Abstract
Background
In advanced systemic mastocytosis the response of neoplastic mast cells to conventional drugs is poor and the prognosis is bad. Current research is, therefore, attempting to identify novel drug targets in neoplastic mast cells. Polo-like kinase-1 is a serine/threonine kinase that plays an essential role in mitosis and has recently been introduced as a new target in myeloid leukemias and solid tumors.
Design and Methods
In the present study, we analyzed the expression and function of Polo-like kinase-1 in neoplastic mast cells in systemic mastocytosis.
Results
As determined by immunostaining, primary neoplastic mast cells as well as the human mast cell leukemia cell line HMC-1 displayed phosphorylated Polo-like kinase-1. In addition, neoplastic mast cells expressed Polo-like kinase-1 mRNA. Polo-like kinase-1-specific small interfering RNA induced apoptosis in neoplastic mast cells, whereas no effect was seen with a control small interfering RNA. BI 2536, a drug targeting Polo-like kinase-1, was found to inhibit proliferation in HMC-1 cells in a dose-dependent manner. BI 2536 also inhibited the growth of primary neoplastic mast cells and cells of the canine mastocytoma cell line C2. The growth-inhibitory effects of BI 2536 on neoplastic mast cells were found to be associated with mitotic arrest and subsequent apoptosis. Finally, BI 2536 was found to synergize with the KIT-targeting kinase inhibitor midostaurin (PKC412) in inhibiting the growth of neoplastic mast cells. In control experiments, BI 2536 did not induce apoptosis in normal cultured mast cells.
Conclusions
Collectively, our data show that Polo-like kinase-1 is a potential therapeutic target in neoplastic mast cells. Targeting Polo-like kinase-1 may be an attractive pharmacological concept in the management of advanced systemic mastocytosis.
Keywords: mastocytosis, KIT, Plk-1, apoptosis, targeted drugs
Introduction
Systemic mastocytosis (SM) is a myeloid neoplasm characterized by abnormal accumulation and growth of neoplastic mast cells (MC) in one or more internal organs, with or without skin-involvement.1–6 Indolent as well as aggressive variants of SM have been described, with variable clinical presentation and course, and different survival times.1–7 In patients suffering from aggressive SM or MC leukemia, the response to conventional drugs is poor and the prognosis is bad.4–7 A number of attempts have, therefore, been made to identify new therapeutic targets in neoplastic MC and to develop targeted drugs for patients with these diseases.8,9
One major growth regulator and key target in neoplastic MC appears to be the KIT tyrosine kinase receptor that is expressed in MC progenitors as well as in mature MC independently of the type of disease.4–9 In SM, neoplastic MC often display mutant forms of KIT that are characterized by autonomous, ligand-independent activation of the receptor, which may contribute to factor-independent growth of MC in SM.10–15 The effects of several KIT-targeting drugs on neoplastic MC have been examined.16–21 However, although some of these drugs have marked effects on in vitro growth of neoplastic MC18–20, so far, no convincing long-lasting effects have been reported in vivo in patients with advanced SM.21 Moreover, it has been described that KIT D816V alone does not induce malignant transformation in SM22, and that apart from KIT other transforming pathways may also play a role in malignant cell growth in aggressive SM and MC leukemia.23 Current research is, therefore, focusing on additional targets in neoplastic MC.
Polo-like kinase-1 (Plk-1) is a serine/threonine kinase that plays an essential role in cell mitosis in various mesenchymal cells. Correspondingly, depletion of Plk-1 is associated with cell cycle arrest and mitotic catastrophe.24–26 A number of recent studies have shown that Plk-1 is expressed in various neoplastic cells including solid tumors, acute myeloid leukemia, chronic myeloid leukemia, and non-Hodgkin’s lymphomas.24–28 More recently, Plk-1 has been proposed as a potential therapeutic target in solid tumors and various hematopoietic malignancies. BI 2536, a drug that targets Plk-1, induces cell cycle arrest and apoptosis in neoplastic cells.29,30 However, Plk-1 has not been examined in the context of mastocytosis or MC leukemia so far.
In the present study, we examined the expression of Plk-1 in neoplastic MC and investigated whether Plk-1 could serve as a therapeutic target in SM.
Design and Methods
Reagents
The Plk-1 inhibitor BI 2536 was kindly provided by Dr. Dorothea Rudolph (Boehringer Ingelheim, Vienna, Austria). The kinase inhibitor PKC412 (midostaurin)31 was kindly provided by Dr. Johannes Roesel and Dr. Paul Manley (Novartis Pharma AG, Basel, Switzerland). Stock solutions of BI 2536 and PKC412 were prepared by dissolution in dimethylsulfoxide (DMSO) (Merck, Darmstadt, Germany). RPMI 1640 medium and fetal calf serum (FCS) were purchased from PAA laboratories (Pasching, Austria), L-glutamine and Iscove’s modified Dulbecco’s medium (IMDM) were obtained from Gibco Life Technologies (Gaithersburg, MD, USA), propidium iodide from Sigma (St. Louis, MO, USA), and 3H-thymidine from GE Healthcare (Buckinghamshire, UK).
Isolation of primary neoplastic cells
Primary neoplastic cells were obtained from 18 patients with KIT D816V-positive SM. Patients were classified as having indolent SM (n=14), smoldering SM (n=1), aggressive SM (n=1), or MC leukemia (n=2) according to published criteria.32,33 Heparinized bone marrow cells were layered over Ficoll to isolate mononuclear cells. Cell viability was greater than 90% in each case. This study was approved by the institutional review board of the Medical University of Vienna and conducted in accordance with the declaration of Helsinki. All patients gave written informed consent before bone marrow puncture or blood donation. Normal MC were generated from cord blood cultures as described elsewhere.34 In seven cases of canine mastocytoma (grade I, n=1; grade II, n=4; grade III, n=2), MC were isolated from primary tumor samples using collagenase following a published protocol.35,36 In brief, tissue specimens were first cut into small pieces, washed thoroughly in Tyrode’s buffer, and then incubated in collagenase type II (Worthington, Lakewood, NJ, USA) at 37°C for 180 min. Isolated cells were recovered by filtration through nytex cloth and collected into FCS-containing tubes. After washing, cells were examined for viability and percentage of MC (Wright-Giemsa staining).
Culture of human and canine cell lines
The human MC leukemia cell line HMC-137 was kindly provided by Dr. J. H. Butterfield (Mayo Clinic, Rochester, MN, USA). Two subclones were used: HMC-1.1 harboring KIT V560G but not KIT D816V, and HMC-1.2 harboring both KIT mutations.16,19 HMC-1 cells were grown in IMDM and 10% FCS, L-glutamine, α-thioglycerol (Sigma, St Louis, MO, USA) and antibiotics (37°C, 5% CO2). In control experiments, the acute myeloid leukemia cell lines MOLM-13, MV4-11, HL-60, U937, and KG1, and the chronic myeloid leukemia cell lines KU812 and K562 were used. The canine mastocytoma cell line C238 was kindly provided by Dr. Warren Gold (University of California, San Francisco, CA, USA). C2 cells were cultured in IMDM supplemented with 5% FCS, α-thioglycerol, L-glutamine, and antibiotics in 5% CO2 at 37°C. Cells were passaged every 3–5 days and re-thawed from an original stock every 6–8 weeks.
Detection of Polo-like kinase-1 mRNA by reverse transcriptase polymerase chain reaction and quantitative polymerase chain reaction
Total RNA was extracted from HMC-1 cells, neoplastic MC, and normal peripheral blood cells (healthy donors, n=3) using Trizol® (Invitrogen, Carlsbad, CA, USA) or an RNeasy® Mini Kit (Qiagen, Hilden, Germany), according to the manufacturers’ instructions. Primary MC were obtained from two patients with MC leukemia. The bone marrow sample from one patient contained more than 80% MC. In the second patient, MC were purified to homogeneity (purity >98%) by cell sorting as described elsewhere.39 Primers for human Plk-1 were: 5′-CCCATCTTCTGGGTCAGCAAG-3′ (forward) and 5′-AAGAG-CACCCCC ACGCTGTT-3′ (reverse). The polymerase chain reaction (PCR) conditions were: annealing 30 s (65°C); extension, 1 min (72°C); denaturation 30 s (94°C); 30 cycles. Equal loading was confirmed by determining β-actin mRNA levels using published primers.39 For quantitative PCR, cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase and random primers (both from Invitrogen Inc, Carlsbad, CA, USA) according to the manufacturer’s instructions. The primers used were: Plk-1: 5′-CCTCCGGATCAAGAAGAATGAA-3′ (forward) and 5′-GCAGTGGGATCTGT CTGAAGCA-3′ (reverse); Abl: 5′-TCTATGATTTTGTGGCCAGTGGAG-3′ (forward) and 5′-GCCTAAGACCCGGAGCTTTTCA-3′ (reverse). The PCR conditions were: denaturation 15 s (95°C); annealing and extension 1 min (60°C). Quantitative PCR was performed on a 7900HT Fast Real-Time PCR System (Applied Biosystems, Darmstadt, Germany) using iTaq SYBR Green Supermix with ROX (Bio-Rad, Hercules, CA, USA).22
Detection of the Polo-like kinase-1 protein by immunostaining
Immunocytochemistry was performed on cytospin preparations of HMC-1 cells using published protocols.39 The mouse monoclonal antibody K50-483 (work dilution 1:20) from Becton Dickinson Pharmingen (San Jose, CA, USA) and a biotinylated goat-anti-mouse IgG antibody (Biocare, San Diego, CA, USA) were applied for detection of phosphorylated Plk-1 (pPlk-1), whereas for detection of total Plk-1, a polyclonal rabbit anti-Plk-1 antibody (1:20; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and biotinylated goat-anti-rabbit IgG (Biocare) were employed. Slides were incubated with anti-Plk-1 antibodies overnight. Streptavidin-alkaline-phosphatase complex (Biocare) served as the chromogen. Antibody-reactivity was made visible using neofuchsin (Nichirei, Tokyo, Japan). In a separate set of experiments, immunocytochemical studies were performed on HMC-1 cells transfected with Plk-1 small interfering RNA (siRNA), or after the anti-Plk-1 antibody had been pre-incubated with a Plk-1-specific blocking peptide (Bethyl Laboratories, Montgomery, TX, USA) at 37°C for 1 h. Immunohistochemical studies were performed on samples from 25 patients with SM (Online Supplementary Table S1), one with a cutaneous mastocyoma, and on normal bone marrow (n=2). Expression of Plk-1 in neoplastic MC was examined on serial sections (2 μm) of paraffin-embedded formalin-fixed bone marrow specimens by indirect immunohistochemistry, as reported elsewhere.40,41 Serial sections were incubated with monoclonal antibody G3 against tryptase (1:500; Santa Cruz Biotechnology), a monoclonal antibody against pPlk-1 (1:100; BD Pharmingen), and a polyclonal rabbit anti-Plk-1 antibody (Santa Cruz Biotechnology). Antibodies were diluted in 0.05 M Tris-buffered saline (pH 7.5) and 1% bovine serum albumin (Sigma). After washing, slides were incubated with biotinylated anti-mouse IgG supplemented with normal horse serum (both from Vector, Burlingame, CA, USA) for 30 min, washed, and exposed to Vectastain ABC KIT (Vector) for 30 min. 3-amino-9-ethyl-carbazole was used as the chromogen.
Silencing of Polo-like kinase-1 with small interfering RNA
To explore the functional role of Plk-1 in neoplastic MC, HMC-1.1 cells and HMC-1.2 cells were transfected with siRNA against Plk-1 (5′-GAUCACCCUCCUUAAA UAUdTdT-3′)42 and siRNA against luciferase (5′-CUUACGCUGAGUACUUC GAdTdT-3′). Both siRNA were synthesized in 2′-deprotected, duplexed, and desalted form and were purchased from Dharmacon Research (Lafayette, CO, USA). The siRNA (200 nM) were transfected into HMC-1 cells using lipofectin (Invitrogen) according to the manufacturer’s instructions. Twenty-four hours after transfection, cells were spun on cytospin slides to determine the number of apoptotic cells by light microscopy. The siRNA-induced knock-down of Plk-1 was confirmed by quantitative PCR. To determine potential cooperative effects of Plk-1 siRNA and PKC412 on survival of neoplastic MC, HMC-1 cells were transfected with 100 nM siRNA and, 4 h later, PKC412 (100 nM) was added. After 24 h (from the time of transfection) cells were spun on cytospin slides and examined for the percentage of apoptotic cells by light microscopy.
Analysis of cell cycle progression by flow cytometry
To evaluate the effects of BI 2536 on cell cycle progression, HMC-1 cells and C2 cells were incubated in control medium or in medium containing 100 nM BI 2536 for 48 h. Thereafter, cells were resuspended in 500 μL staining buffer containing 0.1% sodium acetate and 0.1% Triton X-100 and 0.1 mg/mL RNAse (Sigma Aldrich, St. Louis, MO, USA). Then, 20 μL of propidium iodide (PI, Sigma Aldrich) were added. Cell cycle distribution was analyzed by flow cytometry on a FACSCalibur (Becton Dickinson Biosciences) as reported previously.43
Evaluation of apoptosis by morphology, TUNEL assay, and caspase cleavage
HMC-1 cells and C2 cells were incubated with various concentrations of BI 2536 (1–100 nM) or control medium for 48 or 96 hours. The percentage of apoptotic cells was quantified by Wright-Giemsa staining. Apoptosis was defined according to established cytomorphologic criteria.44 To confirm apoptosis, a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed as reported previously39,45 using HMC-1 cells and C2 cells incubated with BI 2536 (100 nM) or control medium for 48 h. After fixation (with formaldehyde and 70% ethanol) and staining, cells were washed and analyzed with a Nikon Eclipse E 800 fluorescence microscope (Tokyo, Japan). For evaluation of caspase cleavage, HMC-1 cells were incubated with BI 2536 (1–100 nM) or control medium for 48 h. Western blotting was performed essentially as described elsewhere39,43,45 using a polyclonal antibody against cleaved caspase-3, monoclonal antibody 18C8 against cleaved caspase-8, and a polyclonal antibody against cleaved caspase-9 (all from Cell Signaling Technology, Danvers, MA, USA). A polyclonal antibody against β-actin (Sigma) was applied to confirm equal loading. Antibody reactivity was made visible by donkey anti-rabbit IgG (GE Healthcare). Human cord blood-derived mast cells and HMC-1.2 cells were incubated with control medium or medium containing 10 nM or 100 nM BI 2536, for 48 h or 96 h. Thereafter, cell viability was analyzed by annexin V/propidium iodide staining and flow cytometry.43
Measurement of 3H-thymidine uptake
Cell lines and primary neoplastic cells were incubated with various concentrations of BI 2536 (1 nM to 1 μM) in 96-well culture plates (TPP) at 37°C for 48 h. After incubation, 3H-thymidine (0.5 μCi per well) was added at 37°C for 12 h. Cells were then harvested on filter membranes (Packard Bioscience, Meriden, CT, USA) in a Filtermate 196 harvester (Packard Bioscience). Filter-bound radioactivity was counted in a β-counter (Top-Count NXT, Packard Bioscience). To determine potential additive or synergistic drug-effects, HMC-1.1 cells, HMC-1.2 cells, C2 cells, MOLM-13 cells, and primary neoplastic MC were incubated with both BI 2536 and PKC412 at various concentrations (at fixed ratios) for 48 h. Drug interactions (additive, synergistic) were determined by calculating the combination index (CI) values using Calcusyn software (Calcusyn; Biosoft, Ferguson, MO, USA).46
Statistical analysis
To determine the level of significance in drug effects, the Student’s t test for dependent samples was applied. P values less than 0.05 were considered statistically significant.
Results
Neoplastic mast cells express phosphorylated Polo-like kinase-1
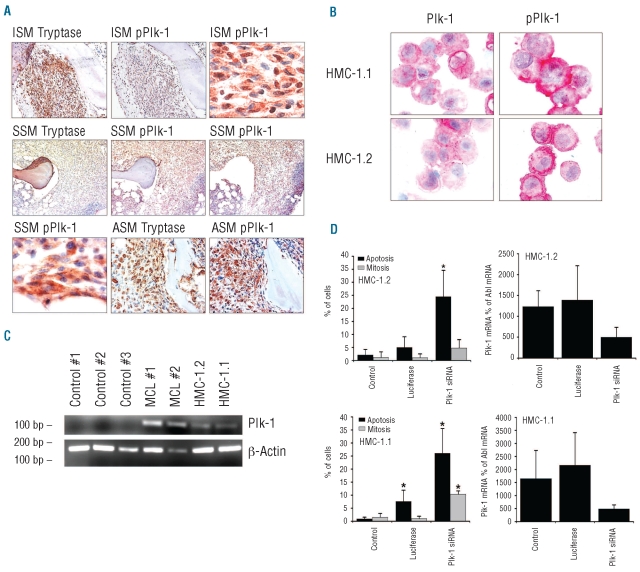
As assessed by immunohistochemistry, neoplastic MC were found to express pPlk-1 in all SM patients examined, without major differences in staining intensity or percentage of positive MC when comparing different variants of SM (Figure 1A and Online Supplementary Table S1). We were also able to detect pPlk-1 and total Plk-1 in primary neoplastic MC from a patient with a localized cutaneous mastocytoma (Online Supplementary Figure S1). Among other cells in the bone marrow (from patients with SM and controls), megakaryocytes and myeloid progenitor cells stained positive for pPlk-1, whereas erythroid cells were not recognized by anti-pPlk-1 antibody. A summary of staining results in bone marrow cells is shown in Online Supplementary Table S2. As assessed by immunocyto-chemistry studies, both HMC-1 subclones were also found to stain positive for total Plk-1 and pPlk-1 (Figure 1B). pPlk-1 was found to be expressed in HMC-1.1 cells lacking KIT D816V as well as in HMC-1.2 cells expressing KIT D816V (Figure 1B). The specificity of the staining reaction was confirmed by using HMC-1.1 cells and HMC-1.2 cells transfected with Plk-1 siRNA (Online Supplementary Figure S2). Moreover, immunocytochemistry staining was blocked by pre-incubating the anti-Plk-1 antibody with a Plk-1-specific blocking peptide (data not shown). Next, we examined the expression of Plk-1 mRNA in neoplastic MC by RT-PCR. As shown in Figure 1C, neoplastic MC obtained from patients with MC leukemia as well as HMC-1.1 cells and HMC-1.2 cells displayed Plk-1 mRNA, whereas only little, if any, Plk-1 mRNA was found in normal blood leukocytes (Figure 1C). Together, these data show that Plk-1 is expressed in an activated form in primary neoplastic MC in SM and MC leukemia.
Figure 1.
Expression and functional role of Plk-1 in neoplastic mast cells. (A) Detection of tryptase, Plk-1, and phosphorylated Plk-1 (pPlk-1) in primary neoplastic mast cells (MC) in serial bone marrow sections from patients with indolent systemic mastocytosis (ISM; patient #2 and #5 in Online Supplementary Table S1), smoldering systemic mastocytosis (SSM; patient #16 in Online Supplementary Table S1) and aggressive systemic mastocytosis (ASM; patient #11 in Online Supplementary Table S1) (magnification: right upper and left lower x100; all other images: x40). (B) Immunocytochemical detection of pPlk-1 and Plk-1 in HMC-1.1 cells and HMC-1.2 cells using antibodies against Plk-1 and pPlk-1 (magnification x100). Images were viewed under an Olympus BX50F4 microscope (Olympus, Hamburg, Germany) and prepared using an Olympus DP11 camera and Adobe Photoshop CS2 software version 11.0 (Adobe Systems, San Jose, CA, USA) to adapt brightness and contrast. Staining protocols are described in the text. (C) RT-PCR analysis of expression of Plk-1 mRNA in mononuclear blood cells from three healthy controls (#1,#2,#3), bone marrow cells obtained from two patients with mast cell leukemia (MCL), HMC-1.1 cells and HMC-1.2 cells. (D) Effects of Plk-1 siRNA on mitosis and viability in HMC-1.2 cells (upper panel) and HMC-1.1 cells (lower panel). Left panels show the percentage of mitotic (gray bars) and apoptotic cells (black bars) after transfection with Plk-1 siRNA or luciferase siRNA. Right panels show siRNA-induced knockdown of Plk-1 mRNA assessed by quantitative PCR. Plk-1 mRNA levels are expressed as percent of Abl mRNA. Results represent the mean±standard deviation of three independent experiments. Asterisk: P<0.05.
Identification of Polo-like kinase-1 as a critical survival molecule in neoplastic mast cells
To explore the functional role of Plk-1 in neoplastic MC, we transfected HMC-1.1 and HMC-1.2 cells with siRNA directed against Plk-1. The siRNA-induced knockdown of Plk-1 was confirmed by quantitative PCR and resulted in a significant increase in the number of apoptotic cells in both HMC-1 subclones (Figure 1D). The Plk-1 knockdown was also found to lead to mitotic arrest of HMC-1.1 cells (Figure 1D) and HMC-1.2 cells (Figure 1D). A control siRNA showed no effect on cell viability in HMC-1.1 cells and HMC-1.2 cells (Figure 1D). Together, these data suggest that Plk-1 acts as a regulator of survival and may thus serve as a potential target in neoplastic MC.
Effects of the Polo-like kinase-1-targeting drug BI 2536 on the growth of neoplastic mast cells
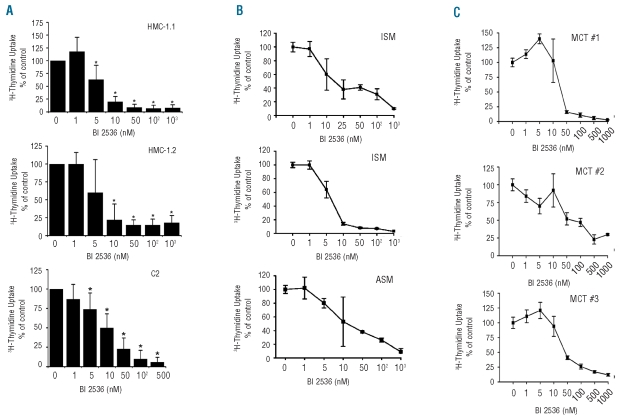
In a next step, we examined the effects of an established pharmacological inhibitor of Plk-1, BI 2536. As assessed by 3H-thymidine uptake, BI 2536 was found to inhibit the proliferation of HMC-1.1 cells, HMC-1.2 cells, and C2 cells in a dose-dependent manner (Figure 2A). IC50 values of BI 2536 were 5–10 nM for HMC-1.1 cells, 5–10 nM for HMC-1.2 cells, and 10–50 nM for C2 cells (Figure 2A). Moreover, BI 2536 counteracted in vitro growth of primary neoplastic human MC and primary neoplastic canine MC (Figure 2B and 2C). The IC50 values for primary MC cells were found to vary from patient to patient. In human SM, the IC50 values ranged between 1 nM and greater than 1 μM (Online Supplementary Table S1) which was initially interpreted as a result of a different content of clonal cells in the sample. However, even in patients in whom virtually all cells in the sample were KIT D816V-positive, the IC50 values of BI 2536 ranged between 10 nM and 1 μM. Similarly, in primary neoplastic canine MC (purity 28%–99%), the IC50 values ranged between 10 nM and greater than 1 μM (Figure 2C). We also compared the effects of BI 2536 in HMC-1 cells with the drug’s effect on various other human leukemic cell lines, including acute myeloid leukemia and chronic myeloid leukemia cell lines. In all cells tested, BI 2536 produced growth inhibition with similar IC50 values (Online Supplementary Table S3).
Figure 2.
Effects of BI 2536 on 3H-thymidine uptake in neoplastic mast cells (MC). (A) Cell lines (HMC-1.1, HMC-1.2, C2), (B) primary human neoplastic MC (indolent SM, ISM n=2; aggressive SM, ASM, n=1), and (C) primary canine neoplastic MC (mast cell tumor, MCT) were incubated in control medium or with various concentrations of BI 2536, as indicated at 37°C for 48 h. After incubation, 3H-thymidine uptake was measured. Results are expressed as percent of control. In Figure 2A, results represent the mean±standard deviation from five independent experiments, in B and C, the mean±standard deviation of triplicate experiments are shown.
Effects of BI2536 on cell cycle distribution in neoplastic mast cells
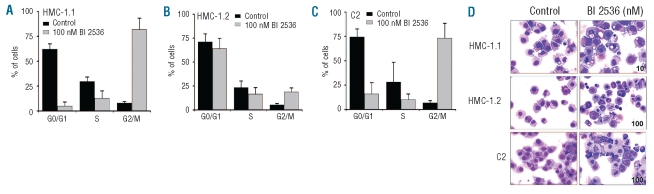
Since Plk-1 is an established cell cycle regulator, we were interested to learn whether BI 2536 affects cell cycle progression in neoplastic MC. As shown in Figure 3, BI 2536 produced a G2/M cell cycle arrest in HMC-1.1 (Figure 3A) cells, HMC-1.2 cells (Figure 3B), and C2 cells (Figure 3C). Corresponding results were obtained from microscopic examinations. In particular, as shown in Figure 3D, BI 2536 produced a mitotic arrest in HMC-1.1 cells, HMC-1.2 cells, and C2 cells. An interesting observation was that the drug-induced cell cycle arrest and mitotic arrest were more pronounced in HMC-1.1 cells lacking KIT D816V than in HMC-1.2 cells expressing KIT D816V (Figure 3A,B).
Figure 3.
Effects of BI2536 on cell cycle distribution in neoplastic mast cells (MC). (A) HMC-1.1 cells, (B) HMC-1.2 cells and (C) C2 cells were incubated in control medium (black bars) or 100 nM BI 2536 (gray bars) at 37°C for 48 h. Thereafter, cell cycle distribution was analyzed by flow cytometry. The percentages of cells in G0/G1-phase, G2/M-phase and S-phase, are shown. Results represent the mean±standard deviation from three independent experiments. (D) HMC-1.1 cells, HMC-1.2 cells and C2 cells were incubated in control medium (left panels) or BI 2536 (10 nM in HMC-1.1; 100 nM in HMC-1.2 and C2; right panel) at 37°C for 48 h. Thereafter, cells were spun on cytospin slides and stained with Wright-Giemsa. Slides were viewed under an Olympus BX50F4 microscope (Olympus, Hamburg, Germany; magnification x 40). Images were prepared using an Olympus DP11 camera and Adobe Photoshop CS2 software version 11.0 (Adobe Systems, San Jose, CA, USA) to adapt brightness and contrast.
BI 2536 induces apoptosis in neoplastic mast cells
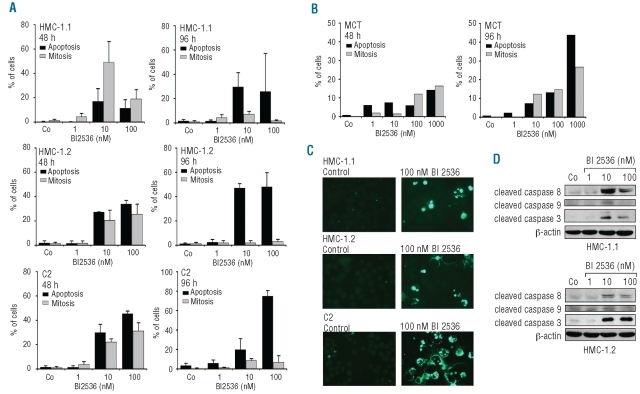
As shown in Figure 4A, BI 2536 induced apoptosis in HMC-1.1 cells, HMC-1.2 cells and C2 cells in a dose- and time-dependent manner. At 96 h (later time point), the numbers of apoptotic cells exceeded the numbers of mitotic cells in all samples examined (Figure 4A). BI 2536 also induced apoptosis in primary canine MC (Figure 4B). The apoptosis-inducing effects of BI 2536 on HMC-1.1 cells, HMC-1.2 cells and C2 cells were confirmed by a TUNEL assay (Figure 4C). Finally, we were able to show that BI 2536 induces cleavage of caspase 3, caspase 8, and caspase 9 in both HMC-1 subclones (Figure 4D). In cultured normal MC, BI 2536 did not induce apoptosis (Online Supplementary Figure S3) although these MC also expressed Plk-1 (data not shown).
Figure 4.
BI 2536 induces apoptosis in neoplastic mast cells (MC). (A) HMC-1.1 cells, HMC-1.2 cells, C2 cells, and (B) primary canine neoplastic MC were incubated in control medium or various concentrations of BI 2536 (as indicated) at 37°C for 48 h and 96 h. The percentages of apoptotic cells and mitotic cells were quantified on Wright-Giemsa-stained cytospin preparations. Results in (A) represent the mean±standard deviation of three independent experiments. Results in (B) are from one experiment. (C) HMC-1.1 cells, HMC-1.2 cells and C2 cells were cultured in control medium (left panel) or with 100 nM BI 2536 (right panel) for 48 h, then apoptotic cells were detected by a TUNEL assay. (D) HMC-1.1 cells and HMC-1.2 cells were cultured in control medium and various concentrations of BI 2536. Western blotting was perfomed using antibodies against cleaved caspases 3, 8, and 9, and β-actin.
BI 2536 synergizes with PKC412 in blocking the growth of neoplastic mast cells
As assessed by 3H-thymidine incorporation, BI 2536 was found to cooperate with midostaurin (PKC412) in inhibiting the growth of HMC-1.1 cells, HMC-1.2 cells, and C2 cells (Online Supplementary Figure 4A). Evaluation of the effects of drug combination using the Calcusyn program revealed clear synergistic effects on all three cell lines (data not shown). In addition, BI 2536 and PKC412 produced synergistic growth-inhibitory effects on primary neoplastic human MC (Online Supplementary Figure 4B) as well as on primary neoplastic canine MC (Online Supplementary Figure 4C). In the FLT3-mutated acute myeloid leukemia cell line MOLM-13, PKC412 and BI 2536 produced additive anti-proliferative effects (Online Supplementary Figure S5). To confirm that Plk-1 is involved in synergistic drug interactions in HMC-1.1 and HMC-1.2 cells, we performed experiments using Plk-1 siRNA and PKC412. In these experiments, we were able to show that Plk-1 siRNA and PKC412 exert cooperative apoptosis-inducing effects on HMC-1.1 cells and HMC-1.2 cells (Online Supplementary Figure 4D).
Discussion
Plk-1 is a serine/threonine kinase that has been implicated in the regulation of mitosis and identified as a potential target in solid tumors and leukemias.24–28 It has also been described that the Plk-1-targeting drug BI 2536 induces cell cycle arrest and apoptosis in various neoplastic cells.29,30,43 Here, we describe that neoplastic MC display pPlk-1 and that both BI 2536 and a Plk-1-specific siRNA induce growth arrest and apoptosis in neoplastic MC. The effects of BI 2536 were dose-dependent and seen in primary human and canine MC, in the human MC line HMC-1, and in the canine mastocytoma cell line C2.
SM is a heterogeneous disease involving MC and their progenitors.1–7 The clinical picture, course, and prognosis vary among patients depending on the SM variant.1–7,47–49 The prognosis of patients with aggressive SM and MC leukemia is bad and responses to most drugs are poor.3–7,47–49 Therefore new drug targets and targeted drugs need to be developed for these patients. In this study, we show that neoplastic MC in indolent SM, aggressive SM, and MC leukemia display pPlk-1. Moreover, pPlk-1 was detectable in the MCL cell line HMC-1. Interestingly, pPlk-1 was not only expressed in the KIT D816V-positive subclone of HMC-1.2, but also in HMC-1.1 cells lacking KIT D816V suggesting that Plk-1 expression in neoplastic MC is not dependent on a particular KIT mutation. This observation is of interest because Plk-1 expression in chronic myeloid leukemia is triggered by the disease-related oncoprotein BCR/ABL.43 One explanation may be that KIT-independent pro-oncogenic signaling pathways in neoplastic MC23 trigger Plk-1 expression. Alternatively, Plk-1 expression is triggered by various oncogenic KIT mutants including KIT V560G expressed in HMC-1.1 cells. Finally, Plk-1 may be a lineage-related antigen expressed during differentiation of MC. In this regard it is worth noting that Plk-1 was not only expressed in neoplastic MC but also in other bone marrow cells, including megakaryocytes and immature granulo-monocytic cells, confirming its role as a regulator of cell cycle progression in immature bone marrow cells.
To confirm that Plk-1 is indeed a relevant growth regulator and thus a potential target in neoplastic MC, we applied a Plk-1 siRNA. In these experiments we were able to show that the siRNA-induced knockdown of Plk-1 in HMC-1.1 cells and HMC-1.2 cells resulted in apoptosis, suggesting that Plk-1 is a potential therapeutic target in neoplastic MC.
In line with this hypothesis, the Plk-1-targeting drug BI 2536 was found to inhibit the growth of neoplastic MC in most patients as well as growth of all MC lines tested, i.e. HMC-1.1, HMC-1.2, and C2 cells. The IC50 values for human and canine MC were within the pharmacologically achievable range of the drug. An interesting observation was that the IC50 values for HMC-1.1 cells and HMC-1.2 cells were identical. By contrast, the IC50 values for primary neoplastic MC varied substantially among human patients and among canine patients. One explanation for the variable effects of the drug on growth of MC may be molecular resistance to BI 2536. Alternatively, in these patients, additional survival- and/or growth-related molecules played a more important role in MC growth and survival.
Plk-1 is involved in mitosis and cell cycle progression in various cells and has been described to be expressed in several myeloid leukemias and other neoplasms.24–28,43,50 We were, therefore, interested in comparing the effects of BI 2536 in various myeloid leukemias. BI 2536 was found to inhibit proliferation in all leukemic cell lines tested, with comparable IC50 values. These data are in good agreement with recently published results,49,50 although we found that HL60 cells responded slightly better to BI 2536 than was reported by Renner et al.50
To study the mechanism(s) of action of BI 2536 on neoplastic MC, we examined drug-exposed cells for signs of mitotic arrest and morphological and biochemical signs of apoptosis. In these experiments, we found impressive and unique morphological changes induced by BI 2536 in HMC-1.1 cells, HMC-1.2 cells and C2 cells, indicating a strong mitotic arrest, and consecutive apoptosis. In particular, whereas at an early time point (48 h) many drug-exposed cells exhibited signs of mitosis, after 96 h most cells exhibited clear signs of apoptosis. Similar results have been reported for chronic myeloid leukemia cells.43 The apoptosis-inducing effect of BI 2536 on both HMC-1 sub-clones was confirmed by a TUNEL assay and caspase cleavage. Together, these data show that inhibition of Plk-1 by BI 2536 is associated with mitotic arrest and apoptosis, suggesting that BI 2536 may be an interesting drug for the treatment of aggressive SM and MC leukemia.
An interesting observation in this study was that BI 2536-induced G2/M cell cycle arrest was more pronounced in HMC-1.1 cells and C2 cells than in HMC-1.2 cells. This observation may be explained by the KIT D816V mutation that is expressed in HMC-1.2 cells and may introduce a relative resistance to the cell-cycle specific effects of BI 2536. Another remarkable observation was that although cell cycle progression was differently affected by BI 2536 in HMC-1.1 cells and HMC-1.2 cells, the drug produced growth inhibition with the same efficacy and comparable IC50 values in both subclones. With regard to apoptosis, the effects of BI 2536 were even more pronounced in HMC-1.2 cells than in HMC-1.1 cells. These data suggest that the primary mechanisms of action of BI 2536 on the HMC-1 subclones may differ in that the drug primarily produces cell cycle inhibition with consecutive apoptosis in HMC-1.1 cells, but primarily induces apoptosis and consecutive growth inhibition in HMC-1.2 cells. In C2 cells, BI 2536 produced both major inhibition of cell cycle progression and marked apoptosis.
KIT is a key regulator of growth of normal and neoplastic MC.5–10 Several studies and trials have, therefore, been initiated to explore the effects of KIT-targeting kinase inhibitors in advanced SM.16–21 One promising agent is PKC412/midostaurin.21 However, the effects of these drugs in aggressive SM and MC leukemia are usually short-lived and often followed by a relapse.21 Current research is, therefore, seeking potential drug partners in order to define optimal drug combinations that exert more profound effects on neoplastic MC in SM. We exposed neoplastic MC to combinations of PKC412 and BI 2536 and found that the two drugs synergized with each other in inhibiting the growth of neoplastic MC. Effects of the drug combination were seen in primary human MC and canine MC as well as in HMC-1.1 cells, HMC-1.2 cells and C2 cells. These data suggest that combinations of KIT inhibitors and Plk-1 inhibitors may be an interesting approach to improve therapy in aggressive SM and MC leukemia.
In summary, Plk-1 is expressed in neoplastic MC and represents a potential new therapeutic target in aggressive SM and MC leukemia. Clinical trials are now warranted to test whether Plk-1 can also serve as a therapeutic target in vivo in patients with aggressive SM and MC leukemia.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
Funding: this work was supported by Fonds zur Förderung der Wissenschaftlichen Forschung in Österreich, and (FWF) grants #P21173-B13 and #SFB-F01820.
References
- 1.Metcalfe DD. Classification and diagnosis of mastocytosis: current status. J Invest Dermatol. 1991;96(3):2S–4S. [PubMed] [Google Scholar]
- 2.Valent P. Biology, classification and treatment of human mastocytosis. Wien Klin Wochenschr. 1996;108(13):385–97. [PubMed] [Google Scholar]
- 3.Horny HP, Valent P. Diagnosis of mastocytosis: general histopathological aspects, morphological criteria, and immunohistochemical findings. Leuk Res. 2001;25(7):543–51. doi: 10.1016/s0145-2126(01)00021-2. [DOI] [PubMed] [Google Scholar]
- 4.Escribano L, Akin C, Castells M, Orfao A, Metcalfe DD. Mastocytosis: current concepts in diagnosis and treatment. Ann Hematol. 2002;81(12):677–90. doi: 10.1007/s00277-002-0575-z. [DOI] [PubMed] [Google Scholar]
- 5.Valent P, Akin C, Sperr WR, Horny HP, Arock M, Lechner K, et al. Diagnosis and treatment of systemic mastocytosis: state of the art. Br J Haematol. 2003;122(5):695–717. doi: 10.1046/j.1365-2141.2003.04575.x. [DOI] [PubMed] [Google Scholar]
- 6.Akin C, Metcalfe DD. Systemic mastocytosis. Annu Rev Med. 2004;55:419–32. doi: 10.1146/annurev.med.55.091902.103822. [DOI] [PubMed] [Google Scholar]
- 7.Pardanani A, Tefferi A. Systemic mastocytosis in adults: a review on prognosis and treatment based on 342 Mayo Clinic patients and current literature. Curr Opin Hematol. 2010;17(2):125–32. doi: 10.1097/MOH.0b013e3283366c59. [DOI] [PubMed] [Google Scholar]
- 8.Valent P, Ghannadan M, Akin C, Krauth MT, Selzer E, Mayerhofer M, et al. On the way to targeted therapy of mast cell neoplasms: identification of molecular targets in neoplastic mast cells and evaluation of arising treatment concepts. Eur J Clin Invest. 2004;34 (Suppl 2):41–52. doi: 10.1111/j.0960-135X.2004.01369.x. [DOI] [PubMed] [Google Scholar]
- 9.Tefferi A, Pardanani A. Clinical, genetic, and therapeutic insights into systemic mast cell disease. Curr Opin Hematol. 2004;11(1):58–64. doi: 10.1097/00062752-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Furitsu T, Tsujimura T, Tono T, Ikeda H, Kitayama H, Koshimizu U, et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of the c-kit product. J Clin Invest. 1993;92(4):1736–44. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci (USA) 1995;92(23):10560–4. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longley BJ, Tyrrell L, Lu SZ, Ma YS, Langley K, Ding TG, et al. Somatic c-kit activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12(3):312–4. doi: 10.1038/ng0396-312. [DOI] [PubMed] [Google Scholar]
- 13.Longley BJ, Metcalfe DD, Tharp M, Tyrrell L, Lu SZ, Heitjan D, et al. Activating and dominant inactivating c-kit catalytic domain mutations in distinct forms of human mastocytosis. Proc Natl Acad Sci USA. 1999;96(4):1609–14. doi: 10.1073/pnas.96.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritsche-Polanz R, Jordan JH, Feix A, Sperr WR, Sunder-Plassmann G, Valent P, et al. Mutation analysis of C-KIT in patients with myelodysplastic syndromes without mastocytosis and cases of systemic mastocytosis. Br J Haematol. 2001;113(2):357–64. doi: 10.1046/j.1365-2141.2001.02783.x. [DOI] [PubMed] [Google Scholar]
- 15.Feger F, Ribadeau Dumas A, Leriche L, Valent P, Arock M. Kit and c-kit mutations in mastocytosis: a short overview with special reference to novel molecular and diagnostic concepts. Int Arch Allergy Immunol. 2002;127(2):110–4. doi: 10.1159/000048179. [DOI] [PubMed] [Google Scholar]
- 16.Akin C, Brockow K, D’Ambrosio C, Kirshenbaum AS, Ma Y, Longley BJ, et al. Effects of tyrosine kinase inhibitor STI571 on human mast cells bearing wild-type or mutated forms of c-kit. Exp Hematol. 2003;31(8):686–92. doi: 10.1016/s0301-472x(03)00112-7. [DOI] [PubMed] [Google Scholar]
- 17.Ma Y, Zeng S, Metcalfe DD, Dimitrijevic S, Butterfield JH, McMahon G, et al. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory type mutations. Blood. 2002;99(5):1741–4. doi: 10.1182/blood.v99.5.1741. [DOI] [PubMed] [Google Scholar]
- 18.Growney JD, Clark JJ, Adelsperger J, Stone R, Fabbro D, Griffin JD, et al. Activation mutations of human c-KIT resistant to imatinib are sensitive to the tyrosine kinase inhibitor PKC412. Blood. 2005;106(2):721–4. doi: 10.1182/blood-2004-12-4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gleixner KV, Mayerhofer M, Aichberger KJ, Derdak S, Sonneck K, Böhm A, et al. The tyrosine kinase-targeting drug PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of kit: comparison with AMN107, imatinib, and cladribine (2CdA), and evaluation of cooperative drug effects. Blood. 2006;107(2):752–9. doi: 10.1182/blood-2005-07-3022. [DOI] [PubMed] [Google Scholar]
- 20.Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C. Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in the majority of patients with systemic mastocytosis. Blood. 2006;108(1):286–91. doi: 10.1182/blood-2005-10-3969. [DOI] [PubMed] [Google Scholar]
- 21.Gotlib J, Berube C, Growney JD, Chen CC, George TI, Williams C, et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood. 2005;106(8):2865–70. doi: 10.1182/blood-2005-04-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayerhofer M, Gleixner KV, Hoelbl A, Florian S, Hoermann G, Aichberger KJ, et al. Unique effects of KIT D816V in BaF3 cells: induction of cluster formation, histamine synthesis, and early mast cell differentiation antigens. J Immunol. 2008;180(8):5466–76. doi: 10.4049/jimmunol.180.8.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gleixner KV, Mayerhofer M, Rix U, Hoermann G, Gruze A, Krauth MT, et al. Delineation of a KIT-independent oncogenic pathway in neoplastic mast cells that involves Lyn and Btk and can be disrupted by the KIT/Lyn/Btk-targeting drug dasatinib. Blood. 2007;110(11):1541. [Google Scholar]
- 24.Takai N, Hamanaka R, Yoshimatsu J, Miyakawa I. Polo-like kinases (Plks) and cancer. Oncogene. 2005;24(2):287–91. doi: 10.1038/sj.onc.1208272. [DOI] [PubMed] [Google Scholar]
- 25.Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24(2):267–76. doi: 10.1038/sj.onc.1208273. [DOI] [PubMed] [Google Scholar]
- 26.Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6(4):321–30. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Zhang M, Zou P. Polo-like kinase 1 as a new target for non-Hodgkin’s lymphoma treatment. Oncology. 2008;74(1–2):96–103. doi: 10.1159/000139137. [DOI] [PubMed] [Google Scholar]
- 28.Didier C, Cavelier C, Quaranta M, Demur C, Ducommun B. Evaluation of Polo-like kinase 1 inhibition on the G2/M checkpoint in acute myelocytic leukaemia. Eur J Pharmacol. 2008;591(1–3):102–5. doi: 10.1016/j.ejphar.2008.06.062. [DOI] [PubMed] [Google Scholar]
- 29.Lénárt P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, et al. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol. 2007;17(4):304–15. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 30.Steegmaier M, Hoffmann M, Baum A, Lénárt P, Petronczki M, Krssák M, et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007;17(4):316–22. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 31.Fabbro D, Ruetz S, Bodis S, Pruschy M, Csermak K, Man A, et al. PKC412 - a protein kinase inhibitor with a broad therapeutic potential. Anticancer Drug Des. 2000;15(1):17–28. [PubMed] [Google Scholar]
- 32.Valent P, Horny H-P, Escribano L, Longley BJ, Li CY, Schwartz LB, Marone G, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Conference Report of “Year 2000 Working Conference on Mastocytosis”. Leuk Res. 2001;25(7):603–25. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 33.Valent P, Horny H-P, Li CY, Longly BJ, Metcalfe DD, Parwaresch R-M, et al. Mastocytosis (Mast cell disease). World Health Organization (WHO) Classification of Tumours. Pathology & Genetics. Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Tumours of Haematopoietic and Lymphoid Tissues. 2001;1:291–302. [Google Scholar]
- 34.Mirkina I, Schweighoffer T, Kricek F. Inhibition of human cord blood-derived mast cell responses by anti-Fc epsilon RI mAb 15/1 versus anti-IgE omalizumab. Immunol Lett. 2007;109(2):120–8. doi: 10.1016/j.imlet.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Valent P, Ashman LK, Hinterberger W, Eckersberger F, Majdic O, Lechner K, Bettelheim P. Mast cell typing: demonstration of a distinct hematopoietic cell type and evidence for immunophenotypic relationship to mononuclear phagocytes. Blood. 1989;73(7):1778–85. [PubMed] [Google Scholar]
- 36.Rebuzzi L, Willmann M, Sonneck K, Gleixner KV, Florian S, Kondo R, et al. Detection of vascular endothelial growth factor (VEGF) and VEGF receptors Flt-1 and KDR in canine mastocytoma cells. Vet Immunol Immunopathol. 2007;115(3–4):320–33. doi: 10.1016/j.vetimm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12(4):345–55. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- 38.DeVinney R, Gold WM. Establishment of two dog mastocytoma cell lines in continuous culture. Am J Respir Cell Mol Biol. 1990;3(5):413–20. doi: 10.1165/ajrcmb/3.5.413. [DOI] [PubMed] [Google Scholar]
- 39.Kondo R, Gleixner KV, Mayerhofer M, Vales A, Gruze A, Samorapoompichit P, et al. Identification of heat shock protein 32 (Hsp32) as a novel survival factor and therapeutic target in neoplastic mast cells. Blood. 2007;110(2):661–9. doi: 10.1182/blood-2006-10-054411. [DOI] [PubMed] [Google Scholar]
- 40.Hsu SM, Raine L, Fanger H. Use of avidinbiotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29(4):577–80. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 41.Cattoretti G, Pileri S, Parravicini C, Becker MH, Poggi S, Bifulco C, et al. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993;171(2):83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- 42.Spänkuch B, Kurunci-Csacsko E, Kaufmann M, Strebhardt K. Rational combinations of siRNAs targeting Plk1 with breast cancer drugs. Oncogene. 2007;26(39):5793–807. doi: 10.1038/sj.onc.1210355. [DOI] [PubMed] [Google Scholar]
- 43.Gleixner KV, Ferenc V, Peter B, Gruze A, Meyer RA, Hadzijusufovic E, et al. Polo-like kinase 1 (Plk1) as a novel drug target in chronic myeloid leukemia: overriding imatinib resistance with the Plk1 inhibitor BI 2536. Cancer Res. 2010;70(4):1513–23. doi: 10.1158/0008-5472.CAN-09-2181. [DOI] [PubMed] [Google Scholar]
- 44.Van Cruchten S, Van Den Broeck W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol. 2002;31(4):214–23. doi: 10.1046/j.1439-0264.2002.00398.x. [DOI] [PubMed] [Google Scholar]
- 45.Hadzijusufovic E, Rebuzzi L, Gleixner KV, Ferenc V, Peter B, Kondo R, et al. Targeting of heat-shock protein 32/heme oxygenase-1 in canine mastocytoma cells is associated with reduced growth and induction of apoptosis. Exp Hematol. 2008;36(11):1461–70. doi: 10.1016/j.exphem.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 47.Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113(23):5727–36. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 48.Escribano L, Alvarez-Twose I, Sánchez-Muñoz L, Garcia-Montero A, Núñez R, Almeida J, et al. Prognosis in adult indolent systemic mastocytosis: a long-term study of the Spanish Network on Mastocytosis in a series of 145 patients. J Allergy Clin Immunol. 2009;124(3):514–21. doi: 10.1016/j.jaci.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Valent P, Akin C, Sperr WR, Escribano L, Arock M, Horny HP, et al. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leuk Res. 2003;27(7):635–41. doi: 10.1016/s0145-2126(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 50.Renner AG, Dos Santos C, Recher C, Bailly C, Créancier L, Kruczynski A, et al. Polo-like kinase 1 is overexpressed in acute myeloid leukemia and its inhibition preferentially targets the proliferation of leukemic cells. Blood. 2009;114(4):659–62. doi: 10.1182/blood-2008-12-195867. [DOI] [PubMed] [Google Scholar]