Abstract
It is now well recognized that tumor cell-host interactions regulate all aspects of cancer development. Amongst the various host response programs that facilitate primary cancer development, an emerging body of literature points to a critical role for leukocytes and their soluble mediators as regulating discrete events during primary tumor development and metastasis. This review focuses on the multiple aspects of leukocytes and their effector molecules as regulators of the metastatic process.
Keywords: leukocytes, inflammation, cancer, metastasis
Dissemination of malignant cancer cells to distant organs is a multistage process requiring detachment and escape from primary tumor sites, extravasation through multiple basement membranes and matrix, survival in peripheral blood or lymphatics, and ability to survive and proliferate in foreign tissue locales. Since, for many tumor types, there is a temporal lag (months to decades) between when malignant cells arrive in ectopic locations and when proliferative capabilities are acquired 1, 2, this implies that in addition to activation of survival programs at the metastatic site, disseminated malignant cells must acquire additional capabilities enabling their survival that likely rely on harnessing embedded regulatory programs at secondary sites. Thus, while cell-intrinsic programs are necessary for successful progression through each of these hurdles 2, 3, cell-extrinsic programs regulated by non-neoplastic cells of mesenchymal, vascular and immune origins are also critical determinants for successful metastatic progression 4.
Chronic infiltration of tissue by leukocytes, aka, chronic inflammation, is associated with predisposition to cancer 5. Chronic inflammation triggered by bacterial and viral infections or by autoimmune disease, is estimated to be linked with 20% of all deaths from cancer worldwide 6. Indeed, epidemiologic studies reporting that non-steroidal anti-inflammatory drugs (NSAIDS) reduce the risk of some cancers provide evidence for a causal link between inflammation and cancer 7–9. Thus, chronic inflammation that precedes neoplasia provides a fertile microenvironment whereby secreted growth factors, reactive oxygen species and cytokines support epithelial proliferation and create a permissive microenvironment to foster ongoing genetic instability and accumulation of genetic alterations that predispose to malignancy 5, 6. Alternatively, inflammation can also be a physiological response to aberrant proliferation and tissue remodeling initiated by mutational activation of intrinsic programs that sustain proliferation and/or block cell death, and thus represent a secondary event enabling progression of neoplastic cells 10. In any event, hallmarks of inflammation, such as the infiltration of neoplastic tissue by innate and adaptive leukocytes, activated angiogenic vasculature, tissue remodeling, and high levels of chemokines and cytokines that regulate these processes, typify most solid tumors 5, 6, 11. This review discusses how key components and pathways of the immune microenvironment are associated with adult solid tumors and thereby promote the multistep cascade of tumor metastasis to distant organs.
Leukocytes implicated in mediating solid tumor metastasis
It has become generally accepted that the chronic presence of activated leukocytes in primary tumors is a “hallmark” of the tumorigenic process, and also represents a predictor of aggressive disease. Tumor-associated macrophages (TAMs) are one of the most abundant innate immune cells present in several types of human cancer 12–16, regulated in part by colony stimulating factor (CSF)1, a key cytokine involved in macrophage maturation, tissue recruitment and activation mediated by the CSF1 receptor (CSF1R/cFMS) 17. A second CSF1R ligand, interleukin (IL)-34, possesses similar binding affinities and also regulates TAM recruitment to tissues, but exhibits distinct tissue distribution 18–20. TAM presence in several types of human cancer correlates with increased vascular density and worse clinical outcome 21–26. Studies in transgenic mouse models of mammary carcinogenesis revealed that TAMs promote tumor growth and enhance pulmonary metastasis by high-level expression of epidermal growth factor (EGF) and activation of EGF-regulated signaling in mammary epithelial cells (MECs) critical for invasive tumor growth and metastatic dissemination15. In mouse models of mammary carcinogenesis, TAMs activated by interleukin (IL)-4 and CSF1 have been identified as essential determinant of pulmonary metastasis due to the pro-metastatic mediators they secrete 27–30. The transcription factor Ets2 was recently implicated in regulating some aspects of these activities as selective deletion of Ets2 in TAMs decreased the frequency and size of pulmonary metastases in mouse models 31.
T lymphocytes were classically studied in the context of their tumor surveillance and anti-tumor capabilities. However, recent investigations have revealed that CD4+ T cells and T regulatory cells instead promote pulmonary metastasis in part by regulating pro- versus anti-tumor bioactivity of innate leukocytes. We reported that interleukin (IL)-4-expressing CD4+ T cells promote invasion and metastasis of mammary adenocarcinomas by directly regulating TAM phenotype, bioeffector function and EGF expression, that in turn regulates invasion, presence of circulating tumor cells (CTCs) and pulmonary metastasis29. Other mediators found to be significant with regards to T cell enhancement of pulmonary metastasis mammary carcinomas are S100A4 and RANKL 32, 33. S100A4 protein mediates T cell attraction to developing neoplasms and premetastatic lungs of tumor-bearing mice, and in turn stimulates T cell production of cytokines, particularly granulocyte colony-stimulating factor and eotaxin-2 33. RANTES stimulates externalization of S100A4 via microparticle shedding from plasma membranes and induces upregulation of fibronectin (FN) from fibroblasts and a number of other cytokines, including RANTES in tumor cells that together enhance tumor cell motility 32. During prostate carcinogenesis, T lymphocyte and macrophage-derived RANKL induces metastasis through activation and nuclear localization of IKKα leading to repression of maspin, a critical suppressor of metastasis 34, 35. Lung metastasis of mammary carcinomas is also regulated by CCR4+ T regulatory cells that can directly kill natural killer (NK) cells 36
Other myeloid cell types implicated in regulating metastasis include neutrophils, mast cells and monocytes harboring T cell suppressive activity 37–40 that are potent suppressors of anti-tumor adaptive immunity and directly facilitate metastasis by regulating angiogenic programs via enhanced metalloproteinase activity 11, 41–43. Cancer-Associated Fibroblasts (CAFs) are implicated in regulating the activities of these myeloid cell types through their secretion of pro-inflammatory chemokines that recruit immune cells to sites of developing neoplasms 33, 44, 45.
Proinflammatory signals that impact exit from primary tumor sites
Regulators of the invasive phenotype
In order for malignant cells to detach from primary tumors and move through their substratum basement membrane, they must transiently acquire a motile and migratory phenotype, sometimes also referred to as Epithelial to Mesenchymal Transition (EMT) 46, 47. This motile state is characterized by loss of homotypic cellular adhesions and apical-basal polarity, and increased migratory capabilities. At the molecular level, this transition is largely driven by intrinsic alterations in gene expression, including suppression of E-Cadherin, mediated by activation of transcriptional repressors Snail, Slug, Twist and Zeb 48.
Extrinsic regulation is also important. Specifically, the chronic inflammatory microenvironment, provided by leukocytes and CAFs plays an important role in regulating the invasive and motile phenotype of would-be metastatic cells. Several leukocyte-regulated mediators have been identified as key to these processes. Notably, tumor necrosis factor (TNF)α secreted by TAMs activates the NF-κB transcription factor in neoplastic (and other) cells, directly leading to expression of Snail1 and Zeb 45, 49, 50. Other leukocyte-derived cytokines (including IL-6 and IL-23) induce activation of intracellular STAT3 that in turn leads to induced expression of Twist 51–53.
Local hypoxia in neoplastic tissue also contributes to induction of motility programs 54, 55 in part by activation of transcription factors hypoxia inducible factor (HIF)-1α and NF-κB, both implicated in EMT via transcription of Snail 49. Moreover, the chemokine receptor CXCR4 is upregulated in mammary carcinomas by hypoxia and is associated with invasive behavior in response to its ligand Stromal-Derived Factor-1 (SDF-1/CXCL12) 56, 57. Thus, hypoxic conditions select for a more metastatic phenotype partially through activation of pro-inflammatory signaling cascades.
Invasion
Movement of malignant cells through basement membrane and stromal matrix requires remodeling of matrix proteins. This process is coordinated by proteolytic enzymes spanning several catalytic classes and includes matrix metalloproteinases (MMPs), cysteine cathepsins and serine proteases 4. Indeed, the increased expression and activity of various proteases has been observed in multiple human and murine tumor types, and can be used as a prognostic indicator of shorter survival rates in patients with breast, ovarian, colorectal and head and neck cancers 58, 59. While many proteolytic enzymes are produced by motile neoplastic cells, the majority of tumor-promoting proteases are produced by activated stromal cells in the local tumor microenvironment 41, 60–63, e.g., fibroblasts 64, or tumor-associated immune cells. Mast cells, neutrophils and macrophages secrete matrix remodeling proteases 65–67 implicated in prometastatic activity, as well as serine proteases that are associated with higher tumor grades and lymph node metastasis in breast cancer 38.
In particular, murine macrophages are known to express elevated levels of the cysteine protease cathepsin B following exposure to IL-4 68. Macrophages at the invasive edge of pancreatic islet cancers express cathepsin B, and this is associated with loss of epithelial E-cadherin on neighboring malignant cells 4. Secretion of proteases by cells within the tumor microenvironment may foster metastatic activity and motility of neoplastic cells through matrix and into vasculature, but also enhance and/or regulate presence and activity of leukocytes: over-expression of cathepsin B in a transgenic mouse model of mammary carcinoma regulates pulmonary metastases, accompanied by increased numbers of B cells, Ig deposition and degranulation of mast cells in the primary tumor site 69
Protease secretion by TAMs is in part regulated by IL-6 emanating from neoplastic cells. Tumor cells-derived IL-6 induces secretion and activation of the cysteine protease cathepsin B, and secretion of matrix metalloproteinases (MMPs) by monocytes 70. Similarly, neoplastic cell and T cell-derived IL-4 induces cathepsin expression and activity in TAMs in several cancer types 30. TAMs in turn regulate neoplastic cell motility by secreting factors such as migration-stimulating factor (MSF) 71. MSF is an oncofetal isoform of fibronectin and is induced in TAMs by macrophage-CSF, IL-4, and transforming growth factor beta (TGFβ). Notably, some immune cell-derived proteases also harbor tumor-suppressive activity: the aspartic proteinase cathepsin E, expressed predominantly by immune cells, including lymphocytes, macrophages, and dendritic cells, mediates neoplastic cell apoptosis by catalyzing the proteolytic release of soluble tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) from the cell surface. Tumor growth is subsequently enhanced by this cascade and survival in tumor-bearing mice impaired72.
Leukocytes and survival of CTCs
Before productive metastatic colonization is possible, circulating tumor cells (CTCs) must develop mechanisms enabling their survival within the circulation. Mechanical shear stress, detachment-induced cell death (anoikis) and cell-mediated cytotoxicity within the microcirculation effectively clear most CTCs 73. It has been estimated that only 0.01% of CTCs survive and eventually extravasate at distal locales 4. Mechanisms that enhance the probability for CTC survival rely on physical interaction with leukocytes. Activated platelets aggregate around CTC and thereby protect them from NK cell-mediated lysis 73 by both thrombin-dependent and -independent mechanisms 74.
Adhesion to capillary walls is largely regulated by the availability of adhesion molecules on CTCs, the endothelium, and the composition of the underlying extracellular matrix (ECM). Platelets 74 and neutrophils facilitate these interactions however, via their production of matrix attachment molecules such as beta(2)-integrin/intracellular adhesion molecule-1 (ICAM-1) 75, 76 and selectins 77. Once CTCs attach to capillary lumens, another obstacle to surmount is inhibition of detachment-induced cell death, or anoikis, which is thought to be a major impediment for productive metastatic spread. Chemokine receptors CXCR4 and CCR7, and their ligands reduce the sensitivity of CTCs to not only arrest on capillary lumens, but also CTC anoikis by selective regulation of pro-apoptotic Bmf and anti-apoptotic Bcl-xL proteins; thus, in the absence of appropriate cell-ECM interactions, selectins and chemokine receptors regulate CTC survival by mediating attachment and blocking cell death 77, 78.
Site-specific colonization
Organ-specific migration
Development of productive metastasis is a highly regulated process that is also subject to organ-specific mechanisms. Some solid tumors metastasize to preferred organ sites, for example, breast cancers metastasize to lung, liver, bone and brain; melanoma to liver, brain and skin; prostate cancer to bone; colorectal cancer to liver and lung, and lung adenocarcinoma metastasizes to bone, liver and brain 2, 4. Several studies have reported that tropism of CTCs to specific organ locales is regulated by the complexity of genetic alterations intrinsic to neoplastic cells 2, while also recognizing that altered expression of important genes can also regulate tropism. Cyclooxygenase (COX)2 (also known as PTGS2), the epidermal growth factor receptor (EGFR) ligand heparin bound (HB)EGF, and the alpha2,6-sialyltransferase (ST6GALNAC5) all act as mediators of malignant cell passage through the blood-brain barrier when breast cancers metastasize to brain 79, 80. In contrast, when breast cancer metastasizes to bone, IL-11 and connective tissue growth factor (CTGF) regulated by transforming growth factor (TGF)β are important 81.
A growing body of literature however also has identified cell-extrinsic mechanisms, in addition to intrinsic, that dictate organ-specificity of metastases, including differential expression of chemokines and their receptors. Chemokines expressed by specific organs promote tumor cell adhesion to microvessel walls, facilitate extravasation into target tissues and induce tumor cell migration. CXCL12-CXCR4, CCR7 and its corresponding chemokine ligands, CCL21 and CCL19, significantly regulate lymph node metastasis, whereas CCR10-CCL27 and CCR4-CCL22 regulate melanoma metastasis 82, 83. Many malignant cells upregulate expression of chemokines receptors during premalignancy, partially as a result of autocrine and paracrine signaling mediated by TNFα, IL-1 and IL-6 at the primary tumor site and subsequent chemokines gradients then in part regulate migration towards specific organs 71. One such functional chemokines-signaling axis involves CXCR4 and its ligand CXCL12. Expression of CXCL12 by mesenchymal bone marrow-derived cells directs migration of metastatic breast cancer cells to bone. These cells constitutively secrete the chemokine stromal cell-derived factor-1 (SDF-1/CXCL12) and thereby attract CXCR4+ malignant cells. Activation of CXCR4 promotes tumor progression by enabling survival and growth programs in malignant cells in ectopic tissues, regulate survival and growth of neoplastic cells in a paracrine manner, and promote tumor angiogenesis by attracting endothelial cells 84. This important axis has been implicated for metastasis of multiple solid tumors including pancreatic, hepatocellular, melanoma, lung and renal cancers 85–92. Other functional chemokine receptors include CCR7, implicated in lymphatic metastasis, and CCR9 that is associated with metastasis to small intestine where its ligand is expressed 93. Other chemokines receptors including CCR10, CXCR1, CXCR2, CXCR3, CXCR5 and CXCR7 expressed by malignant cells by a variety of solid tumors have also been implicated in organ-specific metastasis 11.
Chemokine signaling is also an important feature of site-directed metastasis where neoplastic cell-secreted cytokines and chemokines signal to receptors expressed by various subtypes of myeloid cells, particularly significant with regards to colon carcinoma metastasis to liver 94. Both murine and human colon cancer cells secrete the CC-chemokine ligands CCL9 and CCL15, and thereby induce recruitment of CD34+Gr1− immature myeloid cells that express the CCL9/15 receptor CCR1, activation of which directly induces MMP2 and MMP9 expression. Lack of the Ccr1, Mmp2, or Mmp9 genes in myeloid cells suppresses disseminated tumor growth in the liver and significantly prolongs the survival of tumor-bearing mice 94.
Autocrine signaling loops by malignant cells have also been implicated in organ–specific metastasis. Malignant cell-derived TGFβ induces expression of the cytokine Angiopoietin-like 4 (ANGPTL4) in mammary carcinoma cells, which is critical for carcinoma dissemination and colonization in lungs. TGFβ induces expression of Angptl4 through Smad signaling cascades in carcinoma cells just prior to their entry into circulation. This subsequently enhances their retention in lungs, but not in bone, by disruption of vascular endothelial cell-cell junctions which increases permeability of lung capillaries, and facilitates trans-endothelial passage of tumor cells 95. These results indicate that a cytokine in the primary tumor microenvironment can induce expression of another cytokine in exiting tumor cells thus enabling those cells to disrupt lung capillary walls and seed pulmonary metastases.
Immune cell-support for colonization and organ-specific metastasis is mediated also by non-chemokine mechanisms: The two NF-kB targets, S100A8 and S100A9 are inflammatory mediators with chemotactic activity expressed and secreted by neoplastic cells, as well as by tumor-associated myeloid cells, and are associated with metastasis and a poor outcome in a variety of human tumors 96. Acting through the RAGE receptor (receptor for advanced glycation end-products), these induce migration of myeloid cells with T cell suppressive activity into mammary tumors in murine models of mammary and squamous carcinogenesis. Recruited suppressive myeloid cells facilitate tumor progression by inhibiting T and NK cell activation, and by polarizing immunity toward a tumor-promoting type 2 phenotype 97, 98. Recently, S100A8/S100A9 were also implicated in site-specific colonization of melanoma to lungs: lack of the endogenous anti-inflammatory protein uteroglobin in mice leads to over expression of S100A8/S100A9. Over expression results in induction of MMP expression by neoplastic cells, and chemoattraction of melanoma cells according to the S100A8/S100A9 gradient, thus enhancing colonization of B16 melanoma in lungs 99. Others studies implicate S100A8/S100A9 via secretion into pre-metastatic lungs by neoplastic cells, that in turn induce expression of serum amyloid A (SAA) 3, that acts through Toll-like receptor (TLR) 4 to recruit additional myeloid cells, thus creating an inflammatory environment that accelerates migration of primary tumor cells to lung parenchyma 100.
Leukocyte support for tumor cell survival and colonization at the metastatic organ
The temporal gap between infiltration to distant organs and the ability to colonize and form macrometastases, a process sometimes requiring decades, depending on tumor type, suggests that in order to grow at the metastatic site, disseminated tumor cells must acquire an ability to “educate” their new microenvironment to support their own survival. Although changes in the metastatic microenvironment that enable growth of disseminated cells are poorly defined, emerging data indicate that immune-mediated signaling plays an important role. During the earliest stages of liver metastasis, microvascular arrest of neoplastic cells triggers a local inflammatory response: tumor-secreted vascular endothelial growth factor (VEGF) induces expression of pro-inflammatory cytokines by sinusoidal endothelial cells resulting in upregulation of adhesion molecules such as VCAM-1, allowing arrest of metastatic melanoma cells 101. Another prometastatic mechanism in liver supporting survival of CTCs is mediated by tumor-activated proinflammatory cytokine signaling by liver stellate cells, hepatocytes and myofibroblasts. These are recruited into sites of avascular micrometastases and create a microenvironment that supports metastatic growth through specific release of both proangiogenic factors and tumor cell invasion- and proliferation-stimulating factors provided by tumor-activated hepatocytes and myofibroblasts 102.
An intriguing mechanism by which tumor cells take advantage of immune pathways to increase their metastatic potential is the ectopic expression of FcγRIIB by metastatic melanoma cells. FcγRIIB is an inhibitory low-affinity receptor for IgG that terminates activation signals initiated by antigen cross-linking of the B cell receptor through its inhibitory ITIM motif 103. 40% of human metastatic melanomas gain expression of FcγRIIB, in particular, in liver metastases (69%), suggesting that gain of expression supports their survival in liver by escaping humoral immunity 104, 105. Experimental studies with B16 melanoma cells in immunocompetent mice indicate that tumor-expressed FcγRIIB operates as a decoy receptor inhibiting ADCC (Antibody-Dependent Cell Cytotoxicity) mediated by tissue macrophages, neutrophils and NK cells, that are abundant in liver: anti-tumor antibodies bind tumor cells via Fab domains while the Fc portion is “caught” by the tumor FcγRIIB and cannot be recognized by FcγR of the effector cells106. Thus, liver offers a prometastatic microenvironment that supports metastasis of cancer cells able to resist anti-tumor hepatic defenses, and takes advantage of hepatic cell-derived factors that are key phenotypic properties of liver-metastasizing cancer cells.
The bone and bone marrow are also among the most frequent sites of cancer metastasis. During bone metastasis, breast carcinoma cells, through secretion of IL-6, IL-11, TNFα and parathyroid-hormone-related peptide (PTHRP) are able to activate osteoclasts through RANKL (receptor activator of nuclear factor-B ligand), critical for formation of osteolytic metastases 107. In other cancer types, such as neuroblastomas, IL-6 is secreted by bone marrow stromal cells and promotes osteolysis through the induction of RANKL in osteoblasts as well as in tumor cells 108. NF-κB-regulated signaling in breast carcinoma cells promotes osteolytic bone metastasis by induction of osteoclastogenesis via GM-CSF 109. Late-stage breast cancers also metastasize to brain, where recruitment of glial cells and a brain inflammatory response correlates with tumor cell proliferation and growth in both experimental metastasis in mice and in human brain metastases 110.
A pre-metastatic niche
Several studies suggest that primary tumors can “prepare” the distant target organ of metastasis by creating a pre-metastatic niche 111 whereas others studies indicate that a small number of metastatic cells activate their new microenvironment upon arrival 112. Regardless, neoplastic cells secrete factors that mobilize bone marrow-derived vascular endothelial growth factor receptor (VEGFR)1-expressing hematopoietic progenitor cells to sites of metastasis that induce expression of fibronectin by resident fibroblasts, thus creating favorable conditions for arrival of would-be metastatic cells 113.
Gr1+CD11b+ myeloid cells have also been identified as playing a potential role in mediating changes that activate pre-metastatic lung into a permissive haven by diminishing immune protective programs 114. Mammary tumor cells growing in mammary pads remotely activate expression of TARC/CCL17 and MDC/CCL22 in lungs. These chemokines acting through CCR4 attract both tumor and immune cells 36. Distant primary tumor-derived factors induce the expression of the inflammatory chemoattractants S100A8 and S100A9, which in turn attract Mac1+ myeloid cells to premetastatic lungs mediated by Toll-like receptor 4 (TLR-4) expressing cells that accelerate migration of primary tumor cells to lung tissues 100, 115. Lysyl oxidase (LOX) is a tumor-cell derived factor often induced in primary tumors in response to hypoxia 116. However, systemic secretion of LOX leads to its accumulation in the lung, where it has been found to act on extracellular matrix proteins establishing a permissive niche for infiltrating cancer cells by crosslinking collagen IV in basement membranes, and by recruiting CD11b+ myeloid cells that adhere to crosslinked collagen IV, produce MMP2, and thereby enhance the invasion and recruitment of BMDCs and metastasizing tumor cells 117. These data indicate that, through multiple mechanisms, creation of a pro-inflammatory microenvironment in metastatic organs, whether that be prior to or at the time of malignant cell arrival, enhances the survival and proliferative possibilities for metastatic cells.
Implications for therapy and perspectives
Elucidating the changes in metastatic microenvironments is a significant clinical goal for eradicating cancer-associated death. Immune-based signaling pathways have emerged as central players in facilitating growth of micrometastases into clinically relevant macrometastases. Anti-cancer therapies that target these programs are gaining attention and in a few cases are being evaluated in clinical trials 118. Denosumab, an anti-RANKL antibody, originally developed for treatment of osteoporosis, and has been found effective for inhibiting bone metastasis in prostate cancer 119. Disruption of tumor cell-adhesion to protective stroma by targeting the CXCR4-CXCL12 axis using a small molecule CXCR4 antagonists, such as Plerixafor (AMD3100) is a novel, attractive therapeutic approach being explored in ongoing clinical trials for metastatic multiple myeloma, leukemia and other types of cancer 120, 121
Several therapeutic agents that limit IL-1 activity are approved for treating chronic inflammatory diseases, e.g., recombinant IL-1Rα (anakinra), neutralizing monoclonal antibodies to IL-1β and a soluble receptor to IL-1, that have also been found to exert benefits in animal models of metastasis and tumor-associated angiogenesis. A goal for the future would be to evaluate this activity in clinical trials of IL-1 blockade 122. Despite their critical involvement in invasion and metastasis, there has been conflicting results with anti-proteases, possibly due to of anti-tumorigenic activity of some enzymes 123.
As cancer research and clinical oncology progress increasingly towards a new era of integrative cancer therapy based on combinatorial drug regimens that act synergistically by targeting intrinsic pathways in neoplastic cells, as well as extrinsic pro-oncogenic pathways in the tumor microenvironment, the intensive research in deciphering the role of the metastatic microenvironment and of tumor-promoting inflammation will hopefully result in the coming years in innovative therapeutic strategies.
Figure. Immune signaling in tumor microenvironments facilitates all stages of tumorigenesis.
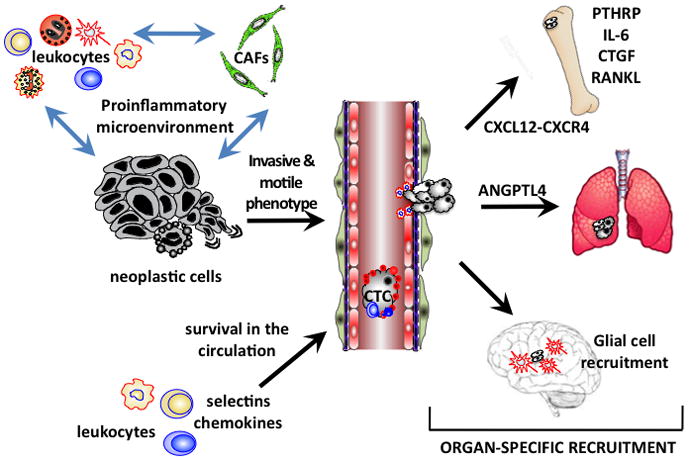
Soluble mediators secreted by infiltrating and resident leukocytes and by carcinoma-associated fibroblasts (CAFs) within primary tumor sites support signaling programs within neoplastic cells that enable motile and invasive growth. Survival of circulating tumor cells (CTCs) in peripheral blood is facilitated by platelets, neutrophils and production of selectins and chemokine receptors. Organ-specific metastasis is directed by differential expression of chemokines and their receptors that together promote extravasation and retention of CTCs in distal organs. Colonization of distal organs is accomplished by mobilization of leukocytes and other stromal cells in ectopic organs, such as activation of osteoclasts in bone, and recruitment of glial cells in brain.
Acknowledgments
The authors thank members of their laboratories for critical discussion and acknowledge support from the NIH/NCI R01CA130980, R01CA132566, R01CA140943, P50CA58207, and the Department of Defense (W81XWH-06-1-0416, PR080717) to LMC.
Contributor Information
Neta Erez, Email: netaerez@post.tau.ac.il.
Lisa M. Coussens, Email: Lisa.Coussens@ucsf.edu.
References
- 1.Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787. doi: 10.1038/427787a. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 3.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 4.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 7.Koehne CH, Dubois RN. COX-2 inhibition and colorectal cancer. Semin Oncol. 2004;31:12–21. doi: 10.1053/j.seminoncol.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 8.Howe LR, Dannenberg AJ. COX-2 inhibitors for the prevention of breast cancer. J Mammary Gland Biol Neoplasia. 2003;8:31–43. doi: 10.1023/a:1025731204719. [DOI] [PubMed] [Google Scholar]
- 9.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–13. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, Santoriello C, Mione M, Hurlstone A, Martin P. Live Imaging of Innate Immune Cell Sensing of Transformed Cells in Zebrafish Larvae: Parallels between Tumor Initiation and Wound Inflammation. PLoS Biol. 2010;8:e1000562. doi: 10.1371/journal.pbio.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 12.DeNardo DG, Brennan D, Rexhapaj E, Ruffel B, Shiao S, Gallagher WM, Wadhani N, Kial SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, et al. Leukocyte complexity in breast cancer predicts overall survival and functionally regulates response to chemotherapy manuscript submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27:11–8. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 14.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 15.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 16.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang R, Beuvon F, Ojeda M, Mosseri V, Pouillart P, Scholl S. M-CSF (monocyte colony stimulating factor) and M-CSF receptor expression by breast tumour cells: M-CSF mediated recruitment of tumour infiltrating monocytes? J Cell Biochem. 1992;50:350–6. doi: 10.1002/jcb.240500403. [DOI] [PubMed] [Google Scholar]
- 18.Wei S, Nandi S, Chitu V, Yeung YG, Yu W, Huang M, Williams LT, Lin H, Stanley ER. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J Leukoc Biol. 2010;88:495–505. doi: 10.1189/jlb.1209822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chihara T, Suzu S, Hassan R, Chutiwitoonchai N, Hiyoshi M, Motoyoshi K, Kimura F, Okada S. IL-34 and M-CSF share the receptor Fms but are not identical in biological activity and signal activation. Cell Death Differ. 2010 doi: 10.1038/cdd.2010.60. [DOI] [PubMed] [Google Scholar]
- 20.Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–11. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- 21.Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep. 2005;14:425–31. [PubMed] [Google Scholar]
- 22.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolat F, Kayaselcuk F, Nursal TZ, Yagmurdur MC, Bal N, Demirhan B. Microvessel density, VEGF expression, and tumor-associated macrophages in breast tumors: correlations with prognostic parameters. J Exp Clin Cancer Res. 2006;25:365–72. [PubMed] [Google Scholar]
- 24.Chen JJ, Lin YC, Yao PL, Yuan A, Chen HY, Shun CT, Tsai MF, Chen CH, Yang PC. Tumor-associated macrophages: the double-edged sword in cancer progression. J Clin Oncol. 2005;23:953–64. doi: 10.1200/JCO.2005.12.172. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Patel L, Pienta KJ. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev. 2010;21:41–8. doi: 10.1016/j.cytogfr.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell MJ, Tonlaar NY, Garwood ER, Huo D, Moore DH, Khramtsov AI, Au A, Baehner F, Chen Y, Malaka DO, Lin A, Adeyanju OO, et al. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–40. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 29.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–55. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zabuawala T, Taffany DA, Sharma SM, Merchant A, Adair B, Srinivasan R, Rosol TJ, Fernandez S, Huang K, Leone G, Ostrowski MC. An ets2-driven transcriptional program in tumor-associated macrophages promotes tumor metastasis. Cancer Res. 2010;70:1323–33. doi: 10.1158/0008-5472.CAN-09-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forst B, Hansen MT, Klingelhofer J, Moller HD, Nielsen GH, Grum-Schwensen B, Ambartsumian N, Lukanidin E, Grigorian M. Metastasis-inducing S100A4 and RANTES cooperate in promoting tumor progression in mice. PLoS One. 2010;5:e10374. doi: 10.1371/journal.pone.0010374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grum-Schwensen B, Klingelhofer J, Grigorian M, Almholt K, Nielsen BS, Lukanidin E, Ambartsumian N. Lung metastasis fails in MMTV-PyMT oncomice lacking S100A4 due to a T-cell deficiency in primary tumors. Cancer Res. 2010;70:936–47. doi: 10.1158/0008-5472.CAN-09-3220. [DOI] [PubMed] [Google Scholar]
- 34.Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, Cheresh DA, Karin M. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–4. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 35.Affara NI, Coussens LM. IKKalpha at the crossroads of inflammation and metastasis. Cell. 2007;129:25–6. doi: 10.1016/j.cell.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 36.Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, Deng J, Xu M, Briest S, Biragyn A. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nozawa F, Hirota M, Okabe A, Shibata M, Iwamura T, Haga Y, Ogawa M. Elastase activity enhances the adhesion of neutrophil and cancer cells to vascular endothelial cells. J Surg Res. 2000;94:153–8. doi: 10.1006/jsre.2000.6002. [DOI] [PubMed] [Google Scholar]
- 38.Xiang M, Gu Y, Zhao F, Lu H, Chen S, Yin L. Mast cell tryptase promotes breast cancer migration and invasion. Oncol Rep. 2010;23:615–9. doi: 10.3892/or_00000676. [DOI] [PubMed] [Google Scholar]
- 39.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16:133–44. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18:11–8. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–90. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 43.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–26. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135–47. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–28. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 47.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119:1417–9. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–14. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maier HJ, Schmidt-Strassburger U, Huber MA, Wiedemann EM, Beug H, Wirth T. NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett. 2010;295:214–28. doi: 10.1016/j.canlet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Cheng GZ, Zhang WZ, Sun M, Wang Q, Coppola D, Mansour M, Xu LM, Costanzo C, Cheng JQ, Wang LH. Twist is transcriptionally induced by activation of STAT3 and mediates STAT3 oncogenic function. J Biol Chem. 2008;283:14665–73. doi: 10.1074/jbc.M707429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–7. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rundqvist H, Johnson RS. Hypoxia and metastasis in breast cancer. Curr Top Microbiol Immunol. 2010;810:121–39. doi: 10.1007/82_2010_77. [DOI] [PubMed] [Google Scholar]
- 55.Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–90. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 56.Cronin PA, Wang JH, Redmond HP. Hypoxia increases the metastatic ability of breast cancer cells via upregulation of CXCR4. BMC Cancer. 2010;10:225. doi: 10.1186/1471-2407-10-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tafani M, Russo A, Di Vito M, Sale P, Pellegrini L, Schito L, Gentileschi S, Bracaglia R, Marandino F, Garaci E, Russo MA. Up-regulation of pro-inflammatory genes as adaptation to hypoxia in MCF-7 cells and in human mammary invasive carcinoma microenvironment. Cancer Sci. 2010;101:1014–23. doi: 10.1111/j.1349-7006.2010.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berdowska I. Cysteine proteases as disease markers. Clin Chim Acta. 2004;342:41–69. doi: 10.1016/j.cccn.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 59.Gocheva V, Joyce JA. Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle. 2007;6:60–4. doi: 10.4161/cc.6.1.3669. [DOI] [PubMed] [Google Scholar]
- 60.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Littlepage LE, Sternlicht MD, Rougier N, Phillips J, Gallo E, Yu Y, Williams K, Brenot A, Gordon JI, Werb Z. Matrix metalloproteinases contribute distinct roles in neuroendocrine prostate carcinogenesis, metastasis, and angiogenesis progression. Cancer Res. 2010;70:2224–34. doi: 10.1158/0008-5472.CAN-09-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gocheva V, Zeng W, Ke D, Klimstra D, Reinheckel T, Peters C, Hanahan D, Joyce JA. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006;20:543–56. doi: 10.1101/gad.1407406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Affara NI, Andreu P, Coussens LM. Delineating protease functions during cancer development. Methods Mol Biol. 2009;539:1–32. doi: 10.1007/978-1-60327-003-8_1. [DOI] [PubMed] [Google Scholar]
- 64.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 65.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–97. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–90. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, Lewis CE, Hanahan D. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10:329–40. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vasiljeva O, Papazoglou A, Kruger A, Brodoefel H, Korovin M, Deussing J, Augustin N, Nielsen BS, Almholt K, Bogyo M, Peters C, Reinheckel T. Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006;66:5242–50. doi: 10.1158/0008-5472.CAN-05-4463. [DOI] [PubMed] [Google Scholar]
- 69.Sevenich L, Werner F, Gajda M, Schurigt U, Sieber C, Muller S, Follo M, Peters C, Reinheckel T. Transgenic expression of human cathepsin B promotes progression and metastasis of polyoma-middle-T-induced breast cancer in mice. Oncogene. 2011 doi: 10.1038/onc.2010.387. [DOI] [PubMed] [Google Scholar]
- 70.Mohamed MM, Cavallo-Medved D, Rudy D, Anbalagan A, Moin K, Sloane BF. Interleukin-6 increases expression and secretion of cathepsin B by breast tumor-associated monocytes. Cell Physiol Biochem. 2010;25:315–24. doi: 10.1159/000276564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L, Pasqualini F, Nebuloni M, Chiabrando C, Mantovani A, Allavena P. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol. 2010;185:642–52. doi: 10.4049/jimmunol.1000413. [DOI] [PubMed] [Google Scholar]
- 72.Kawakubo T, Okamoto K, Iwata J, Shin M, Okamoto Y, Yasukochi A, Nakayama KI, Kadowaki T, Tsukuba T, Yamamoto K. Cathepsin E prevents tumor growth and metastasis by catalyzing the proteolytic release of soluble TRAIL from tumor cell surface. Cancer Res. 2007;67:10869–78. doi: 10.1158/0008-5472.CAN-07-2048. [DOI] [PubMed] [Google Scholar]
- 73.Gassmann P, Haier J. The tumor cell-host organ interface in the early onset of metastatic organ colonisation. Clin Exp Metastasis. 2008;25:171–81. doi: 10.1007/s10585-007-9130-6. [DOI] [PubMed] [Google Scholar]
- 74.Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104:397–401. doi: 10.1182/blood-2004-02-0434. [DOI] [PubMed] [Google Scholar]
- 75.Slattery MJ, Liang S, Dong C. Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. Am J Physiol Cell Physiol. 2005;288:C831–9. doi: 10.1152/ajpcell.00439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slattery MJ, Dong C. Neutrophils influence melanoma adhesion and migration under flow conditions. Int J Cancer. 2003;106:713–22. doi: 10.1002/ijc.11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McDonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, Ferri LE. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer. 2009;125:1298–305. doi: 10.1002/ijc.24409. [DOI] [PubMed] [Google Scholar]
- 78.Kochetkova M, Kumar S, McColl SR. Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell Death Differ. 2009;16:664–73. doi: 10.1038/cdd.2008.190. [DOI] [PubMed] [Google Scholar]
- 79.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massague J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–9. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–23. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–49. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 82.Ben-Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis. 2008;25:345–56. doi: 10.1007/s10585-007-9097-3. [DOI] [PubMed] [Google Scholar]
- 83.Izraely S, Klein A, Sagi-Assif O, Meshel T, Tsarfaty G, Hoon DS, Witz IP. Chemokine-chemokine receptor axes in melanoma brain metastasis. Immunol Lett. 2010;130:107–14. doi: 10.1016/j.imlet.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 84.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 85.Marchesi F, Monti P, Leone BE, Zerbi A, Vecchi A, Piemonti L, Mantovani A, Allavena P. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004;64:8420–7. doi: 10.1158/0008-5472.CAN-04-1343. [DOI] [PubMed] [Google Scholar]
- 86.Monti P, Marchesi F, Reni M, Mercalli A, Sordi V, Zerbi A, Balzano G, Di Carlo V, Allavena P, Piemonti L. A comprehensive in vitro characterization of pancreatic ductal carcinoma cell line biological behavior and its correlation with the structural and genetic profile. Virchows Arch. 2004;445:236–47. doi: 10.1007/s00428-004-1053-x. [DOI] [PubMed] [Google Scholar]
- 87.Zhao FL, Guo W. Expression of stromal derived factor-1 (SDF-1) and chemokine receptor (CXCR4) in bone metastasis of renal carcinoma. Mol Biol Rep. 2010 doi: 10.1007/s11033-010-0200-5. [DOI] [PubMed] [Google Scholar]
- 88.Yoshitake N, Fukui H, Yamagishi H, Sekikawa A, Fujii S, Tomita S, Ichikawa K, Imura J, Hiraishi H, Fujimori T. Expression of SDF-1 alpha and nuclear CXCR4 predicts lymph node metastasis in colorectal cancer. Br J Cancer. 2008;98:1682–9. doi: 10.1038/sj.bjc.6604363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Na IK, Scheibenbogen C, Adam C, Stroux A, Ghadjar P, Thiel E, Keilholz U, Coupland SE. Nuclear expression of CXCR4 in tumor cells of non-small cell lung cancer is correlated with lymph node metastasis. Hum Pathol. 2008;39:1751–5. doi: 10.1016/j.humpath.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 90.Bartolome RA, Ferreiro S, Miquilena-Colina ME, Martinez-Prats L, Soto-Montenegro ML, Garcia-Bernal D, Vaquero JJ, Agami R, Delgado R, Desco M, Sanchez-Mateos P, Teixido J. The chemokine receptor CXCR4 and the metalloproteinase MT1-MMP are mutually required during melanoma metastasis to lungs. Am J Pathol. 2009;174:602–12. doi: 10.2353/ajpath.2009.080636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wagner PL, Hyjek E, Vazquez MF, Meherally D, Liu YF, Chadwick PA, Rengifo T, Sica GL, Port JL, Lee PC, Paul S, Altorki NK, et al. CXCL12 and CXCR4 in adenocarcinoma of the lung: association with metastasis and survival. J Thorac Cardiovasc Surg. 2009;137:615–21. doi: 10.1016/j.jtcvs.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 92.Xiang Z, Zeng Z, Tang Z, Fan J, Sun H, Wu W, Tan Y. Increased expression of vascular endothelial growth factor-C and nuclear CXCR4 in hepatocellular carcinoma is correlated with lymph node metastasis and poor outcome. Cancer J. 2009;15:519–25. doi: 10.1097/PPO.0b013e3181c6aa6b. [DOI] [PubMed] [Google Scholar]
- 93.Richmond A. CCR9 homes metastatic melanoma cells to the small bowel. Clin Cancer Res. 2008;14:621–3. doi: 10.1158/1078-0432.CCR-07-2235. [DOI] [PubMed] [Google Scholar]
- 94.Kitamura T, Fujishita T, Loetscher P, Revesz L, Hashida H, Kizaka-Kondoh S, Aoki M, Taketo MM. Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model. Proc Natl Acad Sci U S A. 2010;107:13063–8. doi: 10.1073/pnas.1002372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massague J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gebhardt C, Nemeth J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol. 2006;72:1622–31. doi: 10.1016/j.bcp.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 97.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–75. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gebhardt C, Riehl A, Durchdewald M, Nemeth J, Furstenberger G, Muller-Decker K, Enk A, Arnold B, Bierhaus A, Nawroth PP, Hess J, Angel P. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205:275–85. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saha A, Lee YC, Zhang Z, Chandra G, Su SB, Mukherjee AB. Lack of an endogenous anti-inflammatory protein in mice enhances colonization of B16F10 melanoma cells in the lungs. J Biol Chem. 2010;285:10822–31. doi: 10.1074/jbc.M109.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, Shibuya M, Akira S, Aburatani H, Maru Y. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10:1349–55. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 101.Mendoza L, Valcarcel M, Carrascal T, Egilegor E, Salado C, Sim BK, Vidal-Vanaclocha F. Inhibition of cytokine-induced microvascular arrest of tumor cells by recombinant endostatin prevents experimental hepatic melanoma metastasis. Cancer Res. 2004;64:304–10. doi: 10.1158/0008-5472.can-03-1829. [DOI] [PubMed] [Google Scholar]
- 102.Vidal-Vanaclocha F. The prometastatic microenvironment of the liver. Cancer Microenviron. 2008;1:113–29. doi: 10.1007/s12307-008-0011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brown KS, Blair D, Reid SD, Nicholson EK, Harnett MM. FcgammaRIIb-mediated negative regulation of BCR signalling is associated with the recruitment of the MAPkinase-phosphatase, Pac-1, and the 3′-inositol phosphatase, PTEN. Cell Signal. 2004;16:71–80. doi: 10.1016/s0898-6568(03)00113-x. [DOI] [PubMed] [Google Scholar]
- 104.Cohen-Solal JF, Cassard L, Fournier EM, Loncar SM, Fridman WH, Sautes-Fridman C. Metastatic melanomas express inhibitory low affinity fc gamma receptor and escape humoral immunity. Dermatol Res Pract. 2010;2010:657406. doi: 10.1155/2010/657406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ran M, Katz B, Kimchi N, Halachmi E, Teillaud JL, Even J, Berko-Flint Y, Atlas E, Fridman WH, Witz IP. In vivo acquisition of Fc gamma RII expression on polyoma virus-transformed cells derived from tumors of long latency. Cancer Res. 1991;51:612–8. [PubMed] [Google Scholar]
- 106.Cassard L, Cohen-Solal JF, Fournier EM, Camilleri-Broet S, Spatz A, Chouaib S, Badoual C, Varin A, Fisson S, Duvillard P, Boix C, Loncar SM, et al. Selective expression of inhibitory Fcgamma receptor by metastatic melanoma impairs tumor susceptibility to IgG-dependent cellular response. Int J Cancer. 2008;123:2832–9. doi: 10.1002/ijc.23870. [DOI] [PubMed] [Google Scholar]
- 107.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 108.Ara T, Declerck YA. Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer. 2010;46:1223–31. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Giordano T, Gu K, Shah V, Pei L, Zarbo RJ, McCauley L, Shi S, et al. NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med. 2007;13:62–9. doi: 10.1038/nm1519. [DOI] [PubMed] [Google Scholar]
- 110.Fitzgerald DP, Palmieri D, Hua E, Hargrave E, Herring JM, Qian Y, Vega-Valle E, Weil RJ, Stark AM, Vortmeyer AO, Steeg PS. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metastasis. 2008;25:799–810. doi: 10.1007/s10585-008-9193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the ‘pre-metastatic niche’: within bone and beyond. Cancer Metastasis Rev. 2006;25:521–9. doi: 10.1007/s10555-006-9036-9. [DOI] [PubMed] [Google Scholar]
- 112.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2009;21:11–9. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, Geng Y, Gray JW, Moses HL, Yang L. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–49. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–75. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 116.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–6. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 117.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–71. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 119.Vallet S, Smith MR, Raje N. Novel bone-targeted strategies in oncology. Clin Cancer Res. 2010;16:4084–93. doi: 10.1158/1078-0432.CCR-10-0600. [DOI] [PubMed] [Google Scholar]
- 120.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 121.Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, Jia X, Wright R, Ospina B, Carlson AL, Alt C, Burwick N, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–51. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dinarello CA. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 2010;29:317–29. doi: 10.1007/s10555-010-9229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Overall CM, Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–39. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]


