian CAFs, and whether this directly impacts ovarian epithelial tumorigenesis, migration, and metastasis, has not been completely addressed and warrants further investigation.
Activation-Associated Fibroblast Factors
Fibroblast activation protein-1α (FAP-1α), a cell surface protease with dipeptidyl peptidase and endopeptidase activity, is expressed by stromal cells in several different cancers [57] and has been used as a clinical therapeutic target by multiple immunoconjugate clinical studies in multiple cancer types [101–104]. FAP-1α is induced in ovarian fibroblasts by exposure to conditioned medium from a metastatic ovarian cancer cell line, HO-8910PM, or to exogenous factors TGF-β1 and IL-1β [105]. Once elevated, FAP-1α promotes proliferation, adhesion, and migration of metastatic ovarian cancer cells [105]. Similarly, a novel category of activated stromal response was identified in OSCC, termed nemosis, that correlates stromal expression of progrowth and proinflammation factors (α-SMA, FAP-1α, and fibroblast-specific protein-1α [FSP-1α]) with in vitro tumor cell function [106]. Thus, FAP-1α presents an interesting opportunity for further study in ovarian tumor stromal biology.
Tenascin-C is a secreted glycoprotein that is elevated in the stromal microenvironment of epithelial cancers, is likely to act by decreasing the formation of cell adhesion complexes thereby promoting proliferation and migration, and is considered a potential oncogene (reviewed in Hsia and Schwarzbauer [107] and Orend [108]). In a powerful multivariate index assay using serum from patients with ovarian cancer and controls, tenascin-C was included as 1 of 11 analytes (selected from 104 candidates) that could distinguish benign from malignant ovarian conditions with sensitivity and specificity of up to 90% [109]. Similarly, tenascin-C was identified as one of four distinguishing factors in an immunohistochemistry-based survival tree model of intrahepatic cholangiocarcinoma [110]. Furthermore, our data show that intense stromal expression of tenascin-C correlates with shorter survival duration in patients with all ovarian cancer histotypes, and specifically in those with high-grade serous tumors (unpublished observations).
Origin of the Ovarian Cancer Tumor Microenvironment
Although the origin of stroma in ovarian cancer is largely unknown, recent evidence from mouse xenograft models of multiple human cancer cell types (including ovarian) points to activation of tissue-resident fibroblasts, recruitment of hematopoietic precursors or mesenchymal stem cells (MSCs) [111,112], and promotion of senescent fibroblasts [63,113]. Using dynamic magnetic resonance imaging, it was observed that recruited fibroblasts formed a functional tumor neovasculature at the rim of ovarian cancer nodules, thereby identifying an activated ovarian fibroblast response with potential therapeutic implications [114]. In ovarian cancer, it was recently reported that ovarian cancer-derived lysophosphatidic acid stimulates differentiation of human adipose tissue-derived MSCs (hADMSC) to CAFs, elevating the expression of SDF-1α through a TGF-β1-mediated autocrine stimulation of Smad2 [115]. Moreover, the authors demonstrated that treating hADMSC with ovarian cancer patient ascites fluid, or with conditioned medium from ovarian cancer cells, induced expression of the reactive stroma marker α-SMA and phosphorylation of Smad2 and that this effect could be abrogated by pretreating with an lysophosphatidic acid receptor antagonist [115]. Also in ovarian cancer, the pro-inflammatory peptide, LL-37, a C-terminal peptide fragment of human cationic antimicrobial protein 18, was shown to be overexpressed in ovarian cancer tissue and to directly stimulate ovarian cancer cell migration in vitro [116]. This same group recently demonstrated that in vivo neutralization of LL-37 significantly inhibited xenograft tumor growth overall, likely through reduced engraftment of MSCs into ovarian tumor xenografts and disruption in the establishment of a tumor fibrovascular network [117]. Thus, LL-37 may facilitate ovarian tumor progression through recruitment of ovarian CAF progenitor cells that express proangiogenic factors. Pertaining to an alternate origin for ovarian cancer tumor microenvironment, senescent ovarian fibroblasts significantly increased in vitro migration of cMyc-mediated early neoplastic ovarian surface epithelial cells compared with coculturing with presenescent ovarian fibroblasts [118]. Further, this study demonstrated that senescent ovarian fibroblasts stimulated early neoplastic ovarian epithelial cell anchorage-independent colony growth, as well as increased proliferation and induced nuclear atypia in a three-dimensional spheroid in vitro model [118]. Thus, the etiology underlying the development of epithelial ovarian neoplasia may depend on the accumulation of senescent (or loss of presenescent) ovarian fibroblasts. Therefore, further work in identifying the likely multifactorial source of ovarian carcinoma-associated stromal cells has therapeutic implications in recognizing and targeting stromal-mediated tumor activation in early-stage ovarian cancer patients.
Tumor Suppressor Status in CAFs
Of the many tumor suppressors characterized, p53 is the only one for which inactivation has been well substantiated in a variety of epithelial cancers and has been shown in a subset of cancer stromal cells [31,119]. The existence of genetic alterations in CAFs is controversial. Studies have identified distinct genetic alterations in breast and squamous CAFs, ranging from mutation of critical tumor-suppressor genes like phosphatase and tensin homolog (PTEN) and TP53, to loss of heterozygosity, or alterations in allelic copy number [120–125] (Figure 3B). Conversely, other studies have not identified similar changes in breast or ovarian CAFs, and no agreement on a unifying genetic alteration in all CAFs exists to date [126–128]. Several reports focusing on breast and prostate CAFs have identified epigenetic mechanisms, such as promoter methylation, that correlate with poor clinical factors [129–131], which need to be addressed in ovarian CAF biology. p53 function is intriguing because it activates non-cell-autonomous functions that likely contribute to tumor suppression through communication with normal fibroblasts. For example, p53-dependent secreted factors such as PTGF, a transforming growth factor-β (TGF-β) family member [132], IGF-BP3 [133], and other factors have been shown to facilitate stromal cell-mediated inhibition of prostate cancer cell growth [134]. Human breast cancer tumor cell injections using p53-null mice resulted in markedly increased tumor growth rates, relative to growth rates after injection into normal, p53-intact control mice [121], indicating that an activated host stroma with incapacitated p53 is tumor promoting. Indeed, p53 inactivation mutations were reported to occur in the fibroblastic stroma of both colon and breast cancers [122,124,135]. Moreover, TP53 mutational status may be a predictor of CAF-mediated resistance to cytotoxic chemotherapeutics, although this response is highly variable across different tumor types [136,137]. Furthermore, breast carcinoma CAFs were recently shown to possess a nonmutated, but functionally deficient, form of p53 [138,139]. The question of whether genetic aberrations in CAFs could be the basis of the cancer-promoting phenotypes of ovarian CAFs remains to be resolved. We believe that irrespective of the tissue source, how close the stromal cell extraction/microcapture site is to the tumor determines whether one observes the genotypic mutational status of true CAFs, or instead normal fibroblasts.
Although there are very few publications addressing p53 regulation of secreted and membrane-bound factors in fibroblasts or CAFs, it is assumed that publications describing p53-mediated mechanisms in epithelial cells will likely translate into similar mechanisms in fibroblast p53 regulation. Elevated expression of both wild-type p53 and wild-type Rb in HeLa cells repressed promoter constructs for IL-6 and c-Fos [140]. Wild-type p53 mediated repression of the chemokine receptor 4 in breast cancer cells, which was negated by the expression of the p53 V143A dominant negative mutant, and cancer-specific p53 phospho-mutants R175H or R280K [141]. p53-mediated repression of EMMPRIN, a transmembrane glycoprotein that promotes survival, invasion, and metastasis through induced MMP expression, led to a decrease in MMP-9 in prostate cancer cells [142]. Specifically addressing p53 mutation in CAFs, colon CAFs overexpressing an alternate human p53 isoform, Δ133p53, displayed repressed miR-34a (a p53-activated microRNA that helps to facilitate senescence) and extended cellular replication in vitro [143]. Therefore, the cause and impact of p53 inhibition in ovarian CAFs, and whether this induces a reciprocal, intercellular communication with neoplastic ovarian epithelial cells to promote tumorigenesis and metastasis, have yet to be characterized.
Anecdotal evidence suggests that, in the absence of focal adhesion kinase (FAK), expression of Pyk2, an inhibitor of p53, prevented cisplatin-mediated apoptosis in human foreskin fibroblasts [144]. Further, a recent preclinical mouse xenograft study using an ATP-competitive reversible inhibitor of FAK and FAK2 (Pyk2) showed a potent inhibition of in vivo metastatic prostate cancer growth [145]. In another mouse model of prostate cancer, increased epithelial tumorigenesis led to a noticeable selection for p53-inhibited stromal cells [146], suggesting that tumor cell behavior may directly control the tumor suppressor status of stromal cells. Furthermore, it was shown recently that immortalized, nontumorigenic lung epithelial cells expressing mutant H-Ras and an siRNA against p53 could reduce p53 levels in human lung cancer CAFs more than in normal lung fibroblasts [147]. These data suggest a CAF-specific susceptibility to secreted factors from preneoplastic cells compared with normal fibroblasts, facilitating inhibition of the p53 non-cell-autonomous tumor suppressor function in CAFs. Similarly, our unpublished data indicate that conditioned medium from mutant H-Ras transformed ovarian surface epithelial cells selectively suppressed p53 in otherwise normal ovarian fibroblasts. Therefore, it can be stated that because tumor suppressor activity in the normal ovarian or fallopian tube stroma exerts an inhibitory influence on ovarian neoplastic initiation and progression, attenuation of p53 activity in the ovarian reactive stroma would strongly favor tumorigenic progression.
Disruption of Stromal Intracellular Signaling Pathways by Cancer Cell Communication: Who Is Implicated?
Epigenetic modification of signaling pathways related to secretion in CAFs is a field in which many questions remain to be answered, but several target pathways in CAFs of various tumor tissue types point to a role for epigenetic modification in ovarian CAFs promoting ovarian cancer development. Within invasive and aggressive gliomas, tenascin-C is upregulated in stromal cells by recombination binding protein Jγ (RBPJγ), a Notch 2 cofactor for transcription in activated Notch signaling [148]. Thus, RBPJ. may facilitate intracellular signaling pathway interpretation of secreted epithelial-stromal cell communication (Figure 3B). In addition to signaling pathways directly activated by molecules secreted by CAFs, the mechanical stress generated during ECM modification by breast CAF-secreted factors may play a role in activating signaling pathways in mammary carcinoma cells, contributing to disease progression and compromised disease treatment [149]. Thus, the changing force exerted by the CAF-remodeled ECM on ovarian cancer cells needs to be considered to fully delineate the process of tumor progression. Interestingly, tenascin-C induction in lung fibroblasts depends on RhoA/RhoA-dependent kinase/integrin-linked kinase-mediated signaling in response to mechanical shear stress, although this pathway does seem to be bypassed through extracellular signal-regulated kinase 1/2 and PKB/Akt signaling [150].
Several recent publications have presented preliminary gene expression profiling data that have identified several secreted target proteins that control microenvironmental cross-talk, guidance, and remodeling (e.g., plasminogen activator inhibitor-2, dickkopf-related protein 1, t-type plasminogen activator) that were upregulated in breast CAFs, which likely to play a role in communicating with, and promoting the aggressiveness of, breast cancer cells [40,151]. It is highly likely that a similar expression profiling study of ovarian CAFs would yield significant potential targets for therapeutic intervention.
Modeling Ovarian Cancer Epithelial-Stromal Cell-Cell Interaction
Despite our familiarity with characterized genetic alternations in ovarian cancer epithelial cells, we do not yet fully recognize how these genetic changes work together to not only transform normal ovarian surface epithelial cells into cancerous cells but also facilitate and recruit an activated stromal microenvironment. In addition, the inherent difficulties in accessing and deriving normal and cancer-associated ovarian fibroblast cell lines makes studying cross-talk between the stroma and epithelium in advanced ovarian cancer very challenging. Similarly, as ovarian carcinoma likely originates from heterogeneous origins, there is a paucity of research investigating stromal-epithelial interactions during tumor initiation in the ovary. Multiple seminal publications using murine model systems for studying ovarian carcinoma development have done so primarily through the introduction mutant K-Ras, c-Myc, or Akt onto a mutated p53 or PTEN background [152–154]. Developing models using human ovarian surface epithelial cells or human fallopian tube epithelial cells has proven a difficult task. Expression of SV40 large T and small t antigens genomic regions [155,156], or human papillomavirus type 16 E6/E7 region [157], extended the cellular replication life of human ovarian surface epithelial cells, yielding marginal transformation and growth in anchorage-independent colony assays as well as nude mouse tumor growth. Recently, a promising ex vivo model was developed using human fallopian tube epithelial cells in the hopes of eventually building a model of late-stage serous ovarian carcinoma [158]. We have developed a genetically defined mouse xenograft model of ovarian carcinoma by expressing the catalytic subunit of human telomerase, oncogenic HRAS or KRAS mutants along with SV40 T/t antigens that yielded subcutaneous tumor formation and intraperitoneal ascites with corresponding CA125/mesothelin staining [62]. We refined this model further by replacing SV40 expression with inhibitory constructs knocking down p53 or Rb, allowing for stepwise delineation of oncogenic and transformative events inmodeling ovarian carcinoma [159,160]. Therefore, existing models such as these allow for investigation into the underlying molecular mechanisms correlated with ovarian or fallopian epithelial cell specification, as well as stage-specific signaling mechanisms, in studying and modeling ovarian cancer.
In murine or in vitro models focusing on the role and contribution of stroma in ovarian cancer development, much work remains. A few existing publications directly address the ovarian stromal mechanisms promoting ovarian tumorigenesis. Recently, a murine ovarian carcinoma cell line stably overexpressing a fluorescent-tagged vascular endothelial growth factor 164 isoform was developed, which demonstrated significantly accelerated tumor growth with ascites formation, elevated tumor angiogenesis, and promotion of tumor cell survival relative to controls [161]. Thus, ovarian tumor cells within this model directly modulate their proximal tumor stromal microenvironment in promoting tumorigenesis and vascular support and may prove useful for future studies with therapeutic agents targeting the endothelial cell-specific stromal microenvironment contribution to tumor growth. Moreover, another recent study used mouse modeling to focus on ovarian stroma contribution and identified that the cell-cell interaction between ovarian epithelial cells and host stroma was an important factor in ovarian tumorigenesis [162]. Furthermore, we have used our Ras-mediated ovarian cancer mouse xenograft model to demonstrate that GRO-α-expressing senescent ovarian fibroblasts significantly promoted xenograft tumorigenesis of preneoplastic ovarian surface epithelial cells [63]. Thus, the scarcity of existing models allowing characterization of the role of ovarian CAFs in promoting ovarian cancer epithelial tumor growth indicates the real need for development in this field.
Conclusions and Future Work
Although progress has been made toward understanding the role of CAFs in ovarian tumorigenesis, many questions remain. Potential therapeutic targets of cancer-activated stromal signaling pathways that act as regulatory switches for tumor-promoting molecules have been identified—for example, IL-1β/IL-1R1, SDF-1α/CXCR4, GROα-1/CXCR-2, NF-γB p65, and tenascin-C—but detailed mechanisms remain to be worked out. An area of critical importance is deciphering the mechanism by which p53 or other tumor suppressors are inhibited in ovarian fibroblasts and the downstream mediators of this inhibition, which activate the expression of secretory tumor cell behavioral modulators like SDF-1α and FAP-1α (Figure 3B). Whether by genetic alteration of tumor suppressors in cancer stroma or by cross-signaling that upregulates key paracrine pathways that stimulate cell growth, successful tumor initiation and evasion of immune surveillance probably depend on proximal stromal activation. Thus, continuing to investigate the role of CAFs in ovarian cancer development should be a high priority for future work. Furthering our understanding of the contribution of activated stromal signaling pathways to ovarian tumorigenesis may yield specific intracellular signaling targets, which effectively suppress the contribution of cancer-associated stromal cells to malignancy, and also novel cancer stromal markers for early detection of ovarian cancer.
Acknowledgments
The authors thank Karen Muller for her insightful manuscript editing and revision;Weiwei Shan, PhD, for helpful comments and discussion; and Kim-Anh Vu for preparation of the graphic art for our figures.
Abbreviations
- CAFs
cancer-associated fibroblasts
- CLIC4
chloride intracellular channel 4
- CXCR
CXC chemokine receptor
- CXCL
chemokine (CXC motif) ligand
- ECM
extracellular matrix
- FAP-1α
fibroblast activation protein-1α
- GRO-α
growth-regulated oncogene α
- IL
interleukin
- NF-γB
nuclear factor γB
- OSCC
oral squamous cell carcinoma
- RBPJγ
recombination binding protein Jγ
- SDF-1α
stromal-derived factor 1α
Footnotes
This work was supported by the National Cancer Institute through a Specialized Program of Research Excellence grant in Ovarian Cancer (CA083639) and R01 grant to J.L., A.K.S., and S.M. and in part by the National Institutes of Health through the UT MD Anderson Cancer Center Support Grant (CA016672). Additional support was provided by an Ovarian Cancer Research Fund Program Developmental Grant.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Hudson LG, Zeineldin R, Stack MS. Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clin Exp Metastasis. 2008;25:643–655. doi: 10.1007/s10585-008-9171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JT, Li M, Nakayama K, Mao TL, Davidson B, Zhang Z, Kurman RJ, Eberhart CG, Shih Ie M, Wang TL. Notch3 gene amplification in ovarian cancer. Cancer Res. 2006;66:6312–6318. doi: 10.1158/0008-5472.CAN-05-3610. [DOI] [PubMed] [Google Scholar]
- 4.Cho KR, Shih Ie M. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auner V, Kriegshauser G, Tong D, Horvat R, Reinthaller A, Mustea A, Zeillinger R. KRAS mutation analysis in ovarian samples using a high sensitivity biochip assay. BMC Cancer. 2009;9:111. doi: 10.1186/1471-2407-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo KT, Guan B, Feng Y, Mao TL, Chen X, Jinawath N, Wang Y, Kurman RJ, Shih Ie M, Wang TL. Analysis of DNA copy number alterations in ovarian serous tumors identifies new molecular genetic changes in low-grade and high-grade carcinomas. Cancer Res. 2009;69:4036–4042. doi: 10.1158/0008-5472.CAN-08-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobel M, Kalloger SE, Boyd N, McKinney S, Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 2008;5:e232. doi: 10.1371/journal.pmed.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobel M, Kalloger SE, Carrick J, Huntsman D, Asad H, Oliva E, Ewanowich CA, Soslow RA, Gilks CB. A limited panel of immunomarkers can reliably distinguish between clear cell and high-grade serous carcinoma of the ovary. Am J Surg Pathol. 2009;33:14–21. doi: 10.1097/PAS.0b013e3181788546. [DOI] [PubMed] [Google Scholar]
- 9.Dubeau L. The cell of origin of ovarian epithelial tumours. Lancet Oncol. 2008;9:1191–1197. doi: 10.1016/S1470-2045(08)70308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilks CB, Prat J. Ovarian carcinoma pathology and genetics: recent advances. Hum Pathol. 2009;40:1213–1223. doi: 10.1016/j.humpath.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Rosen DG, Yang G, Liu G, Mercado-Uribe I, Chang B, Xiao XS, Zheng J, Xue FX, Liu J. Ovarian cancer: pathology, biology, and disease models. Front Biosci. 2009;14:2089–2102. doi: 10.2741/3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KR, Scully RE. Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with “pseudomyxoma peritonei”. Am J Surg Pathol. 2000;24:1447–1464. doi: 10.1097/00000478-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Labiche A, Heutte N, Herlin P, Chasle J, Gauduchon P, Elie N. Stromal compartment as a survival prognostic factor in advanced ovarian carcinoma. Int J Gynecol Cancer. 2010;20:28–33. doi: 10.1111/IGC.0b013e3181bda1cb. [DOI] [PubMed] [Google Scholar]
- 16.Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, Wheeler M, Spitler J, Rowley DR. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res. 2003;9:4792–4801. [PubMed] [Google Scholar]
- 17.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912–2923. [PubMed] [Google Scholar]
- 18.Rowley DR. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev. 1998;17:411–419. doi: 10.1023/a:1006129420005. [DOI] [PubMed] [Google Scholar]
- 19.Seemayer TA, Lagace R, Schurch W, Tremblay G. Myofibroblasts in the stroma of invasive and metastatic carcinoma: a possible host response to neoplasia. Am J Surg Pathol. 1979;3:525–533. doi: 10.1097/00000478-197912000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Mensing H, Pontz BF, Muller PK, Gauss-Muller V. A study on fibroblast chemotaxis using fibronectin and conditioned medium as chemoattractants. Eur J Cell Biol. 1983;29:268–273. [PubMed] [Google Scholar]
- 21.Strauli P, In-Albon A, Haemmerli G. Morphological studies on V2 carcinoma invasion and tumor-associated connective tissue changes in the rabbit mesentery. Cancer Res. 1983;43:5403–5410. [PubMed] [Google Scholar]
- 22.Haemmerli G, Muller-Glauser W, Bruckner P, Hauser-Urfer I, Strauli P. Tumor-associated desmoplasia in the rabbit mesentery characterized by morphological, biochemical and cytophotometric methods. Int J Cancer. 1985;35:527–534. doi: 10.1002/ijc.2910350417. [DOI] [PubMed] [Google Scholar]
- 23.Dabbous MK, Haney L, Carter LM, Paul AK, Reger J. Heterogeneity of fibroblast response in host-tumor cell-cell interactions in metastatic tumors. J Cell Biochem. 1987;35:333–344. doi: 10.1002/jcb.240350408. [DOI] [PubMed] [Google Scholar]
- 24.Dabbous MK, North SM, Haney L, Nicolson GL. Macrophage and lymphocyte potentiation of syngeneic tumor cell and host fibroblast collagenolytic activity in rats. Cancer Res. 1988;48:6832–6836. [PubMed] [Google Scholar]
- 25.Carnemolla B, Balza E, Siri A, Zardi L, Nicotra MR, Bigotti A, Natali PG. A tumor-associated fibronectin isoform generated by alternative splicing of messenger RNA precursors. J Cell Biol. 1989;108:1139–1148. doi: 10.1083/jcb.108.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagy JA, Brown LF, Senger DR, Lanir N, Van de Water L, Dvorak AM, Dvorak HF. Pathogenesis of tumor stroma generation: a critical role for leaky blood vessels and fibrin deposition. Biochim Biophys Acta. 1989;948:305–326. doi: 10.1016/0304-419x(89)90004-8. [DOI] [PubMed] [Google Scholar]
- 27.Angeli F, Koumakis G, Chen MC, Kumar S, Delinassios JG. Role of stromal fibroblasts in cancer: promoting or impeding? Tumour Biol. 2009;30:109–120. doi: 10.1159/000218708. [DOI] [PubMed] [Google Scholar]
- 28.Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev. 2008;18:27–34. doi: 10.1016/j.gde.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 30.Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 31.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth—bystanders turning into key players. Curr Opin Genet Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Kenny PA, Lee GY, Bissell MJ. Targeting the tumor microenvironment. Front Biosci. 2007;12:3468–3474. doi: 10.2741/2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigues-Lisoni FC, Peitl P, Jr, Vidotto A, Polachini GM, Maniglia JV, Carmona-Raphe J, Cunha BR, Henrique T, Souza CF, Teixeira RA, et al. Genomics and proteomics approaches to the study of cancer-stroma interactions. BMC Med Genomics. 2010;3:14. doi: 10.1186/1755-8794-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer. 2003;107:1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- 35.Sneddon JB. The contribution of niche-derived factors to the regulation of cancer cells. Methods Mol Biol. 2009;568:217–232. doi: 10.1007/978-1-59745-280-9_14. [DOI] [PubMed] [Google Scholar]
- 36.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 37.Yao Q, Qu X, Yang Q, Wei M, Kong B. CLIC4 mediates TGF-β1-induced fibroblast-to-myofibroblast transdifferentiation in ovarian cancer. Oncol Rep. 2009;22:541–548. doi: 10.3892/or_00000469. [DOI] [PubMed] [Google Scholar]
- 38.Suh KS, Crutchley JM, Koochek A, Ryscavage A, Bhat K, Tanaka T, Oshima A, Fitzgerald P, Yuspa SH. Reciprocal modifications of CLIC4 in tumor epithelium and stroma mark malignant progression of multiple human cancers. Clin Cancer Res. 2007;13:121–131. doi: 10.1158/1078-0432.CCR-06-1562. [DOI] [PubMed] [Google Scholar]
- 39.Flaberg E, Markasz L, Petranyi G, Stuber G, Dicso F, Alchihabi N, Olah E, Csizy I, Jozsa T, Andren O, et al. High throughput live cell imaging reveals differential inhibition of tumor cell proliferation by human fibroblasts. Int J Cancer. 2010 doi: 10.1002/ijc.25612. Epub ahead of print August 16. [DOI] [PubMed] [Google Scholar]
- 40.Sadlonova A, Bowe DB, Novak Z, Mukherjee S, Duncan VE, Page GP, Frost AR. Identification of molecular distinctions between normal breast-associated fibroblasts and breast cancer-associated fibroblasts. Cancer Microenviron. 2009;2:9–21. doi: 10.1007/s12307-008-0017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kenny HA, Krausz T, Yamada SD, Lengyel E. Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells to the omentum. Int J Cancer. 2007;121:1463–1472. doi: 10.1002/ijc.22874. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Tang H, Cai J, Zhang T, Guo J, Feng D, Wang Z. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. 2011;303(1):47–55. doi: 10.1016/j.canlet.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Ganss R. Tumor stroma fosters neovascularization by recruitment of progenitor cells into the tumor bed. J Cell Mol Med. 2006;10:857–865. doi: 10.1111/j.1582-4934.2006.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 44.Robert M, Costa P, Bressolle F, Mottet N, Navratil H. Percentage area density of epithelial and mesenchymal components in benign prostatic hyperplasia: comparison of results between single biopsy, multiple biopsies and multiple tissue specimens. Br J Urol. 1995;75:317–324. doi: 10.1111/j.1464-410x.1995.tb07342.x. [DOI] [PubMed] [Google Scholar]
- 45.Ueno H, Jones AM, Wilkinson KH, Jass JR, Talbot IC. Histological categorisation of fibrotic cancer stroma in advanced rectal cancer. Gut. 2004;53:581–586. doi: 10.1136/gut.2003.028365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schurch W, Lagace R, Seemayer TA. Myofibroblastic stromal reaction in retracted scirrhous carcinoma of the breast. Surg Gynecol Obstet. 1982;154:351–358. [PubMed] [Google Scholar]
- 47.Shimosato Y, Suzuki A, Hashimoto T, Nishiwaki Y, Kodama T, Yoneyama T, Kameya T. Prognostic implications of fibrotic focus (scar) in small peripheral lung cancers. Am J Surg Pathol. 1980;4:365–373. doi: 10.1097/00000478-198008000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Suhonen KA, Anttila MA, Sillanpaa SM, Hamalainen KM, Saarikoski SV, Juhola M, Kosma VM. Quantification of angiogenesis by the Chalkley method and its prognostic significance in epithelial ovarian cancer. Eur J Cancer. 2007;43:1300–1307. doi: 10.1016/j.ejca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Goswami B, Rajappa M, Sharma M, Sharma A. Inflammation: its role and interplay in the development of cancer, with special focus on gynecological malignancies. Int J Gynecol Cancer. 2008;18:591–599. doi: 10.1111/j.1525-1438.2007.01089.x. [DOI] [PubMed] [Google Scholar]
- 50.Anttila MA, Tammi RH, Tammi MI, Syrjanen KJ, Saarikoski SV, Kosma VM. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 2000;60:150–155. [PubMed] [Google Scholar]
- 51.Voutilainen K, Anttila M, Sillanpaa S, Tammi R, Tammi M, Saarikoski S, Kosma VM. Versican in epithelial ovarian cancer: relation to hyaluronan, clinicopathologic factors and prognosis. Int J Cancer. 2003;107:359–364. doi: 10.1002/ijc.11423. [DOI] [PubMed] [Google Scholar]
- 52.Noskova V, Ahmadi S, Asander E, Casslen B. Ovarian cancer cells stimulate uPA gene expression in fibroblastic stromal cells via multiple paracrine and autocrine mechanisms. Gynecol Oncol. 2009;115:121–126. doi: 10.1016/j.ygyno.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 53.Rask K, Zhu Y, Wang W, Hedin L, Sundfeldt K. Ovarian epithelial cancer: a role for PGE2—synthesis and signalling in malignant transformation and progression. Mol Cancer. 2006;5:62. doi: 10.1186/1476-4598-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Athavale R, Thomakos N, Godfrey K, Kew F, Cross P, de Barros Lopes A, Hatem MH, Naik R. The effect of epithelial and stromal tumor components on FigO stages III and IV ovarian carcinosarcomas treated with primary surgery and chemotherapy. Int J Gynecol Cancer. 2007;17:1025–1030. doi: 10.1111/j.1525-1438.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- 55.Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol. 2010;21(1):33–39. doi: 10.1016/j.semcdb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gonda TA, Varro A, Wang TC, Tycko B. Molecular biology of cancer-associated fibroblasts: can these cells be targeted in anti-cancer therapy? Semin Cell Dev Biol. 2010;21(1):2–10. doi: 10.1016/j.semcdb.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 58.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freedman RS, Deavers M, Liu J, Wang E. Peritoneal inflammation—a microenvironment for epithelial ovarian cancer (EOC) J Transl Med. 2004;2:23. doi: 10.1186/1479-5876-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Wang E, Kavanagh JJ, Freedman RS. Ovarian cancer, the coagulation pathway, and inflammation. J Transl Med. 2005;3:25. doi: 10.1186/1479-5876-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheu BC, Chang WC, Cheng CY, Lin HH, Chang DY, Huang SC. Cytokine regulation networks in the cancer microenvironment. Front Biosci. 2008;13:6255–6268. doi: 10.2741/3152. [DOI] [PubMed] [Google Scholar]
- 62.Liu J, Yang G, Thompson-Lanza JA, Glassman A, Hayes K, Patterson A, Marquez RT, Auersperg N, Yu Y, Hahn WC, et al. A genetically defined model for human ovarian cancer. Cancer Res. 2004;64:1655–1663. doi: 10.1158/0008-5472.can-03-3380. [DOI] [PubMed] [Google Scholar]
- 63.Yang G, Rosen DG, Zhang Z, Bast RC, Jr, Mills GB, Colacino JA, Mercado-Uribe I, Liu J. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc Natl Acad Sci USA. 2006;103:16472–16477. doi: 10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE 2000. 2000 doi: 10.1126/stke.442000re1. re1. [DOI] [PubMed] [Google Scholar]
- 65.Cao Z, Henzel WJ, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996;271:1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 66.Li S, Strelow A, Fontana EJ, Wesche H. IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc Natl Acad Sci USA. 2002;99:5567–5572. doi: 10.1073/pnas.082100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muzio M, Ni J, Feng P, Dixit VM. IRAK(Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 68.Behrens C, Feng L, Kadara H, Kim HJ, Lee JJ, Mehran R, Hong WK, Lotan R, Wistuba II. Expression of interleukin-1 receptor-associated kinase-1 in non-small cell lung carcinoma and preneoplastic lesions. Clin Cancer Res. 2010;16:34–44. doi: 10.1158/1078-0432.CCR-09-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nunez C, Cansino JR, Bethencourt F, Perez-Utrilla M, Fraile B, Martinez-Onsurbe P, Olmedilla G, Paniagua R, Royuela M. TNF/IL-1/NIK/NF-γB transduction pathway: a comparative study in normal and pathological human prostate (benign hyperplasia and carcinoma) Histopathology. 2008;53:166–176. doi: 10.1111/j.1365-2559.2008.03092.x. [DOI] [PubMed] [Google Scholar]
- 70.Liu G, Park YJ, Abraham E. Interleukin-1 receptor-associated kinase (IRAK)-1-mediated NF-γB activation requires cytosolic and nuclear activity. FASEB J. 2008;22:2285–2296. doi: 10.1096/fj.07-101816. [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- 72.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 73.Jaszczynska-Nowinka K, Markowska A. New cytokine: stromal derived factor-1. Eur J Gynaecol Oncol. 2009;30:124–127. [PubMed] [Google Scholar]
- 74.Burger JA, Stewart DJ. CXCR4 chemokine receptor antagonists: perspectives in SCLC. Expert Opin Investig Drugs. 2009;18:481–490. doi: 10.1517/13543780902804249. [DOI] [PubMed] [Google Scholar]
- 75.Fulton AM. The chemokine receptors CXCR4 and CXCR3 in cancer. Curr Oncol Rep. 2009;11:125–131. doi: 10.1007/s11912-009-0019-1. [DOI] [PubMed] [Google Scholar]
- 76.Zlotnik A. Chemokines and cancer. Int J Cancer. 2006;119:2026–2029. doi: 10.1002/ijc.22024. [DOI] [PubMed] [Google Scholar]
- 77.Scotton CJ, Wilson JL, Milliken D, Stamp G, Balkwill FR. Epithelial cancer cell migration: a role for chemokine receptors? Cancer Res. 2001;61:4961–4965. [PubMed] [Google Scholar]
- 78.Gladson CL, Welch DR. New insights into the role of CXCR4 in prostate cancer metastasis. Cancer Biol Ther. 2008;7:1849–1851. doi: 10.4161/cbt.7.11.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luker KE, Luker GD. Functions of CXCL12 and CXCR4 in breast cancer. Cancer Lett. 2006;238:30–41. doi: 10.1016/j.canlet.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 80.Schimanski CC, Bahre R, Gockel I, Muller A, Frerichs K, Horner V, Teufel A, Simiantonaki N, Biesterfeld S, Wehler T, et al. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br J Cancer. 2006;95:210–217. doi: 10.1038/sj.bjc.6603251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsusue R, Kubo H, Hisamori S, Okoshi K, Takagi H, Hida K, Nakano K, Itami A, Kawada K, Nagayama S, et al. Hepatic stellate cells promote liver metastasis of colon cancer cells by the action of SDF-1/CXCR4 axis. Ann Surg Oncol. 2009;16:2645–2653. doi: 10.1245/s10434-009-0599-x. [DOI] [PubMed] [Google Scholar]
- 82.Schimanski CC, Schwald S, Simiantonaki N, Jayasinghe C, Gonner U, Wilsberg V, Junginger T, Berger MR, Galle PR, Moehler M. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743–1750. doi: 10.1158/1078-0432.CCR-04-1195. [DOI] [PubMed] [Google Scholar]
- 83.Yadav VR, Sung B, Prasad S, Kannappan R, Cho SG, Liu M, Chaturvedi MM, Aggarwal BB. Celastrol suppresses invasion of colon and pancreatic cancer cells through the downregulation of expression of CXCR4 chemokine receptor. J Mol Med. 2010;88:1243–1253. doi: 10.1007/s00109-010-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gangadhar T, Nandi S, Salgia R. The role of chemokine receptor CXCR4 in lung cancer. Cancer Biol Ther. 2010;9:409–416. doi: 10.4161/cbt.9.6.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ooi LL, Dunstan CR. CXCL12/CXCR4 axis in tissue targeting and bone destruction in cancer and multiple myeloma. J Bone Miner Res. 2009;24:1147–1149. doi: 10.1359/jbmr.090503. [DOI] [PubMed] [Google Scholar]
- 86.Eck SM, Cote AL, Winkelman WD, Brinckerhoff CE. CXCR4 and matrix metalloproteinase-1 are elevated in breast carcinoma-associated fibroblasts and in normal mammary fibroblasts exposed to factors secreted by breast cancer cells. Mol Cancer Res. 2009;7:1033–1044. doi: 10.1158/1541-7786.MCR-09-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jotzu C, Alt E, Welte G, Li J, Hennessy BT, Devarajan E, Krishnappa S, Pinilla S, Droll L, Song YH. Adipose tissue-derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor-derived factors. Anal Cell Pathol (Amst) 2010;33:61–79. doi: 10.3233/ACP-CLO-2010-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, et al. Autocrine TGF-β and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci USA. 2010;107:20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mishra P, Banerjee D, Ben-Baruch A. Chemokines at the crossroads of tumor-fibroblast interactions that promote malignancy. J Leukoc Biol. 2011;89(1):31–39. doi: 10.1189/jlb.0310182. [DOI] [PubMed] [Google Scholar]
- 90.Furuya M, Suyama T, Usui H, Kasuya Y, Nishiyama M, Tanaka N, Ishiwata I, Nagai Y, Shozu M, Kimura S. Up-regulation of CXC chemokines and their receptors: implications for proinflammatory microenvironments of ovarian carcinomas and endometriosis. Hum Pathol. 2007;38:1676–1687. doi: 10.1016/j.humpath.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 91.Jiang YP, Wu XH, Shi B, Wu WX, Yin GR. Expression of chemokine CXCL12 and its receptor CXCR4 in human epithelial ovarian cancer: an independent prognostic factor for tumor progression. Gynecol Oncol. 2006;103:226–233. doi: 10.1016/j.ygyno.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 92.Pils D, Pinter A, Reibenwein J, Alfanz A, Horak P, Schmid BC, Hefler L, Horvat R, Reinthaller A, Zeillinger R, et al. In ovarian cancer the prognostic influence of HER2/neu is not dependent on the CXCR4/SDF-1 signalling pathway. Br J Cancer. 2007;96:485–491. doi: 10.1038/sj.bjc.6603581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Porcile C, Bajetto A, Barbieri F, Barbero S, Bonavia R, Biglieri M, Pirani P, Florio T, Schettini G. Stromal cell-derived factor-1α (SDF-1α/CXCL12) stimulates ovarian cancer cell growth through the EGF receptor transactivation. Exp Cell Res. 2005;308:241–253. doi: 10.1016/j.yexcr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 94.Scotton CJ, Wilson JL, Scott K, Stamp G, Wilbanks GD, Fricker S, Bridger G, Balkwill FR. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 2002;62:5930–5938. [PubMed] [Google Scholar]
- 95.Kajiyama H, Shibata K, Terauchi M, Ino K, Nawa A, Kikkawa F. Involvement of SDF-1α/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int J Cancer. 2008;122:91–99. doi: 10.1002/ijc.23083. [DOI] [PubMed] [Google Scholar]
- 96.Barbieri F, Bajetto A, Florio T. Role of chemokine network in the development and progression of ovarian cancer: a potential novel pharmacological target. J Oncol. 2010;2010:426956. doi: 10.1155/2010/426956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mehrad B, Burdick MD, Strieter RM. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol. 2009;41:1708–1718. doi: 10.1016/j.biocel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Loh SA, Chang EI, Galvez MG, Thangarajah H, El-ftesi S, Vial IN, Lin DA, Gurtner GC. SDF-1α expression during wound healing in the aged is HIF dependent. Plast Reconstr Surg. 2009;123:65S–75S. doi: 10.1097/PRS.0b013e318191bdf4. [DOI] [PubMed] [Google Scholar]
- 99.Daly AJ, McIlreavey L, Irwin CR. Regulation of HGF and SDF-1 expression by oral fibroblasts—implications for invasion of oral cancer. Oral Oncol. 2008;44:646–651. doi: 10.1016/j.oraloncology.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 100.Moskovits N, Kalinkovich A, Bar J, Lapidot T, Oren M. p53 Attenuates cancer cell migration and invasion through repression of SDF-1/CXCL12 expression in stromal fibroblasts. Cancer Res. 2006;66:10671–10676. doi: 10.1158/0008-5472.CAN-06-2323. [DOI] [PubMed] [Google Scholar]
- 101.Pure E. The road to integrative cancer therapies: emergence of a tumor-associated fibroblast protease as a potential therapeutic target in cancer. Expert Opin Ther Targets. 2009;13:967–973. doi: 10.1517/14728220903103841. [DOI] [PubMed] [Google Scholar]
- 102.Lebeau AM, Brennen WN, Aggarwal S, Denmeade SR. Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin. Mol Cancer Ther. 2009;8(5):1378–1386. doi: 10.1158/1535-7163.MCT-08-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Messerschmidt SK, Musyanovych A, Altvater M, Scheurich P, Pfizenmaier K, Landfester K, Kontermann RE. Targeted lipid-coated nanoparticles: delivery of tumor necrosis factor-functionalized particles to tumor cells. J Control Release. 2009;137:69–77. doi: 10.1016/j.jconrel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 104.Ostermann E, Garin-Chesa P, Heider KH, Kalat M, Lamche H, Puri C, Kerjaschki D, Rettig WJ, Adolf GR. Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin Cancer Res. 2008;14:4584–4592. doi: 10.1158/1078-0432.CCR-07-5211. [DOI] [PubMed] [Google Scholar]
- 105.Chen H, Yang WW, Wen QT, Xu L, Chen M. TGF-β induces fibroblast activation protein expression; fibroblast activation protein expression increases the proliferation, adhesion, and migration of HO-8910PM [corrected] Exp Mol Pathol. 2009;87:189–194. doi: 10.1016/j.yexmp.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 106.Rasanen K, Virtanen I, Salmenpera P, Grenman R, Vaheri A. Differences in the nemosis response of normal and cancer-associated fibroblasts from patients with oral squamous cell carcinoma. PLoS One. 2009;4:e6879. doi: 10.1371/journal.pone.0006879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hsia HC, Schwarzbauer JE. Meet the tenascins: multifunctional and mysterious. J Biol Chem. 2005;280:26641–26644. doi: 10.1074/jbc.R500005200. [DOI] [PubMed] [Google Scholar]
- 108.Orend G. Potential oncogenic action of tenascin-C in tumorigenesis. Int J Biochem Cell Biol. 2005;37:1066–1083. doi: 10.1016/j.biocel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 109.Amonkar SD, Bertenshaw GP, Chen TH, Bergstrom KJ, Zhao J, Seshaiah P, Yip P, Mansfield BC. Development and preliminary evaluation of a multivariate index assay for ovarian cancer. PLoS One. 2009;4:e4599. doi: 10.1371/journal.pone.0004599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Iguchi T, Yamashita N, Aishima S, Kuroda Y, Terashi T, Sugimachi K, Taguchi K, Taketomi A, Maehara Y, Tsuneyoshi M. A comprehensive analysis of immunohistochemical studies in intrahepatic cholangiocarcinoma using the survival tree model. Oncology. 2009;76:293–300. doi: 10.1159/000207506. [DOI] [PubMed] [Google Scholar]
- 111.Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M, Marini F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mishra PJ, Glod JW, Banerjee D. Mesenchymal stem cells: flip side of the coin. Cancer Res. 2009;69:1255–1258. doi: 10.1158/0008-5472.CAN-08-3562. [DOI] [PubMed] [Google Scholar]
- 113.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 114.Granot D, Addadi Y, Kalchenko V, Harmelin A, Kunz-Schughart LA, Neeman M. In vivo imaging of the systemic recruitment of fibroblasts to the angiogenic rim of ovarian carcinoma tumors. Cancer Res. 2007;67:9180–9189. doi: 10.1158/0008-5472.CAN-07-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jeon ES, Moon HJ, Lee MJ, Song HY, Kim YM, Cho M, Suh DS, Yoon MS, Chang CL, Jung JS, et al. Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblast-like cells. Stem Cells. 2008;26:789–797. doi: 10.1634/stemcells.2007-0742. [DOI] [PubMed] [Google Scholar]
- 116.Coffelt SB, Waterman RS, Florez L, Honer zu Bentrup K, Zwezdaryk KJ, Tomchuck SL, LaMarca HL, Danka ES, Morris CA, Scandurro AB. Ovarian cancers overexpress the antimicrobial protein hCAP-18 and its derivative LL-37 increases ovarian cancer cell proliferation and invasion. Int J Cancer. 2008;122:1030–1039. doi: 10.1002/ijc.23186. [DOI] [PubMed] [Google Scholar]
- 117.Coffelt SB, Marini FC, Watson K, Zwezdaryk KJ, Dembinski JL, LaMarca HL, Tomchuck SL, Honer zu Bentrup K, Danka ES, Henkle SL, et al. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc Natl Acad Sci USA. 2009;106:3806–3811. doi: 10.1073/pnas.0900244106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lawrenson K, Grun B, Benjamin E, Jacobs IJ, Dafou D, Gayther SA. Senescent fibroblasts promote neoplastic transformation of partially transformed ovarian epithelial cells in a three-dimensional model of early stage ovarian cancer. Neoplasia. 2010;12:317–325. doi: 10.1593/neo.91948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Olivier M, Petitjean A, Marcel V, Petre A, Mounawar M, Plymoth A, de Fromentel CC, Hainaut P. Recent advances in p53 research: an interdisciplinary perspective. Cancer Gene Ther. 2009;16:1–12. doi: 10.1038/cgt.2008.69. [DOI] [PubMed] [Google Scholar]
- 120.Fukino K, Shen L, Patocs A, Mutter GL, Eng C. Genomic instability within tumor stroma and clinicopathological characteristics of sporadic primary invasive breast carcinoma. JAMA. 2007;297:2103–2111. doi: 10.1001/jama.297.19.2103. [DOI] [PubMed] [Google Scholar]
- 121.Kiaris H, Chatzistamou I, Trimis G, Frangou-Plemmenou M, Pafiti-Kondi A, Kalofoutis A. Evidence for nonautonomous effect of p53 tumor suppressor in carcinogenesis. Cancer Res. 2005;65:1627–1630. doi: 10.1158/0008-5472.CAN-04-3791. [DOI] [PubMed] [Google Scholar]
- 122.Kurose K, Gilley K, Matsumoto S, Watson PH, Zhou XP, Eng C. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet. 2002;32:355–357. doi: 10.1038/ng1013. [DOI] [PubMed] [Google Scholar]
- 123.Moinfar F, Beham A, Friedrich G, Deutsch A, Hrzenjak A, Luschin G, Tavassoli FA. Macro-environment of breast carcinoma: frequent genetic alterations in the normal appearing skins of patients with breast cancer. Mod Pathol. 2008;21:639–646. doi: 10.1038/modpathol.2008.28. [DOI] [PubMed] [Google Scholar]
- 124.Patocs A, Zhang L, Xu Y, Weber F, Caldes T, Mutter GL, Platzer P, Eng C. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N Engl J Med. 2007;357:2543–2551. doi: 10.1056/NEJMoa071825. [DOI] [PubMed] [Google Scholar]
- 125.Weber F, Xu Y, Zhang L, Patocs A, Shen L, Platzer P, Eng C. Microenvironmental genomic alterations and clinicopathological behavior in head and neck squamous cell carcinoma. JAMA. 2007;297:187–195. doi: 10.1001/jama.297.2.187. [DOI] [PubMed] [Google Scholar]
- 126.Qiu W, Hu M, Sridhar A, Opeskin K, Fox S, Shipitsin M, Trivett M, Thompson ER, Ramakrishna M, Gorringe KL, et al. No evidence of clonal somatic genetic alterations in cancer-associated fibroblasts from human breast and ovarian carcinomas. Nat Genet. 2008;40:650–655. doi: 10.1038/ng.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Walter K, Omura N, Hong SM, Griffith M, Goggins M. Pancreatic cancer associated fibroblasts display normal allelotypes. Cancer Biol Ther. 2008;7:882–888. doi: 10.4161/cbt.7.6.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weinberg RA. Coevolution in the tumor microenvironment. Nat Genet. 2008;40:494–495. doi: 10.1038/ng0508-494. [DOI] [PubMed] [Google Scholar]
- 129.Fiegl H, Millinger S, Goebel G, Muller-Holzner E, Marth C, Laird PW, Widschwendter M. Breast cancer DNA methylation profiles in cancer cells and tumor stroma: association with HER-2/neu status in primary breast cancer. Cancer Res. 2006;66:29–33. doi: 10.1158/0008-5472.CAN-05-2508. [DOI] [PubMed] [Google Scholar]
- 130.Hanson JA, Gillespie JW, Grover A, Tangrea MA, Chuaqui RF, Emmert-Buck MR, Tangrea JA, Libutti SK, Linehan WM, Woodson KG. Gene promoter methylation in prostate tumor-associated stromal cells. J Natl Cancer Inst. 2006;98:255–261. doi: 10.1093/jnci/djj051. [DOI] [PubMed] [Google Scholar]
- 131.Hu M, Yao J, Cai L, Bachman KE, van den Brule F, Velculescu V, Polyak K. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 132.Tan M, Wang Y, Guan K, Sun Y. PTGF-β, a type β transforming growth factor (TGF-β) superfamily member, is a p53 target gene that inhibits tumor cell growth via TGF-β signaling pathway. Proc Natl Acad Sci USA. 2000;97:109–114. doi: 10.1073/pnas.97.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Buckbinder L, Talbott R, Velasco-Miguel S, Takenaka I, Faha B, Seizinger BR, Kley N. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature. 1995;377:646–649. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- 134.Komarova EA, Diatchenko L, Rokhlin OW, Hill JE, Wang ZJ, Krivokrysenko VI, Feinstein E, Gudkov AV. Stress-induced secretion of growth inhibitors: a novel tumor suppressor function of p53. Oncogene. 1998;17:1089–1096. doi: 10.1038/sj.onc.1202303. [DOI] [PubMed] [Google Scholar]
- 135.Wernert N, Locherbach C, Wellmann A, Behrens P, Hugel A. Presence of genetic alterations in microdissected stroma of human colon and breast cancers. Anticancer Res. 2001;21:2259–2264. [PubMed] [Google Scholar]
- 136.Sonnenberg M, van der Kuip H, Haubeis S, Fritz P, Schroth W, Friedel G, Simon W, Murdter TE, Aulitzky WE. Highly variable response to cytotoxic chemotherapy in carcinoma-associated fibroblasts (CAFs) from lung and breast. BMC Cancer. 2008;8:364. doi: 10.1186/1471-2407-8-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lafkas D, Trimis G, Papavassiliou AG, Kiaris H. P53 mutations in stromal fibroblasts sensitize tumors against chemotherapy. Int J Cancer. 2008;123:967–971. doi: 10.1002/ijc.23546. [DOI] [PubMed] [Google Scholar]
- 138.Dudley AC, Shih SC, Cliffe AR, Hida K, Klagsbrun M. Attenuated p53 activation in tumour-associated stromal cells accompanies decreased sensitivity to etoposide and vincristine. Br J Cancer. 2008;99:118–125. doi: 10.1038/sj.bjc.6604465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hawsawi NM, Ghebeh H, Hendrayani SF, Tulbah A, Al-Eid M, Al-Tweigeri T, Ajarim D, Alaiya A, Dermime S, Aboussekhra A. Breast carcinoma-associated fibroblasts and their counterparts display neoplastic-specific changes. Cancer Res. 2008;68:2717–2725. doi: 10.1158/0008-5472.CAN-08-0192. [DOI] [PubMed] [Google Scholar]
- 140.Santhanam U, Ray A, Sehgal PB. Repression of the interleukin 6 gene promoter by p53 and the retinoblastoma susceptibility gene product. Proc Natl Acad Sci USA. 1991;88:7605–7609. doi: 10.1073/pnas.88.17.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mehta SA, Christopherson KW, Bhat-Nakshatri P, Goulet RJ, Jr, Broxmeyer HE, Kopelovich L, Nakshatri H. Negative regulation of chemokine receptor CXCR4 by tumor suppressor p53 in breast cancer cells: implications of p53 mutation or isoform expression on breast cancer cell invasion. Oncogene. 2007;26:3329–3337. doi: 10.1038/sj.onc.1210120. [DOI] [PubMed] [Google Scholar]
- 142.Zhu H, Evans B, O'Neill P, Ren X, Xu Z, Hait WN, Yang JM. A role for p53 in the regulation of extracellular matrix metalloproteinase inducer in human cancer cells. Cancer Biol Ther. 2009;8:1722–1728. doi: 10.4161/cbt.8.18.9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fujita K, Mondal AM, Horikawa I, Nguyen GH, Kumamoto K, Sohn JJ, Bowman ED, Mathe EA, Schetter AJ, Pine SR, et al. p53 isoforms Δ133p53 and p53β are endogenous regulators of replicative cellular senescence. Nat Cell Biol. 2009;11:1135–1142. doi: 10.1038/ncb1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lim ST, Miller NL, Nam JO, Chen XL, Lim Y, Schlaepfer DD. Pyk2 inhibition of p53 as an adaptive and intrinsic mechanism facilitating cell proliferation and survival. J Biol Chem. 2010;285:1743–1753. doi: 10.1074/jbc.M109.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sun H, Pisle S, Gardner ER, Figg WD. Bioluminescent imaging study: FAK inhibitor, PF-562,271, preclinical study in PC3M-luc-C6 local implant and metastasis xenograft models. Cancer Biol Ther. 2010;10:38–43. doi: 10.4161/cbt.10.1.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hill R, Song Y, Cardiff RD, Van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123:1001–1011. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 147.Bar J, Feniger-Barish R, Lukashchuk N, Shaham H, Moskovits N, Goldfinger N, Simansky D, Perlman M, Papa M, Yosepovich A, et al. Cancer cells suppress p53 in adjacent fibroblasts. Oncogene. 2009;28:933–936. doi: 10.1038/onc.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sivasankaran B, Degen M, Ghaffari A, Hegi ME, Hamou MF, Ionescu MC, Zweifel C, Tolnay M, Wasner M, Mergenthaler S, et al. Tenascin-C is a novel RBPJ.-induced target gene for Notch signaling in gliomas. Cancer Res. 2009;69:458–465. doi: 10.1158/0008-5472.CAN-08-2610. [DOI] [PubMed] [Google Scholar]
- 149.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maier S, Lutz R, Gelman L, Sarasa-Renedo A, Schenk S, Grashoff C, Chiquet M. Tenascin-C induction by cyclic strain requires integrin-linked kinase. Biochim Biophys Acta. 2008;1783:1150–1162. doi: 10.1016/j.bbamcr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 151.Casey T, Bond J, Tighe S, Hunter T, Lintault L, Patel O, Eneman J, Crocker A, White J, Tessitore J, et al. Molecular signatures suggest a major role for stromal cells in development of invasive breast cancer. Breast Cancer Res Treat. 2009;114:47–62. doi: 10.1007/s10549-008-9982-8. [DOI] [PubMed] [Google Scholar]
- 152.Orsulic S, Li Y, Soslow RA, Vitale-Cross LA, Gutkind JS, Varmus HE. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell. 2002;1:53–62. doi: 10.1016/s1535-6108(01)00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 154.Wu R, Hendrix-Lucas N, Kuick R, Zhai Y, Schwartz DR, Akyol A, Hanash S, Misek DE, Katabuchi H, Williams BO, et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/β-catenin and PI3K/Pten signaling pathways. Cancer Cell. 2007;11:321–333. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 155.Nitta M, Katabuchi H, Ohtake H, Tashiro H, Yamaizumi M, Okamura H. Characterization and tumorigenicity of human ovarian surface epithelial cells immortalized by SV40 large T antigen. Gynecol Oncol. 2001;81:10–17. doi: 10.1006/gyno.2000.6084. [DOI] [PubMed] [Google Scholar]
- 156.Maines-Bandiera SL, Kruk PA, Auersperg N. Simian virus 40-transformed human ovarian surface epithelial cells escape normal growth controls but retain morphogenetic responses to extracellular matrix. Am J Obstet Gynecol. 1992;167:729–735. doi: 10.1016/s0002-9378(11)91579-8. [DOI] [PubMed] [Google Scholar]
- 157.Tsao SW, Mok SC, Fey EG, Fletcher JA, Wan TS, Chew EC, Muto MG, Knapp RC, Berkowitz RS. Characterization of human ovarian surface epithelial cells immortalized by human papilloma viral oncogenes (HPV-E6E7 ORFs) Exp Cell Res. 1995;218:499–507. doi: 10.1006/excr.1995.1184. [DOI] [PubMed] [Google Scholar]
- 158.Levanon K, Ng V, Piao HY, Zhang Y, Chang MC, Roh MH, Kindelberger DW, Hirsch MS, Crum CP, Marto JA, et al. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene. 2010;29:1103–1113. doi: 10.1038/onc.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang G, Rosen DG, Colacino JA, Mercado-Uribe I, Liu J. Disruption of the retinoblastoma pathway by small interfering RNA and ectopic expression of the catalytic subunit of telomerase lead to immortalization of human ovarian surface epithelial cells. Oncogene. 2007;26:1492–1498. doi: 10.1038/sj.onc.1209905. [DOI] [PubMed] [Google Scholar]
- 160.Yang G, Rosen DG, Mercado-Uribe I, Colacino JA, Mills GB, Bast RC, Jr, Zhou C, Liu J. Knockdown of p53 combined with expression of the catalytic subunit of telomerase is sufficient to immortalize primary human ovarian surface epithelial cells. Carcinogenesis. 2007;28:174–182. doi: 10.1093/carcin/bgl115. [DOI] [PubMed] [Google Scholar]
- 161.Zhang L, Yang N, Garcia JR, Mohamed A, Benencia F, Rubin SC, Allman D, Coukos G. Generation of a syngeneic mouse model to study the effects of vascular endothelial growth factor in ovarian carcinoma. Am J Pathol. 2002;161:2295–2309. doi: 10.1016/s0002-9440(10)64505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Greenaway J, Moorehead R, Shaw P, Petrik J. Epithelial-stromal interaction increases cell proliferation, survival and tumorigenicity in a mouse model of human epithelial ovarian cancer. Gynecol Oncol. 2008;108:385–394. doi: 10.1016/j.ygyno.2007.10.035. [DOI] [PubMed] [Google Scholar]