Abstract
Background
Human bone marrow and umbilical cord blood are sources of allogeneic hematopoietic stem cells for transplantation, which is a life-saving treatment in a variety of diseases but is burdened by delayed T-cell reconstitution. Observational studies evaluating T-cell reconstitution in post-transplant recipients suggest that cord blood hematopoietic stem cells have a more effective capacity for T-cell reconstitution. This study focuses on the comparison of the capacity of cord blood and bone marrow hematopoietic stem cells to generate T cells in vitro.
Design and Methods
Hematopoietic stem cells were cultured in OP9-delta-like-1 and OP9-green fluorescent protein co-cultures to estimate T and myeloid generation capacity, respectively. Phenotypic markers of T-lineage or myeloid differentiation were measured by flow cytometry and used to analyze their kinetics as a function of culture time. Hematopoietic stem cells were labeled with carboxyfluorescein diacetate succinamidyl ester and analyzed after culture to track their phenotypic progression in consecutive generations. Mixed OP9-delta-like-1 co-cultures were done with either carboxyfluorescein diacetate succinamidyl ester-labeled bone marrow and unlabeled cord blood hematopoietic stem cells, or vice versa, to evaluate their mutual influence on T-lineage differentiation. The T-cell potential of hematopoietic stem cells was addressed quantitatively by limiting dilution analysis.
Results
Bulk cultures showed faster and more extensive T-cell differentiation by cord blood hematopoietic stem cells. Furthermore, the T-lymphoid differentiation capacity of cord blood and bone marrow hematopoietic stem cells can be discriminated very early based on the coordinated expression of CD34 and CD7. Mixing experiments with cord blood hematopoietic stem cells and bone marrow hematopoietic stem cells showed that these differences are cell intrinsic. Quantitative clonal analyses demonstrated that CD34+CD38−/lo hematopoietic stem cells from cord blood contained a two-fold higher T-lineage generation capacity than CD34+CD38−/lo bone marrow hematopoietic stem cells, whereas the myeloid differentiation was similar.
Conclusions
Our data shows that cord blood hematopoietic stem cells have higher T-lymphoid differentiation potential than bone marrow hematopoietic stem cells and that this property is cell autonomous.
Keywords: cord blood, bone marrow, differentation, hematopoietic stem cells
Introduction
There are currently three possible sources for procuring the hematopoietic stem cells (HSC) used for stem cell transplantation1 These three different sources exhibit specific characteristics. Bone marrow HSC are residents of the bone marrow niche, whereas mobilized peripheral blood HSC have been stimulated by growth factors and are no longer influenced by bone marrow stromal cells. Cord blood HSC not only share some characteristics of mobilized peripheral blood HSC, as they are no longer influenced by the bone marrow niche, but they also have characteristics deriving from the young age of the donor. The ability of human HSC to sustain long-term hematopoiesis in vitro was reported to be higher for cord blood than for adult bone marrow, as illustrated by differences in cloning efficiency for myeloid, erythroid and mixed myeloid-erythroid colony-forming cells.2 Interestingly, there was a gradual shift towards the production of myeloid colony-forming cells upon culture; the lymphoid potential was not investigated. It is, however, of relevance to investigate whether these differences between cord blood and bone marrow HSC also translate into differences in T-cell potential, especially because, after HSC transplantation, the recovery of T cells is delayed in comparison to that of other lineages and this delay is a major cause of life-threatening infections.3
T cells develop within the thymus after entry of circulating hematopoietic progenitor cells with uncertain phenotype. Recent evidence suggests that all T-lineage potential resides within the most primitive CD34+CD38−Lin− subset of cord blood and bone marrow precursors, although earlier studies showed that the CD34+CD38+ subset also has T-lineage capacity in vitro. The identity of the most immature thymocytes remains unclear, but the latest evidence suggests that the CD34+CD10+CD1a−CD7− subset4 contains the most primitive intrathymic T-cell precursors. In any case, as for murine T-cell differentiation, developing human T cells follow a series of stage-specific differentiation events that can be characterized by the coordinate expression of CD4 and CD8, whereby double-negative cells are the early precursor population, double-positive cells represent T cells that undergo TCR-αβ selection and single CD4 and CD8 cells represent the end-stage of intrathymic post-selection naïve T cells.5
Studies of stem cell activity in humans with T-cell lineage capacity have long been hampered by the lack of robust and reproducible in vitro T-cell differentiation assays. Using HSC from fetal liver, we were able to obtain robust T-cell differentiation in vitro when these cells were introduced into fetal thymi from NOD-SCID mice and the fetal organs were cultured in vitro.6 With this hybrid human-mouse fetal thymus organ culture technique (FTOC), the kinetics of the very early steps of T-cell differentiation could be addressed by the sequential appearance of surface and intracellular antigens, including CD4, CD7, HLA-DR and cytoplasmatic CD3ɛ. We showed that the early CD34+CD38−CD4−CD7− precursor cells do not express intracellular cytoplasmic CD3 (cyCD3) but display HLA-DR in varying intensities from negative to strongly positive. These cells differentiate into a CD4+ population that remains CD7−cyCD3−HLA-DR++ and into a CD4− population that expresses CD7 and cyCD3. The CD4+CD7−cyCD3− cells differentiate into phenotypically and functionally mature dendritic cells but do not differentiate into T or NK cells. The CD4−CD7−cyCD3+ population differentiates into a CD4+CD7+cyCD3+HLA-DR− population, which has lost its potential to differentiate into dendritic cells, but is able to differentiate into NK cells and T-cell receptor (TCR)-αβ and TCR-γδ T cells. With this hybrid human-mouse FTOC, we7 and others8,9 were able to show that human HSC from cord blood generate more T cells than bone marrow HSC. However, the migration efficiency of the HSC into the lobes of fetal thymus in this FTOC assay is important and could be responsible for the observed differences. Nowadays, in vitro development of human HSC into T cells can be obtained by co-culture with a murine bone marrow-derived OP9 stromal cell line engineered to express the mouse DLL1 Notch ligand (OP9-DL1).10
Here, we present a study in which we examined the differences in myeloid and T-cell progenitor capacity between cord blood and bone marrow HSC in more detail using the OP9-DL1 co-culture system.
Design and Methods
Cell samples
Samples from cord blood and adult bone marrow were obtained and used according to the guidelines of the Medical Ethical Commission of Ghent University Hospital (Ghent, Belgium), and informed consent was obtained in accordance with the Declaration of Helsinki.
Mononuclear cells were collected after centrifugation over Lymphoprep and were cryopreserved in 10% dimethylsulfoxide, 90% fetal calf serum until required. Cells were thawed and the CD34+ cells were selected using magnetic microbeads (Miltenyi Biotec). Cells were then stained with CD34-APC, CD38-PE, CD14-FITC, CD19-FITC, CD56-FITC (BD Biosciences) and sorted for CD34+38−lin− (cord blood and bone marrow) to a purity of greater than 99% using a FACSAria II cell sorter (BD Biosciences).
Carboxyfluorescein diacetate succinamidyl ester labeling
For carboxyfluorescein diacetate succinamidyl ester (CFSE) labeling,9,11 cord blood or bone marrow CD34+cells were resuspended at a density of 1×106/mL in phosphate-buffered saline with 0.1% bovine serum albumin containing 5 μM CFSE (Molecular Probes). After 4 min at 37°C, further uptake of the dye was blocked by the addition of cold phosphate-buffered saline + 30% fetal bovine serum. The cells were washed three times, with the last wash being performed in serum-free phosphate-buffered saline. Finally, the cells were resuspended at a density of 5×105/mL in α-MEM supplemented with 20% fetal calf serum, and cytokines, stem cell factor, FMS-like tyrosine kinase-3 ligand (FLT3L), and thrombopoietin (20, 10, 10 ng/mL, respectively) and cultured overnight at 37°C in 24-well plates, to allow the efflux of unbound CFSE.
OP9 co-cultures
OP9-GFP and OP9-DL1 cells were maintained in complete medium.10 For limiting dilution experiments, monolayers of OP9 cells were established in 96-well plates or 48-well plates. Bulk cultures were performed in 24-well plates (Falcon, Becton-Dickinson). For CFSE experiments, CD34+ cells were cultured for 4 days in 24-well plates with OP-DL1 cells in complete medium and cytokines: SCF (50 ng/mL), FLT3L (20 ng/mL), and interleukin-7 (5 ng/mL). Experiments were started with 20,000 cells/well. In mixing experiments, 10,000 CFSE-labeled CD34+ cells from cord blood were mixed with 10,000 unlabeled CD34+ cells from bone marrow or vice versa. Some of the CFSE-labeled cells were cultured in the presence of 0.1 μg/mL colcemid as a control for undivided cells. For long-term experiments, co-cultures were started with 4,000–5,000 CD34+ cells/well.
Phenotypic characterization
Cord blood or bone marrow HSC were stained with the following antibodies: CD34-FITC, CD4-PE, CD15-PE, CD14-APC, TCRαβ-PE or APC (Miltenyi, Biotec) CD1-PE, CD7-PE, CD8β (Coulter) CD3-APC-Cy7, HLA-Dr-APC-Cy7, CD4-PE-Cy7, CD5-PE-CY7, CD45-Percp-Cy5.5 5 (E-bioscience), CD34-APC, CD7-V450, TCRγδ-FITC, CD14-FITC, CD19-FITC, CD56-FITC (BD). CD1-FITC (clone OKT6) was cultured; antibody was purified and labeled in our laboratory. Dead cells were excluded with propidium iodide. Multicolor sorting was done with a FACSAria II (Becton Dickinson). Multicolor analyses were done with an LSR II flow cytometer equipped with an HTS plate reader system. FACS data were analyzed using either FACSDIVA, FlowJo software (Tree Star) or ModFit LT (Verity Software).
Cloning analysis of myeloid and T-cell lineage potential
Cord blood or bone marrow CD34+38−/lo cells from three different individual samples each were sorted using the BD Clonecyt Plus Option (BD Biosciences) to deposit 1, 3, 10 or 30 cells (with 30–48 replicates for each donor) directly into individual wells of a 96-well plate containing OP9-DL1 or OP9-GFP cell monolayers and complete medium with the appropriate cytokines.12 Each week cells were transferred to fresh OP9-DL1 or OP9-GFP monolayers in 96-well plates: half of the medium was removed and the complete wells were resuspended and transferred to fresh monolayers and supplied with fresh medium and cytokines. The last week, the cells were transferred to 48-well plates containing OP9-DL1 or OP9-GFP monolayers. Cells in co-cultures on OP9-GFP were analyzed after 19–21 days of co-culture, whereas cells in co-culture on OP9-DL1 cells were analyzed after 28–32 days of co-culture.
Statistical analysis
Data from the limiting dilution assays of each cell source were pooled for statistical analysis using ELDA software (http://bioinf.wehi.edu.au/software/limdil13).
Results
Higher frequency of hematopoietic stem cells with T-cell potential in cord blood than in bone marrow hematopoietic stem cells
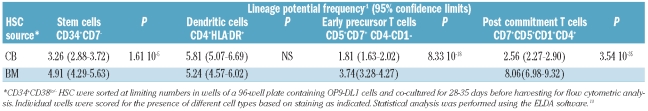
To determine the T-lineage potential of bone marrow and cord blood HSC, limiting dilution assays were performed using OP9-DL1 co-culture assays. Graded numbers of CD34+CD38−Lin− HSC from bone marrow and cord blood were co-cultured with OP9-DL1 stromal cells, and assayed phenotypically after 4–5 weeks for the presence of the following populations: undifferentiated CD34+CD7−HSC, CD4+HLA−DR+ dendritic cells and two populations engaged in two successive steps along the T-lymphoid pathway: uncommitted CD5+CD7+ CD4−CD1− early T-cell precursors and CD5+CD7+ CD1+CD4+ cells, which represent a further stage of committed T-cell progenitors.5,14 As shown in Table 1, the frequency of HSC that have the potential to differentiate into CD34+CD7− cells was higher in cord blood than in bone marrow. There were no significant differences between bone marrow and cord blood HSC regarding the frequency of generation of CD4+HLA-DR+ dendritic cells. Importantly, the frequency was two times higher in cord blood than in bone marrow HSC when the potential to differentiate into CD5+CD7+ early T cells was evaluated, and this increased to a 3-fold difference when CD5+CD7+CD1+CD4+ committed T-lineage precursors were scored at a later stage of differentiation.
Table 1.
Comparative frequency analysis of lineage potential in human hematopoietic stem cells from cord blood (CB) and bone marrow (BM) co-cultured in OP9-DL1 cells.
In parallel, limiting dilution assays were performed to compare the myeloid differentiation capacity of bone marrow and cord blood HSC. OP9-GFP co-culture assays were used for this purpose as they are better suited for the analysis of myeloid development due to the absence of T-lineage-inducing Notch ligands. Graded numbers of CD34+CD38−Lin− HSC from bone marrow and cord blood were co-cultured with OP9-GFP stromal cells and were phenotypically assayed after 2–3 weeks for the presence of the following populations: undifferentiated CD34+ HSC, CD14+HLA-DR+ monocytes and CD15+ granulocytes. As shown in Table 2, the frequency of bone marrow HSC and cord blood HSC differentiating into CD34+ HSC and CD14+ HLA-DR+ monocytes did not differ significantly. However, the potential to develop into CD15+ granulocytes was higher in cord blood HSC than in bone marrow HSC.
Table 2.
Comparative frequency analysis of lineage potential in human hematopoietic stem cells from cord blood (CB) and bone marrow (BM) co-cultured in OP9-GFP cells.
Thus, while little difference was observed with respect to the myeloid differentiation capacities of bone marrow and cord blood HSC, it is clear that the T-lineage potential of bone marrow-derived HSC is dramatically reduced compared to that of cord blood HSC.
Faster and more extensive T-cell differentiation by cord blood hematopoietic stem cells
Given this reduction in T-lineage potential in adult bone marrow HSC compared to cord blood HSC, we decided to investigate this difference further using bulk culture assays as they provide more cells for detailed and kinetic analyses. As shown in Figure 1, a quantitative analysis revealed that cord blood HSC generate more cells in OP9-DL1 co-culture in comparison with bone marrow HSC.
Figure 1.
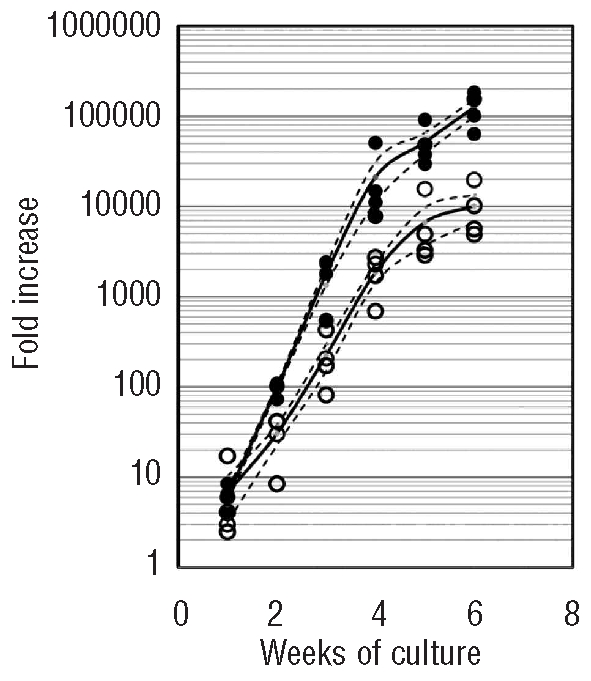
Higher total nucleated cell expansion by cord blood (CB) HSC. HSC cells were co-cultured with OP9-DL1 cells. After incubation during the indicated time period, cells were harvested and total nucleated cell number was analyzed. Individual data are represented as closed circles (CB) or open circles (BM) with mean (black solid line) ± SEM (dashed line).
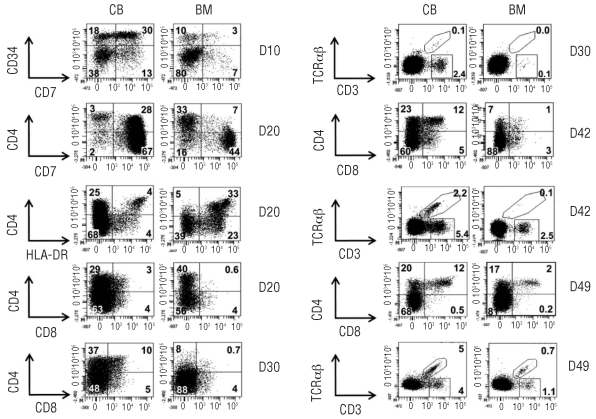
However, a difference in cellular expansion was not sufficient to explain the difference in T-lineage output between the two HSC sources since the frequencies of the developing T-cell subsets were also increased in cultures initiated with cord blood HSC compared to those started with bone marrow HSC (Figure 2).
Figure 2.
Faster and more extensive T-cell differentiation by cord blood (CB) HSC. Sorted CD34+CD38−Lin− HSC from CB and bone marrow (BM) were cultured on OP9-DL1 cells in the presence of interleukin-7, Flt3 ligand and stem cell factor, and their developmental progression was assessed at the indicated time points by flow cytometric analysis for different antigens as indicated. Numbers in quadrants indicate the percentage of cells. TCR-αβ and TCR-γδ (CD3+-αβ cells) are delineated by regions, figures next to those regions indicate the corresponding percentage of cells.
After 10 days, analysis of CD34 versus CD7 showed that cord blood HSC generated cells with a CD34+CD7+ phenotype more efficiently than did bone marrow HSC. In contrast, bone marrow HSC generated a higher frequency of cells with a CD34−CD7− phenotype in comparison with cord blood HSC. When the populations were analyzed on day 20 according to the coordinate expression of CD4 and CD7, we observed that cells co-expressing CD4 and CD7 were clearly present in the OP9-DL1 co-cultures with HSC from cord blood, but this was not the case for the co-cultures started with bone marrow HSC. In these latter cultures, almost none of the CD4 cells expressed CD7 and could be regarded as precursors of CD4+HLA-DR+ monocytic/dendritic population (Figure 2).
These experiments show that there were significant differences between bone marrow and cord blood HSC in the early steps of differentiation after a short period of co-culture in OP9-DL1 cells.
After 30 days of culture, analysis of the coordinated expression of CD4 and CD7 showed that HSC from bone marrow are more efficient in generating a population that is CD4+CD7−. This population also expresses HLA-DR (data not shown). In contrast, the distinguishing feature of HSC from cord blood is a predominance of CD7+ cells. Some of these cells were CD4+ and represented cells that were already CD4 immature or double-positive thymocytes. The other part was CD4− and represented cells at earlier stages of differentiation. We, therefore, may assume that after 30 days of culture the differences in T-cell generating capacity between bone marrow (fewer double-positive, fewer CD7 cells) and cord blood are apparent.
After 6 weeks of co-culture with OP9-DL1 cells, cord blood HSC did not only generate a higher number of cells (Figure 1), but also higher frequencies of double-positive thymocytes, TCR-αβ and TCR-γδ T cells (Figure 2). This indicates that cord blood HSC are more efficient at T-cell generation than bone marrow HSC. When we analyzed the kinetics of the T lymphoid differentiation, we noted that TCR-αβ and TCR-γδ T cells were already present after 30 days when the cultures were initiated with HSC from cord blood, whereas these T cells were nearly absent in corresponding bone marrow HSC cultures. After 30 days of co-culture, HSC from cord blood had already generated a significant number of double-positive thymocytes, whereas this cell population was barely present when HSC from bone marrow were used.
Collectively, the quantitative and qualitative data show that HSC from cord blood have a more robust T-cell differentiation capacity compared to HSC from bone marrow.
The T lymphoid differentiation capacity of cord blood and bone marrow hematopoietic stem cells can be discriminated very early in OP9-DL1 co-cultures
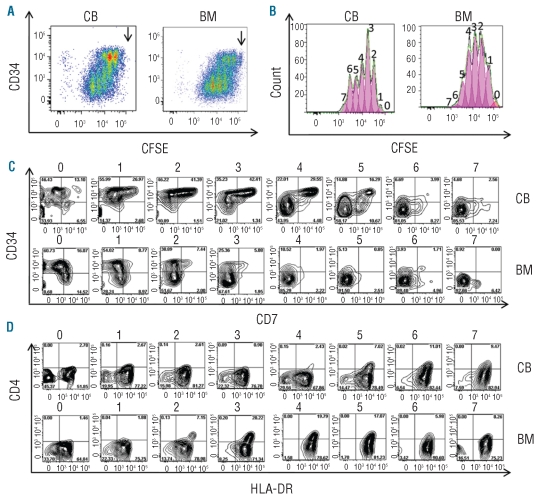
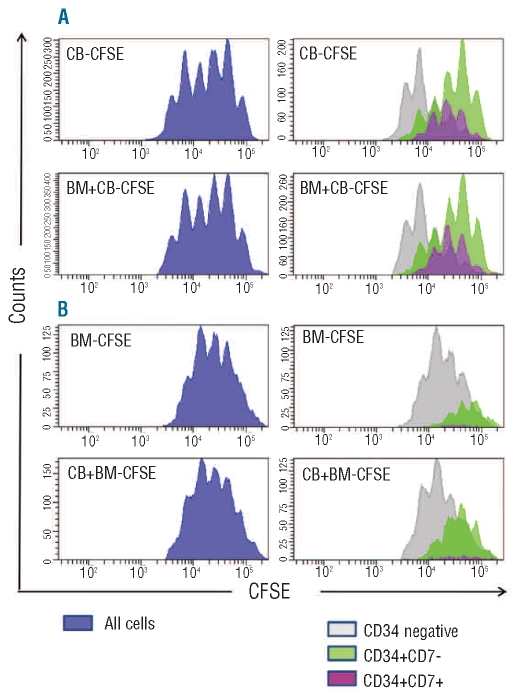
Given the differences in both proliferation and differentiation between cord blood and adult bone marrow HSC with respect to T-cell differentiation, we initiated CFSE experiments in OP9-DL1 co-cultures to investigate the relationship between the two phenomena. The CD34high progeny for both sources divided actively up to six or seven times during a 4-day culture period (Figure 3A,B). The CD34+ cell divisions were developmentally asymmetrical. Most cells in the first to third generations retained the original bright levels of CD34, typically found in human HSC and consistent with self-renewal of CD34highLin− cells which has been described in conditions of high Notch activation.15–17 However, some of the cord blood CD34high progenitor cells started a stepwise differentiation wave, from the first-second generation onwards, in which they retained CD34high expression but also up-regulated CD7 (Figure 3C). These CD34high cells continued to divide but progressively down-regulated CD34 from the third generation onward. Importantly, bone marrow HSC did not up-regulate CD7 to the same extent and instead up-regulated HLA-DR and subsequently also CD4, thus differentiating towards the dendritic cell lineage, despite the presence of high densities of the Notch ligand DLL1 (Figure 3C,D). This wave was more prominent for bone marrow HSC than for cord blood HSC and continued by most of the cells in the fourth to seventh generations and completed in the sixth generation.
Figure 3.
Comparative CFSE profiles and cell surface phenotype of HSC co-cultured on OP9-DL1 for 4 days reveals higher propensity of cord blood (CB) than bone marrow (BM) HSC to generate T lymphocytes. CFSEhighCD34highLin− precursors were purified by FACS (>99.5% pure, n = 3) from CB or BM mononuclear cells loaded with CFSE; labeled with CD34−, Lin-specific antibodies; and co-cultured on OP9-DL1 stroma for 4 days (D4). The CFSEhighCD34highLin− progeny was stained with CD34, CD7, CD5, CD4 and HLA-DR-specific antibodies and submitted to flow cytometry analysis to quantify CFSE content (numbers on top of each peak designate the number of progeny divisions, with 0 representing no division) (A) and surface CD34 levels in each generation dot-plot analysis of CD34 expression versus CFSE content (B), using the same samples as in (A) [position of undivided cells is shown (↓)]. (C) Day 4 expression of CD34 and CD7 in gated CD34+ cells ordered by their progeny generation, as defined by CFSE levels and indicated by numbers on top of the plot. Numbers in quadrants represent the frequency of positive cells. (D) Day 4 expression of CD4 and HLA-DR in gated CD34+ cells ordered by their progeny generation, as defined by CFSE levels and indicated by numbers on top of the plot. Numbers in quadrants represent the frequency of positive cells. Data are representative of the results from three independent experiments. Note that in OP9-DL1 co-cultures, in the first wave of development, CD34highLin− precursors asymmetrically self-renew and differentiate into two distinct populations that down-regulate CD34, up-regulate HLA-DR and acquire CD4 on the one hand and maintain CD34, down-regulate HLA-DR but acquire CD5 and CD7 on the other hand.
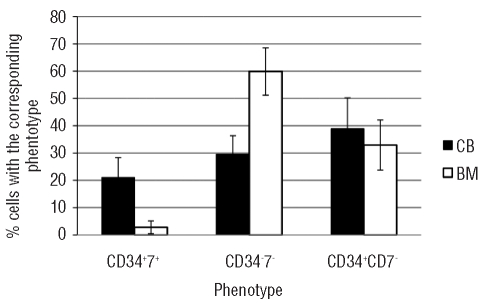
Thus, these data show that in the first 4 days of culture, CD34+Lin− progenitors can differentiate along two distinct developmental pathways: one lymphoid and one myeloid. In the lymphoid pathway CD34 expression is maintained during more cell divisions and CD7 is up-regulated. Finally, CD34 expression is down-regulated but CD7 expression is maintained. Along the myeloid pathway, differentiation is characterized by an immediate down-regulation of CD34 and absence of CD7 expression. Using the in vitro hybrid human-mouse FTOC, we previously showed that, for CD34+ progenitors from human fetal liver, these features allow an early discrimination between CD34+CD7+ progenitor cells that will become lymphoid cells and CD34−CD7− cells that will become dendritic/monocytic cells.6 Consistent with these data and the phenotypic changes observed in the CFSE experiments, we used these parameters to estimate the frequency of lymphoid and myeloid progeny obtained from CD34+Lin− progenitors from bone marrow and cord blood. As shown in Figure 4, CD34+Lin− progenitors from bone marrow have a significantly higher potential than those from cord blood to produce myeloid cells (P=0.007), whereas the potential to produce lymphoid cells is significantly higher for the cord blood progenitor cells (P=0.006).
Figure 4.
Frequency of myeloid and lymphoid cells after 4 days of co-culture of HSC from cord blood (CB) and bone marrow (BM) on OP9-DL1. HSC from CB and BM were co-cultured for 4 days on OP9-DL1 stromal cells and phenotyped for coordinated expression of CD34 and CD7. Bars represent the frequencies of the cells generated by CB (black bars) or BM (white bars). Bars represent the mean with the standard deviation of three independent experiments.
Overall, these results show that adult bone marrow HSC contain a large fraction of precursors that develop along the myeloid pathway but not along the lymphoid T-lineage pathway, despite the abundant presence of Delta-like Notch ligands.
The difference in T-cell developmental potential of cord blood and bone marrow hematopoietic stem cells is cell-autonomous
The superiority of cord blood HSC over bone marrow HSC at engaging in the T-cell developmental pathway in OP9-DL1 co-cultures could be due to non-cell-autonomous phenomena, including differential production of cytokines or other soluble factors, or the emergence of a subpopulation of cells that negatively or positively influences differentiation into T cells. To address this issue, we mixed HSC from cord blood and bone marrow and traced their progeny.
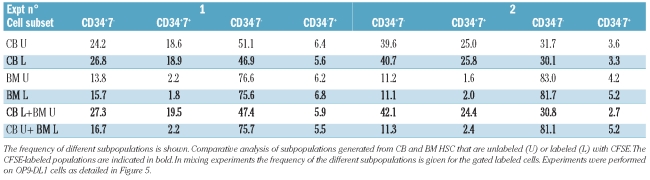
In a first series of control experiments, HSC from cord blood or bone marrow were labeled with CFSE and cultured in OP9-DL1 co-cultures for 4 days. When compared to unlabeled cord blood or bone marrow cells, respectively, the frequencies of the CD34− progenitors engaged in the myeloid pathway, CD34+CD7+ progenitors engaged in the lymphoid pathway and the number of CD34+CD7− progenitors remained unchanged (Table 3), indicating that the cell labeling procedure did not interfere with the cell developmental properties in our co-culture assay. In addition, CFSE analysis demonstrated that the proliferation of the CFSE-labeled cord blood or bone marrow cells was not affected when mixed with unlabeled cord blood or bone marrow cells (data not shown). Moreover, and in agreement with our previous assay (Figure 3A), cells predominantly maintained their original CD34+CD7− phenotype during the first three generations, compatible with self-renewal. Populations in further divisions (fourth and fifth) showed an important proportion of CD34+CD7+ early lymphoid and T-lineage precursors, while populations in the sixth and seventh divisions predominantly contained cells with a CD34− phenotype, indicative of cells engaged in a myeloid pathway. Importantly, as shown in Figure 5 and Table 3, when equal amounts of unlabeled bone marrow HSC and CFSE-labeled cord blood HSC were mixed and cultured on OP9-DL1 cells, the properties of the generated cord blood cells did not change and were similar to those of the OP9-DL1 control co-cultures initiated with only cord blood HSC. As shown in Figure 5 and Table 3, similar results were obtained when the properties of CFSE-labeled bone marrow HSC in OP9-DL1 co-cultures were analyzed in the presence of unlabeled cord blood HSC. In conclusion, these results show that the reduced T-lineage potential of adult bone marrow HSC is due to a cell-intrinsic defect that, furthermore, cannot be rescued by the presence of developing early T-cell precursors from cord blood.
Table 3.
Influence of mixing bone marrow (BM) and cord blood (CB) HSC on differentiation.
Figure 5.
Mixing cord blood (CB) and bone marrow (BM) HSC does not influence the intrinsic difference in T-cell developmental potential. HSC were labeled with CFSE, cultured for 4 days on OP9-DL1 cells and subsequently harvested and stained with CD34 and CD7. In panel (A), left histograms show the CFSE profile of total CB-CFSE HSC alone (top row) or when cultured in the presence of unlabeled BM HSC (bottom row). In panel (B), left histograms show CFSE profiles of total BM-CFSE HSC alone (top) or when cultured in the presence of unlabeled CB HSC (bottom row). The corresponding histograms on the right show an overlay of the CFSE profiles on the cells gated on the three phenotypes as indicated. As the profile of the CFSE-labeled HSC does not change when they are cultured with the HSC from the different sources, these data strongly suggest that the differences in cell proliferation are cell autonomous. Data are representative of two independent experiments shown in Table 3.
Discussion
In this study we investigated the difference in T-lineage capacity between cord blood and adult bone marrow HSC. Clonal experiments revealed a significantly lower T-cell differentiation potential for the bone marrow HSC compared to the cord blood HSC and CFSE experiments in bulk cultures showed that this was due to the preferential adoption of a myeloid differentiation program by the early dividing bone marrow HSC, despite the presence of abundant Notch ligands. Furthermore, mixed cultures of cord blood and adult bone marrow HSC revealed that this reduction in early T-lineage priming in bone marrow HSC was cell-intrinsic and not remedied by the presence of developing T lymphocytes.
Through limiting dilution analysis on OP9-DL1 cells, we determined the frequency of cord blood and bone marrow HSC that had the potential to differentiate into T-cell progenitors and found that this frequency was twice as high for cord blood cells as compared to bone marrow cells. Our data for cord blood HSC reveals a 3-fold higher progenitor potential compared to the data obtained by Awong et al.18, but this is most likely due to kinetic differences with respect to timing of analysis since they looked after 11 days of culture, whereas we scored the cells after 28–35 days. This culture period allowed us to circumvent the issues of differences in developmental kinetics, because we have shown previously19 that at that time bone marrow cells, although they have slower kinetics, have already progressed to this early step of T-lineage differentiation on OP9-DL1 cells. The longer culture period may account for the higher potential in our study since the cells were provided with more time to expand. In a very recent study, Dick’s group 20 analyzed the clonal lineage potentials of a number of different CD34+ hematopoietic progenitor subsets from both cord blood and bone marrow. Since the CD34+CD38− HSC population used in this study, was divided into four different subsets in their study, it is difficult to compare the results from both studies. Using CD34+CD38−/lo HSC, we found a cloning efficiency of 47.9%±15.5 for cord blood and 12.5%±7.2 for bone marrow. In their study, the most robust fraction of CD34+CD38−/lo precursors that contained T-lineage potential also revealed a higher efficiency for cord blood HSC (45%) as compared to bone marrow HSC (27%). Despite the different populations used, the results from both studies are surprisingly similar and the relatively small differences that are observed can easily be attributed to the subsets that were investigated, as well as to differences in culture duration and phenotypic characterization. In each case, from the study by Dick group’s, it is clear that the total CD34+CD38−/lo subset comprises all main precursors for T-lineage cells, indicating that our results are representative for the global T-lineage potential of cord blood and bone marrow HSC.
Remarkably, while we observed a clear difference between cord blood and bone marrow HSC with respect to the generation of uncommitted T-lineage precursors, this difference significantly increased when scoring a further developmental stage along the T-lineage pathway, namely committed T progenitors. Since bulk cultures suggest that the HSC were given sufficient time to differentiate towards this stage, this finding suggests that not only is early commitment different between both HSC sources, but also that other differences are present within the differentiating T-lineage precursors. While this may involve differences in proliferation capacities and cell survival, our results suggest that the defect in T-lineage potential of bone marrow HSC occurs at multiple stages of T-cell development and as a result of different phenomena.
The difference between cord blood and bone marrow HSCs in engagement along the T-lineage pathway was revealed using bulk culture analysis in conjunction with CFSE labeling. With this approach, we were able to track the order of phenotypic changes towards the T cell and myeloid pathway in the early steps of differentiation. Based on our previous studies6 with fetal liver HSC in FTOC and cord blood HSC in OP9-DL1 co-cultures,21 the rapid upregulation of CD7 in CD34+ HSC at high levels can be used as an early marker for engagement towards T-cell differentiation. Since this process is Notch-dependent, the highly significant difference between the abundance of CD34+CD7++ cells in the OP9-DL1 co-cultures initiated with cord blood HSC and the near absence of this population when starting with bone marrow HSC suggests that fewer bone marrow HSC are responsive to the Notch ligand DL1. Since bone marrow HSC, compared to their cord blood counterpart, display a significantly higher frequency of CD34−CD7− cells that represent myeloid lineage differentiation, our findings indicate that very early in the developmental pathway, an important bias for lymphoid cell development discriminates cord blood from bone marrow HSC. Indeed, Delta-Like lig-and induced Notch signaling has been shown to suppress myeloid differentiation, a process that is critical to maintain developing T cells along the T-lineage pathway. The idea of lineage bias is also in accordance with the observation of Panepucci et al.22 who showed that CD34+ and CD133+ cord blood cells had higher HES1 transcript levels compared to their bone marrow counterparts. This could indicate that HSC may have already experienced Notch signaling before migrating to the thymus, a process that may involve Jagged1-mediated Notch signaling. In addition, the observed increased transcript levels of Notch1, TAL1, distinct NF-κB subunits, and other transcription factors on cord blood HSC may prompt these cells to respond more effectively to signals driving lymphopoiesis.
Given the bias of bone marrow HSC to develop along the myeloid pathway, it was important to investigate whether these or other cells that develop in parallel with the T-lineage cells could negatively affect T-cell development, thereby explaining the reduced T-cell potential of bone marrow HSC compared to cord blood HSC. In order to investigate whether HSC from both sources display cell intrinsic differences with respect to early T-cell development, we used CFSE staining to trace the differentiation of HSC from bone marrow and cord blood in mixing experiments. First, we showed that the CFSE staining as such did not influence the differentiation kinetics and characteristics of the HSC, and in addition, that mixing CFSE-labeled cells with unlabeled cells from the same source had no influence on these parameters. Importantly, when either labeled cord blood or bone marrow HSC were mixed with unlabeled bone marrow or cord blood, respectively, no effect was observed on the generation of the different subsets according to the coordinate expression of CD34 and CD7. We, therefore, provide evidence that the differences in T-cell progenitor frequency between cord blood and bone marrow HSC are cell-autonomous and are not due to the production of lymphoid promoting factors by cord blood HSC or of lymphoid-inhibiting factors by soluble factor or bone marrow HSC. These results also illustrate that early developing lymphocytes cannot positively influence other HSC to develop along the T-lineage pathway.
Our finding is in line with new insights into the identification and definition of HSC.23 Recently, it was shown, in mice, that abundant CD150 expression identified a subset of HSC with very potent self-renewal capacity. In addition, CD150 levels predicted myeloid versus lymphoid reconstitution potential with robust myeloid potential for CD150high HSC and superior lymphoid reconstitution for CD150−HSC.24 However, although this CD150high population had an impressive capacity to reconstitute the lymphoid system,24–27 it partially preserved self-renewal and did not express FLT3. These cells are, therefore, different from the lymphoid-primed multipotent progenitors identified by Jacobsen’s group.28 In addition, the population also retained erythroid-megakaryocytic potential.24 These properties are compatible with the view that stem cells may already show propensity to generate preferentially different lineages.29 From our observations, we propose that HSC from cord blood and bone marrow have different differentiation capacities and that cord blood are more lymphocyte-lineage-biased and bone marrow are more myeloid-lineage-biased. It will be important to explore whether markers can be found for human HSC that, analogous with CD150 in mice, can identify these lineage-biased HSC subsets.
While the increased T-cell potential of cord blood HSC is in accordance with the better reconstitution of early and committed hematopoietic progenitors and the higher thymic function and T-cell receptor diversity upon cord blood HSC transplantation, in comparison with bone marrow HSC transplantation,30,31 it is unclear whether this is due to the immaturity of the cord blood HSC, with a status that more closely resembles that of embryonic stem cells, or due to a difference in microenvironment at the time of isolation. The former hypothesis is in line with our preliminary results that show that fetal liver- or fetal bone marrow-derived HSC possess even higher T-cell potential compared to cord blood HSC, while mobilized peripheral blood HSC also show very little T-lineage capacity (data not shown). We, therefore, favor the idea that precursors that are generated earlier during ontogeny might possess a higher capability to differentiate along the T-lineage pathway. This might be the result of greater plasticity of fetal-derived progenitors than of their adult counterparts, resulting in more flexibility to respond to specific environmental cues, such as Notch-activating ligands. It will be of interest to investigate whether this is caused by differences in the epigenetic landscape within the different HSC.
The present study has provided evidence to support the hypothesis that human HSC are composed of heterogeneous cells wherein lymphoid-biased HSC are more enriched in cord blood than in bone marrow. This bias can be rapidly detected by monitoring changes in cell surface proteins and as such, these findings could be of use for exploring human markers, analogous to CD150 in the mouse, which phenotypically discriminate between these lineage-biased HSC. In any case, it will be essential to delineate the molecular mechanisms that account for the defect in early T-lineage differentiation of bone marrow-derived HSC in order to improve immune reconstitution following HSC transplantation.
Acknowledgments
We thank Juan Carlos Zúñiga-Pflücker (University of Toronto, Toronto, ON, Canada) for the OP9-DL1 cells.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
Funding: this work was supported by the Fund for Scientific Research, Flanders (FWO Vlaanderen) and its Odysseus Research program, Stichting tegen Kanker and the Interuniversity Attraction Poles Program (IUAP), supported by the Belgian Science Policy. TK and TT are supported by the Fund for Scientific Research, Flanders (FWO Vlaanderen)
References
- 1.Laghero J, Garcia J, Gluckman E. Genoa, Italy: EBM-ESH Handbook Forum Service Editore; 2008. 2008. Sources and procurement of stem cells; pp. 112–27. [Google Scholar]
- 2.Lansdorp PM, Dragowska W, Mayani H. Ontogeny-related changes in proliferative potential of human hematopoietic cells. J Exp Med. 1993;178(3):787–91. doi: 10.1084/jem.178.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wils EJ, Cornelissen JJ. Thymopoiesis following allogeneic stem cell transplantation: new possibilities for improvement. Blood Rev. 2005;19(2):89–98. doi: 10.1016/j.blre.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Hao QL, George AA, Zhu J, Barsky L, Zielinska E, Wang X, et al. Human intrathymic lineage commitment is marked by differential CD7 expression: identification of CD7- lympho-myeloid thymic progenitors. Blood. 2008;111(3):1318–26. doi: 10.1182/blood-2007-08-106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blom B, Spits H. Development of human lymphoid cells. Annu Rev Immunol. 2006;24:287–320. doi: 10.1146/annurev.immunol.24.021605.090612. [DOI] [PubMed] [Google Scholar]
- 6.Plum J, De Smedt M, Verhasselt B, Offner F, Kerre T, Vanhecke D, et al. In vitro intrathymic differentiation kinetics of human fetal liver CD34+CD38- progenitors reveals a phenotypically defined dendritic/T-NK precursor split. J Immunol. 1999;162(1):60–8. [PubMed] [Google Scholar]
- 7.Offner F, Kerre T, De Smedt M, Plum J. Bone marrow CD34 cells generate fewer T cells in vitro with increasing age and following chemotherapy. Br J Haematol. 1999;104(4):801–8. doi: 10.1046/j.1365-2141.1999.01265.x. [DOI] [PubMed] [Google Scholar]
- 8.Robin C, Bennaceur-Griscelli A, Louache F, Vainchenker W, Coulombel L. Identification of human T-lymphoid progenitor cells in CD34+ CD38low and CD34+ CD38+ subsets of human cord blood and bone marrow cells using NOD-SCID fetal thymus organ cultures. Br J Haematol. 1999;104(4):809–19. doi: 10.1046/j.1365-2141.1999.01266.x. [DOI] [PubMed] [Google Scholar]
- 9.Robin C, Pflumio F, Vainchenker W, Coulombel L. Identification of lymphomyeloid primitive progenitor cells in fresh human cord blood and in the marrow of nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice transplanted with human CD34(+) cord blood cells. J Exp Med. 1999;189(10):1601–10. doi: 10.1084/jem.189.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17(6):749–56. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 11.Taghon T, Van de Walle I, De Smet G, De Smedt M, Leclercq G, Vandekerckhove B, et al. Notch signaling is required for proliferation but not for differentiation at a well-defined beta-selection checkpoint during human T-cell development. Blood. 2009;113(14):3254–63. doi: 10.1182/blood-2008-07-168906. [DOI] [PubMed] [Google Scholar]
- 12.Hoebeke I, De Smedt M, Van de Walle I, Reynvoet K, De Smet G, Plum J, et al. Overexpression of HES-1 is not sufficient to impose T-cell differentiation on human hematopoietic stem cells. Blood. 2006;107 (7):2879–81. doi: 10.1182/blood-2005-05-1815. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347(1–2):70–8. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Vanhecke D, Verhasselt B, De Smedt M, Leclercq G, Plum J, Vandekerckhove B. Human thymocytes become lineage committed at an early postselection CD69+ stage, before the onset of functional maturation. J Immunol. 1997;159(12):5973–83. [PubMed] [Google Scholar]
- 15.Arakawa-Hoyt J, Dao MA, Thiemann F, Hao QL, Ertl DC, Weinberg KI, et al. The number and generative capacity of human B lymphocyte progenitors, measured in vitro and in vivo, is higher in umbilical cord blood than in adult or pediatric bone marrow. Bone Marrow Transplant. 1999;24 (11):1167–76. doi: 10.1038/sj.bmt.1702048. [DOI] [PubMed] [Google Scholar]
- 16.Berenson RJ, Andrews RG, Bensinger WI, Kalamasz D, Knitter G, Buckner CD, et al. Antigen CD34+ marrow cells engraft lethally irradiated baboons. J Clin Invest. 1988;81(3):951–5. doi: 10.1172/JCI113409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oostendorp RA, Audet J, Miller C, Eaves CJ. Cell division tracking and expansion of hematopoietic long-term repopulating cells. Leukemia. 1999;13(4):499–501. doi: 10.1038/sj.leu.2401373. [DOI] [PubMed] [Google Scholar]
- 18.Awong G, Herer E, Surh CD, Dick JE, La Motte-Mohs RN, Zuniga-Pflucker JC. Characterization in vitro and engraftment potential in vivo of human progenitor T cells generated from hematopoietic stem cells. Blood. 2009;114(5):972–82. doi: 10.1182/blood-2008-10-187013. [DOI] [PubMed] [Google Scholar]
- 19.De Smedt M, Hoebeke I, Plum J. Human bone marrow CD34+ progenitor cells mature to T cells on OP9-DL1 stromal cell line without thymus microenvironment. Blood Cells Mol Dis. 2004;33(3):227–32. doi: 10.1016/j.bcmd.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Doulatov S, Notta F, Eppert K, Nguyen LT, Ohashi PS, Dick JE. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol. 2010;11:585–93. doi: 10.1038/ni.1889. [DOI] [PubMed] [Google Scholar]
- 21.Van de Walle I, De Smet G, De Smedt M, Vandekerckhove B, Leclercq G, Plum J, et al. An early decrease in Notch activation is required for human TCR-alphabeta lineage differentiation at the expense of TCR-gammadelta T cells. Blood. 2009;113(13):2988–98. doi: 10.1182/blood-2008-06-164871. [DOI] [PubMed] [Google Scholar]
- 22.Panepucci RA, Oliveira LH, Zanette DL, Viu Carrara, Rde C, Araujo AG, Orellana MD, et al. Increased levels of NOTCH1, NF-kappaB, and other interconnected transcription factors characterize primitive sets of hematopoietic stem cells. Stem Cells Dev. 19(3):321–32. doi: 10.1089/scd.2008.0397. [DOI] [PubMed] [Google Scholar]
- 23.Hock H. Some hematopoietic stem cells are more equal than others. J Exp Med. 207:1127–30. doi: 10.1084/jem.20100950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med. 207(6):1173–82. doi: 10.1084/jem.20091318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kent DG, Copley MR, Benz C, Wohrer S, Dykstra BJ, Ma E, et al. Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood. 2009;113(25):6342–50. doi: 10.1182/blood-2008-12-192054. [DOI] [PubMed] [Google Scholar]
- 26.Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci USA. 107(12):5465–70. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 6(3):265–78. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121(2):295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Ng SY, Yoshida T, Zhang J, Georgopoulos K. Genome-wide lineage-specific transcriptional networks underscore Ikaros-dependent lymphoid priming in hematopoietic stem cells. Immunity. 2009;30(4):493–507. doi: 10.1016/j.immuni.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frassoni F, Podesta M, Maccario R, Giorgiani G, Rossi G, Zecca M, et al. Cord blood transplantation provides better reconstitution of hematopoietic reservoir compared with bone marrow transplantation. Blood. 2003;102(3):1138–41. doi: 10.1182/blood-2003-03-0720. [DOI] [PubMed] [Google Scholar]
- 31.Talvensaari K, Clave E, Douay C, Rabian C, Garderet L, Busson M, et al. A broad T-cell repertoire diversity and an efficient thymic function indicate a favorable long-term immune reconstitution after cord blood stem cell transplantation. Blood. 2002;99(4):1458–64. doi: 10.1182/blood.v99.4.1458. [DOI] [PubMed] [Google Scholar]