Abstract
Chronic lymphatic filarial (LF) infection is associated with suppression of parasite-specific T cell responses that persist even following elimination of infection. While several mechanisms have been implicated in mediating this T cell specific downregulation, a role for alterations in the homeostasis of T effector and memory cell populations has not been explored. Using multiparameter flow cytometry, we investigated the role of persistent filarial infection on the maintenance of T cell memory in patients from the filarial-endemic Cook Islands. Compared to filarial-uninfected endemic normals (EN), microfilaria (mf) positive infected patients (Inf) had a reduced CD4 central memory (TCM) compartment. In addition, Inf patients tended to have more effector memory cells (TEM) and fewer effector cells (TEFF) than did ENs giving significantly smaller TEFF ∶ TEM ratios. These contracted TCM and TEFF populations were still evident in patients previously mf+ who had cleared their infection (CLInf). Moreover, the density of IL-7Rα, necessary for T memory cell maintenance (but decreased in T effector cells), was significantly higher on memory cells of Inf and CLInf patients, although there was no evidence for decreased IL-7 or increased soluble IL7-Rα, both possible mechanisms for signaling defects in memory cells. However, effector cells that were present in Inf and CLInf patients had lower percentages of HLA-DR suggesting impaired function. These changes in T cell populations appear to reflect chronicity of infection, as filarial-infected children, despite the presence of active infection, did not show alterations in the frequencies of these T cell phenotypes. These data indicate that filarial-infected patients have contracted TCM compartments and a defect in effector cell development, defects that persist even following clearance of infection. The fact that these global changes in memory and effector cell compartments do not yet occur in infected children makes early treatment of LF even more crucial.
Introduction
Lymphatic filariasis (LF) is a chronic helminth infection that is associated with a profound parasite-specific T cell hyporesponsiveness in individuals with patent infection. Mechanisms underlying this diminished antigen (Ag)-specific T cell response have included: 1) increased production of IL-10 and/or the expansion of IL-10 producing CD4+ T cells [1], [2], [3], [4]; 2) in utero exposure to the parasite [5], [6] 3) altered Ag presenting cell function [7], [8]; and 4) apoptosis of Ag-activated cells [9]. Furthermore, the increased expression of CTLA-4 [10], [11], PD-1 [10], and Cbl-b [12] as well as modulation of the host response by secreted parasite molecules [13], [14] have also been postulated to play a role in mediating the modulation of T cell responses seen in LF.
The attenuated parasite-specific responses seen in patients with chronic helminth infections have been demonstrated to extend to bystander antigens and to non-filarial infections. Human studies have shown that LF can alter responses to malaria [15], Mycobacterium tuberculosis [16] and HIV [17]. In addition, the presence of schistosome [18] or geohelminth [19] infections has been found to alter allergic responses in children. Of even more broad-reaching importance is the impaired response to parenterally- [20], [21] and orally- [22] administered vaccines seen in those with intestinal or tissue invasive helminth infections.
The mechanisms underlying this bystander suppression in chronic helminth infection remain unclear, although it is probable that some of this suppression is due to the filaria-specific mechanisms mentioned above. However, one area that has not been examined is the role that effector and memory T cells may play. Indeed, the majority of studies on effector and memory T cell populations have been conducted in viral infections [23], [24], [25], [26], [27], [28], [29], [30] and intracellular parasitic infections [31], [32], [33], [34], [35] where clear alterations in the function and phenotype of these T cells have been associated with chronic infection. These types of studies have not generally been extended to extracellular parasites (e.g. helminth infections), though a single study in mice with a GI helminth showed a persistence of memory CD4+ T cells following sterile cure despite the absence of chronic infection [36].
Using recent advances in flow cytometry and the identification of phenotypic markers that identify specific T cell populations, we studied the effects of chronic filarial infection on effector and memory phenotypes in patients. The association of markers that define memory and effector T cell populations, a selection of which are described below for the current study, has been refined based on data from animal studies [26], [30], [36] and from the study of chronic viral infections in humans [37], [38], [39], [40], [41]. Characterization of these T cell populations is often based on the expression of CCR7, a chemokine receptor important for homing to secondary lymphoid organs, and that of the costimulatory molecule CD27. Both markers are found on CD45RA+ naïve cells and long-lived CD45RA− central memory cells, the latter known to proliferate and produce IL-2, displaying effector function only following secondary antigen stimulation. In contrast, the absence of CCR7 characterizes effector memory cells with shorter lifespans, but with the ability to home to sites of inflammation thereby exhibiting immediate effector function [42]. For the purposes of this study, CD45RA+ and CD45RA− effector memory cells were also defined as absent for CD27 expression, a subset similar to the late or fully differentiated effector memory cells described previously [43], [44]. The expression of the chemokine receptor CCR5, present on effector site or effector site seeking cells and downregulated following activation [45], [46] was also used to define effector memory cells [26]. Finally, of most importance to the homeostatic maintenance [47], [48] and survival [49], [50] of memory cells is the cytokine IL-7 and its receptor, IL-7Rα (CD127); therefore, in this study, the expression of IL-7Rα was used to define both stable central and effector memory cell populations.
The patient population in this study was from an LF-endemic island in the South Pacific. This population had been studied longitudinally over an 18 year period with extensive collection of clinical, parasitological and immunological data [51], [52], [53], [54]. Previous work demonstrated that chronically infected individuals had suppressed parasite-specific T cell proliferation and cytokine responses, and moreover, this suppression continued even after the filarial infection (and parasite Ag) had been eliminated [53]. The present study extends these observations to include alterations in effector and memory phenotypes associated with LF, alterations (once established) that remain despite clearance of infection.
Materials and Methods
Ethics Statement
Both studies were approved by the NIAID Institutional Review Board and informed written consent was obtained from all adult subjects; consent from non-adult subjects were obtained through both verbal assent and written consent from the subjects' legal guardian.
Patient Population
The study population was comprised of 29 permanent residents of the island of Mauke in the Cook Islands, a region endemic for the filarial parasite Wuchereria bancrofti. These individuals were part of a larger assessment of filarial infection of the island population over the course of an 18 year period with 2 primary assessments (1975 and 1992; [51], [52], [53], [54]). A subset of those previously evaluated at both timepoints was used for the current study (Table 1). All subjects had been evaluated for clinical (history, physical examination, and complete blood count), parasitologic (filtration of 1 ml of blood through a Nuclepore™ 3 µm filter to quantify microfilariae) and immunologic parameters during both study periods. In addition, serum samples from both 1975 and 1992 were tested for the presence of circulating filarial antigen (CAg; TropBioPty Ltd., Townsville, Australia) that serves as a marker for the presence of adult worms and, therefore, as an indication of an active infection. A group of age and gender matched children was studied separately. The present study utilized cells cryopreserved in1992 and stored in liquid nitrogen until used.
Table 1. Patient Groups.
| GROUP | 1975/1992 INFECTION STATUSa | MALE/FEMALE | AGE RANGE (MEAN) |
| Endemic Normals (EN) | Negative/Negative | 1/5 | 44–72 (56) |
| Infected (Inf) | Positive/Positive | 4/1 | 27–67 (56) |
| Cleared Infected (CLInf) | Positive/Negative | 4/2 | 25–66 (45) |
| Uninfected Children (ENCh) | N/A/Negative | 2/4 | 7–12 (10) |
| Infected Children (InfCh) | N/A/Positive | 2/4 | 6–12 (10) |
Patient groups from the filarial-endemic island of Mauke in the Cook Islands. Filarial infection status was determined at two timepoints (1975 and 1992); age is shown as the range and mean for each group of patients.
All patients positive for infection were microfilaremic; negative status indicates a negative test for circulating antigen (CAg) and microfilaremia.
N/A – Not applicable.
Fluorochrome Antibody Reagents
All fluorochromes were first titrated and negative gates for each were assigned using a “fluorochromes minus one (FMO)” approach [55]. The following antibodies (Ab) were used for each sample: from BD Biosciences (San Jose, CA) – CD3 Pacific Blue, CD4 AmCyan, CD45RA PE-Cy5, CCR7 PE, CD69 FITC, CCR5 APC; from BioLegend (San Diego, CA) – HLA-DR Alexa Fluor 700; from eBioscience (San Diego, CA) – IL-7Rα (CD127) PE-Cy7, CD27 APC-Alexa Fluor 750; and from Molecular Probes (Eugene, OR) – CD8 Qdot 605, Live/Dead Cell Stain Kit UV.
Cell Preparation
Frozen PBMCs were washed, counted with trypan blue, and plated in 6-well plates at a concentration of 1–1.5×106 cells/ml in RPMI/10% FCS. Cells were incubated at 37°C, 5% CO2 for 18 hrs. Prior to harvesting, deoxyribonuclease I (Sigma, St. Louis, MO) was added to the wells for 5 min, 37°C, to prevent clumping. Cells were then washed and resuspended in PBS and stained with the viability dye at RT for 30 min. in the dark. Cells were washed in PBS/1% BSA, blocked with human Ig (Sigma) at RT for 20 min., and subsequently placed into V-bottom plates with fluorochromes for 30 min. on ice in the dark. After labeling, cells were washed, resuspended in PBS/1% BSA, transferred to tubes, and placed on ice in a covered container for immediate flow cytometry.
Flow Cytometry Analysis
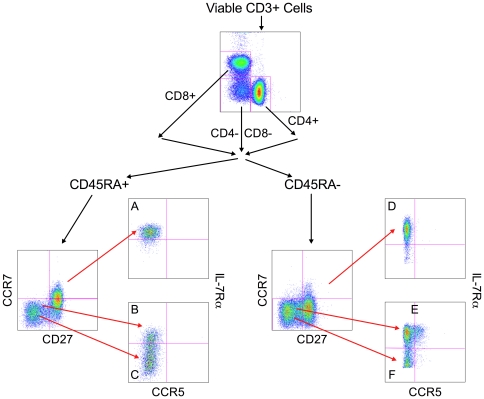
Fluorochrome compensation was accomplished using CompBeads (BD Biosciences) for each Ab. Approximately 200,000 events for each sample were collected on an LSRII flow cytometer (BD Biosciences) and data were analyzed using FlowJo (version 9.0.2; Tree Star, Ashland, OR). Prior to selecting T cell populations, a live cell gate was established after which cells were gated on CD3+ cells followed by separation into CD4+, CD8+, and CD4−CD8− populations. To define the various memory and effector subsets, cells were subsequently divided into CD45RA+ and CD45RA− populations and analyzed for the expression of CD27 versus CCR7 followed by the expression of IL-7Rα versus CCR5. Figure 1 diagrams the gating strategy.
Figure 1. Gating strategy for the 6 major T cell populations beginning with viable CD3+ lymphocytes.
CD4+, CD8+, and CD4−CD8− cells were separated into CD45RA+ and CD45RA− subsets which were subsequently divided into the 6 T cell phenotypes: CD45RA+ cells: A – Naïve cells (CD45RA+CCR7+CD27+IL-7Rα+CCR5−); B – T effector memory RA+ (TEMRA +) cells (CD45RA+CCR7−CD27−IL-7Rα+CCR5−); C – T terminal effector (TTermEff) cells (CD45RA+CCR7−CD27−IL-7Rα−CCR5−); CD45RA- cells: D – T central memory (TCM) cells (CD45RA−CCR7+CD27+IL-7Rα+CCR5−); E – T effector memory (TEM) cells (CD45RA−CCR7−CD27−IL-7Rα+CCR5+/−); F – T effector (TEFF) cells (CD45RA−CCR7−CD27−IL-7Rα−CCR5−).
T cell Populations
Following division into CD3+CD4+, CD3+CD8+, and CD3+CD4−CD8− populations, the following combinations of markers were used to define the naïve, effector and memory subpopulations of T cells (Fig. 1; letters below match the corresponding letters in the figure). CD45RA+ populations were defined as follows: A) Naïve - CD45RA+CCR7+CD27+IL-7Rα+CCR5−; B) T effector memory RA+ (TEMRA +) - CD45RA+CCR7−CD27−IL-7Rα+CCR5−; and C) Terminal Effector cells (TTerm Eff) - CD45RA+CCR7−CD27−IL-7Rα−CCR5−. CD45RA− populations were defined as follows: D) T Central memory (TCM) - CD45RA−CCR7+CD27+IL-7Rα+CCR5−; E) T effector memory (TEM) - CD45RA−CCR7−CD27−IL-7Rα+CCR5+/−; and F) T Effector cells (TEFF) - CD45RA−CCR7−CD27−IL-7Rα−CCR5−.
Effector memory and effector populations (both CD45RA+ and CD45RA−) were then assessed for the presence of the activation markers HLA-DR and CD69. For the purposes of this study, the phenotypes of TEMRA + and TEM cells as described above would predominantly define fully differentiated memory cells that are CD27− but IL-7Rα+. In addition to the populations defined above, there were also transitional effector memory populations in both the CD45RA+ and CD45RA− compartments that differed from the TEMRA + and TEM cells based upon their expression of CD27 and IL-7Rα.
IL-7/Soluble CD127 (sCD127) Assays
Concentrations of IL-7 in patient sera were determined using the IL-7 Quantikine High Sensitivity Elisa according to the manufacturer's instructions (R&D Systems, Inc., Minneapolis, MN). Measurement of the soluble IL-7Rα (sCD127) was modified from a technique recently described [56]. For this assay, 10 µg of goat anti-human IL-7Rα polyclonal Ab (R&D Systems) was covalently coupled to 1×106 carboxylated-modified fluorescent beads (Luminex, Austin, TX). Sera was diluted 1∶5 in assay diluent consisting of PBS, 1% BSA, 0.05% Tween 20, 0.05% NaN3 and 9 mg/ml EDTA and then added to 5000 Ab-coupled beads for 2 hr at RT. Plates were washed with PBS/0.05% Tween 20 and sCD127 was detected with biotinylated mouse anti-human CD127 (1∶500; BD Biosciences) for 1 hr at RT followed by streptavidin PE (Millipore Corporation, Billerica, MA) for 15 minutes. All incubations were done in CoStar (Corning, Lowell, MA) 3357 V-bottom plates covered in foil on a shaking platform. Samples were analyzed on the Bio-Plex 200 System (Bio-Rad, Hercules, CA) and sCD127 levels were determined from a standardized curve generated using recombinant human CD127 Fc Chimera (R&D Systems).
Statistical Analyses
Analysis of the differences between patient groups was accomplished with GraphPad Prism version 5.0 (GraphPad, San Diego, CA). A two-tailed Mann-Whitney U test with a 95% confidence interval was used to compare groups and a p-value≤0.05 was considered significant. The Spearman Rank Test was used to determine correlations between parameters.
Results
EN vs Inf vs CLInf Subjects
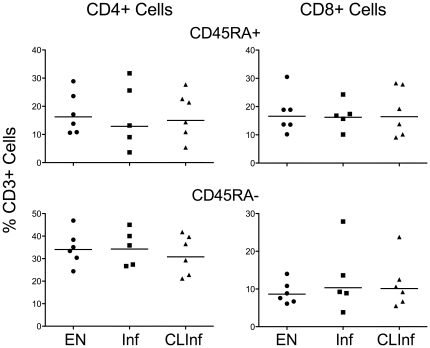
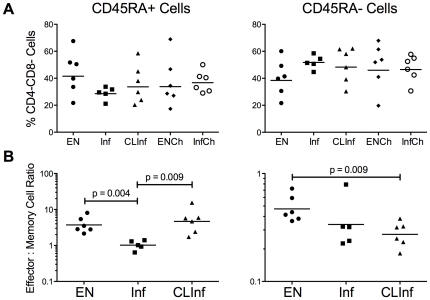
Because the major objectives of this study were to assess the consequences of a chronic helminth infection on effector and memory cell populations and to understand whether the clearance of lingering Ag alters these populations, a comparison was made between endemic normal (EN) individuals (never infected) to those who had clear evidence of long-standing, active filarial infection at both time periods (Inf). This was contrasted to those who had been actively infected in 1975, but who had cleared their infection in the intervening 18 years (CLInf). Initial analysis of CD3+ cells for the EN, Inf, and CLInf groups showed no differences among the groups in the percentages of either CD45RA+ or CD45RA− cells within the CD3+ population (Fig. 2). Therefore, any differences observed in subsequent analyses were not a result of differences within these two major CD3+ cell compartments. There were also no significant differences in the percentage of naïve T cells (defined as CD45RA+CCR7+CD27+IL-7Rα+CCR5−) among the groups (data not shown), although there was an increase in the relative size of the naïve compartment in the EN group compared to either the Inf or CLInf groups, particularly for CD4+ cells (Table 2). Not unexpectedly, the largest percentages of CD45RA+ cells in any of the 3 groups studied were CCR7− (non-naïve).
Figure 2. Total CD45RA+ and CD45RA− cells.
Data for CD45RA+ (top panels) and CD45RA− (bottom panels) cells are expressed as a percent of viable CD3+ cells in endemic normals (EN), filarial-infected patients (Inf), and in patients who had cleared their filarial infection (CLInf) for CD4+ cells (left panels) and CD8+ cells (right panels). Each point represents an individual patient and horizontal lines represent geometric means.
Table 2. Geometric mean % size of each T cell subset relative to all 6 cell phenotypes.
| CD45RA+ | CD45RA− | |||||
| Naive | TEMRA + | TTermEff | TCM | TEFF | TEM | |
| CD3+CD4+ | ||||||
| EN | 14.5 (7.8–28.0) | 2.4 (0.5–4.2) | 1.6 (0.5–4.2) | 7.9 (2.7–15.5) | 5.9 (2.9–9.9) | 63.4 (49.4–75.5) |
| Inf | 7.6 (2.0–26.3) | 4.5 (1.6–12.4) | 2.4 (1.0–5.9) | 4.1 (2.0–13.5) | 3.7 (2.6–4.5) | 69.7 (53.2–83.8) |
| CLInf | 5.7 (1.9–13.1) | 2.8 (1.1–20.8) | 1.5 (0.5–7.5) | 3.5 (0.8–9.9) | 5.0 (3.2–7.3) | 75.0 (64.1–82.9) |
| ENCh | 5.5 (3.1–11.5) | 2.8 (1.6–5.4) | 2.7 (1.2–5.0) | 3.6 (2.2–6.2) | 8.2 (5.8–10.4) | 75.0 (69.3–80.7) |
| InfCh | 6.2 (3.3–10.4) | 3.2 (1.6–6.1) | 4.1 (2.2–6.7) | 3.9 (1.8–6.3) | 12.0 (7.1–25.1) | 67.4 (58.9–76.6) |
| CD3+CD8+ | ||||||
| EN | 7.9 (2.9–18.8) | 6.1 (4.1–9.1) | 29.3 (22.0–39.9) | 0.7 (0.2–1.5) | 28.1 (19.3–36.2) | 23.0 (14.9–35.9) |
| Inf | 4.5 (0.7–18.3) | 9.6 (6.9–14.8) | 25.8 (17.2–45.9) | 0.5 (0.1–1.5) | 21.9 (15.7–30.3) | 30.5 (16.0–42.7) |
| CLInf | 4.8 (3.3–9.0) | 8.2 (4.5–12.2) | 30.5 (22.4–39.3) | 0.5 (0.1–3.0) | 19.2 (15.9–25.1) | 34.1 (26.1–40.9) |
| ENCh | 5.6 (2.2–8.1) | 3.3 (1.1–8.3) | 22.7 (10.5–37.0) | 0.3 (0.1–0.8) | 43.2 (24.7–64.0) | 17.4 (8.3–32.0) |
| InfCh | 8.8 (4.6–15.2) | 3.2 (1.4–7.4) | 15.5 (8.3–34.1) | 0.6 (0.4–0.9) | 45.3 (30.7–65.9) | 20.7 (13.1–37.2) |
| CD3+CD4−CD8− | ||||||
| EN | 0.6 (0.1–12.1) | 10.8 (4.9–17.2) | 39.9 (26.7–52.6) | 0.2 (0.0–1.4) | 13.8 (11.3–19.2) | 29.4 (19.1–40.3) |
| Inf | 0.5 (0.1–3.0) | 27.3 (20.3–36.7) | 27.9 (23.6–36.9) | 0.1 (0.0–0.4) | 10.5 (7.0–22.2) | 31.0 (25.9–39.8) |
| CLInf | 0.6 (0.1–1.4) | 9.5 (3.0–17.6) | 44.5 (30.4–62.3) | 0.1 (0.0–0.6) | 8.8 (6.9–12.0) | 32.1 (18.0–42.8) |
| ENCh | 0.5 (0.2–0.7) | 9.5 (3.6–15.6) | 25.0 (13.4–41.5) | 0.02 (0.0–0.1) | 12.4 (6.7–21.5) | 46.7 (31.3–67.3) |
| InfCh | 0.8 (0.4–1.3) | 7.8 (4.5–13.7) | 24.3 (18.7–36.7) | 0.05 (0.0–0.1) | 13.8 (6.9–37.1) | 46.8 (27.3–65.0) |
The percent of each cell population for the CD45RA+ cells (including naïve, TEMRA +, and TTermEff; A, B, and C in Fig. 1 respectively) and the CD45RA− cells (including TCM, TEM, and TEFF; D, E, and F in Fig. 1 respectively) was calculated for a given patient and the % contribution of each population relative to the six total populations was then determined. The geometric mean percent of each cell phenotype (compartment) for each patient group was then calculated based upon the percentages derived from all of the patients within a group. Ranges for each category are shown in parentheses.
EN – Endemic Normal; Inf – Filarial-infected; CLInf – Cleared Infected; ENCh – Uninfected Children; InfCh – Filarial-infected Children.
Effector and Effector Memory Cells
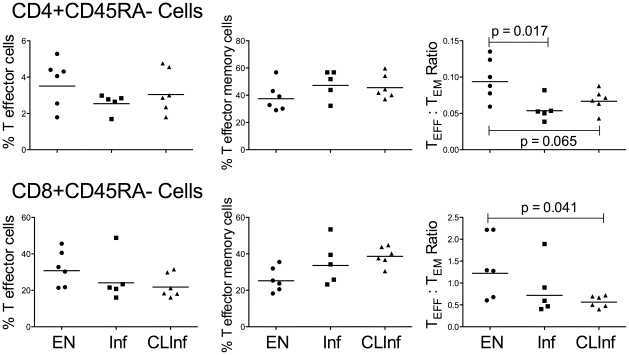
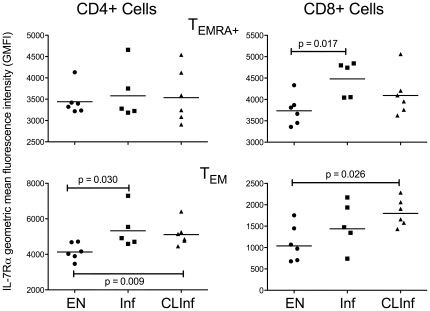
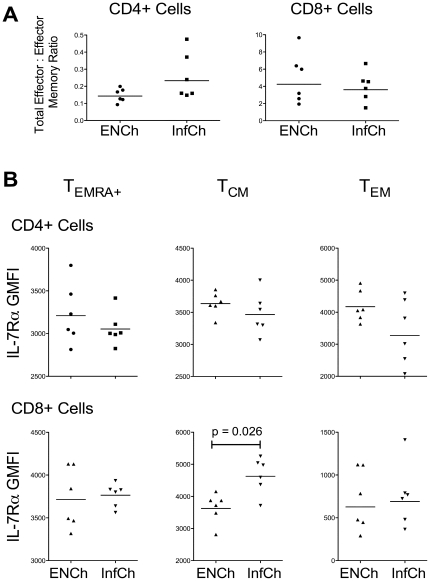
Examination of the TEMRA +, TTermEff, TEM, and TEFF phenotypes in the 3 patient groups showed that while there were no significant differences in the percentage of TEFF and TEM cells among the groups, the EN group had slightly higher numbers of TEFF but fewer TEM cells in the CD45RA− population than those with active (Inf) or previous but cleared (CLInf) infection (Fig. 3). This is most notable when the TEFF to TEM ratio was calculated for either the CD4+ or CD8+ cells (Fig. 3; geometric mean [GM] % for CD4+ cells = 0.094 [EN] vs 0.054 [Inf, p = 0.017] and 0.067 [CLInf, p = 0.065]; for CD8+ cells = 1.22 [EN] vs 0.56 [CLInf, p = 0.041]). Coincidentally, the proportion of the total of the 6 major T cell phenotypes (described in Table 2) that was comprised of either TEFF or TEM cells illustrated that the EN group had a larger TEFF compartment. This contrasted with those with active infection in which the Inf patients had a larger TEM compartment particularly within the CD8+ cell population. A similar comparison between the EN and CLInf groups demonstrated that clearance of parasite antigen did not appear to change the size of either the TEFF or TEM compartment from that seen in patients with active infection. In addition, although it did not reach statistical significance, the ratio of TTermEff to TEMRA + among CD8+CD45RA+ cells was higher in the ENs than in the other 2 groups (GM % = 4.8 [EN] vs 2.7 [Inf] and 3.7 [CLInf]). There was, however, a higher geometric mean fluorescence intensity (GMFI) of IL-7Rα (CD127) on effector memory cells in Inf and CLInf patients compared to ENs. For TEMRA + cells, this increased expression of IL-7Rα was observed in the CD8+ population (EN vs Inf, p = 0.017; Fig. 4), but the disparity in IL-7Rα expression between the EN group compared to either the Inf or CLInf groups was even greater when the CD45RA− TEM compartments were examined (CD4+ cells: EN vs Inf [p = 0.030] and CLInf [p = 0.009]; CD8+ cells: EN vs CLInf [p = 0.026]).
Figure 3. Total TEFF and TEM cells.
Data for TEFF (left panels) and TEM (middle panels) cells are expressed as a % of CD4+CD45RA− cells (top panels) and CD8+CD45RA− cells (bottom panels) in EN, Inf, and CLInf patients. The right panels display the TEFF to TEM ratio for each patient using values derived from percentages of CD45RA− cells for each T cell subset. Each point represents an individual patient and the horizontal lines represent geometric means; the Mann-Whitney U test was used to derive p-values.
Figure 4. Geometric mean fluorescence intensity (GMFI) of IL7-Rα expression.
GMFI of IL-7Rα is shown for EN, Inf, and CLInf patients for TEMRA + cells (top panels) and TEM cells (bottom panels) in CD4+ cells (left panels) and in CD8+ cells (right panels). Each point represents an individual patient and the horizontal lines represent geometric means; the Mann-Whitney U test was used to derive p-values.
Transitional Memory Cells
In addition to the 6 major T cell phenotypes detailed, there were a number of transitional effector memory cell subtypes found within both the CD45RA+ and CD45RA− compartments. The most prevalent of these were CCR7−CD27+IL-7Rα+ with 66–73% found in the CD4+CD45RA+ compartment. However, there were no differences among the 3 patient groups for this presumed transitional effector memory cell subtype. A second transitional phenotype (CCR7−CD27+IL-7Rα−), a probable effector cell recently activated through the CD70 ligand [57], was noticeably reduced in Inf and CLInf patients within the CD8+CD45RA− compartment compared with EN individuals (GM% of CD8+CD45RA− cells = 4.1 and 4.2 vs 8.3 respectively; p = 0.015 CLInf vs EN).
Central Memory Cells (TCM)
Comparison of TCM percentages among the EN, Inf, and CLInf patients demonstrated that EN individuals had an almost 2 fold increase in these CD45RA−CCR7+CD27+IL-7Rα+CCR5− cells within the CD4+ pool compared to either the Inf or the CLInf subjects (GM% of CD4+CD45RA− cells = 4.7 for EN vs 2.8 for Inf or 2.1 for the CLInf). Indeed, the relative contribution of the CD4+ TCM compartment was approximately twice as large in the EN group compared to that of the Inf or CLInf group (GM % TCM compartment size = 7.9 [EN] vs 4.1 [Inf] and 3.5 [CLInf]; Table 2). TCM cells did not differ for the CD8+ compartment and, unlike what was observed for effector memory cells, the GMFI of IL-7Rα did not differ among the patient groups for either CD4+ or CD8+ cells.
Expression of activation markers
When the surface expression of HLA-DR (Table 3) was assessed on each of the cell populations, EN patients had a higher % of CCR7−CD27−HLA-DR+ cells than did the Inf and CLInf individuals for CD4+ TTermEff cells (EN vs Inf, p = 0.018) and TEFF cells (EN vs Inf and CLInf, p = 0.030 and 0.041 respectively). A similar, but not significant, trend was seen for CD8+ TEFF cells (EN vs Inf and CLInf, p = 0.052 and 0.065 respectively; Table 3). There were no significant differences in the expression of CD69 among the groups on any of the cell populations studied (data not shown).
Table 3. HLA-DR expression on Effector Cells.
| TERMINAL EFFECTOR CELLS | EFFECTOR CELLS | |
| CD4+ Cells | ||
| EN | 2.6 (1.3–8.2)a | 3.3 (2.0–4.8)a b |
| Inf | 0.7 (0.3–1.0) | 1.6 (1.2–2.5) |
| CLInf | 1.3 (0.4–4.4) | 1.9 (1.0–2.5) |
| ENCh | 0.9 (0.2–1.5) | 2.6 (2.0–4.0) |
| InfCh | 1.9 (0.6–8.4) | 3.9 (2.4–5.8) |
| CD8+ Cells | ||
| EN | 21.9 (13.8–31.0) | 19.6 (9.0–28.1) |
| Inf | 17.0 (14.9–20.8) | 9.5 (5.9–16.1) |
| CLInf | 19.0 (10.7–30.9) | 11.2 (6.3–17.3) |
| ENCh | 14.2 (7.7–22.7) | 22.4 (12.2–46.9) |
| InfCh | 15.8 (10.4–24.8) | 25.3 (21.9–31.5) |
Values are the geometric mean (range) of HLA-DR expressing cells for each patient group for CD4+ and CD8+ effector cells. HLA-DR+ expression was determined as a % of CCR7−CD27− cells within either the CD45RA+ compartment (Terminal Effector Cells) or CD45RA− compartment (Effector Cells).
EN – Endemic Normal; Inf – Filarial-infected; CLInf – Cleared Infected; ENCh – Uninfected Children; InfCh – Filarial-infected Children.
Indicates a significant difference between EN and Inf.
Indicates a significant difference between EN and CLInf.
Infected vs. Uninfected Children
To examine whether the apparent differences in the effector and effector memory compartments observed in adults with long-standing filarial infection occurs with infection of much shorter duration (e.g. young children), assessments of T cell compartments were made between filarial-infected (InfCh) and uninfected (ENCh), age and gender matched children. Despite the finding that children had a higher frequency of CD4+CD45RA+ cells compared to adults (GM% of CD3+CD4+ cells = 29.4 [EN] vs 53.8 [ENCh] and 45.9 [InfCh]), the frequency of those that were CD4+CD45RA+CCR7+CD27+ was contracted in the children compared to the adults (GM% of CD4+CD45RA+ cells = 9.6 [EN] vs 3.3 [ENCh] and 3.7 [InfCh]).
The most evident finding was that, unlike that seen for adults, the EN (filarial uninfected) and Inf children did not differ in the composition of the CD4+ and CD8+ T cell compartments (Fig. 5, Tables 2 and 3). Indeed, most striking was the fact that, unlike in infected adults, the T cell phenotype of infected children included larger compartments comprised of effector T cells, particularly the CD4+ TTermEff and TEFF compartments (Table 2). Coupled with the finding of an equivalent total (CD45RA+ and CD45RA−) effector∶effector memory ratio in InfCh compared to ENCh in both the CD4+ and CD8+ compartments (Fig. 5A), infection in children was not associated with a shift in effector/effector memory phenotype as seen in adults with filarial infection. Furthermore, there were no differences in IL-7Rα expression on most CD3+ cells in InfCh (compared to ENCh) (Fig. 5B) with the exception of CD8+ TCM cells (GMFI = 4628 [InfCh] vs 3626 [ENCh], p = 0.026).
Figure 5. Comparison of endemic normal children (ENCh) and filarial-infected children (InfCh).
A) Total effector∶effector memory ratios in CD4+ cells (left panel) and CD8+ cells (right panel). Values were calculated for each patient using the percentages of either CD45RA+ or CD45RA− cells and ratios were derived using the following equation: (% TTermEff+% TEFF)/(% TEMRA ++% TEM). B) Geometric mean fluorescence intensity (GMFI) of IL-7Rα expression in TEMRA + cells (left panels), TCM cells (middle panels), and TEM cells (right panels) for CD4+ cells (top panels) and CD8+ cells (bottom panels). Each point represents an individual patient and the horizontal lines represent geometric means; the Mann-Whitney U test was used to derive p-values.
CD4−CD8− Cell Population
Aside from the classical CD3+CD4+ and CD3+CD8+ cells, ∼8.5% (range = 5.8–10.9%) of the viable CD3+ cells were CD4−CD8− in all patient groups studied. This unusual population appeared to be divided between both CD45RA+ and CD45RA− cells (34.7% [range 28.5–41.5] and 45.1% [range 38.4–51.7] respectively; Fig. 6A) and displayed a definitive effector or effector memory phenotype, including a strikingly large contribution (GM = 27.3%) from the CD4−CD8− TEMRA + compartment in Inf patients (Table 2).
Figure 6. T cell populations in the CD3+CD4−CD8− compartment.
A) Total CD4−CD8− cells expressed as a % of CD45RA+ cells (left panel) and CD45RA− cells (right panel) in all 5 patient groups. B) Effector∶Effector memory ratios in adult patient groups for CD45RA+ cells (% TTermEff/% TEMRA +; left panel) and CD45RA− cells (% TEFF/% TEM; right panel). Each point represents an individual patient and the horizontal lines represent geometric means; the Mann-Whitney U test was used to derive p-values.
Given the effector and effector memory nature of these CD4−CD8− cells, the effector∶effector memory ratios were examined for both the CD45RA+ and CD45RA− compartments. Within the CD45RA+ cell pool, Inf patients had a significantly lower TTermEff to TEMRA + ratio compared with the EN group (p = 0.004) within this double negative population; however, the CLInf group was, for the first time, more similar to ENs, displaying a significantly higher TTermEff to TEMRA + ratio than the Inf group (p = 0.009). This latter finding, however, was not seen in the CD4−CD8− CD45RA− cells in which both the Inf and CLInf groups had lower TEFF to TEM ratios in comparison to EN patients. There were no differences among the groups in the expression of IL7-Rα, CD69, or HLA-DR on these CD3+CD4−CD8− cells (data not shown).
IL-7 and sCD127 Levels in Patient Sera
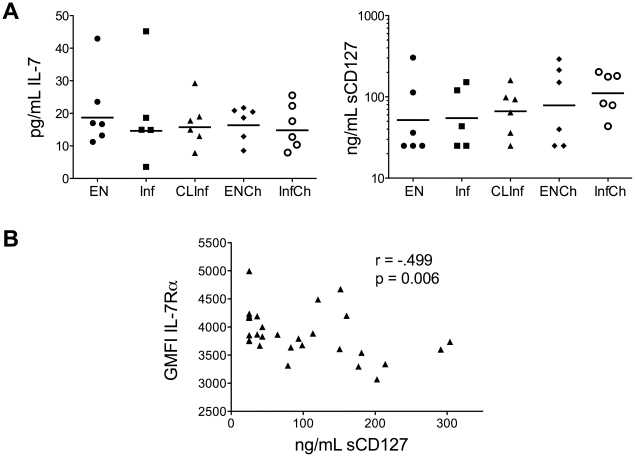
Because of the importance of IL-7 and sCD127 (the soluble IL-7Rα) in chronic viral infections in humans in maintaining T cell homeostasis [23], we compared the serum levels of IL7 and sCD127 (Fig. 7) in all patients. Examination of IL-7 levels showed a broad range but no differences were seen between any of the groups studied. Similar results were found for levels of sCD127, and there was no relationship between serum levels of IL-7 and the levels of sCD127. Comparable findings were seen when an expanded number of subjects from the Cook Islands (n = 68) were examined (data not shown). There was, however, a negative correlation between the GMFI of IL-7Rα on TCM cells and the levels of sCD127 (Fig. 7b), demonstrating a dynamic between the cell bound and the soluble IL-7 receptor.
Figure 7. IL-7 and soluble IL-7Rα in patient sera.
A) IL-7 (left panel) and soluble IL-7Rα (sCD127; right panel) levels as measured in the sera of patients from all 5 patient groups. B) Correlation between sCD127 levels in the sera and geometric mean fluorescence intensity (GMFI) of IL-7Rα expression on CD4+ TCM cells (as measured by flow cytometry).
Discussion
Unlike studies of chronic viral infections and the few studies on intracellular parasites (e.g. Trypanosoma cruzi [33], [58], Toxoplasma gondii [32] and Leishmania spp. [35], [59]), data on effector and memory populations in chronic extracellular parasitic infections are lacking. Study of the Ag-specific populations in human helminth infections is hampered by the lack of robust methods to identify Ag-specific cells because of the intrinsic variability in human MHC Class-II recognition of parasite peptides. Moreover, because of the chronicity (and long-lived nature) of these organisms, continuous exposure of the immune system to the parasite's antigens may perturb memory and effector populations, making it difficult to prove unequivocally what occurs in the absence of infection.
Nevertheless, using a group of well-characterized (and longitudinally followed) patients from the Cook Islands, we have been able to identify memory and effector populations with the goal of determining whether the decrease in parasite-specific T cell responses seen in patients with chronic filarial infection [52], [60], [61] can be attributed, at least in part, to an alteration in the effector and memory populations. While most previous studies on filarial infection have examined parasite-specific responses, it has been clearly demonstrated that patients with filarial infections have modulated immune responses to other pathogens [15], [16], [17]. Thus, while we have not yet defined antigen-specific effector and memory populations, because of the lack of methods to mark the filarial-specific cell populations, we have been able to demonstrate alterations in these cell populations ex vivo that likely contribute to the Ag-specific T cell hyporesponsiveness seen previously in vitro [53].
A limitation of this study was the relatively small size of the patient groups, a limitation based on the paucity of patients within a given study group from whom there were cryopreserved cells. It is also apparent that there was a skewed male∶female ratio among the patient groups. This however reflects the well-known finding that LF is much less common in women of childbearing age. Nevertheless, gender differences in filarial-specific immunological responsiveness have never been seen. In addition, there were no differences in the age ranges among the adult groups studied, a variable that would be more likely to affect infection status and possibly immune responses. Thus, despite these limitations, we were able to show statistically significant differences in memory cell populations in these very well defined patient groups.
The results of this study demonstrated that there were lower numbers of T effector cells in filarial-infected patients in comparison to filarial-uninfected endemic normals not only within the CD4+CD45RA− and CD8+CD45RA− compartments, but also in CD4−CD8− cells (cells thought to contribute to inflammatory responses [62]). Furthermore, a decrease in the population of cells transitioning from a memory to effector state (i.e. CCR7−CD27+IL7-Rα− cells) was also observed in Inf patients. More importantly, this finding of a reduced effector compartment was seen in those who had cleared the infection and with it, the associated circulating parasite Ag. These data parallel those seen in in vitro functional studies on these same patients [53], which demonstrated reduced proliferative and cytokine responses to parasite Ag. In addition, the CD45RA+ and CD45RA− effector cells that were identified in Inf and CLInf patients showed a reduced activation state based on their lower expression of HLA-DR, contrasting with findings seen in HIV-1 infected patients [25].
The reduction in the effector compartment in Inf patients might imply that these cells are turning over more rapidly from activation-induced cell death as a result of the constant exposure to circulating parasite antigens; however, this does not explain why the CLInf patients should have a similarly contracted effector compartment. Moreover, the reduced naïve compartment in both Inf and CLInf patients (Table 2) suggests there should be a larger TEFF compartment in these patients because of constant effector cell recruitment from the naïve compartment. One possible explanation for the reduced effector phenotype in Inf and CLInf patients might be the reduction in IL-2 and IFN-γ in these patients [52], [53], cytokines known to be important for reversal of Ag-induced non-responsiveness and conversion of naïve cells to an activated effector state [63].
Another important finding from our study was that filarial infection was associated with an expanded population of effector memory cells that altered the effector∶effector memory ratio. This altered phenotype was widespread, occurring in each of the CD3+ populations (CD4+, CD8+, CD4−CD8−) and notably, was seen not only in those with active infection but in those who had eliminated the infection (CLInf), suggesting that this may lead to diminished effector cell function. Indeed, it has been hypothesized that chronic Ag stimulation, apparent in older individuals with chronic CMV infection, leads to an increase in senescent dysfunctional cells, primarily within a CD8+CD45RA+CD27− subset [64]. This population, similar to the TEMRA + cells in the current study, was increased in both the Inf and CLInf subjects (Table 2). While these cells could represent those that develop into strong effectors as demonstrated in Mycobacterium infection [65], they could also comprise an accumulating but exhausted CD8+ memory cell population with diminished Ag-responsiveness, found more commonly in older individuals [66]. Such immunosenescence has been demonstrated in CD4+ cells during T. cruzi infection [58]. Further studies of these cell populations for effector function (e.g. granzymes, perforin) will be necessary to elucidate the true nature of these cells.
Unlike TEM cells, the size of the CD4+ TCM compartment was reduced by nearly 50% in both Inf and CLInf patients in comparison to EN individuals. This suggests that filarial infection (past or present) is associated with a defect in long-term memory function that may be reflected in the inability to proliferate and produce IL-2 in response to Ag [52], [53]. The importance of a strong TCM compartment is demonstrated by the ability of these cells to persist longer than TEM cells in vivo, even in the absence of Ag [67], and to mediate protection because of their high proliferative potential [68]. Indeed, protection against the intracellular parasite L. major is mediated not only by short-lived effector cells but also by the long-lived pathogen independent TCM cells [35]. Furthermore, since human TCM and TEM cells do not interconvert [69], the decrease in the numbers of TCM cells (and the increased numbers of TEM cells) cannot be attributed to interconversion.
Surprisingly, data from this study found that not only were there more effector memory cells in Inf and CLInf patients, these cells had a higher mean expression of IL-7Rα (CD127), a finding that differs from what has been seen in chronic viral infections in humans [25], [38], [40] and in mice [70], [71]. Nevertheless, our data support the notion that filarial infection is associated with a diminished ability of effector memory cells to convert to T effectors. Given that IL-7Rα should decrease upon Ag activation [49] and that patient T cells are under constant exposure to parasite Ag, there is an expectation of a larger IL-7Rα− pool. Our data, however, shows just the opposite. There may be several reasons for these differences including: 1) a lack of IL-2 [53] needed for the negative regulation of IL-7Rα [72]; 2) the development of long-term tolerance in cells that are IL-7Rαhi [73]; 3) a slower turnover rate in aged cells [74], given the chronicity of filarial infection and the age of the patients; and 4) a possible signaling defect in the balance between IL-7Rαhi and IL-7Rαlo cells through the interaction of two proteins – GABPα and GF/-1 [75].
Since IL-7 is important for the differentiation and survival of memory cells [32], we examined IL-7 levels in the sera of patients to determine if lower levels in infected patients might be a limiting factor for signaling in memory cells. Our data failed to support this concept. In addition, there was no relationship between the serum levels of IL-7 and the soluble IL-7Rα (sCD127). There were also no differences seen among patient groups in the serum levels of sIL-7Rα suggesting that sCD127 is not preventing the binding of IL-7 to memory cells [23]. There was, however, a negative correlation between sIL-7Rα and the expression of surface IL-7Rα, corroborating the typical dynamic often seen in cell-bound and soluble IL-7R.
Finally, the altered cellular phenotypes seen in adult patients with long-term filarial infection, either past or present, was not seen in young filarial-infected children, despite their lack of T cell proliferation and cytokine responses to parasite Ag ([52], Steel, unpublished). Thus, the question must be asked - are the mechanisms of T cell hyporesponsiveness different in children? Data from this study would suggest they are. More than likely, other immunological mechanisms of immune regulation (e.g. IL-10, APC dysfunction, in utero tolerization) may play a more prominent role in children (or in more recent infection). Indeed, murine LCMV studies predict that if Ag is eliminated during the early stages of infection, memory cells can develop normally [30]; therefore, it might be predicted that, while these children have some loss of T cell function, the global defects in the effector and memory compartments seen with long-term infection might be avoided if the filarial infection is eliminated early in life.
In summary, we have demonstrated a change in the nature of effector and memory populations that characterize patients with chronic filarial infection. Coupled with previous studies that have shown a modulation of effector responses to other pathogens [15], [16], [17] and to parenteral and oral vaccinations [20], [21], [22], the data from this study suggests that there are even more broad-reaching consequences of persistent filarial infection that provides additional impetus to treat filarial infections as early in life as possible.
Acknowledgments
The authors are grateful for the assistance of the NIAID Flow Cytometry Unit, in particular, Kevin Holmes and David Stephany.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by the Intramural Research Program of the NIH/NIAID. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.King CL, Mahanty S, Kumaraswami V, Abrams JS, Regunathan J, et al. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J Clin Invest. 1993;92:1667–1673. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahanty S, Mollis SN, Ravichandran M, Abrams JS, Kumaraswami V, et al. High levels of spontaneous and parasite antigen-driven interleukin-10 production are associated with antigen-specific hyporesponsiveness in human lymphatic filariasis. J Infect Dis. 1996;173:769–773. doi: 10.1093/infdis/173.3.769. [DOI] [PubMed] [Google Scholar]
- 3.Metenou S, Dembele B, Konate S, Dolo H, Coulibaly SY, et al. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J Immunol. 2010;184:5375–5382. doi: 10.4049/jimmunol.0904067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Specht S, Volkmann L, Wynn T, Hoerauf A. Interleukin-10 (IL-10) counterregulates IL-4-dependent effector mechanisms in Murine Filariasis. Infect Immun. 2004;72:6287–6293. doi: 10.1128/IAI.72.11.6287-6293.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malhotra I, Mungai PL, Wamachi AN, Tisch D, Kioko JM, et al. Prenatal T cell immunity to Wuchereria bancrofti and its effect on filarial immunity and infection susceptibility during childhood. J Infect Dis. 2006;193:1005–1013. doi: 10.1086/500472. [DOI] [PubMed] [Google Scholar]
- 6.Steel C, Guinea A, McCarthy JS, Ottesen EA. Long-term effect of prenatal exposure to maternal microfilaraemia on immune responsiveness to filarial parasite antigens [see comments]. Lancet. 1994;343:890–893. doi: 10.1016/s0140-6736(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 7.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Cutting edge: diminished T cell TLR expression and function modulates the immune response in human filarial infection. J Immunol. 2006;176:3885–3889. doi: 10.4049/jimmunol.176.7.3885. [DOI] [PubMed] [Google Scholar]
- 8.Semnani RT, Liu AY, Sabzevari H, Kubofcik J, Zhou J, et al. Brugia malayi microfilariae induce cell death in human dendritic cells, inhibit their ability to make IL-12 and IL-10, and reduce their capacity to activate CD4+ T cells. J Immunol. 2003;171:1950–1960. doi: 10.4049/jimmunol.171.4.1950. [DOI] [PubMed] [Google Scholar]
- 9.Jenson JS, O'Connor R, Osborne J, Devaney E. Infection with Brugia microfilariae induces apoptosis of CD4(+) T lymphocytes: a mechanism of immune unresponsiveness in filariasis. Eur J Immunol. 2002;32:858–867. doi: 10.1002/1521-4141(200203)32:3<858::AID-IMMU858>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 10.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J Immunol. 2006;176:3248–3256. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- 11.Steel C, Nutman TB. CTLA-4 in Filarial Infections: Implications for a Role in Diminished T Cell Reactivity. J Immunol. 2003;170:1930–1938. doi: 10.4049/jimmunol.170.4.1930. [DOI] [PubMed] [Google Scholar]
- 12.Leng Q, Bentwich Z, Borkow G. Increased TGF-beta, Cbl-b and CTLA-4 levels and immunosuppression in association with chronic immune activation. Int Immunol. 2006;18:637–644. doi: 10.1093/intimm/dxh375. [DOI] [PubMed] [Google Scholar]
- 13.Harnett W, Harnett MM. Immunomodulatory activity and therapeutic potential of the filarial nematode secreted product, ES-62. Adv Exp Med Biol. 2009;666:88–94. doi: 10.1007/978-1-4419-1601-3_7. [DOI] [PubMed] [Google Scholar]
- 14.Schierack P, Lucius R, Sonnenburg B, Schilling K, Hartmann S. Parasite-specific immunomodulatory functions of filarial cystatin. Infect Immun. 2003;71:2422–2429. doi: 10.1128/IAI.71.5.2422-2429.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metenou S, Dembele B, Konate S, Dolo H, Coulibaly SY, et al. Patent filarial infection modulates malaria-specific type 1 cytokine responses in an IL-10-dependent manner in a filaria/malaria-coinfected population. J Immunol. 2009;183:916–924. doi: 10.4049/jimmunol.0900257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babu S, Bhat SQ, Kumar NP, Jayantasri S, Rukmani S, et al. Human type 1 and 17 responses in latent tuberculosis are modulated by coincident filarial infection through cytotoxic T lymphocyte antigen-4 and programmed death-1. J Infect Dis. 2009;200:288–298. doi: 10.1086/599797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen NO, Simonsen PE, Dalgaard P, Krarup H, Magnussen P, et al. Effect of diethylcarbamazine on HIV load, CD4%, and CD4/CD8 ratio in HIV-infected adult Tanzanians with or without lymphatic filariasis: randomized double-blind and placebo-controlled cross-over trial. Am J Trop Med Hyg. 2007;77:507–513. [PubMed] [Google Scholar]
- 18.van den Biggelaar AH, van Ree R, Rodrigues LC, Lell B, Deelder AM, et al. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. 2000;356:1723–1727. doi: 10.1016/S0140-6736(00)03206-2. [DOI] [PubMed] [Google Scholar]
- 19.Cooper PJ, Chico ME, Bland M, Griffin GE, Nutman TB. Allergic symptoms, atopy, and geohelminth infections in a rural area of ecuador. Am J Respir Crit Care Med. 2003;168:313–317. doi: 10.1164/rccm.200211-1320OC. [DOI] [PubMed] [Google Scholar]
- 20.Cooper PJ, Espinel I, Paredes W, Guderian RH, Nutman TB. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J Infect Dis. 1998;178:1133–1138. doi: 10.1086/515661. [DOI] [PubMed] [Google Scholar]
- 21.Sabin EA, Araujo MI, Carvalho EM, Pearce EJ. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis. 1996;173:269–272. doi: 10.1093/infdis/173.1.269. [DOI] [PubMed] [Google Scholar]
- 22.Cooper PJ, Chico M, Sandoval C, Espinel I, Guevara A, et al. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect Immun. 2001;69:1574–1580. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crawley AM, Faucher S, Angel JB. Soluble IL-7R alpha (sCD127) inhibits IL-7 activity and is increased in HIV infection. J Immunol. 2010;184:4679–4687. doi: 10.4049/jimmunol.0903758. [DOI] [PubMed] [Google Scholar]
- 24.Lang A, Brien JD, Messaoudi I, Nikolich-Zugich J. Age-related dysregulation of CD8+ T cell memory specific for a persistent virus is independent of viral replication. J Immunol. 2008;180:4848–4857. doi: 10.4049/jimmunol.180.7.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercier F, Boulassel MR, Yassine-Diab B, Tremblay C, Bernard NF, et al. Persistent human immunodeficiency virus-1 antigenaemia affects the expression of interleukin-7Ralpha on central and effector memory CD4+ and CD8+ T cell subsets. Clin Exp Immunol. 2008;152:72–80. doi: 10.1111/j.1365-2249.2008.03610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okoye A, Meier-Schellersheim M, Brenchley JM, Hagen SI, Walker JM, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009;19:47–56. doi: 10.1002/rmv.598. [DOI] [PubMed] [Google Scholar]
- 28.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Waller EC, Day E, Sissons JG, Wills MR. Dynamics of T cell memory in human cytomegalovirus infection. Med Microbiol Immunol. 2008;197:83–96. doi: 10.1007/s00430-008-0082-5. [DOI] [PubMed] [Google Scholar]
- 30.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albareda MC, Laucella SA, Alvarez MG, Armenti AH, Bertochi G, et al. Trypanosoma cruzi modulates the profile of memory CD8+ T cells in chronic Chagas' disease patients. Int Immunol. 2006;18:465–471. doi: 10.1093/intimm/dxh387. [DOI] [PubMed] [Google Scholar]
- 32.Bhadra R, Guan H, Khan IA. Absence of both IL-7 and IL-15 severely impairs the development of CD8 T cell response against Toxoplasma gondii. PLoS One. 2010;5:e10842. doi: 10.1371/journal.pone.0010842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bustamante JM, Bixby LM, Tarleton RL. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat Med. 2008;14:542–550. doi: 10.1038/nm1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu D, Zhang T, Marshall AJ, Okkenhaug K, Vanhaesebroeck B, et al. The p110delta isoform of phosphatidylinositol 3-kinase controls susceptibility to Leishmania major by regulating expansion and tissue homing of regulatory T cells. J Immunol. 2009;183:1921–1933. doi: 10.4049/jimmunol.0901099. [DOI] [PubMed] [Google Scholar]
- 35.Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat Med. 2004;10:1104–1110. doi: 10.1038/nm1108. [DOI] [PubMed] [Google Scholar]
- 36.Zaph C, Rook KA, Goldschmidt M, Mohrs M, Scott P, et al. Persistence and function of central and effector memory CD4+ T cells following infection with a gastrointestinal helminth. J Immunol. 2006;177:511–518. doi: 10.4049/jimmunol.177.1.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 38.Boutboul F, Puthier D, Appay V, Pelle O, Ait-Mohand H, et al. Modulation of interleukin-7 receptor expression characterizes differentiation of CD8 T cells specific for HIV, EBV and CMV. AIDS. 2005;19:1981–1986. doi: 10.1097/01.aids.0000191919.24185.46. [DOI] [PubMed] [Google Scholar]
- 39.Messaoudi I, Warner J, Nikolich-Zugich J. Age-related CD8+ T cell clonal expansions express elevated levels of CD122 and CD127 and display defects in perceiving homeostatic signals. J Immunol. 2006;177:2784–2792. doi: 10.4049/jimmunol.177.5.2784. [DOI] [PubMed] [Google Scholar]
- 40.Shen T, Chen X, Xu Q, Lu F, Liu S. Distributional characteristics of CD25 and CD127 on CD4+ T cell subsets in chronic HCV infection. Arch Virol. 2010;155:627–634. doi: 10.1007/s00705-010-0626-z. [DOI] [PubMed] [Google Scholar]
- 41.Wherry EJ, Day CL, Draenert R, Miller JD, Kiepiela P, et al. HIV-specific CD8 T cells express low levels of IL-7Ralpha: implications for HIV-specific T cell memory. Virology. 2006;353:366–373. doi: 10.1016/j.virol.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 43.Burgers WA, Riou C, Mlotshwa M, Maenetje P, de Assis Rosa D, et al. Association of HIV-specific and total CD8+ T memory phenotypes in subtype C HIV-1 infection with viral set point. J Immunol. 2009;182:4751–4761. doi: 10.4049/jimmunol.0803801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maenetje P, Riou C, Casazza JP, Ambrozak D, Hill B, et al. A steady state of CD4+ T cell memory maturation and activation is established during primary subtype C HIV-1 infection. J Immunol. 2010;184:4926–4935. doi: 10.4049/jimmunol.0903771. [DOI] [PubMed] [Google Scholar]
- 45.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 46.Sallusto F, Kremmer E, Palermo B, Hoy A, Ponath P, et al. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol. 1999;29:2037–2045. doi: 10.1002/(SICI)1521-4141(199906)29:06<2037::AID-IMMU2037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 47.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 48.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Lefrancois L. Development, trafficking, and function of memory T-cell subsets. Immunol Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ottesen EA, Weller PF, Lunde MN, Hussain R. Endemic filariasis on a Pacific Island. II. Immunologic aspects: immunoglobulin, complement, and specific antifilarial IgG, IgM, and IgE antibodies. Am J Trop Med Hyg. 1982;31:953–961. [PubMed] [Google Scholar]
- 52.Steel C, Guinea A, Ottesen EA. Evidence for protective immunity to bancroftian filariasis in the Cook Islands. J Infect Dis. 1996;174:598–605. doi: 10.1093/infdis/174.3.598. [DOI] [PubMed] [Google Scholar]
- 53.Steel C, Ottesen EA. Evolution of immunologic responsiveness of persons living in an area of endemic bancroftian filariasis: a 17-year follow-up. J Infect Dis. 2001;184:73–79. doi: 10.1086/321004. [DOI] [PubMed] [Google Scholar]
- 54.Weller PF, Ottesen EA, Heck L, Tere T, Neva FA. Endemic filariasis on a Pacific island. I. Clinical, epidemiologic, and parasitologic aspects. Am J Trop Med Hyg. 1982;31:942–952. doi: 10.4269/ajtmh.1982.31.942. [DOI] [PubMed] [Google Scholar]
- 55.Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry. 2001;45:194–205. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 56.Faucher S, Crawley AM, Decker W, Sherring A, Bogdanovic D, et al. Development of a quantitative bead capture assay for soluble IL-7 receptor alpha in human plasma. PLoS One. 2009;4:e6690. doi: 10.1371/journal.pone.0006690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arens R, Tesselaar K, Baars PA, van Schijndel GM, Hendriks J, et al. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNgamma-mediated B cell depletion. Immunity. 2001;15:801–812. doi: 10.1016/s1074-7613(01)00236-9. [DOI] [PubMed] [Google Scholar]
- 58.Albareda MC, Olivera GC, Laucella SA, Alvarez MG, Fernandez ER, et al. Chronic human infection with Trypanosoma cruzi drives CD4+ T cells to immune senescence. J Immunol. 2009;183:4103–4108. doi: 10.4049/jimmunol.0900852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colpitts SL, Scott P. The early generation of a heterogeneous CD4+ T cell response to Leishmania major. J Immunol. 2010;185:2416–2423. doi: 10.4049/jimmunol.1000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, et al. Helminth parasites–masters of regulation. Immunol Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 61.Nutman TB, Kumaraswami V, Ottesen EA. Parasite-specific anergy in human filariasis. Insights after analysis of parasite antigen-driven lymphokine production. J Clin Invest. 1987;79:1516–1523. doi: 10.1172/JCI112982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gollob KJ, Antonelli LR, Faria DR, Keesen TS, Dutra WO. Immunoregulatory mechanisms and CD4−CD8− (double negative) T cell subpopulations in human cutaneous leishmaniasis: a balancing act between protection and pathology. Int Immunopharmacol. 2008;8:1338–1343. doi: 10.1016/j.intimp.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 64.Ouyang Q, Wagner WM, Voehringer D, Wikby A, Klatt T, et al. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1). Exp Gerontol. 2003;38:911–920. doi: 10.1016/s0531-5565(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 65.Bruns H, Meinken C, Schauenberg P, Harter G, Kern P, et al. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119:1167–1177. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pawelec G, Koch S, Franceschi C, Wikby A. Human immunosenescence: does it have an infectious component? Ann N Y Acad Sci. 2006;1067:56–65. doi: 10.1196/annals.1354.009. [DOI] [PubMed] [Google Scholar]
- 67.Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo. J Exp Med. 2005;201:579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 69.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 70.Fuller MJ, Hildeman DA, Sabbaj S, Gaddis DE, Tebo AE, et al. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J Immunol. 2005;174:5926–5930. doi: 10.4049/jimmunol.174.10.5926. [DOI] [PubMed] [Google Scholar]
- 71.Lang KS, Recher M, Navarini AA, Harris NL, Lohning M, et al. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur J Immunol. 2005;35:738–745. doi: 10.1002/eji.200425828. [DOI] [PubMed] [Google Scholar]
- 72.Xue HH, Kovanen PE, Pise-Masison CA, Berg M, Radovich MF, et al. IL-2 negatively regulates IL-7 receptor alpha chain expression in activated T lymphocytes. Proc Natl Acad Sci U S A. 2002;99:13759–13764. doi: 10.1073/pnas.212214999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hammerbeck CD, Mescher MF. Antigen controls IL-7R alpha expression levels on CD8 T cells during full activation or tolerance induction. J Immunol. 2008;180:2107–2116. doi: 10.4049/jimmunol.180.4.2107. [DOI] [PubMed] [Google Scholar]
- 74.Zhang X, Fujii H, Kishimoto H, LeRoy E, Surh CD, et al. Aging leads to disturbed homeostasis of memory phenotype CD8(+) cells. J Exp Med. 2002;195:283–293. doi: 10.1084/jem.20011267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chandele A, Joshi NS, Zhu J, Paul WE, Leonard WJ, et al. Formation of IL-7Ralpha high and IL-7Ralpha low CD8 T cells during infection is regulated by the opposing functions of GABPalpha and Gfi-1. J Immunol. 2008;180:5309–5319. doi: 10.4049/jimmunol.180.8.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]