We show that nuclear fragmentation in neurodegeneration is an early trigger for cell death initiated by deregulated Cdk5. Cdk5 induces nuclear dispersion by direct phosphorylation of lamins in neurons and in vivo Alzheimer's disease (AD) mouse models. Inhibition of nuclear fragmentation rescues neurons from cell death, underscoring the significance of this event in AD.
Abstract
Nuclear fragmentation is a common feature in many neurodegenerative diseases, including Alzheimer’s disease (AD). In this study, we show that nuclear lamina dispersion is an early and irreversible trigger for cell death initiated by deregulated Cdk5, rather than a consequence of apoptosis. Cyclin-dependent kinase 5 (Cdk5) activity is significantly increased in AD and contributes to all three hallmarks: neurotoxic amyloid-β (Aβ), neurofibrillary tangles (NFT), and extensive cell death. Using Aβ and glutamate as the neurotoxic stimuli, we show that deregulated Cdk5 induces nuclear lamina dispersion by direct phosphorylation of lamin A and lamin B1 in neuronal cells and primary cortical neurons. Phosphorylation-resistant mutants of lamins confer resistance to nuclear dispersion and cell death on neurotoxic stimulation, highlighting this as a major mechanism for neuronal death. Rapid alteration of lamin localization pattern and nuclear membrane change are further supported by in vivo data using an AD mouse model. After p25 induction, the pattern of lamin localization was significantly altered, preceding neuronal death, suggesting that it is an early pathological event in p25-inducible transgenic mice. Importantly, lamin dispersion is coupled with Cdk5 nuclear localization, which is highly neurotoxic. Inhibition of nuclear dispersion rescues neuronal cells from cell death, underscoring the significance of this event to Cdk5-mediated neurotoxicity.
INTRODUCTION
Alzheimer’s disease (AD) is a debilitating neurological disorder. AD brains consistently show a number of biochemical and molecular abnormalities, including selective cell loss, fragmented nuclei, impaired mitochondria, and various biomarkers of oxidative stress. Nuclear fragmentation in AD is usually viewed as a consequence of apoptotic cell death due to the activation of caspases (Su et al., 1994), p53, and other proapoptotic proteins (Friedlander, 2003). The fact that nuclear fragmentation in AD is irreversible further supports this hypothesis. In contrast, nuclear membrane disassembly during mitosis is a highly orchestrated reversible process, where mitotic nuclear fragments reassemble after cytokinesis. In this study, we show that nuclear disassembly, rather than being a consequence of apoptosis, may be an early and irreversible trigger for apoptosis, which is elicited by cyclin-dependent kinase 5 (Cdk5) deregulation.
Cdk5, a proline-directed kinase, shows significantly higher activity in AD brains compared with nondemented controls (Lee et al., 1999). Cdk5’s kinase activity is tightly regulated by spatial and temporal expression of its activators p35 and p39. These proteins are cleaved into p25 and p29 under a variety of pathological conditions. The formation of p25 and p29 constitutively activates Cdk5 and allows it to access a variety of pathological substrates triggering a cascade of neurotoxic pathways, culminating in neuronal death. Deregulated Cdk5/p25 activity contributes to all three hallmarks of AD: neurotoxic oligomeric amyloid-β (Aβ) and neurofibrillary tangles (NFT) formation, and extensive cell death (Patrick et al., 1998; Wen et al., 2008). Cdk5 promotes neurotoxicity by phosphorylating Cdh1 leading to cyclin B1 neuronal accumulation in excitotoxicity (Maestre et al., 2008). Oxidative stress–induced Cdk5 activation increases neurofilaments’ phosphorylation, leading to inhibition of axonal transport in neurons (Shea et al., 2004a, 2004b). Oxidative stress–induced down-regulation of nestin increases Cdk5 activity and sensitizes neuronal progenitor cells to apoptotic death (Sahlgren et al., 2006). Cdk5 and p25 promote cell death in endoplasmic reticulum (ER)-stressed neurons (Saito et al., 2007). ER stress has been linked to AD pathogenesis. Transgenic mice expressing tetracycline-inducible p25 show significant brain atrophy and NFT formation on p25 induction (Cruz et al., 2003).
Our previous studies have shown that Cdk5 deregulation causes Golgi fragmentation by GM130 phosphorylation (Sun et al., 2008a) and oxidative stress and mitochondrial dysfunction by inactivation of peroxiredoxin-1 and peroxiredoxin-2 (Sun et al., 2008b). We further showed that Cdk5 deregulation triggers direct activation of the c-jun-N-terminal kinase (JNK) pathway by phosphorylation of c-Jun and indirect activation of JNK and p38 cascades by increasing oxidative stress (Sun et al., 2009; Chang et al., 2010). In this study, another mechanism was identified by which Cdk5 deregulation can directly cause neuronal cell death.
RESULTS
A chemical genetic screen identified lamin A and lamin B1 as direct Cdk5 substrates
We recently reported a chemical genetic approach for the identification of direct substrates of Cdk5 (Sun et al., 2008b). This approach consists of engineering a kinase (analogue-sensitive kinase), which in the presence of a radioactive orthogonal ATP analogue (e.g., N6-phenethyl ATP) specifically transfers the radioactive tag to its substrates (Shah et al., 1997; Shah and Shokat, 2002, 2003; Shah and Vincent, 2005; Kim and Shah, 2007; Sun et al., 2008a, 2008b). This unnatural pocket is created by replacing a conserved bulky residue (named gatekeeper) with glycine or alanine in the active site of the kinase. The mutant kinase does not differ from the wild type (WT) in its substrate specificity. Because the ATP analogue is not accepted by WT kinases, it permits unbiased identification of direct substrates of the analogue-sensitive kinase in a global environment.
Lamin A and lamin B1 were identified as novel Cdk5 substrates
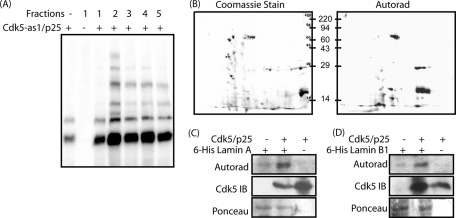
Our previous study revealed N6-phenethyl ATP as the most optimal phosphodonor for analogue-sensitive Cdk5 (Cdk5-as1) (Sun et al., 2008b). Using Cdk5-as1/p25 and orthogonal [γ-32P]N6-phenethyl ATP, an in vitro kinase reaction was performed using fractionated brain lysates (Figure 1A). To isolate these targets, proteins were separated using two-dimensional (2D) gel electrophoresis, isolated, and visualized by autoradiography (Figure 1B). Radiolabeled proteins were excised from the gel, and subsequent to trypsin digestion the peptide cleavage products were identified by tandem mass spectrometry. This study revealed lamin A and lamin B1 as putative substrates of Cdk5.
FIGURE 1:
Lamin A and lamin B1 are direct substrates of Cdk5. (A) Specific labeling of Cdk5 substrates in fractionated brain lysates (1–5 fractions). (B) 2D gel showing Coomassie staining and autoradiography of fraction 5. (C) Lamin A is directly phosphorylated by Cdk5. 6-His-lamin A was phosphorylated by the Cdk5/p25 complex using [γ-32P]ATP as described in Materials and Methods. (D) Recombinant 6-His-lamin B1 was phosphorylated by the Cdk5/p25 complex using [γ-32P]ATP.
Lamin A and lamin B1 are directly phosphorylated by Cdk5 in vitro
Because proteomics screening can often lead to false positives, Cdk5-mediated phosphorylation of lamin A and lamin B1 were examined using an in vitro kinase assay. Lamin A and lamin B1 were generated as 6-His fusion proteins and subjected to in vitro kinase assays with Cdk5/p25. Cdk5 efficiently phosphorylated both lamin A and lamin B1 (Figure 1, C and D), revealing them as direct Cdk5 targets.
Cdk5 deregulation induced by glutamate or Aβ25–35 contributes to the lamin A and lamin B1 dispersion in HT22 cells and primary cortical neurons
Lamin A and lamin B are key components of nuclear lamina that localize close to the inner nuclear membrane. Cdc2-mediated phosphorylation of lamin A causes reversible nuclear disorganization during mitosis (Heald & McKeon, 1990), leading us to hypothesize that deregulated Cdk5 may trigger a similar series of events under neurotoxic conditions albeit irreversibly. We tested this hypothesis initially using HT22 (immortalized mouse hippocampal) cells and later using mature primary cortical neurons using Aβ25–35 and glutamate as the neurotoxic stimuli.
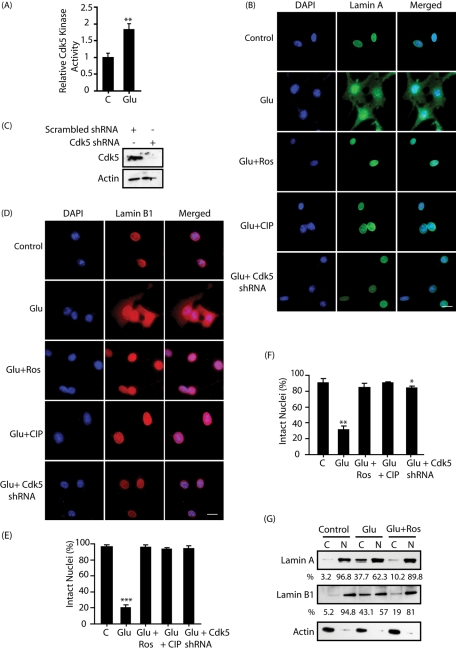
In HT22 cells. Glutamate stimulation. HT22 cells display a significant percentage of fragmented nuclei under normal proliferative conditions due to the cell population undergoing mitosis. To eliminate this background, HT22 cells were arrested at the G1/S phase using thymidine block, followed by glutamate stimulation (without releasing). Analysis of Cdk5 kinase activity revealed almost twofold activation within 30 min (Figure 2A). These results are similar to the previous results obtained using proliferating HT22 cells (Sun et al., 2008a, 2008b, 2009; Chang et al., 2010). Nuclear integrity was next analyzed in glutamate-treated, thymidine-blocked HT22 cells using lamin A and lamin B1 antibodies. Whereas in untreated cells, lamin A and lamin B1 showed exclusive nuclear localization, robust nuclear lamina dispersion was observed with blurred nuclear rims in glutamate-treated cells (Figure 2, B, D, E, and F). Hoechst staining did not show any condensed nuclei, suggesting that Cdk5-mediated nuclear dispersion is an early event and precedes apoptosis.
FIGURE 2:
Glutamate stimulation triggers dispersion of lamin A and lamin B1 in a Cdk5-dependent manner in HT22 cells. (A) Glutamate stimulates Cdk5 kinase activity. Cdk5 was immunoprecipitated from either control (column 1) or glutamate-treated HT22 cells (for 30 min), and the kinase assay was conducted using Cdk5 substrate peptide as described in Materials and Methods. C, control; Glu, glutamate. (B and D) HT22 cells were treated with 5 mM glutamate for 4 h, followed by immunostaining with lamin A (B, green) or lamin B1 (D, red) and DAPI (blue) as described in Materials and Methods. Roscovitine (10 μM) or TAT-CIP (200 nM) was added 0.5 h before glutamate treatment. Cdk5 shRNA lentivirus was added to infect cells for 30 h, followed by glutamate treatment. Representative pictures are shown. Scale bar, 20 μm. (C) Cdk5 levels in HT22 cells on lentiviral infection of Cdk5 shRNA for 30 h. (E) Percentage of cells showing lamin A dispersion was counted as described in Materials and Methods. (F) Percentage of cells showing lamin B1 dispersion was counted. Bar graphs shown are mean ± SD (*p < 0.05, *p < 0.01, ***p < 0.001). (G) Subcellular fractionation of lamin A and lamin B1 in glutamate-treated HT22 cells in the absence or presence of 10 μM roscovitine as described in Materials and Methods. The percentages of nuclear and cytoplasmic lamins were calculated for each sample as shown. Actin is the cytoplasmic marker. N, nuclear fraction; C, cytoplasmic fraction.
Cdk5’s role in this process was initially examined using roscovitine, a potent Cdk5 inhibitor, which prevented nuclear dispersion (Figure 2, B, D, E, and F). Because roscovitine also inhibits Cdc2 appreciably, these results were further verified using a highly Cdk5-selective inhibitor TAT-CIP (TAT-fused Cdk5 inhibitory peptide), which was developed during our previous study (Sun et al., 2008a). In addition, the level of endogenous Cdk5 was depleted using Cdk5 shRNA (Figure 2C), and lamin dispersion was analyzed. Both TAT-CIP and Cdk5 ablation prevented lamin dispersion, underscoring a critical role of Cdk5 in this neurotoxic event (Figure 2, B, D, E, and F).
Subcellular fractionations were subsequently conducted to confirm lamin A and lamin B1 cytoplasmic dispersion in glutamate-treated, thymidine-blocked HT22 cells. Whereas in untreated cells both lamin A and lamin B1 were present in nuclear fraction, an ∼40% increase in lamin A and B1 levels were observed in the cytosolic fraction on glutamate stimulation (4 h) (Figure 2G). Importantly, Cdk5 inhibition largely prevented lamin cytoplasmic distribution in glutamate-treated cells, consistent with the immunocytochemistry data (Figure 2, B, D, E, and F). These results further support the notion that deregulated Cdk5 is responsible for lamin subcellular distribution in neurotoxin-treated neuronal cells.
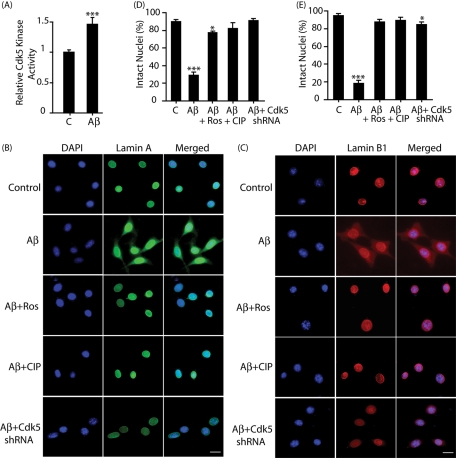
Aβ stimulation. Multiple studies have established that the toxicity and signaling events elicited by Aβ25–35 are comparable to those elicited by the physiological neurotoxic peptide Aβ1–42 (Pike et al., 1995; Klementiev et al., 2007). In this study, Aβ25–35 was used as the neurotoxic signal. Aβ25–35treated, thymidine-blocked HT22 cells showed an increase in Cdk5 kinase activity as expected (Figure 3A). Similar to glutamate, G1/S-arrested HT22 exposed to Aβ25–35 also displayed a robust dissolution of lamin A and lamin B1 into cytoplasm (Figure 3, B–E). Roscovitine, TAT-CIP, and Cdk5 shRNA effectively inhibited Aβ25–35-induced migration of lamin A and lamin B1, further emphasizing a key role of deregulated Cdk5 in the nuclear lamina dispersion.
FIGURE 3:
Aβ stimulation triggers dispersion of lamin A and lamin B1 in a Cdk5-dependent manner in HT22 cells. (A) Aβ stimulates Cdk5 kinase activity. Cdk5 was immunoprecipitated from either control (column 1) or Aβ-treated HT22 cells (for 30 min), and the kinase assay was conducted as described in Materials and Methods. C, (B and C) HT22 cells were treated with 25 μM Aβ25–35 for 4 h, followed by immunostaining with lamin A (B, green) or lamin B1 (C, red), and DAPI (blue). Representative pictures are shown. Scale bar, 20 μm. (D) Percentage of cells showing lamin A dispersion was counted. (E) Percentage of cells showing lamin B1 dispersion was counted. Bar graphs shown are mean ± SD (*p < 0.05, ***p < 0.001).
In primary cortical neurons. To determine Cdk5’s role in nuclear dispersion under more pathologically relevant conditions, mature primary cortical neurons were used. In contrast to HT22 cells, which respond to glutamate toxicity by oxytosis, primary cortical neurons cultured for DIV12–14 develop ionotropic receptors and respond to glutamate via excitotoxicity. Glutamate excitotoxicity directly contributes to neuronal cell loss associated with both acute insults and chronic neurodegenerative disorders.
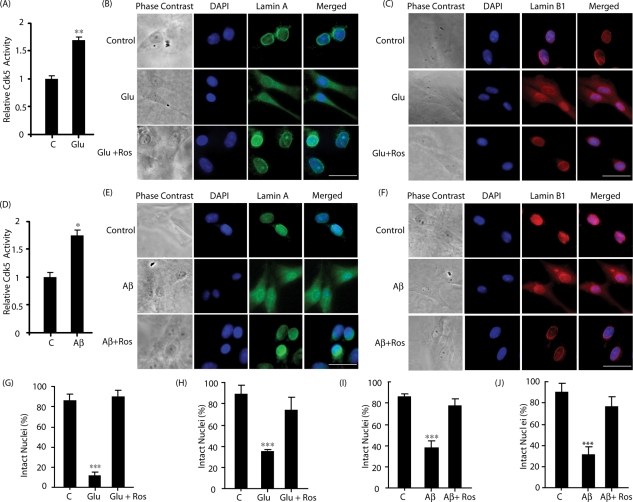
Mature primary cortical neurons were treated with 100 μM glutamate, which caused substantial Cdk5 activation (Figure 4A). Nuclear lamina were next examined in these neurons, which revealed that both lamin A and lamin B1 dispersed in the cytoplasm, which was prevented by Cdk5 inhibition (Figure 4, B, C, G, and H). Similar to glutamate, Aβ treatment in primary cortical neurons also caused Cdk5 activation (Figure 4D) and lamin A and lamin B1 solubilization, which was prevented by Cdk5 inhibition (Figure 4, E, F, I, and J). Together these results show that deregulated Cdk5 is a critical regulator of nuclear dispersion in primary neurons on neurotoxic insults.
FIGURE 4:
Glutamate and Aβ cause dispersion of lamin A and lamin B1 in a Cdk5-dependent manner in primary cortical neurons. (A) Glutamate stimulates Cdk5 kinase activity. Cdk5 immune complex was isolated from either untreated or 100 μM glutamate–treated mature primary cortical neurons (0.5 h), and the kinase assay was conducted using Cdk5 substrate peptide. (B and C) Mature primary cortical neurons were stimulated with 100 μM glutamate for 4 h in the absence or the presence of 10 μM roscovitine, followed by immunostaining with lamin A (green, B) and DAPI (blue) or lamin B1 (red, C) and DAPI (blue). (D) Aβ25–35 stimulates Cdk5 kinase activity in primary cortical neurons. Cdk5 immune complex was isolated from either untreated or 25 μM Aβ25–35–treated mature primary cortical neurons (30 min), and the kinase assay was conducted using Cdk5 substrate peptide. (E and F) Mature primary cortical neurons were stimulated with 25 μM Aβ25–35 for 4 h in the absence or presence of 10 μM roscovitine, followed by immunostaining with lamin A (E, green) and DAPI (blue) or lamin B1 (F, red) and DAPI (blue). Representative pictures are shown. Scale bar, 20 μm. (G) Percentage of glutamate-treated cells showing lamin A dispersion. These were counted as described in Materials and Methods. (H) Percentage of glutamate-treated cells showing lamin B1 dispersion. (I) Percentage of Aβ25–35–treated cells showing lamin A dispersion was counted. (J) Percentage of Aβ25–35-treated cells showing lamin B1 dispersion. Bar graphs shown are mean ± SD (*p < 0.05, *p < 0.01, ***p < 0.001).
Identification of lamin A and lamin B1 phosphorylation sites
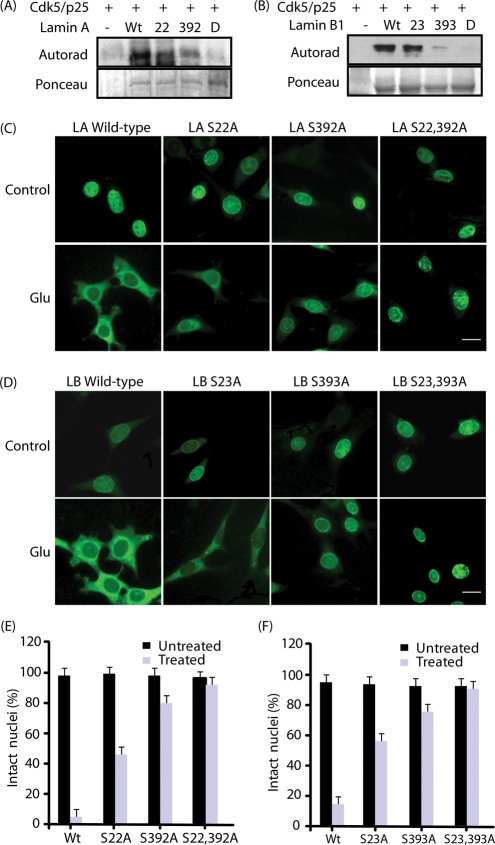
During mitosis, Cdc2 phosphorylates lamin A at S22 and S392 and lamin B1 at S23 and S393. The fact that Cdk5 deregulation caused nuclear lamina dispersion similar to Cdc2 activation during mitosis suggested that Cdk5 may phosphorylate the same sites. Thus, 6-His tagged (S22A) lamin A, (S392A) lamin A, (S22A, S392A) lamin A, (S23A) lamin B1, (S393A) lamin B1, and (S23A, S393A) lamin B1 phosphorylation-resistant mutants were generated, expressed in Escherichia coli, isolated, and subjected to kinase assays using Cdk5/p25. Our results showed that Cdk5 phosphorylates both Ser-22 and Ser-392 in lamin A; however, Ser-392 was the predominant phosphorylation site (Figure 5A). Similarly, in lamin B1, Ser-393 was the major Cdk5 phosphorylation site, although Ser-23 was also phosphorylated (Figure 5B). Importantly, double mutants of lamin A (S22A, S392A) and lamin B1 (S23A, S393A) showed no phosphorylation, suggesting that Cdk5 only phosphorylates these two sites in lamin A and lamin B1, respectively.
FIGURE 5:
Phosphorylation-resistant mutants of lamin A and lamin B show significantly reduced lamina dispersion. (A) Lamin A is directly phosphorylated by Cdk5 at S22 and S392. Recombinant 6-His-tagged WT lamin A, (S22A) lamin A, (S392A) lamin A, and (S22A, S392A) lamin A were phosphorylated by the Cdk5/p25 complex using [γ-32P]ATP as described in Materials and Methods. (B) Lamin B1 is directly phosphorylated by Cdk5 at S23 and S393. Recombinant 6-His-tagged WT lamin B1, (S22A) lamin B1, (S392A) lamin B1, and (S22A, S392A) lamin B1 were phosphorylated by the Cdk5/p25 complex using [γ-32P]ATP. (C) Myc-tagged WT lamin A, (S22A) lamin A, (S392A) lamin A, and (S22, S392A) lamin A–overexpressing HT22 cells were generated as described in Materials and Methods. These cells were exposed to 5 mM glutamate for 4 h, followed by immunostaining with myc antibody (green) and DAPI (blue). Representative pictures are shown. (D) Myc-tagged WT lamin B1, (S23A) lamin B1, (S393A) lamin B1, and (S23, S393A) lamin B1–overexpressing HT22 cells were exposed to 5 mM glutamate for 4 h, followed by immunostaining with myc antibody (green) and DAPI (blue). Representative pictures are shown. (E) Percentage of cells showing lamin A dispersion. (F) Percentage of cells showing lamin B dispersion was counted.
Cdk5 causes nuclear dispersion by direct phosphorylation of lamin A and lamin B1
To determine whether Cdk5-mediated direct phosphorylation of lamin A and lamin B1 contributes to lamina dispersion, myc-tagged WT and phosphorylation-resistant mutants of lamin A and lamin B1 were stably expressed in HT22 cells (Figure 5, C–F). These cells were arrested in the G1/S phase using thymidine block and stimulated with glutamate, and nuclear dispersion was analyzed using myc antibody. While cells expressing (S22A) lamin A mutant showed less nuclear dispersion as compared with WT (Figure 5C, bottom panel), (S392A) lamin A mutant showed minimal lamina dispersion, suggesting that this is the major site responsible for nuclear lamina dispersion. This result is consistent with the Cdk5 phosphorylation site, which also revealed S392 as the major Cdk5 phosphorylation site on lamin A (Figure 5A). (S22A, S392A) lamin A-expressing HT22 cells showed no dispersion on glutamate stimulation, further confirming that S22 and S392 are the only sites responsible for lamin dissolution by deregulated Cdk5 (Figure 5, C and E).
Similar to lamin A, (S393A) lamin B1-HT22 cells showed significantly reduced nuclear dispersion as compared with WT lamin A-HT22 cells, although S23A mutant also contributed to some extent (Figure 5, D and F). (S23A, S393A) lamin B1-HT22 cells showed no dispersion, as expected (Figure 5, D and F). These results strongly suggest that deregulated Cdk5 causes nuclear lamina dispersion by directly phosphorylating lamin A and lamin B1.
Cdk5-mediated nuclear envelope breakdown is an early event that precedes apoptosis
Nuclear irregularities observed in AD brains are often attributed to apoptosis. Our observation of rapid dispersion of nuclear lamina (within 4 h of neurotoxic stimulation) and no obvious abnormalities with Hoechst staining (Figures 2–4) suggests that deregulated Cdk5-mediated nuclear envelope breakdown is an early event that precedes neuronal death.
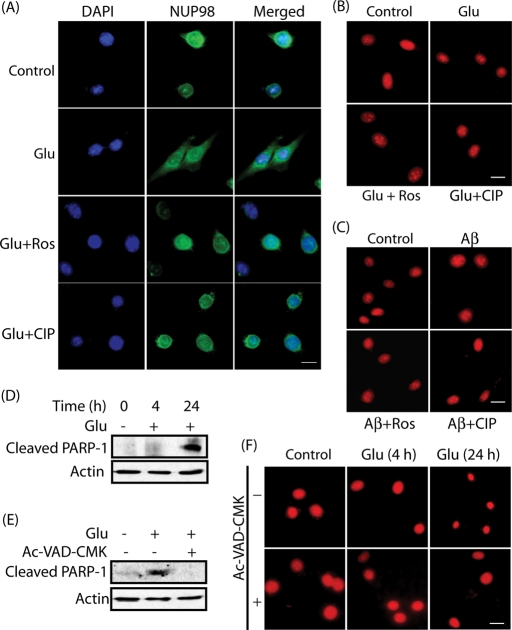
To test this hypothesis, initially nuclear membrane marker NUP98 was used and nuclear dispersion was analyzed in glutamate-treated HT22 cells (4 h) (Figure 6A). Similar to lamin A and lamin B1 (Figures 2 and 3), NUP98 (green) resided in the nucleus of G1/S-arrested HT22 cells and showed clear staining of the nuclear membrane. Glutamate treatment caused significant dispersion of NUP98 in the cytoplasm, which was inhibited using either roscovitine or TAT-CIP (Figure 6A). These findings further support the idea that Cdk5 deregulation triggers nuclear envelope breakdown on neurotoxic insults.
FIGURE 6:
Lamin dispersion precedes apoptosis in glutamate-treated HT22 cells. (A) HT22 cells were treated with 5 mM glutamate for 4 h in the absence and the presence of 10 μM roscovitine or 200 nM TAT-CIP, followed by immunostaining with NUP98 (green) and DAPI (blue). Representative pictures are shown. Scale bar, 20 μm. (B) HT22 cells were treated with 5 mM glutamate for 4 h in the absence and the presence of 10 μM roscovitine or 200 nM TAT-CIP, followed by staining with PI (red). (C) Cells treated with 25 μM Aβ25–35 were stained with PI (red). (D) Cleaved PARP-1 levels in glutamate-treated HT22 cells (4 h and 24 h). (E) Cleaved PARP-1 levels in glutamate-treated HT22 cells in the absence and presence of 40 μM Ac-VAD-CMK. (F) HT22 cells were treated with 5 mM glutamate for 4 h or 24 h in the absence and the presence of 40 μM Ac-VAD-CMK, followed by PI staining (red).
The integrity of nuclei in glutamate- or Aβ25–35-treated, thymidine-blocked HT22 cells was next examined using propidium iodide (PI) staining. Neither glutamate nor Aβ25–35 treatment for 4 h showed condensed or fragmented nuclei (Figure 6, B and C), which is consistent with the results obtained using Hoechst staining (Figures 2–4). To further confirm these results, cleaved poly (ADP-ribose) polymerase 1 (PARP1) levels were analyzed both at 4 and 24 h, which revealed cleaved PARP1 at 24 h, but not at 4 h, suggesting that nuclear lamina dispersion precedes caspase activation (Figure 6D). PARP1 cleavage was effectively inhibited using pan-caspase inhibitor Acetyl-Val-Ala-Asp-Chloromethyl ketone (Ac-VAD-CMK) (Figure 6E). Similar results were obtained using PI staining, which revealed condensed nuclei on longer exposure of glutamate (24 h), which was efficiently inhibited by pan-caspase inhibitor (Figure 6F). Together these results show that Cdk5-mediated solubilization of nuclear lamina occurs early and is not a consequence of caspase activation.
Alteration of lamin localization by induction of Cdk5 in p25 transgenic mice
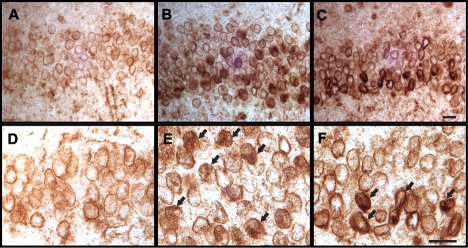
To test whether the alteration of lamin could also be induced by Cdk5 in vivo, we examined the effect of Cdk5 activation on lamin in forebrain neuron–specific, p25-inducible transgenic mice (Cruz et al., 2003). In immunocytochemistry analysis using a lamin A/C specific antibody, we found accumulation and dislocalization of lamin after a 2-wk induction of p25 in hippocampal neurons (Figure 7, B and E), and nuclear irregularity was more evident after a 4-wk induction of p25 (Figure 7, C and F). These data strongly confirm the results of our in vitro studies and suggest that the activation of Cdk5 in p25-inducible mice affect the nuclear membrane structure and function which has been reported in AD (Lee et al., 2006a, 2006b; Sheffield et al., 2006). Because neuronal cell death starts after 8 wk of induction in this p25-inducible transgenic mouse (Cruz et al., 2003), our data also indicate that the alteration of lamin-associated nuclear irregularity is an early event following Cdk5 activation and precedes neuronal cell death in this mouse model.
FIGURE 7:
Cdk5 induction causes the accumulation of lamins at nuclear membrane and nuclear irregularity in p25-inducible transgenic mice. After a 2-wk induction of p25, the nuclear accumulation of lamin is evident in hippocampal neurons (CA1) (B and E) compared with controls (A and D). Further accumulation of lamin and nuclear irregularity is more prominent after 4 wk of induction of p25 (C and F). Scale bar, 20 μm.
(S22A, S392A) lamin A-HT22 and (S23A, S393A) lamin B1-HT22 cells exhibit lower cell proliferation rate and delayed mitosis
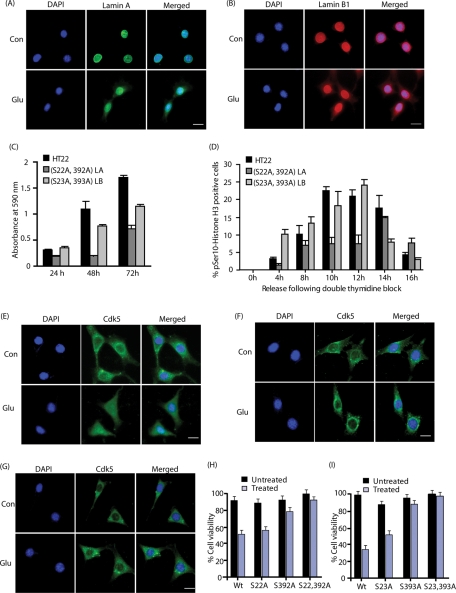
The fact that (S22A, S392A) lamin A– and (S23A, S393A) lamin B1–expressing HT22 cells were viable suggested that endogenous lamin A and lamin B1 may still promote nuclear dispersion. Therefore, endogenous lamin A and lamin B1 dispersions were analyzed in glutamate-treated, thymidine-blocked HT22 cells using lamin A and lamin B1 antibodies (Figure 8, A and B). As expected, untreated cells showed exclusive nuclear localization of lamin A and lamin B1. Unlike HT22 cells, which showed substantial redistribution of lamin A and lamin B1 on glutamate exposure (Figures 2 and 3), double mutant lamin A and lamin B1–expressing HT22 cells showed modest dispersion of lamin A and lamin B1 in the cytosolic compartment (Figure 8, A and B). Most of the lamin A and lamin B1 still remained in the nucleus. This result was expected because lamin A and lamin B1 antibodies recognize both endogenous and exogenously expressed myc-tagged lamins, which are resistant to glutamate treatment (Figure 5, C and D).
FIGURE 8:
Phosphorylation-resistant lamin A and lamin B1 mutants confer resistance to glutamate-mediated toxicity. (A) Glutamate induces endogenous lamin A dispersion in (S22A, S392A) lamin A–expressing HT22 cells. Cells were treated with 5 mM glutamate for 4 h, followed by immunostaining with lamin A (green) and DAPI (blue). Representative pictures are shown. Scale bar, 20 μm. (B) Glutamate induces endogenous lamin B1 dispersion in (S23A, S393A) lamin B1-HT22 cells. Cells were treated with glutamate, followed by staining with lamin B1 (red) and DAPI (blue). (C) (S22A, S392A) lamin A and (S23A, S393A) lamin B1–expressing HT22 cells show reduced proliferation rates. Cells cultured on 96-well plates were incubated for 24 h, 48 h, and 72 h, followed by MTT assay. The absorbance at 595 nm was recorded to reflect cell proliferation rate. (D) HT22 cells expressing (S22A, S293A) lamin A and (S23A, S393A) lamin B1 show delayed mitosis. Double-thymidine-blocked cells were released for various periods (4, 8, 10, 12, 14, and 16 h), fixed, and immunostained using Histone H3 (pSer-10) and DAPI. The graph was plotted as the ratio of Histone H3 (pSer-10)–positive cells vs. total DAPI-stained cells. (E) Glutamate triggers nuclear localization of Cdk5 in HT22 cells. Thymidine-blocked HT22 cells were treated with 5 mM glutamate for 4 h, followed by immunostaining with Cdk5 (green) and DAPI (blue). Representative pictures are shown. More than 95% of cells showed robust Cdk5 nuclear localization. (F) (S22A, S392A) lamin A prevents Cdk5 nuclear dispersion in cells. Thymidine-blocked, myc-tagged (S22A, S392A) lamin A-HT22 cells were treated with 5 mM glutamate for 4 h, followed by immunostaining with Cdk5 (green) and DAPI (blue). Representative pictures are shown. More than 95% of cells revealed no Cdk5 nuclear localization. (G) (S23A, S393A) lamin B1 prevents Cdk5 nuclear dispersion in cells. Thymidine-blocked, myc-tagged (S23A, S393A) lamin B1-HT22 cells were treated with 5 mM glutamate for 4 h, followed by immunostaining with Cdk5 (green) and DAPI (blue). Representative pictures are shown. More than 95% of cells revealed no Cdk5 nuclear localization. (H) Inhibition of Cdk5 phosphorylation sites on lamin A confers a high degree of neuroprotection on glutamate stimulation. Myc-tagged WT lamin A, (S22A) lamin A, (S392A) lamin A, and (S22, S392A) lamin A-HT22 cells were treated with 5 mM glutamate for 24 h, and cell viability was analyzed by MTT assay. This assay was conducted three independent times. Representative results are shown. (I) Inhibition of Cdk5 phosphorylation sites on lamin B1 confers a high degree of neuroprotection on glutamate stimulation. Myc-tagged WT lamin B1, (S23A) lamin B1, (S393A) lamin B1, and (S23, S393A) lamin B1 HT22 cells were treated with 5 mM glutamate for 24 h, and cell viability was analyzed by MTT assay.
To further examine the effect of lamina mutations on HT22 cells, the proliferation rates of HT22, (S22A, S392A) lamin A-HT22, and (S23A, S393A) lamin B1-HT22 cells were investigated using the MTT (3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Interestingly, whereas both lamin A– and lamin B1-double mutant–expressing HT22 cells showed reduced cell proliferation rates, (S22A, S392A) lamin A-HT22 cells displayed the slowest cell growth (Figure 8C), suggesting a more important role of lamin A in cells as compared with lamin B1.
The mitotic status of lamin mutant–expressing HT22 cells was next probed using phospho-Histone H3 (Ser-10) antibody. Cells synchronized at G1/S phase using double thymidine block were released for different time points (4–16 h) and stained with phospho-Histone H3 antibody. Cells displaying intense staining were counted as the mitotic cells. Maximum p-Histone H3–positive cells were observed at 10 h for HT22 cells, 12 h for HT22 cells expressing (S23A, S393A) lamin B1, and 14 h for HT22 cells expressing lamin A double mutant after double thymidine release (Figure 8D). Together, these results show that both lamin A and lamin B1 mutations reduce cell proliferation and delay mitosis; however, lamin A mutant has a more pronounced effect than lamin B1 mutant.
Cdk5 nuclear localization is due to lamin phosphorylation
Cdk5 has no nuclear localization signal but is known to localize in the nucleus on neurotoxic insults. Cdk5 possesses death-promoting activity in the nucleus and prosurvival signaling in the cytoplasm (O’Hare et al., 2005; Sun et al., 2009). Because rapid nuclear dispersion was observed on neurotoxic stimulation, Cdk5 localization was next analyzed. Glutamate-treated HT22 cells showed robust Cdk5 nuclear localization, which was coupled with nuclear dispersion (Figure 8E). In contrast, when phosphorylation-resistant (S22A, S392A) lamin A HT22 cells or (S23A, S393A) lamin B1 HT22 cells were exposed to glutamate, Cdk5 predominantly remained cytoplasmic (Figure 8, F and G). These findings strongly suggest that Cdk5’s nuclear localization is coupled to lamin-mediated nuclear dispersion on neurotoxic insults.
Lamin A and lamin B1 phosphorylation by Cdk5 is highly neurotoxic
Inhibition of Cdk5 nuclear localization in glutamate-treated lamin A and lamin B1 mutant-expressing HT22 cells suggested that lamin phosphorylation by Cdk5 may cause significant neurotoxicity. A cell viability assay was conducted using WT and phosphorylation-resistant lamin mutant–expressing HT22 cells. Glutamate stimulation caused ∼80–90% loss in cell viability) in both WT lamin A– and lamin B1–expressing cells, as expected (Figure 8, H and I). Consistent with phosphorylation data showing S392 as the major Cdk5 phosphorylation site, lamin A (S22A) mutant showed little protection, whereas S392A mutant conferred higher neuroprotection (Figure 8H). Similarly, for lamin B1, S393A was more neuroprotective than S23A on glutamate stimulation (Figure 8I). These results confirm that Cdk5-mediated lamin phosphorylation contributes significantly to cell death under neurotoxic conditions.
DISCUSSION
Lamin A and lamin B associate with several nuclear envelope proteins, such as lamina-associated polypeptides 1 and 2, lamin B receptor, barrier-to-autointegration factor, and chromatin (Stewart et al., 2007). Lamins are involved in various biological functions, including maintaining nuclear morphology, chromatin organization, regulation of mitosis, DNA replication, transcription, gene regulation, and nuclear–cytoskeletal interactions (Dechat et al., 2009).
Previous studies have revealed a pivotal role of Cdc2 in nuclear lamina dispersion during mitosis (Heald and McKeon, 1990). During late stages of apoptosis, lamin B is proteolyzed independent of Cdc2 kinase activity (Oberhammer et al., 1994). Lamin B is also phosphorylated by protein kinase C (PKC)α at S395 and S405 sites, which causes degradation and DNA fragmentation in apoptotic HL60 cells (Hocevar et al., 1993; Shimizu et al., 1998). A recent study showed that lamin A is phosphorylated at S404 by Akt in C2C12 cells, which is presumably a physiological response to insulin (Cenni et al., 2008). There is only one report to date showing lamin cleavage and dissolution of the microtubule network preceding chromatin fragmentation in glutamate-induced cerebellar granule cell apoptosis (Ankarcrona et al., 1996).
AD brains consistently show fragmented nuclei and selective cell loss; however, fragmentation has always been believed to be a consequence of apoptosis. This study elucidates a novel alternative mechanism for nuclear irregularities in neurodegenerative processes. We show that in postmitotic neurons, Cdk5 deregulation causes rapid nuclear dispersion by direct phosphorylation of lamin A and lamin B1. The nuclear dispersion was confirmed using NUP98, which supported Cdk5-mediated breakdown of nuclear envelope on neurotoxic stimulation. S392 in lamin A and S393 in lamin B1, respectively, are the major Cdk5 phosphorylation sites. Consistent with this finding, inhibition of phosphorylation of S392 in lamin A, and S393 in lamin B1, has a more pronounced effect on nuclear lamina dispersion as compared with S22 and S23 sites on lamin A and lamin B1, respectively. Inhibition of Cdk5 activity effectively prevents dispersion of lamins, suggesting that unlike proliferating cells where Cdc2, Akt, and PKCα all contribute to nuclear irregularities, under neurotoxic conditions, Cdk5 dysregulation is the major mechanism causing nuclear disorganization.
Our animal study data also strongly suggest that the activation of Cdk5 affects the nuclear membrane structure and function; a phenomenon reported in AD (Lee et al., 2006a, 2006b; Sheffield et al., 2006). The appearance of nuclear irregularity in p25 transgenic mice looks similar to what has been shown in the vulnerable neurons in AD (Sheffield et al., 2006). Importantly, this alteration in the nucleus in AD was not correlated with apoptosis as evidenced by a lack of association with apoptotic markers (Sheffield et al., 2006). Because the alteration of lamin-associated nuclear alteration appears with a short induction of p25 and precedes neuronal cell death in our study, our data from cell culture and animal models strongly suggest that Cdk5 and lamins may play an important role in nuclear irregularity in AD. Further study is certainly required to confirm these findings.
Our study identified lamin A and B1 as Cdk5 targets, which contribute significantly to Cdk5-induced apoptotic cell death. Our data show that the nuclear envelope dispersion due to phosphorylation of lamins acts as a trigger for apoptosis. No condensed or fragmented nuclei were observed in glutamate- or Aβ25–35–treated neurons at the time of nuclear dispersion (4 h of treatment). Furthermore, inhibition of nuclear dispersion using lamin mutants largely prevents neurotoxicity, underscoring a critical contribution of this process in promoting cell death. This study reveals that nuclear envelope breakdown is yet another key mechanism by which Cdk5 promotes cell death.
Cdk5 is present both in the nucleus and the cytoplasm (Ino and Chiba, 1996; O’Hare et al. 2005). Because Cdk5 has no nuclear localization signal, the dynamics of p35/p25 subcellular localization is considered to be an important mechanism leading to Cdk5 nuclear localization. p25 is localized to the nucleus in neurons undergoing ER stress–induced cell death (Saito et al., 2007). Importin-β/5/7 imports p35 into the nucleus via its nuclear localization signal (Fu et al., 2006). Phosphorylation of Cdk5 by Abl also increases its accumulation in the perinuclear region in Aβ1–42–exposed Drosophila cells (Lin et al., 2007). A recent study showed that p27, a cyclin-dependent kinase inhibitor, is required for the nuclear accumulation of Cdk5 in neurons (Zhang et al., 2010). This study shows that lamin phosphorylation is one of the major mechanisms that promote Cdk5 nuclear localization on neurotoxic insults.
Previous studies have identified multiple neurotoxic pathways by which nuclear Cdk5 induces apoptotic cell death. Cdk5 activates p53 by phosphorylation, inducing apoptosis (Zhang et al., 2002; Lee and Kim, 2007). Cdk5 degrades prosurvival transcription factors MEF2A and MEF2D via phosphorylation, promoting neuronal death (Tang et al., 2005). Cdk5 induces expression of β-secretase by activating Stat3 (Wen et al., 2008). Cdk5 phosphorylates ataxia-telangiectasia mutated and up-regulates its targets p53 and H2AX causing death (Tian et al., 2009). Nuclear Cdk5/p25 promote cell death in ER-stressed neurons (Saito et al., 2007). Cdk5 activates DNA damage signaling by inhibiting histone deacetylase 1 (Kim et al., 2008) and apurinic/apyrimidinic endonuclease 1 (Huang et al., 2010), causing neuronal death. Our study demonstrated that Cdk5-mediated activation of c-Jun is critical in Aβ- and glutamate-mediated neurotoxicity (Sun et al., 2009).
In conclusion, we show that nuclear dispersion in neurodegeneration, rather than being a consequence of apoptosis, may be an early and irreversible trigger for apoptosis. Our data show that nuclear lamina dispersion occurs due to the direct phosphorylation of lamin A and lamin B1 by deregulated Cdk5 in primary neurons and animal models. Prevention of nuclear dispersion by Cdk5 is highly neuroprotective, suggesting that this event is a significant contributor to AD pathology. The structural alteration of the nuclear envelope due to the phosphorylation of lamin A and B1 by Cdk5 might result in functional alteration in nuclear cytoplasmic transportations as shown in AD brains (Lee et al., 2006a, 2006b; Sheffield et al., 2006). Our study supports the idea that inhibition of Cdk5 might be an effective way to prevent/delay the irreversible process of neurodegeneration.
MATERIALS AND METHODS
Materials
Glutamate, MTT, thymidine, and poly-l-lysine were obtained from Sigma (St. Louis, MO). Aβ25–35 was purchased from AnaSpec (Freemont, CA). Roscovitine was purchased from LC Laboratories (Woburn, MA). Antibodies against lamin A (H-102), lamin B (M-20), and actin (C-2) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Fluorescein isothiocyanate (FITC)-labeled goat anti–rabbit and Texas red-labeled goat anti–mouse were bought from Invitrogen (Carlsbad, CA).
Cell culture
HT22 cells were cultured in DMEM supplemented with 10% fetal bovine serum.
Isolation of primary cortical cells
Time pregnant Sprague Dawley rats were purchased from Charles River (Wilmington, MA). Primary cortical neurons were isolated from E17 rat embryos as described previously (Behrens et al., 1999; Sun et al., 2008a, 2008b). Under these conditions, <5% of total cells were astrocytes. Cultures were maintained at 37°C in a humidified 5% CO2 atmosphere. All experiments were conducted after 14 DIV (days in vitro).
Expression plasmids and constructs
WT and mutants lamin A (mouse) and lamin B1 (human) were created by overlapping PCR and cloned into pET-28b-TAT (V2.1) vector to generate His-tagged proteins. They were also cloned into VIP3 retroviral mammalian vector as myc-tagged constructs.
Expression and purification of recombinant proteins
GST-Cdk5/p25, 6-His-tagged lamin proteins were expressed and purified as described previously (Sun et al., 2008a, 2008b). After purification, protein concentration was determined using the Bradford assay, and the protein purity was assessed by gel electrophoresis.
Transfection and retroviral infection
Myc-tagged lamin A and lamin B1 plasmids were transiently transfected into phoenix cells. The viruses were harvested and used to infect HT22 cells as reported previously (Shah and Shokat, 2002). Cells were selected using puromycin.
Cdk5 shRNA preparation and lentivital infection
A Cdk5 mouse shRNA sequence was designed to target the following sequence: 5′-CCGGGAAACTCATGAGATT-3′.It was cloned in pLKO.1 TRC vector (Moffat et al., 2006) according to the manufacturer’s instructions. Scrambled and Cdk5 shRNA lentiviruses were generated in HEK293T cells and used to infect HT22 cells for 30 h. Cells were fixed using 4% formaldehyde for immunofluorescence studies or lysed for immunoblot analysis of endogenous Cdk5 level.
In vitro phosphorylation of lamins by Cdk5/p25 complex
Recombinant WT and mutant lamin A and lamin B1 (5 μg each) proteins were phosphorylated by purified Cdk5/p25 complex (20 ng) at 30°C in a final volume of 30 μl containing 50 mM Tris, pH 8.0, 20 mM MgCl2, and [32P]ATP.
Cdk5 kinase activity
HT22 cells and primary cortical neurons treated with either 5 mM glutamate (for HT22 cells), 100 μM glutamate (for primary neurons), or 25 μM Aβ25–35, were lysed in RIPA buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 1% NP-40, 0.25% deoxycholate, 0.1% SDS, 10 mM NaF, 1 mM Na3VO4, and protease inhibitors). Following Cdk5 immunoprecipitation, a kinase assay was initiated using 10 μM Cdk5 substrate peptide and 2 μCi [γ-32P]ATP for 20 min. The supernatant was spotted on phosphocellulose discs, incubated with 10% acetic acid for 30 min, and finally washed with 0.5% H3PO4, followed by acetone. 32P incorporation on Cdk5 substrate peptide was measured using a scintillation counter.
Western blotting
Cells were harvested and lysed in RIPA buffer. Proteins were separated by SDS–PAGE, transferred to a polyvinylidene fluoride (PVDF) membrane, and incubated with primary and secondary antibodies as reported before (Sun et al., 2008a, 2008b, 2009).
Subcellular fractionation
HT22 cells were treated with 2 mM thymidine for 8 h, followed by 5 mM glutamate for 4 h. Subcellular fractionation buffer (250 mM sucrose, 20 mM HEPES, pH 7.4, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol) was added to lyse the cells and then was passed through a 25 G needle (10×). The cell lysate was further incubated on ice for 20 min. The nuclear pellet was obtained after centrifugation at 3000 rpm for 5 min. The supernatant was centrifuged at 8000 rpm for 10 min. The cytosolic fraction was obtained after the removal of supernatant. Both nuclear and cytosolic fractions were mixed with SDS loading dye. Proteins were separated using SDS–PAGE, then transferred to a PVDF membrane. Actin was used as a cytoplasmic marker.
Immunofluorescence
Rat primary cortical cells were plated on poly-l-lysine–coated coverslips at a density of 50,000 cells per well in 24-well plates. Cells were treated with either 100 μM glutamate or 25 μM Aβ25–35 for 4 h in the absence or presence of 10 μM roscovitine. HT22 cells and myc-tagged, lamin-expressing HT22 cells were plated on poly-l-lysine–coated coverslips at a density of 10,000 cells per well in 24-well plates for 1 d, followed by 2 mM thymidine treatment for 8 h. These cells were then stimulated with 5 mM glutamate or 25 μM Aβ25–35 for 4 h. At the end of the treatment, the medium was aspirated and the cells were fixed with 4% formaldehyde for 15 min, rinsed with phosphate-buffered saline (PBS), and incubated for 20 min with 5% bovine serum albumin (BSA) and 0.1% Triton X-100. Cells were then immunostained using antibodies against lamin A, lamin B, NUP98, Cdk5, or myc for 3 h at room temperature. FITC-labeled goat anti-rabbit and Texas red–labeled goat anti–mouse antibodies were used at a 1:1000 dilution, together with DAPI (1 μg/ml). After three washes with PBS and one wash with water, coverslips were mounted on microscope slides with Mowiol mounting medium. Images were taken using a Nikon Eclipse E600 microscope (Nikon Instruments, Melville, NY). The percentage of cells with intact nuclei was counted in triplicate in at least 100 cells from 10 random frames.
PI staining
HT22 cells plated on poly-l-lysine–coated coverslips at a density of 10,000 cells were treated with 5 mM glutamate or 25 μM Aβ25–35 for 4 h, followed by 2 mM thymidine for 8 h. Cells were fixed with 4% formaldehyde for 15 min at room temperature, permeabilized with 5% BSA and 0.1% Triton X-100 for 20 min, treated with 0.1 μg/ml RNase A for 1 h, and stained with 2.5 μg/ml PI for 1 h. After three washes with PBS and one wash with water, coverslips were mounted on microscope slides with Mowiol mounting medium.
Cell proliferation assay
Cells (1500 cells/well) were seeded on 96-well plates overnight. After incubation for 24, 48, or 72 h, MTT (0.5 mg/ml) was added to each well, and the plate was incubated for an additional 3 h. After the removal of the medium, 100 μl of dimethyl sulfoxide was added to dissolve the cells. The absorbance value was measured at 595 nm in the ULTRA microplate reader (Tecan Research). Experiments were done in triplicate.
Phospho-Histone H3 (Ser-10) assay
Cells were seeded on the poly-l-lysine–coated coverslips at a density of 1 × 104 cells per well in a 24-well plate overnight. Cells were treated with 2 mM thymidine for 18 h. The G1/S-arrested cells were released by replacing the medium with fresh medium without thymidine. After an 8 h incubation, cells were treated with 2 mM thymidine for an additional 18 h, followed by 4, 8, 10, 12, 14, and 16 h of release. Formaldehyde (4%) was added to fix the released cells. Cells were rinsed with PBS and incubated with 5% BSA and 0.1% Triton X-100 for 20 min. Cells were immunostained using antibodies against phospho-Histone H3 (Ser-10) for 3 h at room temperature. The cells were washed with PBS and incubated with FITC-labeled goat anti–rabbit antibodies and DAPI for 1 h at room temperature. Subsequent to washing with PBS, coverslips were mounted on slides with Mowiol mounting medium. The percentage of phospho-Histone H3 (Ser-10)–positive cells was determined by counting in triplicate at least 100 cells from ten random frames.
Immunocytochemistry in p25 transgenic mice
Immunocytochemistry in p25 transgenic mice was conducted according to our published procedure (Sun et al., 2009; Chang et al., 2010).
MTT assay
WT and lamin A– and lamin B1–expressing HT22 cells were seeded onto six-well plates and exposed to glutamate treatment. After 24 h of 5 mM glutamate treatment, the MTT assay was conducted as described previously (Sun et al., 2008a, 2008b).
Statistical significance
Bar graphs are plotted as the average ± SD. Significance was evaluated using Student’s t test, and it is displayed as: *p < 0.05, **p < 0.01, ***p < 0.001.
Acknowledgments
We thank David Root for providing pLKO.1 TRC vector and A. Ramachandran for generating Cdk5 shRNA. This work was supported by the Indiana Alzheimer Disease Center (K.S.).
Abbreviations used:
- 2D
two-dimensional
- Aβ
amyloid-β
- AD
Alzheimer’s disease
- BSA
bovine serum albumin
- Cdk5
cyclin-dependent kinase 5
- Cdk5-as1
analogue-sensitive Cdk5
- ER
endoplasmic reticulum
- FITC
fluorescein isothiocyanate
- JNK
c-jun-N-terminal kinase
- MTT
3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NFT
neurofibrillary tangles
- PARP1
poly (ADP-ribose) polymerase
- PBS
phosphate-buffered saline
- PI
propidium iodide
- PKC
protein kinase C
- PVDF
polyvinylidene fluoride
- Ros
roscovitine
- TAT-CIP
TAT-fused Cdk5 inhibitory peptide
- WT
wild type
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-07-0654) on March 9, 2011.
REFERENCES
- Ankarcrona M, Zhivotovsky B, Holmstrom T, Diana A, Eriksson JE, Orrenius S, Nicotera P. Lamin and β-tubulin fragmentation precede chromatin degradation in glutamate-induced neuronal apoptosis. Neuroreport. 1996;7:2659–2664. doi: 10.1097/00001756-199611040-00050. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Strasser U, Koh JY, Gwag BJ, Choi DW. Prevention of neuronal apoptosis by phorbol ester-induced activation of protein kinase C: blockade of p38 mitogen-activated protein kinase. Neuroscience. 1999;94:917–927. doi: 10.1016/s0306-4522(99)00212-2. [DOI] [PubMed] [Google Scholar]
- Cenni V, et al. Lamin A Ser404 is a nuclear target of Akt phosphorylation in C2C12 cells. J Proteome Res. 2008;7:4727–4735. doi: 10.1021/pr800262g. [DOI] [PubMed] [Google Scholar]
- Chang KH, de Pablo Y, Lee HP, Lee HG, Smith MA, Shah K. Cdk5 is a major regulator of p38 cascade: relevance to neurotoxicity in Alzheimer’s disease. J Neurochem. 2010;113:1221–1229. doi: 10.1111/j.1471-4159.2010.06687.x. [DOI] [PubMed] [Google Scholar]
- Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- Dechat T, Adam SA, Goldman RD. Nuclear lamins and chromatin: when structure meets function. Adv Enzyme Regul. 2009;49:157–166. doi: 10.1016/j.advenzreg.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- Fu X, Choi YK, Qu D, Yu Y, Cheung NS, Qi RZ. Identification of nuclear import mechanisms for the neuronal Cdk5 activator. J Biol Chem. 2006;281:39014–39021. doi: 10.1074/jbc.M512663200. [DOI] [PubMed] [Google Scholar]
- Heald R, McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 1990;61:579–589. doi: 10.1016/0092-8674(90)90470-y. [DOI] [PubMed] [Google Scholar]
- Hocevar BA, Burns DJ, Fields AP. Identification of protein kinase C (PKC) phosphorylation sites on human lamin BPotential role of PKC in nuclear lamina structural dynamics. J Biol Chem. 1993;268:7545–7552. [PubMed] [Google Scholar]
- Huang E, Qu D, Zhang Y, Venderova K, Haque ME, Rousseaux MW, Slack RS, Woulfe JM, Park DS. The role of Cdk5-mediated apurinic/apyrimidinic endonuclease 1 phosphorylation in neuronal death. Nat Cell Biol. 2010;12:563–571. doi: 10.1038/ncb2058. [DOI] [PubMed] [Google Scholar]
- Ino H, Chiba T. Intracellular localization of cyclin-dependent kinase 5 (CDK5) in mouse neuron: CDK5 is located in both nucleus and cytoplasm. Brain Res. 1996;732:179–185. doi: 10.1016/0006-8993(96)00523-9. [DOI] [PubMed] [Google Scholar]
- Kim D, et al. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron. 2008;60:803–817. doi: 10.1016/j.neuron.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Shah K. Dissecting yeast Hog1 MAP kinase pathway using a chemical genetic approach. FEBS Lett. 2007;581:1209–1216. doi: 10.1016/j.febslet.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Klementiev B, Novikova T, Novitskaya V, Walmod PS, Dmytriyeva O, Pakkenberg B, Berezin V, Bock E. A neural cell adhesion molecule-derived peptide reduces neuropathological signs and cognitive impairment induced by Aβ25–35. Neuroscience. 2007;145:209–224. doi: 10.1016/j.neuroscience.2006.11.060. [DOI] [PubMed] [Google Scholar]
- Lee HG, Ueda M, Miyamoto Y, Yoneda Y, Perry G, Smith MA, Zhu X. Aberrant localization of importin α1 in hippocampal neurons in Alzheimer disease. Brain Res. 2006a;1124:1–4. doi: 10.1016/j.brainres.2006.09.084. [DOI] [PubMed] [Google Scholar]
- Lee HG, Ueda M, Zhu X, Perry G, Smith MA. Ectopic expression of phospho-Smad2 in Alzheimer’s disease: uncoupling of the transforming growth factor-β pathway? J Neurosci Res. 2006b;84:1856–1861. doi: 10.1002/jnr.21072. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim KT. Regulation of cyclin-dependent kinase 5 and p53 by ERK1/2 pathway in the DNA damage-induced neuronal death. J Cell Physiol. 2007;210:784–797. doi: 10.1002/jcp.20899. [DOI] [PubMed] [Google Scholar]
- Lee KY, Clark AW, Rosales JL, Chapman K, Fung T, Johnston RN. Elevated neuronal Cdc2-like kinase activity in the Alzheimer disease brain. Neurosci Res. 1999;34:21–29. doi: 10.1016/s0168-0102(99)00026-7. [DOI] [PubMed] [Google Scholar]
- Lin H, Lin TY, Juang JL. Abl deregulates Cdk5 kinase activity and subcellular localization in Drosophila neurodegeneration. Cell Death Differ. 2007;14:607–615. doi: 10.1038/sj.cdd.4402033. [DOI] [PubMed] [Google Scholar]
- Maestre C, Delgado-Esteban M, Gomez-Sanchez JC, Bolanos JP, Almeida A. Cdk5 phosphorylates Cdh1 and modulates cyclin B1 stability in excitotoxicity. EMBO J. 2008;27:2736–2745. doi: 10.1038/emboj.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Oberhammer FA, Hochegger K, Froschl G, Tiefenbacher R, Pavelka M. Chromatin condensation during apoptosis is accompanied by degradation of lamin A+B, without enhanced activation of cdc2 kinase. J Cell Biol. 1994;126:827–837. doi: 10.1083/jcb.126.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare MJ, Kushwaha N, Zhang Y, Aleyasin H, Callaghan SM, Slack RS, Albert PR, Vincent I, Park DS. Differential roles of nuclear and cytoplasmic cyclin-dependent kinase 5 in apoptotic and excitotoxic neuronal death. J Neurosci. 2005;25:8954–8966. doi: 10.1523/JNEUROSCI.2899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick GN, Zhou P, Kwon YT, Howley PM, Tsai LH. p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin-proteasome pathway. J Biol Chem. 1998;273:24057–24064. doi: 10.1074/jbc.273.37.24057. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Walencewicz-Wasserman AJ, Kosmoski J, Cribbs DH, Glabe CG, Cotman CW. Structure-activity analyses of β-amyloid peptides: contributions of the β25–35 region to aggregation and neurotoxicity. J Neurochem. 1995;64:253–265. doi: 10.1046/j.1471-4159.1995.64010253.x. [DOI] [PubMed] [Google Scholar]
- Sahlgren CM, Pallari HM, He T, Chou YH, Goldman RD, Eriksson JE. A nestin scaffold links Cdk5/p35 signaling to oxidant-induced cell death. EMBO J. 2006;25:4808–4819. doi: 10.1038/sj.emboj.7601366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Konno T, Hosokawa T, Asada A, Ishiguro K, Hisanaga S. p25/cyclin-dependent kinase 5 promotes the progression of cell death in nucleus of endoplasmic reticulum-stressed neurons. J Neurochem. 2007;102:133–140. doi: 10.1111/j.1471-4159.2007.04540.x. [DOI] [PubMed] [Google Scholar]
- Shah K, Liu Y, Deirmengian C, Shokat KM. Engineering unnatural nucleotide specificity for Rous sarcoma virus tyrosine kinase to uniquely label its direct substrates. Proc Natl Acad Sci USA. 1997;94:3565–3570. doi: 10.1073/pnas.94.8.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Shokat KM. A chemical genetic screen for direct v-Src substrates reveals ordered assembly of a retrograde signaling pathway. Chem Biol. 2002;9:35–47. doi: 10.1016/s1074-5521(02)00086-8. [DOI] [PubMed] [Google Scholar]
- Shah K, Shokat KM. A chemical genetic approach for the identification of direct substrates of protein kinases. Methods Mol Biol. 2003;233:253–271. doi: 10.1385/1-59259-397-6:253. [DOI] [PubMed] [Google Scholar]
- Shah K, Vincent F. Divergent roles of c-Src in controlling platelet-derived growth factor-dependent signaling in fibroblasts. Mol Biol Cell. 2005;16:5418–5432. doi: 10.1091/mbc.E05-03-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea TB, Yabe JT, Ortiz D, Pimenta A, Loomis P, Goldman RD, Amin N, Pant HC. Cdk5 regulates axonal transport and phosphorylation of neurofilaments in cultured neurons. J Cell Sci. 2004b;117:933–941. doi: 10.1242/jcs.00785. [DOI] [PubMed] [Google Scholar]
- Shea TB, Zheng YL, Ortiz D, Pant HC. Cyclin-dependent kinase 5 increases perikaryal neurofilament phosphorylation and inhibits neurofilament axonal transport in response to oxidative stress. J Neurosci Res. 2004a;76:795–800. doi: 10.1002/jnr.20099. [DOI] [PubMed] [Google Scholar]
- Sheffield LG, Miskiewicz HB, Tannenbaum LB, Mirra SS. Nuclear pore complex proteins in Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:45–54. doi: 10.1097/01.jnen.0000195939.40410.08. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Cao CX, Shao RG, Pommier Y. Lamin B phosphorylation by protein kinase cα and proteolysis during apoptosis in human leukemia HL60 cells. J Biol Chem. 1998;273:8669–8674. doi: 10.1074/jbc.273.15.8669. [DOI] [PubMed] [Google Scholar]
- Stewart CL, Roux KJ, Burke B. Blurring the boundary: the nuclear envelope extends its reach. Science. 2007;318:1408–1412. doi: 10.1126/science.1142034. [DOI] [PubMed] [Google Scholar]
- Su JH, Anderson AJ, Cummings BJ, Cotman CW. Immunohistochemical evidence for apoptosis in Alzheimer’s disease. Neuroreport. 1994;5:2529–2533. doi: 10.1097/00001756-199412000-00031. [DOI] [PubMed] [Google Scholar]
- Sun KH, de Pablo Y, Vincent F, Johnson EO, Chavers AK, Shah K. Novel genetic tools reveal Cdk5’s major role in Golgi fragmentation in Alzheimer’s disease. Mol Biol Cell. 2008a;19:3052–3069. doi: 10.1091/mbc.E07-11-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun KH, de Pablo Y, Vincent F, Shah K. Deregulated Cdk5 promotes oxidative stress and mitochondrial dysfunction. J Neurochem. 2008b;107:265–278. doi: 10.1111/j.1471-4159.2008.05616.x. [DOI] [PubMed] [Google Scholar]
- Sun KH, Lee HG, Smith MA, Shah K. Direct and indirect roles of cyclin-dependent kinase 5 as an upstream regulator in the c-Jun NH2-terminal kinase cascade: relevance to neurotoxic insults in Alzheimer’s disease. Mol Biol Cell. 2009;20:4611–4619. doi: 10.1091/mbc.E09-05-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Wang X, Gong X, Tong M, Park D, Xia Z, Mao Z. Cyclin-dependent kinase 5 mediates neurotoxin-induced degradation of the transcription factor myocyte enhancer factor 2. J Neurosci. 2005;25:4823–4834. doi: 10.1523/JNEUROSCI.1331-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Yang Q, Mao Z. Phosphorylation of ATM by Cdk5 mediates DNA damage signalling and regulates neuronal death. Nat Cell Biol. 2009;11:211–218. doi: 10.1038/ncb1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, et al. Transcriptional regulation of β-secretase by p25/cdk5 leads to enhanced amyloidogenic processing. Neuron. 2008;57:680–690. doi: 10.1016/j.neuron.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li H, Herrup K. Cdk5 nuclear localization is p27-dependent in nerve cells: implications for cell cycle suppression and caspase-3 activation. J Biol Chem. 2010;285:14052–14061. doi: 10.1074/jbc.M109.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McLaughlin R, Goodyer C, LeBlanc A. Selective cytotoxicity of intracellular amyloid β peptide1–42 through p53 and Bax in cultured primary human neurons. J Cell Biol. 2002;156:519–529. doi: 10.1083/jcb.200110119. [DOI] [PMC free article] [PubMed] [Google Scholar]