This study establishes Dyn2 as a novel effector downstream of Src-FAK signaling in mediating FA disassembly. FAK directly binds to and recruits Dyn2 to FAs. The formation of a Src–FAK–Dyn2 complex is essential for Dyn2's phosphoactivation and subsequent endocytic turnover of FAs.
Abstract
Tumor cell migration is supported in part by the cyclic formation and disassembly of focal adhesions (FAs); however, the mechanisms that regulate this process are not fully defined. The large guanosine 5′-triphosphatase dynamin (Dyn) plays an important role in FA dynamics and is activated by tyrosine phosphorylation. Using a novel antibody specific to phospho-dynamin (pDyn–Tyr-231), we found that Dyn2 is phosphorylated at FAs by Src kinase and is recruited to FAs by a direct interaction with the 4.1/ezrin/radizin/moesin domain of focal adhesion kinase (FAK), which functions as an adaptor between Src and Dyn2 to facilitate Dyn2 phosphorylation. This Src–FAK–Dyn2 trimeric complex is essential for FA turnover, as mutants disrupting the formation of this complex inhibit FA disassembly. Importantly, phosphoactivated Dyn2 promotes FA turnover by mediating the endocytosis of integrins in a clathrin-dependent manner. This study defines a novel mechanism of how Dyn2 functions as a downstream effector of FAK–Src signaling in turning over FAs.
INTRODUCTION
Focal adhesions (FAs) are complex structures situated at the cell base that mediate adhesion and motility by connecting the extracellular matrix (ECM) with the actin cytoskeleton (Webb et al., 2002; Wehrle-Haller and Imhof, 2002; Mitra et al., 2005). During migration, FAs form at the leading edge of the cell to anchor cytoplasmic stress fibers and contribute to the generation of a contractile force (Geiger and Bershadsky, 2001; Wehrle-Haller and Imhof, 2002). Concomitant with this localized assembly, FAs at the trailing edge of translocating cells are disassembled to facilitate ECM detachment (Webb et al., 2002). Cells that do not exhibit appropriate FA disassembly are known to leave long cytoplasmic tails that impede migration (Palecek et al., 1998; Ezratty et al., 2009). While the regulated assembly of FAs is generally well understood (Sastry and Burridge, 2000; Webb et al., 2002), the mechanisms that support the regulated turnover of these important structures are less defined.
Focal adhesion kinase (FAK) and Src are both nonreceptor tyrosine kinases viewed as central regulators of FA turnover (Frame et al., 2002; McLean et al., 2005; Mitra et al., 2005; Shattil, 2005). In addition to tyrosine kinase activity, FAK is also known to act as a FA scaffold, where it binds a variety of cytoskeletal and adaptor proteins as well as kinases such as Src (Mitra et al., 2005). A specific tyrosine residue in the SH2 domain of FAK (pTyr-397) is known to both bind and activate Src by disrupting its autoinhibitory conformation (Yeatman, 2004). Subsequently, Src phosphorylates FAK on multiple tyrosine residues, resulting in the recruitment of additional FA proteins (Mitra et al., 2005). In addition to providing a regulated scaffold for FA assembly, both Src and FAK have been implicated in FA turnover, as Src−/− and FAK−/− cells exhibit more stable FAs (Ilic et al., 1995; Yeo et al., 2006). Interestingly, while the phosphorylation of the associated proteins p130Cas and paxillin by a Src–FAK complex is known to promote FA disassembly, FAK kinase activity appears to be dispensable, suggesting that FAK instead functions as a structural scaffold (Ruest et al., 2001). Moreover, the Src–FAK complex is known to promote the expression and activation of matrix metalloproteinases and the calcium-dependent calpain proteases (Palecek et al., 1998; Bhatt et al., 2002) that contribute to FA turnover. While many other FA proteins are substrates for the Src–FAK complex, the effects on FA dynamics are less clear.
The large guanosine 5′-triphosphatase (GTPase) dynamin (Dyn) is well established to participate in a variety of endocytic and membrane trafficking processes (Urrutia et al., 1997; McNiven, 1998; Hinshaw, 2000). The enzymatic activity, polymerization rate, and cellular functions of dynamins have been shown to be modulated by phosphorylation at two specific tyrosine residues, Tyr-231 and Tyr-597, by Src (Ahn et al., 1999; Ahn et al., 2002; Cao et al., 2010; Weller et al., 2010). Recently, Dyn2 has been observed to localize to FAs, where it mediates the clathrin-based internalization of integrins (Chao and Kunz, 2009; Ezratty et al., 2009). Importantly, FAK has been shown by several groups to associate with Dyn2 at FAs (Kharbanda et al., 1995; Ezratty et al., 2005), although it is unclear how this association is regulated and whether it is direct or supported by adaptor proteins such as Grb2 (Kharbanda et al., 1995). In this study we provide new insights into how Src–FAK signaling functions together with Dyn2 to regulate disassembly of FAs. We find that FAK binds to Dyn2 directly through its 4.1/ezrin/radizin/moesin (FERM) domain and recruits Dyn2 into a Src–FAK–Dyn2 trimeric complex that further facilitates Dyn2 tyrosine phosphorylation and activation. We also demonstrate that phospho-Dyn2 (pDyn2) is essential for regulated endocytic internalization of integrins and subsequent FA disassembly. These data define a novel pathway describing how Src–FAK signaling can alter cell adhesion and cell migration.
RESULTS
A population of phosphorylated Dyn2 resides at FAs
Src-based tyrosine phosphorylation of both Dyn1 and Dyn2 has been shown to enhance receptor-mediated endocytosis (Ahn et al., 1999; Cao et al., 2010) and vesicle trafficking from the Golgi apparatus (Weller et al., 2010). To better define the distribution and function of pDyn in these processes, we generated two polyclonal antibodies against phosphotyrosine residues, Tyr-231 and Tyr-597, conserved in all conventional dynamins (see Materials and Methods). While the Dyn pTyr-597 antibody failed to recognize Dyn by Western blot or immunofluorescence (IF) staining, the Dyn pTyr-231 antibody appeared to be very sensitive to phosphorylated Dyn in a variety of cultured cells. IF staining of human pancreatic tumor cells (PANC-1) under resting status showed modest, punctate FA staining (Figure 1, A and A′); however, there was a marked increase in FA staining either when stimulated with 20 ng/ml epidermal growth factor (EGF) for 20 min or with expression of active SrcY530F (Figure 1, B–C′, arrows). To confirm that active Src is required for the phosphorylation of Dyn2 at FAs, a triple-knockout SYF cell line (fibroblasts from Src, Yes, and Fyn knockout mice) was transfected with active Src (Y530F) before staining with the pDyn antibody. The SYF cells showed no FA staining for either Src or pDyn (Figure 1, D and D′), while the adjacent cells expressing SrcY530F displayed substantial pDyn staining at FAs (Figure 1, D and D′, asterisk). Taken together these observations suggest that either physiological stimulation of cells with EGF to activate Src kinase or exogenous expression of active Src is responsible for Dyn phosphorylation at FAs. It should be noted that while the pDyn antibody we made and utilized for this study recognizes all three Dyn forms, epithelial cells express Dyn2 as the most prominent form by far, so we have referred to pDyn as pDyn2.
FIGURE 1:
Src mediates the phosphorylation of Dyn2 at FAs. (A–C′) IF images of PANC-1 cells that were either untreated (A, A′), stimulated with 20 ng/ml EGF for 20 min (B, B′), or transfected with SrcY530F (C, C′) and then fixed and costained with pDyn and vinculin antibodies. While resting cells show modest pDyn staining of FAs, this is markedly increased (arrows) by activation of Src. (D, D′) Fibroblasts from Src, Yes, and Fyn knockout mice (SYF cells) were stained with the pDyn antibody and show virtually no staining of FAs. This staining is increased significantly upon exogenous expression of active Src (*). (E) Quantification of the percentage of PANC-1 cells (A–C′) with pDyn-positive FAs. Values represent the average of three independent experiments. (F, G) Detection of FAK and pDyn association. (F) Immunoprecipitate of FAK from MEF cell lysate was blotted with FAK and pDyn antibodies. A portion of FAK-associated Dyn2 is phosphorylated even under resting conditions. (G) To test whether a phosphomutant of Dyn2 exhibits reduced binding to FAK, 293T cells were cotransfected with SFB-FAK and either WT or Dyn2Y231597F (YF) before IP with S-Sepharose beads and blotted for Dyn2 and Flag (FAK). WT Dyn2 exhibits a significantly higher affinity for FAK than the Dyn2 YF mutant.
To ensure that the pDyn antibody used in these experiments is specific for pDyn, a variety of control experiments were performed. As shown in Supplemental Figure S1A, Western blot analysis of cells expressing the exogenous wild-type (WT) or phosphomutant forms of Dyn2 indicates that the pDyn antibody recognizes both Dyn2 WT and Dyn2 Y597F, but not Y231F. Thus a specific alteration of the phosphoepitope prevents binding of the pDyn antibody. Moreover, expression of a constitutively active Src (SrcY530F) in cells resulted in the marked enhancement in the detection of endogenous Dyn2 by the pDyn antibody (Supplemental Figure S1A). Further, the pDyn2 band was significantly reduced in cells treated with Dyn2 small interfering RNA (siRNA) to reduce the endogenous level of this protein (Supplemental Figure S1C). To test the specificity of this reagent using morphological methods, rat fibroblast (RF) cells were transfected to express Dyn2 WT–green fluorescent protein (GFP) or Dyn2Y231F-GFP and then fixed and stained with the pDyn antibody. Notably, cells expressing Dyn2 WT (*) were brightly stained in comparison to the adjacent nontransfected cells (Supplemental Figure S1, D and D′), while cells expressing the phosphomutant Dyn2Y231F (*) showed no increased staining (Supplemental Figure S1, E and E′). As a final test, Dyn conditional knockout cells (generously provided by Shawn Ferguson and Pietro De Camilli, Yale University, New Haven, CT) were stained with the pDyn antibody and a pan-Dyn antibody (Hudy1). Both antibodies show compromised staining in Dyn-depleted cells (Supplemental Figure S1, F and F′). Taken together, we conclude that the pDynY231 antibody is specific for pDyn. In addition, with this newly characterized antibody we determined that Src is required for Dyn2 phosphorylation at FAs.
FAK recruits Dyn2 to FAs via the FERM domain
FAK is an important kinase and scaffold at FAs and has been implicated to interact with and recruit Dyn2 to FAs (Ezratty et al., 2005). Consistent with this report, we compared the IF staining of pDyn2 in normal and FAK−/− mouse embryonic fibroblasts (MEFs) and observed a substantial reduction of FA staining in FAK−/− cells (Figure 2, A–B′), indicating an essential role for FAK in recruiting Dyn2 to FAs. On the basis of our observation that pDyn2 localizes to FAs, we reasoned that FAK-associated Dyn2 is tyrosine phosphorylated. As shown in Figure 1F, pDyn2 was coimmunoprecipitated (coIP) with FAK. More interestingly, we found that FAK preferentially binds to pDyn2. As shown in Figure 1G, WT Dyn2 or a double phosphomutant Dyn2Y231, 597F (Dyn2 YF) were cotransfected with SFB-FAK (S protein, Flag, and streptavidin binding peptide triple tagged). FAK was immunoprecipitated with S-Sepharose beads and coprecipitated significantly more WT Dyn2 than Dyn2 YF (3.2 ± 0.6-fold). These data provide biochemical evidence supporting the premise that pDyn2 localizes at FAs.
FIGURE 2:
Recruitment of Dyn2 to FAs by the FERM domain of FAK. (A–B′) IF images of WT or FAK−/− MEF cells stained for pDyn and vinculin. MEF cells show significant labeling of pDyn at FAs (arrow) that is diminished in FAK−/− cells (B, B′). (C) Illustration of the FAK constructs utilized to map the domains that interact with Dyn. (D) Western blot analysis of IP from 293T cells that were cotransfected with SFB-Dyn2 and GFP-FAK WT or the indicated FAK mutants. Dyn2 was precipitated with S beads, resolved by SDS–PAGE, and blotted with antibodies against GFP (FAK) and Flag (Dyn2). FAK mutants without the FERM domain do not associate with Dyn2. (E) Far Western blot analysis was performed to test for a direct interaction between Dyn2 and FAK. Purified GST, GST-FERM, and GST-FRNK were resolved by SDS–PAGE, overlaid with purified His-Dyn2, and then blotted with Dyn2 antibody. The FERM domain, but not the GST or the FRNK domain, interacts with Dyn2 directly. (F–H′) IF images of FAK−/− cells expressing WT or mutant FAK to test for restoration of pDyn’s FA staining. FAK−/− cells were transfected with indicated constructs (*) and then fixed and stained with pDyn antibody. The expression of WT FAK, but not ΔFERM or FRNK, rescued FA staining by the pDyn antibody.
To define the mechanism behind the FAK-Dyn2 association, a series of FAK truncation mutants (Figure 2C) were generated and coexpressed along with SFB-Dyn2 in 293T cells. Subsequently, Dyn2 was immunoprecipitated with S beads and blotted with antibodies against GFP (FAK) and Flag (Dyn2). As shown in Figure 2D, removal of the N-terminal FERM domain of FAK abolished the association with Dyn2, while truncation of the C-terminal region (ΔFRNK) did not, suggesting that FAK binds to Dyn2 through the FERM domain. To test whether this binding is direct, purified glutathione S-transferase (GST), GST-FERM, and GST-FRNK were expressed, and Far Western blot analysis was performed by probing these protein fragments with purified His-Dyn2. Consistent with the coIP data described above, His-Dyn2 bound directly to GST-FERM, but not GST or GST-FRNK (Figure 2E), suggesting that the FERM domain of FAK is both necessary and sufficient for Dyn2 binding. As Figure 1G indicates that FAK binds more strongly to pDyn2 in cells, we next tested whether it is true in vitro. To this end we purified WT or YF His-Dyn2 from TKB1 Escherichia coli, which contains a tyrosine kinase capable of generating phosphoproteins (Gomez et al., 2006). We confirmed that His-Dyn2 WT but not YF was phosphorylated with both 4G10 and pDyn2 antibodies (Supplemental Figure S2). By incubating GST-FERM with these Dyn2 proteins, we observed a threefold increase in the level of association between FAK and pDyn2 compared with Dyn2 YF (Supplemental Figure S2). These data suggest that FAK has an increased affinity to pDyn2 through the FERM domain.
On the basis of the role of FAK in recruiting Dyn2 to FAs, we predicted that reexpression of WT FAK in FAK−/− cells, but not Dyn2 binding mutants, should rescue pDyn’s FA staining. This was indeed the case as only modest pDyn2 staining was observed at FAs in FAK−/− cells (Figure 2, B and B′) while the staining was robust in cells rescued with GFP-FAK WT (Figure 2, F and F′) but not GFP-FAKΔFERM or GFP-FRNK (Figure 2, G–H′). Taken together, these data suggest that FAK directly associates with Dyn2 via the FERM domain to target it to FAs.
FAK facilitates the phosphorylation of Dyn2 via a Src–FAK–Dyn2 complex
It is well documented that FAK can act as a scaffold to mediate interactions between Src kinase and a variety of proteins (Polte and Hanks, 1997; Brown et al., 2005; Wu et al., 2005). As FAK binds to Dyn2 and Src via different domains, we hypothesized that both proteins might bind FAK simultaneously and thus form a complex at FAs. To test this premise, we compared the interactions among these proteins between WT and FAK−/− MEF cells by coIP. As shown in Figure 3A, Dyn2 coprecipitated with both FAK and Src from WT MEF cells, while in FAK−/− cells a reduced level of Src associated with Dyn2. This reduced Dyn–Src interaction could be rescued by reexpression of GFP-FAK WT. These data suggest that Src–FAK–Dyn2 exist as a complex, with FAK acting as a scaffold.
FIGURE 3:
Phosphorylation of Dyn2 is amplified when associated with a Src–FAK complex. (A) Western blot of Dyn2 immunoprecipitated from lysates of either WT MEF, FAK−/− cells, or FAK−/− cells reexpressing GFP-FAK. While the IP of Dyn2 brings down a significant amount of FAK and Src in MEF cells, Dyn2-Src association was markedly reduced in FAK−/− cells. This interaction is rescued by FAK reexpression. (B) To test whether FAK participates in the phosphorylation of Dyn2, WT and FAK−/− MEF cells were lysed and blotted for pDyn and Dyn2. There is a significant reduction in pDyn2 level in FAK−/− cells compared with WT MEFs (C). (D) To define the critical domains of FAK that regulate Dyn2 phosphorylation, FAK −/− cells were transfected with either WT or mutant FAK (Figure 2C) unable to bind Src or Dyn2. At 48 h after transfection, cells were lysed and blotted for pDyn and pSrc. While WT FAK–expressing cells exhibit high levels of pDyn and pSrc, this is attenuated in the mutant-expressing cells (E). (F) To test whether Dyn2 is phosphorylated by FAK–Src signaling in vivo, WT and FAK−/− MEF cells were either kept in suspension or plated on 50-μg/ml fibronectin-coated dishes for 1 h and then lysed and blotted with pDyn and Dyn2 antibodies. While there was ∼2.7-fold increase of pDyn2 level in MEF cells after plating on fibronectin, no significant increase was detected in FAK−/− cells (G). *p < 0.05; n.s., not significant. Results represent the average of three independent experiments.
As the Src–FAK complex binds and phosphorylates many different effectors, it seemed likely that a scaffolding function of FAK could accentuate Dyn2 phosphorylation by Src. We first compared pDyn2 levels in normal MEFs that support formation of the trimeric complex to FAK−/− cells that would not form this complex. As shown in Figure 3B, WT MEF cells possessed significantly more pDyn2 compared with FAK−/− cells, suggesting that FAK does play an important role in Dyn2 phosphorylation. To extend these findings we next tested whether reexpression of WT or mutant FAK in these null cells would rescue pDyn2 level. Interestingly, expression of WT FAK increased Dyn2 phosphorylation levels at least twofold higher than expression of either the vector control or the mutant proteins (Figure 3, D and E). Importantly, among the FAK mutants, the ΔFERM protein activated Src at a level equivalent to WT FAK, as this truncation retains the pTyr-397 region that binds to and activates Src. However, expression of the ΔFERM protein failed to rescue pDyn2 levels, suggesting that the FAK-based activation of Src alone, without forming the Src–FAK–Dyn2 complex, is not sufficient for Dyn2 phosphorylation. Last, we tested whether a FAK–Src complex phosphorylates Dyn2 in vivo. Because FAK–Src signaling is activated when integrins bind ECM, pDyn2 levels were compared by Western blot in WT and FAK−/− MEF cells either in suspension or plated on 50 μg/ml fibronectin-coated dishes for 1 h. While there was an ∼2.7-fold increase of pDyn2 levels in MEF cells following plating, no significant difference was detected in the FAK−/− cells (Figure 3, F and G), suggesting that phosphorylation of Dyn2 by Src–FAK signaling is a consequence of physiological stimulation.
The Src–FAK–Dyn2 complex promotes the regulated turnover of FAs
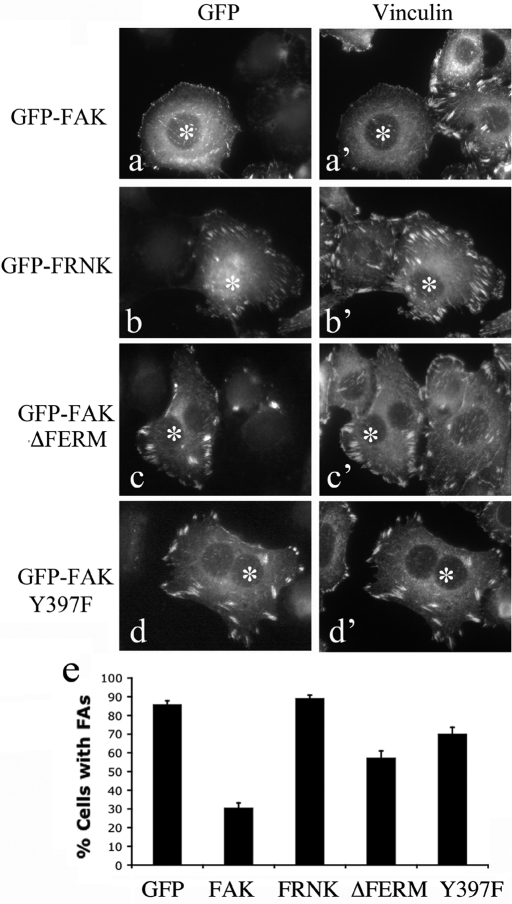
To test whether the physical association of Dyn2 with the FAK–Src complex is important in the regulated turnover of FAs, we implemented a FA disassembly assay (Ezratty et al., 2005) that provides a controllable method to study FA dynamics uniformly across a population of cells. For this assay cells were treated with 10 μM nocodazole for 3.5 h to completely disrupt microtubules (MTs) and then rinsed with serum-free medium to allow the rapid reassembly of tubulin dimers into MTs. On regrowth it is known that the tips of extending MTs reach FAs and subsequently promote FA turnover. In MEF cells, the majority of FAs disassemble within 1 h after nocodazole washout; however, as previously reported, FAK−/− cells are defective in this process (Ezratty et al., 2005). To test whether an intact Src–FAK–Dyn2 complex is essential for FA turnover, we transfected FAK−/− cells with either FAK WT or FAK mutants including FRNK (cannot bind either Dyn2 or Src), Y397F (Src binding defective), and ΔFERM (Dyn2 binding defective). Following a 48-h posttransfection recovery, cells were subjected to the FA disassembly assay. While 70% of WT FAK–reexpressing cells exhibited FA turnover (Figure 4, A and A′), cells expressing FRNK, ΔFERM, and FAK Y397F exhibited greatly reduced FA disassembly (Figure 4, B–D′), suggesting that the integrity of the Src–FAK–Dyn complex is important for FA turnover.
FIGURE 4:
The integrity of the Src–FAK–Dyn2 complex is essential for the turnover of FAs. (A–D′) IF images of FAK−/− cells that were transfected with WT or mutant GFP-FAK as indicated. At 48 h after transfection, cells were subjected to a standard FA disassembly assay by treatment with 10 μM nocodazole for 3.5 h to disrupt MTs followed by a 1-h drug-free rinse, during which MTs repolymerize and promote FA disassembly. Cells were then fixed and stained for vinculin to label FAs. Reexpression of WT FAK (A, A′) promotes FA disassembly compared with expression of the FAK mutants (B–D′) that are unable to form the Src–FAK–Dyn2 complex. Transfected cells are indicated by asterisks (*). (E) Quantification of the percentage of cells that retained FAs following the FA disassembly assay. Results represent the average of three independent experiments.
Phosphorylation of Dyn2 regulates FA turnover by mediating integrin endocytosis
As a Src–FAK–Dyn2 complex appears to facilitate both Dyn2 phosphorylation (Figure 3) and FA disassembly (Figure 4), it was important to test the link between these two events. To this end we utilized a well-characterized Madin Darby canine kidney (MDCK) cell line stably expressing a Src temperature-sensitive (ts) mutant (Behrens et al., 1993) that allows uniform Src activation upon a shift from a restrictive temperature (40.5°C) to a permissive temperature (35°C). Cells were transfected with either Dyn2 WT or Dyn2 YF and cultured at 40.5°C for 48 h. As shown in Supplemental Figure S3, there was no difference in FA size between control and Dyn2 WT– or YF–expressing cells. However, after being switched to 35°C for 1 h, Dyn2 WT–expressing cells exhibited a significant reduction in FA size upon Src activation compared with adjacent untransfected cells (Figure 5, A and A′) or cells expressing the Dyn2 YF mutant (Figure 5, B and B′), suggesting that Dyn2 phosphorylation plays an important role in FA disassembly.
FIGURE 5:
Src-mediated phosphorylation of Dyn2 promotes FA turnover. (A–B′) IF images of MDCK cells, stably expressing a ts-v-Src that is activated at 35°C or repressed at 40.5°C. These cells were transfected with either WT or YF Dyn2 for 24 h; then they were shifted to the permissive 35°C for 1 h and stained with Dyn and vinculin antibody. Transfected cells are indicated by asterisks (*). Dyn2 WT–expressing cells (A, A′) exhibit smaller FAs compared with nontransfected cells, while cells expressing Dyn2 YF (B, B′) maintained larger FAs and were indistinguishable from the surrounding untransfected cells. (C) Quantification of FA sizes in (A–B′). Data represent the average of 50 or more FAs of each group (*p < 0.05; n.s., not significant). (D–F′) The effects of Dyn2 phosphorylation on FA disassembly. IF images of RF cells that were treated with control siRNA (D, D′) or Dyn2 siRNA followed by reexpression of siRNA-resistant Dyn2 WT (E, E′) or YF (F, F′). Cells were subjected to the FA disassembly assay as previously described and stained for Dyn2 and vinculin. While ∼80% of control siRNA–treated cells disassembled FAs (D, D′), more than 60% of Dyn2 siRNA–treated cells retained FAs (E–F′, unlabeled cells). This was rescued by reexpression of Dyn2 WT (E, E′, *) but not Dyn2 YF (F, F′, *). (G) Quantification of the percentage of cells that retained FAs following the disassembly assay. Results represent more than 200 cells over an average of three independent experiments.
The MT-based FA disassembly assay described previously was utilized as a second approach to test the effect of Dyn2 phosphorylation on FA turnover. We reasoned that Dyn2 WT would be more efficacious in turning over FAs upon MT regrowth than the phosphomutant. For these studies RFs were treated with Dyn2 siRNA for 48 h to deplete endogenous Dyn2, followed by reexpression of siRNA-resistant Dyn2 WT or the Dyn2 YF mutant for 24 h. In control siRNA–treated cells, more than 80% disassembled FAs within 1 h after nocodazole washout (Figure 5, D and D′); in contrast, only 35% of Dyn2 siRNA–treated cells exhibited FA loss (Figure 5, E and F, unlabeled cells). Reexpression of Dyn2 WT in Dyn2 siRNA–treated cells resulted in a marked increase of FA disassembly (>75%, Figure 5, E and E′, *), while Dyn YF–expressing cells exhibited only modest effects (<50%, Figure 5, F and F′, *). Taken together, the experimentation using both the ts-v-Src MDCK model and FA disassembly assay suggests that Dyn2 phosphorylation promotes FA disassembly.
Because the internalization of integrins is known to induce FA turnover (Chao and Kunz, 2009; Ezratty et al., 2009), we speculated that Dyn2 may mediate the endocytosis of integrins to promote FA disassembly. To test this hypothesis, we combined the MT-based FA disassembly assay with a cell surface biotinylation approach to monitor the change in β1-integrin surface expression before and after FA disassembly. RF cells were subjected to the FA disassembly assay; then total surface proteins were labeled with biotin at 4°C before cell lysis and pull-down of biotin-labeled proteins with streptavidin beads. The pellet was analyzed by SDS–PAGE and blotted for β1-integrin. As shown in Figure 6A, a significant amount of β1-integrin resided on the surface in resting cells and was not affected by nocodazole treatment alone. However, 1 h after nocodazole washout and subsequent FA disassembly, the surface β1-integrin level dropped by 90% while the total β1-integrin remained constant (Figure 6B), reflecting an internalization concomitant with FA turnover. This experiment was then performed in the context of altering Dyn2 level and function. RF cells were transfected with control or Dyn2 siRNA for 72 h and then subjected to the FA disassembly and biotinylation assays. As shown in Figure 6C, there was about a threefold increase of surface β1-integrin levels in the Dyn2 knockdown cells. Importantly, reexpression of WT Dyn2 in the siRNA-treated cells resulted in a near complete restoration of β1-integrin clearance from the cell surface compared with control cells, while expression of the Dyn2 YF mutant failed to rescue integrin clearance (Figure 6D). All of these observations are consistent with the premise that integrin internalization is dependent upon a tyrosine-based phosphoregulation of Dyn2 at FAs.
FIGURE 6:
pDyn2 promotes clathrin-based integrin endocytosis. (A) Biotinylation assay measuring surface β1-integrin level before and after FA turnover. RF cells were subjected to a FA disassembly assay as previously described; resting cells (NT) and cells without nocodazole washout served as control. The surface proteins were labeled with biotin and precipitated with streptavidin beads. The surface β1-integrin level was determined by immunoblotting with a β1-integrin antibody. While total β1-integrin remained constant under different treatments, the surface level was markedly reduced upon nocodazole washout (B), suggesting that integrins are internalized during FA disassembly. (C) RF cells were transfected with control or Dyn2 siRNA, and 48 h later Dyn2 WT or Dyn2 YF were reexpressed. Following FA disassembly assay, the surface integrin level was determined by biotinylation. Cells with Dyn2 knockdown show significantly more surface integrin than control cells. Expression of Dyn2 WT, but not Dyn2 YF, restored surface β-integrin levels to those in control cells (D). (E) To test whether this pDyn-dependent internalization of integrins requires clathrin or caveolin, cells were treated with siRNA targeting CHC or Cav1 and subjected to FA disassembly assay. CHC KD markedly inhibited integrin internalization, while Cav1 KD had no effect (F) *p < 0.05; n.s., not significant. Results represent the average of three independent experiments. (G) Working model: FAK recruits Dyn and Src to FAs and forms a Src–FAK–Dyn2 complex, which facilitates Dyn2 phosphorylation, activates Dyn2 GTPase activity, and, as a result, promotes integrin endocytosis and FA disassembly.
As Dyn2 has been shown to mediate both clathrin- and caveolin-dependent endocytosis (Takei et al., 1999; Shajahan et al., 2004; Mayor and Pagano, 2007; Cao et al., 2010), we tested for the pathway that mediates integrin internalization. RFs were treated for 72 h with siRNA to reduce levels of either clathrin heavy chain (CHC) or caveolin-1 (Cav1) followed by the FA disassembly assay and cell surface biotinylation. Interestingly, CHC knockdown significantly inhibited β1-integrin internalization, while the Cav1 knockdown had no effect (Figure 6, E and F), suggesting that pDyn2 regulates endocytic turnover of FAs in a clathrin-dependent manner.
DISCUSSION
In this study, we have tested the role of three well-established FA components—Src, FAK, and Dyn2—in the regulated turnover of FAs and have made several novel findings. First, we found that Src, FAK, and Dyn2 exist as a complex at FAs, in which FAK functions as an adaptor to recruit both Src and Dyn2 (Figure 6G). Second, the formation of a Src–FAK–Dyn2 complex facilitates Dyn2’s tyrosine phosphorylation and, as a result, enhances Dyn2’s GTPase activity and promotes the endocytic turnover of FAs. This study establishes Dyn2 as a novel effector downstream of the well-established Src–FAK signaling pathway in mediating FA disassembly.
An essential component of this study was a pDyn antibody made to a conserved phosphotyrosine residue (Tyr-231) present in all three of the conventional dynamin forms. Past studies by others (Ahn et al., 2005, 2002) have convincingly shown that phosphorylation of Tyr-231/597 of Dyn1 by Src kinase markedly enhances the polymerization, GTPase activity, and endocytic potential of this protein. Subsequently we demonstrated that both of these tyrosines are phosphorylated on the more ubiquitously expressed Dyn2 and act to regulate transferrin endocytosis in epithelial cells (Cao et al., 2010). Interestingly, with this pDyn antibody, we observed a basal level of FA staining in a variety of epithelial cells and fibroblasts. This observation confirmed the previous study’s finding that Dyn is localized at FAs, and, importantly, it showed that Dyn is phosphorylated at this spot. The phosphorylation is apparently mediated by Src because expression of constitutively active Src kinase or EGF stimulation that is known to activate Src increases the pDyn’s FA staining; reversely, Src family kinase inhibitor PP2 abolishes the staining. This observation suggests that phosphorylation of this mechanoenzyme may play an important role in regulating FA dynamics and cell migration. To ensure that this localization represented active pDyn2, we conducted a series of biochemical and morphological control experiments (Supplemental Figure S1). All observations were consistent with the premise that the pDyn antibody used here is specific. It is important to note that the peptide used as an antigen for this antibody represents a sequence found in all three conventional forms of dynamin (Dyn1, 2, and 3). As the predominant form of dynamin in most epithelial cells and fibroblasts is Dyn2, we have assumed that this form predominates at FAs and have referred to pDyn as pDyn2 throughout this study.
Previous studies have shown that FAK binds to and recruits Dyn2 to FAs (Ezratty et al., 2005). However, the specific domains involved in this process had not been defined, and it was not clear whether the binding is direct. The most appealing model has been that Grb2 functions as an adaptor between FAK and Dyn2 because Grb2 is known to bind both proteins. However, we have not observed any requirement for Grb2 in mediating the Dyn2-FAK association (unpublished data). Instead, we found that Dyn2 directly binds to the FERM domain of FAK, and IF data confirmed the importance of the FERM domain in recruiting Dyn2 to FAs. The FERM domain has been reported to associate with a wide range of proteins, including EGF receptors, Arp2/3 complex, p53, and MDM2 (Lim et al., 2008), and no conserved FERM-interacting motif has been characterized. While the FERM binding region of Dyn2 is still under investigation, we found that the association between the Dyn2 phosphomutant (Y231F, Y597F) and FAK is reduced compared with WT Dyn2, as assessed by both coIP (Figure 1G) and GST pull-down (Supplemental Figure S2), suggesting that phosphorylation of Dyn2 may enhance its binding to FAK, probably due to a conformational change of Dyn2. This may in part explain why SrcY530F expression or EGF stimulation enhances pDyn2’s FA staining.
Further study has revealed that FAK-Dyn2 is actually part of a Src–FAK–Dyn2 trimeric complex, in which FAK functions as a scaffold between Src and Dyn2. This premise is supported by the fact that FAK and Src are brought down in a Dyn2 IP from MEF cells, and a similar IP from FAK−/− cells showed substantially less prevalent Src-Dyn2 association, which could be restored upon FAK reexpression (Figure 3A), suggesting that Src, FAK, and Dyn2 exist as a complex at FAs. This result is further confirmed by “continuous IP,” using exogenously expressed SFB-FAK, Src, and Dyn2-GFP (unpublished data). It is well documented that Src–FAK form a complex with paxillin and p130Cas and phosphorylate these proteins, so we hypothesize that the formation of this trimeric complex also facilitates Dyn2 phosphorylation. Consistent with this hypothesis is the fact that Dyn2 phosphorylation was ∼twofold higher in MEF cells than in the FAK−/− cells, in which the complex formation is compromised. Our caution toward this result stems from the fact that overall Src activity is reduced in the absence of FAK. However, the reduced pDyn level is not simply due to compromised Src activity because FAK−/− cells reexpressing WT FAK or the Dyn2 binding mutant (ΔFERM) showed no difference in active Src levels, while there was a significant decrease of pDyn2 levels in mutant-expressing cells (Figure 3, D and E), suggesting that FAK plays an essential scaffolding function at the FA to bring both Src and Dyn2 in close proximity to mediate Dyn2 activation.
It is well established that FAK–Src signaling promotes FA turnover by phosphorylating downstream effectors. Dyn2 appears to be such an effector, as others have demonstrated that Dyn2 is involved in FA disassembly, and phosphorylation of dynamin at Tyr-231/597 leads to an amplification in its GTPase activity. By plating WT or FAK−/− MEF cells on fibronectin, a process that activates the integrin-based FAK–Src signaling, we demonstrate that Dyn2 is indeed downstream of this signaling pathway (Figure 3F). To disrupt the Src–FAK–Dyn2 signaling cascade, we utilized a variety of FAK mutants that disrupt the complex formation or Dyn2 tyrosine to phenylalanine mutations on Tyr-231/597 that cannot be phosphorylated by Src–FAK. Both approaches show significant inhibition of FA turnover during the nocodazole-induced FA disassembly assay. Our data strongly suggest the Src–FAK–Dyn cascade as a novel mechanism in regulating FA turnover, which appears to be FA specific as turnover of other receptor–ligand complexes including transferrin and EGF are not affected in FAK−/− cells compared with WT MEF cells (unpublished data).
Finally, we addressed how the phosphorylation of Dyn2 regulates FA disassembly. It is well established that pDyn2 regulates the internalization of the TfR1 and some receptor tyrosine kinases, so we hypothesize that it also regulates integrin endocytosis. Indeed, Dyn2 YF–expressing cells showed a significant block of β1-integrin internalization compared with WT Dyn2–expressing cells during FA disassembly. Endocytic uptake of integrins has been shown to be mediated by either clathrin- or caveolin-based mechanisms (del Pozo et al., 2005; Ezratty et al., 2009). Our findings here using a pancreatic cancer cell model support clathrin-based integrin internalization as the major pathway. A better structural and temporal understanding of how the Dyn2 polymer assembles in conjunction with FAK and the clathrin–adaptor complex in response to receptor activation will prove enlightening.
MATERIALS AND METHODS
Antibodies and reagents
In the generation of pDyn antibody, peptides against the deduced amino acid sequence of rat Dyn2 were synthesized and conjugated to keyhole limpet hemocyanin by the Mayo Protein Core Facility at the Mayo Clinic, suspended in phosphate-buffered saline (PBS) and Freund’s adjuvant, and injected into New Zealand white rabbits, and the antisera were collected after subsequent boost injections. The peptide sequences and corresponding antibody name are PLRRGpYIGVVNRSQKDIEGC (amino acids 226–245; anti-pDyn [Tyr-231]). The crude antisera were affinity purified using an agarose column conjugated with the appropriate high-performance liquid chromatography–purified synthetic peptide two times (first, using PLRRGYIGVVNRSQKDIEGC; second, using PLRRGpYIGVVNRSQKDIEGC) and a low-pH elution buffer, according to the manufacturer’s directions (Pierce Chemical, Rockford, IL). The Dyn2 and MC63 (pan-dynamin) antibodies were generated as previously described (Henley and McNiven, 1996; Henley et al., 1998). The monoclonal dynamin antibody (Hudy-1) was purchased from Upstate Biotechnology (Lake Placid, NY). The monoclonal and polyclonal antibodies against FAK were from BD Biosciences (Franklin Lakes, NJ) and Millipore (Temecula, CA), respectively; anti-Src antibody (sc-18), anti–β1-integrin (sc-8978), and anti-GST (sc-138) were purchased from Santa Cruz Biotechnology (San Diego, CA). The phospho-Src family antibody pTyr-416 was from Cell Signaling Technology (Danvers, MA), the monoclonal vinculin antibody was from Sigma (St. Louis, MO), anti-Flag antibody was from Cell Signaling Technology, and anti-GFP antibody was from Roche (Indianapolis, IN). Goat anti–rabbit or goat anti–mouse secondary antibodies conjugated to either Alexa-488 or -594 were from Invitrogen (Carlsbad, CA). All the other chemicals and reagents unless otherwise stated were from Sigma.
Cell culture
RF, a rat skin fibroblast line (CRL-1213), was purchased from the American Type Culture Collection (ATCC) (Rockville, MD). PANC-1 is an epithelioid carcinoma from pancreas ducts (ATCC CRL-1469). MEFs were a gift from Andras Kapus (St. Michael’s Hospital, Toronto, ON, Canada). FAK−/− cells were a gift from Kun Ling (Mayo Clinic, Rochester, MN). SYF is an embryonic fibroblast cell line isolated from Src, Yes, and Fyn knockout mice (ATCC CRL-2459). The v-Src-ts MDCK cell line was a gift from Yasuyuki Fujita but was produced by Robert Friis’s lab (Bern, Switzerland). Dynamin knockout cells were a gift from Pietro De Camilli (Yale University) (Ferguson et al., 2009). All cells were maintained in DMEM 10% fetal bovine serum (GIBCO BRL, Gaithersburg, MD), 100 U/ml penicillin, and 100 μg/ml streptomycin in 5% CO2 and 95% air at 37°C. v-Src-ts MDCK cells are normally cultured at 40.5°C (restrictive temperature) and switched to 35°C (permissive temperature) to activate v-Src. Cells were cultured in T-75 flasks (Fisher Scientific, Pittsburgh, PA).
Plasmid, siRNA, and transfection
GFP-tagged Dyn2 WT or phosphomutant (Y231F, Y597F) and c-SrcY530F-pCR3.1 were generated as previously described (Cao et al., 1998; Cao et al., 2010). FAK was amplified from a rat brain cDNA library using 5′ primer (5′ ACGCGTCGACATATGGAGAAAAGCGGCTGTAG) and 3′ primer (5′CGGGATCCTCAGTGTGGCCGTGTCTGCC) and inserted into pEGFP-C1 or SFB vector (pIRES2-EGFP with S peptide, Flag tag, and streptavidin binding peptide, a gift from Zhenkun Lou Lab, Mayo Clinic). The FAK Y397F mutant was generated by the PCR-based mutagenesis method. Constructs were transfected with Lipofectamine 2000 (Invitrogen), according to the manufacturer’s instructions. siRNAs targeting Dyn2 (GACATGATCCTGCAGTTTA), Cav1 (AACCAGAAGGGACACACAGUUUU), and CHC (GCAAUGAGCUGUUUGAAGAUU) were purchased from Dharmacon (Lafayette, CO) and transfected with Lipofectamine RNAiMAX (Invitrogen) following the standard protocol.
IP, Far Western blotting, and Western blotting
Cells were lysed with NETN buffer (0.5% NP40, 150 mM NaCl, 50 mM Tris, and 1 mM EDTA) at 4°C. Cell debris was removed by centrifugation, and the supernatant was incubated with 5 μg of the appropriate antibody and protein A beads at 4°C for 4 h. For the S protein IP, cell lysate was incubated with S protein agarose (Novagen, Madison, WI) at 4°C for 2 h. The pellet was washed with NETN buffer three times, eluted in Laemmli sample buffer, and analyzed by Western blot with appropriate antibodies. Far Western blotting was performed as described previously (Horgan et al., 2010). GST alone, GST-FRNK, GST-FERM, or His-Dyn2 proteins were purified from E. coli BL21. GST-tagged proteins were analyzed by SDS–PAGE and transferred to polyvinylidene difluoride membrane, which was blocked by basic buffer (BB: 20 mM HEPES [pH 7.5], 50 mM KCl, 10 mM MgCl2, 1 mM dithiothreitol, and 0.1% Igepal CA-630) plus 5% nonfat dry milk overnight at 4°C. The membrane was incubated in interaction buffer (immunoblot: BB plus 1% nonfat milk) containing 30 μg His-Dyn2 for 2.5 h at 4°C, washed with TBST (Tris-buffered saline and 0.1% Tween-20) three times, and subjected to Western blotting with Dyn2 antibody. pDyn2 protein was purified from TKB1 E. coli (Stratagene, La Jolla, CA) as previously described (Gomez et al., 2006). Western blotting was performed as described (Cao et al., 1998) with appropriated antibodies.
FA disassembly assay
MEFs, FAK−/−, RF, or PANC-1 cells were treated with 10 μM nocodazole for 3.5 h to depolymerize MTs. After washing with serum-free medium, cells were cultured at 37°C for 1 h to allow MT reformation and then either fixed for IF staining or subjected to the biotinylation assay.
Biotinylation assay
The biotinylation assay was performed as described (Cao et al., 2010). Briefly, cells were washed three times with cold PBS and incubated with 0.5 mg/ml N-hydroxysuccinimide–biotin in cold PBS for 30 min on ice. Unbound biotin was removed by rinsing with 50 μM NH4CL in cold PBS three times. Then cells were lysed with NETN buffer and incubated with streptavidin beads, and surface β1-integrin level was determined by Western blotting with a β1-integrin antibody.
Fluorescence microscopy
Cells were grown on 22-mm coverslips for transfections and immunocytochemistry. Cells were fixed in formaldehyde and processed as described (Cao et al., 1998). Images were taken with an Axiovert 35 microscope (Carl Zeiss, Thornwood, NY) and Hamamatsu Orca II camera (Hamamatsu Photonics, Bridgewater, NJ) and analyzed with IPLab software (Scanalytics, Rockville, MD) (Weller et al. 2010).
Supplementary Material
Acknowledgments
This study was supported by grant RO1-DK44650 to M.A.M. and the Optical Morphology Core of the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK84567). Thanks go to Gina Razidlo, Shaun Weller, and Robbin Eppinga for carefully reading the manuscript.
Abbreviations used:
- CHC
clathrin heavy chain
- coIP
coimmunoprecipitation
- ECM
extracellular matrix
- EGF
epidermal growth factor
- FA
focal adhesion
- FAK
focal adhesion kinase
- FERM
4.1/ezrin/radizin/moesin
- GFP
green fluorescent protein
- GST
glutathione S-transferase
- GTPase
guanosine 5′-triphosphatase
- IF
immunofluorescence
- MDCK
Madin Darby canine kidney
- MEF
mouse embryonic fibroblast
- MT
microtubule
- PBS
phosphate-buffered saline
- pDyn
phospho-dynamin
- RF
rat fibroblast
- siRNA
small interfering RNA
- ts
temperature sensitive
- WT
wild-type
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-09-0785) on March 16, 2011.
REFERENCES
- Ahn S, Kim J, Lucaveche CL, Reedy MC, Luttrell LM, Lefkowitz RJ, Daaka Y. Src-dependent tyrosine phosphorylation regulates dynamin self-assembly and ligand-induced endocytosis of the epidermal growth factor receptor. J Biol Chem. 2002;277:26642–26651. doi: 10.1074/jbc.M201499200. [DOI] [PubMed] [Google Scholar]
- Ahn S, Maudsley S, Luttrell LM, Lefkowitz RJ, Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem. 1999;274:1185–1188. doi: 10.1074/jbc.274.3.1185. [DOI] [PubMed] [Google Scholar]
- Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt A, Kaverina I, Otey C, Huttenlocher A. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. J Cell Sci. 2002;115:3415–3425. doi: 10.1242/jcs.115.17.3415. [DOI] [PubMed] [Google Scholar]
- Brown MC, Cary LA, Jamieson JS, Cooper JA, Turner CE. Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness. Mol Biol Cell. 2005;16:4316–4328. doi: 10.1091/mbc.E05-02-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Chen J, Krueger EW, McNiven MA. SRC-mediated phosphorylation of dynamin and cortactin regulates the “constitutive” endocytosis of transferrin. Mol Cell Biol. 2010;30:781–792. doi: 10.1128/MCB.00330-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Garcia F, McNiven MA. Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell. 1998;9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao WT, Kunz J. Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. FEBS Lett. 2009;583:1337–1343. doi: 10.1016/j.febslet.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-Garcia A, Anderson RG, Schwartz MA. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733–747. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7:581–590. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell. 2009;17:811–822. doi: 10.1016/j.devcel.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame MC, Fincham VJ, Carragher NO, Wyke JA. v-Src’s hold over actin and cell adhesions. Nat Rev Mol Cell Biol. 2002;3:233–245. doi: 10.1038/nrm779. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr Opin Cell Biol. 2001;13:584–592. doi: 10.1016/s0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- Gomez TS, et al. HS1 functions as an essential actin-regulatory adaptor protein at the immune synapse. Immunity. 2006;24:741–752. doi: 10.1016/j.immuni.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JR, McNiven MA. Association of a dynamin-like protein with the Golgi apparatus in mammalian cells. J Cell Biol. 1996;133:761–775. doi: 10.1083/jcb.133.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan CP, Hanscom SR, Jolly RS, Futter CE, McCaffrey MW. Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J Cell Sci. 2010;123:181–191. doi: 10.1242/jcs.052670. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Kharbanda S, Saleem A, Yuan Z, Emoto Y, Prasad KV, Kufe D. Stimulation of human monocytes with macrophage colony-stimulating factor induces a Grb2-mediated association of the focal adhesion kinase pp125FAK and dynamin; Proc Natl Acad Sci USA; 1995. pp. 6132–6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer—a new therapeutic opportunity. Nat Rev. 2005;5:505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- McNiven MA. Dynamin: a molecular motor with pinchase action. Cell. 1998;94:151–154. doi: 10.1016/s0092-8674(00)81414-2. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Palecek SP, Huttenlocher A, Horwitz AF, Lauffenburger DA. Physical and biochemical regulation of integrin release during rear detachment of migrating cells. J Cell Sci. 1998;111:929–940. doi: 10.1242/jcs.111.7.929. [DOI] [PubMed] [Google Scholar]
- Polte TR, Hanks SK. Complexes of focal adhesion kinase (FAK) and Crk-associated substrate (p130Cas) are elevated in cytoskeleton-associated fractions following adhesion and Src transformation: requirements for Src kinase activity and FAK proline-rich motifs. J Biol Chem. 1997;272:5501–5509. doi: 10.1074/jbc.272.9.5501. [DOI] [PubMed] [Google Scholar]
- Ruest PJ, Shin NY, Polte TR, Zhang X, Hanks SK. Mechanisms of CAS substrate domain tyrosine phosphorylation by FAK and Src. Mol Cell Biol. 2001;21:7641–7652. doi: 10.1128/MCB.21.22.7641-7652.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry SK, Burridge K. Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp Cell Res. 2000;261:25–36. doi: 10.1006/excr.2000.5043. [DOI] [PubMed] [Google Scholar]
- Shajahan AN, Timblin BK, Sandoval R, Tiruppathi C, Malik AB, Minshall RD. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004;279:20392–20400. doi: 10.1074/jbc.M308710200. [DOI] [PubMed] [Google Scholar]
- Shattil SJ. Integrins and Src: dynamic duo of adhesion signaling. Trends Cell Biol. 2005;15:399–403. doi: 10.1016/j.tcb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Takei K, Slepnev VI, Haucke V, De Camilli P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat Cell Biol. 1999;1:33–39. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- Urrutia R, Henley JR, Cook T, McNiven MA. The dynamins: redundant or distinct functions for an expanding family of related GTPases?; Proc Natl Acad Sci USA; 1997. pp. 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells—over and over and over again. Nat Cell Biol. 2002;4:E97–100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B, Imhof B. The inner lives of focal adhesions. Trends Cell Biol. 2002;12:382–389. doi: 10.1016/s0962-8924(02)02321-8. [DOI] [PubMed] [Google Scholar]
- Weller SG, Capitani M, Cao H, Micaroni M, Luini A, Sallese M, McNiven MA. Src kinase regulates the integrity and function of the Golgi apparatus via activation of dynamin 2; Proc Natl Acad Sci USA; 2010. pp. 5863–5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gan B, Yoo Y, Guan JL. FAK-mediated Src phosphorylation of endophilin A2 inhibits endocytosis of MT1-MMP and promotes ECM degradation. Dev Cell. 2005;9:185–196. doi: 10.1016/j.devcel.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Yeatman TJ. A renaissance for SRC. Nat Rev. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- Yeo MG, Partridge MA, Ezratty EJ, Shen Q, Gundersen GG, Marcantonio EE. Src SH2 arginine 175 is required for cell motility: specific focal adhesion kinase targeting and focal adhesion assembly function. Mol Cell Biol. 2006;26:4399–4409. doi: 10.1128/MCB.01147-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.