Abstract
Oxidative stress leads to lipid peroxidation and may contribute to the pathogenesis of lesions in multiple sclerosis (MS), an autoimmune disease characterized by inflammatory as well as degenerative phenomena. Isoprostanes are prostaglandin-like compounds which are formed by free radical catalysed peroxidation of arachidonic acid esterified in membrane phospholipids. They are a new class of sensitive specific markers for in vivo lipid peroxidation. In this study 26 patients (15 females and 11 males; mean age 48.2 ± 15.2 year; mean disease duration 10.0 ± 6.5 year) with secondary progressive MS (SPMS) and 12 healthy controls were enrolled. In patients with multiple sclerosis the lipid peroxidation as the level of urine isoprostanes and the level of thiobarbituric acid reactive species (TBARS) in plasma were estimated. Moreover, we estimated the total antioxidative status (TAS) in plasma. It was found that the urine isoprostanes level was over 6-fold elevated in patients with SPMS than in control (P < 0.001). In SPMS patients TBARS level was also statistically higher than in controls (P < 0.01). However, we did not observed any difference of TAS level in serum between SPMS patients and controls (P > 0.05). In patients with SPMS the lipid peroxidation and oxidative stress measured as the increased level of isoprostanes was observed. Thus, we suggest that the level of isoprostanes may be used as non-invasive marker for a determination of oxidative stress what in turn, together with clinical symptoms, may determine an specific antioxidative therapy in SPMS patients.
Keywords: Multiple sclerosis, Lipid peroxidation, Oxidative stress, Isoprostanes, Marker, Total antioxidative status
Introduction
Multiple sclerosis (MS) is a complex disease with several pathophysiological processes: inflammation, demyelination, oxidative stress, axonal damage and repair mechanisms that participate in this disorder. These processes are not uniformly represented in patient populations but can selectively predominate in individual patients. Therefore, heterogeneity in phenotypic expression of MS has the effects on the prognosis and response to therapies in MS patients. In MS disease with a complex pathogenesis isoprostanes as a biomarker of lipid peroxidation reflect only one of many ongoing pathogenic processes i.e. oxidative stress. There is a need for developing new therapies especially in progressive phase of MS that are more process-specific and can be used in specific patient subpopulations [1]. There are three main types of MS, defined as: relapsing-remitting (RR), secondary progressive (SP) and primary-progressive (PP) with progressive-relapsing (PR) recently distinguished as an additional subtype [2]. SPMS patients have irreversible disability with a wide range of augmented symptoms.
There is evidence that MS is not only characterized by immune mediated inflammatory reactions but also by neurodegenerative processes. Accumulating data indicate that oxidative stress (OS) plays an important role in this process [3–5]. Oxidative stress leads to lipid peroxidation and may contribute to the pathogenesis of lesions in multiple sclerosis. Polysaturated fatty acids (PUFA) are major components of neuron membranes, which are highly susceptible to reactive oxygen species (ROS) attack and are the source of lipid peroxidation products. The deregulated metabolism of lipids may be of particular importance for central nervous system (CNS) disorders, as this organ has the highest lipid concentration next to adipose tissue [6].
The brain is believed to be particularly vulnerable to oxidative stress as it contains high concentrations of PUFA that are susceptible to lipid peroxidation, consumes relatively large amounts of oxygen for energy production and has lower antioxidant defenses compared to other organs [7, 8]. Of all the brain cells, neurons are particularly vulnerable to oxidative insults due to low levels of reduced glutathione [9]. Peroxidation of lipids can disrupt the organization of the membrane, causing changes in fluidity and permeability, inhibition of metabolic processes and alterations of ion transport. Damage to mitochondria induced by lipid peroxidation can lead to further ROS generation. In the presence of oxygen radicals, double bonds of unsaturated fatty acids of phospholipids are oxidized. A scission of the oxidized polyunsaturated fatty acids results in formation of phospholipid aldehydes such as oxidized phosphatidylcholine (OxPC), and aldehyde cleavage fragments including malonyldialdehyde (MDA), 4-hydroxynonenal (HNE) and acrolein [6].
Free radical catalysed peroxidation of arachidonic acid esterified in membrane phospholipids leads to the formation of isoprostanes which are prostaglandin-like compounds. They are sensitive and specific markers of in vivo lipid peroxidation and oxidative tissue injury [10, 11].
The aim of this study was to evaluate lipid peroxidation as a isoprostanes level in urine and TBARS level in serum of multiple sclerosis patients with secondary progressive clinical course. The measurement of the total antioxidant status (TAS) of SPMS patients was also investigated in our study.
Experimental Procedure
Patients
Subjects with clinically definite MS according to McDonald criteria were included to the study. Secondary progressive course of MS was ascertained as defined by Lubin et al. [12] and patients were observed for one year before. SPMS can be recognized when initial relapsing-remitting course is followed by progression, with or without occasional relapses, minor remissions and plateaux.
Twenty-six of MS patients with secondary progressive disease course (SPMS) and 10 healthy controls (HCs) were enrolled in our study. The MS group consisted of 15 females and 11 males, mean age 48.2 ± 15.2 years and mean EDSS score 6.5 ± 2.0 with a mean disease duration of 10.0 ± 6.5 years (Table 1). The patients were under Neurorehabilitation Ward control for 5 weeks and in that time they received no immunomodulators, immunostimulators, hormons, vitamins, minerals or any other substitutions with antioxidative properties. Prior to the study, all the subjects had undergone medical check-ups including neurological and internist examination. All healthy controls were screened to be free from any neurological or other major medical illnesses and were matched on age to study group (no differences were calculated, P > 0.05) (Table 1).
Table 1.
Characteristics of study subjects
| Healthy controls n = 10 | SPMS n = 26 | |
|---|---|---|
| Mean age (years) | 45.8 ± 12.9 | 48.2 ± 15.2 |
| Female n (%) | 7 (70%) | 15 (73.3%) |
| EDSS* | 6.5 ± 2.0 | |
| Disease duration (years) | 10.0 ± 6.5 |
EDSS* Expanded disability status scale
The protocol and procedures were done according to the Helsinki Declaration and were approved by Ethics Committee of the Medical University of Lodz, Poland. The study was performed at the Neurorehabilitation Division, III General Hospital Lodz, Department of Chemistry and Clinical Biochemistry, Medical University of Lodz, and Department of General Biochemistry, University of Lodz, Poland.
Blood Samples Collection
Blood samples from MS patients and healthy controls were collected into cooled EDTA containing tubes and were centrifuged to isolate plasma and erythrocytes. Erythrocytes were separated from blood plasma by centrifugation (10 min, 710 g) at 4°C and washed 3 times with 0.9% NaCl before examination.
Estimation of 8-isoPGF2α
The level of urinary isoprostane (8-isoPGF2α) was determinated from SPMS patients and controls using an immunoassay kit Randox according to the manufacturer’s instruction. 8-isoPGF2α in samples of urine (100 μl) or standards competes for binding (to the antibody coated on the plate) with 8-isoPGF2α conjugated to horseradish peroxidase (HRP). The peroxidase activity results in colour development in the substrate when added. The intensity of colour is proportional to the amount of HRP-bound 8-isoPGF2α and inversely proportional to the amount of 8-isoPGF2α in the samples (or standards) [13–15].
Estimation of TBARS
TBARS estimation in plasma. Samples of plasma were transferred to an aqual volume of 20% (v/v) cold trichloroacetic acid in 0.6 M HCl and centrifuged at 1,200×g for 15 min. One volume of clear supernatant was mixed with 0.2 vol. of 0.12 M thiobarbituric acid in 0.26 M Tris, pH 7.0 and immersed in a boiling water bath for 15 min. Absorbance at 532 nm was measured and results are expressed as nmol of TBARS.
Evaluation of the Total Antioxidant Status
The determination of the total antioxidant status in blood plasma was performed by spectrophotometric method according to procedure no. NX2332 by Randox (Randox Laboratories Ltd. Ardmore, Diamond Road, Crumlin, Co Antrim, United Kingdom, BT29 4QY). The plasma volume taken to estimation was 5 μl, a total assay volume was 305 μl. Briefly, ABTS (2,2′-Azino-di-[3ethylbenzthiazoline sulphonate]) was incubated with peroxide (metmyoglobin) and H2O2 to produce the radical cation ABTS with a relatively stable blue-green colour. Antioxidants when added in examined sample caused suppression of this colour production measured as decrease of absorbance with a spectrometer (UV/VIS Spectrometer Lambda 14P, Perkin Elmer, USA) at 600 nm. The total antioxidant status was calculated as concentration of antioxidants [mmol/l].
Statistical Analysis
The data point in this study was calculated for three separate experiments from each analyzed patient or control sample. An enzymes activity as well as total antioxidant status was expressed as mean value ± SD. Blinded replicate samples were used for quality control (QC). If no significant differences between variations were found, as assessed by the Snedecor-Fisher test, the differences between means were evaluated by applying the Student’s t test. Otherwise, the Cochran-Cox test was used. The data were analyzed using the STATISTICA (StatSoft, Tulsa, OK) statistical package.
Results
Lipid peroxidation measured as urine isoprostanes (8-EPI) was over 6-fold higher in urine of SPMS patients than in healthy volunteers (Fig. 1). The level of isoprostanes reached a mean value of 624 ng/ml (SD = 6.78) in urine of SPMS patients while for healthy controls the level reached a mean value of 93 ng/ml (SD = 22.11), respectively (Table 2). TBARS level was also statistically significantly elevated in plasma obtained from SPMS patients (P < 0.01) (Fig. 2; Table 2). However, there were no statistical significant difference of TAS level in plasma of SPMS patients according to control subjects in our study (Fig. 3; Table 2).
Fig. 1.
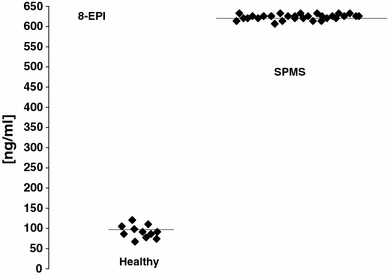
Isoprostanes (8-EPI) in urine of secondary progressive multiple sclerosis (SPMS) patients and controls
Table 2.
Lipid peroxidation measured as urine isoprostanes (8-EPI) and plasma TBARS as well as total antioxidative status (TAS) in secondary progressive multiple sclerosis (SPMS) patients
| SPMS n = 30 | Healthy n = 10 | |||
|---|---|---|---|---|
| X | SD | X | SD | |
| Isoprostanes(8-EPI) [ng/ml] | 624 | 6.78 | 93 | 22.11 |
| TBARS [μM] | 0.553 | 0.145 | 0.336 | 0.063 |
| TAS [mM] | 0.84 | 0.089 | 0.85 | 0.075 |
Fig. 2.
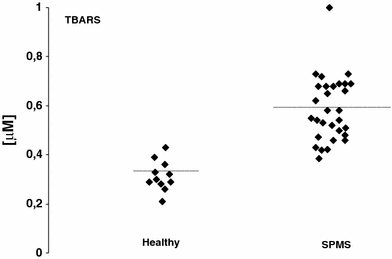
TBARS in plasma of secondary progressive multiple sclerosis (SPMS) patients and controls
Fig. 3.
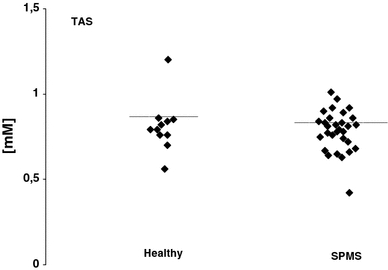
Total antioxidative status (TAS) in plasma of secondary progressive multiple sclerosis (SPMS) patients and controls
Discussion
Disability levels in SPMS patients often worsen despite a stable MRI T(2)(magnetic resonance) lesion burden. The presence of oxidative stress in the absence of measurable inflammation could help explain this phenomenon [16]. Lipid peroxidation has been implicated in the pathogenesis of multiple sclerosis (MS). Isoprostanes, isomers of prostaglandins, are produced by free radical-mediated peroxidation of fatty acids in vivo and can be quantified in biological fluids. In the present study, we estimated the level of lipid peroxidation in urine as well as plasma of MS patients [13–15].
In our study the urine level of the isoprostane 8-epi-prostaglandin (PG)-F2α which is considered as a reliable marker of oxidative stress in vivo was over six times higher in SPMS patients than control subjects. The increased level of 8-epi-prostaglandin (PG)-F2α in cerebrospinal fluid (CSF) of MS patients in several studies has been described [10].
In neurodegenerative diseases, neuronal and axonal loss is mediated by oxidative stress and excitotoxicity that constitute a final common toxic pathway. Most importantly, peroxynitrite is a key mediator of those two intertwined pathomechanisms. In MS, peroxynitrite consistently produces highly toxic nitrating and oxidizing radical species that may alter mitochondrial structure and function by a wide range of lesions including lipid, protein and DNA. During the remitting phase, peroxynitrite participates in neuron and oligodendrocyte damage associated with inflammatory processes. Moreover, in the chronic phase of the disease, peroxynitrite contributes to self-perpetuating mechanisms responsible for its progression [17].
Central nervous system (CNS) is particularly susceptible to ROS-induced damage due to the high oxygen demands of the brain and low concentration of antioxidants, both enzymatic (i.e. catalase, glutathione peroxidase, glutathione reductase, superoxide dismutase) and non-enzymatic antioxidants such as glutathione, vitamins A,C,D, coenzym Q, uric acid etc. It is suggested that that antioxidant status was altered and low activity of antioxidant enzymes were observed in CNS of MS patients [5]. Enzymatic and non-enzymatic antioxidants could regulate function of different immunologic cells in MS. [5]. In addition, an impairment of antioxidant defense systems in MS patients may results in their higher susceptibility to ROS and cause damage of CNS [7, 8]. The antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) are suggested to play a key role in pathogenesis of MS by altered protection against ROS and/or RNS [18, 19]. Recently Choi et al. (2010) has been reported that GSH levels measured in the fronto-parietal regions of the brain were distinctly lower in SPMS patients [19]. Our previous data also presented altered activity of antioxidant enzymes. We found the decrease of SOD [7] and 2-fold higher activity of CAT in erythrocytes of SPMS patients [8]. Moreover, the level of TAS in depressive MS was significantly lower than non-depressive MS as observed in our study (P < 0.0003) [20]. Finally, according to our previously published data we suggest that changes of non-enzymatic antioxidants including glutathione (GSH), uric acid, bilirubin, vitamins C, D and other exogenous antioxidants may be also responsible for a decrease of total antioxidative status in MS patients [7, 8]. In current study we selected only one subtype of MS patients with secondary progressive clinical course. No significant difference in TAS between SPMS patients and controls was observed.
The determination of lipid peroxidation products relies on different methods. The most widely used marker of lipid peroxidation is the level of malonyldialdehyde (MDA) or readily decomposed conjugated dienes in plasma or blood cells. The commonly used assay for MDA based on the reaction with thiobarbituric acid and formation of TBARS is the most widely used method however, it is not sensitive and non-specific since other metabolites can react with thiobarbituric acid [21]. The data of our study presented that lipid peroxidation estimated by TBARS generation in SPMS patients comparing to healthy sex- and age-matched controls were significantly higher.
In order to eliminate these overestimation, we used a sensitive immuneassay dedicated to isoprostanes which are proposed as the specific and reliable clinical marker of lipid peroxidation. In our study isoprostanes were estimated in urine of SPMS and healthy volunteers. Measurement of 8-epi-prostaglandin (PG)-F2α in a single sample of urine represents daily secretion of isoprostanes, which are the end products of peroxidation of arachnoidanate. Elevated levels of 8-epi-prostaglandin (PG)-F2α have been previously described in neurological disorders such as Parkinson’s, Alzhaimer’s, Crotzfeld-Jacob disorders [11, 13]. Then, the elevated level of 8-epi-prostaglandin (PG)-F2α reflects free radicals-induced lipid peroxidation and oxidative stress. The source of 8-epi-prostaglandin (PG)-F2α may be oxidized arachidonic acid derived not only from neuron membranes but also from membrane of different cells including erythrocytes [17].
The neuronal membranes contain a high amount of polyunsturated fatty acid (in phospholipids) that can be the potential site of oxidative stress. Oxidative damage of neuronal membrane may lead to its altered structure and dysfunction which may contribute to specific aspects of MS symptomatology. The peroxidation of membrane lipids changes fluidity of membranes, their permeability and exposition of receptors. It may lead to changes in signal transduction and neurotransmission [6]. In diseases with a complex pathogenesis, such as multiple sclerosis, an individual biomarker is likely to reflect only one of many ongoing pathogenic processes. The data presented in our study indicate that lipid peroxidation and oxidative stress in patients with MS may occur. Thus, we suggest that the level of isoprostanes may be used as non-invasive marker for a determination of oxidative stress in MS patients which in turn may determine an specific antioxidative therapy in SPMS patients. In progressive phase of MS treatment with antioxidants and dietary supplements should be taken into account.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Elżbieta Miller, Phone: +48-42-66677461, FAX: +48-42-6761785, Email: betty.miller@interia.pl.
Małgorzata Mrowicka, Phone: +48-42-6393306, FAX: +48-42-6331113, Email: malgorzata.mrowicka@umed.lodz.pl.
Joanna Saluk-Juszczak, Phone: +48-42-6354482, FAX: +48-42-63554484, Email: juszczak@biol.uni.lodz.pl.
Majsterek Ireneusz, Email: ireneusz.majsterek@umed.lodz.pl.
References
- 1.Bielekova B, Martin R. Development of biomarkers in multiple sclerosis. Brain. 2004;127:1463–1478. doi: 10.1093/brain/awh176. [DOI] [PubMed] [Google Scholar]
- 2.Pokryszko-Dragan A, Gruszka E, Bilińska M. Secondary progressive multiple sclerosis-clinical course and potential predictive factors. Neurol Neurochir Pol. 2008;42:6–11. [PubMed] [Google Scholar]
- 3.Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis. The need for the effective antioxidant therapy. J Neurol. 2004;25:261–268. doi: 10.1007/s00415-004-0348-9. [DOI] [PubMed] [Google Scholar]
- 4.Gonsette R. Oxidative stress and excitotoxicity: a therapeutic issue in multiple sclerosis? Mult Scler. 2008;14:22–34. doi: 10.1177/1352458507080111. [DOI] [PubMed] [Google Scholar]
- 5.Miller E (2011) Multiple sclerosis. In: Shamim I Ahmad. Neurodegenerative diseases. Springer Lands Bioscience. [in press]
- 6.Adibhatla Muralikrishna R, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Subcell Biochem. 2008;49:241–268. doi: 10.1007/978-1-4020-8831-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller E, Mrowicka M, Malinowska, et al. Effects of whole body cryotherapy on total antioxidative status and activities of antioxidative enzymes in blood of patients with multiple sclerosis. J Med Invest. 2010;57:168–173. doi: 10.2152/jmi.57.168. [DOI] [PubMed] [Google Scholar]
- 8.Miller E, Mrowicka M, Malinowska, et al. Effects of whole body cryotherapy on oxidative stress in multiple sclerosis patients. J Therm Biol. 2010;35:406–410. doi: 10.1016/j.jtherbio.2010.08.006. [DOI] [Google Scholar]
- 9.Choi IY, Lee SP, Denney D et al. (2010) Lower levels of gluthatione in the brains of secondary progressive multiple sclerosis patients measured by 1H magnetic resonance chemical shift imaging at 3T. Mult Scler [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 10.Mattsson N, Haghighi S, Andersen O, et al. Elevated cerebrospinal fluid F2- isoprostane levels indicating oxidative stress in healthy siblings of multiple sclerosis patients. Neurosci Lett. 2007;13:233–236. doi: 10.1016/j.neulet.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 11.Awe SO, Opere CA, Harris LC, et al. Effects of isoprostanes on sympathetic neurotransmission in the human isolated iris-ciliary body. Neurochem Res. 2000;25:491–496. doi: 10.1023/A:1007560025570. [DOI] [PubMed] [Google Scholar]
- 12.Lubin FD, Reingold SC. Defining the clinical course of multiple sclerosis results of an international survey. National Multiple Sclerosis Society USA Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 13.Basu S. Isoprostanes: novel bioactive products of lipid peroxidation. Free Radic Res. 2004;38:105–122. doi: 10.1080/10715760310001646895. [DOI] [PubMed] [Google Scholar]
- 14.Greco A, Minghetti L, Levi G. Isoprostanes, novel markers of oxidative injury, help understanding the pathogenesis of neurodegenerative diseases. Neurochem Res. 2000;25:1357–1364. doi: 10.1023/A:1007608615682. [DOI] [PubMed] [Google Scholar]
- 15.Morrow JD. The isoprostanes–unique products of arachidonate peroxidation: their role as mediators of oxidant stress. Curr Pharm. 2006;12:895–902. doi: 10.2174/138161206776055985. [DOI] [PubMed] [Google Scholar]
- 16.Koch J, Mostest AV, Arutjungan M, et al. Plasma lipid peroxidation and progression of disability in mutiple sclerosis. J Europ Neur. 2007;14:529–533. doi: 10.1111/j.1468-1331.2007.01739.x. [DOI] [PubMed] [Google Scholar]
- 17.Onufriev MV. Nitrosative stress in the brain: autoantibodies to nitrotyrosine in liquor as a potential marker. Neurochem J. 2010;4:228–234. doi: 10.1134/S1819712410030116. [DOI] [Google Scholar]
- 18.Wachowicz B, Rywaniak JZ, Nowak P. Apoptic matkers in human blood platelets treated with peroxynitrite. Platelets. 2008;19:624–635. doi: 10.1080/09537100802406646. [DOI] [PubMed] [Google Scholar]
- 19.Choi IY, Lee SP, Denney D et al. (2010) Lower levels of gluthatione in the brains of secondary progressive multiple sclerosis patients measured by 1H magnetic resonance chemical shift imaging at 3T. Mult Scler [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 20.Miller E, Mrowicka M, Malinowska K et al. (2010) Effects of whole body cryotherapy on a total antioxidative status and activity of antioxidant enzymes in blood of depressive multiple sclerosis patients. World J biol Psychiatry [Epub a head of print] [DOI] [PubMed]
- 21.Besler HT, Comoglu S, Okcu Z. Serum levels of antioxidant vitamins and lipid peroxidation in multiple sclerosis. Nutr Neurosci. 2002;5:215–220. doi: 10.1080/10284150290029205. [DOI] [PubMed] [Google Scholar]


