According to the World Health Organization classification, myeloproliferative neoplasms (MPN) include chronic myelogenous leukemia, also known as BCR-ABL1–positive MPN, classic BCR-ABL1-negative MPN including polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF), and non-classic forms (i.e. systemic mastocytosis, chronic eosinophilic leukemia not otherwise specified, chronic neutrophilic leukemia and unclassifiable MPN). All these subtypes are stem cell-derived clonal myeloproliferation, associated with the overproduction of mature blood elements and variable rates of transformation to acute myeloid leukemia (AML).1
JAK2V617F activating mutation is the most prevalent abnormality observed in BCR-ABL1-negative MPN, found in virtually all cases of PV and in about half of ET and PMF (96%, 55% and 65%, respectively). This mutation lies in the pseudokinase-domain of JAK2 and disrupts its regulatory activity. Another mutation affecting JAK2 exon 12 is observed in 3% of all PV cases. Mutations affecting W515 of the thrombopoietin receptor MPL are detected in PMF and ET patients. Additional mutations have been identified in MPN (reviewed in 1). Defects in the control of intracellular signaling involve mutations in LNK and CBL genes. Genetic abnormalities affecting epigenetic regulation, and possibly responsible for disease initiation, concern the ASXL1, EZH2 and TET2 genes. Finally, mutation in IKZF1 and IDH1/2 may be implicated in MPN transformation. In PV, among these additional mutations to JAK2V617F, TET2 mutations are those most frequently reported (16%); the others are only described in small subsets of patients.1
TET2 belongs to a family of three conserved genes in mammals: TET1, TET2 and TET3. The founding member of the family, TET1, has been identified as a fusion partner of MLL in the t(10;11)(q22;q23) translocation of acute leukemia.2,3 The involvement of TET3 in hematologic disorder has not yet been described. The TET proteins are members of the 2-oxoglutarate (2-OG)- and Fe(II)-dependent dioxygenase that are able to convert 5-methyl-cytosine (5-mC) to 5-hydroxymethylcytosine (hmC).4,5 Recent reports indicate an important role for TET1 and TET2 (and, therefore, hmC) in the control of ES cell self-renewal and differentiation.6 TET3 might be involved in genome reprogramming following fecundation.7
5-hydroxymethylation: a novel major player in the epigenetic field
For decades, the implications and impact of 5-mC in human genome has been extensively studied and it is known to be associated with low gene expression. In contrast, little is known about the recently identified hmC. Indeed, the first study reporting a hydroxylated form of 5-mC in mammalian DNA was described in the early 70s.8 However, this modified base did not receive full attention until 2 reports demonstrating that hmC accounts for 0.6% and 0.03% of the total nucleotides in Purkinje cells9 and murine ES cells,5 respectively.
The function of hmC is not yet clear. Several reports have indicated that hmC prevent the binding of proteins interacting with 5-mC and hmC and may represent a step toward demethylation.8 Using a novel chemical method, Song et al.10 showed an enrichment of hmC in maturing murine brain cells that increases with age and is associated with gene expression. Contrasting results have been reported in human samples: TET2 mutation or inhibition of its catalytic activity affect the level of hmC in myeloid malignant samples, but was associated with a decrease of 5-mC in MDS (myelodysplastic syndromes) samples and with an increase of DNA methylation in AML.11,12
TET2 function in normal hematopoiesis and MPN
TET2 is expressed in a wide range of tissues, such as kidney, brain and the hematopoietic system.13 Recent analyses using short-hairpin RNA in murine stem cells from bone marrow have shown that depletion of TET2 promoted an increase in the proportion of hematopoietic stem/progenitor cells with inhibition of normal differentiation of granulocytes and monocytes.12 Using a similar approach, TET2 mRNA depletion was also shown to lead to the expansion of monocyte/macrophage cells in the presence of cytokines stimulating granulocytic differentiation (G-CSF or GM-CSF) but not in the presence of macrophage-colony stimulating factor (M-CSF).11 This study indicates that TET2 may act at many different phases of myeloid differentiation.
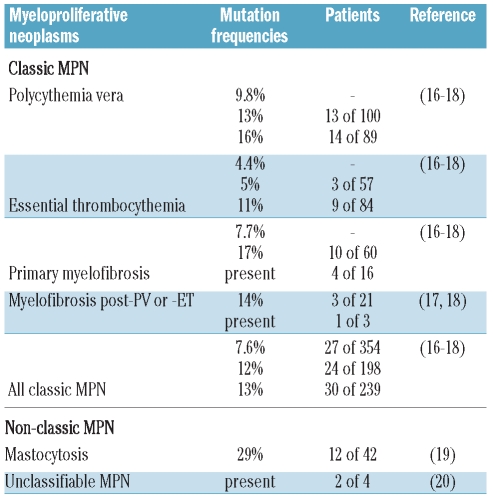
Acquired inactivating mutations of the TET2 gene are frequently observed in human myeloid malignancies (i.e. MDS, de novo and secondary AML) suggesting that they affect a very early myeloid progenitor. TET2 mutations are found in subsets of all subtypes of MPN (Table 1). Inherited TET2 mutations are not responsible for familial MPN,14 but a germline mutation of TET2 was recently described in 2 sisters, one suffering from a JAK2V617F-positive PV, the other healthy.15
Table 1.
TET2 mutations in BCR-ABL1-negative myeloproliferative neoplasms.
In this issue, Swierczek and colleagues21 have investigated the clonality and allele burdens of JAK2V617F and TET2 mutations in patients with sporadic or familial PV. In contrast to other PV patients described in the initial study,17 and in agreement with recent reports,15 they found evidence that TET2 mutations may follow rather than precede the JAK2V617F mutation in some PV patients. Therefore, no strict temporal order of appearance applies to TET2 and JAK2 mutations in PV. Furthermore, they show that clonal in vitro amplifications of mutated erythroid cells only occur from patient samples having both mutations, suggesting that the presence of TET2 mutations increases the aggression of the PV clone. The questions addressed by this work come down to the respective roles of TET2 and JAK2 mutations in the development of human PV.
Animal models using retroviral delivery, transgenic or knock-in technology have proved conclusively that the sole JAK2V617F gene expression in hematopoietic cells is able to induce most of the PV phenotype as soon as the levels match at least that of the JAK2WT allele (heterozygous status). However, does it mean that JAK2V617F is self sufficient for PV in humans? These mouse models are polyclonal and one can argue that JAK2V617F is sufficient for PV as soon as there are enough mutated stem cells. Yet in KI models, the ability of JAK2V617F to amplify the stem cell is controversial.22 Should an additional defect occur for clonal dominance? In human sporadic MPN, other genetic events preceding JAK2V617F are suspected from clonal analysis, using the X chromosome activation or the 20qdel chromosomal abnormality,1 and secondary JAK2WT AML progressing from JAK2V617F MPN.23,24 Indeed, TET2 mutations might be instrumental in amplifying immature myeloid cells, as suggested by several reports on human samples.17,23 However, is it necessary for PV occurrence? The absence of TET2 mutations is reported in most PV patients and in vitro or in vivo mouse SCID clonal amplification seems to be missing in those patients lacking TET2 mutations, as suggested by Swierczek and colleagues.17,21
In this context, TET2 mutations following or preceding JAK2V617F could, therefore, be seen as paradigmatic of events possibly predisposing to PV occurrence and/or accelerating PV evolution. Establishing the importance of JAK2V617F and other TET2-type of mutation orders, their association with transformation and prognosis value, and the precise contribution of each mutation in PV occurrence/evolution will need extensive, in depth clinical, cellular and molecular analyses of a large number of human samples, and animal models demonstrating the precise effect of the identified mutation(s) in a controlled genetic background and their cooperation with JAK2V617F.
Footnotes
Elodie Pronier is a PhD student in unit 1009 of INSERM and works in the search for TET2 function in human cells. Cyril Quivoron is a PhD student in unit U985 of INSERM and works in the search for TET2 function in mouse models. Olivier A. Bernard, PhD, is Director of Research and Head of unit U985 of INSERM « Genetic of tumors », and works in the search for the occurence and roles of genetic abnormalities, especially TET2 mutations, in human malignancies. Jean-Luc Villeval, PhD, is Director of Research in unit 1009 of INSERM «Normal and Pathologic Hematopoiesis» and works in the development of MPN animal models and translational research.
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Tefferi A, Vainchenker W. Myeloproliferative neoplasms: molecular pathophysiology, essential clinical understanding, and treatment strategies. J Clin Oncol. 2011;29(5):573–82. doi: 10.1200/JCO.2010.29.8711. [DOI] [PubMed] [Google Scholar]
- 2.Ono R, Taki T, Taketani T, Taniwaki M, Kobayashi H, Hayashi Y. LCX, leukemia-associated protein with a CXXC domain, is fused to MLL in acute myeloid leukemia with trilineage dysplasia having t(10;11)(q22;q23) Cancer Res. 2002;62(14):4075–80. [PubMed] [Google Scholar]
- 3.Lorsbach RB, Moore J, Mathew S, Raimondi SC, Mukatira ST, Downing JR. TET1, a member of a novel protein family, is fused to MLL in acute myeloid leukemia containing the t(10;11)(q22;q23) Leukemia. 2003;17(3):637–41. doi: 10.1038/sj.leu.2402834. [DOI] [PubMed] [Google Scholar]
- 4.Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renew-al and inner cell mass specification. Nature. 2010;466(7310):1129–33. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324 (5929):930–5. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh KP, Yabuuchi A, Rao S, Huang Y, Cunniff K, Nardone J, et al. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell. 2011;8(2):200–13. doi: 10.1016/j.stem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wossidlo M, Nakamura T, Lepikhov K, Marques CJ, Zakhartchenko V, Boiani M, et al. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat Commun. 2011;2:241. doi: 10.1038/ncomms1240. [DOI] [PubMed] [Google Scholar]
- 8.Dahl C, Gronbaek K, Guldberg P. Advances in DNA methylation: 5-hydroxymethylcytosine revisited. Clin Chim Acta. 2011 Feb 12; doi: 10.1016/j.cca.2011.02.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324(5929):929–30. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song CX, Szulwach KE, Fu Y, Dai Q, Yi C, Li X, et al. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat Biotechnol. 2011;29(1):68–72. doi: 10.1038/nbt.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko M, Huang Y, Jankowska AM, Pape UJ, Tahiliani M, Bandukwala HS, et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature. 2010;468(7325):839–43. doi: 10.1038/nature09586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–67. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langemeijer SM, Kuiper RP, Berends M, Knops R, Aslanyan MG, Massop M, et al. Acquired mutations in TET2 are common in myelodysplastic syndromes. Nat Genet. 2009;41(7):838–42. doi: 10.1038/ng.391. [DOI] [PubMed] [Google Scholar]
- 14.Saint-Martin C, Leroy G, Delhommeau F, Panelatti G, Dupont S, James C, et al. Analysis of the ten-eleven translocation 2 (TET2) gene in familial myeloproliferative neoplasms. Blood. 2009;114(8):1628–32. doi: 10.1182/blood-2009-01-197525. [DOI] [PubMed] [Google Scholar]
- 15.Schaub FX, Looser R, Li S, Hao-Shen H, Lehmann T, Tichelli A, et al. Clonal analysis of TET2 and JAK2 mutations suggests that TET2 can be a late event in the progression of myeloproliferative neoplasms. Blood. 2010;115(10):2003–7. doi: 10.1182/blood-2009-09-245381. [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Wahab O, Mullally A, Hedvat C, Garcia-Manero G, Patel J, Wadleigh M, et al. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood. 2009;114(1):144–7. doi: 10.1182/blood-2009-03-210039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Masse A, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360(22):2289–301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 18.Tefferi A, Pardanani A, Lim KH, Abdel-Wahab O, Lasho TL, Patel J, et al. TET2 mutations and their clinical correlates in polycythemia vera, essential thrombocythemia and myelofibrosis. Leukemia. 2009;23(5):905–11. doi: 10.1038/leu.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tefferi A, Levine RL, Lim KH, Abdel-Wahab O, Lasho TL, Patel J, et al. Frequent TET2 mutations in systemic mastocytosis: clinical, KITD816V and FIP1L1-PDGFRA correlates. Leukemia. 2009;23(5):900–4. doi: 10.1038/leu.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tefferi A, Lim KH, Abdel-Wahab O, Lasho TL, Patel J, Patnaik MM, et al. Detection of mutant TET2 in myeloid malignancies other than myeloproliferative neoplasms: CMML, MDS, MDS/MPN and AML. Leukemia. 2009;23(7):1343–5. doi: 10.1038/leu.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swierczek SI, Yoon D, Bellanné-Chantelot C, Kim S, Saint-Martin C, Delhommeau F, et al. Extent of hematopoietic involvement by TET2 mutations in JAK2V617F polycythemia vera. Haematologica. 2011;96(05):775–8. doi: 10.3324/haematol.2010.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skoda RC. JAK2 impairs stem cell function? Blood. 2010;116(9):1392–3. doi: 10.1182/blood-2010-06-287318. [DOI] [PubMed] [Google Scholar]
- 23.Couronne L, Lippert E, Andrieux J, Kosmider O, Radford-Weiss I, Penther D, et al. Analyses of TET2 mutations in post-myeloproliferative neoplasm acute myeloid leukemias. Leukemia. 2010;24(1):201–3. doi: 10.1038/leu.2009.169. [DOI] [PubMed] [Google Scholar]
- 24.Beer PA, Delhommeau F, LeCouedic JP, Dawson MA, Chen E, Bareford D, et al. Two routes to leukemic transformation after a JAK2 mutation-positive myeloproliferative neoplasm. Blood. 2010;115(14):2891–900. doi: 10.1182/blood-2009-08-236596. [DOI] [PubMed] [Google Scholar]