Abstract
Background
With adoption of Model for End-stage Liver Disease (MELD), the number of simultaneous liver-kidney transplants (SLK) has greatly increased. A recent registry study questioned the equity of allocating kidney transplants (KTx) simultaneously with liver transplantation due to poor outcomes (1).
Methods
To investigate outcome of KTx in SLK, all SLK (n=36) performed at our center from 1/2000–12/2007 were reviewed and KTx outcomes compared to those of kidney transplant alone (KTA) performed during that period (n=1,283). We also reviewed whether pre-transplant panel reactive antibody (PRA) and donor specific antibody (DSA) affected KTx outcome in SLK.
Results
One- and three-year KTx and patient survival were not different between KTA and SLK regardless of sensitization level. There were 348 (27%) KTx failures in KTA vs. 6 (17%) in SLK (NS). Overall freedom from acute cellular rejection (ACR) and antibody-mediated rejection (AMR) in SLK was 93% and 96% at 3 years, compared to 72% and 78% in KTA (p=0.0105 and p=0.0744, respectively). Sensitized KTx recipients had more ACR and AMR (32% and 38%) at three years compared to non-sensitized recipients (28% and 20%) (p=0.23 and 0.0001, respectively). No differences in ACR and AMR were observed when SLK was divided and level of sensitization compared (p=0.17 and 0.65, respectively).
Conclusion
SLK is a life-saving procedure with excellent patient and graft survival. AMR incidence in the KTx appears reduced in SLK compared to KTA regardless of level of preoperative PRA. A high level of DSA should not preclude simultaneous transplantation when clinically indicated.
Keywords: Simultaneous liver-kidney transplantation, kidney transplant outcomes, acute cellular rejection, antibody-mediated rejection
INTRODUCTION
Initially described as a curative operation for patients with inborn errors of metabolism leading to multi-organ failure, simultaneous liver-kidney transplantation (SLK) has been expanded to patients with end-stage liver disease secondary to non-metabolic causes with concomitant renal failure. The adoption of the Model for End-Stage Liver Disease (MELD) system in 2002 and the relative weight of serum creatinine over INR and bilirubin in the MELD calculation have led to an increase in SLK transplantation. Indeed, the incidence of SLK has increased 400% since the adoption of MELD (1).
Recent data have asserted that the outcomes of kidney transplants (KTx) in SLK recipients are inferior to those of patients undergoing kidney transplantation alone (KTA), raising concerns that the current MELD allocation system may disadvantage patients with end-stage kidney disease (ESKD) on the waiting list (1). Although reduced outcomes may be the result of patient selection bias and increased perioperative risk, the impact of immunological factors such as HLA sensitization on the inferior outcomes of KTx in SLK recipients is not known.
In this retrospective single-center study, we sought to determine the outcomes of KTx in SLK recipients and to compare them to those of 1,283 KTA recipients. Additionally, we sought to determine whether pre-transplant panel reactive antibody (PRA) and donor-specific antibody (DSA) affected the outcomes of both renal and liver grafts of SLK recipients, and whether there was any level of pre-transplant sensitization which would contraindicate SLK.
METHODS
An IRB-approved retrospective review was performed utilizing the University of Wisconsin transplant database from 1/1/2000 to 12/31/2007 comparing SLK (n=36) to KTA (n=1,283). All patients had at least two months’ follow-up and a negative complement-dependent cytotoxicity (CDC) assay crossmatch at transplantation. Pediatric recipients were excluded.
Various induction agents, the choice of which was left to the surgeon of record, were used in the KTA cohort. These included alemtuzumab, basiliximab and rabbit anti-thymocyte globulin. All SLK patients received IL2R inhibitor induction in the form of basiliximab, two doses at day 0 and day 4. Most SLK and KTA recipients were maintained on triple drug immunosuppression consisting of prednisone, mycophenolate mofetil, and a calcineurin inhibitor.
Initial antiviral therapy consisted of high-dose acyclovir 800 mg 4 times daily for 12 weeks for low-risk patients. Ganciclovir (Cytovene®, Hoffmann-La Roche Inc., Nutley, NJ), 500–1,000 mg 3 times daily for 12 weeks, was given to high-risk patients. Since 2001, valganciclovir (Valcyte®, Hoffmann-La Roche Inc., Nutley, NJ), 450 mg twice per day for 12 weeks, has replaced ganciclovir in high-risk patients. High-risk patients include CMV-negative recipients of CMV-positive organs and patients being treated for rejection (additional 12 weeks of ganciclovir or valganciclovir according to the era). The drugs were administered intravenously while the patient was not tolerating enteral feedings and converted to oral once the nasogastric tube was removed.
PATIENT POPULATION
Two groups of patients were reviewed: all SLK and all KTA performed at the University of Wisconsin Abdominal Transplant Program between 1/1/2000 and 12/31/2007. Each group was later divided into non-sensitized and sensitized SLK and KTA recipients. Pre-transplant PRA by CDC assay were used as cutoff criteria to define subgroups in regard to level of pre-sensitization. Sensitized cohort is defined as those groups with a pre-transplant PRA >20%. In order to amplify the levels of characterization against the specific donor, determination against HLA antigens was quantified by the presence of detectable DSA in the immediate pre-transplant sera as measured by single antigen bead Luminex® flow cytometry or by a Class I or Class II PRA ≥10% by the same solid phase technique. Patients not meeting the above criteria were considered to be non-sensitized. Patients in both groups were transplanted during the same study period and had at least one year of clinical follow-up. Incidence of acute cellular rejection (ACR), antibody-mediated rejection (AMR) and graft and patient survival of this subpopulation was studied. All patients in this cohort underwent testing of their immediate pre-transplant sera for DSA using Luminex® flow cytometry as described below.
DONOR SPECIFIC ANTIBODY TESTING
Pre-transplant specimens used for cell cytotoxicity crossmatching were analyzed for HLA antibodies using single-antigen bead Luminex®-flow cytometry (LabScreen® Single Antigen Class I and Single Antigen Class II, One Lambda, Inc, Canoga Park, CA). The method was followed as described in the package insert, with the exception of the local implementation of the assay using 3μL instead of 5μL of HLA antigen-coated beads. The resultant Luminex® output files were analyzed with HLA Visual® analysis software (One Lambda, Inc, Canoga Park, CA), and the strength of DSA was measured as normalized mean fluorescence intensity (MFI) of beads representing antigens mismatched to donor antigens. Pre-transplant sera was available for Luminex® analysis in 176 KTA recipients and in 25 SLK recipients.
For the purpose of this study, pre-transplant recipient sera was tested for DSA to HLA Class 1-A and B-locus and Class 2-DR, DRB3, 4, 5(DR51, DR52, and DR53) and DQ-locus. DSA to HLA-Cw and DPB1-locus were tested but not analyzed as the donor HLA tissue typing for these loci is not routinely performed in our laboratory.
DIAGNOSIS OF ACUTE REJECTION
Acute Rejection of Kidney Allografts
All SLK and KTA recipients with an unexplained increase in serum creatinine ≥25% underwent an indication biopsy. No protocol biopsies were performed in the study population.
Fresh core kidney biopsies were fixed in 10% neutral buffered formalin for at least 1½ hours for paraffin embedding. Two- to three-micron thickness sections were obtained and four serial sections per biopsy were stained with hematoxylin-eosin, Masson’s Trichrome, periodic acid shift (PAS), and argyrophilic impregnations (PAMM). Biopsies were interpreted for adequacy and rejection scores according to the Banff 97 criteria (2;3). Immuno-labeling for the complement fraction C4d was performed on four micron acetone-fixed frozen sections and labeled with a 1:600 dilution of an anti-C4d monocloncal antibody (Biogenesis, Kingston, NH). C4d results were interpreted as negative, minimally positive (1–10% of peritubular capillaries), focally positive (10–50% of peritubular capillaries), and diffusely positive (more than 50% of peritubular capillaries labeled) (2; 3).
Acute AMR was diagnosed in patients meeting the following four criteria: 1) allograft dysfunction; 2) evidence of allograft injury [acute tubular necrosis (ATN), peritubular capillaritis and/or vascular fibrinoid necrosis]; 3) peritubular capillaries diffusely positive for C4d; and 4) evidence of circulating DSA. ACR was diagnosed when the kidney biopsy showed evidence of interstitial mononuclear inflammation along with tubulitis and/or endarteritis.
Biopsies were also evaluated for evidence of chronic allograft injury. Transplant glomerulopathy was diagnosed when at least 10% of glomerular capillary walls of the most affected glomeruli showed duplication of capillary walls. Chronic allograft vasculopathy was diagnosed by concentric fibrointimal proliferation of arteries, and trichrome and silver-stained sections were evaluated for the degree of interstitial fibrosis and tubular atrophy. Chronic active AMR was diagnosed when biopsies with chronic changes also showed C4d positive peritubular capillaries. Similarly, chronic active cellular rejection was diagnosed when biopsies showing chronic changes also revealed evidence of ongoing/active mononuclear inflammation of tubulointerstitium or arteries.
Acute Rejection of Liver Allografts
All SLK patients with elevated canalicular enzymes (GGT or alkaline phosphatase) or evidence of transaminitis underwent ultrasound with deep Doppler interrogation of hepatic artery and portal vein as well as a core needle biopsy using ultrasound guidance.
Fresh core liver biopsies were fixed in 10% neutral buffered formalin for at least 1½ hours for paraffin embedding. Two- to three-micron thickness sections were obtained and four serial sections per biopsy were stained with hematoxylin-eosin, Masson’s Trichrome, PAS, and PAMM. Biopsies were interpreted for adequacy and rejection. Immuno-labeling for the complement fraction C4d was performed on four micron acetone-fixed frozen sections and labeled with a 1:600 dilution of an anti-C4d monoclonal antibody (Biogenesis, Kingston, NH). C4d results were interpreted as negative, minimally positive (1–10% of peritubular capillaries), focally positive (10–50% of peritubular capillaries) and diffusely positive (more than 50% of peritubular capillaries labeled) (3).
STATISTICAL ANALYSIS
Recipient and donor characteristics, renal and liver graft and patient outcomes, graft survival, incidence of acute rejection, AMR and ACR were compared. Subgroup analysis was performed comparing SLK patients with pre-transplant DSA (n=5) vs. KTA with DSA (n=163). The incidence of ACR or AMR and graft outcomes were compared between the two groups. Continuous variables were summarized by reporting means and standard deviations and compared between groups using a Wilcoxon-rank sums test. Discrete variables were summarized by reporting percentages and compared between groups using a Fisher’s exact test. Estimated rates of graft survival, patient survival, freedom from acute rejection, freedom from biopsy-proven rejection, and freedom from AMR were based on the methods of Kaplan and Meier and compared between groups using a log-rank test. P-values <0.05 were considered significant. All analyses were performed using SAS statistical software version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
Outcomes of KTx In SLK Versus KTA Alone Recipients
Thirty-six SLK patients were compared to 1,283 KTA recipients. There were no significant differences in the donor (Table 1) or recipient (Table 2) characteristics except the presence of end-stage liver disease in the SLK cohort (Table 1). There was no objective evidence of liver disease in the KTA group. The etiology of renal disease in the SLK cohort was unknown, though patients were not considered for SLK unless they had a pre-existing diagnosis of renal disease or had >8 weeks of dialysis prior to transplantation. The decision to dual list was discussed in a multidisciplinary forum and input from Transplant Nephrology was mandatory. The KTA cohort had the usual indications for KTx (Table 1). In addition, pre-transplant MFI for DSA for class I and II differed between KTA recipients with PRA <20 (50.4±1,375 and 916±2,644, respectively) when compared to KTA with a PRA >20 (1,981.6±2,959 and 5,842.8±6,739, respectively) (p=0.006 for Class I and 0.001 for Class II). Similarly, DSA for Class I and II is significantly lower in SLK recipients with a PRA <20 (1,661±3,647 and 1,364.3±3,196, respectively) when compared to SLK recipients with PRA >20 (6,305.8±5,848 and 8,452±6,374, respectively) (p=0.015 for Class I and 0.0045 for Class II).
Table 1.
Donor Characteristics
| SLK (n=36) | KTA (n=1,283) | p-value | |
|---|---|---|---|
| Gender: | |||
| Male | 19 (53%) | 762 (60%) | 0.395 |
| Female | 17 (47%) | 512 (40%) | 0.39 |
| Ethnicity: | |||
| Caucasian | 30 (83.3%) | 1,195 (93%) | |
| African American | 1 (2.77%) | 41 (4%) | |
| Hispanic | 0 | 18 (1%) | |
| Other | 5 (13.8%) | 29 (2%) | |
| Average donor age (years) | 46±14 | 43.5±17 | 0.49 |
| Donor type: | |||
| SCD | 24 (72%) | 724 (56%) | 0.14 |
| DCD | 2 (5%) | 218 (17%) | |
| ECD | 10 (23%) | 284 (22%) | |
| DCD/ECD | 0 | 57 (4%) | |
| CMV: | |||
| Positive | 11 (31%) | 723 (48%) | 0.13 |
| Negative | 16 (44%) | 554 (37%) | |
| Unknown | 9 (25%) | 6 (0.04%) | |
| Donor creatinine at time of recovery | 1.0± 0.3 (0.6–2.1) | 1.0± 0.53 (range 0.1–3.7) | 0.88 |
Table 2.
Recipient Characteristics
| SLK (n=36) | KTA (n=1,283) | p- value | |
|---|---|---|---|
| Gender: | |||
| Male | 19 (53%) | 772 (60%) | 0.39 |
| Female | 17 (47%) | 511 (40%) | 0.39 |
| Age at transplant (yrs) | 52±13 | 51±13 | 0.33 |
| Weight at transplant (kg) | 85±19 | 80±20 | 0.12 |
| Ethnicity: | |||
| Caucasian | 31 (86%) | 1,006 (79%) | 0.31 |
| African American | 2 (5.5%) | 155 (12%) | |
| Native American | 2 (5.5%) | 27 (2%) | |
| Asian | 1 (3%) | 81 (6%) | |
| Other | 0 | 14 (1%) | |
| MELD | 27± 3.5 | N/A | |
| Cause of kidney failure: | |||
| Chronic glomerulonephritis | 0 | 50 (4%) | |
| Calcineurin toxicity | 2 (6%) | 20 (2%) | |
| Diabetes mellitus | 4 (11%) | 323 (25%) | |
| Unknown | 5 (14%) | 117 (9%) | |
| Focal glomerulonephritis | 0 | 88 (7%) | |
| Hepatorenal syndrome | 10 (3%) | 1 (0.08%) | |
| Hypertension | 3 (8%) | 156 (12%) | |
| Primary IgA nephropathy | 2 (6%) | 49 (4%) | |
| Interstitial nephritis | 0 | 20 (2%) | |
| Membranous glomerulonephritis | 0 | 18 (1%) | |
| Mesangial proliferative glomerulonephritis | 2 (6%) | 11 (0.9%) | |
| Polycystic kidney disease | 1 (3%) | 149 (12%) | |
| Systemic lupus erythematosus | 0 | 46 (4%) | |
| CMV: | |||
| Positive | 26 (72%) | 752 (59%) | 0.13 |
| Negative | 10 (28%) | 517 (40%) | |
| Unknown | 0 | 14 (1%) | |
No statistically significant differences were noted between KTA and SLK recipients with a PRA <20% pre-transplant when pre-transplant MFI for DSA for Class I and Class II were compared (p=0.41 for Class I and 0.07 for Class II). Only the pre-transplant MFI for DSA for Class I was statistically significantly greater for the SLK group, with a PRA >20 when compared to the KTA subgroup with a PRA >20 (p=0.047) (Table 3).
Table 3.
Pre-Transplant Mean Fluorescent Intensity for KTA and SLK.
| Mean Fluorescent Intensity |
|||
|---|---|---|---|
| Class I | Class II | ||
| KTA (n=176) | PRA <20% (n=153) | 504.4±1,375.7 | 916±2,644 |
| PRA >20% (n=23) | 1,981±2,959.4 | 5,842.8±6,739.9 | |
| SLK (n=25) | PRA <20% (n=20) | 1,661±3,647 | 1,364.3±3,196 |
| PRA >20% (n=5) | 6,305.8±5,858 | 8,452±6,374 | |
| KTA | PRA <20 vs. PRA >20 | P<0.006 | P<0.0001 |
| SLK | PRA <20 vs. PRA >20 | P<0.015 | P<0.0045 |
| KTA vs. SLK | PRA <20 | P<0.41 | P<0.067 |
| KTA vs. SLK | PRA >20 | P<0.047 | P<0.307 |
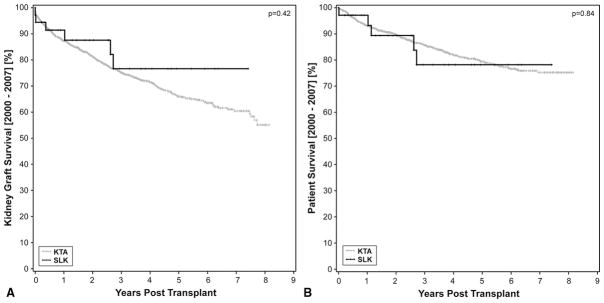
The kidney graft survival in the KTA group at 1, 3, 5 and 7 years was 87.2%, 75.1%, 65.9% and 60%, respectively. In contrast the kidney graft survival in the SLK group was 91% at 1 year and 76% at 3,5 and 7 years. (Figure 1A). Similarly, one-year patient survival was not significantly different between the two groups (97% for SLK vs. 93%, p>0.84) (Figure 1B). There were 348 (27%) KTx failures in the KTA group compared to 6 (17%) in the SLK group. Of the six patient deaths, four were death with a functioning kidney transplant graft. The SLK recipients tended to have more chronic medical conditions and have a worse Eastern Cooperative Oncology Group (ECOG) performance status than KTA recipients. The average MELD of SLK was 27±3.5 vs. 22.6 for liver transplantation alone (LTA) recipients performed during the same time period. In short, SLK recipients were the sickest of the liver recipients and had significantly more comorbidities than KTA recipients. Thirty-five out of 36 SLK recipients had undergone at least eight weeks of dialysis prior to transplantation.
Figure 1.
A) Kaplan-Meier survival curves for SLK (gray) versus deceased donor renal transplant (black) shows no significant differences in renal graft outcome at 1 and 3 years (91% v. 87% and 75% v. 77%, p=0.42). There was a 27% graft loss in the KTA cohort over the course of the study v. 17% in the SLK cohort.
B) Patient survival of SLK (gray) and deceased donor renal transplant recipients (black) is not significantly different between both cohorts (93% v. 97%, p=0.84).
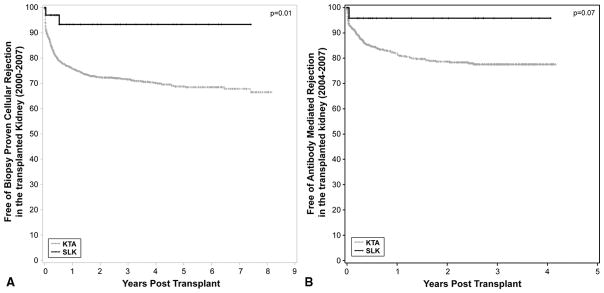
Immunologically, all KTA were performed with a negative CDC crossmatch and 1,120 of the KTA recipients had PRA <20%. There were no episodes of hyperacute rejection of either allograft regardless of crossmatch status. The SLK cohort had significantly more freedom from biopsy-proven acute rejection when compared to KTA (93% vs. 76%, p=0.01) (Figure 2A) at one year. Freedom from AMR in the SLK cohort was 96% vs. 78% in the KTA cohort (p<0.07) at three years (Figure 2B). The small patient sample size is one of the limitations of this study. The data clearly trends toward a significant difference in AMR incidence, but more patients will need to be transplanted before a significant conclusion can be reached. None of the SLK rejections were greater than Banff 1a. The incidence of liver allograft rejection was 13%, compared to 30% in the LTA cohort performed over the same time interval.
Figure 2.
A)Freedom from acute cellular rejection in the SLK cohort (gray) and the deceased donor renal transplant cohort (black). All episodes of rejection were biopsy proven and scored based on the Banff 2007 criteria. The SLK cohort had significantly greater freedom of acute cellular rejection of 93% v. 72% in the KTA cohort at 3 years (p=0.01). All episodes of ACR in the SLK cohort were classified as a Banff 1a.
B)Freedom from acute humoral rejection in SLK (gray) and kidney transplant alone (black). All biopsies were stained for C4d and class I and II donor-specific antibody levels were acquired. The SLK cohort had a greater freedom from acute humoral rejection of 96% vs. 78% in the KTA cohort, p=0.07. All of the rejection episodes in the SLK group had only focal C4d staining.
SLK Transplantation In Sensitized Patients
Recent data from Singh et al. demonstrated that sensitized KTA recipients with detectable pre-transplant DSA with a strength of MFI >100 for Class I and MFI >500 for Class II have worse graft outcomes and lower freedom from AMR, ACR or transplant glomerulopathy (TG) (4). Sensitized SLK recipients were transplanted with pre-transplant DSA of 6,305.8±5,848 for Class I and 8,452±6,374 for Class II. In contrast, the sensitized KTA were transplanted with a pre-transplant DSA and MFI of 1,981.6±2,959 for Class I and 5,842±6,739 for Class II.
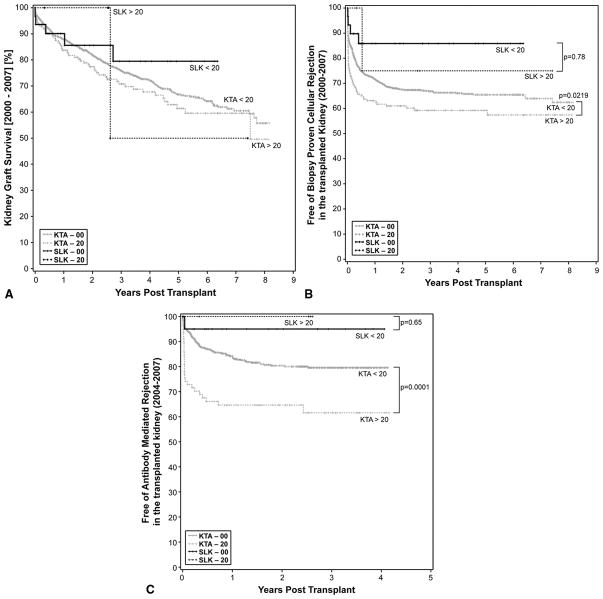
When we compared how the level of sensitization affected just the KTx outcomes in SLK patient outcomes, there was no difference at one year in renal graft outcomes between the non-sensitized (nsSLK) and sensitized cohorts (sSLK; definition of “s” is PRA >20% pre-transplant) (90% vs. 100%, p=0.78) (Figure 3A). Patient survival was not significantly different between the sSLK and either the nsSLK or sKTA group [100% vs. 97% (n=0.65) and 100% vs. 92% (p=0.87)]. Similarly, when we compared the sensitized (sSLK) cohort to the non-sensitized (nsKTA; “ns” defined as PRA <20%) group, there was no difference in KTx survival, demonstrating that the outcomes in SLK, regardless of the level of sensitization, were not affected by pre-transplant PRA (Figure 3A).
Figure 3.
A) Renal allograft survival based on pre-transplant Panel Reactive Antibody (PRA). No differences were seen among the four different groups. When all cohorts were compared to the highly sensitized kidney transplant alone subgroup, graft survival was statistically significantly worse in the kidney transplant alone sub-group with PRA >20% (p=0.02).
B) Incidence of acute cellular rejection stratified by panel reactive antibody (PRA). Ns= no-sensitized (PRA <20%). s= sensitized (PRA>20%). No significant differences were seen when both SLK sub-groups ere compared (p=0.16). In contrast, ACR was significantly reduced in the sKTA group (61%) when compared to the nsKTA (69%) (p=0.02). Freedom from ACR in the nsSLK was 87% v. 69% in the nsKTA cohort (p=0.07). There was a trend toward significance between the sSLK and nsKTA with a freedom from ACR of 80% vs. 68% (p=0.5). In comparing sKTA vs. nsSLK, freedom from ACR was 60% vs. 87% (p=0.0128).
C) Incidence of acute humoral rejection stratified by PRA. Ns=non-sensitized (PRA< 20%). s= sensitized (PRA> 20%). No significant difference was noted when both SLK sub-groups were compared (p=0.65). In contrast, freedom from AMR in sKTA (63%) is significantly reduced when compared to nsKTA (82%) (p=0.0001). Freedom from AMR in nsSLK was 95% vs. 82% in the nsKTA cohort (p=0.17). There was a trend toward significance between sSLK and nsKTA with a freedom from ACR of 0% vs. 82% (p=0.36), reflecting a beta error. Freedom from AMR in nsSLK was statistically significantly greater when compared to the sKTA sub-group (p=0.01).
Freedom from treatment for acute rejection was significant between the sensitized and non-sensitized KTA cohorts at three years (59% vs. 67%, p=0.02) (Figure 3B). nsSLK (PRA <20%) patients had a higher degree of freedom from acute rejection when compared to nsKTA, which showed a trend toward statistical significance at three years (86% vs. 67%, p=0.067) (Figure 3B). This lack of statistical significance likely reflects the small sample size. Moreover, sSLK also had a higher degree of freedom from acute rejection when compared to nsKTA, but this difference was not statistically significant at three years (75% vs. 67%, p=0.55).
When sKTA was compared to nsSLK, the SLK cohort had a significantly improved freedom from acute rejection at three years (59% vs. 86%, p=0.013), as previously described (5–7).
T-Cell and Antibody-Mediated Rejection of KTx in the Study Cohorts
The types of rejection were segregated between ACR and AMR. Freedom from KTx AMR in nsSLK and sKTA was statistically different, favoring the nsSLK cohort at three years (97% vs. 68%, p=0.004) (Figure 3C). In sKTA, there was a 29% incidence of AMR in the first year vs. 0% in the SLK cohort (p=0.18). Though these data only trended toward significance, the small sample size likely affected the outcome of this analysis.
DISCUSSION
SLK transplantation is a life-saving intervention for patients with both renal and hepatic failure. It is clear from multiple studies in the literature that the benefit of SLK is only seen in patients with >8 weeks of dialysis (6–9). These data are based solely on analysis of graft survival curves, but do not address those patients with intrinsic renal disease. There are therefore three groups of patients who need to be considered for this discussion: 1) patients with liver failure and renal failure secondary to hepatorenal syndrome; 2) patients with liver failure and renal failure secondary to intrinsic renal disease not yet on dialysis; and 3) patients with liver failure and renal failure secondary to intrinsic renal disease on dialysis. Each of these groups must be considered separately, not only from the standpoint of patient and graft survival benefit, but also from an immunologic standpoint, as sequential renal transplantation following liver transplantation has been shown to have worse outcomes vs. SLK (10–11). Our data supports the idea that SLK can be performed with equivalent graft and patient outcomes regardless of the level of preoperative sensitization. This is the first study in the literature to address this immunologic component.
Outcomes
A recent registry review has asserted that the renal transplant waiting list is being disadvantaged by the use of renal allografts in SLK patients (1). In addition, Lonze et al. recently showed that the five-year renal allograft survival in kidney after heart recipients and kidney after lung recipients was inferior to matched controls (12). Although this inferior outcome is likely due to the overall health and additional co-morbidities of the recipient, it is clear that the renal allograft provides a survival benefit vs. dialysis. These are the same conclusions that can be reached regarding simultaneous liver and kidney transplantation. Our center’s data clearly demonstrates that a large UNOS review does not represent individual center results. In our cohort of SLK recipients, the renal graft outcomes have graft and patient survival equal to that of >1,200 KTA recipients. These data are even more striking given the fact that SLK patients tend to be sicker, with an average MELD of 27 vs. 20.3±8.24 in our LTA cohort.
It could be argued that the results in our series are biased by a lower MELD score compared to the national average for SLK recipients at the time of transplantation. In order to evaluate the impact of MELD score on patient, liver and kidney graft survival in the SLK population, a sub-analysis was performed, dividing the population into two subgroups based on a MELD score below or above 30 at the time of transplantation. Though the conclusions may be limited by the number of patients in the analysis, in 28 SLK recipients with a MELD score of ≥30 and 8 recipients with a MELD score above 31, patient, kidney and liver graft survival were not different between the two groups (p=0.83, 0.95 and 0.98). This suggests that, even in geographical areas in which the average MELD score is higher at the time of transplantation than at our institution, patient and graft survival could be expected to be equivalent.
In the pre-MELD era, SLK were associated with a high morbidity and mortality, especially in patients over the age of 60 (13), but post-MELD results do not recapitulate these poor outcomes in our series as well as large registry reviews. The key component in this discussion is the long-term outcome of LTA patients on dialysis. Dellon’s data demonstrate that 1-year patient survival is far worse in patients who receive LTA while on dialysis or with a serum creatinine >2.0 mg/dl compared to SLK transplantation (51% vs. 67%). These data become even more skewed toward supporting SLK if age is used as a factor (13;14).
With regard to hepatorenal syndrome, liver dysfunction reduces glomerular filtration rate (GFR) by as much as 50%; therefore, estimates of GFR may be clinically inaccurate. It is important to delineate the etiology of renal dysfunction. Recent UNOS studies have demonstrated that the use of GFR alone shows a crossover of only 1.5% to being listed for renal transplantation after liver transplantation in the first year. Therefore, a GFR <30 mL/min is not enough to qualify for SLK. If this were the only determining factor, the renal transplant waiting list would be disadvantaged. Time on dialysis remains important, and UCLA data clearly demonstrates that patients with end-stage liver disease and hepatorenal syndrome (HRS) have a survival benefit and a resource utilization benefit if they have been hemodialysis-dependent for at least 8 weeks prior to SLK transplantation (9). Moreover, there was no survival benefit if patients underwent SLK with either <8 weeks or no pre-transplant dialysis. In order to test this hypothesis in our liver transplant population, we compared liver transplant recipients with renal failure on dialysis without intrinsic renal disease and less than 8 weeks on dialysis to SLK recipients during the period between 2000 and 2007. MELD score in the liver transplant alone group (20.3±8.24) was statistically significantly lower when compared to recipients of SLK (p=0.04). However when patient and liver graft survival were compared between the two groups, no statistically significant differences were observed (p=0.63 and 0.95, respectively). These results compare favorably to previously published data in which the impact of renal function on patient and graft survival at the time of liver transplantation was clearly demonstrated using the SRTR database (20). For patients with severe renal failure (defined as glomerular filtration rate below 14±3.6 mL/min), the 1-, 2- and 5-year patient survival was 66%, 59% and 40%, respectively, compared to 90.6%, 88.6% and 82% for recipients of a liver alone with severe renal failure requiring dialysis; and 93.5%, 84.3% and 77%, respectively, for patients undergoing SLK. When assessment of liver graft survival was compared in both subgroups using the SRTR database, a greater graft survival was also observed. One- and two-year graft survival for patients with severe renal failure in the SRTR analysis was 62% and 53%, compared to 85% and 83%, respectively, for the groups undergoing liver transplantation alone with severe renal failure on dialysis at our institution, compared to a 90.9% and 82.8%, respectively, for the SLK group.
In addition, SLK is a useful intervention in patients with rapidly progressing HRS or type I HRS, but the outcomes in type 2 HRS are more ambiguous (15), likely due the slow deterioration of renal function which would lead one to postulate that the kidneys are more likely to have a chance of recovery after liver transplantation. In our cohort, patients were approved for SLK if they had a history of chronic renal disease or if liver failure led to concomitant renal failure and dialysis was initiated for at least 6 weeks prior to listing or transplantation.
With regard to patients with intrinsic renal disease on dialysis, there is no doubt that SLK is a better option than LTA with postoperative dialysis followed by sequential renal transplant. This approach maximizes both allograft and patient outcome and is a more equitable utilization of resources. Few would argue with this approach, as these patients would be placed on the renal waiting list regardless of their liver disease. Moreover, there are fewer immunologic concerns in SLK than if one were to receive an LTA with sequential KTA (10).
For patients with intrinsic renal disease who are not on dialysis, the data is clear that the risk of progression to stage 4 or 5 renal failure is quite low within the first year after transplantation (16). Moreover, there is only a 1.5% chance of crossover and listing for kidney transplantation within the first year following LTA. Therefore, there is no data to show that SLK in patients not on renal replacement therapy is of benefit. Of course, there are exceptions to every rule. If a patient presents with a calculated GFR <30 mL/min and a renal biopsy showing intrinsic renal disease, one could better predict the reversibility of the renal disease as recently demonstrated by Tanriover et al. We therefore support the use of native renal biopsy in cases that are ambiguous. In our own center, 1 of the 36 SLK transplants performed was in a patient not yet on dialysis, but this was a retransplant patient with chronic calcineurin toxicity. This patient’s current creatinine is 1.2 and the patient is maintained on full-dose tacrolimus without any incidence of graft rejection.
IMMUNOLOGIC CONCERNS
There is significant data in the literature demonstrating the protective effect of the liver in multi-organ transplantation. Similarly, when the kidney is combined with a heart transplant, the incidence of rejection and outcomes are also excellent (18). Transplantation of the LTx from the same donor as the KTx has been shown to result in improved KTx function and outcomes as demonstrated by a low rate of acute rejection episodes and no incidence of hyperacute rejection, even in the face of a positive solid-phase crossmatch (5;9;19). Similar findings were noted in our series, with a 13% incidence of acute rejection in the low-risk cohort and a 21% incidence in the high-risk cohort vs. 30% in the KTA group. These data may suggest that the liver confers an immune modulating protection against not only ACR but also AMR in SLK recipients, particularly those who are highly sensitized. A retrospective review of our pre-transplant DSA did not predict long-term graft failure as it did in the KTA data (4), indicating that Luminex® MFI cutoffs for Class I and II DSA that would be prohibitive in KTA did not affect post-transplant renal function or the incidence of acute kidney or liver rejection. However, these observations should be interpreted cautiously, since we did not perform protocol biopsies of either graft. Therefore, the true incidence of rejection (i.e., subclinical) may be underestimated. Of our 36 patients, three of seven (43%) renal rejection episodes were C4d positive, even in the face of MFI Class II DSA >5,000. There were nine episodes of LTx rejection in this cohort, two focally positive for C4d and one diffusely positive for C4d. Interestingly, the latter patient died due to septicemia with both allografts functioning well. More important, these Lumine® DSA MFI levels were ten-fold higher than the highest KTA recipient in the comparative group, making this sSLK cohort immunologically impossible to transplant with an isolated renal allograft. To summarize, the presence of C4d or AMR in these patients does not seem to follow the same course as AMR in the KTA cohort. As previously stated, although the mechanism of immune modulation has not been well described, clearly the presence of the LTx in sSLK T recipients modifies the effect of DSA on the kidney allograft. Therefore, our data suggest that the presence of pre-transplant DSA should not be a deterrent to SLK in sensitized patients. We are in the process of investigating this observation further.
Simultaneous liver and kidney transplantation remains a viable option for patients suffering from multiple organ failure. Clearly, the true benefit of SLK is seen in patients on renal replacement therapy for at least 8 weeks. There is conclusive evidence that in the absence of organic renal disease, SLK is not beneficial and only LTA should be offered.
Since the introduction of the MELD score, more SLK transplants have been performed, both in absolute and relative terms, reducing the number of kidneys available and increasing the waiting time for patients in the kidney transplant waiting list. Of course, this problem does not pertain only to SLK recipients but also to combined lung-kidney, combined heart-kidney, and simultaneous pancreas kidney transplantation.
Given the increasing size of the kidney transplant waiting list, is it appropriate that kidneys be allocated for combined transplantation, especially when they will be of a superior quality than much of the remaining (kidney) transplant pool? (21). This quandary is further complicated by the fact that proceeding with kidney transplantation after liver transplantation (KALT) does not lead to be the best utilization of resources based on a lower half life of KALT when compared to SLK (6.6±0.9 vs. 11.7±1.3 years; p<0.001) (22).
We recognize that this is a dilemma faced by transplant programs around the country yet its analysis and potential solutions lay beyond the scope of our study. In conclusion, our outcomes clearly demonstrate that SLK did not disadvantage the expected graft and patient survival as compared to recipients of a KTA.
The incidence of ACR and AMR in the kidney transplant seems to be reduced in SLK compared to KTA regardless of the levels of preoperative PRA and their DSA. A high level of DSA should not preclude SLK when clinically indicated, as this is a life-saving procedure with excellent patient and graft survival. Further studies are required to understand whether the presence of DSA antibodies impacts the long-term expected graft survival of the liver and kidney allografts.
ABBREVIATIONS
- ACR
Acute cellular rejection
- AMR
Antibody-mediated rejection
- CDC
Complement-dependent cytotoxicity
- DSA
Donor-specific antibody
- ECOG
Eastern Cooperative Oncology Group
- ESKD
End-stage kidney disease
- GFR
Glomerular filtration rate
- HRS
Hepatorenal syndrome
- KTA
Kidney transplant alone
- KTx
Kidney transplant
- LTA
Liver transplantation alone
- MELD
Model for End-Stage Liver Disease
- MFI
Mean fluorescence intensity
- nsKTA
Non-sensitized kidney transplant alone
- nsSLK
Non-sensitized simultaneous liver-kidney transplant
- sKTA
Sensitized kidney transplant alone
- SLK
Simultaneous liver-kidney transplant
- sSLK
Sensitized simultaneous liver-kidney transplant
- TG
Transplant glomerulopathy
Footnotes
Steven Hanish conceived and designed the original study and contributed to the analysis of the data. He wrote the original manuscript and read and revised the manuscript in preparation. He provided final approval of the final version. Dr. Hanish received no supporting funds for this study has no conflict of interest to report in relationship to this study.
Milagros Samaniego contributed to the conception and design of the original study and to the analysis of the data. She substantially contributed to the revisions of several draft versions of the manuscript. She substantially contributed to the editorial changes in the final version. Dr. Samaniego received no supporting funds for this study has no conflict of interest to report in relationship to this study.
Joshua Mezrich read and critically revised the manuscript in preparation for important intellectual content. Dr. Mezrich received no supporting funds for this study has no conflict of interest to report in relationship to this study.
David Foley read and critically revised the manuscript in preparation for important intellectual content. Dr. Foley received no supporting funds for this study and has no conflict of interest to report in relationship to this study.
Glen Leverson conceived the original study and read and revised the manuscript in preparation for important intellectual content, providing statistical support and information and analysis of the data. Dr. Leverson received no supporting funds for this study has no conflict of interest to report in relationship to this study.
David Lorentzen provided tissue typing data. He conceived the original study and was involved in its original design, providing tissue typing information and substantially contributing to the interpretation of the data. He read and revised the manuscript in preparation. Mr. Lorentzen received no supporting funds for this study has no conflict of interest to report in relationship to this study.
Hans Sollinger read and critically revised the manuscript in preparation for important intellectual content. Dr. Sollinger received no supporting funds for this study has no conflict of interest to report in relationship to this study.
John Pirsch read and critically revised the manuscript in preparation for important intellectual content Dr. Pirsch received no supporting funds for this study and has no conflict of interest to report in relationship to this study.
Anthony D’Alessandro read and critically revised the manuscript in preparation for important intellectual content. Dr. D’Alessandro received no supporting funds for this study has no conflict of interest to report in relationship to this study.
Luis Fernandez contributed to the conception and design of the original study and to the analysis of the data. He substantially contributed to the revision of several drafts of the initial version of the manuscript and provided final approval of the most recent and final version. Dr. Fernandez received no supporting funds for this study has no conflict of interest to report in relationship to this study.
Contributor Information
Steven I. Hanish, The Emory Transplant Center, Division of Liver Transplantation, Emory University School of Medicine, 1364 Clifton Road NE, Box 7, Atlanta, GA 30322
Milagros Samaniego, Department of Medicine, Division of Nephrology, University of Michigan, 3914 Taubman Health Center, 1500 East Medical Center Drive, Ann Arbor, MI 48109
Joshua D. Mezrich, Department of Surgery, Division of Transplantation, University of Wisconsin School of Medicine and Public Health, 600 Highland Ave., H4/784 CSC, Madison, WI 53792-7375
David P. Foley, Department of Surgery, Division of Transplantation, University of Wisconsin School of Medicine and Public Health, 600 Highland Ave., H4/766 CSC, Madison, WI 53792-7375
Glen E. Leverson, Department of Surgery, University of Wisconsin School of Medicine and Public Health, 600 Highland Ave., H5/301 CSC, Madison, WI 53792-3236
David F. Lorentzen, Clinical Laboratories, University of Wisconsin Hospital and Clinics, 600 Highland Ave., D4/231 CSC, Madison, WI 53792
Hans W. Sollinger, Department of Surgery, Division of Transplantation, University of Wisconsin School of Medicine and Public Health, 600 Highland Ave., H4/780 CSC, Madison, WI 53792-7375
John D. Pirsch, Departments of Surgery and Medicine, Division of Transplantation, University of Wisconsin School of Medicine and Public Health, 600 Highland Ave., H4/772 CSC, Madison, WI 53792-7375
Anthony M. D’Alessandro, Department of Surgery, Division of Transplantation, University of Wisconsin School of Medicine and Public Health, 600 Highland Ave., H5/701 CSC, Madison, WI 53792-7375
Luis A. Fernandez, Department of Surgery, Division of Transplantation, University of Wisconsin School of Medicine and Public Health, 600 Highland Ave., H4/780 CSC, Madison, WI 53792-7375
References
- 1.Locke JE, Warren DS, Singer AL, et al. Declining outcomes in simultaneous liver-kidney transplantation in the MELD era: ineffective usage of renal allografts. Transplantation. 2008;85(7):935–942. doi: 10.1097/TP.0b013e318168476d. [DOI] [PubMed] [Google Scholar]
- 2.Racusen LC, Halloran PF, Solez K. Banff 2003 meeting report: new diagnostic insights and standards. Am J Transplant. 2004;4(10):1562–1566. doi: 10.1111/j.1600-6143.2004.00585.x. [DOI] [PubMed] [Google Scholar]
- 3.Ormonde DG, de Boer WB, Kierath A, et al. Banff schema for grading liver allograft rejection: utility in clinical practice. Liver Transpl Surg. 1999;5(4):261–268. doi: 10.1002/lt.500050418. [DOI] [PubMed] [Google Scholar]
- 4.Singh N, Pirsch J, Lorentzen D, et al. Donor-specific antibodies (DSA) detected by single antigen bead assay (Luminex) as predictors of kidney transplant (KT) outcomes. Am J Transplant. 2008;8(2):273. (published abstract) [Google Scholar]
- 5.Creput C, Durrbach A, Samuel D, et al. Incidence of renal and liver rejection and patient survival rate following combined liver and kidney transplantation. Am J Transplant. 2003;3(3):348–356. doi: 10.1034/j.1600-6143.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez-Banos J, Portillo J, Ballestero R, et al. Renal graft outcome in simultaneous kidney transplantation combined with other organs: experience of a single center. Transplant Proc. 2008;40(10):3424–3427. doi: 10.1016/j.transproceed.2008.06.111. [DOI] [PubMed] [Google Scholar]
- 7.Hadaya K, Ferrari-Lacraz S, Giostra E, et al. Humoral and cellular rejection after combined liver-kidney transplantation in low immunologic risk recipients. Transpl Int. 2009;22(2):242–246. doi: 10.1111/j.1432-2277.2008.00775.x. [DOI] [PubMed] [Google Scholar]
- 8.Mosconi G, Scolari MP, Feliciangeli G, et al. Nephrological indications in combined liver-kidney transplantation. Transplant Proc. 2006;38(4):1086–1088. doi: 10.1016/j.transproceed.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz R, Kunitake H, Wilkinson AH, et al. Long-term analysis of combined liver and kidney transplantation at a single center. Arch Surg. 2006;141(8):735–741. doi: 10.1001/archsurg.141.8.735. [DOI] [PubMed] [Google Scholar]
- 10.Demirci G, Becker T, Nyibata M, et al. Results of combined and sequential liver-kidney transplantation. Liver Transpl. 2003;9(10):1067–1078. doi: 10.1053/jlts.2003.50210. [DOI] [PubMed] [Google Scholar]
- 11.Simpson N, Cho YW, Cicciarelli JC, et al. Comparison of renal allograft outcomes in combined liver-kidney transplantation versus subsequent kidney transplantation in liver transplant recipients: analysis of UNOS database. Transplantation. 2006;82(10):1298–1303. doi: 10.1097/01.tp.0000241104.58576.e6. [DOI] [PubMed] [Google Scholar]
- 12.Lonze BE, Warren DS, Stewart ZA, et al. Kidney transplantation in previous heart or lung recipients. Am J Transplant. 2009;9(3):578–585. doi: 10.1111/j.1600-6143.2008.02540.x. [DOI] [PubMed] [Google Scholar]
- 13.Dellon ES, Galanko JA, Medapalli RK, Russo MW. Impact of dialysis and older age on survival after liver transplantation. Am J Transplant. 2006;6(9):2183–2190. doi: 10.1111/j.1600-6143.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- 14.Gonwa TA. Combined kidney liver transplant in the MELD era: where are we going? Liver Transpl. 2005;11(9):1022–1025. doi: 10.1002/lt.20454. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz R, Barri YM, Jennings LW, et al. Hepatorenal syndrome: a proposal for kidney after liver transplantation (KALT) Liver Transpl. 2007;13(6):838–843. doi: 10.1002/lt.21149. [DOI] [PubMed] [Google Scholar]
- 16.Davis CL. Impact of pretransplant renal failure: when is listing for kidney-liver indicated? Liver Transpl. 2005;11(11 Suppl 2):S35–44. doi: 10.1002/lt.20617. [DOI] [PubMed] [Google Scholar]
- 17.Tanriover B, Mejia A, Weinstein J, et al. Analysis of kidney function and biopsy results in liver failure patients with renal dysfunction: a new look to combined liver kidney allocation in the post-MELD era. Transplantation. 2008;86(11):1548–1553. doi: 10.1097/TP.0b013e31818b22cc. [DOI] [PubMed] [Google Scholar]
- 18.Bertelli R, Nardo B, Cavallari G, et al. Kidney transplantation combined with other organs in Bologna: an update. Transplant Proc. 2008;40(6):1867–1868. doi: 10.1016/j.transproceed.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Mosconi G, Scolari MP, Feliciangeli G, et al. Combined liver-kidney transplantation with preformed anti-HLA antibodies: a case report. Transplant Proc. 2006;38(4):1125–1126. doi: 10.1016/j.transproceed.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 20.Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35 (5):1179. doi: 10.1053/jhep.2002.33160. [DOI] [PubMed] [Google Scholar]
- 21.Andrews P. Combined heart and kidney transplantation. British Journal of Cardiology. 2002;9 (9):501. [Google Scholar]
- 22.Simpson N, Cho YW, Cicciarelli JC, Selby RR, Fong TL. Comparison of renal allograft outcomes in combined liver-kidney transplantation versus subsequent kidney transplantation in liver transplant recipients: Analysis of UNOS Database. Transplantation. 2006;82 (10):1298. doi: 10.1097/01.tp.0000241104.58576.e6. [DOI] [PubMed] [Google Scholar]