Abstract
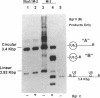
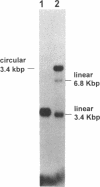
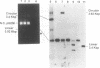
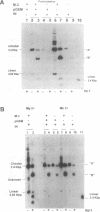
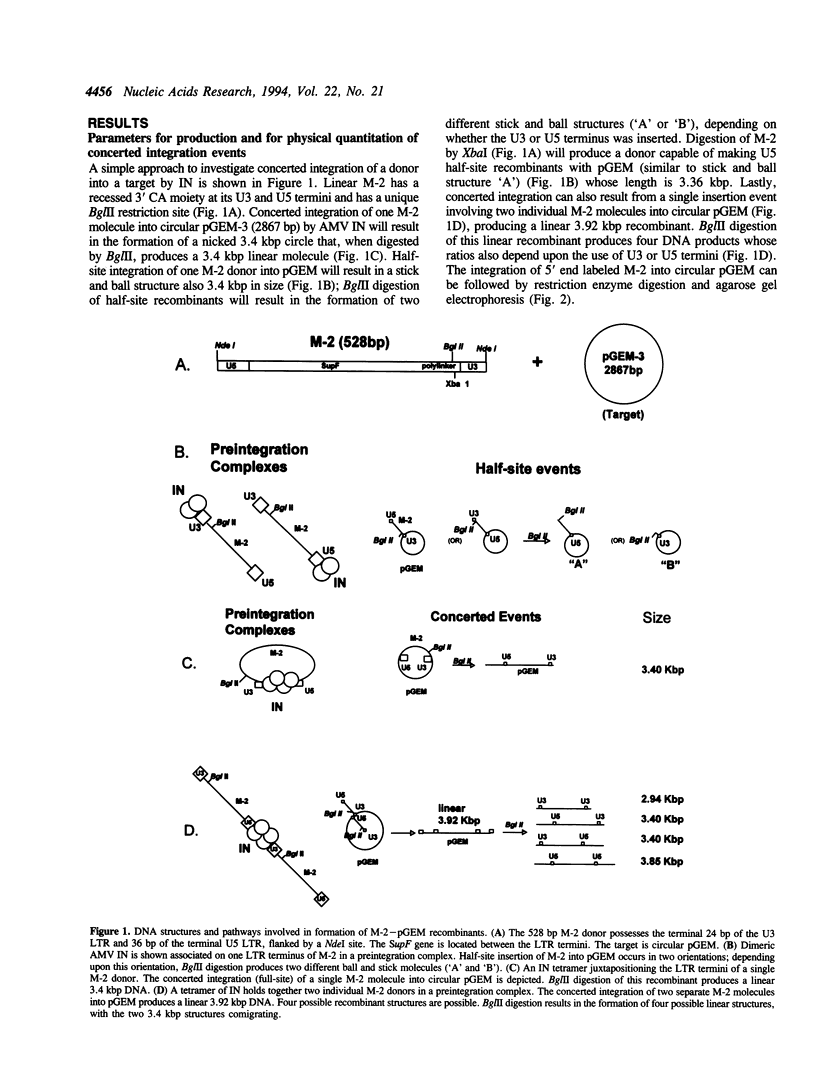
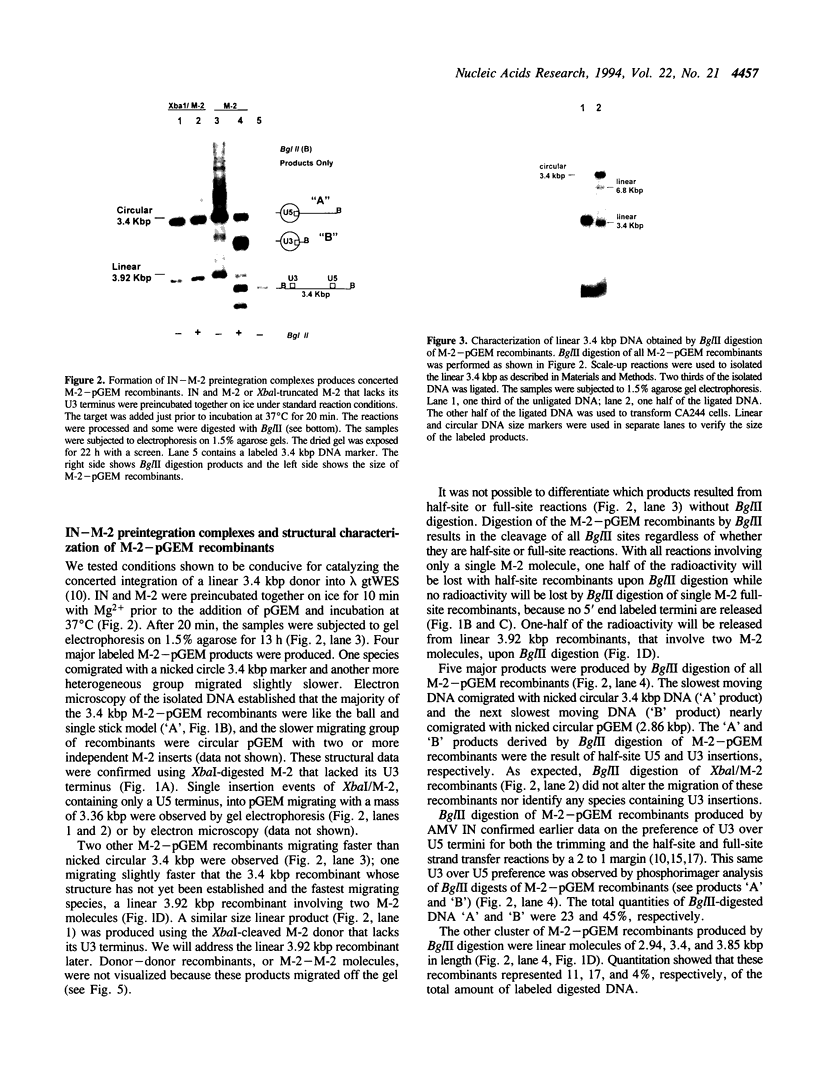
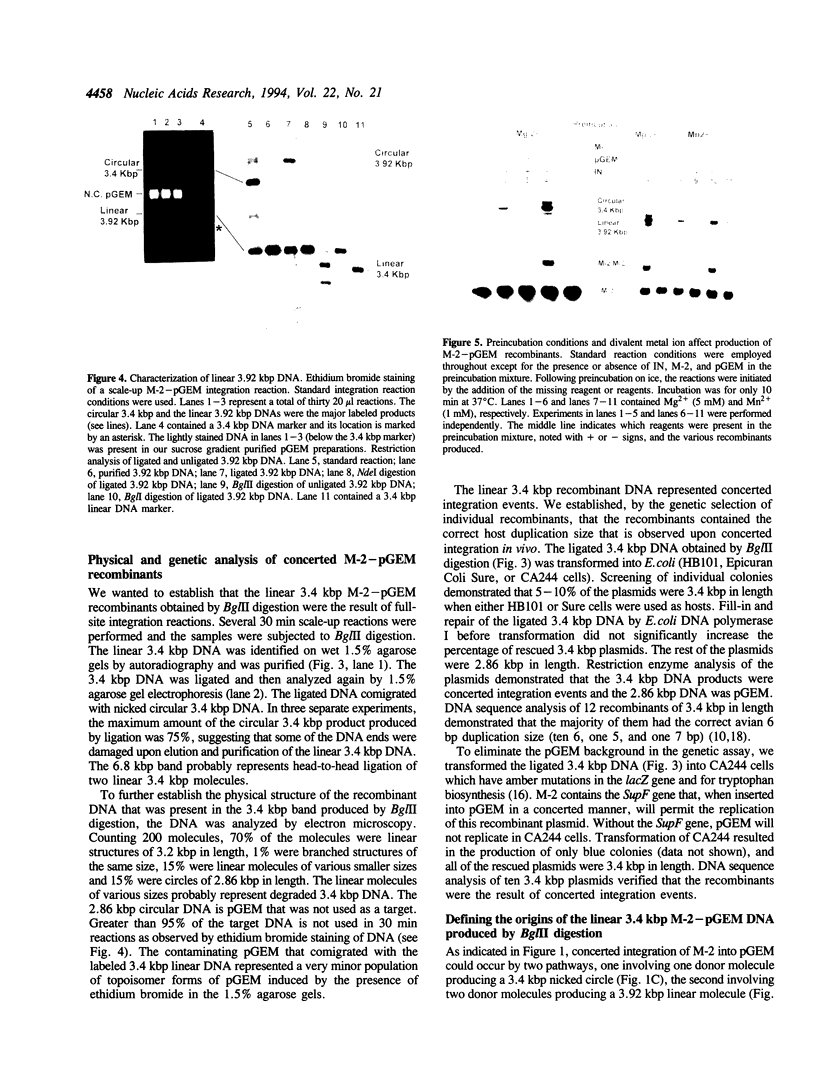
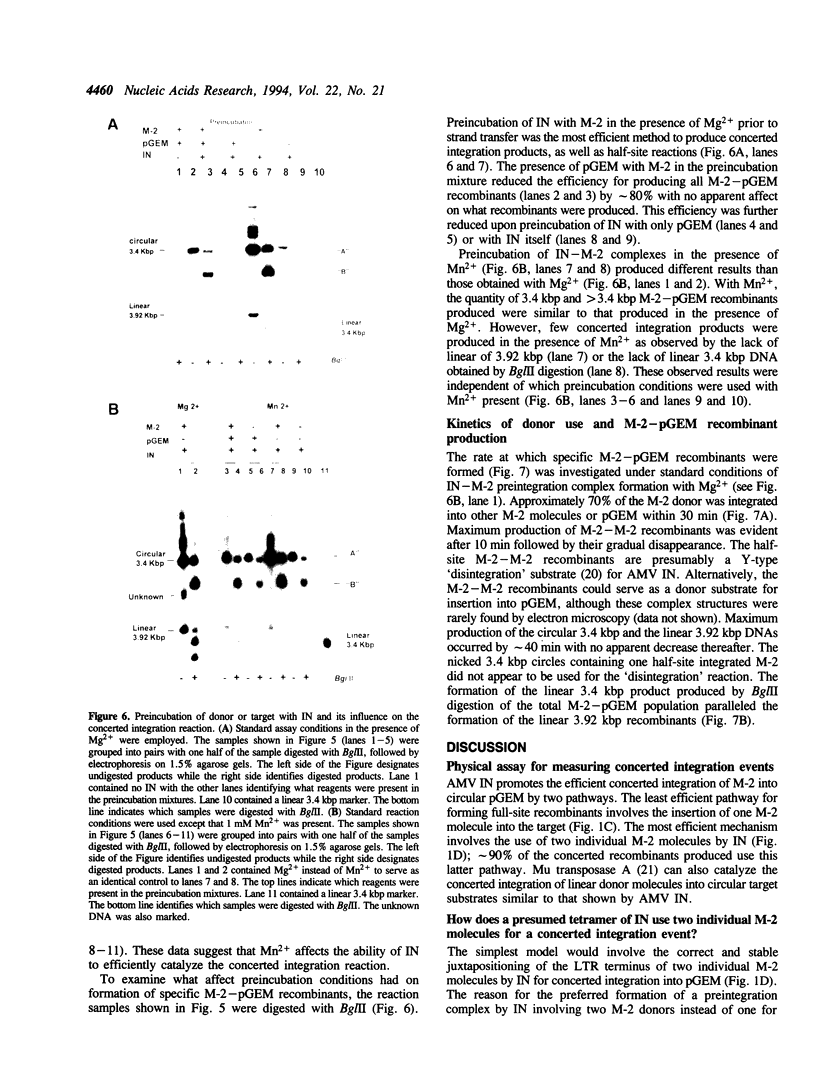
We report the efficient concerted integration of a linear virus-like DNA donor into a 2.8 kbp circular DNA target by integrase (IN) purified from avian myeloblastosis virus. The donor was 528 bp, contained recessed 3' OH ends, was 5' end labeled, and had a unique restriction site not found in the target. Analysis of concerted (full-site) and half-site integration events was accomplished by restriction enzyme analysis and agarose gel electrophoresis. The donor also contained the SupF gene that was used for genetic selection of individual full-site recombinants to determine the host duplication size. Two different pathways, involving either one donor or two donor molecules, were used to produce full-site recombinants. About 90% of the full-site recombinants were the result of using two donor molecules per target. These results imply that juxtapositioning an end from each of two donors by IN was more efficient than the juxtapositioning of two ends of a single donor for the full-site reaction. The formation of preintegration complexes containing integrase and donor on ice prior to the addition of target enhanced the full-site reaction. After a 30 min reaction at 37 degrees C, approximately 20-25% of all donor/target recombinants were the result of concerted integration events. The efficient production of full-site recombinants required Mg2+; Mn2+ was only efficient for the production of half-site recombinants. We suggest that these preintegration complexes can be used to investigate the relationships between the 3' OH trimming and strand transfer reactions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowerman B., Brown P. O., Bishop J. M., Varmus H. E. A nucleoprotein complex mediates the integration of retroviral DNA. Genes Dev. 1989 Apr;3(4):469–478. doi: 10.1101/gad.3.4.469. [DOI] [PubMed] [Google Scholar]
- Brown P. O., Bowerman B., Varmus H. E., Bishop J. M. Correct integration of retroviral DNA in vitro. Cell. 1987 May 8;49(3):347–356. doi: 10.1016/0092-8674(87)90287-x. [DOI] [PubMed] [Google Scholar]
- Bushman F. D., Fujiwara T., Craigie R. Retroviral DNA integration directed by HIV integration protein in vitro. Science. 1990 Sep 28;249(4976):1555–1558. doi: 10.1126/science.2171144. [DOI] [PubMed] [Google Scholar]
- Chow S. A., Vincent K. A., Ellison V., Brown P. O. Reversal of integration and DNA splicing mediated by integrase of human immunodeficiency virus. Science. 1992 Feb 7;255(5045):723–726. doi: 10.1126/science.1738845. [DOI] [PubMed] [Google Scholar]
- Craigie R., Mizuuchi K. Transposition of Mu DNA: joining of Mu to target DNA can be uncoupled from cleavage at the ends of Mu. Cell. 1987 Nov 6;51(3):493–501. doi: 10.1016/0092-8674(87)90645-3. [DOI] [PubMed] [Google Scholar]
- Engelman A., Bushman F. D., Craigie R. Identification of discrete functional domains of HIV-1 integrase and their organization within an active multimeric complex. EMBO J. 1993 Aug;12(8):3269–3275. doi: 10.1002/j.1460-2075.1993.tb05996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnet C. M., Haseltine W. A. Determination of viral proteins present in the human immunodeficiency virus type 1 preintegration complex. J Virol. 1991 Apr;65(4):1910–1915. doi: 10.1128/jvi.65.4.1910-1915.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. L., Grandgenett D. P. Retroviral integration: in vitro host site selection by avian integrase. J Virol. 1994 Jul;68(7):4314–4321. doi: 10.1128/jvi.68.7.4314-4321.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. L., Vora A. C., Grandgenett D. P. Development of an acid-soluble assay for measuring retrovirus integrase 3'-OH terminal nuclease activity. Anal Biochem. 1991 Jul;196(1):19–23. doi: 10.1016/0003-2697(91)90111-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. L., Vora A. C., Zeh W. G., Grandgenett D. P. Concerted integration of viral DNA termini by purified avian myeloblastosis virus integrase. J Virol. 1992 Nov;66(11):6257–6263. doi: 10.1128/jvi.66.11.6257-6263.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Mizuuchi K. Retroviral DNA integration: structure of an integration intermediate. Cell. 1988 Aug 12;54(4):497–504. doi: 10.1016/0092-8674(88)90071-2. [DOI] [PubMed] [Google Scholar]
- Grandgenett D. P., Inman R. B., Vora A. C., Fitzgerald M. L. Comparison of DNA binding and integration half-site selection by avian myeloblastosis virus integrase. J Virol. 1993 May;67(5):2628–2636. doi: 10.1128/jvi.67.5.2628-2636.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Vora A. C., Schiff R. D. A 32,000-dalton nucleic acid-binding protein from avian retravirus cores possesses DNA endonuclease activity. Virology. 1978 Aug;89(1):119–132. doi: 10.1016/0042-6822(78)90046-6. [DOI] [PubMed] [Google Scholar]
- Hochschild A. Detecting cooperative protein-DNA interactions and DNA loop formation by footprinting. Methods Enzymol. 1991;208:343–361. doi: 10.1016/0076-6879(91)08019-e. [DOI] [PubMed] [Google Scholar]
- Katz R. A., Merkel G., Kulkosky J., Leis J., Skalka A. M. The avian retroviral IN protein is both necessary and sufficient for integrative recombination in vitro. Cell. 1990 Oct 5;63(1):87–95. doi: 10.1016/0092-8674(90)90290-u. [DOI] [PubMed] [Google Scholar]
- Katzman M., Katz R. A., Skalka A. M., Leis J. The avian retroviral integration protein cleaves the terminal sequences of linear viral DNA at the in vivo sites of integration. J Virol. 1989 Dec;63(12):5319–5327. doi: 10.1128/jvi.63.12.5319-5327.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y., Lee Y. M., Coffin J. M. Nonrandom integration of retroviral DNA in vitro: effect of CpG methylation. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5532–5536. doi: 10.1073/pnas.89.12.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. E., Goff S. P. A mutation at one end of Moloney murine leukemia virus DNA blocks cleavage of both ends by the viral integrase in vivo. J Virol. 1992 Aug;66(8):5092–5095. doi: 10.1128/jvi.66.8.5092-5095.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P. A., Fyfe J. A. Human immunodeficiency virus integration protein expressed in Escherichia coli possesses selective DNA cleaving activity. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5119–5123. doi: 10.1073/pnas.87.13.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D., Langowski J., Baldwin R. L. DNA flexibility studied by covalent closure of short fragments into circles. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4833–4837. doi: 10.1073/pnas.78.8.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink C., van der Linden K. H., Plasterk R. H. Activities of the feline immunodeficiency virus integrase protein produced in Escherichia coli. J Virol. 1994 Mar;68(3):1468–1474. doi: 10.1128/jvi.68.3.1468-1474.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora A. C., Fitzgerald M. L., Grandgenett D. P. Removal of 3'-OH-terminal nucleotides from blunt-ended long terminal repeat termini by the avian retrovirus integration protein. J Virol. 1990 Nov;64(11):5656–5659. doi: 10.1128/jvi.64.11.5656-5659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gent D. C., Vink C., Groeneger A. A., Plasterk R. H. Complementation between HIV integrase proteins mutated in different domains. EMBO J. 1993 Aug;12(8):3261–3267. doi: 10.1002/j.1460-2075.1993.tb05995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]