Abstract
TET2 mutations are found in polycythemia vera and it was initially reported that there is a greater TET2 mutational burden than JAK2V617F in polycythemia vera stem cells and that TET2 mutations precede JAK2V617F. We quantified the proportion of TET2, JAK2V617F mutations and X-chromosome allelic usage in polycythemia vera cells, BFU-Es and in vitro expanded erythroid progenitors and found clonal reticulocytes, granulocytes, platelets and CD34+ cells. We found that TET2 mutations may also follow rather than precede JAK2V617F as recently reported by others. Only a fraction of clonal early hematopoietic precursors and largely polyclonal T cells carry the TET2 mutation. We showed that in vitro the concomitant presence of JAK2V617F and TET2 mutations favors clonal polycythemia vera erythroid progenitors in contrast with non-TET2 mutated progenitors. We conclude that loss-of-function TET2 mutations are not the polycythemia vera initiating events and that the acquisition of TET2 somatic mutations may increase the aggressivity of the polycythemia vera clone.
Keywords: polycythemia vera, TET2 mutations
Introduction
The TET2 gene mutation was first found in acute myeloid leukemia (AML) and it was suggested that it is a tumor suppressor gene. The diverse loss-of-function mutations, including nonsense, insertion, and deletion of TET2, are found in patients with myeloproliferative disorders/neoplasms (MPNs), AML, and myelodysplastic syndromes (MDS) have been reported.1 Published work has demonstrated that TET2 loss-of-function mutations originate in pluripotent hematopoietic stem cells and that both alleles were affected in many patients.1 In 5 patients with MPN who were also JAK2V617F positive, authors reported that this mutation preceded the JAK2V617F mutation.1,2 Whether or not TET2 mutations alter the severity of MPN is controversial.3,4 While ourselves and others have reported that the JAK2V617F mutation is not a disease-initiating mutation,5,6 the question has arisen whether the TET2 mutation could be the pre-JAK2V617F somatic event responsible for MPN. However, studies of families with multiple MPN members have shown that the TET2 mutations cannot be a disease-initiating mutation in familial MPN as it differs among affected relatives.3
We studied 4 JAK2V617F-positive women with PV with diverse TET2 mutations and 7 additional JAK2V617F-positive PV patients without known TET2 mutations. We determined the mutational burden of JAK2V617F and TET2 in their reticulocytes, granulocytes, platelets, and in CD34+ and CD3+ cells, as well as in BFU-E colonies.
Design and Methods
Study subjects, separation of cells and preparation of DNA and RNA
This study included the following three groups of prospectively recruited subjects: i) 2 sisters, P1 and P2 (family F2 in (3)) and 2 other unrelated PV females P3 and P4, whose TET2 mutations were determined in France. Acid citrate dextrose anticoagulated peripheral blood (~28 ml) was obtained from each subject and shipped to Salt Lake City; ii) 7 JAK2V617F-positive PV patients without known TET2 mutations (TET2 gene sequenced); iii) 47 patients with JAK2V617F-positive MPN patients not evaluated for TET2 mutations, including 31 PV, 12 ET and 4 PMF. All subjects signed the Institutional Approved Informed Consent from University of Utah IRB Board Committee (IRB@hsc.utah.edu. Approved on 6/16/2010. Statement file number: IRB 00035784).
The subjects’ platelets, granulocytes, mononuclear cells, T lymphocytes (CD3), pluripotent progenitors (CD34-positive cells) and reticulocytes were isolated, and genomic DNAs and total RNAs were obtained, as previously described.7,8 CD34+ cells and CD3+ were obtained by FACS sorting.
Clonality studies
All studied females were evaluated for clonality of granulocytes, platelets, CD34+ cells, CD3+ cells and reticulocytes, established by genotyping of 5 X-chromosome exonic polymorphisms followed by determination of the informative heterozygous X-chromosome allelic mRNA usage ratio by quantitative real-time AS-PCR (qAS-PCR), as described.8 We previously established that exonic SNP polymorphic X-chromosome allelic frequency utilization greater than 90% is an indicator of the clonal phenotype.9,10
JAK2 and TET2 mutational analyses
The JAK2V617F mutational burden in separated peripheral blood cells was determined by quantitative allele-specific PCR (qAS-PCR).6 The TET2 coding sequence and intron/exon boundaries were determined, and the allelic burden of each TET2 mutation was established by qAS-PCR utilizing mutation-specific primers containing locked nucleic acid and a mismatched nucleotide, as used for clonality and JAK2V617F assays6,8 (Online Supplementary Table S1). Quantitation of the relative proportion of TET2 alleles was accomplished by subcloning each of the mutants and determining the relative amount of each mutant by serial dilutions of plasmids containing each of the mutations, using the principles previously published.6
Expressional analyses of TET2 and JAK2 transcripts
Granulocytes, platelets and expanded erythroid progenitor TET2 and JAK2 mRNA levels were determined using TaqMan Gene Expression Assays and normalized to an endogenous control HPRT using commercial reagents and following the manufacturer’s reaction conditions (Applied Biosystems, CA, USA).
BFU-E analysis
Erythroid colonies (BFU-E) were grown from peripheral blood mononuclear cells in the absence or presence of erythropoietin (EPO) at 3 U/ml.6,11 Individual BFU-Es were picked, genomic DNA and total RNA isolated, their clonality determined, and their genotype and relative proportions of JAK2V617F and TET2 mutations established. Some colonies were too small for successful analysis or were contaminated by mononuclear cells and/or other colonies, as determined by their clonality assays; approximately 15%-contaminated colonies were excluded from analysis.
In vitro expansion of synchronized peripheral blood erythroid progenitors
In vitro expansion of erythroid cells starting from peripheral blood mononuclear cells was performed, as previously published.12,13 Progenitor cells were harvested at different times from cultures reflecting their progressive erythroid differentiation.
Results and Discussion
TET2 and JAK2V617F mutational burden and clonal analyses in peripheral blood lineages
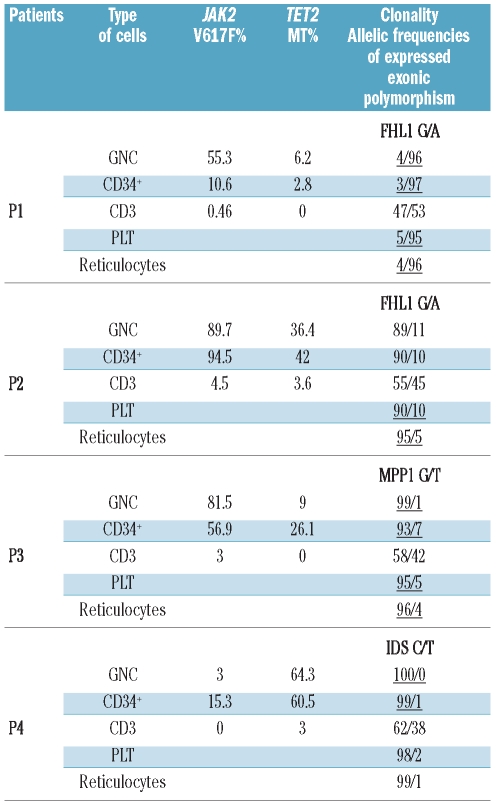
Two PV sisters (P1 and P2) and 2 other unrelated PV females (P3 and P4) were JAK2 positive and had different heterozygous TET2 mutations: P1, a splice-site defect in intron 7 (c.3954+2T>A); P2, deletion of a single nucleotide in exon 3 (c.3138delT, p.Thr1047fs) (3); P3, deletion of a single nucleotide in exon 3 (c.1378delT,p.Ser460fs.); and P4, duplication of a single nucleotide in exon 3 (c.2290dupC,p.Gln764fs.).
We then examined the relative proportions of the above-described TET2 mutants in circulating cells and their clonality. The mutational burden of JAK2V617F and TET2 and clonality were determined in reticulocytes, granulocytes, platelets, CD34+ and CD3+ cells (Table 1). In polyclonal CD3+ cells, we detected no granulocyte contamination by analytical FACS. We found that a small proportion of largely polyclonal T cells also carry TET2 mutation(s). This indicates that loss-of-function TET2 somatic mutations favor myeloid differentiation but do not preclude T-lymphocyte differentiation.
Table 1.
JAK2V617F and TET2 mutational burden and X-chromosome allelic usage transcriptional ratios in platelets (PLT), granulocytes (GNC), CD34-positive cells, T lymphocytes (CD3+), and reticulocytes. The X-chromosome allelic usage ratio indicating clonal or predominantly clonal cell population is depicted in italics and is underlined.
Moreover, CD34-positive cells from 4 analyzed subjects were largely clonal, yet only a proportion carried the TET2 mutation (Table 1) demonstrating that TET2 mutation-bearing progenitors constitute a subclone of PV.
BFU-E analysis
Individual erythroid BFU-E colonies were genotyped. Results of analyses of the individual BFU-E colonies are shown in Figure 1. Patients P1, P2, P3 and P4 had 28, 14, 20 and 16 EPO-independent BFU-Es, respectively, that have either approximately 50% or 100% of JAK2V617F (Online Supplementary Figure S1). In each patient, all colonies grown with or without EPO expressed the same single X-chromosome allele (data not shown). However, several colonies (P1: 61%; P2: 14%; P3: 45%; and P4: 12%) of the total number of BFU-Es had JAK2V617F but not TET2 mutations, demonstrating the primordial origin of the JAK2V617F somatic genetic event in these PV patients. The majority of the analyzed colonies for patients P2, P3 and P4 had heterozygous TET2 mutations. Three BFU-E colonies (two in P1 and one in P3) were homozygous for the TET2 mutation, indicating their genetic instability and formation of a new subclone with loss-of-heterozygosity; unfortunately, a paucity of available DNA precluded determining whether the loss-of-heterozygosity was due to a loss of the wild-type TET2 allele or from uniparenteral disomy.7
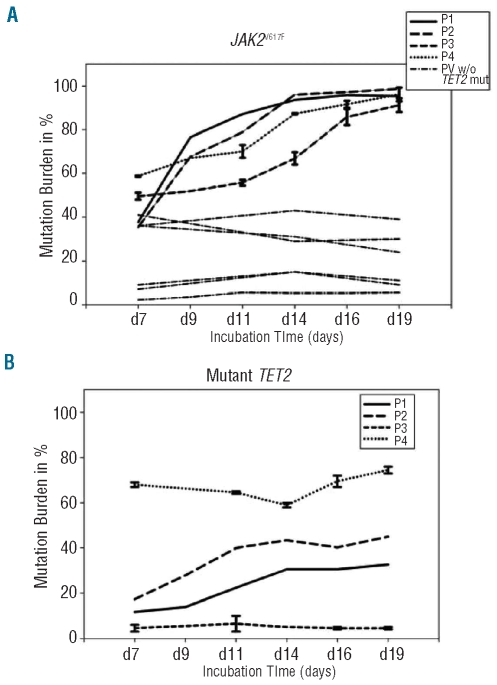
Figure 1.
JAK2V617F and TET2 mutational burden during in vitro erythroid expansion. Peripheral blood-derived erythroid cells were expanded in vitro. At each time point, erythroid progenitors (0.5x106 cells) were harvested and their genomic DNAs isolated and JAK2V617F and TET2 mutational burden established. (A) JAK2V617F mutational burden during in vitro erythroid expansion with Standard Error. PV subjects depicted by solid lines represent JAK2V617F-positive PV patients with TET2 mutations; dashed lines represent a group of JAK2V617F-positive PV patients without known TET2 mutations. (B) TET2 mutational burden during in vitro erythroid expansion for a group of JAK2V617F PV patients with TET2 mutations with Standard Error.
In vitro expansions of erythroid progenitors and their analyses are shown in Figure 1A and B and Figure 2A and C. In our 7 analyzed JAK2 V617F-positive PV patients (P5–P11) without known TET2 mutations, we observed decreased or unchanged JAK2V617F mutational burdens with preferential expansion of polyclonal erythroid progenitors. These data are similar to what we have reported previously; i.e. that in most PV subjects, the erythroid progenitors’ JAK2V617F allelic burden decreased with ongoing erythroid maturation,13 possibly and in contrast to in vivo conditions, due to the high concentrations of EPO and other cytokines that are present in this in vitro system (Figure 1A). In contrast, all 4 PV subjects with TET2 mutations (P1–P4) had a progressive increase of JAK2V617F (Figure 1A) and TET2 mutational burden (Figure 1B) with ongoing maturation of clonal erythroid progenitors; however, the TET2 increase was not noted in patient P3 (Figure 1B) suggesting that JAK2V617F and TET-wild type may preferentially expand as a consequence of the presence of their JAK2V617F and TET-mutated counterparts. These results suggested that the progenitors in the 4 studied patients with TET2 mutations had a selective proliferative advantage under the employed conditions (Online Supplementary Table S2).
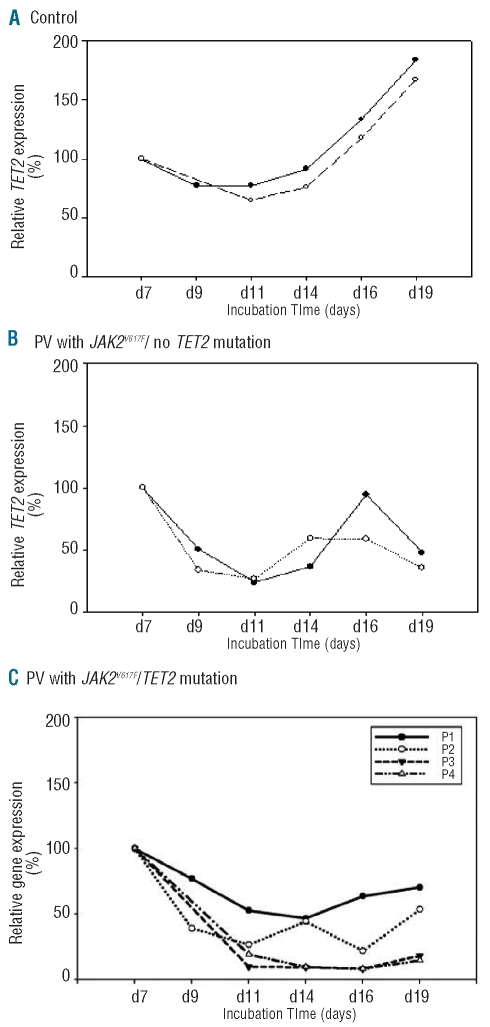
Figure 2.
Expression of TET2 in controls and PV patients during in vitro maturation of erythroid progenitors. (A) Controls; unaffected subjects. (B) Sporadic PV subjects with JAK2V617F mutation without known TET2 mutations. (C) P1–P4 PV subjects with TET2 and JAK2V617F mutations.
TET2 and JAK2 mRNAs transcripts during in vitro expansion of normal and JAK2V617F erythroid progenitors
As shown in Figure 2A, expression of the TET2 tumor suppressor gene increases in normal erythroid progenitors with ongoing maturation. In contrast, in 7 PV JAK2V617F-positive progenitors, expression of TET2 decreases until day 11, when expanded cells still contain non-myeloid cells, and then oscillates around mean from the mid- to late stages of erythroid differentiation (days 14–19)12 concomitant with an increase in differentiated erythroid progenitor cells (TET2-negative, Figure 2B and TET2-positive, Figure 2C; please note that P1 has a TET2 mutation resulting in aberrant splicing that is expected to reduce its mRNA content).
We found no meaningful difference in JAK2 mRNA transcript levels in these conditions in PV, other MPNs, or normal controls (data not shown).
TET2 mRNA in the peripheral blood non-erythroid cells
In contrast to data from PV erythroid progenitors, TET2 mRNA in PV granulocytes and platelets was significantly increased regardless of TET2 mutation, compared with normal controls (Online Supplementary Figure S2), except in patient P1 wherein an intronic TET2 mutation causes aberrant splicing and predictably, based on observations,14 decreased TET2 mRNA (Online Supplementary Figure S2).
We provide further evidence that TET2 in PV is not a disease-initiating mutation. While Delhommeau1 found that the TET2 somatic mutation preceded the JAK2 mutation in several PV patients, three different chronological orders of accumulation of JAK2V617F and TET2 mutations were observed by others.2 The first pattern was compatible with TET2 mutations occurring before JAK2V617F; the second was compatible with JAK2V617F accruing before TET2 mutations; and, in the third, TET2 mutations and JAK2V617F defined two separate clones. This lack of strict order of occurrence suggests that mutations in TET2 are unlikely to represent a predisposition for accruing mutations in JAK2. In our study of 4 JAK2V617F-positive PV patients with TET2 mutations, we also demonstrate that TET2 mutated progenitors constitute a subclone of PV pluripotent and committed hematopoietic progenitors, and that the TET2 somatic mutation often follows the JAK2 somatic mutation. The latter conclusion is further strengthened by the relative proportions of JAK2V617F mutational burdens that were higher than TET2 mutational burdens in clonal hematopoietic progenitors and cells of different lineages. That the TET2 mutation in PV is not a disease-initiating mutation has also been validated by analysis of BFU-E individual colonies in these subjects, which showed several EPO independent colonies that were JAK2V617F-positive and TET2-negative; similar to the conclusions of a study of familial PV.3
Our data provide additional evidence that loss-of-function TET2 mutations are not the PV-initiating events and that, in some PV patients, TET2 mutations follow rather than precede JAK2V617F mutations. Our data also suggest that the concomitant JAK2V617F and TET2 mutations increase proliferation and provide a competitive advantage for the TET2 mutant PV subclone, supporting the notion that TET2 mutations may contribute to the aggressivity of TET2 mutation-positive PV.
Acknowledgments
1P01CA108671-O1A2 (NCI) Myeloproliferative Disorders (MPD) Consortium, project#1 (PI Prchal). FD received funding from the MPD foundation, the Association Laurette Fugain, the Fondation de France, the Institut National du Cancer. Inserm U1009 is supported by grants from the Ligue Nationale Contre le Cancer (équipe labelisée 2009).
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Masse A, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360(22):2289–301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 2.Schaub FX, Looser R, Li S, Hao-Shen H, Lehmann T, Tichelli A, et al. Clonal analysis of TET2 and JAK2 mutations suggests that TET2 can be a late event in the progression of myeloproliferative neoplasms. Blood. 2010;115(10):2003–7. doi: 10.1182/blood-2009-09-245381. [DOI] [PubMed] [Google Scholar]
- 3.Saint-Martin C, Leroy G, Delhommeau F, Panelatti G, Dupont S, James C, et al. Analysis of the ten-eleven translocation 2 (TET2) gene in familial myeloproliferative neoplasms. Blood. 2009;114(8):1628–32. doi: 10.1182/blood-2009-01-197525. [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A, Pardanani A, Lim KH, Abdel-Wahab O, Lasho TL, Patel J, et al. TET2 mutations and their clinical correlates in polycythemia vera, essential thrombocythemia and myelofibrosis. Leukemia. 2009;23(5):905–11. doi: 10.1038/leu.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kralovics R, Teo SS, Li S, Theocharides A, Buser AS, Tichelli A, et al. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006;108(4):1377–80. doi: 10.1182/blood-2005-11-009605. [DOI] [PubMed] [Google Scholar]
- 6.Nussenzveig RH, Swierczek SI, Jelinek J, Gaikwad A, Liu E, Verstovsek S, et al. Polycythemia vera is not initiated by JAK2V617F mutation. Exp Hematol. 2007;35(1):32–8. doi: 10.1016/j.exphem.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Kralovics R, Guan Y, Prchal JT. Acquired uniparental disomy of chromosome 9p is a frequent stem cell defect in polycythemia vera. Exp Hematol. 2002;30(3):229–36. doi: 10.1016/s0301-472x(01)00789-5. [DOI] [PubMed] [Google Scholar]
- 8.Swierczek SI, Agarwal N, Nussenzveig RH, Rothstein G, Wilson A, Artz A, et al. Hematopoiesis is not clonal in healthy elderly women. Blood. 2008;112(8):3186–93. doi: 10.1182/blood-2008-03-143925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen GL, Prchal JT. X-linked clonality testing: interpretation and limitations. Blood. 2007;110(5):1411–9. doi: 10.1182/blood-2006-09-018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prchal JT, Prchal JF, Belickova M, Chen S, Guan Y, Gartland GL, et al. Clonal stability of blood cell lineages indicated by X-chromosomal transcriptional polymorphism. J Exp Med. 1996;183(2):561–7. doi: 10.1084/jem.183.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kralovics R, Stockton DW, Prchal JT. Clonal hematopoiesis in familial polycythemia vera suggests the involvement of multiple mutational events in the early pathogenesis of the disease. Blood. 2003;102(10):3793–6. doi: 10.1182/blood-2003-03-0885. [DOI] [PubMed] [Google Scholar]
- 12.Bruchova H, Yoon D, Agarwal AM, Swierczek S, Prchal JT. Erythropoiesis in polycythemia vera is hyper-proliferative and has accelerated maturation. Blood Cells Mol Dis. 2009;43(1):81–7. doi: 10.1016/j.bcmd.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaikwad A, Nussenzveig R, Liu E, Gottshalk S, Chang K, Prchal JT. In vitro expansion of erythroid progenitors from polycythemia vera patients leads to decrease in JAK2 V617F allele. Exp Hematol. 2007;35(4):587–95. doi: 10.1016/j.exphem.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson P, Yepiskoposyan H, Metze S, Zamudio Orozco R, Kleinschmidt N, Muhlemann O. Nonsense-mediated mRNA decay in human cells: mechanistic insights, functions beyond quality control and the double-life of NMD factors. Cell Mol Life Sci. 2010;67(5):677–700. doi: 10.1007/s00018-009-0177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]