Abstract
When the brain is awake, neurons in the cerebral cortex fire irregularly and the electroencephalogram (EEG) displays low amplitude, high frequency fluctuations. After falling asleep, neurons start oscillating between ON periods, when they fire as during wake, and OFF periods, when they stop firing altogether, and the EEG displays high amplitude slow waves. But what happens to neuronal firing after a long period of wake? We show here in freely behaving rats that, after prolonged wake, cortical neurons can go briefly “OFF line” as they do in sleep, accompanied by slower waves in the local EEG. Strikingly, neurons often go OFF line in one cortical area and not in another. During these periods of “local sleep”, whose incidence increases with wake duration, rats appear awake, active, and display a wake EEG. However, they are progressively impaired in a sugar pellet reaching task. Thus, though both the EEG and behavior indicate wakefulness, local populations of neurons in the cortex may be falling asleep, with negative consequences on performance.
Keywords: slow wave sleep, slow oscillations, EEG, cerebral cortex, multi-unit recording, reaching task, sleep deprivation
Everybody knows the difference between sleep and wake. During wake, the eyes are usually open, animals move around, and they respond to their surroundings. During sleep eyes close, behavior stops, and animals fail to respond to stimuli. Studies of brain activity also show major changes between wake and non-rapid eye movement (NREM) sleep, which makes up ~80% of sleep. During wake neurons in the cerebral cortex fire irregularly, their membrane potential is tonically depolarized, and the EEG shows low voltage high frequency activity. During NREM sleep, due to a decrease in the level of neuromodulators, neurons become bistable: their membrane potential oscillates between a depolarized up state similar to wake, and a hyperpolarized down state during which they cease firing altogether 1. These slow oscillations occur in the range between 0.1 and 6 Hz and they are visible both in multiunit (MUA) activity (ON and OFF periods) and in the EEG (slow waves) 2.
However, we also know that by staying awake too long one becomes tired, and many studies have demonstrated attention lapses, frequent mistakes in various cognitive tasks, and poor judgment, even when we may not feel particularly sleepy 2,3. Moreover, the EEG shows some trace of the sleep/wake history: the longer one has been awake, the higher the spectral power in the slow wave range (SWA, 0.5–4 Hz) of the EEG in subsequent sleep 4, corresponding to larger and more frequent slow waves, and to more intense and synchronous neuronal activity 2. Also, local variations in cortical activity during wake are associated with local sleep changes and sleep-dependent increase in task performance5–7. These changes are reversed progressively in the course of sleep 4. The wake EEG also shows changes that reflect the duration of previous wake, with power increasing in the theta range (5–7 Hz) 8–10. Likewise, neuroimaging studies show blood flow and metabolic changes after sleep deprivation, with some brain regions undergoing decreases and other increases in activation 11. However, it is poorly understood how the underlying neuronal activity may be changing.
Neurons can go OFF-line during prolonged wake
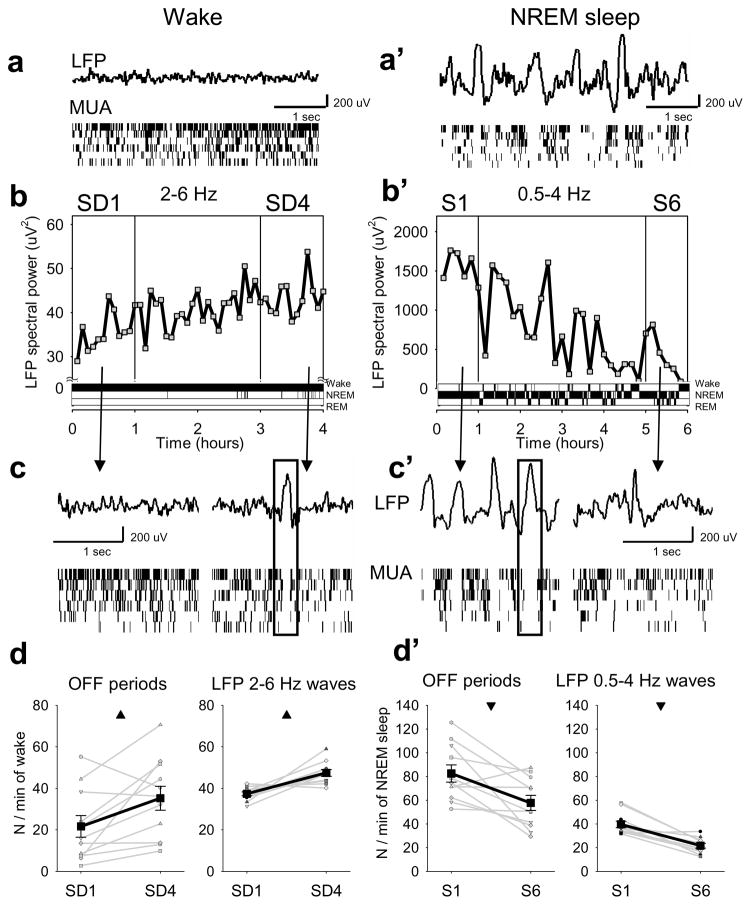
To investigate this question, we implanted a group of adult rats (n=11) with 16-channel microwire arrays in deep layers of the frontal motor cortex and recorded both local field potentials (LFP) and local MUA 2 across spontaneous sleep and wake (Supplementary Information). As expected, the wake LFP was characterized by low amplitude fast and theta waves, accompanied by irregular, tonic MUA, and was readily distinguishable from the LFP of NREM sleep, when high amplitude slow waves occurred concomitantly with synchronous ON and OFF periods at the level of MUA (Fig. 1a,a′).
Figure 1. OFF periods in sleep and wake.
a, a ′, LFP records from frontal cortex and raster plots of corresponding multiunit activity (MUA; 6 putative neurons, each vertical line is a spike). b, time course of wake LFP slow/theta power (2–6 Hz) for consecutive 5-min bins during 4h of sleep deprivation in one rat. b′, time course of LFP slow wave activity (0.5–4 Hz) plotted for consecutive 5-min bins during 6h of recovery after sleep deprivation in the same rat. Note different Y-axis scale in b and b′. Bottom: corresponding hypnograms. c, c′, LFP records in wake at the beginning (SD1) and end (SD4) of sleep deprivation, and in NREM sleep at the beginning (S1) and end (S6) of recovery. Bottom: corresponding MUA raster plots. d, d′, left, changes in OFF periods and 2–6 Hz waves in wake, and of OFF periods and 0.5–4 Hz waves in NREM sleep. Black lines: mean (SEM, n=11 rats); grey lines: individual rats. Triangles depict significant differences (wake, OFF periods: F (1,21)=7.03, p=0.024; 2–6 Hz LFP waves: F (1,21)=18.61, p=0.0015; NREM sleep, OFF periods: F (1,21)=10.40, p=0.009; 0.5–4 Hz LFP waves: F (1,21)=34.83, p=1.5069e-004, fixed-effects model ANOVA).
We then kept rats awake with novel objects for 4 hours starting at light onset 2. As expected, by the end of sleep deprivation the LFP showed an approximately 30% increase in spectral power in the slow/theta range between 2 and 6 Hz (Fig. 1b, Supplementary Fig. 1a). However, close inspection of the recordings revealed an occasional change in firing patterns (Fig. 1c): unlike at the beginning of sleep deprivation (SD1), towards the end (SD4) neuronal activity sporadically showed brief periods of silence, involving all or most of the recorded neurons. These short-lasting population OFF periods were often associated with slow/theta waves in the LFP. An opposite dynamic was observed during 6 hours of recovery sleep, when LFP showed a progressive decline of SWA (Fig. 1b′; Supplementary Fig. 1b). At the beginning of sleep (S1), large LFP slow waves were associated with synchronous ON-OFF oscillations in MUA (Fig. 1c′). At the end of recovery sleep (S6), large slow waves became infrequent and MUA became sparse and irregular. Thus, at the level of neuronal firing, wake under high sleep pressure occasionally resembles late NREM sleep, while low pressure sleep may occasionally resemble wake.
Crucially, we found that the number of OFF periods in wake increased significantly by 57.7±16.5% from SD1 to SD4 (Fig. 1d, left) suggesting that the tendency of neurons to enter a “sleep-like” mode increases with sleep pressure. The number of high amplitude LFP 2–6 Hz waves also increased significantly by 23.3±5.2% (Fig. 1d, right). The initial number of OFF periods or LFP 2–6 Hz waves during SD1 correlated negatively with their increase from SD1 to SD4 (OFF periods, R=−0.53, p<0.1, LFP waves, R=−0.87, p<0.0001), consistent with a saturating increase of sleep pressure4. Again, an opposite dynamic was apparent during recovery sleep: sleep OFF periods and high amplitude LFP slow waves decreased significantly by 36.9±11.2 and 59.5±9.0%, respectively, from S1 to S6 (Fig. 1d′). Of note, OFF periods, as defined here, and 2–6 Hz LFP waves were also observed during baseline spontaneous wake in all rats, but their frequency was lower than that observed during the first hour of sleep deprivation (OFF periods: 7.1±4.1/1 min, p=0.023; 2–6 Hz waves: 25.05±8.1, p=0.06). Thus, high sleep pressure is associated with increased tendency of neurons to go “OFF line” in both wake and sleep, and MUA OFF periods underlie the macroscopic changes in LFP low frequency power.
Wake OFF periods occur asynchronously in distant cortical regions
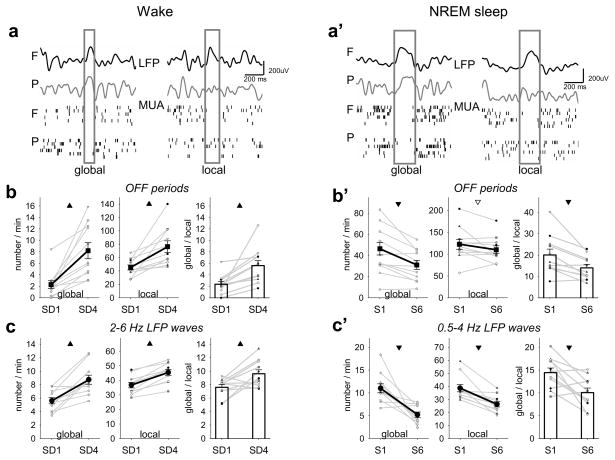
Sleep is usually thought of as a global behavior and a global cortical/EEG state 12, raising the question of whether OFF periods can be detected simultaneously in distant cortical areas. In several animals (n=9), we implanted an additional microwire array in the deep layers of parietal cortex. We found that OFF periods in awake rats were also present in parietal cortex (average duration: 79.02±7.7 ms, incidence: 37.51±6.16/1min) and, similar to the frontal OFF periods, their occurrence increased from SD1 to SD4 (56.6±19.5%, n=9, F(1,17)=6.23, p=0.041). Moreover, during sleep deprivation we found instances in which all recorded neurons in frontal and parietal areas underwent OFF periods near simultaneously, consistent with a global phenomenon (Fig. 2a, left). However, often neurons recorded in one cortical area showed an OFF period, whereas in the other area they stayed ON, as they normally do during wake (Fig. 2a, right). Further analysis revealed that most OFF periods were local, e.g. observed only in one cortical region at a time (frontal: 76.9±2.9%, parietal: 82.8±3.1%; frontal vs parietal: F(1,27)=4.6, p=0.0981). Importantly, both global and local OFF periods increased from SD1 to SD4 (Fig. 2b, left and middle), but the former more than the latter (Fig. 2b, right). Consistent with the MUA findings, most wake 2–6 Hz waves occurred exclusively in the LFP from one of the two areas, while the remaining waves were seen near-simultaneously in both areas. Both local 2–6 Hz waves, and those occurring simultaneously in frontal and parietal areas, became more frequent from SD1 to SD4, but the relative proportion of global waves increased during SD4 compared to SD1 (Fig. 2c), suggesting tha,t as sleep pressure builds up, neuronal activity in wake becomes more synchronized, as it does in sleep.
Figure 2. Local wake OFF periods.
a, top: wake LFP records in frontal (F) and parietal (P) cortex, depicting global or local frontal 2–6 Hz waves (boxed); bottom, raster plots of corresponding MUA. a′, top: LFP records in NREM sleep depicting global or local frontal slow waves (boxed); bottom: raster plots of corresponding MUA. b, left and middle: change in global and local OFF periods during sleep deprivation (mean, n=7 rats, 1–3 experiments/rat). Triangles here and in the next panels depict differences at a significant (filled) or tendency (open) level (global OFF periods: F(1,21)=94.95, p=0.0104; local OFF periods: F(1,21)=20.08, p=0.0464). Right: number of global OFF periods (as % of local, F+P) during SD1 and SD4 (F(1,21)=67.05, p=0.0146, fixed-effects model ANOVA). b′, left and middle: change in global and local OFF periods during NREM sleep (mean, n=7 rats, 1–3 experiments/rat; global OFF periods: F(1,21)=60.72, p=0.0161; local OFF periods: F(1,21)=11.56, p=0.0767). Right: number of global OFF periods (as % of local, F+P) during S1 and S6 (F(1,21)=99.17, p=0.0099, fixed-effects model ANOVA). Note different Y-axis scale in b and b′. c, left and middle: changes in global and local waves from SD1 to SD4 (mean, n=7 rats, 1–3 experiments per rat, SEM; global waves: F(1,21)=34.08, p=0.0281; local waves: F(1,21)=28.54, p=0.0333). Right: number of global waves (as % of local, F+P) during SD1 and SD4 (F(1,21)=52.53, p=0.0185, fixed-effects model ANOVA). c′, left and middle: changes in global and local waves during NREM sleep (mean, n=7 rats, 1–3 experiments/rat, SEM; global waves: F(1,21)=254.42, p=0.0039; local waves: F(1,21)=529.31, p=0.0019). Right: number of global waves (as % of local, F+P) during S1 and S6 (F(1,21)= 37.38, p=0.0253, fixed-effects model ANOVA). Note different Y-axis scale in c and c′.
Since in behaviorally awake animals during sleep deprivation distant brain areas can enter OFF periods independently, we asked next if nearby (~2 mm) neurons can also do so. We found that, even among units recorded with the same microelectrode array, a substantial fraction could stop firing together for up to hundreds of milliseconds, while the remaining neurons maintained their spiking activity at virtually unaltered or even elevated rates (Supplementary Fig. 2a). On average, while a subset of neurons ceased firing abruptly, the remaining neurons increased firing transiently with a ~20 ms delay and then slowed down slightly (~15%, Supplementary Fig. 2b). Controls by shuffling units between subsets indicated that these “hyper-local” OFF periods were unlikely to be an artifact of different firing rates of cortical neurons (Supplementary Information). Hyper-local OFF periods increased by almost 40% from SD1 to SD4, suggesting that they too are related to sleep pressure (Supplementary Fig. 2c).
Having found evidence for local OFF periods during wake, we then asked if sleep OFF periods could also be local. Previous evidence has shown that sleep can be regulated locally13, as demonstrated by a local increase in SWA after manipulations that affect plasticity during wake 12,13. Moreover, high-density EEG studies in humans combined with source localization 14, as well as modeling studies 15, have suggested that sleep slow waves with multiple peaks may result from the summation or interference of separate slow waves originating at different locations. Finally, recent depth recordings in humans have provided evidence that sleep slow waves and OFF periods can be local16. As shown in Fig. 2a′ (left), we found that, in rats, OFF periods during NREM sleep occurred not only synchronously at frontal and parietal areas (Fig. 2a′, left), but also locally, in which case they were associated with local slow waves in the LFP (Fig. 2a′, right). The incidence of both global and local OFF periods in NREM sleep decreased significantly from S1 to S6 (Fig. 2b′, left and middle), accompanied by a relative reduction of global slow waves (Fig. 2b′, right). Thus, just like wake 2–6 Hz waves became more global from SD1 to SD4 (Fig. 2b, right), sleep slow waves became more local from S1 to S6 (Fig. 2b′, right), suggesting that populations of neurons are more easily recruited into synchronous slow oscillations when sleep pressure is high than when it has dissipated 17.
Local OFF periods in wake lead to behavioral deficits
It is common experience that tiredness following prolonged sleep loss can be manifested in “microsleeps”, brief episodes (3–15 seconds) during which a person appears suddenly asleep (eyes closed or closing), may not respond to stimuli, and the EEG shows sleep-like activity 18. Clearly, such microsleeps can be dangerous during tasks requiring alertness, and the detection of sleep-like behavior or EEG changes is being pursued to reduce risks 19. However, careful observation of our rats, which were exposed to a relatively short period of sleep deprivation, did not reveal any indication of sleep: their eyes were open, they responded to stimuli, and their EEG was unambiguously a wake EEG (Fig. 1a, Supplementary Information, Supplementary Figs. 3,4). Moreover, a retrospective analysis of video recordings showed no behavioral signs of sleep specifically during MUA OFF periods.
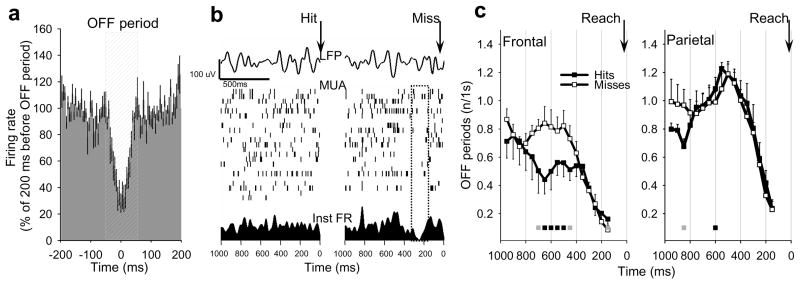
Given that the OFF periods we detected have no overt manifestations, are short-lasting, and are often local, can they have any impact on performance? To investigate potential consequences of neuronal “tiredness”, the rats were trained on a sugar pellet reaching task 13 for 2 hours between SD1 and SD4 (Supplementary Information). Learning the reaching task engages a circumscribed cortical area within the motor cortex, leads to local plasticity in wake 20, and to increased SWA during subsequent sleep 13,20. To investigate directly whether increased incidence of neuronal OFF periods leads to impaired performance, in a subset of animals (n=8) we performed simultaneous video and MUA recordings during the reaching attempts with high temporal resolution. Although the number of OFF periods decreased steadily towards each reaching attempt in both frontal and parietal cortex, possibly reflecting increased global arousal necessary for performing the reach, we found that the occurrence of an OFF period within several hundreds of ms before the reaching attempt was often associated with failure to successfully grasp a sugar pellet (Fig. 3a,b). Specifically, OFF periods occurred more frequently ~300–800 ms before an unsuccessful reaching attempt as compared to successful trials (Fig. 3c, left), and the probability of a successful reach decreased by 37.5% if there was at least one OFF period before the reach (OFF+: 26.1±6.3, OFF−: 41.8±4.1; F(1,11)= 15.6, p=0.01). Importantly, this effect was observed in frontal but not parietal cortex (Fig. 3c, right). We also found that the overall number of misses increased significantly across the training periods (p<0.05), and the behavior became progressively unstable. Thus, while at the beginning of the task hits and misses alternated regularly, as time progressed longer “clusters” of misses became more frequent and had increasingly variable duration (duration: F(2,32)= 4.69, p=0.021; variance: F(2,32)= 4.31, p=0.028, rANOVA). These results suggest that neuronal OFF periods and corresponding increases in low frequency LFP power may be associated with decreased behavioral performance as is typical of sleep deprived individuals.
Figure 3. Wake OFF periods affect performance.
a, average neuronal activity in frontal cortex triggered by the OFF periods (mean, SEM, n=6 rats; shown as % of mean firing rate in the last 200 ms before the OFF period). b, top: individual examples of frontal LFP records immediately preceding a successful or unsuccessful reaching attempts (Hit or Miss, arrows); middle, raster plots of corresponding MUA; bottom: instantaneous firing rates of the entire population (20 putative neurons). Note a generalized suppression of firing before Miss. c, number of OFF periods before Hits or Misses (frontal, n=6 rats; parietal, n=5 rats). Average values are plotted for consecutive overlapping 300ms windows with 50ms shifts against the midpoint of the corresponding window (e.g. the value at 500 ms depicts the number of OFF periods occurring between 350 and 650 ms). Squares show significant differences between Hits and Misses (grey: p<0.1, black: p<0.05, rANOVA).
Discussion
These findings reveal that, in animals kept awake beyond their normal sleep time, populations of neurons in different cortical areas can suddenly go ‘OFF line’ in a way that resembles the OFF periods of NREM sleep. The main differences are that during sleep virtually all cortical neurons show ON-OFF oscillations in the SWA frequency range, the EEG displays typical sleep slow waves and spindles, and the animal is behaviorally immobile, with eyes closed, and unresponsive. During prolonged wake, instead, only subsets of neurons enter OFF periods, usually for shorter durations, the EEG is typical of wakefulness, and the animal appears behaviorally awake, with eyes open, and responsive to stimuli. Also, the number of OFF periods increases with the duration of wakefulness, suggesting that the likelihood of subsets of neurons going OFF line in the context of an otherwise awake cortex increases with sleep pressure. As shown here, the progressive changes observed during sleep deprivation are the mirror-image of changes during recovery sleep: neuronal firing rates during ON periods, number and duration of OFF periods, number of neurons participating synchronously in the OFF periods, and low frequency content of the EEG, all increase during wake just as they decrease during sleep, in agreement with the concept of a homeostatic regulation of sleep need 4.
Perhaps the most striking result of this study is that, in the sleep deprived brain, subsets of neurons may enter an OFF period in one cortical area but not in another, and even within the same cortical area some neurons may be OFF while others are ON, seemingly in a piecemeal fashion. Based on this evidence, the wake behavior of a sleep deprived subject might be better characterized as a covert form of “dormiveglia” 21. Moreover, as shown here using a sugar pellet reaching task, the increasing occurrence of local OFF periods during prolonged wake was associated with worsening performance in the task. While paradigms should be developed to associate more precisely the occurrence of OFF periods in specific subsets of neurons with specific performance failures, these initial findings raise the intriguing possibility that “local sleep” in an awake brain may be responsible for cognitive impairments due to sleep deprivation or restriction3,4,9. It is especially relevant that cognitive impairments, including defective judgment and irritability 22, may occur despite an outward impression of full-fledged wakefulness, the lack of subjective insight 4, and a wake EEG. The sporadic occurrence of local neuronal OFF periods in sleep deprived subjects may be analogous to the sporadic occurrence of local hypersynchronous discharges in partial epilepsy. Such local events can be detected with careful EEG recordings as interictal spikes and may cause momentary lapses (“absence”) without overt behavioral signs 23.
One can only speculate about the mechanisms underlying the local wake OFF periods. A spontaneous slow oscillation of membrane potentials can occur in mouse barrel cortex during quiet wake 24 and affect the amplitude of evoked responses 13, although it is not clear whether such down states occur during active behavior, are local, affect performance and, critically, reflect increasing sleep pressure. While we do not know if the wake OFF periods we observed in freely moving rats are associated with neuronal hyperpolarization, their overall similarity to sleep OFF periods, and the finding that they become more frequent with increasing sleep pressure, suggests that they may be an expression of increasing neuronal bistability 25. Thus, in addition to the global state instability that is a hallmark of sleep deprivation 26, there can also be a local instability, at least in the cerebral cortex. A bistability between ON and OFF periods could be triggered by decreasing levels of arousal-promoting neuromodulators 27, especially since cho-linergic and noradrenergic neurons, for instance, do not always discharge in tight synchrony 30,31, and presynaptic release can be modulated locally 28,29.
Is local sleep in awake rats an adaptive or maladaptive response? In some cetaceans and birds, one hemisphere can remain awake while the other is in slow wave sleep, an adaptive response that permits them to continue swimming, flying, or monitoring the environment 30. The ability to actively control behavior with some neural circuits while others may be idling 31 could also be evolutionary advantageous. However, dissociated behavioral states, such as sleepwalking or REM sleep behavior disorder and other parasomnias, are clearly maladaptive 35,36. Since local wake OFF periods are associated with locally increased excitability after intensive training and with failures in performance, it is more likely that they represent a form of neuronal tiredness due to use-dependent factors, such as synaptic overload 32. A question for the future is whether local OFF periods during wake may also serve a functional role, from energy saving 37,38 to the initiation of a local restorative process.
METHODS SUMMARY
In male WKY rats, local field potentials (LFPs) and multiunit activity (MUA) were recorded from deep layers of frontal (n=11 rats) and/or parietal (n=9) cortex with 16-ch (2×8) polyimide-insulated tungsten microwire arrays. Rats were housed individually in transparent Plexiglas cages (light:dark 12:12, light on at 10am, 23±1°C; food and water ad libitum and replaced daily at 10am, except for the sugar pellet reaching task; see Supplementary Information). Animal protocols followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were in accordance with institutional guidelines.
Data acquisition and online spike sorting were performed with the Multichannel Neurophysiology Recording and Stimulation System (Tucker-Davis Technologies Inc., TDT). MUA was collected continuously (25 kHz, 300 Hz – 5 kHz), concomitantly with the LFPs from the same electrodes and epidural EEGs (both 256 Hz, 0.1–100 Hz). Amplitude thresholds for online spike detection were set manually and allowed only crossings of spikes below −25uV. LFP power spectra were computed by a Fast Fourier Transform (FFT) routine for 4-sec epochs (Hanning window, 0.25 Hz resolution). Sleep stages were scored off-line by visual inspection of 4-sec epochs, where the EEG, LFP, EMG and spike activity were displayed. Spike sorting was performed by PCA followed by SMEM clustering algorithm. Population OFF periods in wake and NREM sleep were defined as periods with suppressed or absent neuronal activity. Recordings were performed continuously for 2–3 weeks. In each animal 2–4 experiments (at least 5 days apart) with 4h of sleep deprivation were performed, one of which combined with the sugar pellet reaching task. For details about the analysis of firing rates and neuronal population OFF periods, see Supplementary Information.
Supplementary Material
Acknowledgments
Supported by NIMH P20 MH077967 (CC), NIH Director’s Pioneer award (GT) and AFOSR FA9550-08-1-0244 (GT). We thank Aaron Nelson, Mike Dash and Dr Ugo Faraguna for help with the experiments, Dr Lisa Krugner-Higby for advice about surgical procedures and Dr. Paolo Frumento for advice on statistical procedures.
Footnotes
COI statement: All authors indicated no financial conflicts of interest.
Author contribution
G.T. and C.C. conceived and directed the study, G.T., C.C. and V.V.V. designed the experiments and wrote the manuscript, V.V.V. and E.C.H. performed the experiments, V.V.V. and U.O performed data analysis, Y.N. contributed to experiments and writing.
References
- 1.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85(5):1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 2.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1(2):112–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 3.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 4.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. W. B. Saunders; Philadelphia: 2005. pp. 405–417. [Google Scholar]
- 5.Dang-Vu TT, et al. Functional neuroimaging insights into the physiology of human sleep. Sleep. 2010;33(12):1589–1603. doi: 10.1093/sleep/33.12.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 7.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430(6995):78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 8.Finelli LA, Baumann H, Borbely AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101(3):523–529. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 9.Vyazovskiy VV, Tobler I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res. 2005;1050(1–2):64–71. doi: 10.1016/j.brainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Leemburg S, et al. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1002570107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maquet P. Functional neuroimaging of normal human sleep by positron emission tomography. J Sleep Res. 2000;9(3):207–231. doi: 10.1046/j.1365-2869.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 12.Sejnowski TJ, Destexhe A. Why do we sleep? Brain Res. 2000;886(1–2):208–223. doi: 10.1016/s0006-8993(00)03007-9. [DOI] [PubMed] [Google Scholar]
- 13.Krueger JM, et al. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9(12):910–919. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riedner BA, et al. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep. 2007;30(12):1643–1657. doi: 10.1093/sleep/30.12.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esser SK, Hill SL, Tononi G. Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep. 2007;30(12):1617–1630. doi: 10.1093/sleep/30.12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nir Y, et al. Regional slow waves and spindles in human sleep. Neuron. doi: 10.1016/j.neuron.2011.02.043. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vyazovskiy VV, Faraguna U, Cirelli C, Tononi G. Triggering slow waves during NREM sleep in the rat by intracortical electrical stimulation: effects of sleep/wake history and background activity. J Neurophysiol. 2009;101(4):1921–1931. doi: 10.1152/jn.91157.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tirunahari VL, Zaidi SA, Sharma R, Skurnick J, Ashtyani H. Microsleep and sleepiness: a comparison of multiple sleep latency test and scoring of microsleep as a diagnostic test for excessive daytime sleepiness. Sleep Med. 2003;4(1):63–67. doi: 10.1016/s1389-9457(02)00250-2. [DOI] [PubMed] [Google Scholar]
- 19.Blaivas AJ, Patel R, Hom D, Antigua K, Ashtyani H. Quantifying microsleep to help assess subjective sleepiness. Sleep Med. 2007;8(2):156–159. doi: 10.1016/j.sleep.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290(5491):533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 21.Moruzzi G, editor. The functional significance of sleep with particular regard to the brain mechanisms underlying consciousness. Springer; Berlin: 1966. [Google Scholar]
- 22.Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 23.Ferrillo F, et al. Sleep-EEG modulation of interictal epileptiform discharges in adult partial epilepsy: a spectral analysis study. Clin Neurophysiol. 2000;111(5):916–923. doi: 10.1016/s1388-2457(00)00246-7. [DOI] [PubMed] [Google Scholar]
- 24.Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454(7206):881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- 25.Hill S, Tononi G. Modeling sleep and wakefulness in the thalamocortical system. J Neurophysiol. 2005;93(3):1671–1698. doi: 10.1152/jn.00915.2004. [DOI] [PubMed] [Google Scholar]
- 26.Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139(3):253–267. [PubMed] [Google Scholar]
- 27.Chauvette S, Volgushev M, Timofeev I. Origin of Active States in Local Neocortical Networks during Slow Sleep Oscillation. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laplante F, Morin Y, Quirion R, Vaucher E. Acetylcholine release is elicited in the visual cortex, but not in the prefrontal cortex, by patterned visual stimulation: a dual in vivo microdialysis study with functional correlates in the rat brain. Neuroscience. 2005;132(2):501–510. doi: 10.1016/j.neuroscience.2004.11.059. [DOI] [PubMed] [Google Scholar]
- 29.Marrocco RT, Lane RF, McClurkin JW, Blaha CD, Alkire MF. Release of cortical catecholamines by visual stimulation requires activity in thalamocortical afferents of monkey and cat. J Neurosci. 1987;7(9):2756–2767. doi: 10.1523/JNEUROSCI.07-09-02756.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6(8):e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pigarev IN, Nothdurft HC, Kastner S. Evidence for asynchronous development of sleep in cortical areas. Neuroreport. 1997;8(11):2557–2560. doi: 10.1097/00001756-199707280-00027. [DOI] [PubMed] [Google Scholar]
- 32.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.