Abstract
Background
Systemic inflammation and endothelial activation are implicated in the development of hypertension. However, epidemiologic studies have yet to compare multiple corresponding biomarkers in relation to risk of hypertension, particularly in multiethnic populations.
Methods
We identified 800 cases of incident hypertension and 800 matched controls with equal numbers of White and Black women in a nested case-control study within the Women’s Health Initiative Observational Study. We measured markers of inflammation (high-sensitivity C-reactive protein [hsCRP], interleukin-6 [IL-6], interleukin-1β [IL-1β], tumor necrosis factor receptor 2 [TNF-r2]) and endothelial activation (soluble intercellular adhesion molecule-1 [sICAM-1]) in baseline blood samples.
Results
Before adjustment for measures of adiposity, higher hsCRP and IL-6 were associated with increased risk of hypertension in both White and Black women, higher TNF-r2 was associated with increased risk of hypertension only in Black women, and IL-1β and sICAM-1 were unassociated with risk of hypertension. All the positive associations were attenuated after adjustment for body mass index. The resulting multivariable-adjusted relative risks (95% CI) of hypertension comparing the highest versus lowest quartile were 1.52 (0.94–2.48) and 1.23 (0.76–1.97) for hsCRP and IL-6 in White women, and 1.30 (0.81–2.07), 1.58 (0.96–2.59), and 1.49 (0.94–2.36) for hsCRP, IL-6, and TNF-r2 in Black women. The results after adjustment for waist circumference were similar.
Conclusions
After adjustment for measures of adiposity, there was no significant association of hsCRP, IL-6, IL-1β, TNF-r2, and sICAM-1 with incident hypertension in either White or Black women. The interrelationships between inflammation and adiposity in development of hypertension need further investigation.
Keywords: inflammation, endothelium-derived factors, hypertension, women, epidemiology
Introduction
Hypertension is the most common chronic disease in the U.S. (1), affecting more than 74.5 million adults in 2003–2006 (2). While research into the etiology of hypertension has yielded an important array of lifestyle, dietary, and psychosocial factors, relevant biochemical markers for risk of hypertension are comparatively less studied.
Both chronic inflammation and endothelial activation are pathophysiologic processes potentially implicated in the development of hypertension (3). Laboratory studies have shown that inflammatory markers attenuate the synthesis of nitric oxide (NO) in endothelial cells (4, 5) and stimulate progressive endothelial dysfunction (6, 7). A diminished bioavailability of NO and impaired vascular tone due to the defective endothelium will result in the elevations of blood pressure (BP) (8, 9). A number of cross-sectional studies have found high circulating levels of inflammatory markers among human subjects with elevated BP or hypertension (10–14). Meanwhile only a few prospective studies examined baseline plasma inflammatory markers, typically including C-reactive protein (CRP), with the subsequent risk of hypertension among initially normotensive individuals (15–18). Similarly, studies on plasma endothelial markers and hypertension have been limited to cross-sectional studies (19–21) and retrospective case-control studies (22–24), in which no causal link can be established.
Hypertension is particularly prevalent, poorly controlled, and causes more severe complications in Blacks versus Whites (25). Differences in inflammatory markers (26, 27) and endothelial markers (28) exist between Blacks and Whites, whereas few studies have examined whether the associations of these biomarkers with risk of hypertension differ by race/ethnicity. We therefore investigated the prospective association of several circulating markers of inflammation and endothelial activation with incident hypertension in a nested case-control study within a multi-ethnic cohort of postmenopausal women.
Materials and Methods
Study subjects
The Women’s Health Initiative-Observational Study (WHI-OS) is a prospective cohort study that examined risk factors for chronic disease among ethnically diverse postmenopausal women. Details of the study rationale and design have been reported previously (29). The study has been reviewed and approved by human subjects review committees at each participating institution, and signed informed consent was obtained from all participants.
Of the 93,676 postmenopausal women enrolled into the WHI-OS, the baseline population for our study consisted of women free of hypertension, cardiovascular disease, peripheral vascular disease, and cancer, and who had provided sufficient baseline blood samples. To minimize the misclassification of borderline hypertension, women free of hypertension were defined as having a measured systolic BP (SBP) <135 mmHg and diastolic BP (DBP) <85 mmHg, no physician diagnosis of hypertension, and no past or current antihypertensive medication use. BP was measured at baseline visit using standard protocols, with subjects sitting for 5 min before measurement. The mean of two BP readings, obtained 30 sec apart, was used for analysis. To minimize the impact of preexisting atherosclerosis, our baseline population was also limited to women aged <70 y. From a baseline population of 36,043 women, incident hypertension was identified during a median follow-up of 5.9 y, defined as the initiation of medication specifically for elevated BP and/or measured BP at the year 3 follow-up visit of either SBP ≥140 mmHg or DBP≥90 mmHg. Antihypertensive medication use was reported on annual follow-up questionnaires and confirmed by the year 3 and/or year 6 follow-up medication inventories review.
For this nested case-control study, we randomly selected 400 White women and 400 Black women who developed incident hypertension among 5,251 eligible cases in the WHI-OS cohort. Based upon risk-set sampling, for each case a control subject was randomly selected from women who maintained SBP <135 mmHg, DBP <85 mmHg and remained free of antihypertensive medications and major morbidity until the case was identified (±3 months). Each control was also matched to respective case by age (±2 year), race/ethnicity (White/Caucasian or Black/African-American), clinical center (geographic location), and time of enrollment (±2 y).
Plasma biomarker assays
Blood samples were obtained from each WHI participant and collected to anticoagulated (citrate and EDTA) tubes. Samples were spun for 10 min at 13000xg in a refrigerated centrifuge within 30 to 45 min of blood collection. Following standardized procedures, all samples were then aliquoted within 15 min after centrifugation and frozen at −70° C for short-term local storage. Samples were batch-shipped on dry ice to a central facility (McKesson BioServices) for long-term storage until analysis.
For our nested case-control study, we measured four inflammatory markers, including high-sensitivity CRP (hsCRP), interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor receptor 2 (TNF-r2), which shows a strong correlation with TNF-α mRNA expression in human adipose tissue (30), plus a marker of endothelial activation, soluble intercellular adhesion molecule-1 (sICAM-1) in plasma. hsCRP was measured by an ultra-sensitive immunotechnique (Dade Behring, Newark, DE); IL-6 was measured by a quantitative sandwich enzyme immunoassay (Quantikine HS Immunoassay Kit); IL-1β was determined by commercially available enzyme-linked immunosorbent assay (Pelikine Compact human IL-1β ELISA kits, CLB, Amsterdam); TNF-r2 was measured by an ELISA kit with immobilized monoclonal antibody to human TNF-r2 (Genzyme, Cambridge, MA); and sICAM-1 was measured also by ELISA (R&D Systems). All investigators and laboratory personnel were blinded to the subjects’ case-control status. All blood samples were handled identically throughout the processes of blood collection, storage, retrieval, and assays. Assay results clearly out of range were considered outliers and excluded, to which 1 subject each was excluded for hsCRP, TNF-r2, and sICAM-1. The intra-assay coefficients of variation were 3.3% for hsCRP, 9.5% for IL-6, 20.5% for IL-1β, 9.9% for TNF-r2, and 5.7% for sICAM-1.
Assessment of other baseline covariates
At baseline in the WHI-OS, participants provided extensive self-reported information on demographic characteristics, education and income, lifestyle factors, diet, medical history, and medication use. Physical activity was assessed based upon the frequency, intensity, and duration of walking and recreational activities, and presented as metabolic equivalents-hr (MET-hr, 1 MET=1 kcal/kg body weight/hr) per week. Hypercholesterolemia was defined by self-reported cholesterol-lowering medication use. Diabetes was defined by self-reported physician diagnosis. Postmenopausal hormone therapy use was also ascertained from self-report. During the baseline clinic visit, body weight was measured using a calibrated balance beam scale, and height was measured using a calibrated, wall-mounted stadiometer, from which body mass index (BMI, in kg/m2) was calculated. Waist circumference was measured at the end of normal expiration over nonbinding undergarments in a horizontal plane at the natural waist.
Statistical analysis
We conducted analyses using SAS version 9.1 (SAS Institute, Cary, NC) for White and Black women separately. We first checked each biomarker for outliers and used logarithmic transformation to normalize the skewed distribution for hsCRP, IL-6 and IL-1β. We compared major hypertension risk factors and baseline circulating biomarker concentrations between hypertension cases and controls. We also divided each biomarker into quartiles based upon its distribution among controls and compared hypertension risk factors. We then calculated relative risks (RRs) and 95% confidence intervals (CIs) of incident hypertension for each quartile of biomarker using conditional logistic regression, with the lowest quartile as the reference. Crude models adjusted for matching factors including age, clinical center, and time of enrollment. Multivariable models additionally adjusted for known lifestyle risk factors for hypertension, including cigarette smoking (current, former, never), alcohol intake (never, former, <1 drink/month, <1 drink/week, 1-<7 drinks/week, ≥7 drinks/week), recreational physical activity (total MET-hr/week), and hormone replacement therapy (never, former, current). We finally adjusted for measures of adiposity (BMI and waist circumference). We also conducted stratified analyses by BMI (<25, 25 to <30, and ≥30 kg/m2) and waist circumference (<77, 77 to <88, ≥88 cm).
Several sensitivity analyses were also performed. First, we excluded women with baseline pre-hypertension (SBP/DBP between 120/80 and 135/85 mmHg); second, we excluded women with baseline diabetes; third, we additionally adjusted for baseline SBP; and fourth, we adjusted for BMI and waist circumference with their quadratic terms to control for possible curvilinear relations. The results were generally similar to main analyses and not presented.
Results
Baseline characteristics of hypertension cases compared with matched controls are shown in Table 1. On average, Black women in this study were younger, heavier, less physically active, and more likely to be diabetic than White women regardless of case/control status. Both White and Black hypertension cases had higher BMI and waist circumference, and engaged in less physical activity compared with respective controls. Black cases were also more likely to have diabetes than controls. Cigarette smoking, alcohol consumption and history of hypercholesterolemia were generally similar in cases and controls. Comparing baseline biomarkers, mean concentrations of hsCRP and IL-6 were higher, while mean concentrations of TNF-r2 and sICAM-1 were lower, in Black cases and controls than in White cases and controls respectively. For both White and Black women, baseline hsCRP and IL-6 were significantly higher in cases than in controls. Baseline TNF-r2 and sICAM-1 were significantly higher in Black cases versus controls, but similar among White case-control pairs. Baseline IL-1β did not differ by case and control for both Whites and Blacks.
TABLE 1.
Baseline characteristics of incident hypertension cases and controls
| Baseline characteristic | White |
Black |
||||
|---|---|---|---|---|---|---|
| Controls (N=400) | Cases (N=400) | Pa | Controls (N=400) | Cases (N=400) | Pa | |
| Age (years), μb | 60.8 | 60.8 | 0.18 | 58.1 | 58.2 | 0.42 |
| Body mass index (kg/m2), μ | 25.2 | 26.8 | < 0.0001 | 28.2 | 30.1 | < 0.0001 |
| Waist circumference (cm), μ | 79.0 | 83.6 | < 0.0001 | 84.6 | 88.8 | < 0.0001 |
| Physical activity (MET-hr/wk), μ | 16.9 | 15.0 | 0.09 | 12.9 | 10.8 | 0.049 |
| Smoking, % | 0.96 | 0.48 | ||||
| Never | 50.9 | 49.1 | 51.4 | 46.5 | ||
| Past | 43.0 | 45.1 | 38.2 | 40.2 | ||
| Current | 6.1 | 5.8 | 10.4 | 13.3 | ||
| Alcohol use, % | 0.24 | 0.35 | ||||
| Never | 8.3 | 8.8 | 17.3 | 13.9 | ||
| Past | 15.0 | 16.8 | 26.0 | 30.1 | ||
| <1 drinks/week | 28.3 | 34.8 | 30.8 | 36.5 | ||
| 1-<7 drinks/week | 35.6 | 27.0 | 20.4 | 14.7 | ||
| ≥7 drinks/week | 12.8 | 12.8 | 5.6 | 4.8 | ||
| Hormone therapy use, % | 0.30 | 0.15 | ||||
| Never | 34.0 | 29.0 | 55.3 | 48.9 | ||
| Past | 12.3 | 10.5 | 10.3 | 11.8 | ||
| Current | 53.8 | 60.5 | 34.5 | 39.4 | ||
| History of diabetes, % | 1.0 | 2.5 | 0.11 | 4.0 | 9.8 | 0.002 |
| Cholesterol-lowering medication use, % | 8.5 | 11.1 | 0.24 | 9.1 | 9.2 | 0.90 |
| Blood pressure (mmHg), μ | ||||||
| Systolic at baseline | 113.3 | 120.9 | < 0.0001 | 115.3 | 121.1 | < 0.0001 |
| Diastolic at baseline | 70.2 | 74.1 | < 0.0001 | 71.9 | 74.7 | < 0.0001 |
| Systolic at year 3 | 113.8 | 130.1 | < 0.0001 | 116.9 | 131.8 | < 0.0001 |
| Diastolic at year 3 | 69.2 | 76.5 | < 0.0001 | 72.6 | 78.1 | < 0.0001 |
| Plasma biomarkers, μ | ||||||
| hsCRP (mg/L)c | 1.40 | 2.08 | < 0.0001 | 1.89 | 2.43 | 0.002 |
| IL-6 (pg/mL)c | 1.29 | 1.50 | 0.006 | 1.71 | 1.99 | 0.007 |
| IL-1β (pg/mL)c | 0.24 | 0.25 | 0.37 | 0.25 | 0.27 | 0.36 |
| TNF-r2 (pg/mL) | 2710 | 2726 | 0.76 | 2405 | 2585 | 0.0009 |
| sICAM-1 (pg/mL) | 294.4 | 301.6 | 0.15 | 250.9 | 268.0 | 0.02 |
P values are derived from paired t-test for continuous variables and McNemar’s test for categorical variables.
Matching variable
Geometric means are shown.
The associations between baseline concentrations of biomarkers and risk of hypertension in White and Black women are shown in Table 2. In White women, using crude models that only adjusted for matching factors, plasma hsCRP and IL-6 were each positively associated with the risk of developing hypertension. In Black women, the crude models showed positive associations of plasma hsCRP, IL-6, and TNF-r2 with the risk of hypertension. Additional multivariable adjustment for other hypertension risk factors did not materially change these associations, with the RRs and 95% CI of hypertension in the highest versus lowest quartile of 2.02 (1.29–3.14) for hsCRP, 1.64 (1.06–2.55) for IL-6 in White women; 1.64 (1.07–2.52) for hsCRP, 1.99 (1.27–3.12) for IL-6, and 1.67 (1.08–2.57) for TNF-r2 in Black women.
TABLE 2.
Relative risks and 95% confidence intervals of hypertension according to race-specific quartiles of biomarkers
| Biomarkers | White |
Black |
||||||
|---|---|---|---|---|---|---|---|---|
| Median | Cases/Controls | Crude modela | Multivariable modelb | Median | Cases/Controls | Crude modela | Multivariable modelb | |
| hsCRP (mg/L) | ||||||||
| 1st quartile | 0.37 | 65/100 | 1.00 (Ref) | 1.00 (Ref) | 0.48 | 82/100 | 1.00 (Ref) | 1.00 (Ref) |
| 2nd quartile | 0.99 | 84/100 | 1.29 (0.82–2.04) | 1.20 (0.74–1.93) | 1.37 | 76/101 | 0.95 (0.63–1.44) | 0.94 (0.60–1.45) |
| 3rd quartile | 2.06 | 96/100 | 1.44 (0.93–2.22) | 1.30 (0.82–2.04) | 2.76 | 101/99 | 1.30 (0.85–1.98) | 1.09 (0.69–1.72) |
| 4th quartile | 4.50 | 154/99 | 2.28 (1.52–3.42) | 2.02 (1.29–3.14) | 7.16 | 140/100 | 1.72 (1.15–2.58) | 1.64 (1.07–2.52) |
| P, trendc | <0.0001 | 0.0007 | 0.001 | 0.003 | ||||
| IL-6 (pg/mL) | ||||||||
| 1st quartile | 0.58 | 74/100 | 1.00 (Ref) | 1.00 (Ref) | 0.74 | 73/100 | 1.00 (Ref) | 1.00 (Ref) |
| 2nd quartile | 0.97 | 99/101 | 1.31 (0.88–1.95) | 1.27 (0.84–1.92) | 1.23 | 91/100 | 1.30 (0.85–1.97) | 1.40 (0.89–2.19) |
| 3rd quartile | 1.54 | 106/99 | 1.49 (0.98–2.28) | 1.37 (0.88–2.13) | 1.94 | 101/100 | 1.47 (0.95–2.28) | 1.59 (0.98–2.57) |
| 4th quartile | 2.78 | 118/99 | 1.69 (1.12–2.57) | 1.64 (1.06–2.55) | 4.39 | 135/100 | 1.92 (1.27–2.91) | 1.99 (1.27–3.12) |
| P, trendc | 0.02 | 0.04 | 0.003 | 0.006 | ||||
| IL-1β (pg/mL) | ||||||||
| 1st quartile | 0.13 | 91/89 | 1.00 (Ref) | 1.00 (Ref) | 0.13 | 65/90 | 1.00 (Ref) | 1.00 (Ref) |
| 2nd quartile | 0.20 | 84/90 | 0.87 (0.55–1.40) | 0.93 (0.56–1.54) | 0.20 | 106/91 | 1.52 (0.97–2.38) | 1.31 (0.81–2.12) |
| 3rd quartile | 0.26 | 91/88 | 0.98 (0.60–1.62) | 0.99 (0.58–1.68) | 0.28 | 98/89 | 1.42 (0.88–2.29) | 1.42 (0.84–2.39) |
| 4th quartile | 0.48 | 89/88 | 1.00 (0.58–1.72) | 1.06 (0.60–1.87) | 0.47 | 89/90 | 1.31 (0.77–2.24) | 1.30 (0.73–2.33) |
| P, trendc | 0.85 | 0.74 | 0.65 | 0.54 | ||||
| TNF-r2 (pg/mL) | ||||||||
| 1st quartile | 1968 | 88/100 | 1.00 (Ref) | 1.00 (Ref) | 1770 | 82/100 | 1.00 (Ref) | 1.00 (Ref) |
| 2nd quartile | 2402 | 103/100 | 1.19 (0.79–1.80) | 1.11 (0.72–1.71) | 2127 | 85/100 | 1.03 (0.69–1.55) | 1.07 (0.69–1.65) |
| 3rd quartile | 2840 | 98/100 | 1.12 (0.75–1.67) | 1.02 (0.67–1.55) | 2516 | 99/100 | 1.26 (0.85–1.88) | 1.29 (0.84–1.97) |
| 4th quartile | 3495 | 109/99 | 1.29 (0.85–1.95) | 1.22 (0.78–1.89) | 3061 | 134/100 | 1.72 (1.14–2.58) | 1.67 (1.08–2.57) |
| P, trendc | 0.29 | 0.45 | 0.005 | 0.01 | ||||
| sICAM-1 (pg/mL) | ||||||||
| 1st quartile | 233 | 87/100 | 1.00 (Ref) | 1.00 (Ref) | 145 | 80/100 | 1.00 (Ref) | 1.00 (Ref) |
| 2nd quartile | 267 | 108/101 | 1.20 (0.81–1.79) | 1.35 (0.89–2.05) | 214 | 88/100 | 1.07 (0.72–1.59) | 1.09 (0.72–1.65) |
| 3rd quartile | 304 | 86/99 | 0.99 (0.65–1.51) | 1.09 (0.70–1.71) | 278 | 113/100 | 1.39 (0.94–2.05) | 1.32 (0.87–1.99) |
| 4th quartile | 356 | 118/99 | 1.39 (0.93–2.08) | 1.53 (0.99–2.37) | 354 | 119/99 | 1.46 (1.00–2.15) | 1.39 (0.92–2.11) |
| P, trendc | 0.19 | 0.11 | 0.03 | 0.09 | ||||
Crude models adjust only for matching variables including age, clinical center, and time of enrollment.
Multivariable models additionally adjust for smoking, alcohol intake, physical activity, and hormone replacement therapy.
Trends across quartiles of biomarkers are tested using median of each quartile as ordinal variable.
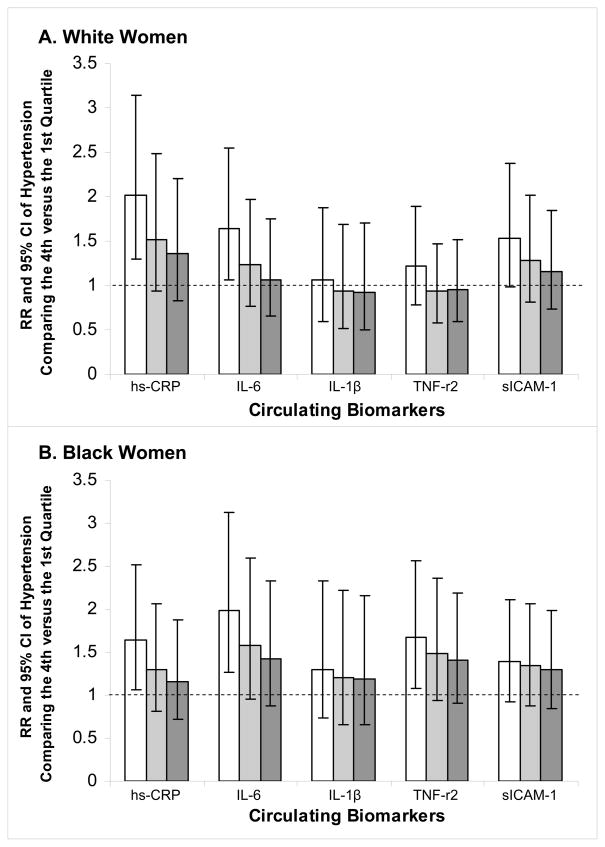
Because all inflammatory markers, except IL-1β, were significantly and positively correlated with BMI and waist circumference in controls (Spearman r ranging from 0.15 to 0.41 in Whites and 0.17 to 0.54 in Blacks), while sICAM-1 was positively correlated with measures of adiposity in White, but not Black, women, we particularly examined how adjustment for measures of obesity impacted the association between each biomarker and risk of hypertension (Fig. 1). Since BMI and waist circumference were highly correlated, subsequent regression models adjusted for these two measures separately. In both White and Black women, adjustment for BMI substantially attenuated the positive associations between inflammatory markers and incident hypertension. The resulting multivariable RRs and 95% CI of hypertension in the highest quartile were 1.52 (0.94–2.48) and 1.23 (0.76–1.97) for hsCRP and IL-6 in White women; 1.30 (0.81–2.07), 1.58 (0.96–2.59), and 1.49 (0.94–2.36) for hsCRP, IL-6, and TNF-r2 in Black women. Adjustment for waist circumference attenuated the RRs similarly.
Figure 1.
Multivariable relative risks of hypertension in the highest quartile of biomarkers among White (panel A) and Black (panel B) women. Open bars show results adjusting for smoking, alcohol intake, physical activity, and hormone replacement therapy. Light grey bars show results with additional adjustment for body mass index. Dark grey bars show results with additional adjustment for waist circumference.
We further evaluated the associations of multiple biomarkers with the risk of hypertension across categories of baseline BMI and waist circumference (Table 3). In White women, there was no association between each biomarker and risk of hypertension in any category of BMI or waist circumference. In Black women, plasma hsCRP was significantly and positively associated with risk of hypertension for those with BMI <25 kg/m2 (RR=2.91, 95% CI: 0.995–8.52) or waist circumference <77 cm (RR=4.43, 95% CI: 1.24–15.9), with no similar associations found for heavier women. Nevertheless, the tests for interactions did not reach statistical significant (p, interaction > 0.05).
TABLE 3.
Multivariablea relative risks of hypertension in the highest quartile versus the lowest quartile of biomarkers, stratified by measures of adiposity.
| Measures of adiposity | hsCRP | IL-6 | IL-1β | TNF-r2 | sICAM-1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| White | Black | White | Black | White | Black | White | Black | White | Black | |
| Body Mass Indexb | ||||||||||
| < 25 | 1.28 (0.68–2.41) | 2.91 (0.995–8.52) | 1.23 (0.66–2.29) | 1.57 (0.59–4.17) | 0.70 (0.38–1.30) | 2.02 (0.78–5.24) | 1.06 (0.56–2.01) | 0.97 (0.43–2.20) | 0.89 (0.48–1.64) | 0.81 (0.30–2.17) |
| 25 to <30 | 1.48 (0.59–3.74) | 1.10 (0.49–2.50) | 1.03 (0.47–2.26) | 0.94 (0.44–2.00) | 1.78 (0.80–3.94) | 1.41 (0.62–3.20) | 0.89 (0.41–1.92) | 1.19 (0.56–2.54) | 1.47 (0.65–3.30) | 1.53 (0.72–3.26) |
| ≥ 30 | 0.40 (0.04–4.06) | 0.76 (0.29–1.98) | 2.80 (0.61–12.9) | 1.79 (0.68–4.76) | 0.59 (0.18–1.88) | 0.65 (0.28–1.50) | 0.25 (0.05–1.35) | 1.91 (0.90–4.04) | 1.86 (0.50–6.92) | 1.43 (0.71–2.86) |
| P, interactionc | 0.25 | 0.88 | 0.94 | 0.60 | 0.28 | 0.48 | 0.74 | 0.65 | 0.65 | 0.42 |
| Waist Circumferenced | ||||||||||
| <77 cm | 1.49 (0.74–2.99) | 4.43 (1.24–15.9) | 1.10 (0.54–2.26) | 1.84 (0.65–5.16) | 0.66 (0.32–1.36) | 2.34 (0.80–6.88) | 1.15 (0.57–2.29) | 1.14 (0.45–2.85) | 0.92 (0.47–1.79) | 0.98 (0.35–2.80) |
| 77 to <88 cm | 1.70 (0.76–3.80) | 0.55 (0.25–1.22) | 1.52 (0.69–3.36) | 1.23 (0.58–2.61) | 1.61 (0.74–3.46) | 1.85 (0.82–4.18) | 1.21 (0.56–2.59) | 1.13 (0.55–2.34) | 1.59 (0.73–3.45) | 0.81 (0.40–1.64) |
| ≥ 88 cm | 0.83 (0.24–2.95) | 0.91 (0.37–2.23) | 0.81 (0.29–2.25) | 0.91 (0.36–2.27) | 0.68 (0.27–1.71) | 0.65 (0.30–1.39) | 0.56 (0.20–1.56) | 1.73 (0.87–3.43) | 0.78 (0.27–2.31) | 1.80 (0.93–3.49) |
| P, interactiond | 0.14 | 0.13 | 0.94 | 0.91 | 0.74 | 0.37 | 0.76 | 0.77 | 0.77 | 0.24 |
Multivariable models adjust for smoking, alcohol intake, physical activity, and hormone replacement therapy.
Models within each category of BMI also include BMI as a continuous variable.
Interactions are tested using Wald chi-square statistics.
Models within each category of waist circumference also include waist circumference as a continuous variable.
Discussion
In this prospective nested case-control study, before adjustment for measures of adiposity, we found positive associations between plasma concentrations of hsCRP, IL-6 and risk of hypertension in both White and Black women, and a positive association between concentrations of TNF-r2 and risk of hypertension in Black, but not White, women. These associations became not significant after adjustment for BMI and waist circumference. Neither IL-1β nor sICAM-1 was associated with the risk of hypertension in any model of White and Black women.
Accumulating evidence suggests that systemic inflammation and endothelial activation underlie the development of hypertension (3). Experimental studies show that CRP, the most extensively studied inflammatory marker, may stimulate endothelial activation by decreasing the expression and activity of NO synthase (4, 5), facilitating release of endothelin-1 (6), and reducing the endothelial progenitor cell survival and differentiation (31). In endothelium activation and subsequent dysfunction, endothelium-dependent vasorelaxation is impaired (9) and vascular tone compromised (32), ultimately leading to increases in BP. On the surface of activated endothelial cells, the expression of cell adhesion molecules such as ICAM-1 and vascular adhesion molecule-1 (VCAM-1) is markedly increased, which is accompanied by release of the soluble forms into the bloodstream (33). The adhesion molecules mediate attraction and migration of immune cells, which would further exacerbate vascular inflammation and endothelial injury (34). Inflammatory cytokines may also promote the development of hypertension through other endothelium-independent mechanisms (35–37).
Previous epidemiologic studies have linked higher plasma concentrations of inflammatory markers, including CRP (10, 12), IL-6 (10, 11), IL-1β (13), and TNF-α (14), to increased SBP and DBP or hypertensive status. With current evidence predominantly from cross-sectional or retrospective studies, it has been unclear whether inflammation is a cause or a consequence of hypertension. The prospective association between CRP and incident hypertension was examined in a few studies. In some (15, 17) but not all (18) studies, a positive association remained significant after controlling for BMI. In a nested case-control study examining both hsCRP and IL-6, the positive associations of both inflammatory markers with hypertension risk were greatly attenuated after adjustment for BMI (16).
Our study extends earlier investigations to other inflammatory markers, including IL-1β and TNF-r2, whose associations with incident hypertension had not been examined in prospective studies to our knowledge. Our study also extends previous cross-sectional and case-control studies of endothelial markers with BP or hypertension (19–24) to prospective study. We found that Black women who developed hypertension had higher plasma concentrations of sICAM-1 at baseline, but this association was attenuated to nonsignificance after multivariable adjustment. Because each adhesion molecule may mediate alterations to the endothelium through different mechanisms (19), our findings for a single adhesion molecule cannot exclude the possibility that other markers of endothelial function might play important roles in the development of hypertension.
Our study underscores the important interrelationships between inflammation, adiposity, and pathogenesis of hypertension. Obesity is a well-known predictor of hypertension and may confound the association between inflammation and hypertension. Meanwhile, inflammation and obesity share common pathways leading to hypertension, such as upregulation of the renin-angiotensin system (35, 38) and disturbance of insulin actions (39). Furthermore, adipose tissue has been characterized as a dynamic endocrine organ that produces pro-inflammatory cytokines (40), which makes it more difficult to distinguish the independent effect of inflammation and obesity on hypertension. In our stratified analyses, there was a significant association between high plasma concentrations of hsCRP and increased risk of hypertension among Black women with either a BMI <25 kg/m2 or waist circumference <77 cm, suggesting that the association between inflammation and hypertension could be stronger among leaner individuals.
Our prospective nested case-control study within the multiethnic WHI-OS simultaneously examined multiple plasma markers of inflammation and endothelial activation in association with risk of hypertension. There are several limitations of this study that deserve attention. First, only a single baseline measurement of each biomarker was available, whereby random misclassification tends to bias the associations towards the null. In addition, some biomarkers, such as IL-1β, had high coefficients of variation that would partly explain the null findings with hypertension. Second, despite comprehensive adjustment for known hypertension risk factors, residual confounding may persist. Third, other potentially important biomarkers of endothelium activation (e.g. VCAM-1 and selectins) and alternative measures of endothelial function were not assessed in our study. Finally, the WHI-OS cohort consisted only of postmenopausal women. Additional studies are necessary to further examine the associations between inflammatory and endothelial markers with risk of hypertension in men and in other ethnically diverse populations.
In conclusion, in this multiethnic study investigating multiple inflammatory and endothelial markers for the risk of hypertension, we found a positive association for plasma hsCRP and IL-6 with hypertension risk in both White and Black women, along with a positive association for plasma TNF-r2 with hypertension risk in Black, but not White, women. However, these associations were weakened upon adjustment for BMI and waist circumference. Additional studies are needed to clarify the interrelationships between inflammation and adiposity in the development of hypertension.
Acknowledgments
We gratefully acknowledge the dedicated efforts of investigators and staff at the WHI clinical centers and coordinating center and extraordinary commitment of WHI participants.
Funding Sources: This study was supported by research grant HL-075455 from the National Institutes of Health, Bethesda, MD. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. These grants provided funding for study conduct and data collection. The funding sources had no role in management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Abbreviations
- NO
nitric oxide
- BP
blood pressure
- WHI-OS
Women’s Health Initiative-Observational Study
- hsCRP
high-sensitivity C-reactive protein
- IL-6
interleukin-6
- IL-1β
interleukin-1β
- TNF-r2
tumor necrosis factor receptor 2
- sICAM-1
soluble intercellular adhesion molecule-1
- BMI
body mass index
- RRs
relative risks
- CIs
confidence intervals
- VCAM-1
vascular adhesion molecule-1
Footnotes
Publisher's Disclaimer: This is an un-copyedited authored manuscript copyrighted by The American Association for Clinical Chemistry (AACC). This may not be duplicated or reproduced, other than for personal use or within the rule of ‘Fair Use of Copyrighted Materials’ (section 107, Title 17, U.S. Code) without permission of the copyright owner, AACC. The AACC disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The final publisher-authenticated version of the article will be made available at http://www.clinchem.org 12 months after its publication in Clinical Chemistry.
References
- 1.Cherry DK, Hing E, Woodwell DA, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2006 summary. Natl Health Stat Report. 2008:1–39. [PubMed] [Google Scholar]
- 2. [Accessed Feb. 09 2010];American Heart Association Statistical Fact Sheet - Disease/Risk Factors 2010 Update. http://www.americanheart.org/presenter.jhtml?identifier=3000946.
- 3.Watson T, Goon PK, Lip GY. Endothelial progenitor cells, endothelial dysfunction, inflammation, and oxidative stress in hypertension. Antioxid Redox Signal. 2008;10:1079–88. doi: 10.1089/ars.2007.1998. [DOI] [PubMed] [Google Scholar]
- 4.Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–9. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 5.Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439–41. doi: 10.1161/01.cir.0000033116.22237.f9. [DOI] [PubMed] [Google Scholar]
- 6.Verma S, Li SH, Badiwala MV, Weisel RD, Fedak PW, Li RK, et al. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation. 2002;105:1890–6. doi: 10.1161/01.cir.0000015126.83143.b4. [DOI] [PubMed] [Google Scholar]
- 7.Devaraj S, Xu DY, Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003;107:398–404. doi: 10.1161/01.cir.0000052617.91920.fd. [DOI] [PubMed] [Google Scholar]
- 8.Cardillo C, Panza JA. Impaired endothelial regulation of vascular tone in patients with systemic arterial hypertension. Vasc Med. 1998;3:138–44. doi: 10.1177/1358836X9800300208. [DOI] [PubMed] [Google Scholar]
- 9.Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–7. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 10.Bermudez EA, Rifai N, Buring J, Manson JE, Ridker PM. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler Thromb Vasc Biol. 2002;22:1668–73. doi: 10.1161/01.atv.0000029781.31325.66. [DOI] [PubMed] [Google Scholar]
- 11.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 12.Sung KC, Suh JY, Kim BS, Kang JH, Kim H, Lee MH, et al. High sensitivity C-reactive protein as an independent risk factor for essential hypertension. Am J Hypertens. 2003;16:429–33. doi: 10.1016/s0895-7061(03)00566-1. [DOI] [PubMed] [Google Scholar]
- 13.Dalekos GN, Elisaf M, Bairaktari E, Tsolas O, Siamopoulos KC. Increased serum levels of interleukin-1beta in the systemic circulation of patients with essential hypertension: additional risk factor for atherogenesis in hypertensive patients? J Lab Clin Med. 1997;129:300–8. doi: 10.1016/s0022-2143(97)90178-5. [DOI] [PubMed] [Google Scholar]
- 14.Skoog T, Dichtl W, Boquist S, Skoglund-Andersson C, Karpe F, Tang R, et al. Plasma tumour necrosis factor-alpha and early carotid atherosclerosis in healthy middle-aged men. Eur Heart J. 2002;23:376–83. doi: 10.1053/euhj.2001.2805. [DOI] [PubMed] [Google Scholar]
- 15.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. Jama. 2003;290:2945–51. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 16.Sesso HD, Wang L, Buring JE, Ridker PM, Gaziano JM. Comparison of interleukin-6 and C-reactive protein for the risk of developing hypertension in women. Hypertension. 2007;49:304–10. doi: 10.1161/01.HYP.0000252664.24294.ff. [DOI] [PubMed] [Google Scholar]
- 17.Wang TJ, Gona P, Larson MG, Levy D, Benjamin EJ, Tofler GH, et al. Multiple biomarkers and the risk of incident hypertension. Hypertension. 2007;49:432–8. doi: 10.1161/01.HYP.0000256956.61872.aa. [DOI] [PubMed] [Google Scholar]
- 18.Lakoski SG, Herrington DM, Siscovick DM, Hulley SB. C-reactive protein concentration and incident hypertension in young adults: the CARDIA study. Arch Intern Med. 2006;166:345–9. doi: 10.1001/archinte.166.3.345. [DOI] [PubMed] [Google Scholar]
- 19.Miller MA, Kerry SM, Cook DG, Cappuccio FP. Cellular adhesion molecules and blood pressure: interaction with sex in a multi-ethnic population. J Hypertens. 2004;22:705–11. doi: 10.1097/00004872-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Rohde LE, Hennekens CH, Ridker PM. Cross-sectional study of soluble intercellular adhesion molecule-1 and cardiovascular risk factors in apparently healthy men. Arterioscler Thromb Vasc Biol. 1999;19:1595–9. doi: 10.1161/01.atv.19.7.1595. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Thompson AM, Tong W, Xu T, Chen J, Zhao L, et al. Biomarkers of inflammation and endothelial dysfunction and risk of hypertension among Inner Mongolians in China. J Hypertens. 2009 doi: 10.1097/HJH.0b013e3283324650. [DOI] [PubMed] [Google Scholar]
- 22.DeSouza CA, Dengel DR, Macko RF, Cox K, Seals DR. Elevated levels of circulating cell adhesion molecules in uncomplicated essential hypertension. Am J Hypertens. 1997;10:1335–41. doi: 10.1016/s0895-7061(97)00268-9. [DOI] [PubMed] [Google Scholar]
- 23.Ferri C, Desideri G, Valenti M, Bellini C, Pasin M, Santucci A, De Mattia G. Early upregulation of endothelial adhesion molecules in obese hypertensive men. Hypertension. 1999;34:568–73. doi: 10.1161/01.hyp.34.4.568. [DOI] [PubMed] [Google Scholar]
- 24.Cottone S, Mule G, Nardi E, Vadala A, Guarneri M, Briolotta C, et al. Relation of C-reactive protein to oxidative stress and to endothelial activation in essential hypertension. Am J Hypertens. 2006;19:313–8. doi: 10.1016/j.amjhyper.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Hall WD, Ferrario CM, Moore MA, Hall JE, Flack JM, Cooper W, et al. Hypertension-related morbidity and mortality in the southeastern United States. Am J Med Sci. 1997;313:195–209. doi: 10.1097/00000441-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Wong ND, Pio J, Valencia R, Thakal G. Distribution of C-reactive protein and its relation to risk factors and coronary heart disease risk estimation in the National Health and Nutrition Examination Survey (NHANES) III. Prev Cardiol. 2001;4:109–14. doi: 10.1111/j.1520-037x.2001.00570.x. [DOI] [PubMed] [Google Scholar]
- 27.LaMonte MJ, Durstine JL, Yanowitz FG, Lim T, DuBose KD, Davis P, Ainsworth BE. Cardiorespiratory fitness and C-reactive protein among a tri-ethnic sample of women. Circulation. 2002;106:403–6. doi: 10.1161/01.cir.0000025425.20606.69. [DOI] [PubMed] [Google Scholar]
- 28.Miller MA, Sagnella GA, Kerry SM, Strazzullo P, Cook DG, Cappuccio FP. Ethnic differences in circulating soluble adhesion molecules: the Wandsworth Heart and Stroke Study. Clin Sci (Lond) 2003;104:591–8. doi: 10.1042/CS20020333. [DOI] [PubMed] [Google Scholar]
- 29.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13:S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 30.Hotamisligil GS, Arner P, Atkinson RL, Spiegelman BM. Differential regulation of the p80 tumor necrosis factor receptor in human obesity and insulin resistance. Diabetes. 1997;46:451–5. doi: 10.2337/diab.46.3.451. [DOI] [PubMed] [Google Scholar]
- 31.Torzewski M, Rist C, Mortensen RF, Zwaka TP, Bienek M, Waltenberger J, et al. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20:2094–9. doi: 10.1161/01.atv.20.9.2094. [DOI] [PubMed] [Google Scholar]
- 32.Todd ME. Hypertensive structural changes in blood vessels: do endothelial cells hold the key? Can J Physiol Pharmacol. 1992;70:536–51. doi: 10.1139/y92-069. [DOI] [PubMed] [Google Scholar]
- 33.Gearing AJ, Hemingway I, Pigott R, Hughes J, Rees AJ, Cashman SJ. Soluble forms of vascular adhesion molecules, E-selectin, ICAM-1, and VCAM-1: pathological significance. Ann N Y Acad Sci. 1992;667:324–31. doi: 10.1111/j.1749-6632.1992.tb51633.x. [DOI] [PubMed] [Google Scholar]
- 34.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 35.Wang CH, Li SH, Weisel RD, Fedak PW, Dumont AS, Szmitko P, et al. C-reactive protein upregulates angiotensin type 1 receptors in vascular smooth muscle. Circulation. 2003;107:1783–90. doi: 10.1161/01.CIR.0000061916.95736.E5. [DOI] [PubMed] [Google Scholar]
- 36.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–14. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto T, Kimura T, Ota K, Shoji M, Inoue M, Ohta M, et al. Effects of interleukin-1 beta on blood pressure, thermoregulation, and the release of vasopressin, ACTH and atrial natriuretic hormone. Tohoku J Exp Med. 1994;173:231–45. doi: 10.1620/tjem.173.231. [DOI] [PubMed] [Google Scholar]
- 38.Engeli S, Feldpausch M, Gorzelniak K, Hartwig F, Heintze U, Janke J, et al. Association between adiponectin and mediators of inflammation in obese women. Diabetes. 2003;52:942–7. doi: 10.2337/diabetes.52.4.942. [DOI] [PubMed] [Google Scholar]
- 39.Yudkin JS. Adipose tissue, insulin action and vascular disease: inflammatory signals. Int J Obes Relat Metab Disord. 2003;27 (Suppl 3):S25–8. doi: 10.1038/sj.ijo.0802496. [DOI] [PubMed] [Google Scholar]
- 40.Sonnenberg GE, Krakower GR, Kissebah AH. A novel pathway to the manifestations of metabolic syndrome. Obes Res. 2004;12:180–6. doi: 10.1038/oby.2004.24. [DOI] [PubMed] [Google Scholar]