Abstract
Resistance to chemotherapy remains a significant obstacle in the treatment of hormone-independent breast cancer. Recent evidence suggests that altered sphingolipid signaling through increased sphingosine kinase activity may be an important mediator of breast cancer drug resistance. Sphingosine kinase-1 (Sphk1) is a proposed key regulator of breast cancer tumorigenesis, proliferation and resistance. There is, however, conflicting data on the role of sphingosine kinase-2 (Sphk2) in cancer biology and resistance, with some suggesting that Sphk2 has an opposing role to that of Sphk1. Here, we studied the effects of the novel selective Sphk2 inhibitor, ABC294640 (3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridin-4-ylmethyl) amide), on human breast cancer. ABC294640 blocked both viability and survival at low micromolar IC50 concentrations in the endocrine therapy-resistant MDA-MB-231 and chemoresistant MCF-7TN-R cell systems. Treatment with the inhibitor significantly reduced proliferation, as seen in immunofluorescence staining of Ki-67 in vitro. Interestingly, pharmacological inhibition of Sphk2 induced apoptosis through the intrinsic programmed cell death pathway. Furthermore, ABC294640 also diminished NFκB survival signaling, through decreased activation of the Ser536 phosphorylation site on the p65 subunit. Xenografts of MCF-7TN-R cells growing in immunocompromised mice were utilized to validate the therapeutic efficacy of the sphingosine kinase-2 inhibitor. Treatment with 50 mg of ABC294640/kg completely blocked tumor volume in this model. These results indicate that pharmacological inhibition of Sphk2 with the orally bioavailable selective inhibitor, ABC294640, has therapeutic potential in the treatment of chemoand endocrine therapy-resistant breast cancer.
Key words: sphingolipids, chemoresistance, sphingosine kinase, NFkappaB, breast cancer, ceramide, TNF, sphingosine-1-phosphate
Introduction
Despite significant improvements in the diagnosis and treatment of breast carcinoma over the past several decades, resistance and toxicity in response to therapy remain the primary causes of breast cancer treatment failure today. Recent studies have indicated that aberrant sphingolipid metabolism, resulting in altered levels of endogenous ceramides and sphingosine-1-phosphate (S1P), is an important mediator of both chemo- and endocrine therapy-resistance.1–3 The balance between these lipids is tightly regulated by the enzyme sphingosine kinase, which converts pro-apoptotic, anti-proliferative ceramide into its pro-survival and mitogenic metabolite, S1P.4 In many cancers, including breast carcinoma, increased expression and activity of sphingosine kinase is associated with malignancy and drug resistance.5 In a single pooled study of 1,269 breast cancer tumor samples, an increase in sphingosine kinase expression directly correlated with loss of ER expression, increased tumor aggressiveness and poor prognosis.6 Similar results have been found in the laboratory as well. Overexpression of sphingosine kinase in MCF-7 breast cancer cells results in increased resistance to doxorubicin, tamoxifen and tumor necrosis factor.3,7
The NFκB transcription factor is known to promote survival and chemoresistance in solid tumor cancers.8 While the components of NFκB are rarely mutated in cancer, the signaling cascade is commonly constitutively activated and the p65 and p50 subunits are frequently overexpressed in breast cancer compared to normal breast tissue.9–11 The activity of the NFκB signaling cascade is associated with mammary carcinogenesis, especially tumors with an aggressive and ER-negative phenotype.12 NFκB promotes malignant proliferation and prevents apoptosis through regulation of Bcl-xL, cFLIP and the inhibitors of apoptosis proteins, cIAP1 and cIAP2.8,13 Both the NFκB and sphingolipid signaling pathways have been implicated as mediators of breast cancer drug resistance. Interestingly, these two pathways are known to intersect and interact with each other at various points. Xia et al. first showed in 1998 that TNF-induced adhesion molecule expression was mediated through sphingosine kinase signaling, leading to speculation on the role of sphingosine kinase in metastasis and downstream NFκB pathway activation.14 It was later shown that Sphk interacts with the TRAF2 component of the TRAF/TRADD complex on the intracellular portion of the TNF receptor. Several studies have utilized this interaction to target downstream NFκB activation in inflammatory bowel disease and certain inflammatory cancers.15–17 Furthermore, in A549 lung carcinoma cells siRNA knockdown of Sphk1 or treatment with the non-specific sphingosine kinase inhibitor, N,N-Dimethylsphingosine (DMS), decreased phosphorylation of IκB, diminished NFκB transcriptional activity and blocked translocation of the p65/p50 complex to the nucleus.18 These results suggest that targeting sphingosine kinase as a means of decreasing NFκB signaling may be of therapeutic interest in the context of inflammatory disease and drug resistant cancers.
There are two differentially expressed isoforms of sphingosine kinase: sphingosine kinase-1 (Sphk1) and sphingosine kinase-2 (Sphk2). These two isoforms have varying substrate specificity, intracellular localization and tissue distributions. Sphk1 is the more extensively studied isoform and has been shown to promote tumor formation, proliferation and resistance in a number of different tissue types, including prostate and breast.19 However, there are conflicting reports as to the function of Sphk2, with some suggesting that Sphk2 has an opposing role to that of Sphk1.20,21 To date, the role of Sphk2 in breast cancer tumor proliferation is under debate. Almost all studies, thus far, on the roles of Sphk1 and Sphk2 have been performed using overexpression and siRNA knockdowns due to a lack of pharmacological agents. However, some have suggested that overexpressing Sphk isoforms may change their subcellular localization and alter endogenous function.21,22 Despite increased understanding of the importance of sphingolipid signaling in anti-cancer drug resistance, pharmacologically targeting this pathway as a therapeutic has been inhibited by a lack of small molecule inhibitors. In this study we use the recently discovered selective sphingosine kinase-2 inhibitor, ABC294640, to elucidate the role of Sphk2 in breast cancer biology.23,24 We have recently analyzed ABC294640 in estrogen receptor (ER)-positive cell systems and catalogued its inhibitory effect on ER signaling.25 Here, we examine and characterize the potential of pharmacological inhibition of Sphk2 as a mechanism to overcome NFκB mediated chemoresistance in triple-negative breast cancer.
Results
Sphingosine kinase is overexpressed in drug resistant breast cancer cells.
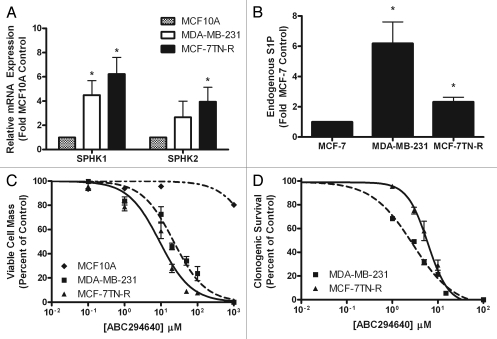
Numerous studies have catalogued variations in the expression of certain sphingolipid metabolizing enzymes and sphingolipid protein levels in human cancers. Here, we compared levels of various enzymes regulating the expression of ceramide and S1P in normal breast epithelium and breast cancers with either de novo or acquired drug resistance. As seen in Figure 1A, both Sphk1 and Sphk2 are overexpressed in drug resistant breast cancers. Sphk1 is overexpressed by 4.49 ± 1.19 fold (p < 0.05) in MDA-MB-231 cells. Both Sphk1 and Sphk2 are overexpressed by 6.22 ± 1.38 fold (p < 0.05) and 3.94 ± 1.28 fold (p < 0.05), respectively, in MCF-7TN-R cells. Furthermore, this alteration in metabolism correlates with increased S1P levels in both drug resistant cancers compared to the drug sensitive MCF-7 cell line, with a 5.19 ± 0.58 (p < 0.05) and 1.33 ± 0.31 fold (p < 0.05) increase in MDA-MB-231 and MCF-7TN-R, respectively (Fig. 1B). Along with an increase in S1P, the changes in sphingolipid metabolizing enzyme expression resulted in altered levels of various endogenous ceramides, with notable increases in C24-ceramide and decreases in C26-Cer (data not shown). These results suggest that altered sphingolipid metabolism may contribute to the increased aggressiveness seen in drug resistant breast cancers.
Figure 1.
Dysregulation of sphingolipid signaling in drug resistant breast cancer cells. (A) PCR analysis for endogenous expression of sphingosine kinase isoforms in drug resistant breast cancer cells. Data are expressed as fold-change relative to MCF10A cell control as normalized to internal β-actin ±SEM. Data points and error bars represent the mean ± SEM of three independent experiments. (B) MCF-7, MDA-MB-231 and MCF-7TN-R cells were measured for cellular levels of various sphingolipid species using ESI/MS/MS. Data points and error bars represent the mean ± SEM of three independent experiments. (C) MCF10A, MDA-MB-231 and MCF-7TN-R cells were plated at 7.5 × 105 cells per 96 well plate. The following day cells were treated with indicated concentrations of ABC294640 for 24 h. Data are presented as percent of vehicle treated samples. Mean values of ±SEM of five different experiments in quadruplicate are reported. (D) MCF10A, MDA-MB-231 and MCF-7TN-R cells were plated at 500 cells per 60 mm2. The following day, cells were treated with ABC294640 for 10–14 days. Colonies ≥50 cells were scored as positive for colony formation. Data are presented as percent of vehicle treated samples. Mean values of ±SEM of three different experiments in duplicate are reported.
Pharmacological inhibition of sphingosine kinase-2 blocks ER-negative breast cancer viability, proliferation and survival.
As mentioned previously, the role of Sphk2 in cancer biology is under debate. There is conflicting data in breast cancer, in particular, with some showing that Sphk2 has similar proliferation and apoptotic effects to that of Sphk1 while others have shown the opposite.20,21,26 However, previous studies were performed using overexpression and siRNA knockdown models without using small molecule inhibitors. Therefore, we determined for ourselves the effect of pharmacological inhibition of Sphk2 on endocrine and chemoresistant ER-negative breast cancers. Utilizing MTT assays, the viability IC50 values were determined in MDA-MB-231 and MCF-7TN-R cell lines. ABC294640 displayed an IC50 value of 22.39 ± 5.11 µM (p < 0.001) in MDA-MB-231 cells and 8.83 ± 1.13 µM (p < 0.001) in MCF-7TN-R cells (Fig. 1C). Selective toxicity for cancer, as opposed to normal cells, is an important characteristic in developing novel experimental therapeutics. Here, we compared the effect of ABC294640 on MCF10A and MCF-7 cell viability. MCF10A are breast epithelial cells that represent an ER-negative, non-cancerous breast cell line.27 Interestingly, while ABC294640 diminished breast cancer viability, there was little effect on MCF10A breast epithelial cell viability. Treatment at the highest concentration of ABC294640 tested, 100 µM, in MCF10A cells did not decrease viability below 50%. These data suggest that ABC294640 targets cancerous breast cells, with little effect on noncancerous breast epithelial cell viability.
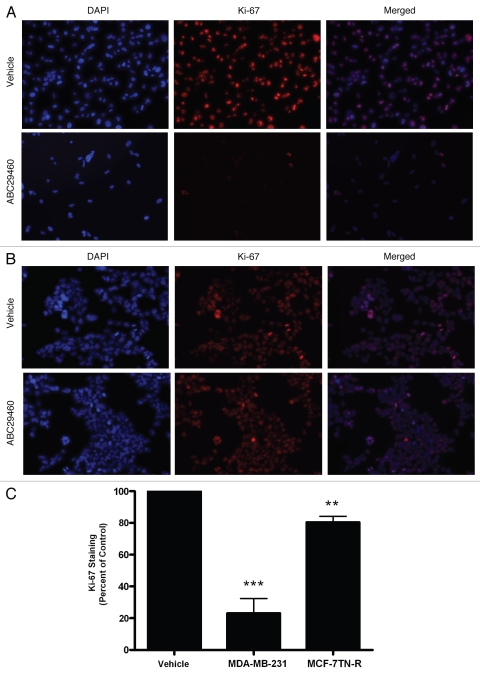
The validity of short-term cytotoxicity assays, such as MTT assays, in predicting therapeutic responses in the clinical patients has been a topic of significant debate in the literature.28 Therefore, we further examined the ability of these compounds to block long-term colony formation in drug resistant breast cancers. As seen in Figure 1D, ABC294640 decreased both endocrine resistant and chemo resistant breast cancer clonogenic survival, with IC50 values in the low micromolar range. ABC294640 exhibited IC50 values of 2.90 ± 1.16 µM (p < 0.001) in MDA-MB-231 cells and 5.21 ± 1.10 µM (p < 0.001) in MCF-7TN-R cells. The ability of a drug to block both survival and proliferation in a resistant cancer is an important characteristic of any new potential chemotherapeutic. Therefore, utilizing Ki-67 immunofluorescence staining we next characterized the anti-proliferative effects of this sphingosine kinase inhibitors. The Ki-67 protein is only expressed during active phases of the cell cycle and is a known proliferative and prognostic marker both in the laboratory and the clinic. Interestingly, ABC294640 had a greater anti-proliferative effect in endocrine resistant breast cancer than in chemoresistant systems. Pharmacological inhibition of Sphk2 decreased Ki-67 staining in MDA-MB-231 by 79.91 ± 9.29% (p < 0.001) (Fig. 2). In the MCF-7TN-R cell line, however, this drug exhibited a much smaller anti-proliferative effect, only decreasing proliferation by 19.54 ± 3.71% (p < 0.001) in this chemoresistant cell line. It should be noted that while the percent of Ki-67 staining was not as significant in MCF-7TN-R compared to control, there were far less total cells following treatment with the sphingosine kinase inhibitor. These results suggest that there is another mechanism other than decreased proliferation that is blocking cell growth in this cell line, consistent with the recent report that ABC294640 can induce autophagy in at least some cancer cell lines.29
Figure 2.
Anti-proliferative effects of ABC294640 on drug resistant breast cancer cells. (A) MDA-MB-231 cells and (B) MCF-7TN-R cells were treated with vehicle or ABC294640 (10 µM) for 48 h. Following treatment, cells were fixed and stained with anti-Ki-67 (red) and nuclei counter stained with DAPI (blue). (A) Representative images of cells at ×250. (B) Quantification of cells positive for Ki-67 staining from ten fields of view per treatment. Data is represented as percent positive cells as compared to total cells normalized to vehicle control. Bars represent mean values ± SEM, (**p < 0.01, ***p < 0.001).
Pharmacological inhibition of sphingosine kinase-2 induces intrinsic apoptosis in chemoresistant breast cancer.
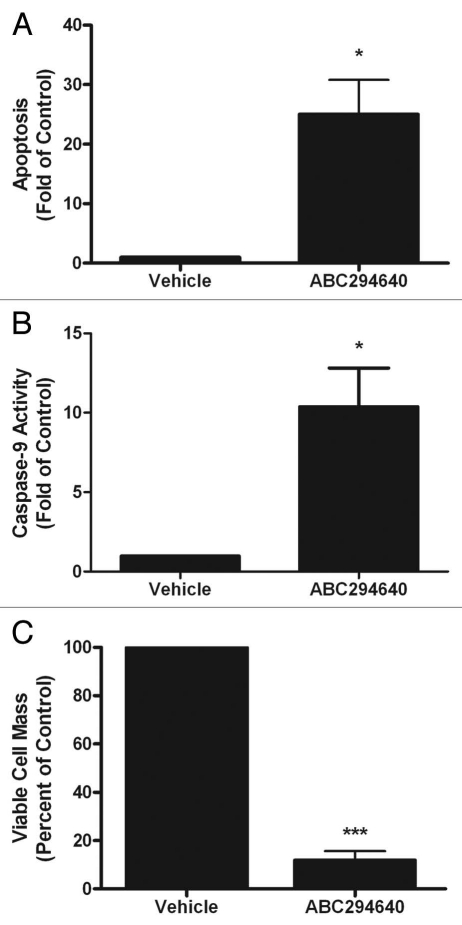
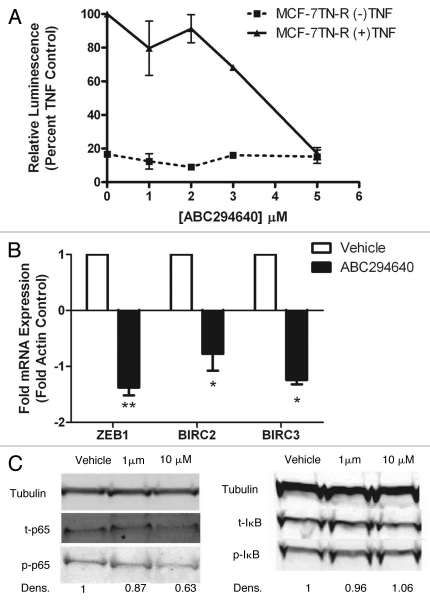
There are conflicting results in the literature as to the function of Sphk2 in regulating programmed cell death, with some suggesting that this isoform is pro-apoptotic while others argue the opposite. Therefore, we determined whether pharmacological inhibition of Sphk2 could induce apoptosis in chemoresistant breast cancer cells. Breast cancer cells were treated for 24 h with a high dose of 50 µM of ABC294640 and analyzed for histone complexed DNA fragmented oligonucleosomes as a measure of apoptosis (Fig. 3A). Interestingly, treatment with ABC294640 increased apoptosis by 20.06 ± 5.75 fold (p < 0.001). This is of particular interest because the MCF-7TN-R cell line is resistant to TNF-induced apoptosis and TNF-induced ceramide accumulation.30 The induction of apoptosis corresponded with a significant decrease in viability, suggesting that programmed cell death may be a primary mechanism for the anti-viability property of this drug (Fig. 3C).
Figure 3.
ABC294640 induces intrinsic apoptosis in drug resistant breast cancer cells. MCF-7TN-R cells were plated at 7.5 × 105 cells per 96 well plate and treated with 50 µM of ABC294640 for 24 h. Following incubation, cells were (A) measured for fragmented DNA oligonucleotides using ELIZA assays, (B) analyzed for caspase-9 activation and (C) measured for viability using MTT analyses. Mean values of ±SEM of four different experiments in duplicate are reported.
Sphingolipids, particularly ceramide, can mediate both the intrinsic and extrinsic apoptosis pathways, though ceramide is primarily involved in mitochondrial-initiated intrinsic programmed cell death. Therefore, we investigated whether sphingosine kinase inhibitor-induced apoptosis in MCF-7TN-R cells functioned via the intrinsic pathway through measurement of caspase-9 activity. Caspase-9 is known to be downstream of cytochrome c release from the mitochondria in the intrinsic apoptosis pathway and is commonly used as a measure of intrinsic apoptosis. As seen in Figure 3B, treatment with ABC294640 increased caspase activation by 10–41 ± 2.41 fold (p < 0.05), suggesting that ABC294640 induced apoptosis, at least in part, through the mitochondrial pathway.
Pharmacologic inhibition of sphingosine kinase-2 enhances the effect of clinical chemotherapeutics in chemoresistant breast cancer.
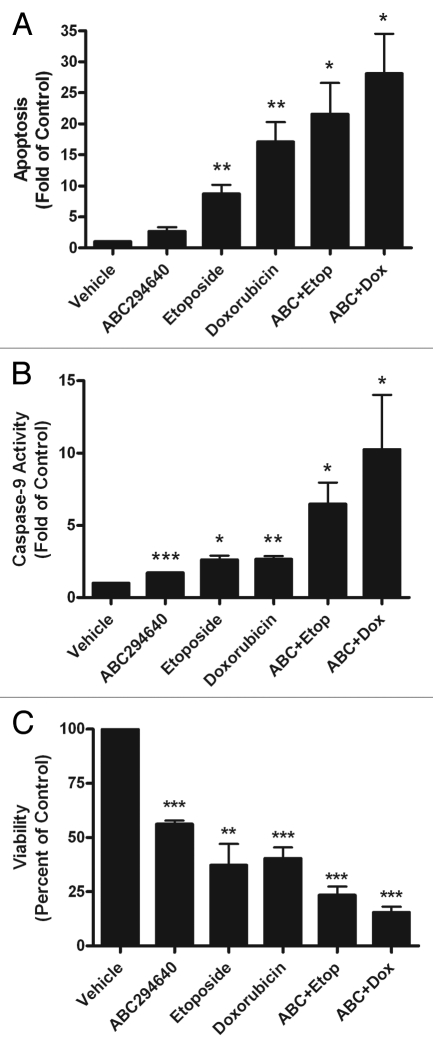
Recent studies suggest that inhibition of sphingosine kinase may sensitize cancer cells to chemotherapeutic treatment. Though few studies have used pharmacological inhibitors, recent reports have shown that using siRNA specific to sphingosine kinase can increase the anti-cancer effects of certain breast cancer therapies, such as tamoxifen and doxorubicin.3,7 Therefore, we determined whether inhibition of Sphk2 could increase the apoptotic and anti-viability effects of the current clinical chemotherapeutics, doxorubicin and etoposide. MCF-7TN-R breast cancer cells were pretreated for 1 h with 10 µM ABC294640 followed by 23 h treatment with a chemotherapeutic agent and compared to isolated treatment with each drug. Following treatment, cells were measured for apoptosis, caspases-9 activity and cell viability. As expected, etoposide and doxorubicin induced apoptosis through the intrinsic pathway (Fig. 4). Treatment with ABC294640 alone showed modest induction of apoptosis and caspase-9 compared to etoposide and doxorubicin alone. However, pretreatment with ABC294640 enhanced the apoptotic effect of both etoposide and doxorubicin in MCF-7TN-R cells. Treatment with ABC294640 and etoposide increased apoptosis and caspase-9 activation by 21.55 ± 5.04 (p < 0.05) fold (p < 0.05) and 6.48 ± 1.47 (p < 0.05), respectively, compared to vehicle control. ABC294640 and doxorubicin treatment also increased apoptosis and caspase-9 activity, with 28.11 ± 6.41 (p < 0.05) fold and 10.25 ± 3.773 (p < 0.05) inductions, respectively (Figs. 4A and 5B). The increase in apoptosis with combination therapy correlates with a greater decrease in viability of MCF-7TN-R cells compared to monotherapy (Fig. 4C). Interestingly, the induction of apoptosis and caspase activity of combination therapy is greater than the additive effect of monotherapy with each drug. As seen in the combination treatment with ABC294640 and doxorubicin, induction of apoptosis is greater than the addition of ABC294640 alone. Taken together, these results suggest that pretreatment with sphingosine kinase inhibitors sensitizes chemoresistant breast cancer cells to chemotherapy-induced apoptosis, and combination therapy with these drugs may result in a synergistic biological effect.
Figure 4.
ABC294640 increases doxorubicin and etoposide-induced intrinsic apoptosis. MCF-7TN-R cells were plated at 15,000 cells per well in a 96 well plate in phenol-free DMEM. The following day cells were treated with ABC294640 (10 µM), chemotherapeutic (etoposide 15 µM, doxorubicin, 0.1 µM) or ABC294640 + chemotherapeutic for 24 h. Following incubation, cells were (A) measured for fragmented DNA oligonucleotides using ELIZA assays, (B) analyzed for caspase-9 activation and (C) measured for viability using MTT analyses. Mean values of ±SEM of four different experiments in duplicate are reported.
Figure 5.
ABC294640 blocks NFκB transcriptional activity in vitro. (A) MCF-7TN-R cells were transiently transfected with pFC-NFκB-luciferase plasmid. Following transfection, cells were treated with vehicle, TNFα, SKI or SKI + TNFα. Cells treated with TNFα were set to 1. Data points and error bars represent the mean ± SEM of three independent experiments. (B) MCF-7TN-R cells were treated with vehicle or ABC294640 for 18 h and analyzed for mRNA levels of ZEB1, BIRC2 and BIRC3 using qRT-PCR. Data are expressed as fold-change relative to vehicle control as normalized to internal β-actin ±SEM (*p < 0.05). Data points and error bars represent the mean ± SEM of three independent experiments. (C) MCF-7TN-R cells were treated with vehicle or ABC294640 (10 µM) for 6 h and analyzed by western blot for phosphorylated and total IκK, IκB and p65 proteins with densitometry analysis. Phosphorylated protein levels were normalized to corresponding tubulin and vehicle control set to 1. Data shown are representative of three independent experiments.
Modulation of the NFκB signaling by sphingosine kinase inhibitors in vitro.
The ability of ABC294640 to induce apoptosis in chemoresistant breast cancer cells led us to examine possible apoptotic and survival pathways that may be targeted by this drug. The MCF-7TN-R cell line was derived from parental MCF-7 cells through prolonged exposure to TNFα until resistance was established. TNF is a potent inducer of the survival transcription factor NFκB. Our laboratory and others have previously established NFκB to be an important mediator of chemoresistance in breast cancer.11,31–33 We have previously shown that blocking NFκB activity in MCF-7TN-R cells can partially restore sensitivity to TNF in these cells. Both the NFκB and sphingolipid signaling pathways have been implicated as mediators of cancer drug resistance. Although ABC294640 has been previously shown to block TNFα-stimulation of NFκB-induced gene transcription in the context of ulcerative colitis, the role of Sphk2 in NFκB signaling in human cancer is not well established.34
In order to analyze the effect of sphingosine kinase inhibitors on NFκB signaling, MCF-7TN-R cells were transiently transfected with a p65-luciferase promoter and treated with increasing concentrations of ABC294640 or TNF and ABC294640. Interestingly, treatment with ABC294640 dose-dependently decreased TNF-induced p65 transcriptional activity (Fig. 5A). To further validate these results, we examined whether pharmacological inhibition of Sphk2 could affect downstream NFκB-mediated gene transcription. NFκB is known to regulate the expression of over 150 different genes, such as inhibitors of apoptosis proteins, IAP1 and IAP2, and the epithelial to mesenchymal transition (EMT) marker Zeb1. IAP1 and IAP2 are coded for by the genes BIRC2 and BIRC3, respectively. Therefore, using RT-PCR analysis, we measured the expression levels of Zeb1, BIRC2 and BIRC3 in MCF-7TN-R cells with and without exposure to ABC294640. As seen in Figure 5B, treatment with ABC294640 decreased mRNA expression of all three of these NFκB-mediated genes. The greatest effect was on Zeb1, with ABC294640 decreasing expression by −1.38 ± 0.14 (p < 0.01) fold. Similarly, expression of IAP1 and IAP2 decreased by −0.77 ± 0.30 (p < 0.05)-fold and −1.24 ± 0.08 (p < 0.01)-fold, respectively. Together, these results suggest that pharmacological inhibition of Sphk2 decreases NFκB transcription activity in vitro.
Though the Sphk1 and NFκB interactions have been previously explored in both epithelial and endothelial cells, to date there are few published studies on the role of Sphk2 in NFκB signaling. It is known that S1P can activate PI3k/Akt signaling and that Akt can phosphorylate the p65 subunit at Ser536 and alter its transcriptional activity. Given these data, we analyzed the ability of ABC294640 to affect phosphorylation of p65 as a means of regulating this NFκB subunit. Treatment with ABC294640 decreased phosphorylation of p65 following a 6 h incubation. This decrease in p65 activity is downstream of IκB activity, as there is no change in phosphorylated levels of IκB following incubation with ABC294640 (Fig. 5C). Taken together, these results suggest that pharmacological inhibition of Sphk2 can alter the NFκB signaling cascade in chemoresistant breast cancer cells.
ABC294640 decreases chemoresistant breast cancer tumor growth in vivo.
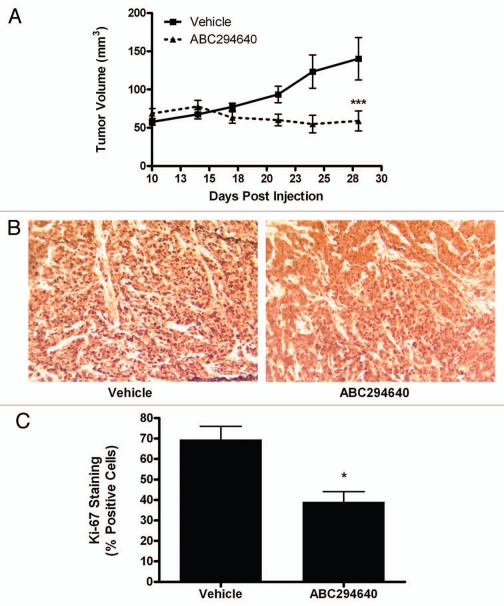
Given the promising therapeutic characteristics of ABC294640 in vitro, we further examined the ability of this drug to block chemoresistant breast cancer tumor growth. Using well-established immunocompromised mouse xenograft models for tumor growth, MCF-7N-R cells were injected subcutaneously into female overiectomized mice and tumor formation and size were documented over the course of 18 days. During the study, mice were treated with either 50 mg/kg of ABC294640 or PBS/DMSO vehicle control. Whereas tumors in vehicle-treated control mice increased in size by approximately 200% in this time, tumors in ABC294640-treated mice did not increase in size during the treatment period (Fig. 6A). Furthermore, ABC294640 treated mice exhibited no obvious toxicity in either internal organs or systemic weight. We previously showed that ABC294640 can diminish proliferation of MCF-7TN-R cells in vitro. Therefore, we next examined whether inhibition of Sphk2 could decrease tumor proliferation in vivo. Tumors excised at endpoint exhibited 43.66 ± 4.98% (p < 0.05) decrease in Ki-67 staining compared than vehicle treated tumors, suggesting that the decrease in proliferation seen in vitro translates into a decrease in tumor proliferation in vivo (Fig. 6B and C).
Figure 6.
ABC294640 decreases chemoresistant breast cancer growth. 5 × 106 MCF-7TN-R cells were injected in the mammary fat pads of female overiectomized mice with exogenous estrogen pellets. Tumors were allowed to form over 7 days. Mice were treated i.p with 50 mg/kg of ABC294640 for 14 days. Tumor volume was measured every 3 days. Diminished volume of treatment tumors at endpoint were statistically significant from vehicle (**p < 0.01). (B) Tumors from vehicle and ABC294640 treated mice were processed stained Ki-67. Representative images of Ki-67 staining in tumor sections are shown. (C) Quantitation of Ki-67 staining is expressed as percent positive of total number of cells per field of view (***p < 0.001, **p < 0.01, *p < 0.05).
Taken together, these results suggest that ABC294640 is a viable antitumor therapeutic in the treatment of chemoresistant and endocrine therapy-resistant breast cancers.
Discussion
Over the past several years, the SK/S1P signaling pathway has been increasingly implicated in both chemo and endocrine therapy resistance. We show here that sphingosine kinase is overexpressed in drug resistant cancer cells, resulting in increased protein levels of S1P in ER-negative, drug resistant breast cancer cells compared to ER-positive, drug sensitive cancer cells. Cross-talk between the sphingosine kinase pathway and the pro-survival transcription factor, NFκB, has been suggested as another possible resistance mechanism.18,35 With resistance to current chemotherapy agents on the rise, there is a growing need for novel therapeutics targeting specific survival and proliferative pathways.
The role of Sphk1 in cancer biology has been well studied in the literature, however the mechanism and function of Sphk2 is currently under debate. The literature suggests that multiple post-transcriptional factors regulate the functional influence of Sphk2 on the cell. For example, the catalytic activity of sphingosine kinase isoforms is markedly increased in cancers. The subcellular location of Sphk2 is tissue type dependent and it has been suggested that altering the nuclear location of Sphk2 (from nucleus to the cytoplasm or vice versa) can change its functional activity and enhance the physiological role of Sphk2. Furthermore, the Spiegel laboratory has shown that the effects of Sphk2 are not solely dependent on its catalytic activity, but also on its BH3 domain. Taken together with our findings here that Sphk2 mRNA is moderately overexpressed in certain breast cancers and not others, it is likely that a combination of post-transcriptional changes in the activity of Sphk2, functional involvement of the BH3 domain, as well as alterations in the subcellular localization of Sphk2, are involved in the proliferative and chemoresistant mechanisms of Sphk2 in cancer.
In this study, we explored the biological effects of the novel Sphk2 inhibitor, ABC294640, on drug resistant breast cancers. Importantly, ABC294640 decreased chemoresistant breast cancer tumor growth in vivo, with no observable toxicity at 50 mg/kg dosage. This inhibitor diminished breast cancer cell viability, proliferation and survival with low micromolar IC50 values in all three assays. Furthermore, pharmacological inhibition of Sphk2 in chemoresistant breast cancer cells can induce apoptosis via the mitochondrial programmed cell death pathway. The role of Sphk2 in apoptosis is a highly debated topic with conflicting results in the literature. Some have reported in studies overexpressing Sphk2, that this isoform induces apoptosis in epithelial cells. However, this may be an artificial finding due to excess Sphk2 undergoing proteolysis in non-endogenous subcellular compartments, releasing BH3 peptides that act in a pro-apoptotic manner. We report that pharmacological inhibition of Sphk2 can induce apoptosis through the intrinsic pathway, however, cell killing through extrinsic pathway and/or autophagy cannot be excluded at this time. With current FDA and NIH standards, the most common path of an anticancer therapeutic from bench to bedside is in combination with FDA approved drugs. Combination therapy has an advantage over monotherapy through dual targeting of cancer cells, resulting in increased response rates and decreased resistance. Therefore, we used combination therapy with ABC294640 and the clinical chemotherapeutics, doxorubicin and etoposide. ABC2946400 can enhance doxorubicin and etoposide-induced programmed cell death through the intrinsic pathway in chemoresistant breast cancer cells. The increase in combination apoptosis appears to be synergistic and correlates with a reduction in cell viability, suggesting that apoptosis may be a primary mechanism of the anti-viability nature of the combination therapies present here.
Previous work in our laboratory demonstrated that the NFκB transcription factor promotes survival and chemoresistance in human breast cancer.31,32,36 Unpublished data also indicates that breast cancer cells with an acquired resistance phenotype have increased p50 and decreased IκB protein levels, as well as increased p65 transcriptional activity. Clinical data supports these findings that increased NFκB signaling may be a key mechanism of chemoresistance in breast cancer.11,12,37 We confirm here the ability of ABC294640 to block the NFκB signaling cascade. Our data reveal that this inhibitor can block p65 transcriptional activity through decreased activation of the Ser536 phosphorylation site. This decrease in p65 activity results in diminished NFκB mediated gene transcription. We propose that this decreased phosphorylation occurs due to SKI blockade of the PI3K/Akt signaling cascade, which is known to phosphorylate Ser536 on p65. Therefore, in both drug sensitive and drug resistant breast cancer, pharmacological inhibition of Sphk2 may have advantageous “off-target” effects that further abrogates survival signaling in addition to SK/S1P blockade. Our data suggest that pharmacological inhibition of Sphk2 can diminish this increased p65 activity and sensitize chemoresistant breast cancer cells to chemotherapy-induced intrinsic apoptosis. This decrease in p65 inactivity is, at least in part, due to decreased phosphorylation at the Ser536 site of p65 following treatment with sphingosine kinase inhibitors. Given that there are nine phosphorylation sites in p65, it is possible other phosphorylation sites are also affected. For example, we know that protein kinase C (PKC) protein levels are upregulated in chemoresistant breast cancer cells and that PKC can activate p65 at Ser311.38 PKC is downstream of both S1P and ceramide and future studies should examine the role of PKC on sphingosine kinase inhibitor function.
Given that all current clinical anti-cancer drugs have reported incidences of drug resistance, development of experimental therapeutic aimed at new proliferative targets are of increasing importance. In conclusion, our studies demonstrate that pharmacological targeting of Sphk2 using orally bioavailable inhibitors has promising potential as drug resistant breast cancer therapeutics.
Materials and Methods
Reagents.
ABC294640 [3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridin-4-ylmethyl)-amide] was provided by Apogee Biotechnology Corporation (Hummelstown, PA).22 Dimethyl sulfoxide (DMSO) and estradiol were purchased from Fisher Scientific.
Cell culture.
ER-negative MCF-7TN-R and MDA-MB-231 cells were cultured as previously described in reference 39 and 40. Briefly, the MCF-7 cell line used is a subclone of MCF-7 cells obtained from the American Type Culture Collection (ATCC, Manassas, VA) generously provided by Louise Nutter (University of Minnesota, MN).41 The MDA-MB-231 and MCF10A cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The MCF-7TN-R cell line is a MCF-7 derived, ER/PR/HER2 negative, chemoresistant breast cancer cell line generated in the laboratory.30,31,39 These cells were derived by growing MCF-7 cells in increasing concentrations of TNFα until resistance was established. The culture flasks were maintained in a tissue culture incubator in a humidified atmosphere of 5% CO2 and 95% air at 37°C. MCF10A cells were obtained from Clontech Corp. (Palo Alto, CA) and cultured in mammary epithelial cell medium (Cambrex, San Diego, CA) supplemented with bovine pituitary extract.
Real time RT-PCR.
Real time RT-PCR was performed as described in previous studies in reference 42 and 43. In brief, total cellular RNA was extracted using the RNeasy® mini column (Qiagen, Valencia, CA), following the manufacturer's instructions. The concentration of RNA was determined using an ultraviolet spectrophotometer. Reverse transcription (RT) was performed using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). The level of Sphk1 and Sphk2 transcripts was determined using the iQ5 real-time quantitative PCR detection system (BioRad Inc., Hercules, CA). Quantification was performed using delta-delta Ct method. RNA was reversed transcribed. The PCR reaction was carried out as follows: step 1: 95°C 3 min, step 2: for 40 cycles 95°C 20 seconds, 60°C 1 min, step 3: 70°C 10 seconds, hold at 4°C. Each reaction tube contained 12.5 µl 2x SYBR Green supermix + 6.5 µl nuclease-free water + 1 µl 0.1 µg/µl primer (pair) + 5 µl cDNA (0.2 µg/µl). Genes were amplified in triplicate. Data was analyzed by comparing relative target gene expression to β-actin control. Relative gene expression was analyzed using the 2−ΔΔCt method. Primers for PCR were designed to span intron/exon junctions to minimize amplification of residual genomic DNA. The primer sequences for Beta-Actin, Sphk1 and Sphk2 are (sense and anti-sense, respectively): Beta-Actin (5′-TGA GCG CGG CTA CAG CTT-3′; 5′-CCT TAA TGT CAC ACA CGA TT-3′) Sphk1 (5′-GTC AGC GGT TGC GTG GAG-3; 5′-GGG TCT CAG AAC AAA CTA GAA GGC-3′), Sphk2 (5′-GCC ACC TAC GAA GAG AAC-3′, 5′-TGA CCA ATA GAA GCA ACC G-3′).
Lipidomics analysis.
Endogenous lipid levels were quantified by mass spectrometry (Lipidomics Core, Medical University of South Carolina, SC) according to published methods.39 Briefly, cells were collected, fortified with internal standards and extracted with ethyl acetate/isopropyl alcohol. Electrospray ionization followed by tandem mass spectrometry (ESI/MS/MS) analyses of sphingoid bases, sphingoid base 1-phosphates, ceramides and sphingomyelins were performed on a Thermo Finnigan TSQ 7000 triple quadrupole mass spectrometer.
Western blot analysis.
Protein analysis was performed as described in reference 41. Cells were plated at 50–60% confluency in 10 cm2 culture flasks in 10% DMEM for 48 h. They were then treated with DMSO or ABC294640 for 1 h. Following treatment, cells were detached with PBS-EDTA and centrifuged. After removing supernatant, cells were lysed in 60–100 µL lysis buffer Mammalian Protein Extraction Reagent, MPER and Halt protease inhibitor (Pierce, Rockford, IL); and PhoSTOP phosphatase inhibitor (Roche, Boulder, CO). Lysed cells were centrifuged for 10 min at 12,000 G at 4°C to separate protein from cell debris. The supernatants were combined with loading buffer 5% 2-mercaptoethanol in 4x LDS Loading Buffer, (Invitrogen), boiled for 5 min and loaded onto a 4–12% Bis Tris Polyacrilamide Gel (Invitrogen) followed by polyacrylamide gel electrophoresis at 150 V for 1.25 h. Protein was transferred to nitrocellulose membranes using the iBlot (Invitrogen) transfer unit. Nitrocellulose membranes were blocked in 5% milk (BioRad Lab) Tris Buffered Saline-Tween 20 (TBS-T) for 1 h at room temperature. Cells were washed briefly with 1x TBS-T (USB, Cleveland, OH) and primary antibodies were diluted in 5% BSA (Bovine Serum Albumin, Sigma-Aldrich, St. Louis, MO) TBS-T according to manufacturer's recommended dilutions. Antibodies for tubulin, p65 (C22B4), phospho-p65 (93H1), IκB (polyclonal), phospho-I IκB (14D4), IκK (L570) and phospho-IκK (16A6) (1:1,000) were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Membranes were incubated in primary antibody overnight at 4°C with gentle agitation. Secondary infrared conjugated antibodies (LI-Cor Biosciences, Lincoln, NE) were diluted in 5% milk-TBST solution at 1:10,000 and membranes were incubated for 1 h under gentle agitation at room temperature. Membranes were scanned using the LI-COR Odyssey imager and software (LI-COR Biosciences, Lincoln, NE) to detect total and phosphorylated protein levels in cell lysates. Western blots and protein quantification were performed following three independent experiments, with representative blots shown. Protein levels were quantified using densitometry analyses.
Clonogenic survival assay.
Colony assays were performed as described in previously published methods in reference 30 and 39. MCF-7 cells were plated in 6-well plates at a density of 1,000 cells per well in full DMEM media. Twenty-four hours later cells were treated with ABC294640 (0.1–10 µM) and then monitored for colony growth. Ten days later the cells were fixed with 3% glutaraldehyde. Following fixation for 15 min, the plates were washed and stained with a 0.4% solution of crystal violet in 20% methanol for 30 min, washed with PBS and dried. Colonies of ≥30 cells were counted as positive. Results were normalized to DMSO vehicle treated control cells. Statistical analysis of IC50 values were calculated from concentration-response curves using GraphPad Prism 5.0 (Graphpad Software, San Diego, CA), using with the equation: Y = Bottom + (Top-bottom)/1 + 10LogEC50-X.
Cell proliferation immunofluorescence assay.
Cells were plated at a density of 10,000 cells per well in a 96 well plate in 10% DMEM media and allowed to attach over night. The following day, cells were treated with DMSO or ABC294640 for 24 h. At endpoint, cells were fixed using 100 µL of 3.7% formaldehyde in PBS for 10 min. Formaldehyde was removed and cells were permeabilized using cold methanol for 5 min at room temperature and washed twice with PBS. 100 µL of 3% FBS in PBS blocking buffer was then added. After 30 min, blocking buffer was removed and cells were incubated for 1 h with Ki-67 (BD Pharmingen, San Diego, CA) antibody. Cells were then washed with PBS and stained with DAPI nuclear stain for 5 min before imaging. For staining quantification, numbers of positively stained cells were expressed as a percentage of the total number of cells per field of view/image. The vehicle control was then set to 1 for comparison with ABC294640 treatment.
Cell viability assay.
Viability assays were performed as previously described in reference 30 and 40. Briefly, cells were plated at a density of 7.5 × 105 cells per well in a 96-well plate in phenolfree DMEM supplemented with 5% FBS and allowed to attach overnight. Cells were then treated with ABC294640 (ranging from 10 nM to 100 µM) for 24 h. Following treatment, 20 µL of 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 5 mg/ml) reagent was incubated in each well for 4 h. Cells were lysed with 20% SDS in 50% dimethylformamide. The pH and absorbances were read on an ELx808 Microtek plate reader (Bio-Tek Instruments, Winooski, VT) at 550 nm, with a reference wavelength of 630 nm.
NFκB-luciferase assay.
As previously described, the cells were seeded in 24-well plates at a density of 5 × 105 cells/well in the same media and allowed to attach overnight.43,44 After 18 h, cells were transfected for 5 h in serum-free DMEM with 10 ng of pFC-NFκB-luciferase plasmid, using 6 µl of Effectene (Qiagen) per µg of DNA, using 6 µl of Effectene (Qiagen) per µg of DNA. After 5 h the transfection medium was removed and replaced with phenol red-free DMEM supplemented with 5% CS-FBS containing vehicle, TNF, SKI or TNF + SKI and incubated at 37°C. After 18 h the medium was removed and 100 µl of lysis buffer was added per well and then incubated for 15 min at room temperature. Cell debris was pelleted by centrifugation at 15,000x g for 5 min. Cell extracts were normalized for protein concentration using reagent according to the manufacturers protocol (BioRad Lab.). Luciferase activity for the cell extracts was determined using luciferase substrate (Promega Corp., Madison, WI) in an Autoluminat Plus luminometer (Berthhold Technologies, Bad Wildbad, Germany).
Animal studies.
Xenograft models were performed in a similar manner to previously reported studies in reference 45. In brief, SCID/Beige immunocompromised female ovariectomized mice (29–32 days old) were obtained from Charles River Laboratories (Wilmington, MA). The animals were allowed a period of adaptation in a sterile and pathogen-free environment ad libitum. MCF-7TN-R cells in the exponential phase of growth were harvested using PBS/EDTA solution and washed. Viable cells (5 × 106) in a 50 µL sterile PBS suspension were mixed with 100 µL Matrigel Reduced Factors (BD Biosciences, Bedford, MA). MCF-7TN-R cells were injected subcutaneously and the incision was closed using staples. All the procedures in animals were performed under anesthesia using a mix of isofluorane and oxygen delivered by mask. Tumors were allowed to form over 10 days and mice were randomized to two treatment groups with 5 mice per group: vehicle control and ABC294640. The ABC294640 mixture was suspended in a solution of DMSO and PBS and was given i.p. at 50 mg/kg/mouse/d for 7 days starting after tumors were measureable. Control mice were injected with vehicle daily for 14 days. Tumor size was measured every 3 days using a digital caliper. The volume of the tumor was calculated using the following formula: 4/3π LS2 (L = larger radius; S = shorter radius). At necropsy, animals were euthanized by cervical dislocation after exposure to a CO2 chamber. Tumors, uteri, livers and lungs were removed and either frozen in liquid nitrogen or fixed in 10% formalin for further analysis. All procedures involving these animals were conducted in compliance with State and Federal laws, standards of the US Department of Health and Human Services and guidelines established by the Tulane University Animal Care and Use Committee. The facilities and laboratory animal program of Tulane University are accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Immunohistochemistry.
Immunohistochemistry was performed as described in previously published methods in reference 25 and 42. Tumor explants were collected at necropsy and fixed in 10% buffered formalin phosphate. FFPE 4-lM-thick tumor sections were analyzed by immunohistochemistry using primary monoclonal antibodies against human Ki-67 (DAKO North America, Inc., Carpinteria, CA). The mouse antibodies on mouse tissue polymer detection kit (Biocare Medical, LLC, Concord, CA) were used to perform IHC. Briefly, FFPE sections were deparaffinized and hydrated in a graded series of ethanol solutions followed by 3% H2O2 for 5 min to inactivate endogenous peroxides then rinsed. Slides were subjected to 10 min incubation in Avidin followed by 10 min incubation in Biotin. For antigen retrieval, sections were exposed to Rodent Decloaker (Biocare Med.) at 95°C for 25 min, rinsed and allowed to cool to room temperature for 20 min. Slides were incubated with Rodent Block for 30 min and then with primary antibodies or serum alone (negative control) for 75 min. Mouse-on-mouse HRP-polymer secondary antibody was added to the sections and incubated for 15 min. After rinsing, DAB solution (Biocare Med.) was applied and incubated for 1 min and sections were counterstained with hematoxylin (Biocare Med.) followed by Tacha Blueing reagent (Biocare Med.) for 30 s each. Slides were then allowed to air dry and then cover slipped using Acrymount (Fisher Scientific Inc., Waltham, MA). Sections were viewed and photographed using the Leica DM IRB Inverted Research microscope and SPOT RT color camera. Five images at x40 were taken of each tumor with care to avoid areas of necrosis. For Ki-67 staining quantification, numbers of positively stained cells were expressed as a percentage of the total number of cells per field of view/image.
Statistical analysis.
Statistical analysis of IC50 values were calculated from concentration-response curves using GraphPad Prism 5.0 (GraphPad Software), using with the equation:
Y = Bottom + (Top-bottom)/1 + 10LogEC50-X.
Assuming a standard slope, where the response goes from 10% to 90% of maximal as X increases over two log units. Differences in IC50 were compared using Student's unpaired t-test with p < 0.05 as the limit of statistical significance. Experiments comparing multiple concentrations to the control were tested with oneway ANOVA with Bonferroni post-test to compare individual concentrations. Combination effects were calculated using the formula; (X1a + X2b)/(X1a + X2b), wherein X1 is the effect of drug 1 at concentration a, X2 is the effect of drug 2 at concentration b and (X1a + X2b) is the effect of the combination of both drugs at concentrations a and b, respectively.46 All statistical analyses were done using GraphPad Prism 5.0 (GraphPad Software).
Acknowledgements
This work was supported by National Institutes of Health Grant CA125806, The Center for Bioenvironmental Research at Tulane and Xavier Universities and the PhRMA Foundation Paul Calabresi Research Fellowship (J.W.A.).
Abbreviations
- ABC294640
3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridin-4-ylmethyl)amide
- BCL-2
B-cell lymphoma 2
- BH3
Bcl-2 homology domain 3
- CER
ceramide
- DAB
diaminobenzidine
- DAP
I 4′,6-diamidino-2-phenylindole
- DMS DCC
dicyclohexylcarbodiimide
- IAP
inhibitor of apoptosis
- IκB inhibitory kappa B
IκK inhibitory kappa B kinase
- MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NFκB
nuclear factor kappa B
- Sphk1
sphingosine kinase-1
- Sphk2
sphingosine kinase-2
- SKI
sphingosine kinase inhibitor
- S1P
sphingosine-1-phosphate
- PKC
protein kinase C
References
- 1.Meacham WD, Antoon JW, Burow ME, Struckhoff AP, Beckman BS. Sphingolipids as determinants of apoptosis and chemoresistance in the MCF-7 cell model system. Exp Biol Med (Maywood) 2009;234:1253–1263. doi: 10.3181/0902-MR-77. [DOI] [PubMed] [Google Scholar]
- 2.Saddoughi SA, Song P, Ogretmen B. Roles of bioactive sphingolipids in cancer biology and therapeutics. Subcell Biochem. 2008;49:413–440. doi: 10.1007/978-1-4020-8831-5_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sukocheva O, Wang L, Verrier E, Vadas MA, Xia P. Restoring endocrine response in breast cancer cells by inhibition of the sphingosine kinase-1 signaling pathway. Endocrinology. 2009;150:4484–4492. doi: 10.1210/en.2009-0391. [DOI] [PubMed] [Google Scholar]
- 4.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 5.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, et al. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–5969. [PubMed] [Google Scholar]
- 6.Ruckhaberle E, Rody A, Engels K, Gaetje R, von Minckwitz G, Schiffmann S, et al. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat. 2008;112:41–52. doi: 10.1007/s10549-007-9836-9. [DOI] [PubMed] [Google Scholar]
- 7.Nava VE, Hobson JP, Murthy S, Milstien S, Spiegel S. Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp Cell Res. 2002;281:115–127. doi: 10.1006/excr.2002.5658. [DOI] [PubMed] [Google Scholar]
- 8.Dai Y, Lawrence TS, Xu L. Overcoming cancer therapy resistance by targeting inhibitors of apoptosis proteins and nuclear factor kappaB. Am J Transl Res. 2009;1:1–15. [PMC free article] [PubMed] [Google Scholar]
- 9.Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, et al. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DW, Sovak MA, Zanieski G, Nonet G, Romieu-Mourez R, Lau AW, et al. Activation of NFkappaB/Rel occurs early during neoplastic transformation of mammary cells. Carcinogenesis. 2000;21:871–879. doi: 10.1093/carcin/21.5.871. [DOI] [PubMed] [Google Scholar]
- 11.Nakshatri H, Goulet RJ., Jr NFkappaB and breast cancer. Curr Probl Cancer. 2002;26:282–309. doi: 10.1067/mcn.2002.129977. [DOI] [PubMed] [Google Scholar]
- 12.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr, Sledge GW., Jr Constitutive activation of NFkappaB during progression of breast cancer to hormoneindependent growth. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez-Vargas H, Rodriguez-Pinilla SM, Julian-Tendero M, Sanchez-Rovira P, Cuevas C, Anton A, et al. Gene expression profiling of breast cancer cells in response to gemcitabine: NFkappaB pathway activation as a potential mechanism of resistance. Breast Cancer Res Treat. 2007;102:157–172. doi: 10.1007/s10549-006-9322-9. [DOI] [PubMed] [Google Scholar]
- 14.Xia P, Gamble JR, Rye KA, Wang L, Hii CS, Cockerill P, et al. Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci USA. 1998;95:14196–14201. doi: 10.1073/pnas.95.24.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawamori T, Kaneshiro T, Okumura M, Maalouf S, Uflacker A, Bielawski J, et al. Role for sphingosine kinase 1 in colon carcinogenesis. Faseb J. 2008;23:405–414. doi: 10.1096/fj.08-117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snider AJ, Kawamori T, Bradshaw SG, Orr KA, Gilkeson GS, Hannun YA, et al. A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. Faseb J. 2008;23:143–152. doi: 10.1096/fj.08-118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chumanevich AA, Poudyal D, Cui X, Davis T, Wood PA, Smith CD, et al. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis. 2010;31:1787–1793. doi: 10.1093/carcin/bgq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1beta and TNFalpha induced production of inflammatory mediators. Cell Signal. 2005;17:1203–1217. doi: 10.1016/j.cellsig.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118–37129. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 21.Sankala HM, Hait NC, Paugh SW, Shida D, Lepine S, Elmore LW, et al. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67:10466–10474. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- 22.French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, et al. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther. 2010;333:129–139. doi: 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beljanski V, Knaak C, Smith CD. A novel sphingosine kinase inhibitor induces autophagy in tumor cells. J Pharmacol Exp Ther. 2010;333:454–464. doi: 10.1124/jpet.109.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, et al. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther. 2010;333:129–139. doi: 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antoon JW, White MD, Meacham WD, Slaughter EM, Muir SE, Elliott S, et al. Antiestrogenic effects of the novel sphingosine kinase-2 inhibitor ABC294640. Endocrinology. 2010;151:5124–5135. doi: 10.1210/en.2010-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem. 2003;278:46832–46839. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- 27.Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, et al. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- 28.Brown JM, Wouters BG. Apoptosis, p53 and tumor cell sensitivity to anticancer agents. Cancer Res. 1999;59:1391–1399. [PubMed] [Google Scholar]
- 29.Beljanski V, Knaak C, Smith CD. A novel sphingosine kinase inhibitor induces autophagy in tumor cells. J Pharmacol Exp Ther. 2010;333:454–464. doi: 10.1124/jpet.109.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Struckhoff AP, Bittman R, Burow ME, Clejan S, Elliott S, Hammond T, et al. Novel ceramide analogs as potential chemotherapeutic agents in breast cancer. J Pharmacol Exp Ther. 2004;309:523–532. doi: 10.1124/jpet.103.062760. [DOI] [PubMed] [Google Scholar]
- 31.Weldon CB, Parker AP, Patten D, Elliott S, Tang Y, Frigo DE, et al. Sensitization of apoptotically-resistant breast carcinoma cells to TNF and TRAIL by inhibition of p38 mitogen-activated protein kinase signaling. Int J Oncol. 2004;24:1473–1480. [PubMed] [Google Scholar]
- 32.Burow ME, Weldon CB, Melnik LI, Duong BN, Collins-Burow BM, Beckman BS, et al. PI3-K/AKT regulation of NFkappaB signaling events in suppression of TNF-induced apoptosis. Biochem Biophys Res Commun. 2000;271:342–345. doi: 10.1006/bbrc.2000.2626. [DOI] [PubMed] [Google Scholar]
- 33.Wu JT, Kral JG. The NFkappaB/IkappaB signaling system: a molecular target in breast cancer therapy. J Surg Res. 2005;123:158–169. doi: 10.1016/j.jss.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Maines LW, Fitzpatrick LR, French KJ, Zhuang Y, Xia Z, Keller SN, et al. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig Dis Sci. 2008;53:997–1012. doi: 10.1007/s10620-007-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnitzer SE, Weigert A, Zhou J, Brune B. Hypoxia enhances sphingosine kinase 2 activity and provokes sphingosine-1-phosphate-mediated chemoresistance in A549 lung cancer cells. Mol Cancer Res. 2009;7:393–401. doi: 10.1158/1541-7786.MCR-08-0156. [DOI] [PubMed] [Google Scholar]
- 36.Weldon CB, Burow ME, Rolfe KW, Clayton JL, Jaffe BM, Beckman BS. NFkappaB-mediated chemoresistance in breast cancer cells. Surgery. 2001;130:143–150. doi: 10.1067/msy.2001.115512. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed KM, Cao N, Li JJ. HER-2 and NFkappaB as the targets for therapy-resistant breast cancer. Anticancer Res. 2006;26:4235–4243. [PMC free article] [PubMed] [Google Scholar]
- 38.Duran A, Diaz-Meco MT, Moscat J. Essential role of RelA Ser311 phosphorylation by zetaPKC in NFkappaB transcriptional activation. EMBO J. 2003;22:3910–3918. doi: 10.1093/emboj/cdg370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antoon JW, Liu J, Gestaut MM, Burow ME, Beckman BS, Foroozesh M. Design, synthesis and biological activity of a family of novel ceramide analogues in chemoresistant breast cancer cells. J Med Chem. 2009;52:5748–5752. doi: 10.1021/jm9009668. [DOI] [PubMed] [Google Scholar]
- 40.Antoon JW, Liu J, Ponnapakkam AP, Gestaut MM, Foroozesh M, Beckman BS. Novel D: -erythro N-octanoyl sphingosine analogs as chemo- and endocrine-resistant breast cancer therapeutics. Cancer Chemother Pharmacol. 2010;65:1191–1195. doi: 10.1007/s00280-009-1233-0. [DOI] [PubMed] [Google Scholar]
- 41.Burow ME, Weldon CB, Tang Y, Navar GL, Krajewski S, Reed JC, et al. Differences in susceptibility to tumor necrosis factor alpha-induced apoptosis among MCF-7 breast cancer cell variants. Cancer Res. 1998;58:4940–4946. [PubMed] [Google Scholar]
- 42.Rhodes LV, Muir SE, Elliott S, Guillot LM, Antoon JW, Penfornis P, et al. Adult human mesenchymal stem cells enhance breast tumorigenesis and promote hormone independence. Breast Cancer Res Treat. 2009;121:293–300. doi: 10.1007/s10549-009-0458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boue SM, Tilghman SL, Elliott S, Zimmerman MC, Williams KY, Payton-Stewart F, et al. Identification of the potent phytoestrogen glycinol in elicited soybean (Glycine max) Endocrinology. 2009;150:2446–2453. doi: 10.1210/en.2008-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bratton MR, Frigo DE, Vigh-Conrad KA, Fan D, Wadsworth S, McLachlan JA, et al. Organochlorine-mediated potentiation of the general coactivator p300 through p38 mitogen-activated protein kinase. Carcinogenesis. 2009;30:106–113. doi: 10.1093/carcin/bgn213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salvo VA, Boue SM, Fonseca JP, Elliott S, Corbitt C, Collins-Burow BM, et al. Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin Cancer Res. 2006;12:7159–7164. doi: 10.1158/1078-0432.CCR-06-1426. [DOI] [PubMed] [Google Scholar]