Abstract
Nephropathy is a common microvascular complication among patients with type 2 diabetes mellitus and a major cause of kidney failure. It is characterized by albuminuria (≥300 mg/d) and a reduced glomerular filtration rate and is often present at the time of diabetes diagnosis after the kidney has been exposed to chronic hyperglycemia during the prediabetic phase. A low glomerular filtration rate (<60 mL/min/1.73 m2) is also an independent risk factor for cardiovascular events and death. Detection of diabetic nephropathy during its initial stages provides the opportunity for early therapeutic interventions to prevent or delay the onset of complications and improve outcomes. An intensive and multifactorial management approach is needed that targets all risk determinants simultaneously. The strategy should comprise lifestyle modifications (smoking cessation, weight loss, increased physical activity, and dietary changes) coupled with therapeutic achievement of blood glucose, blood pressure, and lipid goals that are evidence-based. Prescribing decisions should take into account demographic factors, level of kidney impairment, adverse effects, risk of hypoglycemia, tolerability, and effects on other risk factors and comorbidities. Regular and comprehensive follow-up assessments with appropriate adjustment of the therapeutic regimen to maintain risk factor control is a vital component of care, including referral to specialists, when required.
AACE = American Association of Clinical Endocrinologists; ACCORD = Action to Control Cardiovascular Risk in Diabetes; ACE = angiotensin-converting enzyme; ADA = American Diabetes Association; ARB = angiotensin receptor blocker; BMI = body mass index; CI = confidence interval; CKD = chronic kidney disease; CrCl = creatinine clearance; eGFR = estimated glomerular filtration rate; ESRD = end-stage renal disease; GFR = glomerular filtration rate; HbA1c = hemoglobin A1c; HDL-C = high-density lipoprotein cholesterol; HR = hazard ratio; LDL-C = low-density lipoprotein cholesterol; MDRD = Modification of Diet in Renal Disease; NKF = National Kidney Foundation; RR = relative risk
Diabetes is estimated to affect 23.5 million (10.7%) of Americans 20 years of age or older1; it is a major cause of chronic kidney disease (CKD) and is recognized as the most common cause of end-stage renal disease (ESRD) in the United States.2-4 Approximately 40% of US adults with diagnosed or undiagnosed diabetes had some degree of CKD in the 1999-2006 National Health and Nutrition Examination Survey.4-6 Even among adults with undiagnosed diabetes or prediabetes, the prevalence of kidney damage or dysfunction was substantial (17.7%).6 The presence of CKD also adds considerably to the cost of diabetes management.4,7 For example, a recent analysis of a US managed care database showed that total direct health care costs were significantly higher for patients with diabetes and CKD than for those with diabetes alone (unadjusted annualized mean per patient cost, $18,444 vs $6631; P<.001).7
The mechanisms involved in the pathogenesis of diabetic nephropathy are multiple and complex. Early hemodynamic changes of glomerular hyperperfusion and hyperfiltration are followed by leakage of albumin from the glomerular capillaries and structural changes such as glomerular basement membrane thickening, glomerular hypertrophy, glomerulosclerosis, mesangial cell expansion, and podocyte injury and loss.8 Clinical manifestations of diabetic nephropathy include a decrease in the glomerular filtration rate (GFR) and an increase in levels of urinary albumin excretion (although a substantial proportion of patients with type 2 diabetes mellitus have a low GFR without albuminuria9). Pathologic findings on kidney biopsy in patients with type 2 diabetes are complex and heterogeneous.10 Nevertheless, only about one-third of patients with diabetes develop nephropathy. Poorly controlled glucose levels, blood pressure, and cholesterol activate inflammatory mediators, and patients with a genetic predisposition progress to advanced stage nephropathy.8,11
Besides heralding the onset of deteriorating kidney function in patients with diabetes, albuminuria is an independent risk marker for all-cause mortality and adverse cardiovascular events, including myocardial infarction, stroke, hospitalization for congestive heart failure, and peripheral artery disease.3,12-14 Cardiovascular risk also increases proportionally and independently as the GFR declines in patients with type 2 diabetes mellitus.15,16
The precise pathophysiologic basis for the association between deteriorating kidney function and cardiovascular disease is unclear, although a number of hypotheses have been proposed. Many patients with long-standing diabetes have generalized atherosclerosis (clinical or subclinical). This is manifested in a variety of ways, including increased vascular stiffness with wide pulse pressures and significant
Article Highlights
Early detection of diabetic nephropathy during its initial stages provides opportunity for therapeutic interventions to prevent or delay onset of complications and improve outcomes
Diabetic nephropathy is characterized by albuminuria (≥300 mg/d) and a reduced glomerular filtration rate (GFR) often present at the time of diabetes diagnosis
Presence of stage 3 or higher chronic kidney disease (estimated GFR <60 mL/min/1.73 m2) is associated with a high cardiovascular risk
An intensive and multifactorial management approach is needed that targets all risk determinants simultaneously. The strategy should comprise lifestyle modifications (smoking cessation, weight loss, increased physical activity, and dietary changes) coupled with therapeutic achievement of blood glucose, blood pressure, and lipid goals that are evidence-based
Treating abnormal lipid profiles in people with advanced kidney disease with low doses of 2 different agents reduces cardiovascular mortality
Small limited and sustained increases in serum creatinine of up to 30% after institution of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker should be tolerated because they are associated with slower declines in kidney function if blood pressure is controlled
Micro- or low levels of albuminuria (30-299 mg/d) is NOT indicative of diabetic nephropathy but is associated with endothelial dysfunction and heightened cardiovascular risk
increases in serum creatinine when renin–angiotensin system blockers are given. Although these findings suggest the presence of renal artery stenosis, later investigations show no evidence of renal artery stenosis. This intrarenal hypoperfusion leads to low-grade ischemia and can harm the kidney over the long term.17 In addition, increasing levels of albuminuria reflect systemic vascular endothelial dysfunction and injury to the podocytes.18-20
Hyperglycemic damage to the vascular glycocalyx18,21 may disrupt its function as a barrier between the blood and the endothelium and its role in regulating vascular permeability to macromolecules, adhesion of circulating cells, and flow-mediated dilatation. Other factors implicated include reduced levels of vitamin D that contribute to increased vascular calcifications.22
Diabetes is considered a coronary heart disease risk equivalent (ie, it confers a level of risk for major coronary events equal to that of existing heart disease)23 and, as discussed previously, CKD also imparts a high level of risk for cardiovascular disease.3,24 Thus, for patients with both diabetes and CKD, the risk of cardiovascular events is extremely high. A multifaceted management strategy aimed at controlling CKD risk factors (many of which are also cardiovascular risk factors) is advocated3,25,26 and is associated with improvements in both kidney and cardiovascular outcomes.27-29 Such an approach should target hyperglycemia, hypertension, dyslipidemia, obesity, smoking, and platelet activity according to evidence-based recommendations with appropriate agents and lifestyle modifications.
Primary prevention of CKD, early detection of disease, and prompt intervention with appropriate, evidence-based measures will delay CKD onset or progression, improve kidney and cardiovascular outcomes, and reduce resource utilization.30 Despite these benefits, CKD is both underdiagnosed and undertreated,31-34 and awareness of CKD among patients and providers is low.3 Improvements in CKD screening among patients with diabetes (a high-risk population) and proactive implementation of an early, intensive, and multifactorial management strategy are needed to reduce the burden of CKD in this population.27,35
SCREENING AND DIAGNOSIS
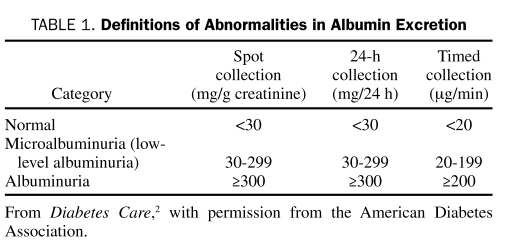
Professional bodies concerned with management of patients with diabetes recommend that all patients with type 2 diabetes be screened annually for CKD, starting at diagnosis.3,25,26 Urinary albumin excretion should be evaluated from the albumin-to-creatinine ratio in a random spot sample (Table 1). Because of fluctuations in urinary albumin excretion, at least 2 of 3 samples collected within a 3- to 6-month time frame should be used to categorize the degree of albuminuria and avoid false-positive results. Common causes of transient increases in albuminuria into microalbuminuria (currently termed low-level albuminuria [30-299 mg/d]) include fever, high-salt diet, vigorous exercise in the previous 24 hours, any infection, dehydration, hematuria, marked hyperglycemia, very high blood pressure, and congestive heart failure.3,26
TABLE 1.
Definitions of Abnormalities in Albumin Excretion
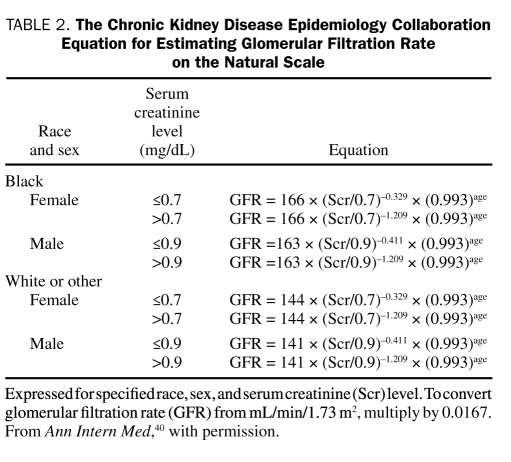
Evaluation of urinary albumin excretion alone is insufficient to assess the presence and severity of CKD because some patients with type 2 diabetes can have advanced stage nephropathy in the absence of albuminuria.36,37 Serum creatinine should also be measured annually in all patients; the GFR is then estimated from serum creatinine and used to stage the level of CKD.3,25,26 Laboratories report estimated GFR (eGFR) alongside serum creatinine. Otherwise, the Modification of Diet in Renal Disease (MDRD) equation can be used to calculate eGFR from the serum creatinine value.38,39 This equation takes into account the variables of age, sex, and ethnicity, which are important determinants of serum creatinine. For example, in elderly patients, the age-related decrease in GFR is not paralleled by an increase in serum creatinine levels because of a concomitant age-related decline in creatinine generation.38 An eGFR calculator based on the MDRD formula is available on the National Kidney Disease Education Program Web site at http://www.nkdep.nih.gov/professionals/gfr_calculators/orig_con.htm. The recently proposed Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation40 appears to provide a more accurate estimate of GFR from serum creatinine than the MDRD equation, especially at higher GFR values.41 This equation more closely resembles the actual measured GFR, in that is has reduced the bias in the equation. It has made the equation far more accurate, especially in people with a GFR greater than 60 mL/min/1.73 m2. Measurements of GFR using the older MDRD equation, currently used by most laboratories, have been compared with GFR values obtained using the CKD-EPI equation.41 For individuals with an eGFR of 30 to 59 mL/min/1.73 m2, bias was decreased from 4.9 to 2.1 mL/min/1.73 m2 (57% improvement); for an eGFR of 60 to 89 mL/min/1.73 m2, bias was decreased from 11.9 to 4.2 mL/min/1.73 m2 (61% improvement); and for an eGFR of 90 to 119 mL/min/1.73 m2, bias was decreased from 10.0 to 1.9 mL/min/1.73 m2 (75% improvement). A limitation of this comparison of equations was the inclusion of a limited number of elderly and racial/ethnic minorities with measured GFR. Table 2 shows how to calculate GFR from the CKD-EPI equation, taking into account age, sex, race, and serum creatinine level.40
TABLE 2.
The Chronic Kidney Disease Epidemiology Collaboration Equation for Estimating Glomerular Filtration Rate on the Natural Scale
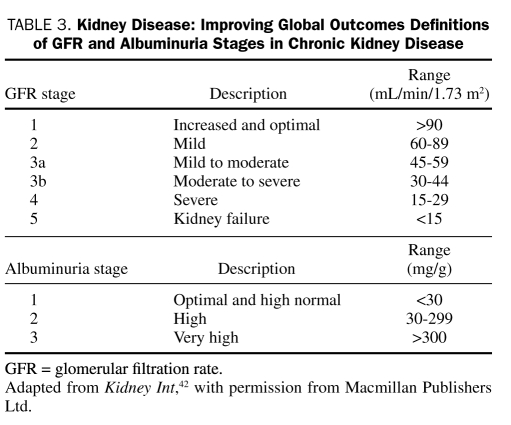
A new classification system for CKD has been proposed by Kidney Disease: Improving Global Outcomes (KDIGO), which defines different stages of GFR and categories of albuminuria (Table 3).42 Stages 1 and 2 are defined according to the GFR and presence of kidney damage on urinalysis, imaging, or biopsy; stages 3 through 5 are defined on the basis of GFR alone. These abnormalities must be present for at least 3 months to exclude cases of acute kidney injury. The likelihood of certain clinical diagnoses, including diabetic nephropathy, can be evaluated from consideration of both the GFR and the level of albuminuria.42 Findings suggesting an alternative cause of CKD include an absence of diabetic retinopathy, a low or rapidly decreasing GFR, rapidly increasing proteinuria or nephrotic syndrome, active urinary sediment, signs or symptoms of other systemic disease, or a greater than 30% decrease in GFR within 2 to 3 months after angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) initiation in the absence of dehydration, bilateral renal artery stenosis, or heart failure.3
TABLE 3.
Kidney Disease: Improving Global Outcomes Definitions of GFR and Albuminuria Stages in Chronic Kidney Disease
Detection of diabetic kidney disease is only of value if it triggers initiation and continual assessment of an effective management strategy by the primary care physician. Referral to a nephrologist is appropriate if the GFR deteriorates rapidly or if difficult management issues are encountered.
LIFESTYLE MODIFICATIONS
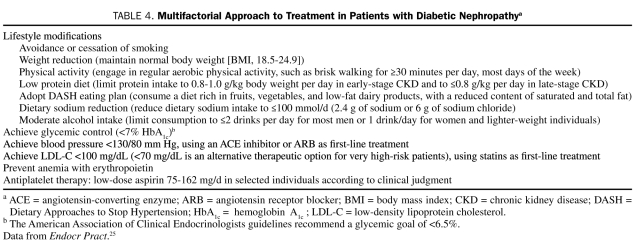
Lifestyle improvements are a first step in diabetes management, irrespective of the presence of CKD.25,26 These should comprise measures to encourage smoking cessation, weight loss, and increased physical activity, as well as dietary changes (Table 4).
TABLE 4.
Multifactorial Approach to Treatment in Patients with Diabetic Nephropathya
Smoking
Smoking appears to promote onset and progression of diabetic kidney disease.43-45 Several studies have reported a higher prevalence of microalbuminuria and macroalbuminuria and reduced GFR (<60 mL/min/1.73 m2) among patients with type 2 diabetes who smoked compared with their nonsmoking counterparts.45 A range of structural and functional kidney changes have been identified in smokers with diabetes, although the precise mechanisms by which smoking exerts its nephrotoxic effects require clarification. One hypothesis is that activation of multiple cellular pathways in smokers with diabetes results in accumulation of reactive oxygen species and a loss of kidney redox homeostasis.45 Smoking cessation appears to be effective at preventing progression of early nephropathy in patients with type 2 diabetes.44,46 Consequently, support should be provided to help patients quit smoking through education and counseling, smoking cessation delivery systems, and use of antismoking medications (eg, nicotine-replacement therapy).43,47 Smoking cessation will also reduce the cardiovascular risk.
Weight Loss
Many patients with type 2 diabetes are overweight (body mass index [BMI], calculated as the weight in kilograms divided by the height in meters squared, 25.0-29.9) or obese (BMI ≥30.0). These conditions are associated with an increased incidence and rate of progression of CKD as well as an increased risk of renal cell carcinoma and nephrolithiasis.48,50 In particular, obesity appears to independently heighten CKD risk and progression in the setting of diabetes.48 Adverse hemodynamic, structural, and functional changes are observed in the kidneys of obese individuals.48 However, as with smoking, the mechanisms behind such obesity-induced kidney injury, especially in a diabetic milieu, are complex and yet to be fully elucidated.48,49 Weight loss, by nonsurgical or surgical interventions, has been shown to reduce proteinuria and microalbuminuria and stabilize kidney function in various populations, including those with type 2 diabetes.51 These findings are likely to be attributable, in part, to a concomitant reduction in blood pressure. Additional long-term studies are needed to evaluate the durability of the effects of weight loss on the kidneys and whether they translate into improved outcomes, such as slowing the development of ESRD. Nevertheless, weight loss is recommended for overweight or obese patients with diabetes.52 Weight loss will not only improve glycemic control but will also reduce the risk of cardiovascular disease through beneficial effects on blood pressure, dyslipidemia, and serum markers of inflammation.52 The weight loss goal should be individualized to the patient and should be both achievable and maintainable; the National Kidney Foundation (NKF) recommends a target BMI of 18.5-24.9 (ie, within the normal range) for patients with diabetes and CKD.3 However, this target is unrealistic for most overweight or obese patients with type 2 diabetes and is rarely achieved. Weight management programs should comprise lifestyle measures (dietary restriction and increased physical activity) and antiobesity medications if needed, coupled with appropriate support and counseling.26,52 Bariatric surgery should be considered only for patients with type 2 diabetes with a BMI greater than 35.26
Dietary Modifications
Protein restriction may have benefits in patients with diabetic nephropathy in terms of slowing the progression of albuminuria, the decline in GFR, and the development of ESRD.3,25,26 Patients with early-stage CKD should be advised to limit their protein intake to 0.8 g/kg body weight per day; the target for those with late-stage CKD is 0.8 g/kg or lower per day.25,26 Adopting the Dietary Approaches to Stop Hypertension (DASH) diet, reducing sodium intake (≤2.4 g/d of sodium or ≤6 g of salt), and limiting alcohol consumption (≤2 drinks/day for most men or 1 drink/day in women and lighter-weight individuals) will have a positive effect on blood pressure.53,54 Dietary changes to improve diabetic dyslipidemia should comprise reductions in saturated fat, trans fat, and cholesterol intake, together with increases in omega-3 fatty acids (eg, 1 g/d of fish oil), viscous fiber, and plant stanols/sterols.25,26 Weight loss and increased physical activity also have beneficial effects on the lipid profile.
PRESERVING KIDNEY FUNCTION THROUGH GLYCEMIC CONTROL
Hyperglycemia is a contributing factor to diabetic complications, including diabetic nephropathy. Thus, good glycemic control is one logical measure that will help prevent development of kidney disease and may slow progression of existing CKD in patients with type 2 diabetes (Table 4).
Evidence for the Benefits of Glycemic Control on Kidney Function
In the UK Prospective Diabetes Study (UKPDS 33), patients with newly diagnosed type 2 diabetes were randomized to receive intensive glycemic control or conventional treatment.55 For 10 years, hemoglobin A1c(HbA1c) values were significantly lower with intensive vs conventional treatment (7.0% vs 7.9%), and reductions were also observed in the intensive treatment group in microalbuminuria (relative risk [RR] at 9 years for intensive strategy vs conventional strategy, 0.76; 99% confidence interval [CI], 0.62-0.91; P<.001), proteinuria (RR, 0.67; 99% CI, 0.42-1.07; P=.026), and a doubling of serum creatinine (RR, 0.40; 99% CI, 0.14-1.20; P=.027). Furthermore, early intensive glycemic control was associated with a long-term decrease in the risk of a composite microvascular end point, which included kidney failure (24% relative reduction at 10 years; P=.001).56
Intensive glucose control (HbA1c 6.5% vs 7.3% in the standard care comparator group) was also associated with a significant reduction in renal events in patients with type 2 diabetes in the Action in Diabetes and Vascular Disease–Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE).57 This included new or worsening nephropathy (4.1% vs 5.2%; hazard ratio [HR], 0.79; 95% CI, 0.66-0.93; P=.006), notably, the development of macroalbuminuria (2.9% vs 4.1%; HR, 0.70; 95% CI, 0.57-0.85; P<.001), as well as new-onset microalbuminuria (23.7% vs 25.7%; HR, 0.91; 95% CI, 0.85-0.98; P=.02). A trend toward a reduction in the need for renal replacement therapy or death from renal causes (0.4% vs 0.6%; HR, 0.64; 95% CI, 0.38-1.08; P=.09) was also observed, although there was no effect on doubling of serum creatinine (1.2% vs 1.1%; HR, 1.15; 95% CI, 0.82-1.63; P=.42). Similarly, in the Veterans Affairs Diabetes Trial (VADT), worsening of albuminuria (9.1% vs 13.8%; P=.01) and progression from normo- to micro- to macroalbuminuria (2.9% vs 5.1%; P=.04) was also significantly less in patients with type 2 diabetes who were assigned to intensive glycemic control vs standard therapy (HbA1c 6.9% vs 8.4%).58 However, the more intensive regimen had minimal (nonsignificant) effects on severe kidney complications (doubling of serum creatinine, creatinine >3 mg/dL, or GFR <15 mL/min) or on the rate of GFR decline.
In the Diabetes Control and Complications Trial (DCCT), significantly lower HbA1c levels were achieved in patients with type 1 diabetes who were receiving intensive insulin therapy compared with conventional insulin therapy.59 This superior glycemic control with intensive therapy was associated with significant reductions in the occurrence of microalbuminuria (39% reduction; 95% CI, 21%-52%) and albuminuria (54% reduction; 95% CI, 19%-74%). Follow-up studies showed persistence of the benefits in previously intensively treated patients, even though their glycemic control during the follow-up period was equivalent to that of patients receiving conventional therapy.60
Results of these clinical trials suggest that glycemic control has a beneficial effect on albuminuria in patients with type 1 and type 2 diabetes. It also plays a greater role as primary prevention in the early stages of microvascular disease development and a lesser role as secondary intervention after complications are more advanced. Thus, early attainment and maintenance of glycemic control would be expected to reduce the cumulative burden of chronic hyperglycemia and result in an even greater reduction in the risk of complications such as nephropathy.
Glycemic Goals
The primary target for hyperglycemia management is HbA1c, which should be controlled without inducing clinically important hypoglycemia.26 The American Diabetes Association (ADA) and NKF both recommend achieving an HbA1c level of 7.0% in most patients with diabetes, irrespective of the presence of CKD.3,26,61 Guidelines from the American Association of Clinical Endocrinologists (AACE) vary slightly, endorsing a more stringent HbA1c goal of 6.5% or less.25 The early termination of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial generated debate about whether lower is always better. Increased mortality was observed in this trial among patients assigned to intensive therapy (target HbA1c <6.0%) vs those assigned to standard therapy (target HbA1c 7.0%-7.9%).62 A post hoc analysis of the ACCORD data conducted to explore this finding showed that a higher average on-treatment HbA1c was a strong predictor of death.63 In the intensive therapy arm, the mortality risk increased approximately linearly over an average HbA1c range of 6.0% to 9.0%; the excess risk vs standard therapy was observed only among patients whose average on-treatment HbA1c remained higher than 7.0% (ie, the ADA HbA1c goal). Severe hypoglycemia was higher among patients receiving intensive therapy64 and was associated with an increased risk of death with both strategies.65 However, it did not appear to account for the excess mortality in the intensive treatment arm.65 Although a retrospective cohort analysis of data from patients with type 2 diabetes indicated a U-shaped relationship between HbA1c decile and mortality,66 no evidence from prospective studies supports this correlation.
The HbA1c targets proposed by the ADA, NKF, and AACE are general recommendations, and clinical judgment should be used to individualize each patient's goal.25,26,61,67 A balance should be sought between lowering HbA1c and the anticipated long-term benefits vs specific safety issues, taking into account the duration of diabetes, age/life expectancy, comorbidities, macro- or microvascular complications (including diabetic nephropathy), and patient awareness of hypoglycemia.
Antidiabetic Drug Treatment
Tight glycemic control, rather than use of any specific antidiabetic agent, appears to be the principal factor in decreasing the risk of microvascular complications in clinical trials.55 A treatment regimen tailored to the individual will maximize his or her likelihood of achieving and maintaining glycemic control while reducing the risk of adverse events. The choice of antidiabetic medication should take into account adverse effects, risk of hypoglycemia, tolerability, ease of use, long-term compliance, cost, nonglycemic effects on comorbidities or risk factors (eg, body weight, blood pressure, and the lipid profile), and any other specific patient considerations.
The presence of kidney disease brings an additional layer of complexity to the management of patients with type 2 diabetes. Kidney function should be assessed before initiation of antidiabetic therapy because the stage of CKD, if present, will influence antidiabetic drug selection.68 Any potentially harmful or protective effects on the kidneys beyond glucose lowering will also need to be considered in the prescribing decision. Patients with decreased kidney function (CKD stages 3-5) are particularly susceptible to hypoglycemia.3,69 Impaired clearance of insulin (which is excreted renally) and decreased insulin degradation in peripheral tissues predispose patients with CKD to hypoglycemic episodes.69,70 Hypoglycemia is also a concern among patients with diminished kidney function, especially at GFR levels below 30 mL/min/1.73 m2, who are receiving treatment with oral antidiabetic drugs that are primarily eliminated by the kidneys.3,69 Furthermore, CKD is associated with impaired kidney gluconeogenesis due to a decrease in tissue mass.3 Therefore, patients with CKD must monitor their glucose levels closely and reduce their dose of antidiabetic medication to prevent hypoglycemia, if necessary.
Metformin. Patients with mild to moderate CKD can be treated with metformin, but it is contraindicated at serum creatinine concentrations of 1.5 mg/dL or higher in men or 1.4 mg/dL or higher in women because of an increased risk of lactic acidosis.3,71,72 However, recent studies report use of metformin in patients with eGFRs as low as 45 mL/min/1.73 m2 with no problems.73 Thus, although use of metformin is reasonable in a young person with a creatinine concentration of 1.5 mg/dL, elderly patients with a creatinine concentration of 1.3 mg/dL may have an eGFR lower than 50 mL/min/1.73 m2 and hence have substantially decreased clearance of metformin. Consequently, a wiser approach is to assess eGFR and not use metformin if the eGFR is lower than 45 mL/min/1.73 m2. Hypoglycemia is unlikely with metformin monotherapy.3,71,72
Sulfonylureas. As kidney function declines, the clearance of sulfonylureas or their active metabolites also declines and increases the risk of hypoglycemic reactions.3 Therefore, the initial dose, subsequent dose titrations, and maintenance dosage should be conservative in patients with any degree of kidney dysfunction. First-generation sulfonylureas (acetohexamide, tolazamide, and tolbutamide) should generally be avoided in patients with stage 3 to 5 CKD; chlorpropamide can be used at a reduced dose in patients with a GFR of 50 to 70 mL/min/1.73 m2 but should be avoided if the GFR is lower than 50 mL/min/1.73 m2.3 Glipizide is the preferred second-generation sulfonylurea for patients with stage 3 to 5 CKD, although glimepiride can also be used if initiated at a low dose.3 Glyburide is substantially excreted by the kidney and should be avoided in patients with stage 3 to 5 CKD to avoid hypoglycemic reactions.3
Meglitinides. Both nateglinide and repaglinide can be given to patients with type 2 diabetes and CKD. No dose adjustment is required for nateglinide in patients with mild to severe kidney impairment74 or for repaglinide in patients with mild to moderate kidney impairment.75 However, the initial dose of repaglinide should be reduced in patients with severe kidney dysfunction.75
Alpha-Glucosidase Inhibitors. Acarbose and miglitol are suitable for patients with mild to moderate CKD but are not recommended for patients with severe kidney dysfunction (serum creatinine >2 mg/dL).3,76,77
Thiazolidinediones. No dose adjustment is necessary with either pioglitazone or rosiglitazone in patients with kidney impairment.78,79 Furthermore, these drugs have been shown to significantly decrease urinary albumin and protein excretion in patients with diabetes.80 Whether this translates into improved kidney outcomes in patients with diabetic nephropathy has yet to be investigated.
Glucagon-Like Peptide-1 Mimetics. Exenatide may be used without dose adjustment in patients with mild kidney dysfunction (creatinine clearance [CrCl], 50-80 mL/min),81 although care is necessary when initiating or increasing exenatide doses in patients with moderate kidney impairment (CrCl, 30-50 mL/min). Exenatide is not recommended for patients with severe kidney impairment (CrCl <30 mL/min) or ESRD. Experience with liraglutide in patients with kidney impairment is limited, and it should be used with caution in this population; no dose adjustment is required.82
Dipeptidyl Peptidase-4 Inhibitors. Reduction of the sitagliptin dose is unnecessary in patients with mild kidney impairment (CrCl ≥50 mL/min) but is recommended in patients with moderate (CrCl ≥30 to <50 mL/min) or severe (CrCl <30 mL/min) kidney impairment or ESRD.3,83 Likewise, a reduced dose of saxagliptin is advised for patients with moderate or severe kidney dysfunction (CrCl ≤50 mL/min) or ESRD.84 Vildagliptin (which is approved in Europe but not the United States) does not require dose reduction in patients with mild renal impairment; however, its use is not recommended in those with moderate or severe renal impairment or in hemodialysis patients with ESRD. Linagliptin, alogliptin, and dutogliptin are dipeptidyl peptidase -4 inhibitors currently in clinical development. Of these, linagliptin may hold promise as a new antidiabetic agent for patients with reduced kidney function because excretion via the kidneys is only a minor route of elimination.85
Insulin. Doses of insulin are not based on the degree of kidney function but should be titrated to achieve glycemic control without inducing hypoglycemia.3 Whichever hypoglycemic agent is selected, HbA1c levels and kidney function should be monitored regularly and the antidiabetic regimen adjusted accordingly. Because of the progressive nature of type 2 diabetes,86 most patients will require a combination of agents to maintain glycemic control over time. These combinations should be selected with care in patients with CKD.
PRESERVING KIDNEY FUNCTION THROUGH BLOOD PRESSURE CONTROL
Hypertension and diabetes are commonly associated, such that most individuals with diabetes also have hypertension.3,26,53 High blood pressure is a key pathogenic factor that contributes to kidney deterioration, and treatment of hypertension is probably the most important intervention in CKD management. Indeed, there is indisputable evidence from many large, randomized clinical trials that correct blood pressure control will delay the development and progression of diabetic kidney disease.87-91 Early studies such as the UKPDS 38 study provided some evidence that intensive blood pressure control in patients with type 2 diabetes and hypertension has a beneficial effect on kidney function.87 Patients (N=1148) were randomized to 2 levels of blood pressure control; mean blood pressure over 9 years in the 2 groups was 144/82 mm Hg vs 154/87 mm Hg (P<.0001). However, the lower blood pressure group showed no additional benefit with regard to the risk of proteinuria, fatal or nonfatal renal failure, or doubling of serum creatinine concentrations. Keep in mind that this achieved blood pressure level is well above the currently recommended target of less than 130/80 mm Hg. Nevertheless, blood pressure levels lower than 140/90 mm Hg have uniformly been shown to reduce cardiovascular risk.92
Blood Pressure Goals
Current guidelines recommend a blood pressure goal of 130/80 mm Hg for patients with type 2 (and type 1) diabetes, irrespective of the presence of CKD (Table 4).3,25,26,53 This target is based on the results of randomized clinical trials, such as the UKPDS studies87,88 and the Hypertension Optimal Treatment trial,93 that demonstrated the benefits of lowering blood pressure on both macro- and microvascular outcomes in patients with diabetes. Furthermore, early studies equated low levels of albuminuria (microalbuminuria) with kidney disease, and lower levels of blood pressure reduced albuminuria.89-91,94 We now know that low levels of albuminuria do not equate to the presence of kidney disease, but rather vascular inflammation and cardiovascular risk.8,95,96
Recent clinical trials suggest the presence of a J-shaped relationship between blood pressure and cardiovascular outcomes.97-99 Among patients with diabetes who participated in the International Verapamil SR-Trandolapril study,97 the risk of the primary end point (all-cause death, nonfatal myocardial infarction, or nonfatal stroke) was increased in the subset of patients with a diastolic blood pressure lower than 70 mm Hg. In the ACCORD blood pressure trial, no additional benefit on a similar composite end point (nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes) was observed at systolic blood pressures below 120 mm Hg.98 Furthermore, intensive antihypertensive therapy in ACCORD was associated with a higher incidence of serious adverse events.98 Thus, the current blood pressure goal of less than 130/80 mm Hg cannot be supported by data from prospective studies. A notable exception, however, is patients with advanced proteinuric kidney disease who have eGFRs lower than 50 mL/min/1.73 m2 with greater than 500 mg/d of proteinuria. Data from long-term follow-up of 2 prospective studies support a blood pressure lower than 130/80 mm Hg in this subgroup to slow nephropathy progression.100,101
There is some concern that lower diastolic blood pressure may impair myocardial perfusion.102 Thus, future evidence-based guidelines are not likely to recommend lower targets for patients with proteinuric kidney disease.
Antihypertensive Drug Selection
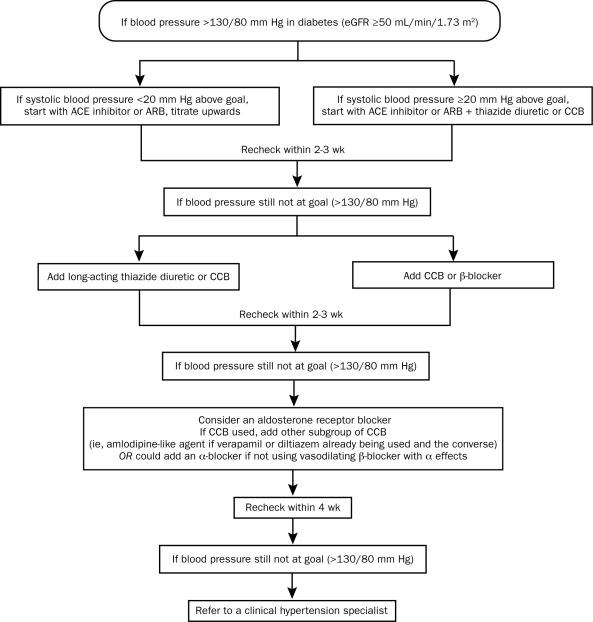
In addition to lifestyle modifications, individuals with type 2 diabetes will generally require 2 or more antihypertensive agents at maximal doses to achieve their blood pressure target. The Figure shows an algorithm proposed by the American Society of Hypertension for the treatment of blood pressure in patients with diabetes and an eGFR of 50 mL/min/1.73 m2 or higher.54,103
FIGURE.
A suggested approach to achieve blood pressure goal in patients with diabetes. An estimated glomerular filtration rate (eGFR) of ≥50 mL/min/1.73 m2 generally responds well to thiazide diuretics. Chlorthalidone is the suggested thiazide-like diuretic because it is the diuretic used in clinical trials and forms the basis for the cardiovascular outcomes data. Vasodilating β-blockers have a better tolerability profile and fewer metabolic consequences compared with older agents such as atenolol. ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CCB = calcium channel blocker.
From J Am Soc Hypertens,54 with permission from Elsevier.
Guidelines generally support first-line treatment of patients with diabetes and hypertension, regardless of the presence of CKD, with an agent that targets the renin–angiotensin system (ie, an ACE inhibitor or ARB).3,26 Large-scale outcomes trials of patients with advanced proteinuric kidney disease demonstrate that inhibitors of the renin–angiotensin system delay the decline in kidney function independent of the benefit attributable to the blood pressure reduction.104-106 A meta-analysis by Kunz et al107 supports the concept that combined administration of an ACE inhibitor and an ARB is more effective than either drug alone to further reduce proteinuria in patients with or without diabetes. However, benefit with these agents vs other agents that simply achieve current blood pressure goals has been proven only in patients with advanced proteinuric nephropathy or in those with heart failure but not in those with early nephropathy.108,109 The ADA also recommends that normotensive patients with diabetes and early-stage nephropathy receive treatment with an ACE inhibitor or ARB.26 Note, however, that the practice of treating low levels of albuminuria with a blocker of the renin–angiotensin system in normotensive patients cannot be justified on the basis of findings of a recent double-blind, placebo-controlled trial that found no benefit on nephropathy progression.110
Initial combination therapy is recommended for patients whose blood pressure is greater than 20/10 mm Hg above the goal (ie, >150/90 mm Hg for those with diabetes).53,54,111 The combination should comprise an ACE inhibitor or ARB and a second drug, such as a thiazide diuretic, calcium channel blocker, or β-blocker.26 These can be given either as a fixed or free combination. A loop diuretic rather than a thiazide diuretic should be used in patients with lower levels of kidney function (eGFR <50 mL/min/1.73 m2).54
If the blood pressure goal is not reached and maintained with 2 drugs, other antihypertensive agents from different therapeutic classes should be added, as needed. ACE inhibitor/ARB combinations are not advised on the basis of results of the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET).112,113 In this trial of patients at high risk of vascular events, 38% of whom had diabetes, combination treatment with telmisartan plus ramipril reduced progression of albuminuria compared with ramipril alone.113 However, the combination also increased the risk of hyperkalemia, syncope, hypotension, and kidney dysfunction compared with ramipril monotherapy.112 The definitive answer regarding this question will arise from the currently ongoing VA NEPHRON-D trial.114
General Considerations
Recommendations in hypertension management guidelines provide general advice and should not supplant good clinical judgment; the antihypertensive drug regimen should be tailored to each patient according to specific comorbidities and needs. In addition, blood pressure should be monitored regularly and the antihypertensive regimen adjusted, either through uptitration of doses or use of additional agents, to maintain blood pressure control. Serum creatinine and potassium levels should be monitored if ACE inhibitors, ARBs, or diuretics are used in order to detect development of acute kidney disease and hyperkalemia.26
PRESERVING KIDNEY FUNCTION THROUGH CHOLESTEROL CONTROL
Dyslipidemia is another comorbidity commonly associated with type 2 diabetes. Diabetic dyslipidemia is characterized by elevated triglyceride levels, low high-density lipoprotein cholesterol (HDL-C) levels, and an excess of highly atherogenic, small, dense low-density lipoprotein cholesterol (LDL-C) particles (Table 4).3,25 Studies in animal models have shown that lipoprotein abnormalities can contribute to the initiation and progression of CKD, regardless of its underlying cause, through a number of pathophysiologic mechanisms.115,116 Several clinical studies also suggest that dyslipidemia can contribute to CKD progression. In particular, high triglycerides and low HDL-C levels (characteristic features of diabetic dyslipidemia) appear to be associated with a deterioration in kidney function.117-120 Recently presented data from the Study of Heart and Renal Protection (SHARP) trial121 indicate that lowering of LDL-C slows the progression of nephropathy and reduces cardiovascular events.
The primary role of effective lipid management in patients with diabetes is to reduce the very high cardiovascular risk. Aggressive management of LDL-C levels with statins has been shown to stabilize kidney function in patients with cardiovascular disease122-125 and to decrease the risk of major cardiovascular events in patients with coronary heart disease and CKD.126 Current evidence also suggests that statins can significantly improve the eGFR or delay eGFR decline in patients with type 2 diabetes.127,128 Statins appear to have little effect on events in patients with diabetes and advanced kidney impairment, indicating a need to prescribe these agents before kidney function declines substantially.128
Lipid Goals
Guidelines issued by the AACE and ADA endorse the National Cholesterol Education Program Adult Treatment Panel III lipid targets.23,25,26
LDL-C. The goal for LDL-C, the primary target of lipid-lowering therapy, is lower than 100 mg/dL (<2.6 mmol/L). The updated goal of lower than 70 mg/dL (<1.8 mmol/L) in very high-risk patients, such as those with diabetes and overt cardiovascular disease, is a therapeutic option. If pharmacologic treatment with a maximum tolerated dose of statin fails to achieve these levels, an alternative goal is to reduce LDL-C by approximately 30% to 40%.
HDL-C. Although no specific goals exist for HDL-C, levels greater than 40 mg/dL (1.0 mmol/L) in men and greater than 50 mg/dL (1.3 mmol/L) in women are desirable.
Triglycerides. No formal goal is given for triglycerides; a concentration lower than 150 mg/dL (1.7 mmol/L) is optimal. If triglyceride levels are 200 mg/dL or higher (2.2 mmol/L), non–HDL-C should be a secondary therapeutic target; the non–HDL-C goal is 30 mg/dL higher than the LDL-C goal.
Selection of Lipid-Lowering Therapy
First-line Therapy. If lipid targets are not achieved with lifestyle measures alone, statins are the lipid-lowering agents of choice for patients with stage 1 to 4 CKD.3,25,26 Statins should also be initiated at the time of lifestyle interventions in patients with diabetes at very high cardiovascular risk, regardless of baseline lipid levels. This includes all patients with documented cardiovascular disease and those without cardiovascular disease who are older than 40 years and have at least 1 other cardiovascular risk factor. For patients undergoing maintenance hemodialysis, a statin should not be initiated unless there is a specific cardiovascular indication for treatment.3 Ezetimibe is an appropriate alternative first-line agent for patients who cannot tolerate statins,25 and fibrates should be used as primary therapy in patients whose triglyceride levels exceed 400 mg/dL.25
Additional Therapy. If patients do not attain their lipid goals despite treatment with maximally tolerated doses of statin, addition of other lipid-lowering agents should be considered. Options to further reduce LDL-C levels include ezetimibe, niacin (especially in patients with low HDL-C), a fibrate, or a bile acid sequestrant (eg, colesevelam).25,26 Statin/fibrate combinations should be used with caution because of an increased risk of rhabdomyolysis. This risk appears to be greater among patients with kidney impairment and appears to be lower with fenofibrate than with gemfibrozil.25,26
Continued and regular monitoring of the lipid profile is necessary and should direct appropriate adjustments to the therapeutic regimen to maintain lipid control and reduce the cardiovascular, and possibly renal, risk.
OTHER INTERVENTIONS
Several other therapeutic measures require consideration in patients with type 2 diabetes, although their effects on the progression of diabetic nephropathy are currently unclear.
Prevention of Anemia
Patients with stage 4 diabetic nephropathy will develop erythropoietin deficiency as the disease progresses, resulting in anemia. Therefore, patients should be screened regularly for onset of anemia and corrective treatment with erythropoietin initiated to increase the hemoglobin level to 11 g/dL (Table 4).25 A randomized controlled trial has shown that initiating erythropoietin early in nondiabetic predialysis patients with nonsevere anemia slowed the progression of kidney disease and delayed the initiation of renal replacement therapy.129 However, whether this practice will delay the course of diabetic nephropathy is currently unknown.
Antithrombotic Therapy
A review of trials suggests that aspirin may offer a modest benefit in reducing cardiovascular risk in patients with diabetes, although the evidence is equivocal.130 Nevertheless, current ADA diabetes management guidelines,26 endorsed by the American Heart Association and American College of Cardiology,130 recommend low-dose aspirin (75-162 mg/d) for primary prevention in patients at increased cardiovascular risk (ie, a 10-year risk of >10%) and for those with diabetes and existing cardiovascular disease (Table 4). Use of aspirin in other individuals for primary prevention should be governed by clinical judgment, dependent on the level of cardiovascular risk. Any contraindications to aspirin and the likelihood of bleeding must also be considered. Clopidogrel at 75 mg/d is a useful alternative for patients unable to tolerate aspirin, and dual antiplatelet therapy for 1 year with aspirin plus clopidogrel should be considered for patients experiencing an acute coronary syndrome.
CONCLUSION
Nephropathy is a common complication among patients with type 2 diabetes. Surveys indicate that CKD often develops during the prediabetic stage, secondary to hypertension and other factors, and is present in up to a third of adults at the time diabetes is diagnosed. Screening for CKD should be performed annually, starting at the time of diabetes diagnosis, and should include measures of the eGFR in addition to urinary albumin excretion. Conscientious screening will facilitate detection of kidney impairment earlier, when interventions to prevent or delay progression are more effective.
Optimal management of diabetic nephropathy requires implementation of a multifactorial approach, comprising lifelong lifestyle modification strategies, glycemic control, blood pressure control, and cholesterol management. Physicians should aim to achieve target levels for all risk determinants simultaneously through careful and individualized selection of antidiabetic, antihypertensive, and lipid-lowering agents according to degree of kidney impairment, patient characteristics, and effects on comorbidities. Moreover, continual monitoring and adjustment of the treatment regimen to maintain control of all risk factors over the long term is needed because of the progressive nature of diabetes. Such a coordinated approach to management should now be intuitive based on our increased understanding of the complex interactions among these risk factors in the development of diabetes complications, especially cardiovascular disease and nephropathy. Therefore, it is disappointing to note that substantial proportions of patients with type 2 diabetes do not attain their glycemic, blood pressure, and LDL-C goals, and the prevalence of obesity continues to increase.
Acknowledgments
Writing and editorial assistance was provided by Elaine Griffin, MA, DPhil, of Envision Scientific Solutions, and was contracted by Boehringer Ingelheim Pharmaceuticals, Inc, for these services.
Footnotes
This work was supported by Boehringer Ingelheim Pharmaceuticals, Inc.
REFERENCES
- 1. Centers for Disease Control and Prevention (CDC) National Diabetes Fact Sheet, 2007. www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf Accessed January 28, 2011
- 2. Molitch ME, DeFronzo RA, Franz MJ, et al. Nephropathy in diabetes. Diabetes Care. 2004;27(suppl 1):S79-S83 [DOI] [PubMed] [Google Scholar]
- 3. National Kidney Foundation KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2)(suppl 2):S12–S154 [DOI] [PubMed] [Google Scholar]
- 4. US Renal Data System (USRDS) Annual data report: 2009. Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009. http://www.usrds.org/adr_2009.htm Accessed January 28, 2011 [Google Scholar]
- 5. Koro CE, Lee BH, Bowlin SJ. Antidiabetic medication use and prevalence of chronic kidney disease among patients with type 2 diabetes mellitus in the United States. Clin Ther. 2009;31(11):2608-2617 [DOI] [PubMed] [Google Scholar]
- 6. Plantinga LC, Crews DC, Coresh J, et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010;5(4):673-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laliberté F, Bookhart BK, Vekeman F, et al. Direct all-cause health care costs associated with chronic kidney disease in patients with diabetes and hypertension: a managed care perspective. J Manag Care Pharm. 2009;15(4):312-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4(8):444-452 [DOI] [PubMed] [Google Scholar]
- 9. Kramer CK, Leitao CB, Pinto LC, Silveiro SP, Gross JL, Canani LH. Clinical and laboratory profile of patients with type 2 diabetes with low glomerular filtration rate and normoalbuminuria. Diabetes Care. 2007;30(8):1998-2000 [DOI] [PubMed] [Google Scholar]
- 10. Gambara V, Mecca G, Remuzzi G, Bertani T. Heterogeneous nature of renal lesions in type II diabetes. J Am Soc Nephrol. 1993;3(8):1458-1466 [DOI] [PubMed] [Google Scholar]
- 11. Balakumar P, Arora MK, Reddy J, Anand-Srivastava MB. Pathophysiology of diabetic nephropathy: involvement of multifaceted signalling mechanism. J Cardiovasc Pharmacol. 2009;54(2):129-138 [DOI] [PubMed] [Google Scholar]
- 12. Weir MR. Microalbuminuria and cardiovascular disease. Clin J Am Soc Nephrol. 2007;2(3):581-590 [DOI] [PubMed] [Google Scholar]
- 13. Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(4):421-426 [DOI] [PubMed] [Google Scholar]
- 14. Mann JF, Yi QL, Gerstein HC. Albuminuria as a predictor of cardiovascular and renal outcomes in people with known atherosclerotic cardiovascular disease. Kidney Int Suppl. 2004. November;(92):S59-S62 [DOI] [PubMed] [Google Scholar]
- 15. Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20(8):1813-1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. So WY, Kong AP, Ma RC, et al. Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes Care. 2006;29(9):2046-2052 [DOI] [PubMed] [Google Scholar]
- 17. El Nahas M. Cardio-kidney-damage: a unifying concept. Kidney Int. 2010;78(1):14-18 [DOI] [PubMed] [Google Scholar]
- 18. Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol. 2006;17(8):2106-2111 [DOI] [PubMed] [Google Scholar]
- 19. Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage: the Steno hypothesis. Diabetologia. 1989;32(4):219-226 [DOI] [PubMed] [Google Scholar]
- 20. Khosla N, Sarafidis PA, Bakris GL. Microalbuminuria. Clin Lab Med. 2006;26(3):635-653 [DOI] [PubMed] [Google Scholar]
- 21. Nobel MIM, Drake-Holland AJ. Hyperglycaemia and the vascular glycocalyx: the key to microalbuminuria and cardiovascular disease in diabetes mellitus? Br J Diabetes Vasc Dis. 2010;10(2):66-70 [Google Scholar]
- 22. Mizobuchi M, Ogata H, Koiwa F, Kinugasa E, Akizawa T. Vitamin D and vascular calcification in chronic kidney disease. Bone. 2009;45(suppl 1):S26-S29 [DOI] [PubMed] [Google Scholar]
- 23. Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497 [DOI] [PubMed] [Google Scholar]
- 24. Lloyd-Jones D, Adams RJ, Brown TM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46-e215 [DOI] [PubMed] [Google Scholar]
- 25. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(suppl 1):1-68 [DOI] [PubMed] [Google Scholar]
- 26. American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(suppl 1):S11-S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353(9153):617-622 [DOI] [PubMed] [Google Scholar]
- 28. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383-393 [DOI] [PubMed] [Google Scholar]
- 29. Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580-591 [DOI] [PubMed] [Google Scholar]
- 30. Saran R, Hedgeman E, Plantinga L, et al. Establishing a national chronic kidney disease surveillance system for the United States. Clin J Am Soc Nephrol. 2010;5(1):152-161 [DOI] [PubMed] [Google Scholar]
- 31. Schoolwerth AC, Engelgau MM, Hostetter TH, et al. Chronic kidney disease: a public health problem that needs a public health action plan. Prev Chronic Dis. 2006;3(2):A57 [PMC free article] [PubMed] [Google Scholar]
- 32. Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16(1):180-188 [DOI] [PubMed] [Google Scholar]
- 33. Plantinga LC, Tuot DS, Powe NR. Awareness of chronic kidney disease among patients and providers. Adv Chronic Kidney Dis. 2010;17(3):225-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Radbill B, Murphy B, LeRoith D. Rationale and strategies for early detection and management of diabetic kidney disease. Mayo Clin Proc. 2008;83(12):1373-1381 [DOI] [PubMed] [Google Scholar]
- 35. Bilous R. Microvascular disease: what does the UKPDS tell us about diabetic nephropathy? Diabet Med. 2008;25(suppl 2):25-29 [DOI] [PubMed] [Google Scholar]
- 36. Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA. 2003;289(24):3273-3277 [DOI] [PubMed] [Google Scholar]
- 37. MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care. 2004;27(1):195-200 [DOI] [PubMed] [Google Scholar]
- 38. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461-470 [DOI] [PubMed] [Google Scholar]
- 39. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254 [DOI] [PubMed] [Google Scholar]
- 40. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stevens LA, Schmid CH, Greene T, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56(3):486-495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Levey AS, de Jong PE, Coresh J, et al. The definition, classification and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. doi: 10.1038/ki.2010.483. [published online ahead of print December 8, 2010] doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 43. Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care. 1999;22(11):1887-1898 [DOI] [PubMed] [Google Scholar]
- 44. Phisitkul K, Hegazy K, Chuahirun T, et al. Continued smoking exacerbates but cessation ameliorates progression of early type 2 diabetic nephropathy. Am J Med Sci. 2008;335(4):284-291 [DOI] [PubMed] [Google Scholar]
- 45. Cignarelli M, Lamacchia O, Di Paolo S, Gesualdo L. Cigarette smoking and kidney dysfunction in diabetes mellitus. J Nephrol. 2008;21(2):180-189 [PubMed] [Google Scholar]
- 46. Chuahirun T, Simoni J, Hudson C, et al. Cigarette smoking exacerbates and its cessation ameliorates renal injury in type 2 diabetes. Am J Med Sci. 2004;327(2):57-67 [DOI] [PubMed] [Google Scholar]
- 47. Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care. 2004;27(suppl 1):S74-S75 [DOI] [PubMed] [Google Scholar]
- 48. Eknoyan G. Obesity, diabetes, and chronic kidney disease. Curr Diab Rep. 2007;7(6):449-453 [DOI] [PubMed] [Google Scholar]
- 49. Ting SM, Nair H, Ching I, Taheri S, Dasgupta I. Overweight, obesity and chronic kidney disease. Nephron Clin Pract. 2009;112(3):c121-c127 [DOI] [PubMed] [Google Scholar]
- 50. Kramer H, Cao G, Dugas L, Luke A, Cooper R, Durazo-Arvizu R. Increasing BMI and waist circumference and prevalence of obesity among adults with type 2 diabetes: the National Health and Nutrition Examination Surveys. J Diabetes Complications. 2010;24(6):368-374 [DOI] [PubMed] [Google Scholar]
- 51. Afshinnia F, Wilt TJ, Duval S, Esmaeili A, Ibrahim HN. Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant. 2010;25(4):1173-1183 [DOI] [PubMed] [Google Scholar]
- 52. Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care. 2004;27(8):2067-2073 [DOI] [PubMed] [Google Scholar]
- 53. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252 [DOI] [PubMed] [Google Scholar]
- 54. Bakris GL, Sowers JR, American Society of Hypertension Writing Group Treatment of hypertension in patients with diabetes—an update. J Am Soc Hypertens. 2010;4(2):62-67 [DOI] [PubMed] [Google Scholar]
- 55. UK Prospective Diabetes Study (UKPDS) Group Intensive bloodglucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853 [PubMed] [Google Scholar]
- 56. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589 [DOI] [PubMed] [Google Scholar]
- 57. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560-2572 [DOI] [PubMed] [Google Scholar]
- 58. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139 [DOI] [PubMed] [Google Scholar]
- 59. The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986 [DOI] [PubMed] [Google Scholar]
- 60. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342(6):381-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Riddle MC, Ambrosius WT, Brillon DJ, et al. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010;33(5):983-990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340(5):b4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA1c in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375(9713):481-489 [DOI] [PubMed] [Google Scholar]
- 67. Cheung BMY, Ong KL, Cherny SS, Sham PC, Tso AW, Lam KS. Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med. 2009;122(5):443-453 [DOI] [PubMed] [Google Scholar]
- 68. Kramer H, Molitch ME. Screening for kidney disease in adults with diabetes. Diabetes Care. 2005;28(7):1813-1816 [DOI] [PubMed] [Google Scholar]
- 69. Moen MF, Zhan M, Hsu VD, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1121-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Snyder RW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial. 2004;17(5):365-370 [DOI] [PubMed] [Google Scholar]
- 71. Glucophage (metformin hydrochloride) tablets [package insert]. Princeton, NJ: Bristol-Myers Squibb; January 2009. http://packageinserts.bms.com/pi/pi_glucophage.pdf Accessed January 28, 2011 [Google Scholar]
- 72. Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334(9):574-579 [DOI] [PubMed] [Google Scholar]
- 73. Vasisht KP, Chen SC, Peng Y, Bakris GL. Limitations of metformin use in patients with kidney disease: are they warranted? Diabetes Obes Metab. 2010;12(12):1079-1083 [DOI] [PubMed] [Google Scholar]
- 74. Starlix (nateglinide) tablets [prescribing information]. Stein, Switzerland: Novartis Pharmaceuticals; July 2008. www.pharma.us.novartis.com/product/pi/pdf/Starlix.pdf Accessed January 28, 2011 [Google Scholar]
- 75. Prandin (repaglinide) tablets [package insert]. Princeton, NJ: Novo Nordisk Pharmaceuticals; May 2010. www.prandin.com/docs/prandin_insert.pdf Accessed January 28, 2011 [Google Scholar]
- 76. Precose (acarbose tablets) [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals; August 2008. www.univgraph.com/Bayer/inserts/Precose.pdf Accessed January 28, 2011 [Google Scholar]
- 77. Glyset miglitol tablets [prescribing information]. New York, NY: Pfizer; revised October 2010. www.pfizer.com/files/products/uspi_glyset.pdf Accessed January 28, 2011 [Google Scholar]
- 78. Actos (pioglitazone hydrochloride) tablets [prescribing information]. Deerfield, IL: Takeda Pharmaceuticals America; August 2008. http://general.takedapharm.com/content/file/pi.pdf?applicationcode=8a9c4571-a123-4477-91de-b9cafe7d07e3&filetypecode=actospi Accessed January 28, 2011 [Google Scholar]
- 79. Avandia (rosiglitazone maleate) tablets [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; October 2008. http://us-gsk.com/products/assets/us_avandia.pdf Accessed January 28, 2011 [Google Scholar]
- 80. Sarafidis PA, Stafylas PC, Georgianos PI, Saratzis AN, Lasaridis AN. Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: a meta-analysis. Am J Kidney Dis. 2010;55(5):835-847 [DOI] [PubMed] [Google Scholar]
- 81. Byetta (exenatide injection) [prescribing information]. San Diego, CA: Amylin Pharmaceuticals; October 2009http://pi.lilly.com/us/byetta-pi.pdf Accessed January 28, 2011 [Google Scholar]
- 82. Victoza (liraglutide [rDNA origin] injection), solution for subcutaneous use [prescribing information]. Princeton, NJ: Novo Nordisk Pharmaceuticals; January 2010. www.victozapro.com/pdf/victoza_comboPI_5.24.pdf Accessed January 28, 2011 [Google Scholar]
- 83. Januvia (sitagliptin) tablets [prescribing information]. Pavie, Italy: Merck Sharp & Dohme (a subsidiary of Merck & Co); February 2010. http://www.merck.com/product/usa/pi_circulars/j/januvia/januvia_pi.pdf Accessed January 28, 2011 [Google Scholar]
- 84. Onglyza (saxagliptin) tablets [package insert]. Princeton, NJ: Bristol-Myers Squibb; July 2009. http://packageinserts.bms.com/pi/pi_onglyza.pdf Accessed January 28, 2011 [Google Scholar]
- 85. Heise T, Graefe-Mody EU, Huttner S, Ring A, Trommeshauser D, Dugi KA. Pharmacokinetics, pharmacodynamics and tolerability of multiple oral doses of linagliptin, a dipeptidyl peptidase-4 inhibitor in male type 2 diabetes patients. Diabetes Obes Metab. 2009;11(8):786-794 [DOI] [PubMed] [Google Scholar]
- 86. Wajchenberg BL. β-Cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28(2):187-218 [DOI] [PubMed] [Google Scholar]
- 87. UK Prospective Diabetes Study (UKPDS) Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703-713 [PMC free article] [PubMed] [Google Scholar]
- 88. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321(7258):412-419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Maki DD, Ma JZ, Louis TA, Kasiske BL. Long-term effects of antihypertensive agents on proteinuria and renal function. Arch Intern Med. 1995;155(10):1073-1080 [PubMed] [Google Scholar]
- 90. Parving HH, Andersen AR, Smidt UM, Hommel E, Mathiesen ER, Svendsen PA. Effect of antihypertensive treatment on kidney function in diabetic nephropathy. Br Med J (Clin Res Ed). 1987;294(6585):1443-1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mogensen CE, Keane WF, Bennett PH, et al. Prevention of diabetic renal disease with special reference to microalbuminuria. Lancet. 1995;346(8982):1080-1084 [DOI] [PubMed] [Google Scholar]
- 92. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351(9118):1755-1762 [DOI] [PubMed] [Google Scholar]
- 94. Nelson RG, Bennett PH, Beck GJ, et al. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. N Engl J Med. 1996;335(22):1636-1642 [DOI] [PubMed] [Google Scholar]
- 95. Kalaitzidis R, Bakris G. Pathogenesis and treatment of microalbuminuria in patients with diabetes: the road ahead. J Clin Hypertens (Greenwich). 2009;11(11):636-643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Steinke JM, Sinaiko AR, Kramer MS, Suissa S, Chavers BM, Mauer M. The early natural history of nephropathy in type 1 diabetes; III: predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes. 2005;54(7):2164-2171 [DOI] [PubMed] [Google Scholar]
- 97. Bakris GL, Gaxiola E, Messerli FH, et al. Clinical outcomes in the diabetes cohort of the INternational VErapamil SR-Trandolapril study. Hypertension. 2004;44(5):637-642 [DOI] [PubMed] [Google Scholar]
- 98. The ACCORD Study Group Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575-1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kalaitzidis R, Bakris GL. Lower blood pressure goals for cardiovascular and renal risk reduction: are they defensible? J Clin Hypertens (Greenwich). 2009;11(7):345-347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005;142(5):342-351 [DOI] [PubMed] [Google Scholar]
- 101. Appel LJ, Wright JT, Jr, Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363(10):918-929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Roy M, Mahmood N, Rosendorff C. Evidence for aggressive blood pressure-lowering goals in patients with coronary artery disease. Curr Atheroscler Rep. 2010;12(2):134-139 [DOI] [PubMed] [Google Scholar]
- 103. Ruilope L, Kjeldsen SE, de la Sierra A, et al. The kidney and cardiovascular risk–implications for management: a consensus statement from the European Society of Hypertension. Blood Press. 2007;16(2):72-79 [DOI] [PubMed] [Google Scholar]
- 104. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851-860 [DOI] [PubMed] [Google Scholar]
- 105. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861-869 [DOI] [PubMed] [Google Scholar]
- 106. Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870-878 [DOI] [PubMed] [Google Scholar]
- 107. Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148(1):30-48 [DOI] [PubMed] [Google Scholar]
- 108. Hopkins KA, Bakris GL. Lower blood pressure goals in high-risk cardiovascular patients: are they defensible? Cardiol Clin. 2010;28(3):447-452 [DOI] [PubMed] [Google Scholar]
- 109. Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care. 2000;23(suppl 2):B54-B64 [PubMed] [Google Scholar]
- 110. Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361(1):40-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gradman AH, Basile JN, Carter BL, Bakris GL. Combination therapy in hypertension. J Am Soc Hypertens. 2010;4(1):42-50 [DOI] [PubMed] [Google Scholar]
- 112. The ONTARGET Investigators Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547-1559 [DOI] [PubMed] [Google Scholar]
- 113. Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372(9638):547-553 [DOI] [PubMed] [Google Scholar]
- 114. Fried LF, Duckworth W, Zhang JH, et al. Design of combination angiotensin receptor blocker and angiotensin-converting enzyme inhibitor for treatment of diabetic nephropathy (VA NEPHRON-D). Clin J Am Soc Nephrol. 2009;4(2):361-368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lacquaniti A, Bolignano D, Donato V, Bono C, Fazio MR, Buemi M. Alterations of lipid metabolism in chronic nephropathies: mechanisms, diagnosis and treatment. Kidney Blood Press Res. 2010;33(2):100-110 [DOI] [PubMed] [Google Scholar]
- 116. Dalrymple LS, Kaysen GA. The effect of lipoproteins on the development and progression of renal disease. Am J Nephrol. 2008;28(5):723-731 [DOI] [PubMed] [Google Scholar]
- 117. Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 2000;58(1):293-301 [DOI] [PubMed] [Google Scholar]
- 118. Schaeffner ES, Kurth T, Curhan GC, et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol. 2003;14(8):2084-2091 [DOI] [PubMed] [Google Scholar]
- 119. Zoppini G, Targher G, Chonchol M, Perrone F, Lippi G, Muggeo M. Higher HDL cholesterol levels are associated with a lower incidence of chronic kidney disease in patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2009;19(8):580-586 [DOI] [PubMed] [Google Scholar]
- 120. Ozsoy RC, van der Steeg WA, Kastelein JJ, Arisz L, Koopman MG. Dyslipidaemia as predictor of progressive renal failure and the impact of treatment with atorvastatin. Nephrol Dial Transplant. 2007;22(6):1578-1586 [DOI] [PubMed] [Google Scholar]
- 121. SHARP Collaborative Group Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J. 2010;160(5):785-794e10. [DOI] [PubMed] [Google Scholar]
- 122. Shepherd J, Kastelein JJ, Bittner V, et al. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol. 2007;2(6):1131-1139 [DOI] [PubMed] [Google Scholar]
- 123. Athyros VG, Mikhailidis DP, Papageorgiou AA, et al. The effect of statins versus untreated dyslipidaemia on renal function in patients with coronary heart disease: a subgroup analysis of the Greek atorvastatin and coronary heart disease evaluation (GREACE) study. J Clin Pathol. 2004;57(7):728-734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tonelli M, Moyé L, Sacks FM, Cole T, Curhan GC. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol. 2003;14(6):1605-1613 [DOI] [PubMed] [Google Scholar]
- 125. Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol. 2006;17(7):2006-2016 [DOI] [PubMed] [Google Scholar]
- 126. Shepherd J, Kastelein JJ, Bittner V, et al. Intensive lipid lowering with atorvastatin in patients with coronary heart disease and chronic kidney disease: the TNT (Treating to New Targets) study. J Am Coll Cardiol. 2008;51(15):1448-1454 [DOI] [PubMed] [Google Scholar]
- 127. Luk AO, Yang X, Ma RC, et al. Association of statin use and development of renal dysfunction in type 2 diabetes-The Hong Kong Diabetes Registry. Diabetes Res Clin Pract. 2010;88(3):227-233 [DOI] [PubMed] [Google Scholar]
- 128. Athyros VG, Mitsiou EK, Tziomalos K, Karagiannis A, Mikhailidis DP. Impact of managing atherogenic dyslipidemia on cardiovascular outcome across different stages of diabetic nephropathy. Expert Opin Pharmacother. 2010;11(5):723-730 [DOI] [PubMed] [Google Scholar]
- 129. Gouva C, Nikolopoulos P, Ioannidis JP, Siamopoulos KC. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int. 2004;66(2):753-760 [DOI] [PubMed] [Google Scholar]
- 130. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Circulation. 2010;121(24):2694-2701 [DOI] [PubMed] [Google Scholar]