Abstract
Recombinant adenovirus vectors (Ad) have been recognized as effective in vivo gene delivery vehicles and utilized as gene therapy agents for a number of cancers. The elucidation of viral entry mechanisms has allowed the development of recombinant vectors that exploit existing cell surface receptors to achieve entry into the cell. B lymphocytes are normally resistant to infection by adenovirus 5, likely due to the lack of the Coxsackie and Adenovirus receptor (CAR). Using reverse-transcriptase PCR and flow cytometry, the CD40 receptor has been shown to be expressed on many lymphoma cells. We exploited this finding to develop a gene therapy strategy for treatment of canine B-cell lymphoma. Ad5 was targeted to cells expressing CD40 via CD40 ligand (CD40L) and was effective in infecting CD40-expressing control cells; however, both primary canine lymphoma cells and cell lines demonstrated limited evidence of transduction. Following receptor binding, adenovirus entry into cells may require interaction with αvβ3/5 integrins; we demonstrate that canine lymphoma cells are deficient in these integrins. Reduced αvβ3 integrin expression may render these cells incapable of internalizing Ad vectors. Thus, any viral targeting approaches for treatment of canine lymphoma must also take into account the potential lack of internalization signals.
Key words: gene therapy, adenovirus, canine, lymphoma, transduction, CD40, integrins
Introduction
The successful development of a gene therapy approach to B-cell lymphoma requires the construction of vectors that specifically target cancer cells and the use of an appropriate model for evaluation of these vectors. Canine lymphoma represents an excellent model of human non-Hodgkin lymphoma. The canine disease has a similar etiology and presentation to the human disease, the model has an intact immune system and the size of the animal model allows for extrapolation to humans.1,2 Recombinant Adenoviral (Ad) vectors derived from human serotypes 2 and 5 are the most promising vehicles for successful in vivo gene delivery, based on previously demonstrated efficiency in successful gene delivery in a number of cancer gene therapy applications, including gastric neoplasms and cancers of the head and neck.3,4 The use of adenoviral vectors for gene transfer has a number of benefits: viral stocks can be produced easily and quickly, there is robust gene expression with little risk of insertional mutagenesis, and infection and subsequent gene expression can occur in quiescent cells.5 Potential limitations to the use of recombinant adenoviruses as agents for gene therapy include the potential lack of transduction of target tissue by Ad vectors due to the paucity of native receptors on target cells, and the possibility of transfer to normal cells as the native Ad receptor, CAR, is present on a wide range of human tissue types.6,7
Entry of Ad into cells is a multi-step process, requiring first the binding of the native CAR by the viral fiber knob, followed by interaction of an RGD motif in the penton base of Ad with αvβ3/5 integrins on the cell surface.8–10 Cells of the lymphocyte lineage have proven refractory to Ad infection, which has been hypothesized to be due to the lack of cell surface expression of the primary Ad receptor CAR.11 Ad vectors have been suggested as suitable vectors for transducing lymphocytes and CAR-expressing lymphocytes have demonstrated efficient transduction by recombinant Ad.12,13 Ad has been used in conjunction with a bispecific conjugate to achieve cell entry in a CAR-independent fashion in human B lymphocytes via CD70;14 murine B lymphocytes activated with lipopolysaccharide (LPS) were transduced with Ad modified to contain a polylysine heparin binding motif in the fiber protein.15 This same modification was also successful in transducing human myeloma and myeloid leukemia cells in the presence of growth factors.16 Therefore, the development of an adenoviral vector to target B-cell lymphoma requires the modification of native viral tropism either by modification of the viral capsid or by the use of a bispecific conjugate that binds a cell surface receptor. To this end, CD40 was recognized as a potential candidate molecule for retargeting. The CD40 molecule is expressed on B lymphocytes and the interaction between CD40 and its ligand promotes growth and differentiation of these cells.17
As a model for B-cell lymphoma targeted therapy, capsid-modified vectors retargeted to human and canine CD40 and a bispecific conjugate comprised of the soluble CAR ectodomain (sCAR) and CD40L were utilized. Both human- and canine-derived cell lines and primary canine lymphoma cells were used to evaluate the efficacy of the retargeted vectors in transducing cells in a CAR-independent manner. The following report demonstrates the feasibility of retargeting Ad to CD40, and identifies that other cellular components necessary for internalization of the vector may be lacking in canine lymphoma. In particular, cells that lack αvβ3 integrins fail to internalize virus after it is bound by the CD40-CD40L interaction.
Results
Ad targeting to CD40-positive cells.
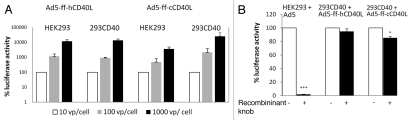
In order to evaluate the efficiency of transduction with adenovirus particles targeting CD40 with CD40L, 293CD40 cells expressing canine CD40 were created. These cells and control parental HEK293 cells were used to assess the ability to provide receptor-specific delivery to target cells. Transduction by control and CD40 targeted vectors was assessed by measuring the luciferase activity in cell lysates following incubation (Fig. 1A). Both HEK293 and 293CD40 cells express CAR and were efficiently transduced by untargeted virus with luciferase activity increasing in a dose-dependent manner (data not shown). While transduction was achieved in both cell lines with retargeted virus, the level of transduction in 293CD40 cells was higher than parental cells with either retargeted vector. To further demonstrate this transduction was achieved in a CAR-independent manner, a recombinant knob protein which binds to and blocks CAR was utilized. Incubation of cells with recombinant knob blocked transduction of HEK293 cells with wild-type (wt) virus, and luciferase activity was reduced to 2% of that observed without blocking. By contrast, blocking with recombinant knob prior to infection had less effect on 293CD40 cells. Luciferase activity remained almost unchanged at 94.7% for Ad5-ff-hCD40L and 85% for Ad5-ff-cCD40L (Fig. 1B). While the reduction in luciferase with the canine CD40L vector achieved statistical significance, it was far less of an effect than was seen in HEK293 cells. This data indicates targeting CD40 via CD40L is an effective method of redirecting adenovirus tropism, and that the interaction is specific to CD40 and does not involve CAR. Additionally, this data suggests that human CD40L may serve as a better ligand for canine CD40 than native ligand.
Figure 1.
(A) Transduction of CD40-positive cells by targeted vector. CD40-negative HE K293 cells and CD40-positive 293CD40 cells were infected with either Ad5-ff-hCD40L or Ad5-ff-cCD40L at MOI of 10, 102 or 103 vp/cell. Transduction was indicated by luciferase activity; this was measured from cell lysates and is reported as relative light units. Transduction increased with both vectors in both cell lines in a dose dependent manner; the highest level of transduction as indicated by luciferase activity was seen with the Ad5-ff-hCD40L vector. The values were shown as relative infectivity by taking the level of luciferase at 10 vp/cell as 100% and expressing other readings as relative percentage increase. (B) Incubation of cells with recombinant knob blocked transduction by untargeted virus. Cells were incubated with recombinant knob to block CAR prior to infection with wt virus and luciferase activity was used to measure transduction. In CD40-negative cells infected with wt virus, luciferase activity was reduced to 2% while in CD40-positive cells infected with targeted virus, there was little reduction in transduction efficiency as determined by luciferase activity. Error bars indicate standard deviation. *p < 0.05; ***p < 0.001.
CD40 mediated gene delivery in canine cell lines.
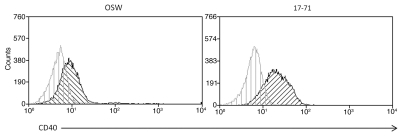
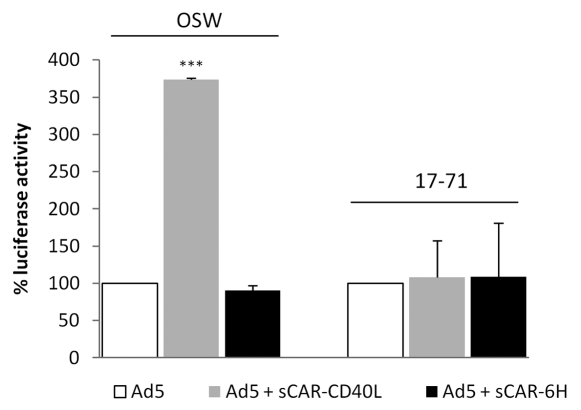
Following the success of targeting 293CD40 cells with modified vectors, demonstration of this process in CD40 positive canine cell lines was attempted. Initial examination was performed in DH82, a canine histiocytoma line determined to be CD40-positive by RT-PCR.26 These cells were poorly infected by CD40 targeted virus. Subsequent flow cytometry with anti-CD40 antibody showed these cells to be devoid of cell-surface CD40 (data not shown). Canine lymphoid lines OSW and 17–71 were then obtained for this task. Both lymphoma cell lines were shown to be CD40 positive by flow cytometry (Fig. 2). To evaluate transduction with the retargeted vectors, OSW and 17–71 cells were infected with Ad5, Ad5 ff hCD40L and Ad5 ff cCD40L at MOIs ranging from 10.103 virus particles per cell and incubated for 24 h. Both lymphoma cell lines showed low-level transduction by wt virus at a moi of 103 vp/cell indicated by luciferase activity. This was approximately 10-fold higher in OSW cells than 17–71 cells. There was no transduction detected in either cell line in response to the retargeted vectors Ad5-ff-hCD40L or Ad4-ff-cCD40L. The efficacy of the sCAR-CD40L biphasic adapter in achieving gene transfer via CD40 was also evaluated in these cell lines. Adaptor use increased luciferase expression 3.7-fold in OSW cells. To confirm that the increase in luciferase expression was due to the interaction of CD40L and CD40 and not via another viral component, the sCAR-6His adapter was used as a negative control and luciferase activity was reduced below background levels (Fig. 3).
Figure 2.
Expression of CD40 by lymphoma cell lines. The canine lymphoma cell lines OSW and 17–71 were analyzed for CD40 expression using anti-CD40 monoclonal antibody by flow cytometry. Cells were incubated with a primary anti-CD40 monoclonal antibody labeled with Zenon Alexa 610/RPE.
Figure 3.
Use of a bispecific adapter to increase CD40 targeted transduction in lymphoma cell lines. Cell lines were infected with untargeted virus alone or in conjunction with a biphasic adapter targeted to CD40 (sCAR-CD40L) or a negative control (sCAR-6His). The use of the targeted adapter increased transduction in both cell lines and this was reduced to background levels with sCAR-6His. The values were shown as relative infectivity by taking the level in untargeted virus as 100% and expressing other readings as relative percentage change. Error bars indicate standard deviation. ***p < 0.001.
These experiments demonstrated canine lymphoma lines positive for surface CD40 by flow cytometry were resistant to infection by CD40-targeted adenoviruses. Moderate levels of transduction were achieved, however, when these cells were infected with wt virus and bispecific adapter.
CD40-mediated gene delivery in primary lymphoma cells.
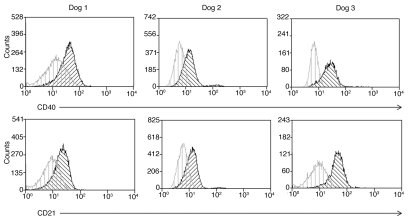
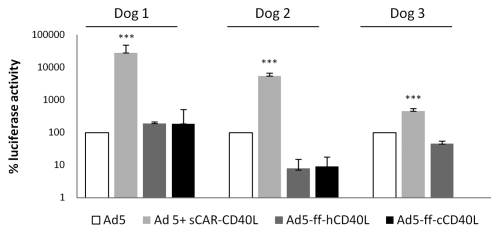
Following experiments to demonstrate transduction of canine cell lines via CD40, we attempted to transduce primary canine lymphoma cells with the targeted viruses. Following isolation, cells were analyzed by flow cytometry and chosen for transduction experiments on the basis of positive CD21 and CD40 staining (Fig. 4). Cells isolated from five individual cases of primary lymphoma met these criteria and were tested. These cells were extracted from lymph nodes following excision and virus was added within 24 h of isolation. Cells were infected with control untargeted virus, targeted virus (Ad5-ff-hCD40L and Ad5-ffc-CD40L), and wt virus complexed with sCAR-hCD40L, incubated at 37°C at times ranging from 24.72 h, and then luciferase activity was measured. Two of the five cases showed no transduction at any point; however, luciferase activity was detected in three cases. In these three cases, the highest level of transduction achieved was with the use of the bispecific adapter plus wt virus (Fig. 5). In dog 1, the use of the bispecific adapter with untargeted virus increased luciferase activity by 200-fold when compared to untargeted virus alone. While there was an increase in transduction with the recombinant vectors Ad5-ff-hCD40L and Ad5-ff-cCD40L compared to untargeted vectors (1.8- and 7-fold, respectively), this was less dramatic. The level of transduction with Ad5-ff-cCD40L was comparable to that observed in control cells. In dog 2, transduction increased 54-fold with the bispecific adapter plus untargeted virus, however, while transduction was observed with the targeted viruses, it was less than that observed with the wt virus and approximately 100-fold less than in control cells in the case of Ad5-ff-hCD40L and 10-fold less with ad5-ff-cCD40L. In dog 3, transduction was increased 4-fold with the use of the bispecific adapter over wt alone, and again there was very low luciferase activity in response to transduction with Ad5-ff-hCD40L and none with Ad5-ff-cCD40L. Interestingly, in both dogs 1 and 2, transduction was higher with the canine CD40 targeted virus than with human sequence, which was an effect opposite to that observed in control cells. Overall, this data is consistent with that observed in OSW cells where transduction via the sCAR-CD40L adapter was more efficient than retargeted viruses and retargeted Ad were much less efficient than hypothesized.
Figure 4.
CD21 and CD40 expression by primary canine lymphoma cells. Cells extracted from lymph nodes of canine patients diagnosed with lymphoma where transduction was attempted with retargeted vectors. Cells were stained with anti-CD40 antibody labeled with Zenon Alexa 610/RPE and antiCD21 antibody labeled with Zenon Alexa 660. Top panel shows CD40, lower panel CD21.
Figure 5.
CD40-targeted transduction in primary cells. Three primary cultures of cells obtained from lymph nodes of lymphoma-affected dogs were infected within 24 h of collection with untargeted virus alone (control), untargeted virus and sCAR-CD40L, Ad5-ff-hDC40L or Ad5-ff-cCD40L and luciferase activity was measured from cell lysates. The values were shown as relative infectivity by taking the level of luciferase expression of Ad5 transduced cells as 100% and expressing other readings as relative percentage change. Error bars indicate standard deviation. ***p < 0.001.
The limited levels of transduction by CD40 targeted virus observed in both primary canine lymphoma cells and canine lymphoma cell lines raises the potential for an absent component or inoperative step in the viral entry pathway.
Integrin analysis of cell lines.
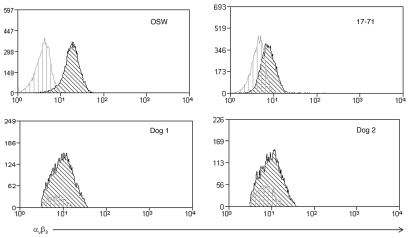
The cellular uptake of Ad is a multi-step process requiring viral binding to a cell surface receptor followed by interaction with surface integrins which mediate internalization of the virus.8,10,15,27 Since there are differences in cell line and primary cell transduction efficiencies, the role of αv;3 integrin in adenoviral infection of canine lymphoma cells was evaluated. The canine lymphoma cell lines OSW and 17–71 were analyzed by flow cytometry for αv;3 integrin levels to determine if differential expression might help explain the disparities in transduction activity demonstrated between these cell lines (Fig. 6). OSW cells have a detectable level of αv;3, approximately 10-fold above background; however, expression was much lower on 17–71 cells (approximately 2-fold). Primary lymphoma cell lines also exhibited very low surface expression levels of integrin αv;3. Thus, low levels of αvβ3 integrin appear to be an obstacle to efficient adenoviral infection of canine lymphoma cells.
Figure 6.
Expression of αvβ3 receptors on lymphoma cell lines and primary cells. Staining of αvβ3 receptors on OSW and 17–71 cell lines and two of the primary lymphoma cases where transduction was observed.
Discussion
A major advance in the design of Ad vectors to treat tumors was the development of methods to selectively transduce target cells in a CAR-independent manner. This approach also circumvents the lack of native CAR receptors on the surface of many tumor cells, coupled with the need to de-target Ad from normal cells that are susceptible to infection due to the presence of CAR.6,7,11 The results of the above experiments confirm most primary canine B-cell lymphomas and many primary T-cell lymphomas express high levels of CD40 on their surface making this protein attractive as a potential molecule for retargeted antitumor Ad vectors.
Retargeting of Ad has been demonstrated using a number of approaches, including genetic modification of the fiber knob or the use of bispecific adapters to redirect the virus.28 In the experiments described herein, successful retargeting of Ad to CD40 using CD40L in the 293cCD40 cell line with capsid-modified virus was demonstrated. However, CD40-positive lymphoma cell lines and CD40-positive primary lymphomas proved generally resistant to transduction by modified Ad, with both OSW and 17–71 cells showing no luciferase activity in response to infection with either Ad5-ff-hCD40L or Ad5-ff-cCD40L at a range of MOIs. No luciferase activity was present in the majority of primary lymphoma cells infected, with only one case showing moderate luciferase expression following infection with Ad5-ff-cCD40L. The use of an sCAR-CD40L bispecific adapter complexed with wt Ad5 was more efficient at transducing cells in the OSW cell line and two of the primary canine cell cultures.
As canine B-cell lymphoma cells, proven to express adequate levels of CD40, are refractory to infection with CD40 targeted Ad vectors, the failure of infection must be due to some mechanism occurring after initial binding of the virus to the cell surface. Potential points of infection failure may include virus internalization, un-coating, and/or nuclear trafficking. Virus internalization has been shown to depend on the presence of cell surface integrins, namely αvβ3/5 integrins,27 which may not be present on some tumors. Human neoplastic B lymphocytes have been shown to have low level expression of αvβ3 and αvβ5 integrins.29,30 Additionally, previous work has suggested that lack of integrin seems to be a crucial barrier to transducing B-lymphocyte cell lines.30 While the level of αvβ3 integrin on canine lymphoma cells was evaluated in this study, the lack of an available reagent prevented examination of the expression of αvβ5 integrins.
The differing levels of αvβ3 integrin expression on the OSW and 17–71 cell lines correlated with the transduction efficiency of wt virus, alone and in conjunction with a bispecific adapter, in these cells. This data suggests that, in this model, the expression of αvβ3 integrin may play a role inensuring internalization of the virus.
Our bispecific adapter molecule was partially successful transducing both cell lines and primary neoplastic cells. In fact, the highest level of luciferase activity detected was in two of the primary cell assays that had not been cryopreserved and were infected with wt Ad and the bispecific adapter sCARCD40L. This may indicate that either the modified viruses are not as efficient as wt at transducing cells, or the use of the bispecific adapter is responsible for this increased transduction. Previous research has demonstrated αvβ3 integrins in neoplastic B lymphocytes can be upregulated following engagement of CD40 with its ligand. This interaction leads to a five-fold increase in Ad-mediated gene transfer in these cells.29 Additionally, B-lymphocyte proliferation has been demonstrated following CD40-CD40L interaction in the presence of IL-4.31,32 As the experiments were conducted in cells extracted from lymph nodes and were not enriched for B lymphocytes, the combination of the CD40-CD40L engagement in this multi-cellular environment, with the potential release of endogenous growth factors, may have contributed to the upregulation of cell surface proteins necessary for viral internalization. Although lymphoma cells have low levels of surface CAR, interaction with other receptors, including the α2 domain of MHC class I molecules, have been implicated with virus binding,33 which may also indicate an increased ability of cells to internalize Ad. Based on these studies, the retargeting of adenovirus to selectively transduce lymphoma cells will require overcoming the inability of these cells to internalize Ad based on a paucity of cell surface integrins. Interestingly, in the above experiments, the bispecific sCAR adapter was found to be the most effective method of achieving transduction in both lymphoma cell lines and primary cells. The mechanism by which this is achieved needs to be examined further and could be important in designing a targeted therapy for B-cell lymphoma.
Methods
Cells lines and primary cells.
HEK293 and DH82 (canine histiocytic cell line) cells were purchased from ATCC and grown in DMEM/F12 supplemented with 10% fetal bovine serum (FBS) (Hyclone), 1% antibiotic/antimycotic solution containing penicillin, streptomycin and amphotericin (Cellgro), and grown at 37°C in 5% CO2. 293CD40 cells were created by stable transfection of HEK293 cells with a linearized plasmid expressing full-length canine CD40 cDNA. HEK293 cells were stably transfected with a pcDNA3 vector (Invitrogen, San Diego, CA) into which a full-length cDNA amplicon of the canine CD40 open reading frame had been ligated. Amplicons were confirmed for identity and sequence fidelity by direct DNA sequencing (Auburn University DNA Sequencing Core) prior to ligation. Plasmids were linearized outside the CD40 and Neo coding and control regions prior to transfection and then transfected and clones were selected in G418 media as previously described in reference 18.
The OSW canine lymphoma cell line was provided by Dr. William C. Kisseberth, The Ohio State University, and the 17–71 cell line was provided by Dr. Steven Suter, North Carolina State University, and have been previously described in reference 19 and 20. These cell lines were grown in RPMI supplemented with 10% FBS, 2 mM L-glutamine and 1% antibiotic/antimycotic solution and maintained at 37°C and 5% CO2.
Primary lymphoma cells were obtained by mechanical disruption of tissue obtained following excision of lymph nodes surgically removed from untreated canine lymphoma patients and submitted for histologic analysis as part of standard medical care. Briefly, lymph node tissue was placed in a sterile 6 cm dish, with enough complete media (RPMI with 10% FBS, 2 mM Glutamax, 1% antibiotic/antimycotic solution) to cover the tissue and cut into sections approximately 25 mm2. These pieces were then passed through a sterile 70 µm nylon filter. Media was added to a total of approximately 10 ml and the cells were centrifuged at 250x g for 5 min. Cells were either re-suspended in complete media and incubated at 37°C prior to infection or analysis by flow cytometry or cryopreserved by re-suspending at 1 × 107 cells/ml in Gibco Recovery Cell Culture Freezing Media (Invitrogen).
Flow cytometry.
The following antibodies were used to detect cell surface expression of receptors: rat anti-canine CD4-FITC (clone YKIX302.9, AbD Serotec, Oxford UK), rat anti-canine CD8-PE (clone YCATE55.9, AbD Serotec), mouse anti-canine CD21 (Clone CA2.1D6, AbD Serotec) labeled with Zenon anti-mouse Alexa Fluor 610/R-PE (Invitrogen), mouse anti-human CD40 antibody (Clone B-B20, Diaclone Bensancon, France) labeled with Zenon anti-mouse Alexa Fluor 700 (Invitrogen), mouse anti-human αvβ3 (Clone MAB1976, Millipore, Bellerica, MA) conjugated to PE or labeled with Zenon anti-mouse Alexa Fluor 700, and monoclonal antibody to human CAR RmcB (produced using a hybridoma purchased from ATCC and kindly provided by Joanne Douglas, University of Alabama, Birmingham, AL). Cells were suspended (1 × 106) in 100 µl of staining buffer (SB: PBS, 1% BSA, 0.1% sodium azide) and incubated for 20–30 min on ice. Antibodies were then added and cells incubated on ice for 40–60 min in the dark. Following this incubation, cells were washed two times in 2 ml PBS, and secondary antibodies were added at this stage if needed, followed by 40–60 min incubation and 2x PBS wash. Cells were then re-suspended in 500 µl flow wash buffer (PBS, 0.1% BSA) and filtered to 50 µm prior to analysis. All flow cytometry assays were performed on a MoFlo Flow Cytometer and Cell Sorter (Beckman Coulter). The expression profiles were determined using Summit 4.3 software (Beckman Coulter).
Adenovirus constructs.
Luciferase-expressing vectors encoding artificial fiber proteins containing human (Ad5-ff-hCD40L) or canine (Ad5-ff-cCD40L) CD40L were constructed as previously described in reference 21. Luciferase-expressing Ad5 vectors encoding the native fiber protein were constructed as previously described in reference 22 and 23.
All Ad5 vectors were isolated from infected HEK293 cells and purified by equilibrium centrifugation in CsCl gradients according to a standard protocol.24 The protein concentrations in the viral preparations were determined using the DC protein assay (Bio-Rad, Hercules, CA) with purified bovine serum albumin (BSA) as a standard. The virus titers were calculated using the formula: 1 µg of protein = 4 × 109 viral particles (vp). The fusion proteins sCAR-His6 and sCAR-CD40L were constructed as previously described in reference 25.
Gene transfer assays.
Transduction by the panel of recombinant Ad5 vectors (Ad5CMV, Ad5-ff-hCD40L, Ad5-ff-cCD40L) was examined using luciferase as the reporter gene. Cells were grown in triplicate at a density of 1 × 105 cells/well in a 24 well plate. Virus dilutions were made in multiplicities of infection (moi) ranging from 101–104 vp/cell in media supplemented with 2% FBS and 200 µl aliquots were added to cell monolayers. The cells were incubated with virus for 1 h at 37°C to allow virus internalization after which 300 µl of complete media was added to each well. Between 24 and 72 h post infection, cells were collected, lysed and luciferase activity measured using the Luciferase Assay System (Promega, Madison, WI) following the manufacturer's directions. To determine efficiency of Ad-mediated transfer using an sCAR-CD40L or sCAR-His6 biphasic adapter, aliquots of Ad5CMVLuc were mixed with 20.100 µg of the biphasic adapter and incubated at room temperature for 30 min. The virus-sCAR complexes were diluted with media supplemented with 2% FBS and infection proceeded as described above. Blocking assays with soluble Ad5 knob were conducted by diluting the soluble knob to 100 µg in 200 µl cold media followed by addition to each well. Cells were then incubated on ice for 1 h, recombinant knob removed, washed and infected with the appropriate virus dilution as described above. All experiments were conducted in triplicate and repeated at least two times.
Acknowledgements
This work was supported by NIH grant 5R01CA113454-04. The authors thank Allison Church Bird for flow cytomety assistance and Maninder Sandey for statistical analysis.
Abbreviations
- Ad
adenovirus
- BSA
bovine serum albumin
- CAR
coxsackie and adenovirus receptor
- FBS
fetal bovine serum
- LPS
lipopolysaccharide
- MOI
multiplicity of infection
- PBS
phosphate buffered saline
- RT-PCR
reverse-transcriptase polymerase chain reaction
- wt
wild type
References
- 1.Vail DM, MacEwen EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000;18:781–792. doi: 10.3109/07357900009012210. [DOI] [PubMed] [Google Scholar]
- 2.Hansen K, Khanna C. Spontaneously and genetically engineered animal models; use in preclinical cancer drug development. Eur J Cancer. 2004;40:858–880. doi: 10.1016/j.ejca.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Khalighinejad N, Hariri H, Behnamfar O, Yousefi A, Monemi A. Adenoviral gene therapy in gastric cancer: A review. World J Gastroenterol. 2008;14:180–184. doi: 10.3748/wjg.14.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vattemi E, Claudio PP. Adenoviral gene therapy in head and neck cancer. Drug News Perspect. 2006;19:329. doi: 10.1358/dnp.2006.19.6.1015352. [DOI] [PubMed] [Google Scholar]
- 5.McConnell MJ, Imperiali MJ. Biology of adenovirus and its use as a vector for gene therapy. Hum Gene Ther. 2004;15:1022–1033. doi: 10.1089/hum.2004.15.1022. [DOI] [PubMed] [Google Scholar]
- 6.Cichon F, Schmidt HH, Benhidjeb T, Löser P, Siemer S, Haas R, et al. Intravenous administration of recombinant adenoviruses causes thrombocytopenia, anemia and erythroblastosis in rabbits. J Gene Med. 1999;1:360–371. doi: 10.1002/(SICI)1521-2254(199909/10)1:5<360::AID-JGM54>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 7.Smith JS, Tian J, Muller J, Byrnes AP. Unexpected pulmonary uptake of adenovirus vectors in animals with chronic liver disease. Gene Ther. 2004;11:431–438. doi: 10.1038/sj.gt.3302149. [DOI] [PubMed] [Google Scholar]
- 8.Bergelson JM, Cunningham JA, Drouguett G, Kurt-Jones EA, Krithivas A, Hong JS, et al. Isolation of a common receptor for coxsackie B virus and adenoviruses 2 and 5. Science. 1997;275:1320132–1320133. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 9.Huang S, Endo RI, Nemerow GR. Upregulation of integrins alphavbeta3 and alphavbeta5 on human monocytes and T lymphocytes facilitates adenovirusmediated gene delivery. J Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang S, Kamata T, Takada Y, Ruggeri ZM, Nemerow GR. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J Virol. 1996;70:4502–4508. doi: 10.1128/jvi.70.7.4502-4508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marini FC, Yu Q, Wichnam T, Kovesdi I, Andreeff M. Adenovirus as a gene therapy vector for hematopoietic cells. Cancer Gene Ther. 2000;7:816–825. doi: 10.1038/sj.cgt.7700174. [DOI] [PubMed] [Google Scholar]
- 12.Leon RP, Hedlund T, Meech SJ, Li S, Schaack J, Hunger SP, et al. Adenoviral-mediated gene transfer in lymphocytes. Proc Nat Acad Sci USA. 1998;95:13159–13164. doi: 10.1073/pnas.95.22.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matto M, Nuutinen UM, Hakkarainen T, Tallone T, Wahlfors J, Pelkonen J. hCAR-EGFP fusion receptor in human follicular lymphoma B cells—a model for adenoviral gene therapy for B cell malignancies. Int J Mol Med. 2006;17:1057–1062. [PubMed] [Google Scholar]
- 14.Israel BF, Pickles RJ, Segal DM, Gerard RD, Kenney SC. Enhancement of adenovirus vector entry into CD70-positive B cell lines by using a bispecific CD70-adenovirus fiber antibody. J Virol. 2001;75:5215–5221. doi: 10.1128/JVI.75.11.5215-21.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Wickham TJ, Keegan AD. Efficient transduction of murine B lymphocytes and B lymphoma cell lines by modified adenoviral vectors: enhancement via targeting to FcR and heparan-containing proteins. Gene Ther. 2001;8:938–945. doi: 10.1038/sj.gt.3301487. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez R, Vereecque R, Wickham TJ, Vanrumbeke M, Kovesdi I, Bauters F, et al. Increased gene transfer in acute myeloid leukemic cells by an adenovirus vector containing a modified fiber protein. Gene Ther. 1999;6:314–320. doi: 10.1038/sj.gt.3300836. [DOI] [PubMed] [Google Scholar]
- 17.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 18.DeInnocentes P, Agarwal P, Bird RC. Phenotype-rescue of cyclin-dependent kinase inhibitor p16/INK4A defects in a spontaneous canine cell model of breast cancer. J Cell Biochem. 2009;106:491–505. doi: 10.1002/jcb.22034. [DOI] [PubMed] [Google Scholar]
- 19.Kisseberth WC, Nadella MVP, Breen M, Thomas R, Duke SE, Murahari S, et al. A novel canine lymphoma cell line: a translational and comparitive model for lymphoma research. Leuk Res. 2007;31:1709–1720. doi: 10.1016/j.leukres.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Steplewski Z, Jeglum KA, Rosales C, Weintrub N. Canine lymphoma-associated antigens defined by murine monoclonal antibodies. Cancer Immunol Immunother. 1987;24:197–201. doi: 10.1007/BF00205629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belousova N, Korokhov N, Krendelschchikova V, Simonenko V, Mikheeva G, Triozzi PL, et al. Genetically targeted adenovirus vector directed to CD40-expressing cells. J Virol. 2003;77:11367–11377. doi: 10.1128/JVI.77.21.11367-77.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korokhov N, Mikheeva G, Krendelshchikov A, Belousova N, Simonenko V, Drendelshchikova V, et al. Targeting of adenovirus via genetic modification of the viral capsid combined with a protein bridge. J Virol. 2003;77:12931–12940. doi: 10.1128/JVI.77.24.12931-40.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krasnykh VN, Mikheeva GV, Douglas JT, Curiel DT. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70:6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham FL, Prevec L. Methods for construction of adenovirus vectors. Mol Biotechnol. 1995;3:207–220. doi: 10.1007/BF02789331. [DOI] [PubMed] [Google Scholar]
- 25.Dmitriev I, Kashentseva E, Rogers BE, Krasnykh V, Curiel DT. Ectodomain of Coxsackie and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor positive cells. J Virol. 2000;74:6875–6884. doi: 10.1128/jvi.74.15.6875-6884.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bird RC, DeInnocentes P, Lenz S, Thacker EE, Curiel DT, Smith BF. An allogeneic hybrid-cell fusion vaccine against canine mammary cancer. Vet Immunol Immunopath. 2008;123:289–304. doi: 10.1016/j.vetimm.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alphavbeta3 and alphavbeta5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-E. [DOI] [PubMed] [Google Scholar]
- 28.Majhen D, Ambriovic-Ristov A. Adenoviral vectors—how to use them in cancer gene therapy? Virus Res. 2006;119:121–133. doi: 10.1016/j.virusres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Cantwell MJ, Sharma S, Friedmann T, Kipps TJ. Adenovirus vector infection of chronic lymphocytic leukaemia B cells. Blood. 1996;88:4676–4683. [PubMed] [Google Scholar]
- 30.Ebert O, Wilbert D, Buttgereit P, Ziske C, Flieger D, Schmidt-Wolf IGH. Effects of recombinant adenovirus-mediated expression of IL-2 and IL-12 in human B lymphoma cells on co-cultured PBMC. Genet Vaccines Ther. 2004;2:15. doi: 10.1186/1479-0556-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mason NJ, Coughlin CM, Overley B, Cohen JN, Mitchell EL, Colligon TT, et al. RNA-loaded CD40-activated B cells stimulate antigen-specific T cell responses in dogs with spontaneous lymphoma. Gene Ther. 2008;15:955–965. doi: 10.1038/gt.2008.22. [DOI] [PubMed] [Google Scholar]
- 32.Rousset F, Garcia E, Banchereau J. Cytokine-induced proliferation and immunoglobulin production of human B lymphocytes triggered through their CD40 antigen. J Exp Med. 1991;173:705–710. doi: 10.1084/jem.173.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong SS, Karayan L, Tournier J, Curiel DT, Boulanger PA. Adenovirus type 5 fiber knob binds to MHC class I alpha2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]