Abstract
A diastereoselective synthesis of trans-1,2-substituted cyclopropanols is outlined. Bimetallic CH2(ZnI)2 was found to react with α-chloroaldehydes to give cyclopropanols in yields of 64–89% and dr’s ≥ 10:1. The high trans-selectivity resulted from equilibration of the cyclopropoxide intermediates.
Cyclopropanes form an integral part of over 100 therapeutic agents1-3 and exhibit a broad spectrum of biological properties.1,4-6 They are frequently found in natural products, including pheromones, steroids, terpenes, fatty acid metabolites, and amino acids.1,6 Their medicinal properties and synthetic utility have inspired numerous preparations.1,4,7-12 One class of cyclopropanes that has received less attention is cyclopropanols.
Previous approaches to cyclopropanols include the Kulinkovich reaction using titanium tetraisopropoxide, Simmons-Smith cyclopropanation of silyl-enol ethers,13 chromium(II) mediated cyclopropanation of α,β-unsaturated ketones,14 and reaction of samarium with diiodomethane and ketones or esters.15 Recently, there has been significant interest in the stereoselective synthesis of cyclopropyl boronates as precursors to cyclopropanols. Pietruszka and coworkers have studied diastereoselective cyclopropanation of vinyl boronates with stoichiometric chiral auxiliaries on boron10 while the groups of Ito and Gevorgyan have both developed metal-catalyzed asymmetric methods.11 Ito’s method involves Cu(I) catalyzed reaction of bis(pinacolato)diboron with allylic carbonates and phosphates to form cyclopropyl boronates while Gevorgyan employs rhodium catalyzed hydroborations of cyclopropenes. We developed highly diastereoselective cyclopropanation of 2-B(pin)-substituted allylic alcohols and their oxidation to cyclopropanols.16
In this Letter, we describe a highly diastereoselective conversion of α-chloroaldehydes into trans-cyclopropanols using the readily prepared dizinc reagent CH2(ZnI)2. We demonstrate that the high trans-diastereoselectivity arises from equilibration of the cis-and trans-cyclopropoxide intermediates via a proposed transient homoenolate.
Methylene bis(iodozinc) derivatives have attracted attention,17-22 because the two Zn–C bonds can be used to form two C–C bonds. Methylene bis(iodozinc) is conveniently prepared by mixing diiodomethane, zinc dust, and a catalytic amount of lead chloride.23 Matsubara and coworkers have demonstrated that α-diketones and α-ketoimines react with CH2(ZnI)2 to form cis-cyclopropan-1,2-diols and cis-2-aminocyclopropanols, respectively (Scheme 1).19
Scheme 1.

Matsubara’s methylene bis(iodozinc) addition to α-diketones and α-ketoimines.19
The same group reported that treatment of enantioenriched α-sulfonyloxy ketones with CH2(ZnI)2 gave a zinc cyclopropoxide intermediate that furnished cyclopropanol with low dr on workup (dr = 76:24 to 67:33).20 An important feature of cyclopropoxy zinc and copper complexes is their reversible ring opening to generate homoenolate intermediates. Thus, despite the low diastereoselectivity of cyclopropoxide formation in Scheme 2a, addition of Cu(CN)•2LiCl allowed trapping of the homoenolate with allyl bromide. The ee of the sulfonyloxy ketone is conserved in the homoenolate formation and the subsequent reaction with electrophiles.20b In contrast, when CH2(ZnI)2 reacted with α,β-epoxyketones (Scheme 2b), formation of the cyclopropanol occurred with high diastereoselectivity. The authors proposed that initial attack on the ketone occurred with high diastereoselectivity by a chelation-controlled pathway.21
Scheme 2.

Reaction of CH2(ZnI)2 with α-sulfonyloxy ketones20 and α,β-epoxy ketones.21
Based on the reversible formation of homoenolates from cyclopropyl alkoxide derivatives,24 we envisioned that trans-disubstituted cyclopropanols could be accessed with high diastereoselectivity by addition of CH2(ZnI)2 to α-haloaldehydes.
Racemic α-bromo- and α-chloroaldehydes were prepared according to the methods of Pagnoni25 and Jørgensen and coworkers.26 The optimization of the cyclopropanol formation was performed as outlined in Table 1. Initial studies were conducted with 2-bromooctanal. Reaction with one equiv of CH2(ZnI)2 at 0 °C resulted in the formation of the desired cyclopropanol in 58% yield with a dr of 6:1 (entry 1). The initial assignment of the major diastereomer as the trans isomer was based on coupling constants in the 1H NMR spectrum. Interestingly, lowering the temperature to −20 °C and doubling the equiv of CH2(ZnI)2 resulted in no diastereoselectivity (entry 2). Initiating the reaction at 0 °C and allowing it to warm slowly to rt with 2.0 or 2.5 equiv CH2(ZnI)2 resulted in an increase in the diastereoselectivity to >18:1 and yields reaching 71% (entries 3 and 4). Employing CH2(ZnBr)2 under similar conditions led to olefination product (1H NMR, entry 5). Olefination products were not observed by 1H NMR in the crude reaction mixtures throughout these studies when CH2(ZnI)2 was used. Maintaining the temperature at 0 °C with CH2(ZnI)2 resulted in an increase in the yield to 81% with high diastereoselectivity (16:1, entry 6).
Table 1.
Optimization of reaction of CH2(ZnI)2 with α-haloaldehydes.

| R | X | dizinc:aldehyde | temp (°C) | time | yield (dr) | |
|---|---|---|---|---|---|---|
| 1 | n-hexyl | Br | 1:1 | 0 | 1 h | 58% (6:1) |
| 2 | Br | 2:1 | −20 | 40 min | 32% (1:1) | |
| 3 | Br | 2:1 | 0 to rt | 8 h | 64% (>20:1) | |
| 4 | Br | 2.5:1 | 0 to rt | 8 h | 71% (18:1) | |
| 5 | Br | 2.5:1 | 0 to rt | 8 h | 0%a | |
| 6 | Br | 2:1 | 0 | 1 h | 81% (16:1) | |
| 7 | Cl | 2:1 | 0 | 1 h | 88% (18:1) | |
| 8 | Cl | 2:1 | −20 | 1.5 h | 48% (2:1) | |
| 9 | Cl | 2:1 | −40 | 1.5 h | 24% (1:1) | |
| 10 | benzyl | Br | 2:1 | 0 | 1 h | 74% (13:1) |
| 11 | Br | 2:1 | 0 to rt | 8 h | 0%a | |
| 12 | Cl | 2:1 | 0 | 1 h | 73% (12:1) |
Reactions employing CH2(ZnBr)2. 1H NMR indicated mostly methylenation product.
We then chose to examine 2-chlorooctanal. Addition of 2 equiv CH2(ZnI)2 to the chloroaldehyde at 0 °C resulted in formation of the desired product with high diastereoselectivity (18:1) and 88% yield. Lowering the temperature again resulted in a significant drop in diastereoselectivity (entries 8 and 9). The reaction was also optimized with α-halodihydrocinnamaldehydes. Using the α–bromo derivatives, the reaction with CH2(ZnI)2 gave the desired product with 74% yield and 13:1 dr (entry 10). The α-chloro analogue gave comparable results with CH2(ZnI)2. Based on the results in Table 1, we elected to pursue the chemistry with the more readily available α-chloro aldehydes.
With suitable conditions in hand, we examined a variety of α-chloroaldehydes (Table 2). In addition to 2-chlorooctanal and 2-chlorodihydrocinnamaldehyde, other simple alkyl substituted chloroaldehydes were amenable to this method (entries 1–5). The dr’s of the cyclopropanol products were ≥11:1 and yields ranged from 64–88%. The compound in entry 2 is previously known27 and our NMR data matched the literature values, supporting the assignment of the trans stereochemistry. In addition, the cyclopropanols were independently synthesized as a mixture of diastereomers by Takai’s method28 to facilitate the NMR assignments and determination of dr (see SI for more details). 2-Chloroaldehydes with β- or γ-hydroxy groups protected as silyl or trityl ethers gave cyclopropanols in good yields (61–89%) and with high diastereoselectivities (dr≥10:1, entries 6–10). To further support the stereochemical assignment of the cyclopropanols, the structure of the product in entry 10 was confirmed by X-ray analysis (see SI). As shown in entry 11, the indole-containing substrate furnished the cyclopropanol with 11:1 dr in 66% yield. Reaction with α-bromoketones was largely unsuccessful as previously reported.20b Enantioenriched 2-chlorodihydrocinnamaldehyde29 (95% ee) underwent reaction to give 2-benzylcyclopropanol of 95% ee.
Table 2.
Diastereoselective synthesis of trans-cyclopropanols using CH2(ZnI)2.

| R = | product | yield (dr)a | |
|---|---|---|---|
| 1) | n-hexyl |
|
88% (18:1) |
| 2) | n-butyl |
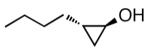
|
87% (11:1) |
| 3) | allyl |

|
65% (13:1) |
| 4) | benzyl |

|
73% (12:1) |
| 5) | cyclohexyl |

|
64% (16:1) |
| 6) |

|
|
83% (10:1) |
| 7) | TIPSOCH2 |
|
71% (12:1) |
| 8) | TBDPSOCH2 |
|
89% (11:1) |
| 9) |

|

|
86% (13:1) |
| 10) |

|

|
61% (>19:1) |
| 11) |

|

|
66% (11:1) |
dr determined by 1H NMR of the crude reaction mixture.
We envisioned two possible mechanisms to explain the observed predominance of the trans diastereomer. The first involves diastereoselective carbonyl addition by the dizinc reagent. Addition must occur with chelation-controlled to favor the trans product.30 This seemes unlikely, because chelation of α-halo carbonyl groups is improbable, especially in THF.31 A second mechanism involves addition of the dizinc reagent to α-halo aldehydes to generate mixtures of diastereomers (Scheme 3). Subsequent SN2 displacement of the halide gives a mixture of the cis- and trans-cyclopropoxides. Ring opening generates a transient homoenolate20,32 and allows equilibration of the diastereomers. The equilibrium favors the trans-cyclopropoxide on steric grounds.
Scheme 3.

Proposed mechanism for equilibration of cis- and trans-cyclopropoxides.
To test the latter mechanism, a 3:1 mixture of cis- and trans-2-hexylcyclopropanol was treated with 1.0 equiv of CH2(ZnI)2 at 0 °C to form the corresponding zinc alkoxide. Upon quenching the reaction mixture, trans cyclopropanol was observed by 1H NMR (16:1 trans:cis and 69% isolated yield). In a separate experiment, a 1:2 mixture of cis- and trans-2-hexylcyclopropanol was added to 2-chlorodihydrocinnamaldehyde and 2.0 equiv of dizinc reagent under conditions similar to those in Table 2. The 1H NMR spectrum of the crude reaction mixture showed that the dr of the 2-hexylcyclopropanol had increased to 12:1. The dr of 2-benzylcyclopropanol was 8:1. We hypothesize that the low dr’s in Table 1 (entries 2, 8, and 9) are due to the slow equilibration of the isomeric cyclopropoxides at low temperature and the high diastereoselectivity arises from equilibration of the cyclopropoxides, which favor the trans-isomer to minimize steric interactions.
In summary, a simple and highly diastereoselective route to trans-cyclopropanols was developed using CH2(ZnI)2 and α -chloro and α-bromoaldehydes. Experimental observations indicate that the high diastereoselectivity arises from equilibration of the diastereomeric cyclopropoxides to the more stable trans isomer via a transient homoenolate intermediate.
Supplementary Material
Acknowledgment
We thank the NSF (CHE-0848467) and NIH (National Institute of General Medical Sciences GM58101) for support of this work. Funds for instrumentation were provided by the NIH for a Waters LCTOF-Xe Premier ESI mass spectrometer (1S10RR023444) and NSF for an X-ray diffractometer (CHE–0840438). We thank Gretchen Stanton (University of Pennsylvania) for the preparation of some α-haloaldehyde substrates.
Footnotes
Supporting Information Available: Procedures and full characterization are available. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1).Pellissier H. Tetrahedron. 2008;64:7041–7095. [Google Scholar]
- 2).Liu HW, Walsh CT. In: The Chemistry of the Cyclopropyl Group. Rappoport Z, editor. John Wiley; New York, NY: 1997. p. 959. [Google Scholar]
- 3).Djerassi C, Doss GA. New J. Chem. 1990;14:713–719. [Google Scholar]
- 4) (a).Pietruszka J. Chem. Rev. 2003;103:1051–1070. doi: 10.1021/cr010027g. [DOI] [PubMed] [Google Scholar]; (b) Lebel H, Marcoux JF, Molinaro C, Charette AB. Chem. Rev. 2003;103:977–1050. doi: 10.1021/cr010007e. [DOI] [PubMed] [Google Scholar]; (c) Wessjohann LA, Brandt W, Thiemann T. Chem. Rev. 2003;103:1625–1648. doi: 10.1021/cr0100188. [DOI] [PubMed] [Google Scholar]
- 5).Donaldson WA. Tetrahedron. 2001;57:8589–8627. [Google Scholar]
- 6).Salaun J. Top. Curr. Chem. 2000;207:1–67. [Google Scholar]
- 7).Charette AB, Marcoux JF. Synlett. 1995:1197–1207. [Google Scholar]
- 8).Simmons HE, Smith RD. J. Am. Chem. Soc. 1959;81:4256–4264. [Google Scholar]
- 9).Rubin M, Rubina M, Gevorgyan V. Chem. Rev. 2007;107:3117–3179. doi: 10.1021/cr050988l. [DOI] [PubMed] [Google Scholar]
- 10) (a).Hohn E, Palecek J, Pietruszka J, Frey W. Eur. J. Org. Chem. 2009:3765–3782. [Google Scholar]; (b) Luithle JEA, Pietruszka J. J. Org. Chem. 2000;65:9194–9200. doi: 10.1021/jo0056601. [DOI] [PubMed] [Google Scholar]; (c) Luithle JEA, Pietruszka J. Eur. J. Org. Chem. 2000:2557–2562. [Google Scholar]; (d) Luithle JEA, Pietruszka J, Witt A. Chem. Commun. 1998:2651–2652. [Google Scholar]; (e) Pietruszka J, Widenmeyer M. Synlett. 1997:977–979. [Google Scholar]; (f) Imai T, Mineta H, Nishida S. J. Org. Chem. 1990;55:4986–4988. [Google Scholar]
- 11) (a).Zhong C, Kunii S, Kosaka Y, Sawamura M, Ito H. J. Am. Chem. Soc. 2010;132:11440–11442. doi: 10.1021/ja103783p. [DOI] [PubMed] [Google Scholar]; (b) Ito H, Kosaka Y, Nonoyama K, Sasaki Y, Sawamura M. Angew. Chem. Int. Ed. 2008;47:7424–7427. doi: 10.1002/anie.200802342. [DOI] [PubMed] [Google Scholar]; (c) Rubina M, Rubin M, Gevorgyan V. J. Am. Chem. Soc. 2003;125:7198–7199. doi: 10.1021/ja034210y. [DOI] [PubMed] [Google Scholar]
- 12) (a).Díez D, García P, Marcos IS, Garrido NM, Basabe P, Broughton HB, Urones JG. Org. Lett. 2003;5:3687–3690. doi: 10.1021/ol035451d. [DOI] [PubMed] [Google Scholar]; (b) Díez D, García P, Marcos IS, Garrido NM, Basabe P, Urones JG. Synthesis. 2003;1:53–62. doi: 10.1021/ol035451d. [DOI] [PubMed] [Google Scholar]; (c) Díez D, García P, Pacheco MP, Marcos IS, Garrido NM, Basabe P, Urones JG. Synlett. 2002;2:355–357. [Google Scholar]
- 13) (a).Rubottom GM, Lopez MI. J. Org. Chem. 1973;38:2097–2099. [Google Scholar]; (b) Murai S, Aya T, Sonoda N. J. Org. Chem. 1973;38:4354–4356. [Google Scholar]; (c) Du H, Long J, Shi Y. Org. Lett. 2006;8:2827–2829. doi: 10.1021/ol0609659. [DOI] [PubMed] [Google Scholar]
- 14).Toratsu C, Fujii T, Suzuki T, Takai K. Angew. Chem., Int. Ed. 2000;39:2725–2727. [PubMed] [Google Scholar]
- 15) (a).Imamoto T, Takiyama N. Tetrahedron Lett. 1987;28:1307–1308. [Google Scholar]; (b) Imamoto T, Kamiya Y, Hatajima T, Takahashi H. Tetrahedron Lett. 1989;30:5149–5152. [Google Scholar]
- 16).Hussain MM, Li H, Hussain N, Ureña M, Carroll PJ, Walsh PJ. J. Am. Chem. Soc. 2009:6516–6524. doi: 10.1021/ja900147s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17) (a).Zimmer LE, Charette AB. J. Am. Chem. Soc. 2009;131:15624–15626. doi: 10.1021/ja906033g. [DOI] [PubMed] [Google Scholar]; (b) Fournier JF, Mathieu S, Charette AB. J. Am. Chem. Soc. 2005;127:13140–13141. doi: 10.1021/ja054328+. [DOI] [PubMed] [Google Scholar]; (c) Charette AB, Mathieu S, Fournier JF. Synlett. 2005:1779–1782. [Google Scholar]; (d) Fournier JF, Charette AB. Eur. J. Org. Chem. 2004:1401–1404. [Google Scholar]; (e) Charette AB, Lemay J. Angew. Chem. Int. Ed. 1997;36:1090–1092. [Google Scholar]; (f) Charette AB, Lebel H. J. Org. Chem. 1995;60:2966–2967. [Google Scholar]
- 18) (a).Matsubara S, Kawamoto K, Utimoto K. Synlett. 1998:267–268. [Google Scholar]; (b) Matsubara S, Mizuno T, Otake T, Kobata M, Utimoto K, Takai K. Synlett. 1998;12:1369–1371. [Google Scholar]; (c) Matsubara S, Arioka D, Utimoto K. Synlett. 1999;8:1253–1254. [Google Scholar]; (d) Matsubara S, Toda N, Kobata M, Utimoto K. Synlett. 2000:987–988. [Google Scholar]; (e) Hirayama T, Oshima K, Matsubara S. Angew. Chem. Int. Ed. 2005;44:3293–3296. doi: 10.1002/anie.200500133. [DOI] [PubMed] [Google Scholar]; (f) Nomura K, Hirayama T, Matsubara S. Chem. Asian J. 2009;4:1298–1303. doi: 10.1002/asia.200900123. [DOI] [PubMed] [Google Scholar]; (g) Sada M, Matsubara S. J. Am. Chem. Soc. 2010;132:432–433. doi: 10.1021/ja910428y. [DOI] [PubMed] [Google Scholar]; (h) Sada M, Komagawa S, Uchiyama M, Kobata M, Mizuno T, Utimoto K, Oshima K, Matsubara S. J. Am. Chem. Soc. 2010;132:17452–17458. doi: 10.1021/ja104439w. [DOI] [PubMed] [Google Scholar]; (i) Takada Y, Nomura K, Matsubara S. Org. Lett. 2010;12:5204–5205. doi: 10.1021/ol102237b. [DOI] [PubMed] [Google Scholar]
- 19) (a).Ukai K, Oshima K, Matsubara S. J. Am. Chem. Soc. 2000;122:12047–12048. [Google Scholar]; (b) Matsubara S, Ukai K, Fushimi H, Yokota Y, Yoshino H, Oshima K, Omoto K, Ogawa A, Yasunori H, Fujimoto H. Tetrahedron. 2002;58:8255–8262. [Google Scholar]; (c) Nomura K, Oshima K, Matsubara S. Tetrahedron Lett. 2004;45:5957–5959. [Google Scholar]; (d) Nomura K, Asano K, Kurahashi T, Matsubara S. Heterocycles. 2008;76:1381–1399. [Google Scholar]
- 20) (a).Nomura K, Matsubara S. Chem. Lett. 2007;36:164–165. [Google Scholar]; (b) Nomura K, Matsubara S. Chem. Asian J. 2010;5:147–152. doi: 10.1002/asia.200900289. [DOI] [PubMed] [Google Scholar]
- 21) (a).Nomura K, Oshima K, Matsubara S. Angew. Chem. 2005;117:6010–6013. doi: 10.1002/anie.200501644. [DOI] [PubMed] [Google Scholar]; (b) Nomura K, Matsubara S. Chem. Commun. 2009:2212–2213. doi: 10.1039/b901043b. [DOI] [PubMed] [Google Scholar]
- 22).For a mini-review, see: Matsubara S, Oshima K, Utimoto K. J. Organomet. Chem. 2001;617-618:39–46.
- 23) (a).Takai K, Kakiuchi T, Kataoka Y, Utimoto K. J. Org. Chem. 1994;59:2668. [Google Scholar]; (b) Takai K, Kakiuchi T, Utimoto K. J. Org. Chem. 1994;59:2671. [Google Scholar]; (c) Matsubara S, Yoshino H, Yamamoto Y, Oshima K, Matsuoka H, Matsumoto K, Ishikawa K, Matsubara E. J. Organomet. Chem. 2005;690:5546–5551. [Google Scholar]; (d) Matsubara S, Oshima K, Matsuoka H, Matsumoto K, Ishikawa K. Matsubara Chem. Lett. 2005;34:952–953. [Google Scholar]
- 24) (a).Nickon A, Lambert JL. J. Am. Chem. Soc. 1962;84:4604–4605. [Google Scholar]; (b) Nakamura E, Kuwajima I. J. Am. Chem. Soc. 1977;99:7360–7362. [Google Scholar]; (c) Hirai Y, Terada T, Yamazaki T. J. Am. Chem. Soc. 1988;110:958–960. [Google Scholar]; (d) Martins EO, Gleason JL. Org. Lett. 1999;1:1643–1645. [Google Scholar]
- 25).Bellesia F, Ghelfi F, Grandi R, Pagnoni UM. J. Chem. Res. (S) 1986:428–429. [Google Scholar]
- 26).Halland N, Braunton A, Bachmann S, Marigo M, Jørgensen KA. J. Am. Chem. Soc. 2004;126:4790–4791. doi: 10.1021/ja049231m. [DOI] [PubMed] [Google Scholar]
- 27) (a).Imai T, Mineta H, Nishida S. J. Org. Chem. 1990;55:4986–4988. [Google Scholar]; (b) Pietruszka J, Widenmeyer M. Synlett. 1997:977–979. [Google Scholar]; (c) Luithle JEA, Pietruszka J. Liebigs Ann. Recl. 1997:2297–2302. [Google Scholar]; (d) Luithle JEA, Pietruszka J. Eur. J. Org. Chem. 2000:2557–2562. [Google Scholar]
- 28).Takai K, Toshikawa S, Inoue A, Kokumai R, Hirano M. J. Organomet. Chem. 2007;692:520–529. [Google Scholar]
- 29).Amatore M, Beeson TD, Brown SP, MacMillan DWC. Angew. Chem. Int. Ed. 2009;48:5121–5124. doi: 10.1002/anie.200901855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Cee VJ, Cramer CJ, Evans DA. J. Am. Chem. Soc. 2006;128:2920–2930. doi: 10.1021/ja0555670. [DOI] [PubMed] [Google Scholar]
- 31).Stanton GR, Johnson CN, Walsh PJ. J. Am. Chem. Soc. 2010;132:4399–4408. doi: 10.1021/ja910717p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Examples of zinc homoenolates include: Nakamura E, Kuwajima I. J. Am. Chem. Soc. 1977;99:7360–7362. Nakamura E, Aoki S, Sekiya K, Oshino H, Kuwajima I. J. Am. Chem. Soc. 1987;109:8056–8066. Tamaru Y, Ochiai H, Nakamura T, Yoshida Z. Angew. Chem. Int. Ed. 1987;26:1157–1158. Nakamura E, Sekiya K, Kuwajima I. Tetrahedron Lett. 1987;28:337–340. Oshino H, Nakamura E, Kuwajima I. J. Org. Chem. 1985;50:2804–2805. Nakamura E, Kuwajima I. J. Am. Chem. Soc. 1984;106:3368–3370.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



