Abstract
Patients with type 2 diabetes mellitus have an increased risk of fracture that can be further exacerbated by thiazolidinediones. A new class of antidiabetic agents control glucose through reduction of dipeptidyl peptidase-4 (DPP-4) activity; however the importance of DPP-4 for the control of bone quality has not been extensively characterized. We compared the effects of the thiazolidinedione pioglitazone and the DPP-4 inhibitor sitagliptin on bone quality in high-fat diet (HFD)-fed wild-type mice. In complementary studies, we examined bone quality in Dpp4+/+ vs. Dpp4−/− mice. Pioglitazone produced yellow bones with greater bone marrow adiposity and significantly reduced vertebral bone mechanics in male, female, and ovariectomized (OVX) HFD fed female mice. Pioglitazone negatively affected vertebral volumetric bone mineral density, trabecular architecture, and mineral apposition rate in male mice. Sitagliptin treatment of HFD-fed wild-type mice significantly improved vertebral volumetric bone mineral density and trabecular architecture in female mice, but these improvements were lost in females after OVX. Genetic inactivation of Dpp4 did not produce a major bone phenotype in male and female Dpp4−/− mice; however, OVX Dpp4−/− mice exhibited significantly reduced femoral size and mechanics. These findings delineate the skeletal consequences of pharmacological and genetic reduction of DPP-4 activity and reveal significant differences in the effects of pioglitazone vs. sitagliptin vs. genetic Dpp4 inactivation on bone mechanics in mice.
The skeleton is a highly specialized and dynamic organ that maintains bone mass by undergoing continuous remodeling over time. Osteoblastic bone formation and osteoclastic bone resorption are closely coordinated processes that enable bone to adapt to and repair the mechanical loads and age-related hormonal changes that it will endure throughout adult life. A sustained imbalance of bone formation and resorption can reduce bone quality and result in an increased susceptibility to fracture. Osteoporosis results when osteoclastic bone resorption outpaces osteoblastic bone formation leading to low bone mass, microarchitectural deterioration of the skeleton, increased bone fragility, and fracture risk (1). In addition to senile or postmenopausal osteoporosis that occur with increasing age or sex steroid deficiency, patients with type 2 diabetes mellitus (T2DM) have an increased risk of fracture, despite average or elevated bone mineral density (BMD) (2–5).
Recent information demonstrates that antidiabetic agents may influence bone density and fracture rates. Long-term treatment with thiazolidinediones (TZDs) is associated with an increased risk of fracture in T2DM patients compared with treatment with other antidiabetic agents (6–9). A widely used TZD, rosiglitazone, promotes differentiation of mesenchymal stromal cells into adipocytes rather than osteoblasts and inhibits bone formation through suppression of osteogenic transcription factors (10–14). Additionally, peroxisome proliferator-activated receptor (PPAR)-γ has been shown to have a proosteoclastogenic role and activation of PPAR-γ by rosiglitazone enhanced osteoclast differentiation in a receptor-dependent manner (15). However, not all PPAR-γ ligands exhibit the same skeletal effects, possibly due to differences in receptor binding affinity or affinity for other PPAR subtypes (16). Although pioglitazone is a widely used PPAR-γ agonist, the skeletal effects of pioglitazone treatment are much less understood. Nevertheless, the available data suggest that pioglitazone treatment leads to reduced BMD, bone formation, and mechanical strength in rodents (17).
Dipeptidyl peptidase-4 (DPP-4) inhibitors are a comparatively newer class of antidiabetic agents that improve glycemic control via enhancement of incretin hormone action (18). Several DPP-4 substrates, including glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide (GLP)-1, and GLP-2 exert anabolic actions on the skeleton in rodents (19). However, the potential contribution of multiple DPP-4 substrates to putative changes in bone quality after DPP-4 inhibition is complex and has not been previously examined. We have now investigated and compared the effects of pioglitazone vs. sitagliptin on bone quality in high-fat-diet (HFD) fed male and female mice and in ovariectomized (OVX) HFD fed mice. Additionally, we evaluated bone quality in male and female and OVX Dpp4−/− mice.
Materials and Methods
Animals
Male and female C57BL/6 mice obtained from Taconic laboratories (Hudson, NY) were housed in the animal facility at the Toronto Centre for Phenogenomics (Toronto, Ontario, Canada). Animals were housed four to five in a cage with free access to water and food and were maintained at a constant temperature on a 12-h light, 12-h dark cycle. All mice were maintained on a standard rodent diet until 3 months of age when all mice were fed a HFD ad libitum (40% of kilocalories from fat; Research Diets, New Brunswick, NJ) until the animals were killed at 7 months of age. At 4 months of age, high-fat chow was supplemented with pioglitazone (Takeda Pharmaceuticals, Japan) or sitagliptin (Merck Frosst, Québec, Canada) at concentrations of 0.28 and 4 g/kg chow, respectively. The control groups were maintained on nonsupplemented high-fat chow. Selected female mice were OVX at 3 months of age and were fed chow supplemented with or without pioglitazone or sitagliptin. Lean and fat mass and water content were assessed by mouse magnetic resonance imaging as previously described (20). Similarly, oral glucose tolerance testing (1 mg glucose per gram body weight) and insulin tolerance testing (1.2 U insulin per kilogram body weight) were carried out as described (20).
Dpp4−/− and Dpp4+/+ littermate controls were generated on a C57BL/6 background as described (21). Female Dpp4−/− and Dpp4+/+ mice were OVX at 3 months of age, and all mice were killed for analysis at 7 months of age. All experiments conformed to specific protocols approved by the University Health Network and Mount Sinai Hospital (Toronto, Ontario).
Preparation of bones
The right and left femora and L5 and L6 vertebrae were excised, cleaned of adherent soft tissue, and stored at −20 C in saline-soaked gauze. The processes were carefully removed from all vertebrae. Bones were thawed at room temperature before any experimental testing. The L3 and L4 vertebrae were excised and immediately fixed in 10% neutral-buffered formalin or 70% ethanol, respectively, for histomorphometry. The L3 and L4 vertebrae were allowed to fix at room temperature for 5 d before further processing for embedding purposes.
Bone mineral assessment and imaging tools
The right and left femora and L5 and L6 vertebrae were evaluated for areal BMD (aBMD; grams per square centimeter) and bone mineral content (BMC; grams) using a dual-energy x-ray absorptiometry (DEXA) PIXImus mouse densitometer (GE Medical Systems, Madison, WI) and software version 2.0. The instrument was calibrated daily using the manufacturer’s phantom mouse. Bones were precisely positioned at the center of the x-ray cone beam and scanned in air on the Plexiglas platform provided. A grid on the Plexiglas platform allowed the position of each femora and vertebrae to remain consistent between samples.
Volumetric BMD (vBMD; grams per cubic centimeter) of the right femora and L5 vertebra was evaluated using a SkyScan 1174 compact microcomputed tomography machine (Micro-CT; Skyscan, Kontich, Belgium). The latter instrument was also used to evaluate the femoral geometrical properties of the right femora and trabecular architecture of the L5 vertebrae. The Skyscan CT-An software (version 1.5.0) was used for all microcomputed tomography (micro-CT) measurements and calculations (22).
Mechanical testing
Mechanical testing was performed using a 100 N load cell on an Instron 4465 testing machine (Instron Corp., Canton, MA). Load-deformation curves were generated for all mechanical tests using Labview Acquisition software (LabVIEW 5.0; National Instruments, Austin, TX). The vertebrae were tested to evaluate the lattice network of trabecular bone, whereas femora were tested to evaluate cortical bone. For three-point bending testing, each femur was positioned, posterior face down, on two supports separated by a constant 6-mm gauge length. A load was vertically applied to the midshaft of the femur at a rate of 1.0 mm/min until failure occurred. The proximal end of femora tested by three-point bending were immediately embedded in a jig with polymethyl-methacrylate and used for femoral neck fracture testing. The femoral head-neck structure was tested to failure by loading the head parallel to the shaft at a rate of 1 mm/min. For the vertebral compression testing, vertebral processes were carefully removed and L6 vertebrae were loaded at a rate of 0.5 mm/min. The distal end of the vertebral body was secured in an upright position with cyanoacrylate-based adhesive and unilaterally compressed until a failure point or 10% drop in measured load was reached. Micro-CT and Image J analysis (National Institutes of Health, Bethesda, MD) were used to determine the geometrical properties necessary for normalization of three-point bending and vertebral compression testing, respectively (23).
Bone histomorphometry
The Leitz Bioquant morphometry system was used to quantify dynamic histomorphometry and osteoclast-staining parameters. Dynamic histomorphometry was performed to determine bone formation parameters. Two single ip injections of calcein green (0.6% caclein green; 30 mg/kg rodent) were given at 12 and 2 d before the animals were killed. L4 vertebrae were excised and immediately fixed in 70% ethanol and embedded in Spurr resin. Spurr blocks were cut in 7-μm-thick coronal sections using a Leica RM2265 rotary microtome (Leica, Wetzlar, Germany).
Tartrate-resistant acid phosphatase (TRAP)-staining for osteoclasts was performed on L3 vertebrae to quantify osteoclast numbers and surface parameters. Excised L3 vertebrae were immediately fixed in 10% buffered formalin. Samples were then decalcified using EDTA at 4 C with daily solution changes. Complete decalcification was determined by Faxitron imaging (Munich, Germany). Decalcified samples underwent processing (series of formalin, 70% ethanol, 90% ethanol, 100% ethanol, 100% xylene, and paraffin) before embedding in bone-specific paraffin. Samples were cut in 5-μm-thick coronal sections using a Leica Reichert Jung 2030 microtome (Heidelberg, Germany) and were mounted on Superfrost glass slides (Menzel-Glaser, Braunschweig, Germany). The acid phosphatase leukocyte kit and protocol (procedure no. 386; Sigma-Aldrich, St. Louis, MO) were used to prepare and perform TRAP staining.
Backscattered electron microscopy (BSE)
BSE was used for mineralization profile and strut analysis using a scanning electron microscope (Philips XL 300 SEM; Philips, Best, The Netherlands). Spurr blocks sectioned for histomorphometry were polished to a 1-μm finish. Samples were examined at a 20-kV accelerating voltage, 15-mm working distance, and a beam spot size of 6. A BSE detector (Centaurus detector) was used to image the entire polished bone surface at ×120 magnification. The backscattered signal was calibrated by observing the histogram of a silicon dioxide (SiO2) and magnesium fluoride (MgF) standard. Gray levels represent varying degrees of bone mineralization, in which brighter intensities are indicative of increased degrees of mineralization. The mineralization profile of a specimen is represented by a histogram of gray-level intensities, and the logit function describes this mineralization distribution. Mineralization profiles were created for total (trabecular + cortical), trabecular, and cortical bone area as described (23).
Strut analysis was performed to evaluate trabecular connectivity. BSE images were digitized into binary images and skeletonized to obtain connectivity parameters. The free ends of trabeculae were described as end points; the points at which three struts met constituted nodes, and the points at which trabeculae met the cortex were designated cortical points. Parameters of connectivity included total strut length, number of nodes, length of node-node struts and node-free struts, and parameters describing disconnectivity included number of free ends and length of free-free struts (22).
Statistics
All results were analyzed using SPSS 17.0 statistical analysis software (SPSS Inc., Chicago, IL). Independent Student t tests were performed separately for sitagliptin-treated males (sitagliptin vs. vehicle) and pioglitazone-treated males (pioglitazone vs. vehicle). A one-way ANOVA was used separately for female (vehicle, sitagliptin, and pioglitazone) and OVX female (vehicle, sitagliptin, and pioglitazone) mice. Pairwise comparisons between groups were carried out using a Fisher least significant difference post hoc test if the group populations passed the homogeneity of variance testing. The nonparametric Dunnet’s T3 post hoc test was performed if the variances were not homogeneous. The data were considered to be statistically significant at a confidence level of 95% (P = 0.05). Data are presented as mean ± SE.
Results
Metabolic effects of treatment with pioglitazone vs. sitagliptin
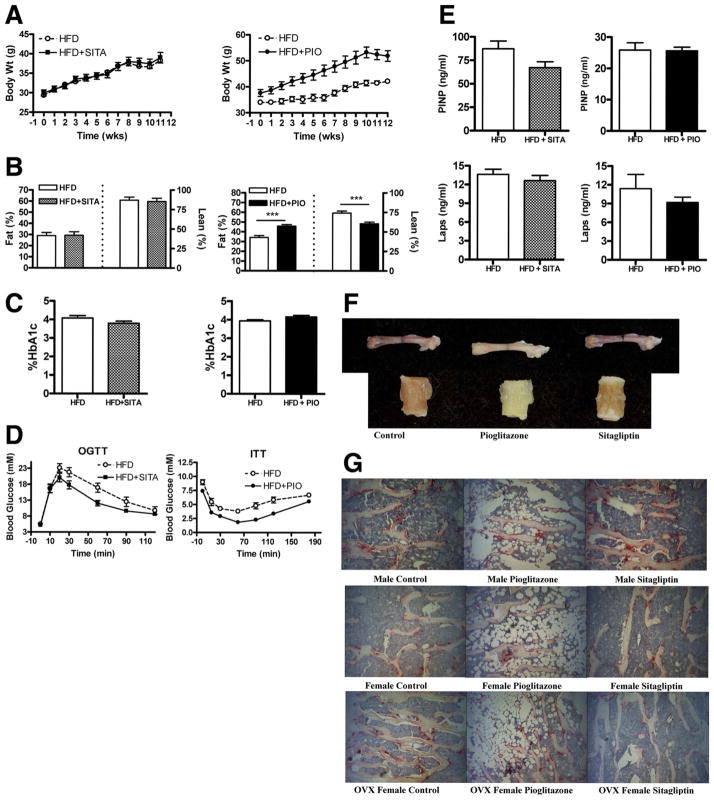
To mimic the metabolic scenario under which antidiabetic agents such as pioglitazone and sitagliptin are commonly used, we fed wild-type mice a HFD known to induce obesity, insulin resistance, and β-cell dysfunction (24). Mice maintained on a HFD supplemented with pioglitazone (HFD+PIO) gained significantly more weight (Fig. 1A) and exhibited increased body fat and relatively reduced lean body mass (Fig. 1B) compared with HFD-fed controls. In contrast, body weight gain, lean body mass, and fat content were not different between control mice and mice fed a HFD containing sitagliptin (HFD-SITA) (Fig. 1, A and B). Random blood glucose (data not shown) and levels of hemoglobin A1c were comparable in pioglitazone- vs. sitagliptin-treated mice (Fig. 1C). Consistent with the known mechanism of action of sitagliptin, which enhances enteral glucose tolerance through potentiation of incretin action (25), oral glucose tolerance was improved in HFD+SITA mice vs. controls (Fig. 1D). Likewise, blood glucose levels were lower in an insulin tolerance test in HFD+PIO mice compared with HFD-fed controls, in keeping with the insulin-sensitizing effect of pioglitazone (Fig. 1D). No differences in markers of bone formation or resorption were observed in either HFD+PIO or HFD+SITA mice compared with controls (Fig. 1E).
FIG. 1.
Differential effects of sitagliptin vs. pioglitazone on metabolic parameters and bone in HFD male mice. Body weight change over time (A), body fat and lean body mass assessed by magnetic resonance imaging (B), hemoglobin A1c (HbA1c) (C), oral glucose (OGTT) or insulin tolerance testing (ITT) (D), and markers of bone formation (PINP) or resorption (Laps) (E) in wild-type male mice maintained on either a HFD or a HFD supplemented with sitagliptin (HFD+SITA) or pioglitazone (HFD+PIO) for 12 wk. Visible color change of bone (F) and increased bone marrow adiposity (TRAP staining) (G) due to pioglitazone treatment. Magnification, × 10 (n = 8–16 mice/group). For A, the AUC body weight was P < 0.05 for HFD+PIO vs. HFD. ***, P < 0.001 for HFD+PIO vs. HFD (B). For D, the AUC blood glucose was P < 0.05 and P < 0.001 for HFD+SITA or HFD+PIO, respectively, vs. HFD. PINP, N terminal propeptide of type I procollagen; Laps, C-telopeptide degradation products of type I collagen.
Effect of pioglitazone on bone quality
Bones excised from pioglitazone-treated mice appeared more yellow in color than bones from untreated or sitagliptin-treated mice (Fig. 1F). Histological analysis revealed greater bone marrow adiposity in pioglitazone-treated relative to control or sitagliptin-treated mice (Fig. 1G). DEXA revealed significant decreases in femoral aBMD of pioglitazone-treated mice, but these changes were not seen in vBMD as measured by micro-CT (Table 1). Femoral geometry, as measured by micro-CT, and femoral mechanical testing by three-point bending and femoral neck fracture testing did not reveal significant differences due to pioglitazone treatment (Supplemental Tables 1–3, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org.). Vertebral aBMD was significantly reduced in pioglitazone-treated OVX mice but was not significantly lower when measured volumetrically by micro-CT. However, pioglitazone-treated male mice exhibited significantly reduced vertebral vBMD (Table 1) and significantly reduced trabecular architecture (Table 2). Pioglitazone treatment produced significant reductions in trabecular bone volume (BV/TV), thickness (Tb.Th.), and number (Tb.N.) and connectivity (Table 2) with reduced total strut length, number of nodes, and length of node-node struts. Vertebral compression revealed mechanical reductions for pioglitazone-treated mice, with significant reductions in stiffness, ultimate stress, and Young’s modulus for male mice; energy to failure and toughness in female mice; and ultimate load, ultimate stress, and Young’s modulus for OVX mice (Table 3). Histomorphometric analysis revealed pioglitazone treatment resulted in a significantly reduced mineral apposition rate in male mice (Supplemental Fig. 1). Quantification of osteoclast staining (Supplemental Table 4) did not reveal significant changes with the exception of reduced osteoclast number per osteoclast surface in pioglitazone-treated female mice, possibly due to increased marrow adiposity.
TABLE 1.
DEXA and micro-CT results for pioglitazone-treated and control mice
| Male
|
Female
|
OVX
|
||||
|---|---|---|---|---|---|---|
| Vehicle | Pio | Vehicle | Pio | Vehicle | Pio | |
| n | 8 | 11 | 8 | 12 | 9 | 11 |
| Femoral aBMD (g/cm2) | 0.0586 ± 0.0015 | 0.0520 ± 0.0014a | 0.0587 ± 0.0008 | 0.0556 ± 0.0006a | 0.0463 ± 0.0007 | 0.0433 ± 0.0005a |
| Femoral BMC (g) | 0.0311 ± 0.0013 | 0.0262 ± 0.0014a | 0.0297 ± 0.0007 | 0.0275 ± 0.0004a | 0.0191 ± 0.0006 | 0.0188 ± 0.0007 |
| Femoral vBMD (g/cm3) | 1.151 ± 0.005 | 1.142 ± 0.005 | 1.182 ± 0.009 | 1.200 ± 0.004 | 1.184 ± 0.005 | 1.183 ± 0.005 |
| Vertebral aBMD (g/cm2) | 0.0292 ± 0.0010 | 0.0263 ± 0.0010 | 0.0303 ± 0.0009 | 0.0277 ± 0.0011 | 0.0240 ± 0.0008 | 0.0207 ± 0.0003a |
| Vertebral BMC (g) | 0.0023 ± 0.0002 | 0.0018 ± 0.0001 | 0.0026 ± 0.0001 | 0.0022 ± 0.0001 | 0.0015 ± 0.0001 | 0.0009 ± 0.0001a |
| Vertebral vBMD (g/cm3) | 0.386 ± 0.015 | 0.311 ± 0.019a | 0.268 ± 0.018 | 0.288 ± 0.025 | 0.288 ± 0.025 | 0.247 ± 0.028 |
Values reported as mean ± SE. Pio, Pioglitazone.
Significant (P ≤ 0.05) compared with vehicle-treated wild-type control.
TABLE 2.
Vertebral trabecular architecture and connectivity for pioglitazone-treated and control mice
| Male
|
Female
|
OVX
|
||||
|---|---|---|---|---|---|---|
| Vehicle | Pio | Vehicle | Pio | Vehicle | Pio | |
| n | 8 | 11 | 8 | 12 | 9 | 11 |
| BV/TV (%) | 28.0 ± 0.8 | 24.1 ± 0.6a | 19.6 ± 0.9 | 21.6 ± 1.5 | 20.5 ± 1.7 | 18.3 ± 1.7 |
| Tb.Th. (μm) | 65.1 ± 0.7 | 61.0 ± 0.9a | 69.7 ± 1.9 | 67.7 ± 1.0 | 64.7 ± 0.7 | 63.7 ± 1.4 |
| Tb.N. (mm−1) | 4.3 ± 0.1 | 3.9 ± 0.1a | 2.8 ± 0.1 | 3.2 ± 0.2 | 3.2 ± 0.3 | 2.8 ± 0.3 |
| Tb.Sp. (μm) | 154.9 ± 8.2 | 166.1 ± 5.4 | 250.0 ± 16.3 | 218.9 ± 16.5 | 220.0 ± 22.6 | 248.2 ± 23.6 |
| n | 8 | 11 | 8 | 11 | 9 | 11 |
| Measures of connectivity | ||||||
| Total strut length (mm/mm2) | 6.23 ± 0.17 | 5.63 ± 0.52a | 4.96 ± 0.28 | 4.82 ± 0.30 | 4.19 ± 0.36 | 4.02 ± 0.22 |
| Number of nodes (mm−2) | 13.8 ± 0.9 | 10.7 ± 0.9a | 12.4 ± 1.0 | 10.7 ± 1.0 | 10.6 ± 2.2 | 7.7 ± 0.7 |
| Length of node-node struts (mm/mm2) | 2.49 ± 0.23 | 1.62 ± 0.17a | 2.18 ± 0.34 | 1.66 ± 0.23 | 1.28 ± 0.34 | 0.95 ± 0.14 |
| Length of node-free struts (mm/mm2) | 1.75 ± 0.20 | 1.92 ± 0.10 | 1.23 ± 0.15 | 1.39 ± 0.12 | 1.37 ± 0.13 | 1.40 ± 0.17 |
| Measures of disconnectivity | ||||||
| Number of free ends (mm−2) | 14.8 ± 0.9 | 16.2 ± 0.7 | 9.7 ± 0.7 | 11.6 ± 0.7 | 16.2 ± 0.9 | 15.0 ± 0.8 |
| Length of free-free struts (mm/mm2) | 0.45 ± 0.07 | 0.71 ± 0.13 | 0.35 ± 0.12 | 0.42 ± 0.08 | 0.62 ± 0.11 | 0.77 ± 0.13 |
Values reported as mean ± SE.
Significant (P ≤ 0.05) compared with vehicle-treated control.
TABLE 3.
Vertebral compression of pioglitazone-treated and control mice
| Male
|
Female
|
OVX
|
||||
|---|---|---|---|---|---|---|
| Vehicle | Pio | Vehicle | Pio | Vehicle | Pio | |
| n | 8 | 10 | 8 | 12 | 8 | 10 |
| Structural properties | ||||||
| Ultimate load (n) | 24.9 ± 2.5 | 19.8 ± 1.7 | 25.8 ± 2.5 | 22.8 ± 2.3 | 19.9 ± 2.1 | 12.5 ± 0.8a |
| Failure displacement (mm) | 0.31 ± 0.03 | 0.38 ± 0.10 | 0.42 ± 0.07 | 0.27 ± 0.03 | 0.37 ± 0.05 | 0.38 ± 0.04 |
| Energy to failure (mJ) | 4.6 ± 0.6 | 4.8 ± 0.8 | 6.9 ± 1.4 | 3.4 ± 0.3a | 4.3 ± 0.8 | 3.4 ± 0.57 |
| Stiffness (N/mm) | 131.0 ± 8.8 | 100.0 ± 9.7a | 113.3 ± 11.1 | 148.9 ± 23.2 | 108.8 ± 17.6 | 66.7 ± 6.7 |
| Material properties | ||||||
| Ultimate stress (MPa) | 10.7 ± 1.0 | 7.9 ± 0.8a | 10.3 ± 1.1 | 9.7 ± 1.0 | 9.3 ± 1.0 | 5.3 ± 0.2a |
| Failure strain (%) | 9.5 ± 0.9 | 11.7 ± 1.6 | 13.1 ± 2.2 | 8.2 ± 0.8 | 11.9 ± 1.6 | 12.3 ± 1.2 |
| Toughness (MPa) | 0.59 ± 0.07 | 0.57 ± 0.10 | 0.87 ± 0.18 | 0.44 ± 0.05a | 0.64 ± 0.12 | 0.44 ± 0.06 |
| Young’s modulus (MPa) | 183.8 ± 13.5 | 130.1 ± 15.1a | 145.9 ± 15.8 | 209.3 ± 31.5 | 162.9 ± 29.2 | 87.9 ± 8.5a |
Values reported as mean ± SE. MPa, Megapascals.
Significant (P ≤ 0.05) compared with vehicle-treated control.
Effect of sitagliptin on bone quality
Sitagliptin treatment had no effect on body weight and did not affect bone color or marrow adiposity (Fig. 1, A, F, and G). Additionally, sitagliptin treatment did not affect femoral aBMD, vBMD (Table 4), geometry, or mechanical properties in male, female, or OVX mice (Supplemental Tables 5–7). Sitagliptin-treated male mice exhibited significantly improved vertebral aBMD, but this was not significantly different when measured volumetrically by micro-CT (Table 4). Vertebral vBMD was significantly improved in sitagliptin-treated female mice despite a lack of improvement in aBMD (Table 4). No changes in BMD, volumetric or areal, were seen in sitagliptin-treated OVX mice (Table 4). Sitagliptin-treated female mice also exhibited significant improvements in trabecular architecture with increased BV/TV, Tb.Th., and Tb.N. and reduced trabecular separation (Tb.Sp.) (Table 5). Improvements in trabecular architecture were not seen in sitagliptin-treated male or OVX mice. Vertebral compression was generally not affected by sitagliptin treatment in male and female mice; however, Young’s modulus was significantly decreased in sitagliptin-treated OVX mice (Supplemental Table 8). Histomorphometric analysis did not reveal significant changes in mineral apposition rate (Supplemental Fig. 2) or osteoclast number due to sitagliptin treatment (Supplemental Table 9). BSE analysis of trabecular bone mineralization profiles revealed significantly increased mineralization in sitagliptin-treated male, female, and OVX mice (Supplemental Fig. 3).
TABLE 4.
DEXA and micro-CT results for sitagliptin-treated and control mice
| Male
|
Female
|
OVX
|
||||
|---|---|---|---|---|---|---|
| Vehicle | Sitagliptin | Vehicle | Sitagliptin | Vehicle | Sitagliptin | |
| n | 11 | 10 | 8 | 8 | 9 | 12 |
| Femoral aBMD (g/cm2) | 0.0541 ± 0.0010 | 0.0548 ± 0.0010 | 0.0587 ± 0.0008 | 0.0587 ± 0.0006 | 0.0463 ± 0.0007 | 0.0462 ± 0.0006 |
| Femoral BMC (g) | 0.0289 ± 0.0012 | 0.0311 ± 0.0011 | 0.0297 ± 0.0007 | 0.0299 ± 0.0007 | 0.0191 ± 0.0006 | 0.0198 ± 0.0004 |
| Femoral vBMD (g/cm3) | 1.090 ± 0.016 | 1.117 ± 0.019 | 1.182 ± 0.009 | 1.195 ± 0.010 | 1.184 ± 0.005 | 1.186 ± 0.004 |
| Vertebral aBMD (g/cm2) | 0.0258 ± 0.0009 | 0.0295 ± 0.0006* | 0.0303 ± 0.0009 | 0.0321 ± 0.0011 | 0.0240 ± 0.0008 | 0.0240 ± 0.0010 |
| Vertebral BMC (g) | 0.0020 ± 0.0002 | 0.0028 ± 0.0001* | 0.0026 ± 0.0001 | 0.0026 ± 0.0001 | 0.0015 ± 0.0001 | 0.0014 ± 0.0001 |
| Vertebral vBMD (g/cm3) | 0.225 ± 0.011 | 0.253 ± 0.012 | 0.268 ± 0.018 | 0.390 ± 0.022* | 0.288 ± 0.025 | 0.313 ± 0.018 |
Values reported as mean ± SE.
Significant (P ≤ 0.05) compared with vehicle-treated wild-type control.
TABLE 5.
Vertebral trabecular architecture for sitagliptin-treated and control mice
| Male
|
Female
|
OVX
|
||||
|---|---|---|---|---|---|---|
| Vehicle | Sitagliptin | Vehicle | Sitagliptin | Vehicle | Sitagliptin | |
| n | 11 | 10 | 8 | 8 | 9 | 11 |
| BV/TV (%) | 28.2 ± 1.1 | 30.8 ± 1.0 | 19.6 ± 0.9 | 27.6 ± 1.9a | 20.5 ± 1.7 | 21.7 ± 1.4 |
| Tb.Th. (μm) | 71.4 ± 0.4 | 70.9 ± 0.6 | 69.7 ± 1.9 | 76.1 ± 1.4a | 64.7 ± 0.7 | 63.9 ± 0.8 |
| Tb.N. (mm−1) | 3.9 ± 0.2 | 4.3 ± 0.1 | 2.8 ± 0.1 | 3.6 ± 0.2a | 3.2 ± 0.3 | 3.4 ± 0.2 |
| Tb.Sp. (μm) | 201.3 ± 7.4 | 204.1 ± 6.1 | 250.0 ± 16.3 | 178.5 ± 20.6a | 220.0 ± 22.6 | 190.6 ± 19.8 |
Values reported as mean ± SE.
Significant (P ≤ 0.05) compared with vehicle-treated control.
Effect of genetic inactivation of DPP-4 on bone quality
Body weight was not significantly different in male or female Dpp4−/− vs. Dpp4+/+ control mice; however, Dpp4−/− OVX females weighed significantly less than their OVX Dpp4+/+ female controls (Supplemental Fig. 4). DEXA revealed significant reductions in femoral and vertebral aBMD and BMC for OVX knockout mice, but no changes were observed in male or female Dpp4−/− mice (Table 6). Femoral and vertebral vBMD evaluated by micro-CT did not reveal any changes in male, female, or OVXDpp4+/+ vs. Dpp4−/− mice (Table 6). OVX Dpp4−/− mice had significantly reduced femoral geometry with reductions in anterior-posterior diameter, moment of inertia, cortical thickness, and cross-sectional bone area (Table 7). Accordingly, three-point bending revealed a decrease in stiffness (Supplemental Table 10), and femoral neck fracture revealed decreases in ultimate load, energy to failure, and stiffness for OVX Dpp4−/− mice (Supplemental Table 11). No changes were seen in the femoral mechanics of male and female Dpp4−/− mice (Supplemental Table 11). Small changes were seen in male Dpp4−/− mice with reductions in vertebral ultimate load, Tb.Th. (Supplemental Tables 12 and 13 and Supplemental Fig. 6). Female Dpp4−/− mice exhibited an increase in vertebral Young’s modulus but had reductions in Tb.N. and length of node-free strut, a measure of trabecular connectivity (Supplemental Tables 12–14). Osteoclast staining revealed no significant differences for male, female, or OVX groups (Supplemental Table 15), and no major changes were seen in the trabecular mineralization profiles (Supplemental Fig. 5) or the mineral apposition rates (Supplemental Fig. 6) across Dpp4 genotypes in male or female mice.
TABLE 6.
DEXA and micro-CT results for Dpp4−/− (KO) and WT mice
| Male
|
Female
|
OVX
|
||||
|---|---|---|---|---|---|---|
| WT | KO | WT | KO | WT | KO | |
| n | 15 | 23 | 14 | 19 | 10 | 13 |
| Femoral aBMD (g/cm2) | 0.0548 ± 0.0009 | 0.0523 ± 0.0010 | 0.0538 ± 0.0007 | 0.0547 ± 0.0010 | 0.0498 ± 0.0011 | 0.0446 ± 0.0004a |
| Femoral BMC (g) | 0.0276 ± 0.0008 | 0.0263 ± 0.0008 | 0.0251 ± 0.0006 | 0.0263 ± 0.0008 | 0.0240 ± 0.0007 | 0.0197 ± 0.0003a |
| Femoral vBMD (g/cm3) | 1.264 ± 0.008 | 1.259 ± 0.006 | 1.285 ± 0.006 | 1.273 ± 0.004 | 1.265 ± 0.005 | 1.251 ± 0.026 |
| Vertebral aBMD (g/cm2) | 0.0267 ± 0.0009 | 0.0247 ± 0.0007 | 0.0262 ± 0.0009 | 0.0272 ± 0.0007 | 0.0203 ± 0.0012 | 0.0150 ± 0.0010a |
| Vertebral BMC (g) | 0.0020 ± 0.0002 | 0.0017 ± 0.0001 | 0.0019 ± 0.0002 | 0.0021 ± 0.0001 | 0.0011 ± 0.0001 | 0.0005 ± 0.0001a |
| Vertebral vBMD (g/cm3) | 0.265 ± 0.020 | 0.265 ± 0.011 | 0.279 ± 0.022 | 0.265 ± 0.013 | 0.292 ± 0.022 | 0.260 ± 0.015 |
Values reported as mean ± SE. WT, Wild type; KO, knockout.
Significant (P ≤ 0.05) compared with wild-type control.
TABLE 7.
Geometrical properties of right femora for Dpp4−/− (KO) and WT mice
| Male
|
Female
|
OVX
|
||||
|---|---|---|---|---|---|---|
| WT | KO | WT | KO | WT | KO | |
| n | 15 | 23 | 14 | 18 | 10 | 13 |
| A-P diameter (mm) | 1.38 ± 0.02 | 1.42 ± 0.02 | 1.35 ± 0.01 | 1.36 ± 0.01 | 1.39 ± 0.01 | 1.33 ± 0.01a |
| Moment of inertia (mm4) | 0.173 ± 0.011 | 0.177 ± 0.007 | 0.141 ± 0.004 | 0.147 ± 0.005 | 0.155 ± 0.007 | 0.120 ± 0.003a |
| Cross-sectional bone area (mm2) | 0.895 ± 0.022 | 0.877 ± 0.016 | 0.849 ± 0.017 | 0.847 ± 0.016 | 0.808 ± 0.028 | 0.688 ± 0.016a |
| Cortical thickness (mm) | 0.173 ± 0.009 | 0.166 ± 0.003 | 0.182 ± 0.003 | 0.183 ± 0.003 | 0.167 ± 0.005 | 0.151 ± 0.004a |
Values reported as mean ± SE. WT, Wild type; KO, knockout; A-P, anterior-posterior.
Significant (P ≤ 0.05) compared with wild-type control.
Discussion
Effects of pioglitazone treatment on bone quality
Pioglitazone treatment led to increased weight gain and marrow fat in male, female, and OVX mice. These findings are consistent with previous reports of weight gain and increased bone marrow fat (13, 14, 26) associated with TZD treatment. Pioglitazone-treated male, female, and OVX mice experienced significant reductions in femoral aBMD and BMC; however, these changes were not seen when femoral BMD was measured volumetrically by micro-CT. Additionally, no changes were seen in the femoral geometry or mechanics of pioglitazone-treated mice. The lack of changes seen in femoral mechanics were unexpected, given reports of fractures of the distal upper and lower limbs and not of the spine in human subjects exposed to TZDs for several years (7, 27, 28). Because cortical bone has a lower surface area and overall slower turnover rate than trabecular bone, a longer treatment period may be required to detect greater effects of pioglitazone on the skeleton. Consistent with this possibility, reductions in three-point bending mechanics were detected in rats treated with a higher dose of pioglitazone for 4 months, yet no changes were seen in femoral neck fractures (17).
Although pioglitazone negatively affected vertebral mechanics of all treated mice, male mice exhibited the greatest sensitivity to the metabolic and skeletal effects of pioglitazone with reductions in trabecular architecture and connectivity as well as reduced bone formation. The results obtained here with pioglitazone are consistent with observations reporting reductions in vertebral strength (29), trabecular architecture (13, 29), and mineral apposition (13, 29) in male mice treated with rosiglitazone.
Pioglitazone-treated female mice did not exhibit changes in vertebral vBMD, trabecular architecture, and connectivity, suggesting that the reductions in vertebral mechanics are not likely due to adverse effects on bone mineral. Energy to failure and toughness are largely influenced by collagen and not necessarily by changes in the mineral phase or density of bone (30–32). Furthermore, no significant changes were seen in bone formation parameters in female mice treated with pioglitazone. This finding was unexpected, given that clinical studies revealed an increased risk of fracture in women treated with pioglitazone (27, 28, 33). However, a study by Sottile et al. (34) reported a dissociation between the doses of rosiglitazone required to generate metabolic effects without producing significant differences in BMD or histomorphometric parameters in female rats. The reductions in energy to failure and toughness in pioglitazone-treated female mice are consistent with negative effects on the collagen network (30, 31). Interestingly, TZD activation of PPAR-γ resulted in suppression of type 1 collagen in a stromal cell line (10), and PPAR-γ-mediated reductions in collagen biosynthesis are dependent on levels of estrogen (35, 36). Nevertheless, the effects of PPAR-γ activation on collagen and bone strength are not fully understood.
Effects of sitagliptin on bone quality
We originally hypothesized that sitagliptin may produce positive effects on bone due to its regulation of multiple gut hormones such as GIP and GLP-2, known to enhance bone formation and/or prevent bone resorption (37). The GIP receptor is expressed in osteoblasts (38), and GIP increased collagen type 1 expression and alkaline phosphatase activity in osteoblast-like cells (38) as well as protected osteoblasts from apoptosis (39). GIP receptors have also been found on osteoclasts and GIP inhibits bone resorption in vitro (40). Gipr−/− mice exhibit low bone mass due to decreased bone formation and increased bone resorption (39, 41).
The proglucagon-derived peptide, GLP-2, is also a DPP-4 substrate (42, 43). Exogenous GLP-2 administration reduced serum and urine markers of bone resorption and increased hip BMD in a dose-dependent manner in postmenopausal women and improved spinal BMD in short-bowel patients with no colon (44–46). The skeletal role of the proglucagon-derived peptide, GLP-1, cosecreted together with GLP-2, is less understood, but the GLP-1 receptor is expressed in rodent thyroid C cells, and GLP-1 increases calcitonin secretion and gene expression in mice and rats (47, 48). Glp1r−/− mice exhibit cortical osteopenia and reduced levels of calcitonin, suggesting that GLP-1 may have an indirect role in murine bone metabolism, possibly through a calcitonin-mediated pathway (48).
Female mice treated with sitagliptin exhibited significant improvements in vertebral vBMD and trabecular architecture. Whether these positive changes reflect the cumulative actions of GIP, GLP-1, and GLP-2, or actions of sitagliptin on other substrates acting on the skeleton, cannot be determined from the present study. The improvements seen in vertebral vBMD and trabecular architecture in female mice were lost in sitagliptin-treated OVX female mice, suggesting that partial DPP-4 inhibition does not offset the adverse skeletal effects arising from a marked decline in estrogen production. The beneficial effects seen with sitagliptin treatment in female mice were not seen in male mice.
Interestingly, BSE analysis of total and trabecular bone area mineralization profiles revealed significant shifts toward increased mineralization for all sitagliptin-treated mice, independent of gender or OVX. These increases suggest that inhibition of DPP-4 activity reduces the resorptive rate of bone. GLP-2 administration has been associated with an acute suppression in bone resorption based on bone marker evaluation (49). Suppressed bone resorption could allow more time for secondary mineralization to occur, resulting in a more mineralized bone. Another explanation for increased mineralization could be improved calcium deposition on bone, which may be linked to GIP action (39). Taken together, sitagliptin treatment appears to have a neutral effect on femoral bone mechanics in mice with only modest effects on vertebral bone mechanics. Because we cannot completely exclude the possibility that sitagliptin may exert effects on bone independent of its inhibition of DPP-4 catalytic activity, attribution of these results to the DPP-4-inhibitory properties of sitagliptin will require additional studies using chemically distinct DPP-4 inhibitors.
Effects of genetic inactivation of DPP-4 on bone quality
Sitagliptin treatment reduces but does not completely abrogate DPP-4 activity; hence, we assessed bone quality in mice with complete genetic disruption of the Dpp4 gene to identify a potential skeletal role for the transmembrane or soluble forms of DPP-4. Because Dpp4−/− mice are resistant to HFD-induced obesity (50), we studied Dpp4−/− mice fed a regular chow diet to avoid the confounding effects of differential weight gain in the analysis of bone quality. Genetic inactivation of Dpp4 results in modest defects in bone quality in male and female mice. Male Dpp4−/− mice exhibited significantly reduced ultimate load, Tb.Th. and mineral apposition rate, whereas female Dpp4−/− mice exhibited significantly reduced Tb.N. and length of node-free strut (a measure of connectivity) but exhibited a significantly increased vertebral Young’s modulus. It is important to note that the skeletal changes seen in male and female Dpp4−/− mice were quite modest, given the wide range of tests performed to quantify bone quality. The majority of analyses found no significant differences comparing male and female Dpp4+/+ vs. Dpp4−/− mice, demonstrating that genetic inactivation of Dpp4 does not produce a striking bone phenotype in normal mice.
On the other hand, Dpp4−/− OVX mice exhibited reductions in femoral geometry and femoral structural properties. The interpretation of these data is complicated by the observation that Dpp4−/− mice gain less weight after OVX relative to their Dpp4+/+ littermate controls. Weight gain is an undesirable and complicating effect of OVX in rodent models because it provides partial protection against OVX-induced bone loss (51). Interestingly, the positive effects of sitagliptin treatment on bone quality in female mice were lost in sitagliptin-treated OVX mice, and sitagliptin treatment was associated with less weight gain compared with non-sitagliptin-treated OVX controls. Hence, the effects of DPP-4 inhibition on prevention of weight gain may partially offset the positive effects of DPP-4 inhibition on potentiation of gut hormone action in a postmenopausal estrogen-deficient model.
The motivation for examining the effects of DPP-4 inhibition on the skeleton was partly based on studies that suggested positive skeletal effects from gut hormones that are also DPP-4 substrates, principally GIP, GLP-1, GLP-2, and peptide YY. Nevertheless, the anabolic and antiresorptive effects of gut hormones have usually been demonstrated in studies administering pharmacological doses of gut hormones (49), whereas DPP-4 inhibition and genetic inactivation of Dpp4 would be expected to produce only modest changes in the levels of active gut hormones. Direct comparison of the phenotypes arising in mice treated with sitagliptin vs. Dpp4−/− mice is difficult for several reasons. First, sitagliptin-treated mice were studied on a HFD known to produce multiple changes in insulin secretion, insulin action, and levels of circulating adipokines, independent metabolic parameters that may also influence bone quality. Furthermore, sitgaliptin produces partial but incomplete reduction of DPP-4 activity, whereas Dpp4−/− mice exhibit complete disruption of enzyme activity and the potential for compensatory changes in related genes and proteins that may mask a skeletal phenotype. Nevertheless, mice subjected to DPP-4 inhibition with sitagliptin and Dpp4−/− mice exhibit only modest skeletal phenotypes, notably increasing mineralization and skeletal effects that are reduced in females after OVX.
In summary, pioglitazone negatively affects trabecular bone mechanics in male and female wild-type mice and in estrogen-deficient OVX mice, whereas sitagliptin produces very few changes in bone quality. Although TZDs also increase fracture risk in human subjects, it is difficult to extrapolate results obtained with sitagliptin in studies of rodent bone quality to human subjects due to species-specific differences in skeletal and gut hormone biology. For example, although both Gipr−/− and Glp1r−/− mice exhibit skeletal phenotypes, and exogenous administration of GIP and GLP-1 reduce bone resorption in rodents, acute administration of GIP or GLP-1 has no effect on markers of bone turnover in human subjects (39, 47–49, 52, 53). Nevertheless, because T2DM is a chronic disease associated with reduced bone quality and an increased risk of bone fractures, additional studies examining the effects of antidiabetic agents potentiating incretin action on bone formation, quality, and resorption are clearly warranted.
Supplementary Material
Acknowledgments
We thank Michelle Chan, Sara Che, Vesna Mihajlovic, Natasha Szabolc and Rushika Perera for technical assistance.
This work was supported in part by Canadian Institutes for Health Research Grant NMD-86919, Canadian Diabetes Association Grant OG-3-08-2565-DD, and a grant from Merck Research Laboratories Inc. K.A.K. was supported in part by graduate student awards from the Canadian Institutes for Health Research and Banting and Best Diabetes Centre. D.J.D. is supported by a Canada Research Chair in Regulatory Peptides.
Abbreviations
- aBMD
Areal BMD
- BMC
bone mineral content
- BMD
bone mineral density
- BSE
backscattered electron microscopy
- BV/TV
trabecular bone volume
- DEXA
dual-energy x-ray absorptiometry
- DPP-4
dipeptidyl peptidase-4
- GIP
glucose-dependent insulinotropic polypeptide
- GLP
glucagon-like peptide
- HFD
high-fat-diet
- micro-CT
microcomputed tomography
- OVX
ovariectomized
- PPAR
peroxisome proliferator-activated receptor
- Tb.N
trabecular number
- Tb.Sp
trabecular separation
- Tb.Th
trabecular thickness
- T2DM
type 2 diabetes mellitus
- TRAP
tartrate-resistant acid phosphatase
- TZD
thiazolidinedione
- vBMD
volumetric BMD
Footnotes
Disclosure Summary: D.J.D. has been a consultant to Merck Research Laboratories. The other authors have nothing to declare.
References
- 1.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 2.de Liefde I, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int. 2005;16:1713–1720. doi: 10.1007/s00198-005-1909-1. [DOI] [PubMed] [Google Scholar]
- 3.Hofbauer LC, Brueck CC, Singh SK, Dobnig H. Osteoporosis in patients with diabetes mellitus. J Bone Miner Res. 2007;9:1317–1328. doi: 10.1359/jbmr.070510. [DOI] [PubMed] [Google Scholar]
- 4.Kwon DJ, Kim JH, Chung KW, Lee JW, Kim SP, Lee HY. Bone mineral density of the spine using dual energy X-ray absorptiometry in patients with non-insulin-dependent diabetes mellitus. J Obstet Gynaecol Res. 1996;22:157–162. doi: 10.1111/j.1447-0756.1996.tb00959.x. [DOI] [PubMed] [Google Scholar]
- 5.Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Zmuda JM, Bauer DC, Tylavsky FA, de Rekeneire N, Harris TB, Newman AB. Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2004;19:1084–1091. doi: 10.1359/JBMR.040311. [DOI] [PubMed] [Google Scholar]
- 6.Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, Reid IR. The peroxisome-proliferator-activated receptor-γ agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2007;92:1305–1310. doi: 10.1210/jc.2006-2646. [DOI] [PubMed] [Google Scholar]
- 7.Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, Kravitz BG, Yu D, Heise MA, Aftring RP, Viberti G. Rosiglitazone associated fractures in type 2 diabetes: an analysis from ADOPT. Diabetes Care. 2008;31:845–851. doi: 10.2337/dc07-2270. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka-Czernik B, Feingold KR, Strotmeyer ES, Resnick HE, Carbone L, Beamer BA, Park SW, Lane NE, Harris TB, Cummings SR. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab. 2006;91:3349–3354. doi: 10.1210/jc.2005-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med. 2008;168:820–825. doi: 10.1001/archinte.168.8.820. [DOI] [PubMed] [Google Scholar]
- 10.Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARγ2. J Cell Biochem. 1999;74:357–371. [PubMed] [Google Scholar]
- 11.Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-γ 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143:2376–2384. doi: 10.1210/endo.143.6.8834. [DOI] [PubMed] [Google Scholar]
- 12.Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146:1226–1235. doi: 10.1210/en.2004-0735. [DOI] [PubMed] [Google Scholar]
- 13.Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145:401–406. doi: 10.1210/en.2003-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazarenko OP, Rzonca SO, Suva LJ, Lecka-Czernik B. Netoglitazone is a PPAR-γ ligand with selective effects on bone and fat. Bone. 2006;38:74–84. doi: 10.1016/j.bone.2005.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan Y, Chong LW, Evans RM. PPAR-γ regulates osteoclastogenesis in mice. Nat Med. 2007;13:1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- 16.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 17.Syversen U, Stunes AK, Gustafsson BI, Obrant KJ, Nordsletten L, Berge R, Thommesen L, Reseland JE. Different skeletal effects of the peroxisome proliferator activated receptor (PPAR)α agonist fenofibrate and the PPARγ agonist pioglitazone. BMC Endocr Disord. 2009;9:10. doi: 10.1186/1472-6823-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 19.Walsh JS, Henriksen DB. Feeding and bone. Arch Biochem Biophys. 2010;503:11–19. doi: 10.1016/j.abb.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Hansotia T, Maida A, Flock G, Yamada Y, Tsukiyama K, Seino Y, Drucker DJ. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest. 2007;117:143–152. doi: 10.1172/JCI25483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marguet D, Baggio L, Kobayashi T, Bernard AM, Pierres M, Nielsen PF, Ribel U, Watanabe T, Drucker DJ, Wagtmann N. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci USA. 2000;97:6874–6879. doi: 10.1073/pnas.120069197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magalhaes JK, Grynpas MD, Willett TL, Glogauer M. Deleting Rac1 improves vertebral bone quality and resistance to fracture in a murine ovariectomy model. Osteoporos Int. doi: 10.1007/s00198-010-1355-6. [DOI] [PubMed] [Google Scholar]
- 23.Mousny M, Omelon S, Wise L, Everett ET, Dumitriu M, Holmyard DP, Banse X, Devogelaer JP, Grynpas MD. Fluoride effects on bone formation and mineralization are influenced by genetics. Bone. 2008;43:1067–1074. doi: 10.1016/j.bone.2008.07.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 25.Hansotia T, Baggio LL, Delmeire D, Hinke SA, Yamada Y, Tsukiyama K, Seino Y, Holst JJ, Schuit F, Drucker DJ. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes. 2004;53:1326–1335. doi: 10.2337/diabetes.53.5.1326. [DOI] [PubMed] [Google Scholar]
- 26.Sorocéanu MA, Miao D, Bai XY, Su H, Goltzman D, Karaplis AC. Rosiglitazone impacts negatively on bone by promoting osteoblast/osteocyte apoptosis. J Endocrinol. 2004;183:203–216. doi: 10.1677/joe.1.05723. [DOI] [PubMed] [Google Scholar]
- 27.Bilik D, McEwen LN, Brown MB, Pomeroy NE, Kim C, Asao K, Crosson JC, Duru OK, Ferrara A, Hsiao VC, Karter AJ, Lee PG, Marrero DG, Selby JV, Subramanian U, Herman WH. Thiazolidinediones and fractures: evidence from translating research into action for diabetes. J Clin Endocrinol Metab. 2010;95:4560–4565. doi: 10.1210/jc.2009-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aubert RE, Herrera V, Chen W, Haffner SM, Pendergrass M. Rosiglitazone and pioglitazone increase fracture risk in women and men with type 2 diabetes. Diabetes Obes Metab. 2010;12:716–721. doi: 10.1111/j.1463-1326.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- 29.Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148:2669–2680. doi: 10.1210/en.2006-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Bank RA, TeKoppele JM, Hubbard GB, Athanasiou KA, Agrawal CM. Effect of collagen denaturation on the toughness of bone. Clin Orthop Relat Res. 2000;371:228–239. doi: 10.1097/00003086-200002000-00027. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 32.Wang XD, Masilamani NS, Mabrey JD, Alder ME, Agrawal CM. Changes in the fracture toughness of bone may not be reflected in its mineral density, porosity, and tensile properties. Bone. 1998;23:67–72. doi: 10.1016/s8756-3282(98)00071-4. [DOI] [PubMed] [Google Scholar]
- 33.Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ. 2009;180:32–39. doi: 10.1503/cmaj.080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sottile V, Seuwen K, Kneissel M. Enhanced marrow adipogenesis and bone resorption in estrogen-deprived rats treated with the PPARγ agonist BRL49653 (rosiglitazone) Calcif Tissue Int. 2004;75:329–337. doi: 10.1007/s00223-004-0224-8. [DOI] [PubMed] [Google Scholar]
- 35.Kociecka B, Surazynski A, Miltyk W, Palka J. The effect of Telmisartan on collagen biosynthesis depends on the status of estrogen activation in breast cancer cells. Eur J Pharmacol. 2010;628:51–56. doi: 10.1016/j.ejphar.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 36.Surazynski A, Jarzabek K, Miltyk W, Wolczynski S, Palka J. Estrogen-dependent regulation of PPAR-γ signaling on collagen biosynthesis in adenocarcinoma endometrial cells. Neoplasma. 2009;56:448–454. doi: 10.4149/neo_2009_05_448. [DOI] [PubMed] [Google Scholar]
- 37.Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. 2007;30:1335–1343. doi: 10.2337/dc07-0228. [DOI] [PubMed] [Google Scholar]
- 38.Bollag RJ, Zhong Q, Phillips P, Min L, Zhong L, Cameron R, Mulloy AL, Rasmussen H, Qin F, Ding KH, Isales CM. Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology. 2000;141:1228–1235. doi: 10.1210/endo.141.3.7366. [DOI] [PubMed] [Google Scholar]
- 39.Tsukiyama K, Yamada Y, Yamada C, Harada N, Kawasaki Y, Ogura M, Bessho K, Li M, Amizuka N, Sato M, Udagawa N, Takahashi N, Tanaka K, Oiso Y, Seino Y. Gastric inhibitory polypeptide as an endogenous factor promoting new bone formation after food ingestion. Mol Endocrinol. 2006;20:1644–1651. doi: 10.1210/me.2005-0187. [DOI] [PubMed] [Google Scholar]
- 40.Zhong Q, Itokawa T, Sridhar S, Ding KH, Xie D, Kang B, Bollag WB, Bollag RJ, Hamrick M, Insogna K, Isales CM. Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am J Physiol Endocrinol Metab. 2007;292:E543–E548. doi: 10.1152/ajpendo.00364.2006. [DOI] [PubMed] [Google Scholar]
- 41.Xie D, Cheng H, Hamrick M, Zhong Q, Ding KH, Correa D, Williams S, Mulloy A, Bollag W, Bollag RJ, Runner RR, McPherson JC, Insogna K, Isales CM. Glucose-dependent insulinotropic polypeptide receptor knockout mice have altered bone turnover. Bone. 2005;37:759–769. doi: 10.1016/j.bone.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 42.Drucker DJ, Shi Q, Crivici A, Sumner-Smith M, Tavares W, Hill M, DeForest L, Cooper S, Brubaker PL. Regulation of the biological activity of glucagon-like peptide 2 in vivo by dipeptidyl peptidase IV. Nat Biotechnol. 1997;15:673–677. doi: 10.1038/nbt0797-673. [DOI] [PubMed] [Google Scholar]
- 43.Hartmann B, Thulesen J, Kissow H, Thulesen S, Orskov C, Ropke C, Poulsen SS, Holst JJ. Dipeptidyl peptidase IV inhibition enhances the intestinotrophic effect of glucagon-like peptide-2 in rats and mice. Endocrinology. 2000;141:4013–4020. doi: 10.1210/endo.141.11.7752. [DOI] [PubMed] [Google Scholar]
- 44.Haderslev KV, Jeppesen PB, Hartmann B, Thulesen J, Sorensen HA, Graff J, Hansen BS, Tofteng F, Poulsen SS, Madsen JL, Holst JJ, Staun M, Mortensen PB. Short-term administration of glucagon-like peptide-2. Effects on bone mineral density and markers of bone turnover in short-bowel patients with no colon. Scand J Gastroenterol. 2002;37:392–398. doi: 10.1080/003655202317316006. [DOI] [PubMed] [Google Scholar]
- 45.Henriksen DB, Alexandersen P, Byrjalsen I, Hartmann B, Bone HG, Christiansen C, Holst JJ. Reduction of nocturnal rise in bone resorption by subcutaneous GLP-2. Bone. 2004;34:140–147. doi: 10.1016/j.bone.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Henriksen DB, Alexandersen P, Hartmann B, Adrian CL, Byrjalsen I, Bone HG, Holst JJ, Christiansen C. Four-month treatment with GLP-2 significantly increases hip BMD: a randomized, placebo-controlled, dose-ranging study in postmenopausal women with low BMD. Bone. 2009;45:833–842. doi: 10.1016/j.bone.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, Jacobsen SD, Moses AC, Molck AM, Nielsen HS, Nowak J, Solberg H, Thi TD, Zdravkovic M. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473–1486. doi: 10.1210/en.2009-1272. [DOI] [PubMed] [Google Scholar]
- 48.Yamada C, Yamada Y, Tsukiyama K, Yamada K, Udagawa N, Takahashi N, Tanaka K, Drucker DJ, Seino Y, Inagaki N. The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology. 2008;149:574–579. doi: 10.1210/en.2007-1292. [DOI] [PubMed] [Google Scholar]
- 49.Henriksen DB, Alexandersen P, Bjarnason NH, Vilsboll T, Hartmann B, Henriksen EE, Byrjalsen I, Krarup T, Holst JJ, Christiansen C. Role of gastrointestinal hormones in postprandial reduction of bone resorption. J Bone Miner Res. 2003;18:2180–2189. doi: 10.1359/jbmr.2003.18.12.2180. [DOI] [PubMed] [Google Scholar]
- 50.Conarello SL, Li Z, Ronan J, Roy RS, Zhu L, Jiang G, Liu F, Woods J, Zycband E, Moller DE, Thornberry NA, Zhang BB. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:6825–6830. doi: 10.1073/pnas.0631828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wronski TJ, Schenck PA, Cintrón M, Walsh CC. Effect of body weight on osteopenia in ovariectomized rats. Calcif Tissue Int. 1987;40:155–159. doi: 10.1007/BF02555700. [DOI] [PubMed] [Google Scholar]
- 52.Bollag RJ, Zhong Q, Ding KH, Phillips P, Zhong L, Qin F, Cranford J, Mulloy AL, Cameron R, Isales CM. Glucose-dependent insulinotropic peptide is an integrative hormone with osteotropic effects. Mol Cell Endocrinol. 2001;177:35–41. doi: 10.1016/s0303-7207(01)00405-1. [DOI] [PubMed] [Google Scholar]
- 53.Xie D, Zhong Q, Ding KH, Cheng H, Williams S, Correa D, Bollag WB, Bollag RJ, Insogna K, Troiano N, Coady C, Hamrick M, Isales CM. Glucose-dependent insulinotropic peptide-overexpressing transgenic mice have increased bone mass. Bone. 2007;40:1352–1360. doi: 10.1016/j.bone.2007.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.