Abstract
Introduction
The effects of exogenous BNP post-myocardial infarction (MI) are not known. We tested the hypothesis that in-vivo infusion of BNP would improve cardiac function and affect left ventricular (LV) remodeling in an experimental model of MI.
Methods
MI was induced by LAD ligation in rats and confirmed by echocardiography. 19 rats were randomized to 1 of 3 groups: Sham; MI + saline; MI + BNP (400 ng/kg/min). Infusions were delivered for 7 days via venous catheters tunneled to an infusion pump. Rats were followed for 8 wks. Echocardiography, hemodynamics and in vivo and ex vivo pressure-volume relationships were then obtained.
Results
Rats with MI developed HF vs. Sham surgery rats, with a reduced fractional shortening (FS) and increased LV end-diastolic pressure. BNP attenuated the decline in FS vs. HF. LV systolic pressure, dP/dtmax, dP/dtmin, heart weight and myocyte size improved with BNP treatment compared to the control MI group. Serum BNP levels increased during BNP therapy, confirming delivery of BNP vs. other groups.
Conclusion
We demonstrate beneficial effects on LV function and decreased LV remodeling with BNP infusion in an experimental model of acute MI.
Keywords: natriuretic peptides, myocardial infarction, remodeling
Introduction
B-type (BNP), atrial natriuretic (ANP) and C-type natriuretic peptides function to regulate intravascular volume through effects on vascular tone and renal filtration. These peptides bind to guanylyl cyclase receptors found in smooth muscle cells in blood vessels, the adrenals, the kidneys, spleen, and the brain, but also in cardiomyocytes, cardiac fibroblasts and cardiac mesangial cells [1], producing cellular effects via cyclic GMP production. Expression of BNP has been found in the heart - BNP is secreted by ventricular myocytes in response to increased wall stress and pressure-volume overload [2–4]. In addition, BNP has been found to have important anti-proliferative properties that suggest a direct role in the function of myocytes, as well as the myocyte response to injury [5].
BNP levels have been widely utilized to assess chronic heart failure (HF) severity and predict long-term mortality [6]. Its application as a therapeutic agent has been established for the treatment of acute decompensated HF via natriuresis, via arterial and venous vasorelaxation, and via decreases in neurohormonal activation [7,8]. To date, little study has focused on the use of BNP immediately following myocardial ischemia to prevent ventricular remodeling or the development of HF. Recent studies suggest that BNP may show promise for this indication; results from the NAPA trial have expanded the use of BNP to the post-coronary bypass patients, who were found to have improved serum creatinine, greater urine output, and lower 28-day mortality [9]. Similarly, ANP has been given to patients as a 24 hour infusion post-percutaneous reperfusion after myocardial infarction, producing significant favorable changes in ejection fraction and ventricular size at one month [10]. ANP differs in structure and function from BNP, however, and recombinant ANP is not approved for use in most countries, except Japan. Accordingly, the primary objective of this study was to evaluate the long-term effects of recombinant BNP infusion on ventricular function, hemodynamics, and myocardial histology in a rat model of acute myocardial infarction (AMI) and ischemic HF.
Methods
Study Design
A well-established experimental rat model of AMI and ischemic HF described previously [11] utilizing left anterior descending coronary artery (LAD) ligation, was employed to create acute ischemia. Rats were randomized to Sham surgery (n=7), heart failure (HF) (PBS solution) (n=5), or recombinant rat BNP (400 ng/kg/min, Scios Inc., Fremont, CA) (n=7). LAD ligation in the HF and BNP groups was followed by infusion pump insertion in all rats, and, 60 minutes after ligation, by continuous intravenous PBS or BNP infusion for 7 days. Rats were then followed for a total of 8 weeks. Echocardiographic studies were conducted at baseline (1 hour post-LAD ligation but prior to BNP infusion) and at 8 weeks, while invasive hemodynamics, in-vivo and ex-vivo left ventricular (LV) pressure-volume relationships and myocardial tissue harvest were obtained at 8 weeks (study termination).
Studies were performed in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication 85-23, revised 1996) and were approved by the Institutional Animal Care and Use Committee of Columbia University.
Animal Model and Surgical Procedures
Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 250 to 300 g were used for all experiments. After induction with intraperitoneal ketamine (75 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (5 mg/kg; Lloyd Laboratories, Shenandoah, IA), endotracheal intubation with an angiocathether was performed. Rats were supported by a small animal ventilator (Harvard Apparatus, Holliston, MA). After performing a left thoracotomy, a sham operation (pericardiectomy only) or LAD ligation was performed in the HF and BNP rats, as previously described [11]. After coronary ligation, the wounds were closed, and the animals were recovered. A baseline echo was performed, the infusion pump was inserted, and infusion of PBS or BNP was subsequently begun 60 minutes after completion of LAD ligation for the rats randomized to the HF or BNP treatment groups.
At study conclusion at week 8, rats were again anesthetized lightly after intubation with the use of inhaled isoflurane anesthesia (Abbott Laboratories, Abbott Park, IL). A 2 Fr volume conductance catheter (Millar Instruments, Houston, TX) was advanced from the aorta into the LV for continuous pressure-volume tracings immediately following right carotid artery cannulation. Upon completion of hemodynamic measurements, the heart was excised and the LV was weighed and sectioned for histology.
BNP Administration
A continuous intravenous BNP infusion was chosen as the mode of administration, similar to current clinical use in humans. Under light isofluorane anesthesia, the right internal jugular vein was exposed, distally ligated, and proximally cannulated using a polyethylene catheter that was passed to the superior vena cava and right atrial junction. This catheter was tunneled to the back of the animal subjects. The catheter were then connected to disposable continuous infusion pumps (Infu-Disk pumps, Med-e-cell, San Diego, CA), which were attached to the back of the rats using commercially-available protective jackets (Infu-jacket, Med-e-cell). All animal groups underwent catheter placement and received recombinant rat BNP (400 ng/kg/min, Scios Inc) or PBS solution infusion (Sham and HF groups) for a total of 7 days. New infusion pumps containing freshly prepared BNP or PBS were connected to the indwelling catheters on day 4, in order to ensure the stability of the BNP solutions. The rat BNP was generously provided by Scios Inc. and freshly dissolved in PBS solution on the day of infusion pump loading. Delivery was confirmed by measuring serum BNP levels during infusion (data not shown).
Echocardiographic Studies
Trans-thoracic echocardiographic studies (Sonos 5500, 12 MHz transducer) were performed on lightly anesthetized animals (2% Isoflurane) in a left lateral decubitus position by an observer blinded to the study group. From the cardiac short axis (papillary level), an M-mode trace of the LV was obtained, and LV end-diastolic diameter (LVEDD), end-systolic diameter (LVESD), and LV end-diastolic (LVDa) and end-systolic areas (LVSa) were measured. Fractional shortening (FS) and fractional area change (FAC) were subsequently calculated.
Hemodynamics and In-Vivo LV Pressure-Volume Relationship Analysis
Hemodynamic determinations were made on all rats at study termination. LV pressure (end-systolic (LVSP), end-diastolic (LVEDP)) and LV volume were measured using the Millar conductance catheter placed into the LV across the aortic valve, and aortic pressures were measured after withdrawal of the catheter into the aorta. End-systolic volume (ESV) and end-diastolic volume (EDV) were measured using conductance catheter standard techniques, normalized to relative volume units (RVU). LV pressure-volume relationship loops were measured at rest (steady-state) and during a transient preload reduction induced by inferior vena cava occlusion, as previously described [12]. The end-systolic pressure-volume relationship (ESPVR) and end-diastolic pressure-volume relationship (EDPVR) was calculated as previously described [12]. Ejection fraction (EF), cardiac output (CO), arterial elastance (Ea), stroke work (SW), and preload-adjusted maximum power (PAMP) were computed using a pressure-volume analysis program (PVAN v. 3.2, Millar Instruments, Houston, TX). The time constant of LV isovolemic pressure relaxation, τ, was calculated using the logarithmic method described by Raff and Glantz.
Ex-vivo EDPVR and Stiffness Calculation
Ex-vivo passive LV pressure-volume relationships were measured as previously described [11]. Briefly, LV pressures were measured using a 5-F Millar micromanometer as volume was infused into the balloon (located in the LV) in 0.050 μl increments until reaching an LV pressure of 40 mmHg.
Chamber stiffness constant (α), normalized to heart weight, was calculated according to the equation described by Mirsky: P = β*e[α*(V/Vm), where Vm is LV myocardial volume (=0.943·LV weight), where α is a chamber stiffness constant, and β is a scaling constant.
Histologic Studies
Anterior LV wall samples were obtained post ex-vivo measurements for histology [11]. Samples were placed in a 2% paraformaldehyde fixative and then in 75% ethanol. These samples were subsequently stained for trichrome and hematoxylin & eosin. In addition, cross-sections of the LV at maximal infarction were taken from each rat in order to quantify infarct size. Histologic analysis was performed by a blinded researcher. Myocyte size (average of 10 cells measured at the level of the nucleus) at 20X, percent collagen deposition of 4 representative sections at 10X, and infarct size (percentage of LV infarct area to total LV area) were analyzed for each rat using digital software (Image Pro Plus, v. 4.5.1, Silver Spring, MD).
Statistical Analysis
Continuous variables are expressed as Mean ± standard error and compared using two-way analysis of variance with post-hoc Bonferroni correction. Comparisons of echocardiographic measurements between pre- and post-treatment timepoints were performed with the use of repeated measures analysis of variance with the group and timepoint as fixed factors. For all analyses, a p-value of less than 0.05 was considered statistically significant. All analysis was performed using SPSS software (v. 11.5, Chicago, Ill).
Results
Echocardiography
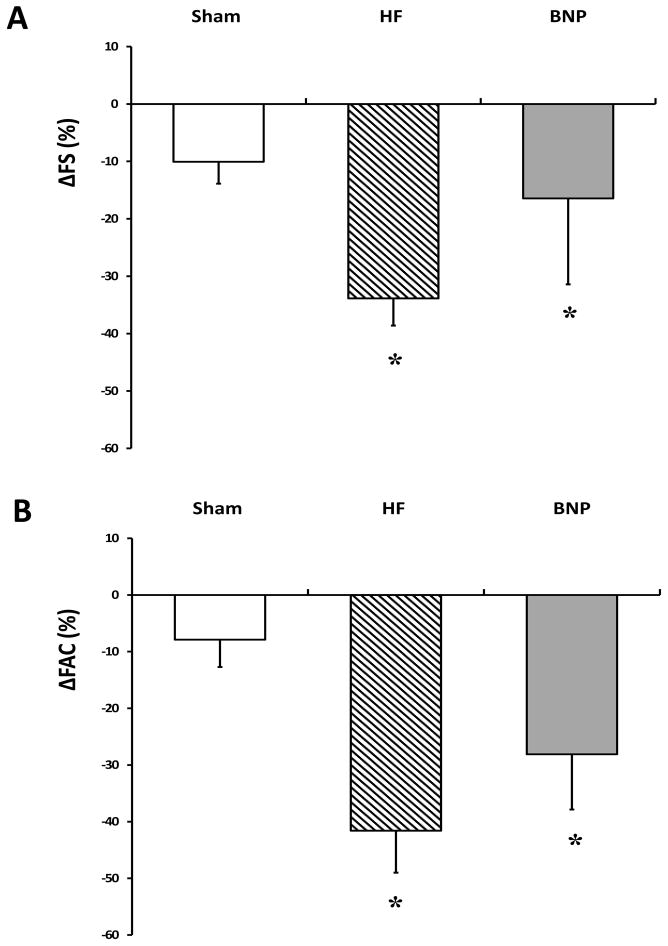
The presence of AMI and effectiveness of LAD ligation was confirmed in all HF and BNP-treated rats undergoing coronary ligation based on baseline echocardiography - FS was significantly and equally reduced in these animals when compared to Sham rats (Figure 1 Panel A). FAC was also significantly decreased after coronary ligation, further confirming the validity and reliability of the experimental heart failure model (Figure 1 Panel B). Although statistical significance was not reached, both FS and FAC trended slightly towards improvement in BNP in comparison to HF rats. Ventricular dimensions, although an insensitive measure in small animals, were comparable across groups, although trends toward a decrease in BNP and increases in HF rats were seen that may have reached significance with a larger sample size (Table 1).
Figure 1.
Echocardiographic Changes. Changes in fractional shortening (FS) (Panel A) and fractional area change (FAC) (Panel B) from Baseline to study termination were less severe in BNP rats, although all groups experienced a significant decline versus Sham rats. (* p<0.05 vs. Sham)
Table 1.
Echocardiography
| Sham (n=7) | HF (n=5) | BNP (n=7) | |
|---|---|---|---|
| LVEDD (mm) | 8.6 ± 0.2 | 10.2 ± 0.3* | 10.4 ± 0.4** |
| LVESD (mm) | 4.4 ± 0.3 | 8.3 ± 0.2*** | 7.7 ± 0.7*** |
| LVDa (mm2) | 562 ± 16 | 876 ± 63** | 838 ± 54** |
| LVSa (mm2) | 237 ±15 | 639 ± 47*** | 566 ± 66*** |
p<0.05 vs Sham, ** p<0.01 vs Sham, *** p<0.001 vs Sham,
HF-Heart Failure, LVEDD-Left Ventricular End-Diastolic Diameter, LVESD-Left Ventricular End-Systolic Diameter, LVDa-Left Ventricular Diastolic Area, LVSa-Left Ventricular Systolic Area
Hemodynamic Measurements
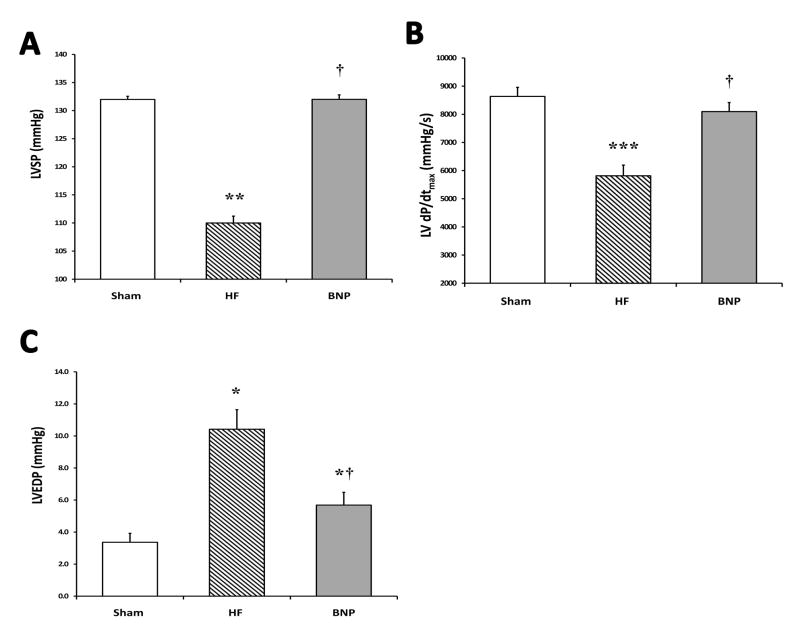
A summary of terminal hemodynamics is shown in Table 2. Systolic dysfunction was evident in HF rats as dP/dtmax was reduced by 33% versus Sham (p<0.01), as well as a reduction in LVSP (p<0.05). In contrast, rats treated with BNP maintained LVSP, comparable to Sham rats, and a preserved dP/dtmax was apparent (p<0.05 vs. HF) (Figures 2 Panels A–C). The pressure-volume overload state was apparent in HF rats, who demonstrated a significant increase in LVEDP, but was reduced in BNP rats (p<0.05 vs. HF) (Figure 2 Panel D). No overall differences in HR were seen among groups. Although not statistically significant, a trend towards preservation of CO (conductance catheter-based calculations) was apparent in BNP treated rats (p=0.2 vs. HF).
Table 2.
Hemodynamic Measurements
| Sham (n=7) | HF (n=5) | BNP (n=7) | |
|---|---|---|---|
| HR (bpm) | 304 ± 10 | 301 ± 20 | 327 ± 21 |
| ESV (RVU) | 15.1 ± 1.5 | 15.3 ± 1.3 | 14.8 ± 1.5 |
| EDV (RVU) | 19.5 ± 1.5 | 17.4 ± 1.3 | 18.8 ± 2.3 |
| SV (RVU) | 5.4 ± 1.0 | 4.1 ± 0.8 | 5.5 ± 2.1 |
| EF (%) | 26.7 ± 4.3 | 21.7 ± 4.2 | 25.6 ± 6.6 |
| CO (RVU/min) | 1622 ± 331 | 1242 ± 235 | 1631 ± 446 |
HF-Heart Failure, HR-Heart Rate, LVEDP-Left Ventricular End-Diastolic Pressure, ESV-End-Systolic Volume, EDV-End-Diastolic Volume, SV-Stroke Volume, EF-Ejection Fraction, CO-Cardiac Output, RVU-Relative Volume Units
Figure 2.
Terminal Hemodynamics. Panel A shows a significant reduction in left ventricular systolic pressure (LVSP) in HF rats versus Sham, and preservation in BNP rats in comparison to HF rats. A significant improvement in dP/dtmax was seen in rats treated with BNP, in contrast to the HF group (Panel B). Finally, in Panel C, left ventricular end-diastolic pressure (LVEDP) was elevated in HF rats versus BNP rats. (* p<0.05 vs. Sham, ** p<0.01 vs. Sham, *** p<0.001 vs. Sham, † p<0.05 vs. HF)
In-Vivo Pressure Volume Relationship and Analysis
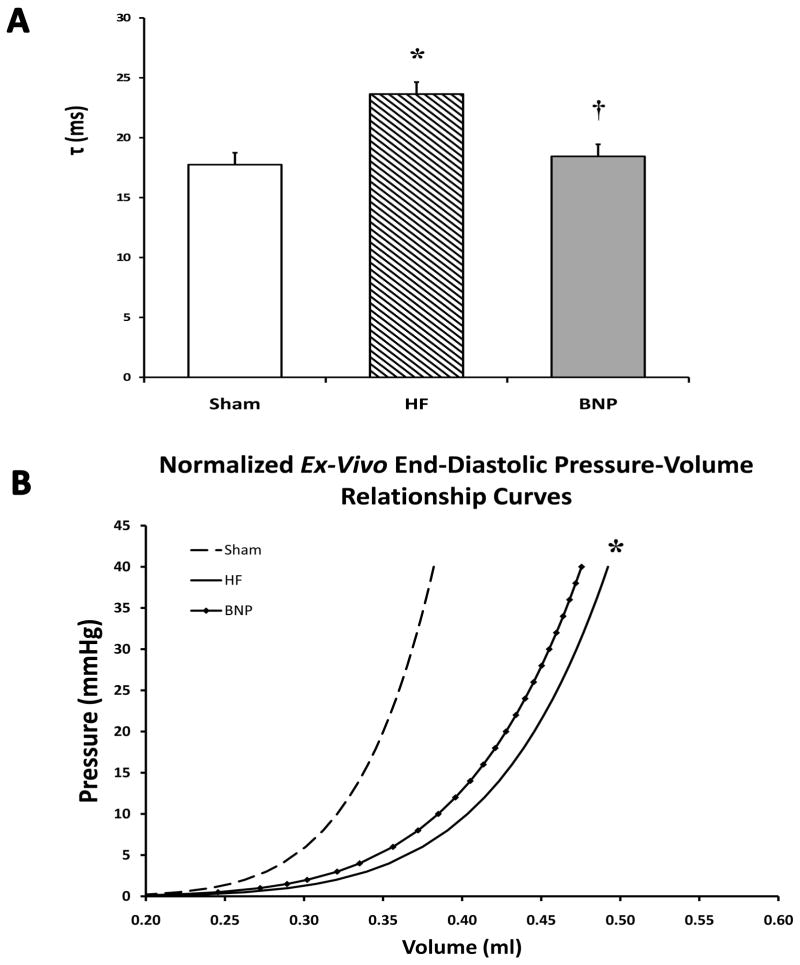
No changes in SW, PAMP, or Ea were seen among treatment groups (Table 3) on steady-state, in-vivo pressure-volume analysis. Significant increases were found only in ventricular isovolemic relaxation (τ) in HF rats, indicative of passive diastolic dysfunction. However, isovolemic relaxation was significantly reduced in BNP rats vs HF rats, and comparable to Sham rats (p<0.05 vs. HF) (Figure 3 Panel A). Data from end-systolic pressure-volume relationships are shown in Table 3. No significant change in end-systolic elastance (Ees) was seen.
Table 3.
End-Systolic and End-Diastolic Pressure-Volume Relationship and Analysis
| Sham (n=7) | HF (n=5) | BNP (n=7) | |
|---|---|---|---|
| In-Vivo ESPVR | |||
| Ees (mmHg/ml) | 9.53 ± 1.26 | 8.12 ± 3.14 | 21.09 ± 11.01 |
| V0 (ml) | 3.00 ± 2.30 | −50.06 ± 56.34* | 4.77 ± 4.26 |
| SW (mmHg*RVU) | 498 ± 108 | 295 ± 235 | 379 ± 144 |
| PAMP (mWatts/RVU2) | 56.0 ± 9.8 | 52.4 ± 24.1 | 52.1 ± 12.2 |
| Ea (mmHg/RVU) | 28.1 ± 5.9 | 32.9 ± 9.8 | 33.3 ± 8.3 |
| Ex-Vivo EDPVR | |||
| α (unitless) | 37.3 ± 4.70 | 25.5 ± 2.0 | 27.5 ± 4.0 |
| β (mmHg) | 0 | 0 | 0 |
| V30 (ml) | 0.51 ± 0.04 | 0.85 ± 0.11* | 0.77 ± 0.07 |
p<0.05 vs Sham, ** p<0.01 vs Sham
ESPVR-End-Systolic Pressure-Volume Relationship, EDPVR-End-Diastolic Pressure-Volume Relationship, HF-Heart Failure, Ees-End-systolic Elastance, V0-Volume Intercept, SW-Stroke Work, PAMP-Preload-Adjusted Maximum Power, Ea-Arterial Elastance, Ea-Arterial Elastance, RVU-Relative Volume Unit, α-Myocardial Stiffness, β-Stiffness Constant, V30-Volume at Pressure 30 mmHg
Figure 3.
Pressure-Volume Analysis. In Panel A, τ, the isovolemic ventricular relaxation constant, was significantly lower in the BNP group vs HF rats and comparable to Sham rats. In Panel B, ex-vivo EDPVR curves demonstrate an attenuated shift to the right after BNP therapy vs. Sham. HF rats had a slightly greater shift to the right, representative of worsened diastolic properties, that was also confirmed on in-vivo pressure-volume analysis. (* p<0.05 vs. Sham, † p<0.05 vs HF)
Ex-Vivo End-Diastolic Pressure Volume Relationship
Ex-vivo end-diastolic pressure-volume curves for each study group are displayed in Fig 3 Panel B. Ex-vivo pressure-volume curves from HF rats show a larger shift to the right when compared to BNP rats (p<0.05), representative of improved ventricular geometry and reverse remodeling in BNP rats. This is in contrast to end-stage ventricular dilatation apparent in the curves of HF rats. The slope of the all curves remained stable, indicating minimal change in ventricular compliance, despite BNP therapy. This finding was confirmed on quantitative analysis, as V30 was increased in HF rats (p<0.05) (i.e. increased ventricular size for a given pressure), but no changes were seen in α (myocardial stiffness constant) compared to Sham (Table 3).
Body and Heart Weight
Changes in heart weight to body weight ratio are an important measure of degree of volume overload and failure. In the current study, rats treated with BNP (0.35 ± 0.05) and Sham rats (0.30 ± 0.02) were found to have significantly lower LV/body weight when compared to HF rats (0.38 ± 0.06) (both p<0.05). Lung weights in BNP and Sham rats were also lower than HF, possibly consistent with less pulmonary edema (all p<0.05).
Histology
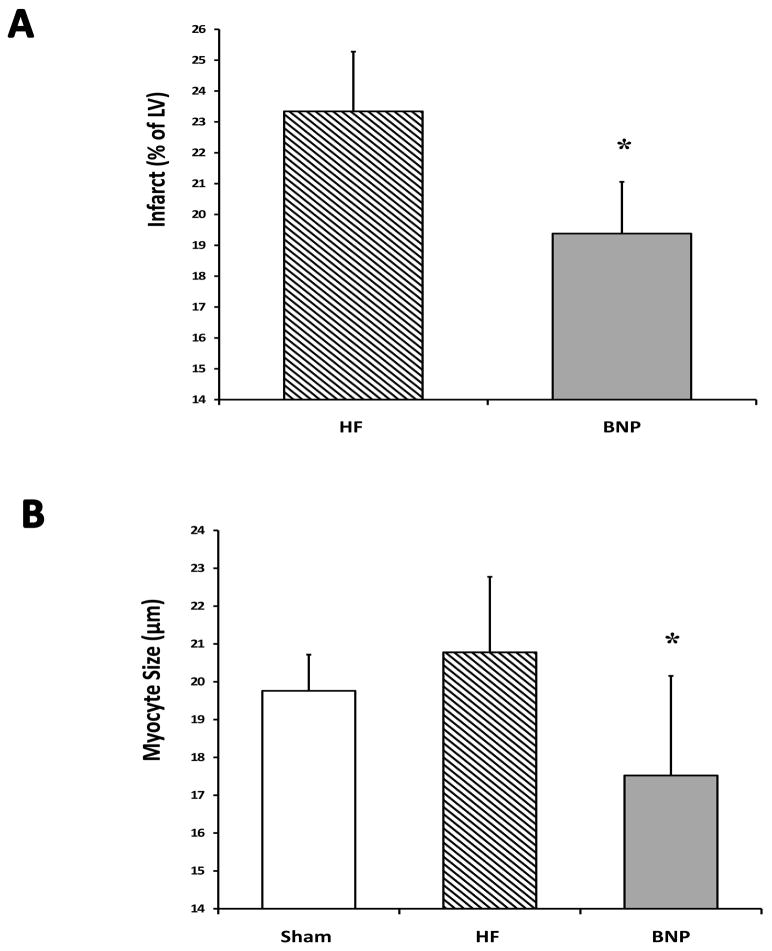
Moderate-sized myocardial infarction was confirmed on trichome staining of LV tissues samples on all rats undergoing coronary ligation. BNP therapy was associated with significantly less LV infarction (as a percentage of LV) when compared with HF rats (p<0.05) (Figure 4 Panel A). Myocyte size was also significantly less in BNP treated rats when compared with HF rats (p<0.05) (Panel B). No changes in collagen deposition were seen.
Figure 4.
Histologic Changes. Representative left ventricular samples (LV) are shown in Panel A from Sham, Heart Failure (HF), and BNP-treated groups. BNP was associated with lower levels of LV infarction, as a percentage of total LV (Panel A) and reduced myocyte size after therapy (Panel B). (* p<0.05 vs. HF)
Discussion
Despite improvements in reperfusion strategies for AMI, complications secondary to prolonged ischemia and infarction pose considerable risk and mortality due to the development of congestive heart failure, with a one year mortality of up to 33% in patients with HF [13]. The process of ventricular remodeling, a series of progressive pathologic changes consisting of contractile dysfunction with hemodynamic derangement, organ hypoperfusion, ventricular dilatation, and neurohormonal activation, occurs frequently after AMI, even with timely reperfusion. The combination of natriuretic, lusiotropic, and anti-fibrotic properties makes BNP a potentially attractive drug in the AMI setting to attenuate or even reverse ventricular remodeling and impede progression of the HF state. In our study, administration of BNP was evaluated in a well-established rat model of AMI and ischemic HF, and doses given reflect higher doses than typically given in human use to maximize benefit. A BNP infusion time 1 hour after the start of ischemia was chosen as an optimal timing for this experiment to produce maximal benefit within a realistic treatment timepoint, as over 30% of patients suffering an AMI have symptom onset to hospital presentation times under 2 hours [14]. Observation was continued for a total of 8 weeks after AMI and 7 weeks post-drug infusion to study the long-term effects of BNP on cardiac remodeling. The primary findings of the study were: 1) moderate heart failure was induced by coronary ligation after 8 weeks; 2) systolic contractile dysfunction, as measured by dP/dtmax, ventricular pressures, and echocardiography, was lessened in rats treated with BNP; 3) ex-vivo diastolic function curves indicated attenuated diastolic injury in BNP rats; and 4) ventricular remodeling was partially reversed after BNP therapy as seen by a smaller myocyte size and lower heart weight.
The hemodynamic effects of BNP have been well-documented. When given to human subjects, BNP infusion lowers mean arterial pressure [15], decreases systemic vascular resistance [16] increases stroke volume index [17,18], and greatly reduces pulmonary capillary wedge pressure [19]. Studies in dogs with pacing-induced heart failure also suggest the ability to increase cardiac output after BNP infusion [20]. In the current study, therapy with BNP immediately after AMI was associated with moderate improvements in systolic function on long-term follow-up. The contractile function of this group was equal to Sham by most invasive hemodynamic measures, although this was not confirmed echocardiographically. BNP rat hearts generated higher systolic, mean pressures and LV dP/dtmax, and signs of ventricular failure, such as pulmonary edema (evidenced by lung weight), elevated LVEDP, and excessive myocyte hypertrophy, were absent. A number of explanations for these findings exist. First, volume unloading, secondary to BNP natriuretic properties, can serve to unload the left ventricle, thus reducing wall stress, myocardial consumption [21], and thus improve systolic function over time. Additionally, BNP reduces levels of catecholamines implicated in sympathetic stimulation of pathologic compensatory mechanisms [22]. The inhibition of norepinephrine, aldosterone, renin, angiotensin II, and endothelin supplies strong support for the rationale that BNP can affect ventricular function after ischemic injury [23]. Finally, we found slightly smaller infarct sizes on histology after BNP, similar to previous investigators, although its effects may have been modest [24]. The cytoprotective properties of natriuretic peptides in after ischemic injury requires further investigation to clarify specific cellular mechanisms involved, which have not yet been elucidated.
Diastolic function was likewise improved – ex-vivo EDPVR curves of BNP rats demonstrated less stiff and less dilated hearts when compared to HF, and shorter isovolemic relaxation, τ, allowed greater diastolic filling per cardiac cycle. This can partially be explained due to volume unloading, but may also be due to intrinsic effects of natriuretic peptides on lusiotropy. Natriuretic peptides increase cGMP by binding to natriuretic peptide A (NPRA) – antagonism of these receptors by a synthetic compound greatly slowed diastolic ventricular relaxation in dogs with chronic heart failure, whereas BNP has the opposite effect [25]. These changes in isovolemic relaxation are relatively load independent and are not confounded by diuretic effects of BNP.
Reverse remodeling was evident by the end of the study in BNP rats, which was expected given the in-vitro effects of BNP. Rat LV mass and heart to body ratios were lower after BNP treatment, accompanied by favorable changes in myocyte size and infarct size. The potent anti-proliferative properties of BNP may ultimately be the most important clinically in the context of this study. Despite being a 7-day infusion after AMI, BNP effectively produced cellular changes in myocardium that persisted after 8 weeks. The biologic basis for these changes lies in cGMP activity in regulation and growth of cardiac tissue. BNP secreted by cardiac fibroblasts after AMI inhibits de novo collagen synthesis, and expression of MMP-1, -2, and -3 increases in response to BNP [26]. Mice with genetic disruption of natriuretic peptide receptors display cardiac hypertrophy, fibrosis, hypertension, and have increased expression of TGFβ1, TGFβ3, and collagen 1 [27]. Excessive fibrosis is seen with pressure-volume overload in mice lacking the BNP gene, as well [2]. BNP given to cultured cardiac fibroblasts inhibited TGF-b induced cell proliferation, production of collagen-1 and fibronectin proteins, and opposed genes related to fibrosis [5]. These mechanisms may also be involved in limiting scar formation in infarcted tissue. These anti-proliferative mechanisms highlight the importance of addressing the acute stress state after injury that propagates ventricular remodeling. In our study, BNP, even for a short infusion period, halted the cycle of worsening hemodynamics, ventricular hypertrophy, and thus, compensatory pathway activation, such as the renin-angiotensin or endothelin systems, that ultimately lead to end-stage HF.
The limitations of echocardiography in the rat model were apparent in our experiment – echocardiographic results, although trending towards improvement in BNP rats, failed to reach significance likely due to its imprecision and inaccuracy in small animals. Echocardiography was used primarily to grossly confirm the presence of HF after coronary ligation, while much more highly sensitive invasive measurements were taken to fully quantify myocardial function at study termination.
In conclusion, a one-week infusion of BNP following AMI altered the development of ischemic HF in rats. Treatment with BNP was associated with lessened myocardial injury, and both systolic and diastolic dysfunction associated with pressure-volume overload was reduced. Changes in cardiac function were accompanied with histologic evidence of reverse remodeling, suggesting a role for BNP in the treatment of acute myocardial infarction.
Table 4.
Heart and Body Weight
| Sham (n=7) | HF (n=5) | BNP (n=7) | |
|---|---|---|---|
| LV Weight (g) | 1.37 ± 0.09 | 1.76 ± 0.21* | 1.68 ± 0.16* |
| Body Weight (g) | 452 ± 39 | 466 ± 52 | 482 ± 21 |
| LV/Body Weight Ratio | 0.30 ± 0.02 | 0.38 ± 0.06* | 0.35 ± 0.05 |
| Lung Weight (g) | 1.68 ± 0.19 | 2.84 ± 1.27* | 1.99 ± 0.68 |
LV-Left Ventricle
p<0.05 vs Sham
Acknowledgments
This research was supported by an unrestricted research grant from Scios, Inc. (Fremont, CA).
Funding: This research was supported by an unrestricted research grant from Scios, Inc. (Fremont, CA).
References
- 1.Lincoln TM, Cornwell TL. Intracellular cyclic GMP receptor proteins. FASEB. 1993;7:328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- 2.Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, Itoh H, Saito Y, Tanaka I, Otani H, Katsuki M. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Nat Acad Sci USA. 2000;97:4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lattion AL, Michel JB, Arnauld E, Corvol P, Soubrier F. Myocardial recruitment during ANF mRNA increase with volume overload in the rat. Am J Physiol. 1986;251:H890–H896. doi: 10.1152/ajpheart.1986.251.5.H890. [DOI] [PubMed] [Google Scholar]
- 4.Saito Y, Nakao K, Arai H, Nishimura K, Okumura K, Obata K, Takemura G, Fujiwara H, Sugawara A, Yamada T, Itoh H, Mukoyama M, Hosoda K, Kawai C, Ban T, Yasue H, Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama H, Itoh H, Saito Y, Tanaka I, Otani H, Katsuki M. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci USA. 2000;97:4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapoun AM, Liang F, O’Young G, Damm DL, Quon D, White RT, Munson K, Lam A, Schreiner GF, Protter AA. B-Type Natriuretic Peptide Exerts Broad Functional Opposition to Transforming Growth Factor-β in Primary Human Cardiac Fibroblasts. Circ Res. 2004;94:453–461. doi: 10.1161/01.RES.0000117070.86556.9F. [DOI] [PubMed] [Google Scholar]
- 6.Troughton RW, Prior DL, Pereirra JJ, Martin M, Fogarty A, Morehead A, Yandle TG, Richards AM, Starling RC, Young JB, Thomas JD, Klein AL. Plasma B-Type Natriuretic Peptide Levels in Systolic Heart Failure. J Am Coll Cardiol. 2004;43:416–422. doi: 10.1016/j.jacc.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 7.Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD, Wagoner LE, Givertz MM, Liang GS, Neibaur M, Haught WH, LeJemtel TH. Intravenous Nesiritide, a Natriuretic Peptide, in the Treatment of Decompensated Congestive Heart Failure. N Eng J Med. 2000;343:246–53. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- 8.Fonarow GC. B-Type Natriuretic Peptide: Spectrum of Application. Nesiritide (Recombinant BNP) for Heart Failure. Heart Fail Rev. 2003;8:321–325. doi: 10.1023/a:1026170228469. [DOI] [PubMed] [Google Scholar]
- 9.Mentzer RM, Oz MC, Sladen RN, Graeve AH, Hebeler RF, Luber JM, Smedira NG. Effects of Perioperative Nesiritide in Patients with Left Ventricular Dysfunction Undergoing Cardiac Surgery. J Am Coll Cardiol. 2007;49:716–26. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi M, Tsutamoto T, Wada A, Maeda K, Mabuchi N, Tsutsui T, Horie H, Ohnishi M, Kinoshita M. Intravenous Atrial Natriuretic Peptide Prevents Left Ventricular Remodeling in Patients with First Anterior Acute Myocardial Infarction. J Am Coll Cardiol. 2001;37:1820–6. doi: 10.1016/s0735-1097(01)01233-5. [DOI] [PubMed] [Google Scholar]
- 11.Xydas S, Kherani AR, Chang JS, Klotz S, Hay I, Mutrie CJ, Moss GW, Gu A, Schulman AR, Gao D, Hu D, Wu EX, Wei C, Oz MC, Wang J. beta(2)-Adrenergic stimulation attenuates left ventricular remodeling, decreases apoptosis, and improves calcium homeostasis in a rodent model of ischemic cardiomyopathy. J Pharm Exp Therap. 2006;317:553–556. doi: 10.1124/jpet.105.099432. [DOI] [PubMed] [Google Scholar]
- 12.He KL, Yi GH, Sherman W, Zhou H, Zhang GP, Gu A, Kao R, Haimes HB, Harvey J, Roos E, White D, Taylor DA, Wang J, Burkhoff D. Autologous skeletal myoblast transplantation improved hemodynamics and left ventricular function in chronic heart failure dogs. J Heart Lung Transplant. 2005;24:1940–9. doi: 10.1016/j.healun.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 13.Jong P, Vowinckel E, Liu PP, Gong Y, Tu JV. Prognosis and determinants of survival in patients newly hospitalized for heart failure: a population-based study. Arch Int Med. 2002;162(15):1689–94. doi: 10.1001/archinte.162.15.1689. [DOI] [PubMed] [Google Scholar]
- 14.Zijlstra F, Patel A, Jones M, Grines CL, Ellis S, Garcia E, Grinfeld L, Gibbons RJ, Ribeiro EE, Ribichini F, Granger C, Akhras F, Weaver WD, Simes RJ. Clinical characteristics and outcomes of patients with early (<2h), intermediate (2–4h), and late (>4h) presentation treated by primary coronary angioplasty or thrombolytic therapy for acute myocardial infarction. Eur Heart J. 2002;23:550–557. doi: 10.1053/euhj.2001.2901. [DOI] [PubMed] [Google Scholar]
- 15.Van der Zander K, Houben AJHM, Hofstra L, Kroon AA, de Leeuw PW. Hemodynamic and renal effects of low-dose brain natriuretic peptide infusion in humans: a randomized, placebo-controlled crossover study. Am J Physiol Heart Circ Physiol. 2003;285:H1206–H1212. doi: 10.1152/ajpheart.00085.2003. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura M, Yasue H, Morita E, Sakaino N, Jougasaki M, Kurose M, Mukoyama M, Saito Y, Nakao K, Imura H. Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure. Circulation. 1991;84:1581–1588. doi: 10.1161/01.cir.84.4.1581. [DOI] [PubMed] [Google Scholar]
- 17.Marcus LS, Hart D, Packer M, Yushak M, Medina N, Danziger RS, Heitjan DF, Katz SD. Hemodynamic and renal excretory effects of human brain natriuretic peptide infusion in patients with congestive heart failure. A double-blind, placebo-controlled, randomized crossover trial. Circulation. 1996;94(12):3184–9. doi: 10.1161/01.cir.94.12.3184. [DOI] [PubMed] [Google Scholar]
- 18.Yasue H, Yoshimura M. Natriuretic peptides in the treatment of heart failure. J Card Failure. 1996;2:S277–S285. doi: 10.1016/s1071-9164(96)80088-1. [DOI] [PubMed] [Google Scholar]
- 19.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Eng J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 20.Chen HH, Grantham A, Schirger JA, Jougasaki M, Redfield MM, Burnett JC. Subcutaneous Administration of Brain Natriuretic Peptide in Experimental Heart Failure. J Am Coll Cardiol. 2000;36:1706–12. doi: 10.1016/s0735-1097(00)00911-6. [DOI] [PubMed] [Google Scholar]
- 21.Michaels AD, Klein A, Madden JA, Chatterjee K. Effects of Intravenous Nesiritide on Human Coronary Vasomotor Regulation and Myocardial Oxygen Uptake. Circulation. 2003;107:2697–2701. doi: 10.1161/01.CIR.0000070547.88378.EA. [DOI] [PubMed] [Google Scholar]
- 22.Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitiz RL, LeJemtel TH, Cheng ML, Wynne J ADHERE Scientific Advisory and Investigators ADHERE Study Group. In-Hospital Mortality in Patients with Acute Decompensated Heart Failure Requiring Intravenous Vasoactive Medications. J Am Coll Cardiol. 2005;46:57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 23.Kohno M, Yokokawa K, Horio T, Yasunari K, Maurakawa K, Takeda T. Atrial and brain natriuretic peptides inhibit the endothelin-1 secretory response to angiotensin II in porcine aorta. Circ Res. 1992;70:241–7. doi: 10.1161/01.res.70.2.241. [DOI] [PubMed] [Google Scholar]
- 24.D’Souza SP, Yellon DM, Martin C, Schulz R, Heusch G, Onody A, Ferdinandy P, Baxter GF. B-type natriuretic peptide limits infarct size in rat isolated hearts via KATP channel opening. Am J Physiol Heart Circ Physiol. 2003;284(5):H1592–600. doi: 10.1152/ajpheart.00902.2002. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto K, Burnett JC, Jr, Redfield MM. Effect of the endogenous natriuretic peptide system on ventricular and coronary function in the failing canine heart. Am J Physiol. 1997;273:H2406–14. doi: 10.1152/ajpheart.1997.273.5.H2406. [DOI] [PubMed] [Google Scholar]
- 26.Tsuruda T, Boerrigter G, Huntley BK, Noser JA, Cataliotti A, Costello-Boerrigter LC, Chen HH, Burnett JC., Jr Brain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circulation Research. 2002;91(12):1127–34. doi: 10.1161/01.res.0000046234.73401.70. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Kishimoto I, Saito Y, Harada M, Kuwahara K, Izumi T, Takahashi N, Kawakami R, Tanimoto K, Nakagawa Y, Nakanishi M, Adachi Y, Garbers DL, Fukamizu A, Nakao K. Guanylyl cyclase-A inhibits angiontensin II type 1A receptor-mediated cardiac remodeling, an endogenous protective mechanism in the heart. Circulation. 2002;106:1722–1728. doi: 10.1161/01.cir.0000029923.57048.61. [DOI] [PubMed] [Google Scholar]