Abstract
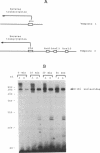
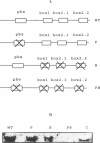
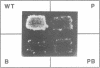
Reverse transcription of the yeast retrotransposon Ty1 is primed by the cytoplasmic initiator methionine tRNA (tRNA(iMet)). The primer tRNA(iMet) is packaged inside virus-like particles (VLPs) and binds to a 10 nucleotides minus-strand primer binding site, the (-)PBS, complementary to its 3' acceptor stem. We have found that three short sequences of the Ty1 RNA (box 1, box 2.1 and box 2.2) located 3' to the (-)PBS are complementary to other regions of the primer tRNA(iMet) (T psi C and DHU stems and loops). Reconstitution of reverse transcription in vitro with T7 transcribed Ty1 RNA species and tRNA(iMet) purified from yeast cells shows that the boxes do not affect the efficiency of reverse transcription. Thus the role of the boxes on packaging of the primer tRNA(iMet) into the VLPs was investigated by analysing the level of tRNA(iMet) packaged into mutant VLPs. Specific nucleotide changes in the (-)PBS or in the boxes that do not change the protein coding sequence but disrupt the complementarity with the primer tRNA(iMet) within the VLPs. We propose that base pairing between the primer tRNA(iMet) and the Ty1 RNA is of major importance for tRNA(iMet) packaging into the VLPs. Moreover the intactness of the boxes is essential for retrotransposition as shown by the transposition defect of a Ty1 element harboring an intact (-)PBS and mutated boxes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyar A., Cobrinik D., Ge Z., Kung H. J., Leis J. Interaction between retroviral U5 RNA and the T psi C loop of the tRNA(Trp) primer is required for efficient initiation of reverse transcription. J Virol. 1992 Apr;66(4):2464–2472. doi: 10.1128/jvi.66.4.2464-2472.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barat C., Lullien V., Schatz O., Keith G., Nugeyre M. T., Grüninger-Leitch F., Barré-Sinoussi F., LeGrice S. F., Darlix J. L. HIV-1 reverse transcriptase specifically interacts with the anticodon domain of its cognate primer tRNA. EMBO J. 1989 Nov;8(11):3279–3285. doi: 10.1002/j.1460-2075.1989.tb08488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barat C., Schatz O., Le Grice S., Darlix J. L. Analysis of the interactions of HIV1 replication primer tRNA(Lys,3) with nucleocapsid protein and reverse transcriptase. J Mol Biol. 1993 May 20;231(2):185–190. doi: 10.1006/jmbi.1993.1273. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Eichinger D., Castrillon D., Fink G. R. The Saccharomyces cerevisiae genome contains functional and nonfunctional copies of transposon Ty1. Mol Cell Biol. 1988 Apr;8(4):1432–1442. doi: 10.1128/mcb.8.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K. B., Byström A. S., Boeke J. D. Initiator methionine tRNA is essential for Ty1 transposition in yeast. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3236–3240. doi: 10.1073/pnas.89.8.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrès J., Keith G., Kuo K. C., Gehrke C. W. Presence of phosphorylated O-ribosyl-adenosine in T-psi-stem of yeast methionine initiator tRNA. Nucleic Acids Res. 1989 Feb 11;17(3):865–882. doi: 10.1093/nar/17.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichinger D. J., Boeke J. D. The DNA intermediate in yeast Ty1 element transposition copurifies with virus-like particles: cell-free Ty1 transposition. Cell. 1988 Sep 23;54(7):955–966. doi: 10.1016/0092-8674(88)90110-9. [DOI] [PubMed] [Google Scholar]
- Hansen L. J., Chalker D. L., Sandmeyer S. B. Ty3, a yeast retrotransposon associated with tRNA genes, has homology to animal retroviruses. Mol Cell Biol. 1988 Dec;8(12):5245–5256. doi: 10.1128/mcb.8.12.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isel C., Marquet R., Keith G., Ehresmann C., Ehresmann B. Modified nucleotides of tRNA(3Lys) modulate primer/template loop-loop interaction in the initiation complex of HIV-1 reverse transcription. J Biol Chem. 1993 Dec 5;268(34):25269–25272. [PubMed] [Google Scholar]
- Kohlstaedt L. A., Steitz T. A. Reverse transcriptase of human immunodeficiency virus can use either human tRNA(3Lys) or Escherichia coli tRNA(2Gln) as a primer in an in vitro primer-utilization assay. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9652–9656. doi: 10.1073/pnas.89.20.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. E., Goff S. P. Construction and analysis of deletion mutations in the U5 region of Moloney murine leukemia virus: effects on RNA packaging and reverse transcription. J Virol. 1989 Jan;63(1):319–327. doi: 10.1128/jvi.63.1.319-327.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., Haseltine W. A., Baltimore D., Peters G., Harada F., Dahlberg J. E. Specific binding of tryptophan transfer RNA to avian myeloblastosis virus RNA-dependent DNA polymerase (reverse transcriptase). Proc Natl Acad Sci U S A. 1975 Jul;72(7):2535–2539. doi: 10.1073/pnas.72.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. G., Hu J. Reverse transcriptase as the major determinant for selective packaging of tRNA's into Avian sarcoma virus particles. J Virol. 1980 Dec;36(3):692–700. doi: 10.1128/jvi.36.3.692-700.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochart P., Agoutin B., Fix C., Keith G., Heyman T. A very poorly expressed tRNA(Ser) is highly concentrated together with replication primer initiator tRNA(Met) in the yeast Ty1 virus-like particles. Nucleic Acids Res. 1993 Apr 11;21(7):1517–1521. doi: 10.1093/nar/21.7.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochart P., Agoutin B., Rousset S., Chanet R., Doroszkiewicz V., Heyman T. Biochemical and electron microscope analyses of the DNA reverse transcripts present in the virus-like particles of the yeast transposon Ty1. Identification of a second origin of Ty1DNA plus strand synthesis. Nucleic Acids Res. 1993 Jul 25;21(15):3513–3520. doi: 10.1093/nar/21.15.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarih-Cottin L., Bordier B., Musier-Forsyth K., Andreola M. L., Barr P. J., Litvak S. Preferential interaction of human immunodeficiency virus reverse transcriptase with two regions of primer tRNA(Lys) as evidenced by footprinting studies and inhibition with synthetic oligoribonucleotides. J Mol Biol. 1992 Jul 5;226(1):1–6. doi: 10.1016/0022-2836(92)90117-3. [DOI] [PubMed] [Google Scholar]
- Sawyer R. C., Hanafusa H. Comparison of the small RNAs of polymerase-deficient and polymerase-positive Rous sarcoma virus and another species of avian retrovirus. J Virol. 1979 Mar;29(3):863–871. doi: 10.1128/jvi.29.3.863-871.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucka R., Schwarzlose C., Lochmüller H., Häcker U., Feldmann H. Molecular analysis of the yeast Ty4 element: homology with Ty1, copia, and plant retrotransposons. Gene. 1992 Dec 1;122(1):119–128. doi: 10.1016/0378-1119(92)90039-r. [DOI] [PubMed] [Google Scholar]
- Varmus H. Retroviruses. Science. 1988 Jun 10;240(4858):1427–1435. doi: 10.1126/science.3287617. [DOI] [PubMed] [Google Scholar]
- Warmington J. R., Waring R. B., Newlon C. S., Indge K. J., Oliver S. G. Nucleotide sequence characterization of Ty 1-17, a class II transposon from yeast. Nucleic Acids Res. 1985 Sep 25;13(18):6679–6693. doi: 10.1093/nar/13.18.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Boeke J. D. High-frequency deletion between homologous sequences during retrotransposition of Ty elements in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8553–8557. doi: 10.1073/pnas.84.23.8553. [DOI] [PMC free article] [PubMed] [Google Scholar]