Abstract
One of the primary functions of any epithelium is to act as a barrier. To maintain integrity, epithelia migrate rapidly to cover wounds and there is intense interest in understanding how wounds are detected. Numerous soluble factors are present in the wound environment and epithelia can sense the presence of adjacent denuded extracellular matrix. However, the presence of such cues is expected to be highly variable, and here we focus on the presence of edges in the epithelial sheets as a stimulus, since they are universally and continuously present in wounds. Using a novel tissue culture model, free edges in the absence of any other identifiable cues were found to trigger activation of the epidermal growth factor receptor and increase cell motility. Edges bordered by inert physical barriers do not activate the receptor, indicating that activation is related to mechanical factors rather than to specific cell-cell interactions.
Key words: cell migration, wound, healing, mechanotransduction, epithelial, edges, chronic ulcers, contact inhibition, sheet movement
The fundamental role of epithelia is to provide barriers between different compartments of the organism and to the outside environment. During development and in adulthood, epithelial cells employ their inherent ability to migrate as a collective sheet to generate or restore barrier function. Collective migration is essential for processes such as organogenesis and wound healing, and similar migratory mechanisms can go awry and contribute to cancer metastasis. Therefore, a considerable amount of research has been directed at understanding the cellular signals that initiate and sustain epithelial migration.1–3
In numerous epithelia, the epidermal growth factor receptor (EGFR) is activated by wounding, and blocking the activity of the receptor pharmacologically or by genetic techniques inhibits healing. Conversely, experimental stimulation of the EGFR results in enhancement of wound healing in many instances, underscoring the central role of the EGFR in the healing process.4–6 Wounding induces proteolytic release of ligands, such as heparin-binding EGF-like growth factor (HB-EGF), from precursors located in the cell membrane in a mechanism that resembles EGFR transactivation by G-protein coupled receptors.7–9 In a mammalian model of epithelial morphogenesis, eyelid closure in mice, epithelial sheet movement is also dependent on the proteolytic release of HB-EGF, which activates the EGFR.10 Therefore, not only are the biomechanical processes that control epithelial movements during morphogenesis and wound healing similar, but the signals that induce this motility are similar as well.
Given its importance, it is not surprising that many mechanisms have evolved to regulate epithelial wound healing. Starting immediately after wounding, the epithelium is inundated with a large number of growth factors and cytokines produced by bordering tissues and infiltrating inflammatory cells.1,11,12 In addition, epithelial cells themselves possess mechanisms that detect the presence of wounds. Epithelial cells in a monolayer are not stationary, but appear to move around in a lively fashion, which could theoretically produce wound closure because the cells could simply fill up the space that is opened up after wounding. In support of this, computer modeling has shown that the behavior of individually randomly moving cells can approximate the observed collective migration as a sheet.13 However, human corneal limbal epithelial (HCLE) and other cells react to wounding by increasing their velocities near edges,14 so they respond to wounds by changes in behavior and must therefore contain appropriate detection mechanisms.
Different Roles of Stimuli during Wound Healing
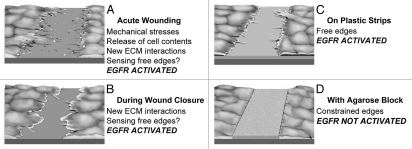
Tissue culture models have been useful in understanding molecular mechanisms in healing of wounds in epithelial cell sheets. Although some important aspects of wound healing are lost, for instance effects of blood-derived factors and other interactions with adjacent tissues, the models do reproduce the closure of gaps introduced in the cell layer and important features of signaling in the induction of movement are retained. Even in culture, wounding is a complex event and generates many potential stimuli that can be detected by cells. In the most commonly used model, scratching a cell layer with a pipette tip or similar instrument (Fig. 1A), there is inevitably cell breakage that results in release of intracellular components such as ATP. In addition, the initial trauma induces mechanical perturbation, the extracellular matrix is laid bare and free edges are created. Some of the potential stimuli may act only at the time of infliction of wounds. This is clearly the case for the initial mechanical perturbation. Also, wounding induces an instant Ca2+ signal at the edge, but the signal is extinguished after a couple of minutes.15 Signaling by extracellular ATP is also likely to be transient. It is mainly generated from broken cells and is expected to be removed by exonucleases or washed out.
Figure 1.
Models to study cues that initiate and sustain migration of epithelial cell sheets. (A) A wound induced acutely by scraping for instance with a pipette tip. Mechanical stresses and cell breakage are prominent and released molecules can act as stimuli. (B) Migration at later stages. New interactions can be formed continuously with extracellular matrix as the cells move. (C) Cells grown at a non-adhesive interphase. Free edges are present and the cells can extend various protrusions. Whether free edges are also detected in (A and B) is not known. Plastic is depicted as very dark grey, polyHEMA light grey. (D) A cell sheet bordering a physical barrier (agarose, textured grey). Notably, EGFR activation and increased cell motility is increased in all situations where physically unconstrained edges are present (A–C).
Many of these immediate, transient stimuli can undoubtedly contribute to promote cell migration. For instance, stimulation of cells with ATP clearly induces activation of the EGFR,16–18 and ATP accelerates healing when present continuously at high concentrations in the culture medium. However, no single one of these signals seems to be necessary for induction of movement. For instance, neither wounding sheets of epithelial cells under conditions that minimize cell breakage,8,19,20 nor the effective removal of extracellular ATP with apyrase has any detectable effect on healing of wounds in sheets of corneal epithelial cells.18 In addition, the early activation of the EGFR, which occurs after a few seconds, is not absolutely required for induction of movement because blocking the receptor by a chemical inhibitor (tyrphostin AG 1478) at the time of wounding and subsequently washing it out at later time points does not impede healing.14
Some stimuli are expected to be present continuously as cell sheets migrate to cover a wound or during development (Fig. 1B). For instance, an epithelial cell sheet that migrates over a basement membrane is expected to constantly form new interactions with cell surface integrins, which is known to induce activation of the EGFR.21 Blocking EGFR signaling at various times after infliction of wounds with either tyrphostin AG 1478 or neutralizing antibodies has shown that continuous activity of the EGFR is required for progression of wound closure.14
A Model to Determine the Effects of Free Edges on Signaling in Epithelial Cell Sheets
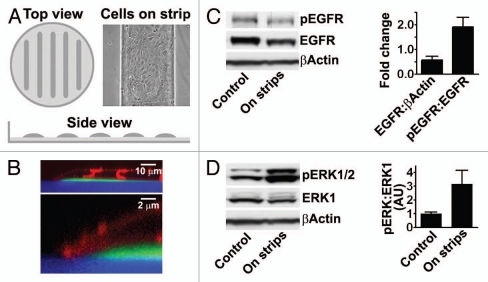
Wounds are very heterogeneous in nature, and the presence of individual stimuli is expected to be highly inconsistent. To decipher the roles of different stimuli, we and others have developed models that reduce the number of signaling inputs that may influence healing.8,14,19,20 A common factor shared by epithelial sheets during wound healing and development is the presence of free edges, and we therefore created a new model to test the effects edges in the absence of other cues in epithelial cell sheets.14 Petri dishes are coated with poly(2-hydroxyethyl methacrylate) (polyHEMA), which does not allow cell adherence and cells are seeded on 0.5 mm wide plastic strips cast on top of this layer (Figs. 1C and 2A). Examination by confocal microscopy reveals that the cells extend over the edges of the plastic strips and are thus physically unconstrained (Fig. 2B). In this model there is no acute cell damage and new adhesions cannot form with adjacent extracellular matrix. Also, activation is not due to extracellular ATP signaling or to breakdown of segregation of EGFR and its ligands at edges. As controls, cells are seeded on dishes that are totally coated with plastic, and signaling can therefore be compared in cultures that contain many free edges with cultures that contain no introduced edges.
Figure 2.
A model to determine the effects of free edges in epithelial cell sheets (cf. Fig. 1C).14 (A) Schematic of plates covered with polyHEMA and plastic strips. Light gray, polyHEMA; dark gray, plastic; inset, phase contrast microscopy of HCLE cells grown on plastic strips. (B) x–z section of a confocal image of HCLE cells at the edge of a plastic strip. The strips and poly-HEMA were labeled with fluorophores (green and blue, respectively), and the cells were labeled with the membrane dye Vybrant DiD (depicted in red). (C) Immunoblot of extracts with an antibody against EGFR phosphorylated on tyr-1173. The blots were stripped and reprobed with antibodies that recognize total amounts of the EGFR. The same blots were also probed with an antibody against β-actin as load control. (D) Immunoblot of extracts with an antibody against activated ER K1/2. The blots were stripped and reprobed with antibodies that recognize total amounts of the ER K1. The columns in (C and D) are means of six determinations ±SD.
Using this model, we found that edges induce activation of the EGFR and its down-stream effectors extracellular signal-regulated kinases 1 and 2 (ERK1/2) in HCLE and MDCK cells (Fig. 2C and D). Activation results from proteolytic cleavage of precursors of ligands for the receptor, as is the case after acute wounding, and similarly to wounding, secretion of the ligands is under the control of Src family kinases. In HCLE cells, the EGFR appears downregulated, which is in agreement with the chronic nature of the stimulation. Cells near the edges migrate at increased velocities thus demonstrating a similar biological response as is seen after acute wounding. This shows that the presence of free edges in itself is a signal that is detected by the cells.
Sensing Free Edges
It is significant that the presence of edges that are mechanically constrained do not cause activation of the EGFR. When HCLE cells are physically constrained by agarose (Fig. 1D), the EGFR is not activated. Because edges constrained by these acellular barriers block activation, the free edge sensor is unlikely to be the absence of cell-cell interaction mediated by specific molecules as has been suggested in classical formulations of contact inhibition.22,23 More likely, cells sense free edges by the lack of mechanical forces opposing the cells at the free edges, which can be provided by other cells in the cell sheets or by the presence of agarose barriers.
It is increasingly apparent that cells are exquisitely responsive to mechanical forces.24,25 At a molecular level, cells can sense the presence of forces by partial unfolding of proteins revealing cryptic sites that may serve to initiate signaling.26 Candidate proteins are in many instances part of, or associated with the cytoskeleton. Actin-based protrusions could be associated with sensory functions and, for instance, filopodia have been suggested to have a major role in probing the environment.27,28 Also, cells can sense mechanical signals in the plasma membrane through stress-activated ion channels,29 and it is possible that the part of the cell membrane at the free edge has very different levels of tensions compared to the membrane of cells interior in the cell sheet. Clearly, a major focus for future research should be to identify the molecular sensor that triggers edge signaling.
Perspectives
Wounding typically induces many potential cues that promote damaged epithelia to migrate and cover areas that are laid bare. The cues commonly induce activation of the EGFR and they therefore seem to have at least partially redundant effects. Wounding is a messy event and the extent and duration of each cue is expected to be highly variable. We therefore speculated that the very presence of edges, which per definition are always present in wounds, might in itself be a cue for induction of EGFR activation and migration. This is not a new thought; in 1915 Herbert W. Rand formulated the famous dictum that “an epithelium will not tolerate a free edge”30 and that epithelia tend to move to form uninterrupted sheets during development or after wounding. Indeed, with very few exceptions epithelia in adult organisms are continuous. However, when edges form after wounding or during development, subsequent movement is now known to be guided by interplay of many types of cues. We developed a simple model that allows analysis of signaling induced by free edges in epithelial cell sheets with a minimal influence of other cues including molecules released from broken cells or formation of new connections to extracellular matrix. We found that the very presence of edges is sufficient to induce activation of the EGFR and to increase motility of cells. Although the details may vary (for instance, other receptor systems could be involved), this type of mechanism could explain the universal propensity of epithelia to migrate at edges.
Elucidation of the signaling pathway that detects free edges should be valuable for answering questions concerning its roles. This will likely allow monitoring and specifically blocking the pathway even when other signals that trigger the EGFR are activated, because edges appear to induce unique intracellular signaling. For instance, exogenous ATP or interaction with extracellular matrix signals through Pyk2 (a kinase related to focal adhesion kinase31) but free edges do not signal through Pyk2 (Block and Klarlund,32 data not shown).
Many questions need to be addressed: Is the pathway activated acutely after wounding? The model (Fig. 1C) reflects a chronic stimulation and it is possible that rearrangements within the cell layer after wounding are necessary for the appropriate tensions to develop to trigger signaling. The size and geometry of wounds are known to determine whether wounds heal by a formation of a contractile actin cable or by lamellipodia-dependent migration, which are regulated by the small GTPases rho, rac and cdc42. Which GTPases are activated through the pathway, and can the signaling stimulate both mechanisms of healing? Finally, elucidation of the pathway should allow gene knockout strategies to study the role of the pathway in vivo.
Chronic wounds are characterized by continued defects in epithelial coverage and since the model provides a persistent stimulation, it should be a useful in vitro tool to study defects in signaling at edges in such wounds. It is noteworthy that the EGFR is downregulated at the edges of the epidermis in chronic venous ulcers,33 as is predicted by the model. During acute wounding, or when movement of the epithelial sheet is progressing (Fig. 1A and B), additional stimuli are present that can further enhance motility and fine-tune the motile phenotype of the epithelial cells. For instance, the presence of edges alone results in slightly enhanced secretion of MMP9 in HCLE cells, but the levels of MMP9 production are greatly increased when the cells are allowed to spread on adjacent tissue culture space (Fig. 1C vs. B).14 Production of MMP9 is known to be influenced by integrins binding to extracellular matrix proteins34 and signals derived from such interactions are probable cues that induce maximal MMP9 production in combination with signals from the EGFR.
The response to edges is quite possibly graded, and the level of activation of the pathway could be influenced by the mechanical properties of the tissues through which an epithelium migrates. The biological effects of matrix metalloproteases and other extracellular proteolytic enzymes in tumors is extremely complicated, but is thought overall to promote progression of malignancies.35 Proteolysis is expected to soften the extracellular matrix in the microenvironment of the tumor cells, and decreased mechanical resistance could provide a mechanism that promotes activation of the EGFR in invading epithelial tumor cells. Many proteases are associated with the cell membrane, and such a mechanism could act very locally at the surface of the cells, and may act in conjunction with additional mechanisms that register other mechanical properties of the tumor environment.36 Clearly, the existence of a detection mechanism implies that attention should be given to the mechanical properties at edges in epithelia.
Acknowledgements
We thank Kira Lathrop for creating Figure 1. This work was supported by the National Institutes of Health grant EY008098, Research to Prevent Blindness and The Eye and Ear Foundation (Pittsburgh, PA, USA).
Abbreviations
- EGFR
epidermal growth factor receptor
- polyHEMA
poly(2-hydroxyethyl methacrylate)
- ERK1/2
extracellular signal-regulated kinases 1 and 2
- HB-EGF
heparin-binding epidermal growth factor-like growth factor
- HCLE
human corneal limbal epithelial
References
- 1.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 2.Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122:3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biname F, Pawlak G, Roux P, Hibner U. What makes cells move: requirements and obstacles for spontaneous cell motility. Mol Biosyst. 2010;6:648–661. doi: 10.1039/b915591k. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura Y, Sotozono C, Kinoshita S. The epidermal growth factor receptor (EGFR): role in corneal wound healing and homeostasis. Exp Eye Res. 2001;72:511–517. doi: 10.1006/exer.2000.0979. [DOI] [PubMed] [Google Scholar]
- 5.Repertinger SK, Campagnaro E, Fuhrman J, El-Abaseri T, Yuspa SH, Hansen LA. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004;123:982–989. doi: 10.1111/j.0022-202X.2004.23478.x. [DOI] [PubMed] [Google Scholar]
- 6.Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol. 2008;128:1365–1374. doi: 10.1038/sj.jid.5701184. [DOI] [PubMed] [Google Scholar]
- 7.Tokumaru S, Higashiyama S, Endo T, Nakagawa T, Miyagawa JI, Yamamori K, et al. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J Cell Biol. 2000;151:209–220. doi: 10.1083/jcb.151.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Block ER, Matela AR, SundarRaj N, Iszkula ER, Klarlund JK. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: role of heparin-binding epidermal growth factor-like growth factor signaling. J Biol Chem. 2004;279:24307–24312. doi: 10.1074/jbc.M401058200. [DOI] [PubMed] [Google Scholar]
- 9.Xu KP, Ding Y, Ling J, Dong Z, Yu FS. Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:813–820. doi: 10.1167/iovs.03-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mine N, Iwamoto R, Mekada E. HB-EGF promotes epithelial cell migration in eyelid development. Development. 2005;132:4317–4326. doi: 10.1242/dev.02030. [DOI] [PubMed] [Google Scholar]
- 11.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 12.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 13.Bindschadler M, McGrath JL. Sheet migration by wounded monolayers as an emergent property of single-cell dynamics. J Cell Sci. 2007;120:876–884. doi: 10.1242/jcs.03395. [DOI] [PubMed] [Google Scholar]
- 14.Block ER, Tolino MA, Lozano JS, Lathrop KL, Sullenberger RS, Mazie AR, et al. Free edges in epithelial cell sheets stimulate epidermal growth factor receptor signaling. Mol Biol Cell. 2010;21:2172–2181. doi: 10.1091/mbc.E09-12-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klepeis VE, Cornell-Bell A, Trinkaus-Randall V. Growth factors but not gap junctions play a role in injury-induced Ca2+ waves in epithelial cells. J Cell Sci. 2001;114:4185–4195. doi: 10.1242/jcs.114.23.4185. [DOI] [PubMed] [Google Scholar]
- 16.Boucher I, Yang L, Mayo C, Klepeis V, Trinkaus-Randall V. Injury and nucleotides induce phosphorylation of epidermal growth factor receptor: MMP and HB-EGF dependent pathway. Exp Eye Res. 2007;85:130–141. doi: 10.1016/j.exer.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin J, Xu K, Zhang J, Kumar A, Yu FS. Wound-induced ATP release and EGF receptor activation in epithelial cells. J Cell Sci. 2007;120:815–825. doi: 10.1242/jcs.03389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Block ER, Klarlund JK. Wounding sheets of epithelial cells activates the epidermal growth factor receptor through distinct short- and long-range mechanisms. Mol Biol Cell. 2008;19:4909–4917. doi: 10.1091/mbc.E08-01-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikolic DL, Boettiger AN, Bar-Sagi D, Carbeck JD, Shvartsman SY. Role of boundary conditions in an experimental model of epithelial wound healing. Am J Physiol Cell Physiol. 2006;291:68–75. doi: 10.1152/ajpcell.00411.2005. [DOI] [PubMed] [Google Scholar]
- 20.Poujade M, Grasland-Mongrain E, Hertzog A, Jouanneau J, Chavrier P, Ladoux B, et al. Collective migration of an epithelial monolayer in response to a model wound. Proc Natl Acad Sci USA. 2007;104:15988–15993. doi: 10.1073/pnas.0705062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem J. 2009;418:491–506. doi: 10.1042/BJ20081948. [DOI] [PubMed] [Google Scholar]
- 22.Abercrombie M. Contact inhibition and malignancy. Nature. 1979;281:259–262. doi: 10.1038/281259a0. [DOI] [PubMed] [Google Scholar]
- 23.Carmona-Fontaine C, Matthews HK, Kuriyama S, Moreno M, Dunn GA, Parsons M, et al. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature. 2008;456:957–961. doi: 10.1038/nature07441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingber DE. Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Biol. 2008;97:163–179. doi: 10.1016/j.pbiomolbio.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- 28.Albiges-Rizo C, Destaing O, Fourcade B, Planus E, Block MR. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J Cell Sci. 2009;122:3037–3049. doi: 10.1242/jcs.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sachs F. Stretch-activated ion channels: what are they? Physiology (Bethesda) 2010;25:50–56. doi: 10.1152/physiol.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rand HW. Wound closure in actinian tentacles with reference to the problem of organization. Rouxs Arch Entwicklungsmech Organismen. 1915;41:151–214. [Google Scholar]
- 31.Lipinski CA, Loftus JC. Targeting Pyk2 for therapeutic intervention. Expert Opin Ther Targets. 2010;14:95–108. doi: 10.1517/14728220903473194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Block ER, Tolino MA, Klarlund JK. Pyk2 activation triggers epidermal growth factor receptor signaling and cell motility after wounding sheets of epithelial cells. J Biol Chem. 2010;285:13372–13379. doi: 10.1074/jbc.M109.083089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brem H, Stojadinovic O, Diegelmann RF, Entero H, Lee B, Pastar I, et al. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med. 2007;13:30–39. doi: 10.2119/2006-00054.Brem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamar JM, Pumiglia KM, DiPersio CM. An immortalization-dependent switch in integrin function upregulates MMP-9 to enhance tumor cell invasion. Cancer Res. 2008;68:7371–7379. doi: 10.1158/0008-5472.CAN-08-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]