Abstract
Filamin A (FLNa), the first non-muscle actin filament crosslinking protein, was identified in 1975. Thirty-five years of FLNa research has revealed its structure in great detail, discovered its isoforms (FLNb and c), and identified over 90 binding partners including channels, receptors, intracellular signaling molecules, and even transcription factors. Due to this diversity, mutations in human FLN genes result in a wide range of anomalies with moderate to lethal consequences. This review focuses on the structure and functions of FLNa in cell migration and adhesion.
Key words: filamin, actin-binding protein, actin, cytoskeleton, adhesion, migration, FilGAP, integrin, cell mechanics, mutation
Introduction
Filamin A (FLNa) is the first actin filament cross-linking protein or gelation factor identified in non-muscle cells,1 and we now understand its structure in great detail. FLN is part of a family of three proteins (FLNb and c) that are products of distinct genes and are known to serve as scaffolds for over 90 binding partners including channels, receptors, intracellular signaling molecules, and even transcription factors (Table 1 summarizes the partners involved in cell adhesion and migration and Sup. Table 1 presents the other partners). Because of this extensive array of associated proteins, mutations in human FLN genes result in a wide range of cell and tissue anomalies.2,3 In this review, we focus on the structure and functions of FLNa in cell migration and adhesion. Other functions of FLNs are described in more detail in several recent review articles.4–6
Table 1.
Filamin binding partners involved in cell adhesion, spreading and migration
| Partners | Binding sites* | Significance |
| F-actin | ABD, rod-1 | FLN induces orthogonal F-actin networks with unique mechanical and physiological properties1,12 |
| Calmodulin | ABD | Regulates F-actin binding in vitro95 |
| R-Ras | 3 | Enhances integrin activation98 |
| Syk | 5 | Flna is required for ITAM-mediated receptor signaling in platelet99 |
| Vimentin | 1–8 | Expression of IgFLNa1-8 restores spreading of filamin-deficient HEK-293 cells, vimentin phosphorylation, and the cell surface expression of β1 integrins90 |
| Supervillin | 8–10, 20–22 | Overexpression of IgFLNa8–10, but not 20–22 decreases spreading of Hela cells on fibronectin100 |
| Pro-Prion | 10,16–18, 20, 21, 23 | FLNa interacts with the GPI anchor peptide signal sequence of pro-PrP that is expressed in some cancer cells This interaction also promotes cell spreading and migration of melanoma cells22,101 |
| FAP52(PASCIN2/Syndapin II) | 15–16 | Formation of focal adhesion102 |
| ECSM2 | 15–16, 19–21 | Endothelial chemotaxis and tube formation103 |
| FILIP | 15–18 | Downregulates FLNa and controls polarity and migration of neocortical cell104 |
| GPIbα (CD42b) | 17(A/B) | Platelet adhesion and activation Genomic instability15,105,106 |
| ICAM-1 | 19–24(A/B) | Transendothelial migration No binding to splice variant-1107 |
| Integrin β | 21 (A/B/C) | Adhesion Mechanoprotection Negative regulation of integrin activation16, 47 |
| Migfilin (FBLP-1) | 21 (A/B/C), 10–13 (B) | Disconnects FLNa from integrin and promotes talin-integrin binding17,18,108,109 |
| Sphingosine kinase 1 | 22–24 | FLNa-dependent kinase activity110 |
| Tissue Factor | 22–24 | Phosphorylation of TF enhances the interaction111 |
| CEACAM1 (CD66a) | 23–24 | Reduces cell migration112 |
| Trio | 23–24 | GEF for RhoG/Rac1 and RhoA Required for ruffling82 |
| FilGAP | 23 (A specific) | Rho- and ROCK-regulated GAP for Rac. FLNa-binding is required for cell spreading and stimulates GAP activity19,96 |
| Rho | 24 | Remodeling of cytoskeleton113 |
| Rac | 24 (B: 20–21) | Remodeling of cytoskeleton113–115 |
| Cdc42 | 24 | Remodeling of cytoskeleton113 |
| RalA | 24 | Filopodia formation113 |
| ROCK | 24 | Remodeling of cytoskeleton116 |
| FILIP-1L (downregulated in ovarian cancer 1)** | ? | Overexpression of FILIP1L inhibits cell proliferation and migration and increased apoptosis117 |
| IKAP (ELP1) | ? | Loss-of-function mutations in the IKBKAP gene, which encodes IKAP, cause familial dysautonomia118 |
| Lbc** | ? | RhoGEF Co-immunorecipitated with calcium-sensing receptor, RhoA, and Gαq119 |
| P190RhoGAP** | ? | Expression of calpain-insensitive FLNa excludes p190RhoGAP from the lipid raft, thereby increase Rho activity46 |
| Vav-2** | ?(B) | Guanine nucleotide exchange factor for the Rho family Complexes with Rac-1114 |
| p311** | ? | Highly expressed in invasive glioma cells and enhances glioma cell migration120 |
Binding site on FLNa from N-terminal (top) to C-terminal (bottom) unless otherwise noted,
direct interaction has not been confirmed, cytoskeleton (red), small GTP-binding proteins and their regulators (yellow), kinases (green), transmembrane proteins (cyan), others (white).
Structure of FLNa
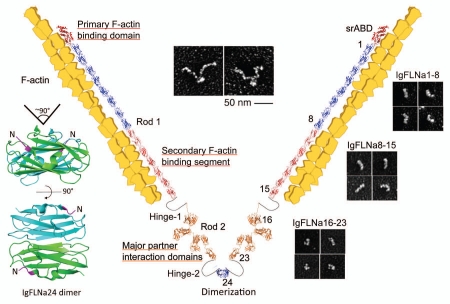
FLNa consists of two 280 kDa subunits that self-associate to form a 160 nm long semi-flexible strand (Fig. 1). Each FLN subunit has an N-terminal spectrin-related actin-binding domain (srABD) followed by 24 repeat β-pleated sheet units. Two intervening calpain-sensitive “hinges” separate the repeats into rod 1 (repeats 1–15), rod 2 (repeats 16–23) and the self-association domain (repeat 24).4,7 FLN repeats are Ig-like (IgFLN),8 each a β-barrel structure assembled from seven runs of (A∼G) β-strands.9,10 The most C-terminal repeat, IgFLNa24, mediates dimerization and bestows a V-shape to the dimeric molecules11 that results in the perpendicular branching of F-actin12 (Fig. 1 and left part). A secondary F-actin-binding domain of lower affinity resides in the rod-1 segment of FLNa. Rod 2, on the other hand, does not interact with F-actin, leaving it free to associate with partner proteins, and most partner interactions occur within the rod 2 domain.12 Binding and positioning of multiple partners in close proximity on rod 2 facilitates signal transduction at FLNa-enriched sites in cells.
Figure 1.
A schematic structure of FLNa molecule and the F-actin crosslink. This model was generated on PyMOL (www.pymol.org) by assembling Ig domains uploaded on protein data bank and by fitting them within rotary shadowed images of FLNa molecules. Structures were modeled using the Swiss model database (http://swissmodel.expasy.org). Rotary shadowed images of full-length FLNa and its subfragments are adopted from reference 12. The atomic structure of IgFLNa24 was generated on PyMOL (PDB accession number: 3CNK).
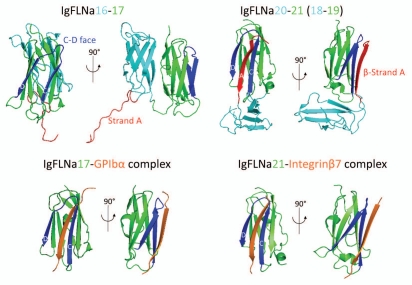
Detailed studies of purified FLNa and its subfragments in the electron microscope have shown rod 2 to have a folded structure (Fig. 1).12 Rod 1 is an extended chain. Its 58 nm contour length, encompassing Ig repeats 1–15, corresponds to the predicted end-to-end lengths of 15 IgFLNa repeats (3.5 nm each from the N-terminal to the C-terminal). However, the eight Ig repeats in the rod 2 domain (repeats 16–23) form a structure that is far more compact (19 nm) than those of rod 1 segments containing equivalent numbers of FLNa Ig repeats (Fig. 1). This compact structure is generated when the even-numbered repeats 16, 18 and 20 pair with neighboring repeats 17, 19 and 21, respectively (Fig. 2).13,14 For example, IgFLNa16 and 17 interact through their B-G and A-G faces, respectively, while strand A of IgFLNa16 protrudes from its normal position in the Ig domain and elongates hinge-1. Strands A of IgFLNa18 and 20 are also excluded from their normal positions and interact with the CD faces of neighboring IgFLNa19 and 21, respectively. As a result, the overall configuration of repeats in rod 2 is nonlinear providing rod 2 with a more globular structure. The exact configuration of paired repeats relative to each other in the rod 2 domain remains to be solved at the atomic level.
Figure 2.
Atomic structures of FLNa rod 2 subdomains and the binding interfaces of known FLNa-partner complexes. The models were generated on PyMOL (PDB accession number: IgFLNa16–17, 2K7P; IgFLNa20–21, 2J3S; IgFLNa18–19, 2K7Q; IgFLNa17-GPIbβ, 2BP3; IgFLNa21-integrinβ7, 2BRQ). The CD faces of repeats and strand A are indicated with blue and red, respectively.
Structure of the FLNa-Partner Complex
The atomic structures of the binding interfaces between FLNa and GPIbα (a subunit of the GPIb complex or von Willebrand factor receptor), FLNa and the β integrin cytoplasmic tail or FilGAP and FLNa reveal that the C and D β-strands of the interacting IgFLNa repeats form a binding pocket for an opposing β-strand, donated by the binding partner (Fig. 2).15,16 In similar fashion, the FLNa binding sites in migfilin, CFTR (cystic fibrosis transmembrane conductance regulator), and pro-prion protein are related to those of GPIbα, β integrins and FilGAP.17–22 Each binding interface is a β-strand run of nine residues that can correctively position itself in the CD repeat groove of a FLNa repeat (Fig. 2). Alternating residues of these β-strands, thus, either face or look away from the groove. Those facing the groove are restricted to hydrophobic amino acids, which suggests primarily a hydrophobic interaction.
A single binding event between FLNa and a given binding partner, however, fails to account for the measured binding specificity or strength of the native proteins' interaction. For example, a GPIbα peptide containing all the FLNa binding sequence binds to not one but seven FLNa repeats (IgFLNa 4, 9, 12, 17, 19, 21 and 23) that are structurally similar, although point mutations in IgFLNa17 in full-length FLNa are sufficient to disrupt all binding to the GPIb complex when it is expressed in CHO cells.15,23 The same holds true for the FLNa-binding site of FilGAP which is similar to that of other binding partners, and potentially interacts with other repeats, although FilGAP binds only to IgFLNa23 in cells.19 Thus, there are structural features in the intact FLNa molecule or in the partner molecule that limit this promiscuous binding. Establishment of high affinity interactions between FLNa and partners requires binding between two or more binding sites, i.e., one on each FLNa subunit and two on the partner protein, as the binding affinities, determined with peptides encompassing the binding strands of GPIbα, β integrins or FilGAP, for an individual FLNa Ig repeat are weak.15,19 Taken together, the quaternary structure made by FLN bound to its partner protein, therefore, defines the overall binding specificity and strength. Thus, bivalent reagents should be considered when designing inhibitors for partner binding sites.
Since the CD faces of repeats 19 and 21 that are used to bind the integrin cytoplasmic tail or other partner proteins are occupied with strands A of their precedent repeats (Fig. 2), binding of partners within these cryptic sites may require conformational changes in the rod 2 domain. The CD faces of repeats 17 and 23, on the other hand, which interact with the GPIb complex and FilGAP, respectively, are not masked. Partner binding to and between the two FLNa subunits may also crosslink the FLNa molecule and stabilize its structure.15,19
Regulation of FLNa-Partner Interactions
FLNa-partner interactions are regulated by: (1) mechanical forces, (2) phosphorylation, (3) proteolysis, (4) competition between binding partners and/or (5) multimerization of partners.
Mechanical force-induced conformational changes.
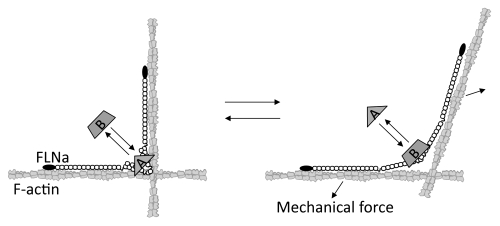
Insight into the molecular structure of FLNa, its abundant C-terminal binding and interacting partners, and its mode of binding F-actin into orthogonal branches, all suggest that it is ideally suited for mechanical signal transduction. Deformation of FLNa's C-terminal, transduced through its actin filament connections, could alter interactions between neighboring repeat pairs (Fig. 3),13,19 and computational simulation analyses suggest that physiologically relevant forces are sufficient to expose the cryptic integrin binding site.24 In vivo, FLNa is necessary for mechanosensing behavior of cells and tissue generation. FLNa-null cells are more susceptible to force-induced apoptosis,25 and FLNa is required for ductal morphogenesis of breast epithelial cells induced by a mechanically compliant collagen matrix.26 FLNa is also critical for stretch activation of ion channels regulated by polycystin-1/-2 whose genes are mutated in autosomal-dominant polycystic kidney disease, the most frequent monogenic cause of kidney failure.27
Figure 3.
Model for how mechanical force regulates FLNa-partner interactions. Mechanical force changes the conformation of the rod 2 domain to release partner A by changing the geometry between the two FLNa subunits, while exposing the cryptic binding for partner B by unfolding the A strand interaction between repeats 20 and 21.
Phosphorylation.
FLNa is phosphorylated at multiple sites by several protein kinases (Table 1 and Sup. Table 1).28–32 A heterodimer of Cyclin B1/Cdk1 has been reported to phosphorylate FLNa in vitro and thereby reduce its ability to gel actin.33 Calpain-mediated cleavage of FLNa in the hinge regions disconnects rod 2 from actin filaments and monomerizes it. Phosphorylation of FLNa by cAMP-dependent protein kinase has been reported to stabilize it against proteolysis.28,34 Many protein kinases phosphorylate FLNa at Ser2152, which is located near the A-strand of repeat 20.29,31,35 This phosphorylation site is believed to regulate integrin binding to FLNa, but neither a phospho-mimicking FLNa mutant S2152D or a non-phospho S2152A mimic has been found to effect integrin binding.36 Consistent with these negative results, a molecular dynamics simulation suggested that phosphorylation at Ser2152 does not impact the “opening” of the cryptic binding site of repeat 21 by mechanical force.24 In contrast, a recent computer simulation concluded that this phosphorylation event might facilitate force-induced dissociation of auto inhibition by decreasing the force requirement.37
Phosphorylation of partners' binding sites can also regulate their interaction with FLN. Phosphorylation of the integrin β2 tail on Thr758 dissociates it from FLNa and promotes binding of 14-3-3 and talin to the integrin,38 thereby maintaining it in an active state.
Proteolysis.
Cleavage within the two hinges of FLNa by calpain generates rod1, rod2 and self-association domain (repeat 24) subfragments.8 Following proteolysis, C-terminal-derived FLNa fragments (IgFLNa16-23 or 16-24) translocate to the nucleus together with FLNa-binding transcription factors such as androgen receptor and FOXC1.39–41 Catastrophic proteolysis of FLN is mediated by an ankyrin repeat-containing protein with a suppressor of cytokine signaling box 2 (ASB2), which targets FLNs for proteosomal degradation at discrete stages of development and differentiation in cells.42,43 Proteolysis of FLN by calpain or ASB2 inhibits cell spreading, but not migration, presumably by altering the dynamics of focal adhesions.42–46
Competition with other molecules.
All FLN isotypes interact with β integrin cytoplasmic tails albeit with different affinities, which can be predicted by their atomic structures.16,47–49 The FLN-binding site on integrin overlaps the site used by other integrin-binding proteins such as talin, kindlins, β3 endonexin, ICAP1, 14-3-3, CD98 and Shc.50 Conversely, certain FLN-binding partners such as migfilin compete with the integrin for repeat 21 binding (Table 1 and Sup. Table S1). Therefore, partner competition for FLN or for the integrin tail regulate focal adhesion complexes16,23,38 making the expression profile and concentration of each reactant critical in understanding focal adhesion turnover.
Oligomerization and/or clustering of partners.
While the topology of the binding groove in the FLNa repeat and that on opposing partner β-strand site determines the binding kinetics for a given site, high avidity binding requires engagement of two or more sites as discussed above: binding interactions with both FLNa subunits greatly increase binding avidities by ∼100 fold. For example, the kapps for the binding of polypeptide mimics of the GPIbα and FilGAP binding to IgFLNa17 and IgFLN23, respectively, are over 10 µM.15,19,51 However, the binding affinity of the von Willebrand receptor complex for FLNa or FilGAP to FLNa are estimated for other experiments to be at 100 nM and ∼200 nM.19,51 In similar fashion, integrins clustered at focal adhesion sites may generate much higher avidities for FLNa.18 Partner oligomerization through clustering, therefore, can potentially regulate the interaction with FLNa.52
Filamin Mutations and Diseases
FLNa, encoded on the X chromosome,8 is the most abundant and widely distributed member of the filamin family proteins. FLNb, a nonmuscle filamin, is encoded on human chromosome 3.53–55 The FLNc gene resides on chromosome 7, and is expressed primarily in adult cardiac, smooth and striated muscle tissues.56,57 FLN isoforms have both common and distinctive binding partners and features in their structures, expression level and localization.3 Mutations and deletions in the FLN genes that prevent normal FLN protein expression result in wide range of congenital anomalies.3,58,59 FLN mutations are also common in human breast and colon cancers.60 Nuclear FLNa fragments produced by proteolysis are significantly more abundant in benign prostate than metastatic prostate cancers.41 Additionally, FLNa mutations have been identified as the cause of the most common genetic heart valvular disorder, familiar cardiac valvular dystrophy.61 In mice, complete loss of Flna expression causes embryonic lethality with severe defects in cardiovascular formation and bone development.62–64 Mutations or depletions of FLN orthologs in other organisms, such as Dictyostelium,65,66 Drosophila and C. elegans,67 also result in developmental defects. Since numerous binding partners interact with FLNs (Table 1 and Sup. Table S1), the pathological mechanisms of the diseases are most likely attributed to loss of partner binding or aberrant interactions caused by mutations. However, the affect of most of these mutations on partner interactions remains to be elucidated at the molecular level.19,21,68
Role of FLNa in Cell Mechanics
Cell adhesion and migration inevitably rely on active and reversible changes in the mechanical properties of cells. Leukocytes, for example, need to generate internal force to crawl through connective tissues to hunt and ingest pathogens. Cells also respond to external mechanical forces imposed by their environment. Vascular cells undergo morphological changes in response to alterations in the fluid and mechanical shear stresses that are imparted on their apical surface by blood flow and their basal-lateral surfaces by pulsatile vascular stetch and retraction, and these responses have profound implications in the physiological function of blood vessels, and can lead to disorders such as atherosclerosis and thrombosis.69 In addition, depending on the stiffness of substrates, stem cells differentiate into distinct cells.70 Therefore, understanding of cell mechanics is essential for elucidating many of the fundamental aspects of cell behavior from motility to differentiation and development.
Unlike most conventional materials, cells behave in a highly nonlinear fashion (strain vs. stress) as strain increases. When a small force is applied on a short time scale, cells deform linearly and reversibly in proportion to the applied force. However, as the strain increases, cells deform less, a process called “strain-stiffening,” which extends the range of forces a cell can endure before undergoing mechanical collapse. When a relatively small continuous stress is applied on longer time scale, cells respond by deforming slowly and irreversibly. These behaviors demonstrate that cells have both elastic and viscous characteristics, and behaves as nonlinear viscoelastic materials.
The mechanical properties of cells are generated by the combined interactions of the cytoskeletal elements. One of the polymer systems, filamentous actin, concentrates underneath the plasma membrane and is necessary for both cell motility and the maintenance of cell shape. Purified F-actin forms entangled networks in solution that deform linearly. Hence, additional cohesive proteins are required to stiffen the networks to reconstitute cell like properties.71 ABPs that can cross-link actin filaments account for this discrepancy.72 FLNa-actin networks reconstitute many aspects of cell mechanics. They behave as weak elastic solids under low shear stress due to the flexible nature of actin-FLNa crosslinks, yet can support large shear stresses and have pronounced nonlinear strain-stiffening behaviors.73–75 These mechanical properties are attributed to FLNa's unique structure and how it interacts with F-actin to form orthogonal branches. High avidity binding to F-actin due to dimerization and multiple binding to F-actin through FLNa ABD and rod 1 confers strain-stiffening on actin networks.12 The hinges account for the flexibility of FLNa molecule.73 Orthogonal branching is the most efficient way to form the largest volume of actin gel with a minimal of material to support cellular integrity.
Effect of Genetic Loss of Filamin on Cell Migration and Development
Cultured FLNa-deficient melanoma cells fail to polarize and move because they have highly unstable surfaces that continuously expand and contract circumferential blebs.76,77,89 Restoring normal levels of FLNa in these deficient cells rescues motility. In humans, null mutations in the FLNa gene disrupt long-range directed neuronal migration within the cerebral cortex in X-linked periventricular heterotopia.78 Overexpression of Flna can also prevent migration,79 presumably by sequestering signaling molecules from their normal position and by altering F-actin and focal adhesion turnover to enhance adhesion. Flna knock-out mice, however, do not develop periventricular heterotopia, and embryonic fibroblasts isolated from these animals do not bleb or have defects in migration and growth.62,63 Since FLNb is also ubiquitously expressed in these cells, FLNc is expressed in some nonmuscle cells during development63 and many FLNa-binding partners also interact with these isoforms (Table 1 and Sup. Table 1), they could compensate for FLNa deficiency. In fact, expression of an shRNA-resistant FLNa in FLNab double knockdown cells completely rescues their spreading defect.44 However, even some FLNa knockdown cells migrate at speeds comparable to wild-type cells once they overcome their diminished ability to spread on substrates and become attached.44 Whether this is due to the residual expression of other FLN isotypes, or if there is a FLN-independent migration mode, remains to be elucidated. Additionally, developmental differences between humans and mice should also be considered. The mouse brain is small and less complex than its human counterpart. Neuronal migration in humans may also require different cues. However, Flna-null mice do exhibit a thinning of the cerebral cortex that may indicate a migration defect.63
Cooperation of Filamin with its Partners in Cell Adhesion and Migration
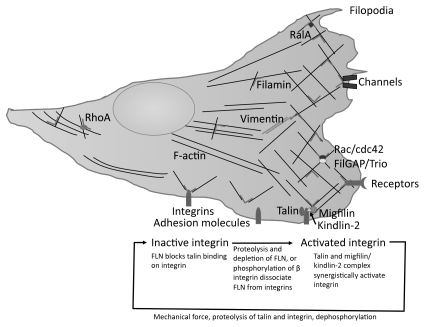
FLN-binding partners interactions regulate cell adhesion, spreading and migration (Table 1 and Fig. 4). The molecular mechanism by which FLN coordinates these partners is complex. Directed migration requires temporal polarization and spatial enrichment of molecules at specific sites. How does FLN, particularly when bound to actin that is dispersed through cells, perform this task? Immunofluorescent microscopy reveals FLNa and b to be enriched at the cell periphery and in the focal adhesions of cultured cells, while FLNc localizes in the muscle Z-disc.56,80,81 One mechanism for the enrichment of FLNs at these sites is their recruitment by binding partners, such as receptors and adhesion molecules that preferentially reside at these sites (Fig. 4).82 A second mechanism concentrating FLNa in newly assembled actin networks in lamellipodia occurs because of higher avidity for the branched F-actin junctions in these regions (Fig. 4).12,83 FLNa recruitment by one partner could allow it to scaffold additional signaling molecules and enhance the efficiency of signal transduction. In addition, while FLN-binding partners may potentially collect FLN molecules to specific locations within the cell, their function may require the conscription of additional partners by FLNa. It is unlikely that all FLN-binding partners are expressed by a given cell, as partner expression levels are tightly regulated in different cells and/or different developmental stages (Table 1 and Sup. Table 1). Therefore, the phenotype of FLN mutations must be studied within different cell types and/or developmental stages.
Figure 4.
Schematic of how certain FLN-partner complexes regulate cell adhesion and motility. Top part: FLN crosslinks actin filaments and attaches them to signaling molecules, membrane proteins and the extracellular matrix through adhesion molecules such as integrins. FLN is required for the recycling, trafficking and stabilization of membrane proteins. FLN also scaffolds multiple partners in close proximity on rod 2, thereby facilitating signal transduction at specific locations within cells. Note that the expression levels of FLN-binding partners are dependent on the cell type and, therefore not all partners necessarily participate in cell adhesion and migration in the same cell. Bottom part: Model for how FLN regulates integrin activation. FLN acts as a negative regulator for integrin activation by blocking talin binding to the β integrin tail. Perturbation of this interaction allows talin to interact with β integrin, thereby activating integrin at the leading edge of a migrating cell.
Role of Filamin in Integrin-Dependent Cell Adhesion and Migration
Although certain cells can migrate without integrins or swim without attaching to a substrate,84,85 most cells migrate in an integrin-dependent manner. Our current understanding of integrin function at focal adhesions in moving cells is summarized in Figure 4. Since depletion of FLN results in integrin activation, FLN appears to act primarily as a negative regulator of integrin activation.16 Therefore, any event that alters the FLN-integrin binding affects integrin activation status.42,86 Consistent with this notion, diminished expression of FLNa increases the invasiveness of human breast cancer cells.87 Since FLNa silencing also induces activation of calpain, which leads to degradation of focal adhesion proteins,88,89 suppression of FLNa expression may facilitate focal adhesion turnover, thereby promoting the invasion. Recycling of integrin is also essential for sustained cell migration. The trafficking of β1 integrins to the cell membrane is regulated by PKCε-mediated phosphorylation of vimentin bound on the N-terminal of FLNa, as vimentin phosphorylation is impaired in FLNa knockdown cells.90 Taken together, FLN regulates not only integrin activation, but also its recycling to coordinate integrin-dependent migration.
Despite increasing evidence supporting a FLN's suppressor function for integrin activation, degradation of FLN is not likely to be the only mechanism to affect cell migration, and it is not surprising that an opposite effect of FLNa expression on cell migration has been reported. This result indicates that the role of FLN in cell migration is cell type-dependent and that balanced FLNa/integrin interactions generate normal cell adhesion and migration.
Does FLNa Regulate Blebbing Migration?
Blebbing has recently received renewed attention in motility, as cancer cells can switch between mesenchymal (elongated) and amoeboid (blebbing or rounded) migration modes in three-dimensional environments.91,92 Since amoeboid cells can squeeze into the extracellular matrix, these cells escape and metastasize. Elucidating blebbing mechanisms and the conversion between migration modes is therefore of great importance.
Blebbing is believed to initiate following local disruption of plasma membrane-F-actin interactions and occurs because cells are under internal hydrostatic pressure powered by myosin contraction, as blebbistatin, a myosin II inhibitor, quenches most cellular blebbing. Blebbing is a prominent feature of FLNa-deficient melanoma cells93 that have weak cortical actin networks. In normal cells, both Ca2+ activated-gelsolin and Ca2+-calmodulin signaling can reduce stiffness of an FLNa-actin gel.94,95 The blebbing mode is also regulated by the RhoA-ROCK pathway that induces myosin contraction and antagonizes the Rac1-WAVE pathway, which induces actin polymerization-dependent protrusions (mesenchymal migration mode). Suppression of Rac1 activity is catalyzed by ARHGAP22 whose activity is regulated by actinomyosin contractility through an unknown mechanism, rather than by direct phosphorylation by ROCK.91 Given that overexpression of FilGAP (ARHGAP24), a Rac-specific GAP that interacts with FLNa, induces blebbing,96 and that the FLNa-FilGAP interaction is potentially regulated by mechanical force,19,97 force-induced regulation of the FLNa-FilGAP interaction could provide a simple mechanism for the conversion between mesenchymal and amoeboid migration modes.
Conclusion and Outlook
Since its discovery in 1975, studies on FLN have substantially advanced an understanding of its structure and functions. Nevertheless, important questions still remain: (1) How many of the FLN-partner interactions are regulated? (2) What is the quaternary structure formed between FLN and partners? and (3) What are the biochemical mechanisms of disease caused by FLN mutations? Disease-causing mutations distribute throughout the FLN molecule, but why do only a small number of binding partners interact in FLN's N-terminal rod 1 domain, although repeat 10 is a mutational hot spot?3
The schematic FLNa model depicted in Figure 1 is consistent with all currently reported data. However, a more detailed structural analysis of FLNs, particularly complexed with partners, is required to understand how FLN mutations cause disease. Structural information will enable investigators to generate point mutations in FLNs or their partner proteins that lack only partner-related specific activity. Probing cell function with these molecules in vivo will provide deeper understanding of the dynamics of FLN-partner interaction during migration.
Acknowledgements
This work was supported by by National Institutes of Health Grants HL-19429 (T.P.S.) and HL-56252 (J.H.H.), the HUSEC Seed Fund for Interdisciplinary Science (T.P.S. and F.N.).
Abbreviations
- FLNa
filamin A
- IgFLN
immunoglobulin-like filamin repeat
- ABP
actin-binding protein
- ABD
actin-binding domain
- GAP
GTPase-activating protein
Supplementary Material
References
- 1.Hartwig JH, Stossel TP. Isolation and properties of actin, myosin and a new actinbinding protein in rabbit alveolar macrophages. J Biol Chem. 1975;250:5696–5705. [PubMed] [Google Scholar]
- 2.Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol. 2004;6:1034–1038. doi: 10.1038/ncb1104-1034. [DOI] [PubMed] [Google Scholar]
- 3.Robertson SP, Filamin A. phenotypic diversity. Curr Opin Genet Dev. 2005;15:301–307. doi: 10.1016/j.gde.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, et al. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2:138–145. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- 5.Popowicz GM, Schleicher M, Noegel AA, Holak TA. Filamins: promiscuous organizers of the cytoskeleton. Trends Biochem Sci. 2006;31:411–419. doi: 10.1016/j.tibs.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Zhou AX, Hartwig JH, Akyurek LM. Filamins in cell signaling, transcription and organ development. Trends Cell Biol. 2010;20:113–123. doi: 10.1016/j.tcb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 7.van der Flier A, Sonnenberg A. Structural and functional aspects of filamins. Biochim Biophys Acta. 2001;1538:99–117. doi: 10.1016/s0167-4889(01)00072-6. [DOI] [PubMed] [Google Scholar]
- 8.Gorlin JB, Yamin R, Egan S, Stewart M, Stossel TP, Kwiatkowski DJ, et al. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J Cell Biol. 1990;111:1089–1105. doi: 10.1083/jcb.111.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fucini P, Renner C, Herberhold C, Noegel AA, Holak TA. The repeating segments of the F-actin cross-linking gelation factor (ABP-120) have an immunoglobulin-like fold. Nat Struct Biol. 1997;4:223–230. doi: 10.1038/nsb0397-223. [DOI] [PubMed] [Google Scholar]
- 10.Pudas R, Kiema TR, Butler PJ, Stewart M, Ylanne J. Structural basis for vertebrate filamin dimerization. Structure. 2005;13:111–119. doi: 10.1016/j.str.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Seo MD, Seok SH, Im H, Kwon AR, Lee SJ, Kim HR, et al. Crystal structure of the dimerization domain of human filamin A. Proteins. 2009;75:258–263. doi: 10.1002/prot.22336. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura F, Osborn TM, Hartemink CA, Hartwig JH, Stossel TP. Structural basis of filamin A functions. J Cell Biol. 2007;179:1011–1025. doi: 10.1083/jcb.200707073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lad Y, Kiema T, Jiang P, Pentikainen OT, Coles CH, Campbell ID, et al. Structure of three tandem filamin domains reveals auto-inhibition of ligand binding. EMBO J. 2007;26:3993–4004. doi: 10.1038/sj.emboj.7601827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heikkinen OK, Ruskamo S, Konarev PV, Svergun DI, Iivanainen T, Heikkinen SM, et al. Atomic structures of two novel immunoglobulin-like domain pairs in the actin cross-linking protein filamin. J Biol Chem. 2009;284:25450–25458. doi: 10.1074/jbc.M109.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura F, Pudas R, Heikkinen O, Permi P, Kilpelainen I, Munday AD, et al. The structure of the GPIb-filamin A complex. Blood. 2006;107:1925–1932. doi: 10.1182/blood-2005-10-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiema T, Lad Y, Jiang P, Oxley CL, Baldassarre M, Wegener KL, et al. The molecular basis of filamin binding to integrins and competition with talin. Mol Cell. 2006;21:337–347. doi: 10.1016/j.molcel.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Lad Y, Jiang P, Ruskamo S, Harburger DS, Ylanne J, Campbell ID, et al. Structural basis of the migfilin-filamin interaction and competition with integrin beta tails. J Biol Chem. 2008;283:35154–35163. doi: 10.1074/jbc.M802592200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ithychanda SS, Das M, Ma YQ, Ding K, Wang X, Gupta S, et al. Migfilin, a molecular switch in regulation of integrin activation. J Biol Chem. 2009;284:4713–4722. doi: 10.1074/jbc.M807719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura F, Heikkinen O, Pentikainen OT, Osborn TM, Kasza KE, Weitz DA, et al. Molecular basis of filamin A-FilGAP interaction and its impairment in congenital disorders associated with filamin A mutations. PLoS ONE. 2009;4:4928. doi: 10.1371/journal.pone.0004928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith L, Page RC, Xu Z, Kohli E, Litman P, Nix JC, et al. Biochemical basis of the interaction between cystic fibrosis transmembrane conductance regulator and immunoglobulin-like repeats of filamin. J Biol Chem. 2010;285:17166–17176. doi: 10.1074/jbc.M109.080911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Playford MP, Nurminen E, Pentikainen OT, Milgram SL, Hartwig JH, Stossel TP, et al. Cystic fibrosis transmembrane conductance regulator interacts with multiple immunoglobulin domains of filamin A. J Biol Chem. 2010;285:17156–17165. doi: 10.1074/jbc.M109.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Yu S, Nakamura F, Pentikainen OT, Singh N, Yin S, et al. Pro-prion binds filamin A facilitating its interaction with integrin beta1 and contributes to melanomagenesis. J Biol Chem. 2010;285:30328–30339. doi: 10.1074/jbc.M110.147413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ithychanda SS, Hsu D, Li H, Yan L, Liu D, Das M, et al. Identification and characterization of multiple similar ligand-binding repeats in filamin: implication on filamin-mediated receptor clustering and cross-talk. Biol Chem. 2009;284:35113–35121. doi: 10.1074/jbc.M109.060954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pentikainen U, Ylanne J. The regulation mechanism for the auto-inhibition of binding of human filamin A to integrin. J Mol Biol. 2009;393:644–657. doi: 10.1016/j.jmb.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 25.Glogauer M, Arora P, Chou D, Janmey PA, Downey GP, McCulloch CA. The role of actin-binding protein 280 in integrin-dependent mechanoprotection. J Biol Chem. 1998;273:1689–1698. doi: 10.1074/jbc.273.3.1689. [DOI] [PubMed] [Google Scholar]
- 26.Gehler S, Baldassarre M, Lad Y, Leight JL, Wozniak MA, Riching KM, et al. Filamin A-beta1 integrin complex tunes epithelial cell response to matrix tension. Mol Biol Cell. 2009;20:3224–3238. doi: 10.1091/mbc.E08-12-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, et al. Polycystin-1 and -2 dosage regulates pressure sensing. Cell. 2009;139:587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 28.Chen M, Stracher A. In situ phosphorylation of platelet actin-binding protein by cAMP-dependent protein kinase stabilizes it against proteolysis by calpain. J Biol Chem. 1989;264:14282–14289. [PubMed] [Google Scholar]
- 29.Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, et al. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol. 2002;4:681–690. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- 30.Tigges U, Koch B, Wissing J, Jockusch BM, Ziegler WH. The F-actin cross-linking and focal adhesion protein filamin A is a ligand and in vivo substrate for protein kinase C alpha. J Biol Chem. 2003;278:23561–23569. doi: 10.1074/jbc.M302302200. [DOI] [PubMed] [Google Scholar]
- 31.Woo MS, Ohta Y, Rabinovitz I, Stossel TP, Blenis J. Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Mol Cell Biol. 2004;24:3025–3035. doi: 10.1128/MCB.24.7.3025-3035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong Z, Yeow WS, Zou C, Wassell R, Wang C, Pestell RG, et al. Cyclin d1/cyclin-dependent kinase 4 interacts with filamin a and affects the migration and invasion potential of breast cancer cells. Cancer Res. 2010;70:2105–2114. doi: 10.1158/0008-5472.CAN-08-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cukier IH, Li Y, Lee JM. Cyclin B1/Cdk1 binds and phosphorylates Filamin A and regulates its ability to cross-link actin. FEBS Lett. 2007;581:1661–1672. doi: 10.1016/j.febslet.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 34.Fox JE, Reynolds CC, Phillips DR. Calcium-dependent proteolysis occurs during platelet aggregation. J Biol Chem. 1983;258:9973–9981. [PubMed] [Google Scholar]
- 35.Jay D, Garcia EJ, de la Luz Ibarra M. In situ determination of a PKA phosphorylation site in the C-terminal region of filamin. Mol Cell Biochem. 2004;260:49–53. doi: 10.1023/b:mcbi.0000026052.76418.55. [DOI] [PubMed] [Google Scholar]
- 36.Travis MA, van der Flier A, Kammerer RA, Mould AP, Sonnenberg A, Humphries MJ. Interaction of filamin A with the integrin beta7 cytoplasmic domain: role of alternative splicing and phosphorylation. FEBS Lett. 2004;569:185–190. doi: 10.1016/j.febslet.2004.04.099. [DOI] [PubMed] [Google Scholar]
- 37.Chen HS, Kolahi KS, Mofrad MR. Phosphorylation facilitates the integrin binding of filamin under force. Biophys J. 2009;97:3095–3104. doi: 10.1016/j.bpj.2009.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takala H, Nurminen E, Nurmi SM, Aatonen M, Strandin T, Takatalo M, et al. Integrin {beta}2 phosphorylation on THR758 acts as a molecular switch to regulate 14-3-3 and filamin binding. Blood. 2008;112:1853–1862. doi: 10.1182/blood-2007-12-127795. [DOI] [PubMed] [Google Scholar]
- 39.Loy CJ, Sim KS, Yong EL. Filamin-A fragment localizes to the nucleus to regulate androgen receptor and coactivator functions. Proc Natl Acad Sci USA. 2003;100:4562–4567. doi: 10.1073/pnas.0736237100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry FB, O'Neill MA, Coca-Prados M, Walter MA. FOXC1 transcriptional regulatory activity is impaired by PBX1 in a filamin A-mediated manner. Mol Cell Biol. 2005;25:1415–1424. doi: 10.1128/MCB.25.4.1415-1424.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Kreisberg JI, Bedolla RG, Mikhailova M, deVere White RW, Ghosh PM. A 90 kDa fragment of filamin A promotes Casodex-induced growth inhibition in Casodex-resistant androgen receptor positive C4-2 prostate cancer cells. Oncogene. 2007;26:6061–6070. doi: 10.1038/sj.onc.1210435. [DOI] [PubMed] [Google Scholar]
- 42.Heuze ML, Lamsoul I, Baldassarre M, Lad Y, Leveque S, Razinia Z, et al. ASB2 targets filamins A and B to proteasomal degradation. Blood. 2008;112:5130–5140. doi: 10.1182/blood-2007-12-128744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bello NF, Lamsoul I, Heuze ML, Metais A, Moreaux G, Calderwood DA, et al. The E3 ubiquitin ligase specificity subunit ASB2beta is a novel regulator of muscle differentiation that targets filamin B to proteasomal degradation. Cell Death Differ. 2009;16:921–932. doi: 10.1038/cdd.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baldassarre M, Razinia Z, Burande CF, Lamsoul I, Lutz PG, Calderwood DA. Filamins regulate cell spreading and initiation of cell migration. PLoS ONE. 2009;4:7830. doi: 10.1371/journal.pone.0007830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwak KB, Chung SS, Kim OM, Kang MS, Ha DB, Chung CH. Increase in the level of m-calpain correlates with the elevated cleavage of filamin during myogenic differentiation of embryonic muscle cells. Biochim Biophys Acta. 1993;1175:243–249. doi: 10.1016/0167-4889(93)90212-8. [DOI] [PubMed] [Google Scholar]
- 46.Mammoto A, Huang S, Ingber DE. Filamin links cell shape and cytoskeletal structure to Rho regulation by controlling accumulation of p190RhoGAP in lipid rafts. J Cell Sci. 2007;120:456–467. doi: 10.1242/jcs.03353. [DOI] [PubMed] [Google Scholar]
- 47.Sharma CP, Ezzell RM, Arnaout MA. Direct interaction of filamin (ABP-280) with the beta2-integrin subunit CD18. J Immunol. 1995;154:3461–3470. [PubMed] [Google Scholar]
- 48.van der Flier A, Kuikman I, Kramer D, Geerts D, Kreft M, Takafuta T, et al. Different splice variants of filamin-B affect myogenesis, subcellular distribution and determine binding to integrin [beta] subunits. J Cell Biol. 2002;156:361–376. doi: 10.1083/jcb.200103037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gontier Y, Taivainen A, Fontao L, Sonnenberg A, van der Flier A, Carpen O, et al. The Z-disc proteins myotilin and FATZ-1 interact with each other and are connected to the sarcolemma via muscle-specific filamins. J Cell Sci. 2005;118:3739–3749. doi: 10.1242/jcs.02484. [DOI] [PubMed] [Google Scholar]
- 50.Legate KR, Fassler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci. 2009;122:187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- 51.Andrews RK, Fox JE. Interaction of purified actin-binding protein with the platelet membrane glycoprotein Ib-IX complex. J Biol Chem. 1991;266:7144–7147. [PubMed] [Google Scholar]
- 52.Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15:547–556. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Takafuta T, Wu G, Murphy GF, Shapiro SS. Human beta-filamin is a new protein that interacts with the cytoplasmic tail of glycoprotein Ibalpha. J Biol Chem. 1998;273:17531–17538. doi: 10.1074/jbc.273.28.17531. [DOI] [PubMed] [Google Scholar]
- 54.Krakow D, Robertson SP, King LM, Morgan T, Sebald ET, Bertolotto C, et al. Mutations in the gene encoding filamin B disrupt vertebral segmentation, joint formation and skeletogenesis. Nat Genet. 2004;36:405–410. doi: 10.1038/ng1319. [DOI] [PubMed] [Google Scholar]
- 55.Zhou X, Tian F, Sandzen J, Cao R, Flaberg E, Szekely L, et al. Filamin B deficiency in mice results in skeletal malformations and impaired microvascular development. Proc Natl Acad Sci USA. 2007;104:3919–3924. doi: 10.1073/pnas.0608360104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson TG, Chan YM, Hack AA, Brosius M, Rajala M, Lidov HG, et al. Filamin 2 (FLN2): A muscle-specific sarcoglycan interacting protein. J Cell Biol. 2000;148:115–126. doi: 10.1083/jcb.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dalkilic I, Schienda J, Thompson TG, Kunkel LM. Loss of FilaminC (FLNc) results in severe defects in myogenesis and myotube structure. Mol Cell Biol. 2006;26:6522–6534. doi: 10.1128/MCB.00243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferland RJ, Gaitanis JN, Apse K, Tantravahi U, Walsh CA, Sheen VL. Periventricular nodular heterotopia and Williams syndrome. Am J Med Genet A. 2006;140:1305–1311. doi: 10.1002/ajmg.a.31259. [DOI] [PubMed] [Google Scholar]
- 59.Unger S, Mainberger A, Spitz C, Bahr A, Zeschnigk C, Zabel B, et al. Filamin A mutation is one cause of FG syndrome. Am J Med Genet A. 2007;143:1876–1879. doi: 10.1002/ajmg.a.31751. [DOI] [PubMed] [Google Scholar]
- 60.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 61.Kyndt F, Gueffet JP, Probst V, Jaafar P, Legendre A, Le Bouffant F, et al. Mutations in the gene encoding filamin A as a cause for familial cardiac valvular dystrophy. Circulation. 2007;115:40–49. doi: 10.1161/CIRCULATIONAHA.106.622621. [DOI] [PubMed] [Google Scholar]
- 62.Hart AW, Morgan JE, Schneider J, West K, McKie L, Bhattacharya S, et al. Cardiac malformations and midline skeletal defects in mice lacking filamin A. Hum Mol Genet. 2006;15:2457–2467. doi: 10.1093/hmg/ddl168. [DOI] [PubMed] [Google Scholar]
- 63.Feng Y, Chen MH, Moskowitz IP, Mendonza AM, Vidali L, Nakamura F, et al. Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis. Proc Natl Acad Sci USA. 2006;103:19836–19841. doi: 10.1073/pnas.0609628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou X, Boren J, Akyurek LM. Filamins in cardiovascular development. Trends Cardiovasc Med. 2007;17:222–229. doi: 10.1016/j.tcm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 65.Khaire N, Muller R, Blau-Wasser R, Eichinger L, Schleicher M, Rief M, et al. Filamin-regulated F-actin assembly is essential for morphogenesis and controls phototaxis in Dictyostelium. J Biol Chem. 2007;282:1948–1955. doi: 10.1074/jbc.M610262200. [DOI] [PubMed] [Google Scholar]
- 66.Annesley SJ, Bandala-Sanchez E, Ahmed AU, Fisher PR. Filamin repeat segments required for photosensory signalling in Dictyostelium discoideum. BMC Cell Biol. 2007;8:48. doi: 10.1186/1471-2121-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kovacevic I, Cram EJ. FLN-1/Filamin is required for maintenance of actin and exit of fertilized oocytes from the spermatheca in C. elegans. Dev Biol. 2010;347:247–257. doi: 10.1016/j.ydbio.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vorgerd M, van der Ven PF, Bruchertseifer V, Lowe T, Kley RA, Schroder R, et al. A mutation in the dimerization domain of filamin c causes a novel type of autosomal dominant myofibrillar myopathy. Am J Hum Genet. 2005;77:297–304. doi: 10.1086/431959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces and atherogenesis. Ann NY Acad Sci. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- 70.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 71.Janmey PA, Hvidt S, Kas J, Lerche D, Maggs A, Sackmann E, et al. The mechanical properties of actin gels. Elastic modulus and filament motions. J Biol Chem. 1994;269:32503–32513. [PubMed] [Google Scholar]
- 72.Matsudaira P. Actin crosslinking proteins at the leading edge. Semin Cell Biol. 1994;5:165–174. doi: 10.1006/scel.1994.1021. [DOI] [PubMed] [Google Scholar]
- 73.Gardel ML, Nakamura F, Hartwig JH, Crocker JC, Stossel TP, Weitz DA. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc Natl Acad Sci USA. 2006;103:1762–1767. doi: 10.1073/pnas.0504777103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kasza KE, Koenderink GH, Lin YC, Broedersz CP, Messner W, Nakamura F, et al. Nonlinear elasticity of stiff biopolymers connected by flexible linkers. Phys Rev E Stat Nonlin Soft Matter Phys. 2009;79:41928. doi: 10.1103/PhysRevE.79.041928. [DOI] [PubMed] [Google Scholar]
- 75.Kasza KE, Nakamura F, Hu S, Kollmannsberger P, Bonakdar N, Fabry B, et al. Filamin A is essential for active cell stiffening but not passive stiffening under external force. Biophys J. 2009;96:4326–4335. doi: 10.1016/j.bpj.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cunningham CC, Gorlin JB, Kwiatkowski DJ, Hartwig JH, Janmey PA, Byers HR, et al. Actin-binding protein requirement for cortical stability and efficient locomotion. Science. 1992;255:325–327. doi: 10.1126/science.1549777. [DOI] [PubMed] [Google Scholar]
- 77.Simpson KJ, Selfors LM, Bui J, Reynolds A, Leake D, Khvorova A, et al. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol. 2008;10:1027–1038. doi: 10.1038/ncb1762. [DOI] [PubMed] [Google Scholar]
- 78.Fox JW, Lamperti ED, Eksioglu YZ, Hong SE, Feng Y, Graham DA, et al. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron. 1998;21:1315–1325. doi: 10.1016/s0896-6273(00)80651-0. [DOI] [PubMed] [Google Scholar]
- 79.Sarkisian MR, Bartley CM, Chi H, Nakamura F, Hashimoto-Torii K, Torii M, et al. MEKK4 signaling regulates filamin expression and neuronal migration. Neuron. 2006;52:789–801. doi: 10.1016/j.neuron.2006.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pavalko FM, Otey CA, Burridge K. Identification of a filamin isoform enriched at the ends of stress fibers in chicken embryo fibroblasts. J Cell Sci. 1989;94:109–118. doi: 10.1242/jcs.94.1.109. [DOI] [PubMed] [Google Scholar]
- 81.Calderwood DA, Huttenlocher A, Kiosses WB, Rose DM, Woodside DG, Schwartz MA, et al. Increased filamin binding to beta-integrin cytoplasmic domains inhibits cell migration. Nat Cell Biol. 2001;3:1060–1068. doi: 10.1038/ncb1201-1060. [DOI] [PubMed] [Google Scholar]
- 82.Bellanger JM, Astier C, Sardet C, Ohta Y, Stossel TP, Debant A. The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin. Nat Cell Biol. 2000;2:888–892. doi: 10.1038/35046533. [DOI] [PubMed] [Google Scholar]
- 83.Urban E, Jacob S, Nemethova M, Resch GP, Small JV. Electron tomography reveals unbranched networks of actin filaments in lamellipodia. Nat Cell Biol. 2010;12:429–435. doi: 10.1038/ncb2044. [DOI] [PubMed] [Google Scholar]
- 84.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 85.Barry NP, Bretscher MS. Dictyostelium amoebae and neutrophils can swim. Proc Natl Acad Sci USA. 2010;107:11376–11380. doi: 10.1073/pnas.1006327107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O'Connell MP, Fiori JL, Baugher KM, Indig FE, French AD, Camilli TC, et al. Wnt5A activates the calpain-mediated cleavage of filamin A. J Invest Dermatol. 2009;129:1782–1789. doi: 10.1038/jid.2008.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu Y, Bismar TA, Su J, Xu B, Kristiansen G, Varga Z, et al. Filamin A regulates focal adhesion disassembly and suppresses breast cancer cell migration and invasion. J Exp Med. 2010;207:2421–2437. doi: 10.1084/jem.20100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6:977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 89.Flevaris P, Stojanovic A, Gong H, Chishti A, Welch E, Du X. A molecular switch that controls cell spreading and retraction. J Cell Biol. 2007;179:553–565. doi: 10.1083/jcb.200703185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim H, Nakamura F, Lee W, Hong C, Perez-Sala D, McCulloch CA. Regulation of cell adhesion to collagen via beta1 integrins is dependent on interactions of filamin A with vimentin and protein kinase C epsilon. Exp Cell Res. 2010;316:1829–1844. doi: 10.1016/j.yexcr.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 91.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 92.Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181:879–884. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ. Non-equilibration of hydrostatic pressure in blebbing cells. Nature. 2005;435:365–369. doi: 10.1038/nature03550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yin HL, Zaner KS, Stossel TP. Ca2+ control of actin gelation. Interaction of gelsolin with actin filaments and regulation of actin gelation. J Biol Chem. 1980;255:9494–9500. [PubMed] [Google Scholar]
- 95.Nakamura F, Hartwig JH, Stossel TP, Szymanski PT. Ca2+ and calmodulin regulate the binding of filamin A to actin filaments. J Biol Chem. 2005;280:32426–32433. doi: 10.1074/jbc.M502203200. [DOI] [PubMed] [Google Scholar]
- 96.Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol. 2006;8:803–814. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- 97.Shifrin Y, Arora PD, Ohta Y, Calderwood DA, McCulloch CA. The role of FilGAP-filamin A interactions in mechanoprotection. Mol Biol Cell. 2009;20:1269–1279. doi: 10.1091/mbc.E08-08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gawecka JE, Griffiths GS, Ek-Rylander B, Ramos JW, Matter ML. R-Ras regulates migration through an interaction with filamin A in melanoma cells. PLoS One. 2010;5:11269. doi: 10.1371/journal.pone.0011269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Falet H, Pollitt AY, Begonja AJ, Weber SE, Duerschmied D, Wagner DD, et al. A novel interaction between FlnA and Syk regulates platelet ITAM-mediated receptor signaling and function. J Exp Med. 2010;207:1967–1979. doi: 10.1084/jem.20100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith TC, Fang Z, Luna EJ. Novel interactors and a role for supervillin in early cytokinesis. Cytoskeleton (Hoboken) 2010;67:346–364. doi: 10.1002/cm.20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li C, Yu S, Nakamura F, Yin S, Xu J, Petrolla AA, et al. Binding of pro-prion to filamin A disrupts cytoskeleton and correlates with poor prognosis in pancreatic cancer. J Clin Invest. 2009;119:2725–2736. doi: 10.1172/JCI39542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nikki M, Merilainen J, Lehto VP. FAP52 regulates actin organization via binding to filamin. J Biol Chem. 2002;277:11432–11440. doi: 10.1074/jbc.M111753200. [DOI] [PubMed] [Google Scholar]
- 103.Armstrong LJ, Heath VL, Sanderson S, Kaur S, Beesley JF, Herbert JM, et al. ECSM2, an endothelial specific filamin a binding protein that mediates chemotaxis. Arterioscler Thromb Vasc Biol. 2008;28:1640–1646. doi: 10.1161/ATVBAHA.108.162511. [DOI] [PubMed] [Google Scholar]
- 104.Nagano T, Yoneda T, Hatanaka Y, Kubota C, Murakami F, Sato M. Filamin A-interacting protein (FILIP) regulates cortical cell migration out of the ventricular zone. Nat Cell Biol. 2002;4:495–501. doi: 10.1038/ncb808. [DOI] [PubMed] [Google Scholar]
- 105.Fox JE. Identification of actin-binding protein as the protein linking the membrane skeleton to glycoproteins on platelet plasma membranes. J Biol Chem. 1985;260:11970–11977. [PubMed] [Google Scholar]
- 106.Li Y, Lu J, Prochownik EV. c-Myc-mediated genomic instability proceeds via a megakaryocytic endomitosis pathway involving Gp1balpha. Proc Natl Acad Sci USA. 2007;104:3490–3495. doi: 10.1073/pnas.0610163104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kanters E, van Rijssel J, Hensbergen PJ, Hondius D, Mul FP, Deelder AM, et al. Filamin B mediates ICAM-1-driven leukocyte transendothelial migration. J Biol Chem. 2008;283:31830–31839. doi: 10.1074/jbc.M804888200. [DOI] [PubMed] [Google Scholar]
- 108.Tu Y, Wu S, Shi X, Chen K, Wu C. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell. 2003;113:37–47. doi: 10.1016/s0092-8674(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 109.Takafuta T, Saeki M, Fujimoto TT, Fujimura K, Shapiro SS. A new member of the LIM protein family binds to filamin B and localizes at stress fibers. J Biol Chem. 2003;278:12175–12181. doi: 10.1074/jbc.M209339200. [DOI] [PubMed] [Google Scholar]
- 110.Maceyka M, Alvarez SE, Milstien S, Spiegel S. Filamin A links sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 at lamellipodia to orchestrate cell migration. Mol Cell Biol. 2008;28:5687–5697. doi: 10.1128/MCB.00465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ott I, Fischer EG, Miyagi Y, Mueller BM, Ruf W. A role for tissue factor in cell adhesion and migration mediated by interaction with actin-binding protein 280. J Cell Biol. 1998;140:1241–1253. doi: 10.1083/jcb.140.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klaile E, Muller MM, Kannicht C, Singer BB, Lucka L. CEACAM1 functionally interacts with filamin A and exerts a dual role in the regulation of cell migration. J Cell Sci. 2005;118:5513–5524. doi: 10.1242/jcs.02660. [DOI] [PubMed] [Google Scholar]
- 113.Ohta Y, Suzuki N, Nakamura S, Hartwig JH, Stossel TP. The small GTPase RalA targets filamin to induce filopodia. Proc Natl Acad Sci USA. 1999;96:2122–2128. doi: 10.1073/pnas.96.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Del Valle-Perez B, Martinez VG, Lacasa-Salavert C, Figueras A, Shapiro SS, Takafuta T, et al. Filamin B plays a key role in VEGF-induced endothelial cell motility through its interaction with Rac-1 and Vav-2. J Biol Chem. 2010;285:10748–10760. doi: 10.1074/jbc.M109.062984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jeon YJ, Choi JS, Lee JY, Yu KR, Ka SH, Cho Y, et al. Filamin B serves as a molecular scaffold for type I interferon-induced c-Jun NH2-terminal kinase signaling pathway. Mol Biol Cell. 2008;19:5116–5130. doi: 10.1091/mbc.E08-06-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ueda K, Ohta Y, Hosoya H. The carboxy-terminal pleckstrin homology domain of ROCK interacts with filamin-A. Biochem Biophys Res Commun. 2003;301:886–890. doi: 10.1016/s0006-291x(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 117.Kwon M, Hanna E, Lorang D, He M, Quick JS, Adem A, et al. Functional characterization of filamin a interacting protein 1-like, a novel candidate for antivascular cancer therapy. Cancer Res. 2008;68:7332–7341. doi: 10.1158/0008-5472.CAN-08-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Johansen LD, Naumanen T, Knudsen A, Westerlund N, Gromova I, Junttila M, et al. IKAP localizes to membrane ruffles with filamin A and regulates actin cytoskeleton organization and cell migration. J Cell Sci. 2008;121:854–864. doi: 10.1242/jcs.013722. [DOI] [PubMed] [Google Scholar]
- 119.Pi M, Spurney RF, Tu Q, Hinson T, Quarles LD. Calcium-sensing receptor activation of rho involves filamin and rho-guanine nucleotide exchange factor. Endocrinology. 2002;143:3830–3838. doi: 10.1210/en.2002-220240. [DOI] [PubMed] [Google Scholar]
- 120.McDonough WS, Tran NL, Berens ME. Regulation of glioma cell migration by serine-phosphorylated P311. Neoplasia. 2005;7:862–872. doi: 10.1593/neo.05190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.